Chapter 30 Percutaneous Dilational Cricothyrotomy and Tracheostomy
I. Definitions and Classifications of Percutaneous Cricothyrotomy and Tracheostomy
IV. Indications and Contraindications for Percutaneous Dilational Cricothyrotomy and Tracheostomy
V. Percutaneous Dilational Cricothyrotomy
VI. Percutaneous Dilational Tracheostomy
VII. Postoperative Considerations
I Definitions and Classifications of Percutaneous Cricothyrotomy and Tracheostomy
A Cricothyrotomy and Percutaneous Dilational Cricothyrotomy
Cricothyrotomy is a technique for providing an opening in the space between the anterior inferior border of the thyroid cartilage and the anterior superior border of the cricoid cartilage for the purpose of gaining access to the airway. This area is considered to be the most accessible part of the respiratory tree below the glottis.1–6
Cricothyrotomy can be classified in several ways. Based on the urgency of the clinical situation, the procedure has been classified as emergent or elective. Emergent cricothyrotomy may be done in the prehospital setting, emergency room, intensive care unit (ICU), or operating room. Elective cricothyrotomy is usually done before surgery in the operating room. It also may be performed in critically ill patients in the ICU at the bedside.7 Depending on the technique used, the procedure may also be classified as nonsurgical or surgical. The nonsurgical approach can be achieved by needle puncture or percutaneously over a guidewire, with or without a cricothyroid membrane (CTM) incision.8
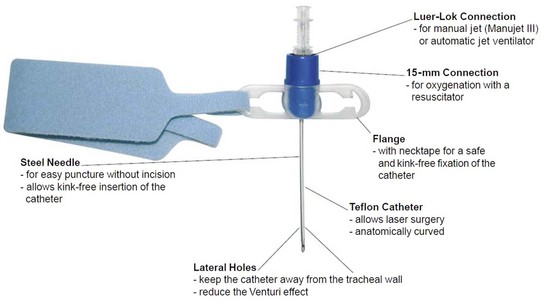
A practical and clinical classification of cricothyrotomy techniques includes three categories. The first category includes techniques that use a needle or over-the-needle catheter placed directly into the cricothyroid space. The needle technique is used for transtracheal catheter ventilation or, more properly, transcricoid ventilation.9 The cricothyrotomy needle (Fig. 30-1) and the Ravussin cannula (Fig. 30-2) are examples of these devices. Transtracheal catheter ventilation cannulas are also available, but they are inserted as described for the second category (Fig. 30-3).
The third category is surgical cricothyrotomy, which involves the use of a scalpel and other surgical instruments to create an opening between the skin and the cricothyroid space. It is discussed in Chapter 31.
B Tracheostomy and Percutaneous Dilational Tracheostomy
1 Open Tracheostomy
Surgical tracheostomy, as described by Chevalier Jackson,10 is a surgical procedure that provides an airway through the cervical trachea. It remains the standard against which all other procedures with the same aim must be compared in terms of success and complications rates. Classically, the procedure is performed in the operating room under general or local anesthesia, as dictated by the clinical situation. An open tracheostomy may be performed at the bedside in the ICU or in the emergency room in urgent situations. After an initial skin incision, sharp dissection is carried out to the thyroid isthmus, which is divided. The cervical trachea is then incised and a tracheostomy tube inserted.
2 Percutaneous Tracheostomy
Percutaneous tracheostomy is performed by means of a skin puncture into the trachea that is subsequently dilated to form a stoma, rather than creating a stoma by surgical incision.11 Although there are many techniques for performing a percutaneous tracheostomy, the initial part of the procedure always involves a puncture through the skin into the trachea, which is then enlarged by dilation or with forceps to spread the puncture to a size that allows placement of an appropriate tracheostomy tube. These techniques are typically used in patients with an established airway (i.e., endotracheal tube [ETT] or laryngeal mask airway [LMA]) and are mostly used for intubated ICU patients.
Emergency situations were traditionally considered absolute contraindications to the use of this technique, but in the past few years, some reports have supported its safety and feasibility in selected emergent cases.12,13 The Ciaglia technique, first described in 1985, is the original technique now described as percutaneous dilational tracheostomy (PDT). It involves making a very small skin incision, introducing a needle into the trachea, and dilating the opening with sequentially larger dilators to allow insertion of a tracheostomy tube of the selected size. As originally described, this procedure was performed blind, but it is increasingly performed under continuous endoscopic guidance.
3 Percutaneous Dilational Cricothyrotomy and Tracheostomy in Airway Control
a the Problem of Airway Control
Adverse outcomes related to respiratory events account for one of the two largest classes of injury in the American Society of Anesthesiologists (ASA) Closed Claims Project. As reported by Caplan and colleagues and Cheney and associates, the two major categories of anesthesia-related events or mechanisms causing death or brain damage between 1975 and 2000 were respiratory and cardiovascular difficulties, which together made up 68% of damaging events.14,15 Three mechanisms of injury were responsible for most of the adverse respiratory events: difficult ETT placement (23%), inadequate ventilation (22%), and esophageal intubation (13%).
In an analysis of claims against the National Health System in England between 1995 and 2007,16 airway and respiratory claims accounted for 12% of anesthesia-related claims, 53% of deaths, 27% of cost, and 10 of the 50 most expensive claims in the dataset. These claims most frequently described events at induction of anesthesia, involved airway management with a tracheal tube, and typically led to hypoxia and the patient’s death or brain injury.
In the operating room, in the ICU (or in other hospital areas), and in the prehospital setting, three difficult scenarios have been repeatedly observed during attempts to control the airway: (1) the airway can be easily controlled by mask ventilation, but endotracheal intubation is not possible; (2) the airway cannot be mask ventilated but can be intubated; and (3) rarely, the airway cannot be mask ventilated or intubated. It is every anesthesiologist’s nightmare to encounter a true difficult airway as depicted in the third scenario.17 Five to 35 of 10,000 patients (0.05% to 0.35%) reportedly cannot be endotracheally intubated, and approximately 0.01 to 2.0 of 10,000 patients are difficult to mask ventilate and intubate.18,19
b Roles of the Anesthesiologist, Otolaryngologist, and Emergency Medicine Physician
The anesthesiologist may be called immediately or after other physicians have attempted unsuccessfully to secure the airway or failed to recognize the futility of standard intubation techniques. In rare instances, the availability of a physician skilled in the technique of cricothyrotomy or percutaneous tracheostomy may be life-saving. This individual should be the anesthesiologist. Appropriate equipment for cricothyrotomy should be available throughout the hospital or as part of an emergency airway kit. No data exist regarding how frequently anesthesiologists are called to secure an airway outside the operating room, but in many hospitals, this responsibility seems to be handled more frequently by other physicians.20–23
Each institution should have a clear plan for alerting qualified individuals when emergency airway support is required in different areas of the hospital.24 Many publications describe the use of various types of advanced airway equipment, report the availability of such devices, and explain the use of simulators to teach difficult airway management skills, and many articles identify the need to educate residents in advanced airway techniques.
Organizing resources and staff to manage a difficult airway and maintaining appropriate training are important for patient safety and clinical quality.25,26 In 2000, Showan and Sestito proposed that the components of a successful airway management system include personnel, training, an emergency response system, an oversight process, standardized equipment, and patient education. As with any type of emergency, preparedness is the key when planning for response.27–30
In 1996, a comprehensive airway program was introduced at Johns Hopkins.26 The core components of the comprehensive difficult airway program were communication and electronic medical record information (including airway documentation), equipment, personnel, and education. Investigators based their implementation on the causes that required a surgical airway and the inability of an anesthesiologist to intubate and ventilate. The causes were an inability to access the written medical record, resulting in a lack of preoperative information about the patient’s airway; lack of immediate access to equipment and supplies necessary to manage a difficult airway; and lack of availability of trained personnel to help manage and secure the airway.
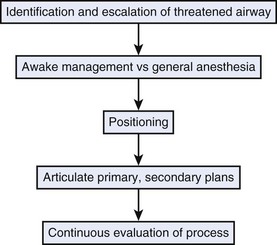
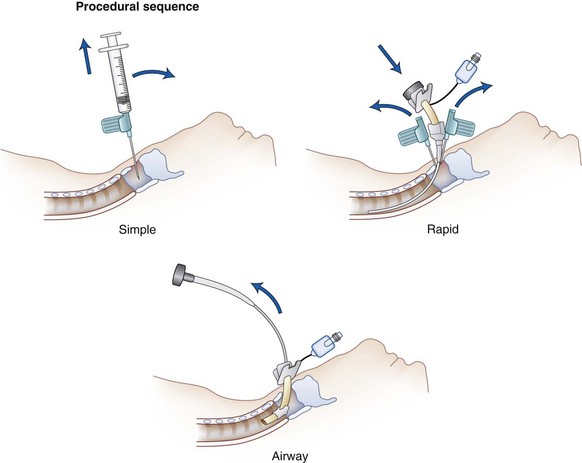
A threatened airway protocol has been proposed for implementing an escalation-based model at The University of Texas Medical School at Houston.31 The model is based on seven general principles to guide physicians in the identification and management of situations in which hospitalized patients may have rapid deterioration of a condition affecting the upper airway that requires immediate intervention to maintain or reestablish ventilation and oxygenation. These seven principles are concerned with appropriate communication among providers, maintenance of oxygenation, avoidance of sedation until the patient is in a safe environment, complete airway assessment, maintenance of spontaneous ventilation as long as possible, and avoidance of rapid-sequence induction (i.e., administration of a muscle relaxant without a prior attempt to ventilate), unless an easy airway and a full stomach are expected. Four main features constitute the cornerstones of management of a patient with a threatened airway: identification of the airway emergency and escalation of the approach to management, choice of appropriate sedation or anesthesia technique, positioning, and articulation of plans for intervention. A progressive algorithm (Fig. 30-4) that guides the progression of the necessary steps has been inspired by others’ work.31,32 The use of specific airway devices or tools is mandated in the primary and secondary plans by the success in securing the airway or depending on changes in airway viability (Fig. 30-5).
The otolaryngologist plays a critical role in airway management by contributing a skill set that is different from but complementary to that of the anesthesiologist. Circumstances may range from well-controlled elective situations to near-panic, last-ditch attempts to establish an airway when all else has failed.33 The otolaryngologist possesses an excellent knowledge of the three-dimensional anatomy of the upper aerodigestive tract and the variations encountered in pathologic circumstances. This knowledge and expert endoscopy skills can assist the anesthesiologist in determining a difficult airway.
II Historical Perspective
In 1909, Chevalier Jackson,10 a laryngologist at the Jefferson Medical School in Philadelphia, described the technical details of surgical tracheostomy and standardized the procedure. Jackson’s technique was for years considered the preferred method of surgical airway management. Jackson later published the results of 30 years’ observation of his own tracheotomized patients and reported a very high incidence of laryngeal and subglottic stenosis in patients who underwent a procedure that he referred to as high tracheostomy, involving division of the cricoid or thyroid cartilage.34 As a consequence, the high tracheostomy technique was abandoned for many decades.
In 1969, Toye and Weinstein described a technique for percutaneous tracheostomy based on the premise that a functional tracheal airway could be more rapidly and safely achieved percutaneously than with Jackson’s method of surgical dissection.35 The technique involved inserting a needle into the trachea and dilating the resultant needle tract to allow placement of a breathing catheter.
Cricothyrotomy, which differed from high tracheostomy because it involved opening of the CTM instead of dissection of the cricoid cartilage, was proposed again in 1976 by two Denver cardiothoracic surgeons, Brantigan and Grow. They published the results of 655 consecutive cricothyrotomies in which there were minimal complications and no reported incidence of subglottic stenosis. Subsequently, other clinical and experimental series have been reported, and cricothyrotomy has become generally accepted. The procedure was found to be faster, simpler, less invasive, and less likely to cause bleeding than tracheostomy. It is associated with lower morbidity and mortality rates than emergency tracheostomy, making it desirable as an emergency technique for gaining immediate airway control. Various modifications of the original technique have been developed. The use of the Seldinger technique for insertion, as described by Corke and Cranswick in 1988, enhances the safety of the procedure.8
The Ciaglia technique, first described in 1985, is the original technique now described as PDT. In this technique, insertion of the guidewire is followed by serial dilations performed with multiple, progressively larger dilators.36 The Rapitrach method, proposed in 1989 by Schachner and coworkers, entails the use of dilating forceps and a single-step dilating technique.37
In 1990, Griggs and colleagues presented the guidewire dilating forceps method, which was similar to the Rapitrach method and based on a one-step dilating techinque38 that used a modified forceps. In 1997, Fantoni proposed the translaryngeal tracheostomy technique based on retrograde dilation of an initial tracheal puncture by means of a conic cannula inserted through the oral cavity.39
The Ciaglia Blue Rhino, a modified version of the Ciaglia technique, was introduced by Cook Medical (Bloomington, IN) in 2000.40 In this technique, the series of sequentially larger dilators of the original Ciaglia technique is replaced by a single, curved dilator with a hydrophilic coating, the Blue Rhino, that progressively dilates the stoma in one step.
In 2002, Frova and Quintel described the Percutwist tracheostomy technique. A “rotating dilation” is performed in a single step by means of a screwlike, rotating device.41 In 2008, a further development of the Ciaglia technique was presented by Cook Medical: the Ciaglia Blue Dolphin balloon percutaneous tracheostomy introducer. This device combines balloon dilation and tracheal tube insertion into one step.
Many of the techniques proposed for PDC and PDT are performed over guidewires. Although anesthesiologists are familiar with the Seldinger technique for the insertion of vascular catheters, many are unacquainted with airway management techniques that use airway devices based on the same technology and concept.27,42
III Anatomy and Physiology
Safe and rapid performance of cricothyrotomy requires a thorough knowledge of cricothyroid space anatomy (Fig. 30-6) and its relation to other structures in the neck.1,6,43–47 The CTM ligament is 10 mm long and 22 mm wide and is composed mostly of yellow elastic tissue. It covers the cricothyroid space and is located in the anterior neck between the thyroid cartilage superiorly and the cricoid cartilage inferiorly. The cricothyroid space can be readily identified by palpating a slight dip or indentation in the skin immediately below the laryngeal prominence.
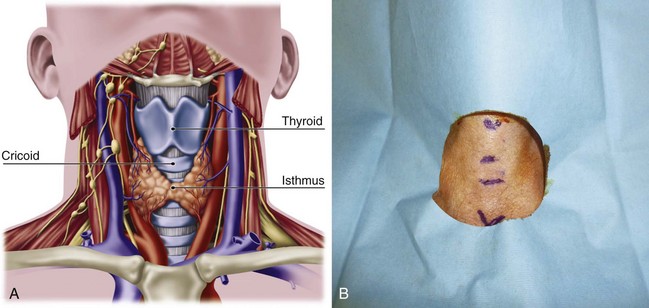
Figure 30-6 A, Dissection anatomy. B, External landmarks.
(A, From De Leyn P, Bedert L, Delcroix M: Tracheotomy: Clinical review and guidelines. Eur J Cardiothorac Surg 32:412–421, 2007.)
The CTM consists of a central anterior triangular portion (i.e., conus elasticus) and two lateral parts. The thicker and stronger conus elasticus narrows above and broadens below, connecting the thyroid to the cricoid cartilage. It lies subcutaneously in the midline and is often crossed horizontally in its upper third by the superior cricothyroid vessels. To minimize the possibility of bleeding, the CTM should be incised at its inferior-third portion. The two lateral parts are thinner, lie close to the laryngeal mucosa, and extend from the superior border of the cricoid cartilage to the inferior margin of the true vocal cords. On either side, the CTM is bordered by the cricothyroid muscle. Lateral to the membrane are venous tributaries from the inferior thyroid and anterior jugular veins. Because the vocal cords usually lie 1 cm above the cricothyroid space, they are not commonly injured, even during emergency cricothyrotomy.48 The anterior jugular veins run vertically in the lateral aspect of the neck and are rarely injured, but tributaries may occasionally course over the cricothyroid space and be damaged during the procedure. Characteristically, the CTM does not calcify with age and lies immediately underneath the skin.
Conscious effort to identify these landmarks reduces the possibility of committing this preventable error (see Fig. 30-6B). When the normal anatomy is distorted, identification of these landmarks is difficult. In these cases, the suprasternal notch may be used as an alternative marker. The small finger of the right hand should be placed in the patient’s suprasternal notch, followed by placement of the ring, long, and index fingers adjacent to each other in a stepwise fashion up the neck, with each finger touching the one below it. When the head is in the neutral position, the index finger is usually on or near the CTM.
IV Indications and Contraindications for Percutaneous Dilational Cricothyrotomy and Tracheostomy
A Cricothyrotomy
Cricothyrotomy is considered by many to be the standard approach to airway management when orotracheal or nasotracheal intubation and fiberoptic approaches have failed.5,18,49 In the emergency room or prehospital setting,50,51 cricothyrotomy is indicated for immediate airway control in patients with maxillofacial, cervical spine, head, neck, and multiple trauma and in patients in whom endotracheal intubation is impossible to perform or contraindicated. It is also used for the immediate relief of upper airway obstruction. In the operating room and in the ICU, the technique is indicated when conventional methods of intubation fail, such as in patients with traumatic facial injuries in whom other techniques of airway access are difficult or impossible to perform. Cricothyrotomy can also be used as an alternative to tracheostomy in patients with recent sternotomy who need airway access because the incision does not communicate with the mediastinal tissue planes. A needle-size cricothyrotomy with a Luer-Lok connection (for jet ventilation) or an anesthesia circuit–size connection is used for thoracic and other procedures involving the airways, especially the trachea, larynx, epiglottis, and base of the tongue.
Emergency cricothyrotomy has largely replaced emergency tracheostomy in the emergency department because of its simplicity, rapidity, and minimal morbidity, and percutaneous techniques are replacing surgical approaches.52,53 Use of emergency tracheostomy is limited and indicated only when laryngeal trauma may be accompanied by local edema, hemorrhage, subcutaneous emphysema, and damage to the thyroid or cricothyroid cartilage, precluding the performance of cricothyrotomy.
Cricothyrotomy is technically problematic to perform in the pediatric population and should be performed with extreme caution in children younger than 10 years. It should not be performed at all in children younger than 6 years unless a wire can be placed in the cricothyroid space and placement within the trachea can be verified.54 Emergency tracheostomy under controlled conditions is the preferred choice.55 Physicians who are unfamiliar or inexperienced with the technique are discouraged from performing the procedure without adequate supervision from a more senior or knowledgeable member of the medical team. Inexperience has been implicated as the most important factor contributing to cricothyroid complications.56–58 Accuracy in identifying anatomic landmarks significantly depends on the physician’s experience but is poor overall, justifying the percutaneous technique in emergency conditions but supporting the use of ultrasound or video-enhanced visualization during elective procedures.
B Percutaneous Dilational Tracheostomy
PDT is mainly indicated in adult intubated patients (Box 30-1). In this patient population, the main indications for performing a PDT are the same as those for surgical tracheostomy:
• Preventing upper airway damage due to prolonged intubation
• Facilitating pulmonary toilet
• Providing a stable airway in patients requiring long-term mechanical ventilation and oxygen support
Box 30-1 Indications and Contraindications to Percutaneous Tracheostomy in Intubated Adults in the Intensive Care Unit
Several benefits of performing a tracheostomy in patients who require prolonged ventilation have been postulated and are supported by different levels of evidence.59 Shorter ICU and hospital stays and less need for sedation are the most widely recognized benefits, whereas improved patient comfort, decreased work of breathing, improved oral hygiene, better long-term laryngeal function, faster weaning from mechanical ventilation, lower risk of ventilator-associated pneumonia, and lower mortality rates have also been reported but are supported by a lower level of evidence.59
For the population of critically ill adult patients, PDT has been recommended as the procedure of choice for performing elective tracheostomy.59,60 PDT is recommended on the basis of a lower risk of wound infection, being able to perform it at the bedside rather than transferring critically ill patients to the operating room, and better cost-effectiveness compared with surgical tracheostomy.60
Upper airway obstruction due to tumor, edema, infection, stenosis, or trauma represents the other major category of indications for tracheostomy. However, the overall safety of performing PDT in emergent situations and with unprotected airways is extremely controversial, and, in these conditions, the procedure should be reserved for selected patients and performed only by experienced providers.13 Anatomic suitability for this procedure must be determined preoperatively with the patient’s neck extended. Maximum neck extension increases the length of the cervical trachea and defines critical anatomic landmarks, such as the cricoid cartilage and sternal notch. A contraindication to the procedure is the inability to palpate the cricoid cartilage above the sternal notch. Similarly, the patient with a midline neck mass, high innominate artery, or large thyroid gland should undergo open surgical tracheostomy in the operating room. Coagulopathies should be corrected preoperatively. Ideally, the functional platelet count should be 50,000 or greater, and the international normalized ratio (INR) should be corrected to 1.5 or less. However, there have been reports of PDT safely performed in patients with severe thrombocytopenia.61
Patients requiring a positive end-expiratory pressure (PEEP) of 15 cm H2O or higher are at high risk for complications such as subcutaneous emphysema and pneumothorax, and when possible, the procedure should be postponed for these patients. PDT is relatively contraindicated in nonintubated patients with acute airway compromise and in the pediatric population. For airway compromise in nonintubated patients, the risks are related to the length of the procedure and the inability to perform the procedure under direct endoscopic visualization without an ETT. Reasons to avoid PDT in children include the different airway anatomy and dimensions and the technical difficulties of maintaining adequate ventilation with a bronchoscope within a small ETT. Selected cases may present an exception to these contraindications, depending on the experience of the providers.13
V Percutaneous Dilational Cricothyrotomy
A Principles and Planning
This chapter focuses on percutaneous dilational techniques. Surgical cricothyrotomy and transtracheal catheter ventilation are discussed elsewhere in Chapter 31.
PDC is fast and usually easy to perform, even on patients with short necks or with spinal injury. Cricothyrotomy may be performed for elective airway management in trauma patients with technically challenging neck anatomy in lieu of tracheostomy, because it does not require a surgeon’s skill to gain airway access and has fewer operative and postoperative complications.62–65 Several commercially available devices use this technology. These devices have in common the insertion of an airway catheter over a dilator, which is usually introduced over a guidewire. The guidewire is inserted through a needle or over-the-needle catheter (i.e., Seldinger technique) after making an initial skin incision. This technique, often used for the insertion of catheter introducer sheaths and central lines, is familiar to anesthesiologists. An airway over a dilator and guidewire is preferable because of the inherent safety of this technique and the ability to insert an airway of far greater diameter than the initial catheter.
The over-the-wire technique offers several advantages. Even if the over-the-needle or direct dilational technique, such as the Quicktrach, PCK, or the Melker military version, may be faster to perform, the reported difficulties and complications are greater. This is also true for the Nu-Trake device. Complications have included failure to gain airway access, multiple attempts at cannulation, mediastinal injury, pneumothorax, and severe bleeding. The wire-guided technique has the disadvantage of the wire kinking. Several clinical and cadaver-based studies have established the safety and efficacy of the percutaneous over-the-needle or -cannula, wire-guided technique.42,66–71 For some of the devices, their use, diffusion, and success seem to have been influenced by local availability, original country of manufacturing, preliminary animal studies, and marketing, despite scarce clinical evidence of efficacy.
B Insertion Techniques
1 Percutaneous Dilational Cricothyrotomy Device
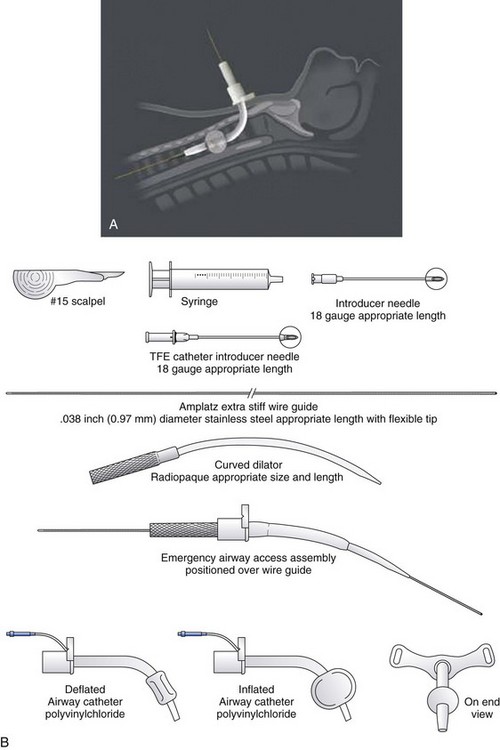
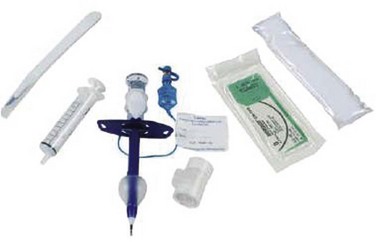
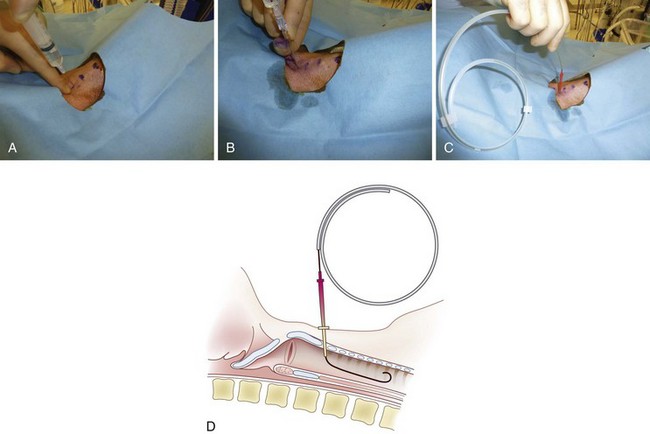
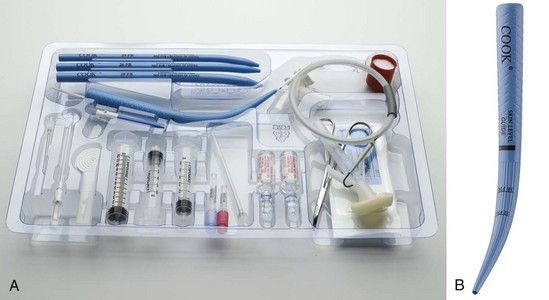
The PDC device manufactured by Melker contains a scalpel blade; a syringe with an 18-G over-the-needle catheter or a thin-walled introducer needle, or both; a guidewire; a dilator of appropriate length and diameter; and a polyvinyl airway catheter with or without a cuff (Fig. 30-7). A universal kit combines open cricothyrotomy and percutaneous tools in a single tray. (Although it defeats the concept of a percutaneous approach, it may be useful in remote or austere locations.) Detailed insertion instructions for this type of device are available from the manufacturer’s Website, brochure, and CD. A description of the Melker insertion technique (Fig. 30-8) follows:
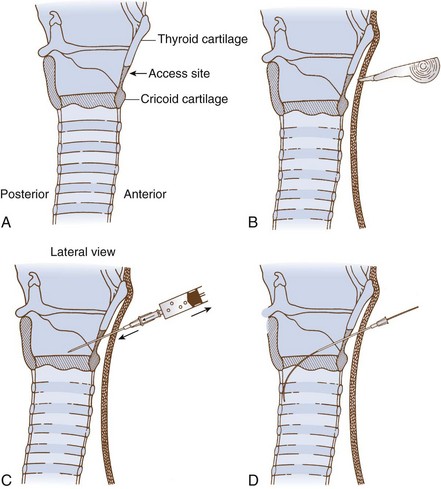
1. Position the patient supine, and if there is no contraindication, slightly extend the neck by using a roll under the neck or shoulders. If cervical spine injury is suspected, properly immobilize the head and neck, and maintain a neutral position.
2. Open the prepackaged cricothyrotomy set, and assemble the components. Whenever possible and appropriate, use aseptic technique and local anesthetic.
3. Identify the CTM between the cricoid and thyroid cartilages.
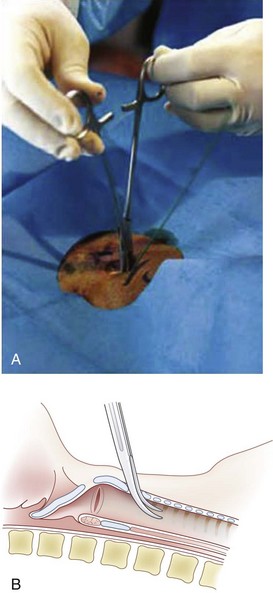
4. Carefully palpate the CTM, and while stabilizing the cartilage, make a vertical or horizontal skin incision using the scalpel blade (can also be performed after the Seldinger technique). Make a stab incision (vertical or horizontal) through the lower third of the CTM. An adequate incision eases introduction of the dilator and airway, but the incision can follow the placement of the guidewire.
5. Attach the supplied syringe to the 18-G introducer needle–plastic catheter (over the needle technique) system (same that you would use to place an angio-catheter), or alternatively attach the syringe to the introducer needle only (having removed the plastic catheter) if you prefer or are concerned the plastic catheter may kink. Insert the syringe-needle-catheter or syringe-needle only, and advance it through the incision into the airway at a 45-degree angle to the frontal plane in the midline in a caudad direction. When advancing the needle forward, entrance into the airway can be confirmed by aspiration with the syringe resulting in free air return or air bubbles in a saline-filled syringe.
6. Remove the syringe and needle, leaving the plastic catheter or introducer needle in place. Do not attempt to advance the plastic catheter completely into the airway, which may result in kinking of the catheter and an inability to pass the guidewire. Advance the soft, flexible end of the guidewire through the catheter or needle and several centimeters into the airway.
7. Remove the plastic catheter or needle, leaving the guidewire in place.
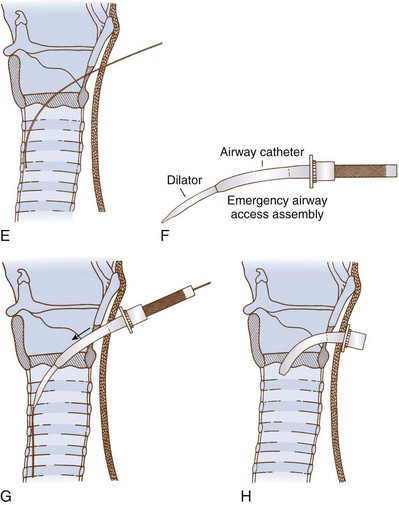
8. Advance the handled dilator inside the airway catheter (single dilation if a preincision was made), tapered end first, into the connector end of the airway catheter until the handle stops against the connector. With other sets, insert the dilator to the recommended depth, or insert the dilator over the guidewire for a preinsertion dilation (recommended if a preincision was not made). Use of lubrication on the surface of the dilator may enhance the fit and placement of the emergency airway catheter.
9. Advance the emergency airway access assembly over the guidewire until the proximal stiff end of the guidewire is completely through and visible at the handle end of the dilator. Always visualize the proximal end of the guidewire during the airway insertion procedure to prevent its inadvertent loss into the trachea. Maintaining the guidewire position, advance the emergency airway access assembly over the guidewire with an in-and-out motion.
10. As the airway catheter is fully advanced into the trachea, remove the guidewire and dilator simultaneously.
11. If a cuffed tube is inserted, inflate it with 10 mL of air with the syringe provided.
12. Fix the emergency airway catheter in place with the cloth tracheostomy tape strip in a standard fashion.
13. Using its standard 15- to 22-mm adapter, connect the emergency airway catheter to an appropriate ventilatory device.
5 Percutaneous Dilational Cricothyrotomy
a Arndt
The Arndt cricothyrotomy cannula (Cook Medical) is technically a percutaneous dilational wire-guided cannula designed for transtracheal jet ventilation (Fig. 30-9; see Fig. 30-3).
b Pertrach
The Pertrach (Fig. 30-10) is similar to the previously described devices, except that the guidewire and dilator are a single unit. The introducer needle must be split after the distal end of the guidewire is advanced so that the dilator can be introduced (Fig. 30-11). This is cumbersome, especially in emergency situations, and requires the guidewire and dilator to be advanced far down the airway. A study in cadavers showed equal success for PDC with the Pertrach and surgical cricothyrotomy. Surgical cricothyrotomy was faster, but it was impossible to predict whether bleeding complications would have been higher with the surgical cricothyrotomies. The manufacturer and the users have reported problems with the needle, which is occasionally difficult or impossible to split.
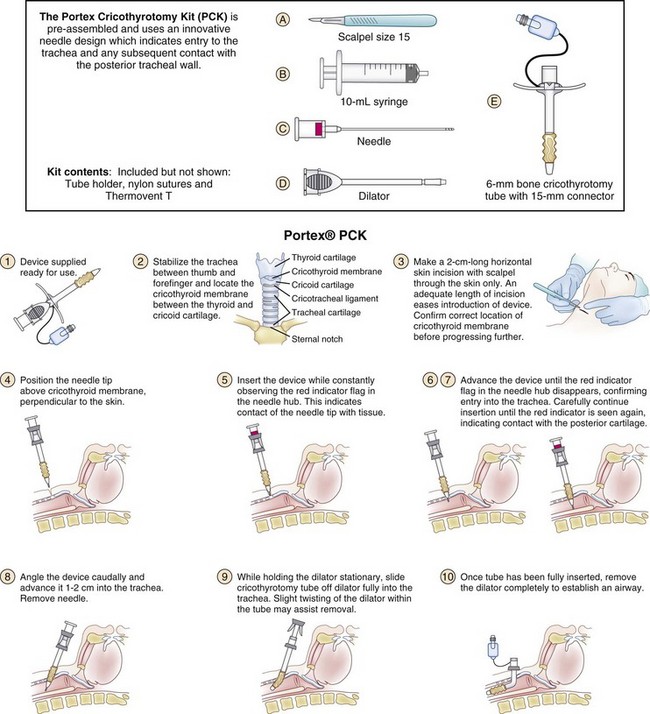
d Quicktrach and Portex Cricothyroidotomy Kit
The Quicktrach (Rüsch, VBM) (Fig. 30-12) and PCK (Portex) (Fig. 30-13) offer a single-step technique that is preceded by a skin incision, proceeds over a needle, and is not guided by a wire. The devices are technically faster to use but overall are less safe, carrying a higher complication rate (e.g., multiple attempts, inability to advance the cannula, false pas sage) than the Seldinger technique. The PCK (Fig. 30-14) has a Veress needle system, which is designed to detect pressure on the posterior wall of the trachea. The PCK is inserted directly through the CTM after a skin incision (Fig. 30-15).
VI Percutaneous Dilational Tracheostomy
A Principles and Planning
PDT is an accepted alternative to surgical tracheostomy, and it is gaining in popularity, particularly for patients in the ICU who have been intubated or are expected to need endotracheal intubation for extended periods.59,72–76 PDT is a mostly elective procedure, although there have been reports of PDT safely performed in selected emergent situations.13 Cricothyrotomy is a preferred route for emergent airway access.
Many kits are commercially available for this procedure. The most widely used are those based on the original Ciaglia technique (Fig. 30-16A) and subsequent modifications that led to the single-dilator kits. Included in this category is the Ciaglia Blue Rhino G2 Advanced Percutaneous Tracheostomy Kit (Cook Medical), the Portex ULTRAperc Single-Stage Dilator (Smith Medical), and the Ciaglia Blue Dolphin balloon percutaneous tracheostomy kit (Cook Medical).
B Insertion Techniques
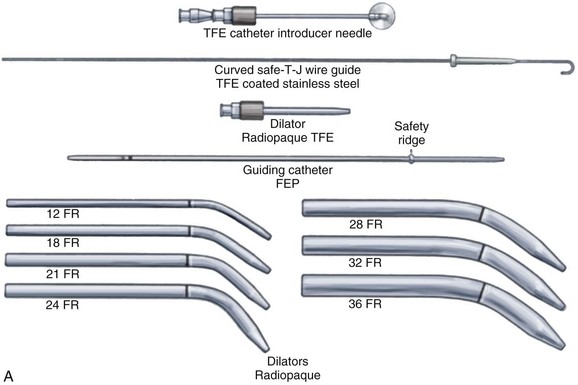
1 Seldinger Guidewire and Single-Dilator Kit
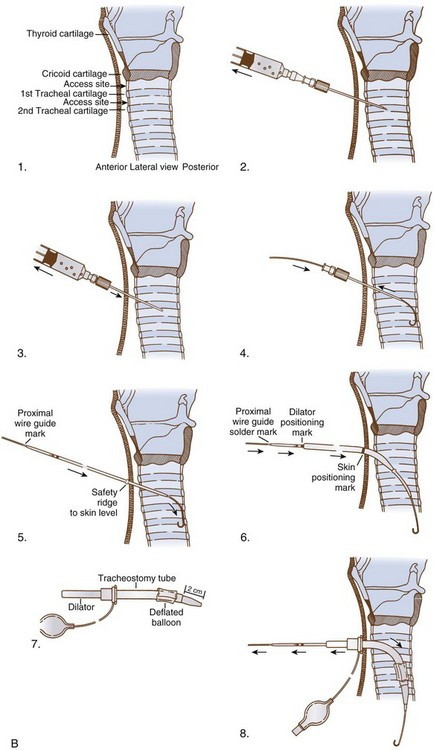
1. The patient is positioned as for conventional tracheostomy with the head extended on the neck, and anatomic landmarks are marked (see Fig. 30-6, B). A standard preparation and drape are applied.
2. The skin and subcutaneous tissues are infiltrated with 2% lidocaine and a 1 : 100,000 solution of epinephrine one or two finger breadths below the previously palpated and marked cricoid cartilage (Fig. 30-17, A).
3. A 1.5-cm horizontal skin incision is made, and the subcutaneous tissues are bluntly separated with a curved hemostat. No attempt is made to manipulate the strap muscles or thyroid gland.
4. At this point, the fiberoptic bronchoscope (FOB) is advanced until its tip is aligned with the lower margin of the ETT (advance in a supraglottic device until the cricoid is visualized). External manipulation and transtracheal illumination can facilitate structure recognition.
5. Any ties securing the ETT are loosened, and the FOB and ETT are withdrawn slowly in unison until the incision is maximally transilluminated.
6. The FOB, which may be connected to a monitor, is maintained in this position throughout the procedure, allowing direct visualization of every step.
7. The 16- or 17-gauge introducer needle is inserted between the first and second or second and third tracheal rings (see Fig. 30-17, B).
8. A midline intercartilaginous placement is verified bronchoscopically.
9. The needle is withdrawn, leaving the overlying catheter sheath through which the guidewire can be inserted (see Fig. 30-17, C and D).
10. The sheath is removed and replaced by a 14-F introducer dilator, which is advanced over the guidewire (several times); this maneuver enlarges the tracheal aperture sufficiently to allow easy placement of the 12-F guiding catheter.
11. The guiding catheter and guidewire are left in place and form the backbone over which the single dilator is used.
12. The single dilator with the hydrophilic coating moistened is advanced over this unit (see Fig. 30-16, B), several times if necessary, until resistance is minimal. The chosen depth of dilation also depends on the size of the cannula to be inserted.
13. The dilator is replaced by the preloaded tracheostomy tube, which is advanced into the trachea. Some resistance may be encountered at the interface between the dilator and tracheostomy tube.
14. The guidewire, guiding catheter, and dilator are removed and replaced by the inner cannula.
15. The ventilatory apparatus is connected to the tracheostomy, which is secured with four corner sutures.
16. When ventilation is adequate, the ETT is removed while examining the vocal folds. A postoperative chest radiograph is obtained to rule out a pneumothorax. In a patient with a large, thick neck, a longer tracheostomy tube should be used to prevent accidental displacement of the tube into the pretracheal soft tissue. In the event of accidental decannulation within 5 days of the procedure, the ICU staff is advised to reintubate the patient orally rather than attempt to reinsert the tracheostomy tube.
Precautions in the performance of this technique include the following:
• Always confirm access into trachea by air bubble aspiration.
• Maintain safety positioning marks of the guidewire, guiding catheters, and dilator during the dilating procedure to prevent trauma to the posterior wall of the trachea.
• Tracheostomy tubes should fit snugly to the dilator for insertion. Generous lubrication of the surface of the dilator enhances fit and placement of the tracheostomy tube.
2 Ciaglia Blue Rhino G2 Advanced Percutaneous Tracheostomy Kit
The Ciaglia Blue Rhino G2 Advanced Percutaneous Tracheostomy Kit (Cook Medical) (Fig. 30-18, A) has a curved dilator that is advanced over a guiding catheter and creates a tracheostomy opening in one pass, obviating the need for multiple dilators as with previous kits (see Fig. 30-18, B). The softness of the dilator, the hydrophilic coating, and the one-passage technique are the main advantages of this widely used percutaneous tracheostomy system, which has been at least as safe as the PDT techniques in multiple trials.40,77–81
3 Ciaglia Blue Dolphin Balloon Percutaneous Tracheostomy Kit
The Ciaglia Blue Dolphin Balloon percutaneous tracheostomy kit (Cook Medical) offers an improvement on the single-dilator PDT technique. This system combines balloon dilation and tracheal tube insertion in a single step (Fig. 30-19). The underlying principle is that balloon dilation should minimize pressure on the anterior tracheal wall and deliver an even and controlled radial dilation. Given the novelty of this device, data from the literature are insufficient to determine any advantage of this system compared with established methods and devices.
4 Portex ULTRAperc Single-Stage Dilator
The Portex ULTRAperc Single-Stage Percutaneous Dilation Tracheostomy Kit (Smiths Medical) is based on the widely accepted Seldinger guidewire technique. The kits are available with the Blue Line Ultra Suctionaid Tracheostomy Tube that features an integrated suction lumen for removal of pooled secretions (Fig. 30-20).
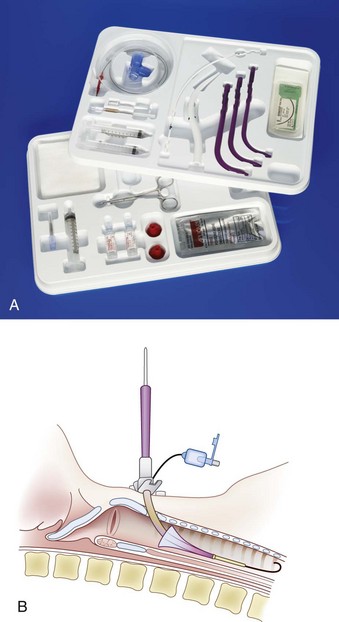
Figure 30-20 A, Portex ULTRAperc kit. B, Insertion of the ULTRAperc cannula with a handle introducer.
(A, Courtesy of Smiths Medical, Dublin, OH.)
In a study comparing the ULTRAperc device with the Blue Rhino in mannequin and porcine models, dilation with the ULTRAperc set was subjectively easier and required less force, and the time for tracheostomy tube insertion was shorter.82 The investigators suggest that the ULTRAperc set is subjectively easier to use, quicker, and causes less anterior-posterior tracheal compression during tracheostomy tube insertion compared with the Blue Rhino set in mannequin and porcine airway models. This advantage may be from the tracheostomy tube introducer in the ULTRAperc set that allows smooth passage of the tracheostomy tube through the dilated stoma.
5 Portex Griggs Percutaneous Dilation Tracheostomy Kit
The Portex Griggs Percutaneous Dilation Tracheostomy Kit (Smiths Medical) features guidewire dilating forceps that are central to the Griggs PDT technique (Fig. 30-21). Dilating forceps specially designed to slide along a prepositioned guidewire are used to open pretracheal tissue and the anterior tracheal wall in preparation for tube insertion. Use of the forceps causes less compression compared with the cone-shaped, single-stage dilators. In a prospective, randomized comparison of progressive-dilational versus forceps-dilational percutaneous tracheostomy,83 the progressive-dilational tracheostomy took longer, caused more hypercapnia, and caused more minor and major difficulties than forceps-dilational tracheostomy. We agree that the guidewire dilating forceps technique is safe and easy to learn, and it may be quicker than the progressive-dilational technique. Sometimes, the forceps cannot be inserted to the full length due to thick subcutaneous tissue of the anterior neck. Switching from forceps to a progressive dilator allows completion of the procedure without complications.31,84 The Portex Griggs Percutaneous Dilation Tracheostomy Kit also may be used in combination with Suctionaid tracheostomy tubes with an integral suction lumen to aid suctioning of secretions from above the cuff.
6 Percutwist
Increased control over the dilating maneuver from start to finish is one of the advantages of the Percutwist (Rüsch-Teleflex Medical) (see Fig. 46-19 in Chapter 46). Another is the possibility to lift the anterior tracheal wall, facilitating the endoscopic view of the dilation site during the procedure. Gradual application of the forces applied to produce dilation may increase the safety of this technique, although cases of posterior tracheal wall injury have been reported.78,85
7 Translaryngeal Tracheostomy Kit
The Translaryngeal Tracheostomy Kit (Mallinckrodt, Mirandola, Italy) is used mostly in European countries to perform translaryngeal tracheostomy according to the technique introduced by Fantoni in 1997. The critical steps of this procedure and use of this system are summarized in Figure 46-16 (see Chapter 46). Percutaneous tracheostomy has a learning curve and requires appropriate training. All studies comparing different methods should take into consideration potential differences in training of the personnel performing PDT with each proposed technique. Different environments and patient characteristics may dictate the choice of a specific technique or determine the preference for a specific system.
8 Controversies and Questions
a Use of Bronchoscopy and Ultrasound
Opponents argue that bronchoscopy increases the length and cost of the procedure and does not add to safety in experienced hands. Moreover, the presence of the bronchoscope within the ETT may result in CO2 retention and difficulty ventilating the patient. The accumulated weight of evidence in the literature points to the advantages of bronchoscopy for safety reasons.86,87 Nonetheless, a large meta-analysis was not able to demonstrate a clear advantage of bronchoscope-guided procedures in terms of decreasing overall mortality and major perioperative complications.88
Ultrasound has been proposed as a useful tool to identify the anatomy of airway, blood vessels, and other structures, such as the thyroid gland and isthmus. Ultrasound has in some cases replaced bronchoscopic guidance.89 However, a combination of the two (depending on resource availability) may offer better results. Whether ultrasound can reduce the incidence of tracheal-innominate fistulas (a rare but deadly complication) needs further investigation.90
b Patient’s Habitus
Accidental decannulation in these patients is more likely to occur because of the displacement of the tube in the abundant soft tissues. Using proximally extended tracheostomy tubes dramatically reduces the risk of this complication. Ben Nun and colleagues presented a case series of 154 critically ill adult patients in whom percutaneous tracheostomy using the Griggs technique was performed at the bedside.91 Eighteen of these patients had a short, fat neck as their only risk factor for PDT. Short, fat neck was defined as a neck circumference greater than 46 cm, with a distance between the cricoid cartilage and the sternal notch of less than 2.5 cm and distance between the cricoid cartilage and the pretracheal soft tissues of more than 2.5 cm. No complications were reported in this group. Heyrosa and coworkers reported their results for a series of 89 obese patients (body mass index [BMI] >35 kg/m2) undergoing PDT and 53 obese patients (same BMI) undergoing open tracheostomy, and they found the same complication rate (6.5%) for the two groups.92
Aldawood and colleagues performed PDT in 50 obese patients, mostly without bronchoscopic guidance.93 They reported an increased rate of major complications compared with nonobese patients (12% versus 2%, P = 0.04) but a similar rate of minor complications for the two groups.93 They defined obesity as a BMI of 30 kg/m2 or higher. The most frequent major complication was “procedure aborted, not otherwise specified,” but no surgical conversion, pneumothorax, or death occurred in either group. The investigators concluded that PDT could be performed safely in most obese patients.
In a prospective evaluation of endoscopic PDT in 500 consecutive intubated adults in the ICU, patients with a BMI of 30 kg/m2 or greater had a significantly greater (P < 0.06) number of complications (15%) than the patients (8%) with a BMI less than 30 kg/m2.94 This risk was even more significant for patients with a BMI of 30 or more who were also in ASA physical status class 4 (11 of 56 [20%]) (P < 0.02). Byhahn and colleagues reported an extremely high complication rate (43.8%) for 73 obese patients and found that obese patients (BMI >27.5 kg/m2) had a 2.7-fold increased risk for perioperative complications and a 4.9-fold increased risk for serious complications compared with nonobese patients.95 The researchers concluded that percutaneous tracheostomy in obese patients was associated with a considerably increased risk for perioperative complications, especially for serious adverse events. Comparison of the results and conclusions from these studies is problematic because of the different criteria used in defining obesity, the dissimilar primary and secondary end points, and the use of different percutaneous techniques for PDT.
VII Postoperative Considerations
A Cricothyrotomy
Cricothyrotomy is usually performed emergently to secure a difficult airway, but it can be performed electively.7 When cricothyrotomy is performed under less than ideal circumstances, it should be considered a temporary measure, and when the patient is stabilized, endotracheal intubation with or without an FOB or a tracheostomy should be performed. The FOB affords an opportunity to evaluate the airway, especially at the site of the cricothyrotomy. The cricothyrotomy site should be examined frequently for signs of infection, and all patients should have a careful neurologic and airway evaluation before discharge from the hospital to ensure that there has been no damage to the vocal cords or other proximate structures. There is no consensus of opinion on what work-up is necessary after emergency cricothyrotomy, but any complaints by the patient of difficulty swallowing or phonating should be carefully evaluated. Complication rates from properly performed emergent cricothyrotomy are acceptably low.
B Percutaneous Tracheostomy
1 Complications and Outcome Data
a Cricothyrotomy
The reported complication rate is 6% to 8% for elective cricothyrotomy and 10% to 40% for emergent procedures.96,97 The morbidity and mortality rates for elective cricothyrotomy are similar to those for elective tracheostomy. Boyd and colleagues found 10 complications (6.8%) in 147 cricothyrotomies, but no differentiation was made between elective and emergency procedures. In 1976, Brantigan and Grow reported a 6.1% complication rate for 655 cases, most of which were correctable and self-limited, and this compared favorably with the complication rate associated with tracheostomy. The same investigators implicated the presence of acute laryngeal pathology (especially from prolonged intubation before cricothyrotomy) as the predisposing factor in the subsequent development of subglottic obstruction.98
Adverse effects of cricothyrotomy can be categorized as those that occur early and those that occur late in the postoperative period. Early complications include asphyxia related to failure to establish the airway, hemorrhage, improper or unsuccessful tube placement, subcutaneous and mediastinal emphysema, prolonged procedure time, pneumothorax, and airway obstruction. Esophageal or mediastinal perforation, vocal cord injury, aspiration, and laryngeal disruption may also occur.99 Long-term complications include tracheal and subglottic stenosis (especially in the presence of preexisting laryngeal trauma or infection), aspiration, swallowing dysfunction, tube obstruction, tracheal-esophageal fistula, and voice changes.100 Voice change is the most common complication, occurring in up to 50% of cases.20 Voice problems include hoarseness, weak voice, or decreased pitch. The voice dysfunction may be caused by injury to the external branch of the superior laryngeal nerve, decreased cricothyroid muscle contractility, or mechanical obstruction related to narrowing of the anterior parts of the thyroid and cricoid cartilages.101 Infection, late bleeding, persistent stoma, and tracheomalacia have also been reported. Although subglottic stenosis is the most frequently reported major complication after cricothyrotomy,102 it is rare after tracheostomy. Pneumothorax and major blood vessel erosion are also associated with tracheostomy. Other complications associated with tracheostomy include mediastinal emphysema, accidental extubation, cardiac arrest, and death.
The complication rate for cricothyrotomy is higher in the pediatric population. Pneumothorax is the most common complication in children (5%to 7%) and is rarely seen in adults. Between 1% and 2% of adults develop subglottic stenosis after tracheostomy, compared with 2% to 8% of children. Children undergoing cricothyrotomy have a mortality rate up to 8.7%. Prehospital cricothyrotomy performed by emergency medical services (EMS) personnel carries a higher risk of morbidity than the in-hospital procedure. Spaite and Joseph reported an overall acute complication rate of 31% for 20 emergency patients. Failure to secure the airway accounted for the major complication rate (12%). Minor complications included right main stem intubation, infrahyoid placement, and thyroid cartilage fracture. Sixty surgical cricothyrotomies performed by trained aeromedical system personnel had a complication rate of 8.7%.103 These complications included significant hemorrhage or soft tissue hematoma and incorrect placement. All the previous complications are from surgical or mixed surgical and percutaneous cricothyrotomy studies. Problems and complications associated specifically with percutaneous cricothyrotomy include difficulties with insertion, esophageal or mediastinal misplacement, and bleeding.104 The overall reported complication rate is 5%. Complications included CTM calcification, blockage by secretions, dystrophic ossification, and heterotrophic bone formation. Displacement of the tube into the mediastinum may occur and can cause emphysema, respiratory distress, and pneumothorax.105 Bleeding occurs in 2% of cases, but significant hemorrhage requiring surgical intervention is rare. The Seldinger technique appears to lessen the incidence of bleeding and promote a more precise technique of insertion.106
b Percutaneous Tracheostomy
Most studies report excellent success and low complication rates with PDT.90,107,108 Since PDT became common clinical practice, data on the utility of this procedure and its potential advantages over standard tracheostomy have been reported in many publications.36,73,90,107,109,110
Potential complications of tracheostomy, whether performed openly or percutaneously, can be described as intraoperative or postoperative and as early or late; they are listed in Box 30-2. The overall complication rate reported for PDT in large studies, systematic reviews, and meta-analyses ranges from about 6% to 15%.85,111–114 It has been suggested that bronchoscopy-guided PDT might have a lower incidence of complications compared with PDT performed without bronchoscopy,86,87,115 but the data on this issue are mixed, and other studies have not confirmed this hypothesis.88 Among intraoperative complications, premature extubation and bleeding are most concerning and reported more frequently. Early postoperative complications include tube malpositioning, bleeding, subcutaneous emphysema, and pneumothorax. The most significant late complications are glottic and tracheal stenosis and stomal infection. A rare but deadly late complication is tracheal-innominate fistula.90 Whether this complication is related to any specific technique or anatomic variants (e.g., tracheostomy performed too low, cannula not appropriately chosen, variant blood vessel anatomy) must be determined, as well as the possible contribution of ultrasound to reduce the incidence. However, because the incidence of this complication is low, a very large number of observations would be required to evaluate the value of an intervention to decrease it.
The differences between techniques used, operators’ experience, patient selection criteria, and indications and timing of the procedures make it difficult to perform an accurate comparison of the data available from the literature on the overall safety of PDT with that for standard open tracheostomy. However, there seems to be a significant amount of evidence to support that PDT has a lower incidence of peristomal bleeding and wound infection compared with open tracheostomy.73–7688 For these reasons, it has been advocated that PDT should be considered the procedure of choice for performing elective tracheostomy in critically ill patients.74,88 The decreased incidence of bleeding may be related to the lack of sharp dissection and the tamponade effect of the dilator. The much smaller wound created with the PDT technique reduces the surface area available for bacterial colonization and may explain the low rate of wound infection. Late outcome studies evaluating serious long-term complications associated with PDT indicate that the incidence of clinically significant tracheomalacia or stenosis requiring corrective intervention is low.116
After decannulation, 16 patients were evaluated by means of physical examination, standardized interviews, and fiberoptic laryngotracheoscopy. The subjective rating was good for all patients. Laryngotracheoscopy showed incidental tracheal changes in two patients consisting of soft tissue swelling and a membranous scar, respectively. Neither of these findings required treatment.117 Histologic studies were conducted on 21 laryngotracheal specimens from patients who had undergone PDT or standard open tracheostomy. In the percutaneous group, cartilage fractures associated with a strong inflammatory response were found in one third of cases, compared with a more limited inflammatory response in the standard group. There was no clinical evidence of laryngotracheal stenosis in either group.
Carrer and associates, in a prospective observational study of 181 ICU patients receiving PDT over a 6-year period, reported a 0.7% rate of tracheal stenosis requiring tracheal stent placement and a 1.4% rate of recurrent granuloma of the stoma that was treated with laser resection. Late decannulation seemed the major risk factor for these infrequent but clinically significant late complications.107
2 Practical Applications of Cricothyrotomy
Another important issue is establishing indications based on patients’ injuries and habitus and on clinical scenarios.118,119 Percutaneous techniques are increasingly used in the prehospital setting and for major facial injuries, neck abnormalities, or challenging anatomy. Many patients can be assisted or ventilated with a bag-valve-mask if definitive cricothyrotomy is performed, and they can be jet ventilated or ventilated mechanically if a needle cannula has been placed.
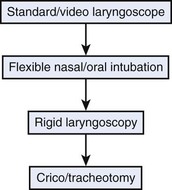
Cricothyrotomy is an important tool for managing the impossible airway or the threatened airway, and it often is the last and only way to avoid anoxia. In the previous edition of this textbook, a simplified protocol was proposed for prehospital emergency trauma airway control (Fig. 30-22). The protocol can be expanded by including an in-hospital approach (see Fig. 30-23 and the Difficult Airway Society’s “cannot ventilate, cannot intubate” scenario (http://www.das.uk.com/guidelines/cvci.html) or the ASA algorithm.49 Both scenarios converge on an emergency pathway, in which the option of awake airway control or awakening the patient is not possible. In this emergency pathway, as interpreted by a modified ASA difficult airway algorithm (Fig. 30-24), cricothyrotomy performed surgically or percutaneously plays a fundamental role.
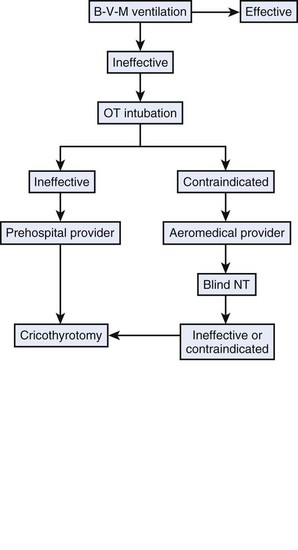
Figure 30-22 Emergency airway protocol for prehospital care. B-V-M, Bag-valve-mask; NT, nasotracheal; OT, orotracheal.
Figure 30-23 The Difficult Airway Society cannot intubate algorithm.
(Courtesy of the Difficult Airway Society, London, United Kingdom.)
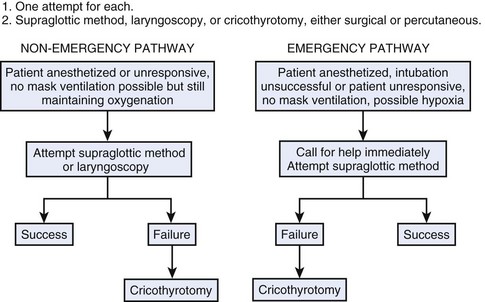
Figure 30-24 Modified American Society of Anesthesiologists cricothyrotomy airway algorithm.
(Modified from the American Society of Anesthesiologists, Park Ridge, IL.)
In the past few years, many changes in airway management occurred as a result of better airway equipment availability and implementation of airway protocols. As observed by Timmerman and colleagues and Combes and associates, cricothyrotomy is rare, even in emergency airway conditions, if an airway protocol is used (assuming no major neck trauma).120,121 However, a careful reading of the literature shows a significant number of complications that may question the underuse of more invasive techniques and argue for the earlier use of these techniques, depending on the level of training, the setting, and clinical conditions. Appropriate training is fundamental to maintain proficiency in the technical skills required to perform these invasive procedures safely, rapidly, and effectively.
VIII Training Models
Because PDC is rarely performed, there is a need for quality teaching and training aids.25,122 Although the technique closely mimics over-the-wire vascular insertion methods, it is sufficiently different that anesthesiologists ideally should practice on a regular basis.27,57 Simple and inexpensive models can be made for training residents and inexperienced personnel, as well as maintaining the skills and proficiency of the trainees and experts.
Of the available animal models, dogs appear to be most similar to humans. The canine CTM, muscles, and cricothyroid area are similar to those in humans. The tracheal dimensions of the 25-kg dog are comparable to those of the adult human.2 Cricothyrotomy has been performed on other animals, including pigs, sheep, and goats. The larynx is significantly smaller in these animal models, and 3.5- or 4.0-mm-ID sets must be used for teaching. In pigs, attempts to pass a needle or over-the-needle catheter into the cricothyroid space may result in hitting cartilage. The space can be entered only by directing the needle cephalad, not caudad. Dissection of the larynx revealed a projection on the inferior surface of the thyroid cartilage that articulated with the cricoid cartilage. This cornu had been previously described and had to be removed to perform cricothyrotomy studies.101 The pig trachea model (professionally isolated and prepared) is used for airway training and is combined with manikin simulations for the education of residents and faculty in surgical and percutaneous cricothyrotomy in teaching institutions, workshops, and airway management courses (e.g., Society for Airway Management, Difficult Airway Workshop, American Society of Anesthesiologists Annual Meeting).
Fresh and embalmed cadaver specimens can be used.67,123–125 The former are superior because the laryngeal structures of embalmed specimens are somewhat constricted because of muscle contraction, and it may be more difficult to discern the cricothyroid space. Mannequins can also be an acceptable model, and several products are available, but cheaper and simple models can also be used for skill maintenance and simulation.126 Simulation and practice-workshops are used in teaching programs or as part of dedicated airway management courses or meetings.56,127–129
IX Miscellaneous Considerations
A Cuff Pressure
• Having a pressure-controlled cuff with a pressure pop-off valve, which prevents inflation beyond 20 mm Hg (although less than 20 mm Hg has been associated with increased risk of leakage of bacterial pathogens around the cuff into the lower respiratory tract)130
• Regular measurement of intracuff pressure with a manometer attached to a three- or four-way stopcock (the latter gives more accurate results)
• Cuff deflation for as long as safely possible in patients who do not require mechanical ventilation
B Infections
2 Infection Sites
a Stomal Infection
Colonization of the surgical wound after tracheostomy occurs within 24 to 48 hours with primarily gram-negative organisms, including Klebsiella, P. aeruginosa, Escherichia coli, and occasionally S. aureus.7,131 Wound edges may demonstrate mild erythema, and yellow or green secretions from the area may be copious, particularly in the first 7 to 10 days. These findings are more marked after standard open tracheostomy than PDT, probably because of the very small incision and tight tract in the latter procedure. Frequent and meticulous wound care with mechanical débridement, if necessary, is the best way to deal with this situation. Progressive cellulitis, despite aggressive local care, indicates infection, usually polymicrobial, and warrants systemic antibiotics. Rarely, necrotizing stomal infections may occur, with substantial loss of soft tissue down to and including the tracheal wall. This may create difficulties in maintaining adequate mechanical ventilation. Progression of the process may result in carotid artery exposure, with its attendant risks. Management involves replacing the tracheostomy tube with an ETT and aggressive wound débridement and cleaning with antiseptic dressings. Rarely, local flaps may be necessary to provide soft tissue coverage for vital structures.
c Hospital-Acquired and Ventilator-Associated Pneumonia
Hospital-acquired pneumonia (HAP) accounts for up to 25% of all ICU infections and for more than 50% of the antibiotics prescribed.132 Ventilator-associated pneumonia (VAP) occurs in 9% to 27% of all intubated patients.133,134 In ICU patients, almost 90% of HAP episodes occur during mechanical ventilation. In mechanically ventilated patients, the incidence increases with duration of ventilation.130 Important risk factors for HAP include exposure to invasive respiratory devices and aspiration of oropharyngeal pathogens or leakage of secretions containing bacteria around the ETT.130
d Cleaning and Suctioning
Under normal circumstances, the nose efficiently warms, humidifies, and filters inspired air; in the patient with a tracheostomy, these functions must be restored artificially. Dehydration of the respiratory tract results in impaired mucociliary function, causing inspissated secretions and atelectasis.135 Providing adequate humidification is essential for all patients. Suctioning of secretions to maintain pulmonary toilet and patency of the tracheostomy tube constitutes an integral component of the care of the tracheostomy patient receiving mechanical ventilation and should be carried out according to the patient’s needs.
e Swallowing and Communication
In patients with tracheostomies in place who require mechanical ventilation, the incidence of swallowing dysfunction approaches 80%.136,137 The cause in most cases is multifactorial and may include the following:
• Glottic injury from previous orotracheal or nasotracheal intubation, or both
• Limitation of normal laryngeal excursion by the tethering effect of the tracheostomy tube
• Compression of the esophagus, particularly in the presence of an inflated cuff
• Desensitization of the larynx and loss of protective reflexes related to chronic diversion of air through the tube
• Impaired vocal fold adduction
• The use of anxiolytics or neuromuscular blocking agents, or both
XI Clinical Pearls
• Clinicians should quickly recognize a severe airway obstruction and a hypoxic condition to allow fast intervention.
• Proper airway anatomy recognition, even with an ultrasound-assisted evaluation, is the first step in successful management and avoidance of complications.
• The anesthesiologist should always consider the danger of piercing the posterior wall of the trachea and midline neck blood vessels.
• Cricothyrotomy is the technique of choice to secure the airway in emergencies and pending airway obstruction.
• Percutaneous dilational tracheostomy (PDT) is better performed under direct visualization (e.g., flexible fiberoptic bronchoscopy) below first tracheal ring.
• Training should be maintained by performing at least one procedure twice each year on live models and specialized mannequins.
• Percutaneous dilational techniques do not prevent complications. Disasters can be prevented by proper knowledge, preparation, anatomic identification, procedural indications, and skill acquisition and maintenance.
All references can be found online at expertconsult.com.
8 Corke C, Cranswick P. A Seldinger technique for minitracheostomy insertion. Anaesth Intensive Care. 1988;16:206–207.
11 Al-Ansari MA, Hijazi MH. Clinical review: Percutaneous dilatational tracheostomy. Crit Care. 2006;10:202.
14 Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
16 Cook TM, Scott S, Mihai R. Litigation related to airway and respiratory complications of anaesthesia: An analysis of claims against the NHS in England 1995–2007. Anaesthesia. 2010;65:556–563.
18 Benumof JL. Management of the difficult airway: With special emphasis on the awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
20 Cole RR, Aguilar EA. Cricothyroidotomy versus tracheostomy: An otolaryngologist’s perspective. Laryngoscope. 1988;98:131–135.
26 Berkow LC, Greenberg RS, Kan KH, et al. Need for emergency surgical airway reduced by a comprehensive difficult airway program. Anesth Analg. 2009;109:1860–1869.
36 Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy: A new simple bedside procedure. Preliminary report. Chest. 1985;87:715–719.
91 Ben Nun A, Altman E, Best LA. Extended indications for percutaneous tracheostomy. Ann Thorac Surg. 2005;80:1276–1279.
123 Latif R, Chhabra N, Ziegler C, et al. Teaching the surgical airway using fresh cadavers and confirming placement nonsurgically. J Clin Anesth. 2010;22:598–602.
1 Caparosa RJ, Zavatsky AR. Practical aspects of the cricothyroid space. Laryngoscope. 1957;67:577–591.
2 Ruhe DS, Williams GV, Proud GO. Emergency airway by cricothyroid puncture or tracheostomy. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:182–203.
3 Safar P, Penninckx JJ. Cricothyroid membrane puncture with special cannula. Anesthesiology. 1967;28:943–948.
4 Boyd AD, Romita MC, Conlan AA, et al. A clinical evaluation of cricothyroidotomy. Surg Gynecol Obstet. 1979;149:365–368.
5 Vanner R. Emergency cricothyrotomy. Curr Anaesth Crit Care. 2001;12:238–243.
6 Gulsen S, Unal M, Dinc AH, et al. Clinically correlated anatomical basis of cricothyrotomy and tracheostomy. J Korean Neurosurg Soc. 2010;47:174–179.
7 Holst M, Hedenstiema G, Kumlein JA, et al. Five years’ experience with elective coniotomy. Intensive Care Med. 1985;11:202–206.
8 Corke C, Cranswick P. A Seldinger technique for minitracheostomy insertion. Anaesth Intensive Care. 1988;16:206–207.
9 Mace SE, Khan N. Needle cricothyrotomy. Emerg Med Clin North Am. 2008;26:1085–1101.
10 Jackson C. Tracheostomy. Laryngoscope. 1909;18:285–290.
11 Al-Ansari MA, Hijazi MH. Clinical review: Percutaneous dilatational tracheostomy. Crit Care. 2006;10:202.
12 Ault MJ, Ault B, Ng PK. Percutaneous dilatational tracheostomy for emergent airway access. J Intensive Care Med. 2003;18:222–226.
13 Ben-Nun A, Altman E, Best LA. Emergency percutaneous tracheostomy in trauma patients: An early experience. Ann Thorac Surg. 2004;77:1045–1047.
14 Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–833.
15 Cheney FW, Posner KL, Lee LA, et al. Trends in anesthesia-related death and brain damage: A closed claims analysis. Anesthesiology. 2006;105:1081–1086.
16 Cook TM, Scott S, Mihai R. Litigation related to airway and respiratory complications of anaesthesia: An analysis of claims against the NHS in England 1995–2007. Anaesthesia. 2010;65:556–563.
17 Tunstall ME. Failed intubation in the parturient [editorial]. Can J Anaesth. 1989;36:611–613.
18 Benumof JL. Management of the difficult airway: With special emphasis on the awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
19 Bair AE, Filbin MR, Kulkarni RG, Walls RM. The failed intubation attempt in the emergency department: Analysis of prevalence, rescue techniques, and personnel. J Emerg Med. 2002;23:131–140.
20 Cole RR, Aguilar EA. Cricothyroidotomy versus tracheostomy: An otolaryngologist’s perspective. Laryngoscope. 1988;98:131–135.
21 O’Connor JV, Reddy K, Ergin MA, et al. Cricothyroidotomy for prolonged ventilatory support after cardiac operations. Ann Thorac Surg. 1988;39:353–354.
22 Wain JC, Wilson DJ, Mathisen DJ. Clinical experience with minitracheostomy. Ann Thorac Surg. 1990;49:881–886.
23 Wong E, Ng YY. The difficult airway in the emergency department. Int J Emerg Med. 2008;1:107–111.
24 Zugai BM, Eley V, Mallitt KA, Greenland KB. Practice patterns for predicted difficult airway management and access to airway equipment by anaesthetists in Queensland, Australia. Anaesth Intensive Care. 2010;38:27–32.
25 Hagberg CA, Greger J, Chelly JE, Saad-Eddin HE. Instruction of airway management skills during anesthesiology training. J Clin Anesth. 2003;15:149–153.
26 Berkow LC, Greenberg RS, Kan KH, et al. Need for emergency surgical airway reduced by a comprehensive difficult airway program. Anesth Analg. 2009;109:1860–1869.
27 Ezri T, Szmuk P, Warters RD, et al. Difficult airway management practice patterns among anesthesiologists practicing in the United States: Have we made any progress? J Clin Anesth. 2003;15:418–422.
28 Walsh K, Cummins F. Difficult airway equipment in departments of emergency medicine in Ireland: Results of a national survey. Eur J Anaesthesiol. 2004;21:128–131.
29 Marcolini EG, Burton JH, Bradshaw JR. A standing-order protocol for cricothyrotomy in prehospital emergency patients. Prehosp Emerg Care. 2004;8:23–28.
30 Green L. Can’t intubate, can’t ventilate! A survey of knowledge and skills in a large teaching hospital. Eur J Anaesthesiol. 2009;26:480–483.
31 Cattano D, Alexander R, Lambert E, et al: Recommendations for management of the threatened airway: A proposed model at UTMS at Houston. Presented at the 14th Annual Society for Airway Management Scientific Meeting (SAM), Chicago, IL, September 24–26, 2010.
32 Rosenblatt WH. The airway approach algorithm: A decision tree for organizing preoperative airway information. J Clin Anesth. 2004;16:312–316.
33 Shapiro SL. Emergency airway for acute laryngeal obstruction. Eye Ear Nose Throat Mon. 1970;49:35–40.
34 Jackson C. High tracheostomy and other errors: The chief causes of chronic laryngeal stenosis. Surg Gynecol Obstet. 1921;32:392–398.
35 Toye FJ, Weinstein JD. A percutaneous tracheostomy device. Surgery. 1969;65:384–389.
36 Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy: A new simple bedside procedure. Preliminary report. Chest. 1985;87:715–719.
37 Schachner A, Ovil Y, Sidi J, et al. Percutaneous tracheostomy: A new method. Crit Care Med. 1989;17:1052–1056.
38 Griggs WM, Worthley LIG, Gilligan JE, et al. A simple percutaneous tracheostomy technique. Surgery. 1990;170:543–545.
39 Fantoni A, Ripamonti D. A non-derivative, non-surgical tracheostomy: The translaryngeal method. Intensive Care Med. 1997;23:386–392.
40 Byhahn C, Wilke HJ, Halbig S, et al. Percutaneous tracheostomy: Ciaglia Blue Rhino versus the basic Ciaglia technique of percutaneous dilational tracheostomy. Anesth Analg. 2000;91:882–886.
41 Frova G, Quintel M. A new simple method for percutaneous tracheostomy: Controlled rotating dilation. A preliminary report. Intensive Care Med. 2002;28:299–303.
42 Ratnayake B, Langford RM. A survey of emergency airway management in the United States. Anaesthesia. 1996;51:908–911.
43 Breatnach E, Abbott GC, Fraser RG. Dimensions of the normal human trachea. AJR Am J Roentgenol. 1983;14:903–906.
44 Little CM, Parker MG, Tarnopolsky R. The incidence of vasculature at risk during cricothyroidostomy. Ann Emerg Med. 1986;15:805–807.
45 Bennett JDC, Guha SC, Sankar AB. Cricothyrotomy: The anatomical basis. J R Coll Surg Edinb. 1996;41:57–60.
46 Ellis H. Applied anatomy of cricothyrotomy and tracheostomy. Br J Hosp Med (Lond). 2009;70:M148–M149.
47 Elliott DS, Baker PA, Scott MR, et al. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia. 2010;65:889–894.
48 Greisz H, Qvarnstorm O, Willen R. Cricothyroidotomy: A clinical and histopathological study. Crit Care Med. 1982;10:387–389.
49 An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway: Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
50 Spaite D, Joseph M. Prehospital cricothyrotomy: An investigation of indications, technique, complications and patient outcome. Ann Emerg Med. 1990;19:279–285.
51 Fortune JB, Judkins DG, Scanzaroli D, et al. Efficacy of prehospital surgical cricothyrotomy in trauma patients. J Trauma. 1997;42:832–836. discussion 837–838
52 Chan TC, Vilke GM, Bramwell KJ, et al. Comparison of wire-guided cricothyrotomy versus standard surgical cricothyrotomy technique. J Emerg Med. 1999;17:957–962.
53 Bair AE, Sakles JC. A comparison of a novel cricothyrotomy device with a standard surgical cricothyrotomy technique. Acad Emerg Med. 1999;6:1172–1174.
54 Coté CJ, Hartnick CJ. Pediatric transtracheal and cricothyrotomy airway devices for emergency use: Which are appropriate for infants and children? Paediatr Anaesth. 2009;19(suppl 1):66–76.
55 McLaughlin J, Iserson KV. Emergency pediatric tracheostomy: A usable technique and model for instruction. Ann Emerg Med. 1986;15:463–465.
56 Friedman Z, You-Ten KE, Bould MD, Naik V. Teaching lifesaving procedures: The impact of model fidelity on acquisition and transfer of cricothyrotomy skills to performance on cadavers. Anesth Analg. 2008;107:1663–1669.
57 Chang RS, Hamilton RJ, Carter WA. Declining rate of cricothyrotomy in trauma patients with an emergency medicine residency: Implications for skills training. Acad Emerg Med. 1998;5:247–251.
58 Heffner JE, Sahn SA. The technique of tracheostomy and cricothyroidotomy. J Crit Illness. 1987;2:79–87.
59 Durbin CG. Tracheostomy: Why, when, and how? Respir Care. 2010;55:1056–1068.
60 De Leyn P, Bedert L, Delcroix M. Tracheotomy: Clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32:412–421.
61 Kluge S, Meyer A, Kuhnelt P, et al. Percutaneous tracheostomy is safe in patients with severe thrombocytopenia. Chest. 2004;126:547–551.
62 Van Hasselt EJ, Bruining HA, Hoeve LJ. Elective cricothyroidotomy. Intensive Care Med. 1985;11:207–209.
63 Bair AE, Panacek EA, Wisner DH, et al. Cricothyrotomy: A 5-year experience at one institution. J Emerg Med. 2003;24:151–156.
64 Eisenburger P, Laczika K, List M, et al. Comparison of conventional surgical versus Seldinger technique emergency cricothyrotomy performed by inexperienced clinicians. Anesthesiology. 2000;92:687–690.
65 Sulaiman L, Tighe SQ, Nelson RA. Surgical vs wire-guided cricothyroidotomy: A randomised crossover study of cuffed and uncuffed tracheal tube insertion. Anaesthesia. 2006;61:565–570.
66 Schaumann N, Lorenz V, Schellengowski P, et al. Evaluation of Seldinger technique emergency cricothyrotomy versus standard surgical cricothyrotomy in 200 cadavers. Anesthesiology. 2005;102:7–11.
67 Benkhadra M, Lenfant F, Nemetz W, et al. A comparison of two emergency cricothyroidotomy kits in human cadavers. Anesth Analg. 2008;106:182–185.
68 Dimitriadis JC, Paoloni R. Emergency cricothyrotomy: A randomized crossover study of four methods. Anaesthesia. 2008;63:1204–1208.
69 Salah N, Saigh I, Haynes N, McCaul C. Airway injury during emergency transcutaneous airway access: A comparison at cricothyroid and tracheal sites. Anesth Analg. 2009;109:1901–1907.
70 Mariappa V, Stachowski E, Balik M, et al. Cricothyrotomy: Comparison of three different techniques on a porcine airway. Anaesth Intensive Care. 2009;37:961–967.
71 Murphy C, Rooney SJ, Maharaj CH, et al. Comparison of three cuffed emergency percutaneous cricothyrotomy devices to conventional surgical cricothyrotomy in a porcine model. Br J Anaesth. 2011;106:57–64.
72 Moe KS, Stoeckli SJ, Schmid S, Weymuller EA, Jr. Percutaneous tracheostomy: A comprehensive evaluation. Ann Otol Rhinol Laryngol. 1999;108:384–391.
73 Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412–1418.
74 Freeman BD, Isabella K, Cobb JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001;29:926–930.
75 Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243.
76 Mallick A, Bodenham AR. Tracheostomy in critically ill patients. Eur J Anaesthesiol. 2010;27:676–682.
77 Westphal K, Byhahn C, Wilke HJ, Lischke V. Percutaneous tracheostomy: A clinical comparison of dilatational (Ciaglia) and translaryngeal (Fantoni) techniques. Anesth Analg. 1999;89:938–943.
78 Byhahn C, Westphal K, Meininger D, et al. Single-dilator percutaneous tracheostomy: A comparison of PercuTwist and Ciaglia Blue Rhino techniques. Intensive Care Med. 2002;28:1262–1266.
79 Divisi D, Altamura G, Di Tommaso S, et al. Fantoni translaryngeal tracheostomy versus Ciaglia Blue Rhino percutaneous tracheostomy: A retrospective comparison. Surg Today. 2009;39:387–392.
80 Anon JM, Escuela MP, Gomez V, et al. Percutaneous tracheostomy: Ciaglia Blue Rhino versus Griggs’ guide wire dilating forceps: A prospective randomized trial. Acta Anaesthesiol Scand. 2004;48:451–456.
81 Fikkers BG, Staatsen M, Lardenoije SG, et al. Comparison of two percutaneous tracheostomy techniques, guide wire dilating forceps and Ciaglia Blue Rhino: A sequential cohort study. Crit Care. 2004;8:R299–R305.
82 Patel PB, Ferguson C, Patel A. A comparison of two single dilator percutaneous tracheostomy sets: The Blue Rhino and the Ultraperc. Anaesthesia. 2006;61:182–186.
83 Kaiser E, Cantais E, Goutorbe P, et al. Prospective randomized comparison of progressive dilational vs forceps dilational percutaneous tracheostomy. Anaesth Intensive Care. 2006;34:51–54.
84 Cattano D, Abramson S, Buzzigoli S, et al. The use of the laryngeal mask airway during guidewire dilating forceps tracheostomy. Anesth Analg. 2006;103:453–457.
85 Westphal K, Maeser D, Scheifler G, et al. Percutwist: A new single-dilator technique for percutaneous tracheostomy. Anesth Analg. 2003;96:229–232.
86 Polderman KH, Spijkstra JJ, de Bree R, et al. Percutaneous dilatational tracheostomy in the ICU: Optimal organization, low complication rates, and description of a new complication. Chest. 2003;123:1595–1602.
87 Oberwalder M, Weis H, Nehoda H, et al. Videobronchoscopic guidance makes percutaneous dilational tracheostomy safer. Surg Endosc. 2004;18:839–842.
88 Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit Care. 2006;10:R55.
89 Sustić A, Kovac D, Zgaljardić Z, et al. Ultrasound-guided percutaneous dilatational tracheostomy: A safe method to avoid cranial misplacement of the tracheostomy tube. Intensive Care Med. 2000;26:1379–1381.
90 Dempsey GA, Grant CA, Jones TM. Percutaneous tracheostomy: A 6 yr prospective evaluation of the single tapered dilator technique. Br J Anaesth. 2010;105:782–788.
91 Ben Nun A, Altman E, Best LA. Extended indications for percutaneous tracheostomy. Ann Thorac Surg. 2005;80:1276–1279.
92 Heyrosa MG, Melniczek DM, Rovito P, Nicholas GG. Percutaneous tracheostomy: A safe procedure in the morbidly obese. J Am Coll Surg. 2006;202:618–622.
93 Aldawood AS, Arabi YM, Haddad S. Safety of percutaneous tracheostomy in obese critically ill patients: A prospective cohort study. Anaesth Intensive Care. 2008;36:69–73.
94 Kost KM. Endoscopic percutaneous dilatational tracheotomy: A prospective evaluation of 500 consecutive cases. Laryngoscope. 2005;115:1–30.
95 Byhahn C, Lischke V, Meininger D. Peri-operative complications during percutaneous tracheostomy in obese patients. Anaesthesia. 2005;60:12–15.
96 Kress TD, Balasbramanian S. Cricothyroidotomy. Ann Emerg Med. 1982;11:197–201.
97 Sise MJ, Shackford SR, Cruickshank JC, et al. Cricothyroidotomy for long-term tracheal access: A prospective analysis of morbidity and mortality in 76 patients. Ann Surg. 1984;200:13–17.
98 Brantigan CO, Grow JB. Cricothyroidotomy revisited again. Ear Nose Throat J. 1980;59:289–295.
99 Kuriloff DB, Setzen M, Portnoy W, Gadaleta D. Laryngotracheal injury following cricothyroidotomy. Laryngoscope. 1989;99:125–130.
100 Isaacs JH, Jr. Emergency cricothyrotomy: Long-term results. Am Surg. 2001;67:346–349. discussion 349–350
101 Holst M, Halbig I, Persson A, Schiratzki H. The cricothyroid muscle after cricothyroidotomy. Acta Otolaryngol. 1989;107:136–140.
102 Kirchner JA. Cricothyroidotomy and subglottic stenosis. Plast Reconstr Surg. 1981;68:828–829.
103 Boyle MF, Hatton D, Sheets C. Surgical cricothyrotomy performed by air ambulance flight nurses: A 5-year experience. J Emerg Med. 1993;11:41–45.
104 Abbrecht PH, Kyle RR, Reams WH, Brunette J. Insertion forces and risk of complications during cricothyroid cannulation. J Emerg Med. 1992;10:417–426.
105 Pedersen J, Lou H, Schurizek BA, et al. Ossification of the cricothyroid membrane following minitracheostomy. Intensive Care Med. 1989;15:272–273.
106 Jackson IJB, Choudhry AK, Ryan DW, et al. Minitracheostomy: Seldinger-assessment of a new technique. Anaesthesia. 1991;46:475–477.
107 Carrer S, Basilico S, Rossi S, et al. Outcomes of percutaneous tracheostomy. Minerva Anestesiol. 2009;75:607–615.
108 Cattano D, Buzzigoli S, Genovesi M, et al. Percutaneous tracheostomy: Prospective practice. Br J Anaesth. 106, 2011. 278-278. author reply
109 Hazard PB, Garrett HE, Jr., Adams JW, et al. Bedside percutaneous tracheostomy: Experience with 55 elective procedures. Ann Thorac Surg. 1988;46:63–67.
110 Hazard P, Jones C, Benitone J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Crit Care Med. 1991;19:1018–1024.
111 Anon JM, Gomez V, Escuela MP, et al. Percutaneous tracheostomy: Comparison of Ciaglia and Griggs techniques. Crit Care. 2000;4:124–128.
112 Kearney PA, Griffen MM, Ochoa JB, et al. A single-center 8-year experience with percutaneous dilational tracheostomy. Ann Surg. 2000;231:701–709.
113 Angel LF, Simpson CB. Comparison of surgical and percutaneous dilational tracheostomy. Clin Chest Med. 2003;24:423–429.
114 Pandian V, Vaswani RS, Mirski MA, et al. Safety of percutaneous dilational tracheostomy in coagulopathic patients. Ear Nose Throat J. 2010;89:387–395.
115 Steele APH, Evans HW, Afaq MA, et al. Long-term follow-up of Griggs percutaneous tracheostomy with spiral CT and questionnaire. Chest. 2000;117:1430–1433.
116 Fischler MP, Kuhn M, Cantieni R, Frutiger A. Late outcome of percutaneous dilatational tracheostomy in intensive care patients. Intensive Care Med. 1995;21:475–481.
117 Stoeckli SJ, Breitbach T, Schmid S. A clinical and histologic comparison of percutaneous dilational versus conventional surgical tracheostomy. Laryngoscope. 1997;107:1643–1646.
118 Blankenship DR, Kulbersh BD, Gourin CG, et al. High-risk tracheostomy: Exploring the limits of the percutaneous tracheostomy. Laryngoscope. 2005;115:987–989.
119 Rehm CG, Wanek SM, Gagon EB, et al. Cricothyroidotomy for elective airway management in critically ill trauma patients with technically challenging neck anatomy. Crit Care. 2002;6:531–535.
120 Timmermann A, Eich C, Russo SG, et al. Prehospital airway management: A prospective evaluation of anaesthesia trained emergency physicians. Resuscitation. 2006;70:179–185.
121 Combes X, Jabre P, Margenet A, et al. Unanticipated difficult airway management in the prehospital emergency setting: Prospective validation of an algorithm. Anesthesiology. 2011;114:105–110.
122 Borges BC, Boet S, Siu LW, et al. Incomplete adherence to the ASA difficult airway algorithm is unchanged after a high-fidelity simulation session. Can J Anaesth. 2010;57:644–649.
123 Latif R, Chhabra N, Ziegler C, et al. Teaching the surgical airway using fresh cadavers and confirming placement nonsurgically. J Clin Anesth. 2010;22:598–602.
124 Tabas JA, Rosenson J, Price DD, et al. A comprehensive, unembalmed cadaver-based course in advanced emergency procedures for medical students. Acad Emerg Med. 2005;12:782–785.
125 Johnson DR, Dunlap A, Mcfeeley P, et al. Cricothyrotomy performed by prehospital personnel: A comparison of two techniques in a human cadaver model. Am J Emerg Med. 1993;11:207–209.
126 Varaday SS, Yentis SM, Clarke S. A homemade model for training in cricothyrotomy. Anaesthesia. 2004;59:1012–1015.
127 Abou-Elhamd KE, Al-Sultan AI, Rashad UM. Simulation in ENT medical education. J Laryngol Otol. 2010;124:237–241.
128 Grober ED, Hamstra SJ, Wanzel KR, et al. The educational impact of bench model fidelity on the acquisition of technical skill: The use of clinically relevant outcome measures. Ann Surg. 2004;240:374–381.
129 Siu LW, Boet S, Borges BC, et al. High-fidelity simulation demonstrates the influence of anesthesiologists’ age and years from residency on emergency cricothyroidotomy skills. Anesth Analg. 2010;111:955–960.
130 American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
131 Brantigan CO, Grow JB. Cricothyroidotomy: Elective use in respiratory problems requiring tracheostomy. J Thorac Cardiovasc Surg. 1976;71:72–81.
132 Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical ICUs in the United States: National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892.
133 Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
134 Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121.
135 Au J, Walker WS, Inglis D, Cameron EW. Percutaneous cricothyroidostomy (minitracheostomy) for bronchial toilet: Results of therapeutic and prophylactic use. Ann Thorac Surg. 1989;48:850–852.
136 Romero CM, Marambio A, Larrondo J, et al. Swallowing dysfunction in nonneurologic critically ill patients who require percutaneous dilatational tracheostomy. Chest. 2010;137:1278–1282.
137 DeVita MA, Spierer-Rundback L. Swallowing disorders in patients with prolonged orotracheal intubation or tracheostomy tubes. Crit Care Med. 1990;18:1328–1330.