Chapter 60 Percutaneous and Minimally Invasive Approaches to Decompression and Arthrodesis of the Thoracolumbar Spine
While still considered a burgeoning field, minimally invasive surgery (MIS) of the spine boasts a storied history spanning several decades. Since its inception, many MIS techniques have been developed to treat a wide variety of spinal pathologic conditions. The history of this field, the philosophy and rationale for its use, and an overview of clinical outcomes achieved are thoroughly covered elsewhere in this book. Additionally, Chapter 49 in this book is devoted to MIS of the cervical spine. Herein, we examine surgical approaches limited to the lumbar spine and the thoracic spine, and we analyze their clinical outcomes.
Lumbar Spine
Dorsal Approaches to Arthrodesis
Minimally Invasive Posterior Lumbar Interbody Fusion
The traditional approach for posterior lumbar interbody fusion (PLIF) requires a large dorsal midline exposure with substantial manipulation of overlying ligamentous and muscular processes. When compared to other approaches to the lumbar spine, PLIF results in the highest complication rate, possibly owing to the broad exposure that is traditionally used.1 This rate makes the possibility of successful minimally invasive approaches particularly attractive. Few studies have examined MIS PLIF, and none have directly compared traditional PLIF to MIS PLIF.
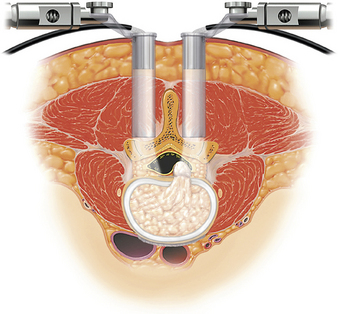
One of the first and most illustrative reports of the use of MIS to limit collateral soft tissue damage during PLIF was presented in 2002 by Khoo et al.2 Briefly, small incisions were made between 2 and 4 cm from the midline bilaterally at the level of interest (Fig. 60-1). Under fluoroscopic guidance, sequentially larger dilators were then placed over a K-wire to ultimately place a tubular retractor that provided a working corridor 15 to 20 mm in diameter.3,4 Decompression, hemilaminotomy, and discectomy were then performed endoscopically. During interbody graft placement, the authors used endoscopic visualization to ensure neural element retraction while working within the tubular system.
Percutaneous pedicle screw-rod systems were used in concert to complete the instrumented arthrodesis. Generally (and as reported in all patients in the cited study), cannulation of the pedicles can be performed by using the same incisions that are made for the purpose of decompression after removal of the tubular retractor and endoscope.2 Fluoroscopy was utilized while a Jamshidi needle was advanced to the pedicle and subsequently swapped for a K-wire using the Seldinger technique. In lieu of traditional fluoroscopy, a fluoroscopic navigation system was used to track the needle in the sagittal, coronal, and axial planes. A tap was passed over the K-wire, and the multiaxial pedicle screws (Sextant, Medtronic Sofamor Danek) were attached to screw extender sleeves and passed over the K-wire into the screw pathway.2 The remainder of the screw-rod assembly involved mating of the ipsilateral pedicle pairs using Sextant precontoured rods.
Another clinical series in 2002 reported positive outcomes in 10 patients who underwent MIS PLIF with pedicle screw fixation.5 Again, operative time was found to be greatest during the familiarization period and declined with time. Half of the patients were discharged on postoperative day 1 or 2, the remainder being discharged on the third postoperative day. Solid fusions were documented in all patients at 13.8 months of mean follow-up time. In 2007, Park and Ha compared 32 patients who had been treated with traditional one-level PLIF with 29 who had undergone MIS PLIF.6 Consistent with the aforementioned comparison, there was no statistical difference in fusion rates at 1-year minimum follow-up. The MIS group did show statistically significant differences in postoperative and intraoperative metrics. These included decreased blood loss, postoperative pain, recovery time, and hospital stay.
Interestingly, a recent study has examined the MIS placement of percutaneous pedicle screws using a miniature robotic system.7 In this procedure, 31 patients received a PLIF with decompression, discectomy, and polyetheretherketone (PEEK) cage implantation along with percutaneous pedicle screw fixation. The SpineAssist (MAZOR Surgical Technologies Ltd., Israel) system was utilized, in which an image-correlated framing system provided an edifice for spinous process clamping and screw guidance. The authors used a modified frame to support pedicle screw fixation and computer-based trajectories. In 29 of 31 cases, seamless percutaneous pedicle screw placement was achieved, with deviations from surgical trajectories of less than 2 mm in the vast majority of cases. While this study was an interesting proof of concept and demonstrated precision in percutaneous screw placement, it utilized a modified MIS approach and did not provide outcomes for the patient population. Further work with this or similar guided systems may be of interest in the future to expand MIS PLIF approaches.
Transforaminal Lumbar Interbody Fusion
As a variation of dorsal lumbar fusion, transforaminal lumbar interbody fusion (TLIF) utilizes a far lateral, or transforaminal, approach that can be applied throughout the lumbar spine. Additionally, complications related to neural element retraction are minimized in comparison to PLIF1 as TLIF does not necessitate retraction of the traversing nerve root during the discectomy and cage/graft placement. Furthermore, TLIF is not limited to levels L3-4 and below, as is the case with PLIF. When compared to MIS PLIF, MIS TLIF uses a similar but more lateral approach, giving way to an eventual facetectomy, along with complete resection of the inferior and superior articulating facets of the inferior and superior vertebrae, respectively. Discectomy, preparation of end plates, pedicle/facet screw placement, and interbody graft placement are then performed in the usual manner.
Schwender et al.8 first reported the results of 49 patients who had undergone TLIF with percutaneous pedicle screw placement. Fusion was reported in all patients at a minimum follow-up of 18 months, and narcotic use was discontinued on average between 2 and 4 weeks postoperatively. Other clinical indicators were similarly favorable, with an average blood loss of 140 mL and an average Oswestry Disability Index (ODI) score that dropped from 46 preoperatively to 14 at last follow-up. Jang and Lee also presented a cohort of 23 patients undergoing MIS TLIF with both ipsilateral pedicle screw and contralateral facet screw fixation.9 In this series, 21 of 24 patients achieved fusion at follow-up (mean of 19 months), with an average blood loss of 310 mL and ODI scores dropping from 33.1 to 7.6 after surgery.
In 2009, Peng et al. presented one of the most comprehensive comparisons of MIS versus open spine surgeries, comparing 29 MIS TLIF procedures to 29 open TLIF procedures.10 The benefits of MIS TLIF were most pronounced in the immediate postoperative period and included less blood loss, a shorter hospitalization period, diminished postoperative pain, and subsequent lower analgesic use (P < .05 for all parameters). However, the MIS procedure also required longer operative and fluoroscopic exposure time. Importantly, follow-up at 6 months and follow-up at 2 years were statistically equivalent when quality-of-life metrics and fusion rates were compared. The authors concluded that MIS TLIF had “retained” positive long-term outcomes associated with TLIF but with fewer immediate postoperative complications (13.8% vs. 6.9%, open vs. MIS). It should be noted that three quarters of complications in the open group likely arose from immobility (two urinary tract infections and one case of atelectasis) and were not directly attributable to the surgical procedure.
Ventral and Lateral Approaches to Arthrodesis
Lateral Interbody Fusion
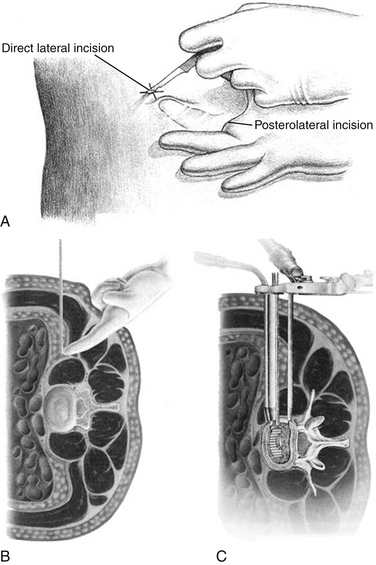
In 2004, Bergey et al. described an endoscopic lateral transpsoas approach to the lumbar spine in 21 patients undergoing discectomy and fusion.11 A modification of McAffee’s endoscopic approach,11 the true lateral approach provides a retroperitoneal approach to the lumbar spine without trespassing into the peritoneum or dissecting the great vessels.12 The exposure typically requires the patient to be in the right lateral decubitus position (Fig. 60-2). An incision is made along the lateral border of the lumbar paraspinal muscle, and a finger dissection is performed through the retroperitoneal space to the psoas.3 With the use of fluoroscopy to determine the proper starting point along the flank, a counterincision is made directly lateral to the disc space(s) of interest. The dilators are guided to the psoas using the initial incision, and a corridor is developed through the psoas to the disc space of interest. Continuous electromyogram monitoring is utilized to avoid injuring the exiting lumbar nerve roots, which also pass through the psoas muscle. A final expandable retractor is then placed over the final dilator, providing adequate access to the disc space. The discectomy and placement of the interbody graft are then followed by placement of a side plate and/or dorsal fixation of some kind (i.e., pedicle screws, translaminar or facet screws).
While a lateral approach decreases the likelihood of complications arising from anterior lumbar interbody fusion, it presents a unique set of challenges. First, it is essential to note the location of the lumbosacral plexus, as it migrates ventrally from L1 to L513 and is at significant risk when approaching the L4-5 interspace.14 Injury to the genitofemoral nerve is still possible and was noted in 30% of patients in Bergey et al.’s 2004 study.11 This complication was not found in a 2006 study,15 but staying within the ventral third of the psoas and visualizing the genitofemoral nerve has been recommended.3 The far lateral approach provides excellent access to the L1-4 levels and ability to restore disc height, but further dissection is necessary to expose any caudal disc spaces and may even require removal of a portion of the iliac crest.16 Clinical outcomes of extreme lateral interbody fusion are also notable; a recent study shows MIS extreme lateral interbody fusion, unlike traditional open lumbar procedures, to be no more likely to result in complications in the obese patient.17
Ventral Limited, Laparoscopic Approaches
Ventral laparoscopic approaches to the lumbar spine have fallen out of favor with the spine surgery community. As late as the mid-1990s, prospective studies demonstrated equivalent rates of complications between laparoscopic and open approaches, encouraging further study.18 However, the past decade has revealed that complication rates for video-assisted procedures were higher than those in open surgeries.19 In one series, the laparoscopic approach, utilizing techniques similar to those of abdominal laparoscopy, resulted in higher rates of retrograde ejaculation and new-onset radicular pain. In 11% of cases, the laparoscopic approach required conversion to a more open approach for reasons that included major vessel lacerations and peritoneal tears. The laparoscopic techniques also favored the L4 vertebra and below, as higher approaches were deemed even more technically demanding. Therefore, the authors of that study advocated abandonment of the laparoscopic technique for ventral lumbar fusion.
Nonlaparoscopic, MIS approaches to the lumbar spine have faired far better. In 1997, Mayer introduced a muscle-splitting approach that allowed easy access to the entire lumbar spine.20 After exposure of the target level, disc removal and graft placement can proceed. In his study, Mayer reported uniform fusion at follow-up, low intraoperative blood loss and postoperative morbidity, and a short recovery period. A 2004 study retrospectively compared 33 patients who had undergone the traditional extraperitoneal approach to 23 who had undergone Mayer’s minimally invasive approach.21 First, unlike the case with most MIS procedures, the authors reported a significantly shorter operative time when compared to the open procedure. Blood loss was significantly less in the MIS group, and complication and fusion rates were similar. In light of these findings and a similar study showing higher complication rates in the open group,22 it is reasonable to conclude that the MIS approach may be preferred to the traditional extraperitoneal approach. A variety of grafting options can be used for an MIS ALIF approach. The Lumbar Tapered (LT)-Cage (Medtronic Sofamor Danek, Memphis, TN) is a stand-alone tapered titanium cage that is approved by the Food and Drug Administration (FDA)23 for single-level ALIF with the concomitant use of bone morphogenetic protein-2 (BMP-2) (INFUSE Bone Graft, Medtronic Sofamor Danek, Memphis, TN), with fusion rates reported at 94.5% at 12 months. Other graft options for MIS (analogous to those in open procedures) include the autogenous iliac crest corticocancellous graft, allograft (e.g., femoral ring), and PEEK, carbon fiber, bioabsorbable, and other metallic interbody cages. These materials, when used as stand-alone devices (i.e., no dorsal fixation), result in suboptimal fusion rates and clinical outcomes. Therefore, it is recommended that they be used in conjunction with dorsal fixation. Equally nebulous at the time of this writing is the ideal grafting substrate. The ideal substrate (i.e., iliac crest autograft, allograft, tricalcium phosphate, BMP-2) to be used with the various interbody structural components (non-LT metallic cages, allograft rings, PEEK, and carbon fiber cages) have not been defined clearly in the literature. Appropriately designed and executed studies are necessary to define the ideal cage, graft substrate, quantity, and, in the case of BMP-2, dose for the use of ALIF surgery.
Minimally Invasive Decompression
A thorough treatise on MIS lumbar decompression could fill several volumes. The use of laser dehydration, chemical application, percutaneous extraction, and MIS implants have been evaluated repeatedly and found to be inferior to direct decompression.24,25 While implants may be an attractive alternative for select patients, it is still not entirely known how patient selection affects outcome, and several studies have shown high failure rates or inconsistent radiographic and clinical factors predicting clinical outcomes.26,27 Therefore, this chapter focuses on MIS surgical intervention for decompression of the thoracolumbar spine.
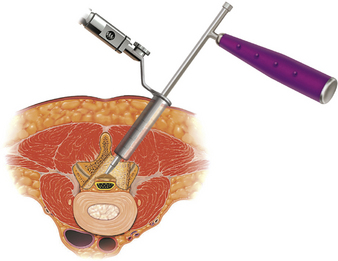
The approach to MIS decompression (i.e., laminotomy, laminectomy, foraminotomy) is similar to that of MIS PLIF in that muscle-splitting techniques are used with tubular retractor systems to provide targeted access to the affected vertebra. The placement of the tubular or expandable retractor relative to midline is predicated on the type of decompression being performed (e.g., ipsilateral foraminotomy vs. bilateral decompression via a unilateral approach; Fig. 60-3).25 A microscope or endoscope can be used for visualization; however, the latter has ergonomic restraints, particularly in working through a narrow retractor. A recent study retrospectively compared 38 patients who had undergone MIS decompression with 126 patients who had been treated with open laminectomy.28 MIS procedures were associated with shorter operative times, less blood loss, shorter length of hospital stay, and fewer complications. Several other recent studies have also reported excellent clinical results in single-level and multilevel MIS laminotomy or midline decompression.29–31 A biomechanical study examined lumbar segments with regard to axial compression, flexion, extension, and lateral bending after intervention of isolated fenestration, bilateral decompression via unilateral approach, and medial or total facetectomy.32 These preliminary results found that bilateral decompression had less effect on stiffness, as did medial facetectomy. This supports the use of a minimally invasive bilateral decompression due to preservation of facet joints.
Thoracic Spine
Minimally Invasive Surgical Approaches to Arthrodesis and Decompression
Application of endoscopic technologies for ventral exposure of the thoracic spine was first reported in the mid-1990s.33–35 However, both the high complication rate and the steep learning curve, as well as limited access to more cephalad vertebrae, have limited the use of MIS ventral approaches in favor of open approaches that allow greater visibility.36
Dorsal MIS approaches were until recently limited to trauma, metastasis, deformity, and infection, entities that are outside the scope of this chapter. In the past few years, however, progress in thoracic MIS approaches has accelerated. In 2006, Ringel et al.37 assessed the feasibility of MIS percutaneous dorsal pedicle screws in 104 patients with instability in the thoracic and lumbar spine. The authors found “good” screw placement in 87% of cases, and a majority of patients proceeded to ventral MIS fusion; of those patients, all but two reported resolution of radiculopathy. However, this study lacked a control arm or matched comparison to open thoracic screw placement. In 2009, Haufe et al.38 performed isolated laminoforaminoplasty for the treatment of thoracic radiculopathy in 12 patients. When compared to traditional laminotomy, the authors reported a shorter hospital stay and lower complication rates. Overall, while clinical data for MIS approaches to arthrodesis and decompression are sparse, initial reports dating as far back as 2006 provide positive outcomes and a solid foundation for further study.
Discussion
Minimally invasive surgery has become increasingly popular among both spine surgeons and patients. Over the past decade, MIS technology (i.e., retractors, instrumentation, interbody cages, pedicle and facet screws) has advanced at a rate that has exceeded the literature on the topic. The fundamental premise of MIS surgery is that it is better for the patient because it reduces the amount of tissue trauma associated with open procedures. Certainly, short-term results indicate a benefit for patients following decompression and fusion surgery in regard to narcotic use and hospital stays. However, there is a paucity of articles that define long-term outcomes. There are many studies that have demonstrated that open midline spine approaches are associated with paraspinal muscle damage, and proponents of MIS surgery use this as a springboard to promote MIS techniques.39–47 However, there is currently a lack of evidence that substantiates less soft tissue damage with MIS techniques. Simple observation may lead one to believe that MIS causes less tissue damage, but this has not been quantified and remains an aspect of MIS surgery that needs to be defined further.
Large-scale, systematic, well-designed and well-executed studies are necessary in order to define the optimal retractor size for each type of MIS procedure, the ideal type of interbody cage (i.e., metal vs. PEEK vs. autograft vs. allograft), the ideal graft substrate, the adverse event profile for the various techniques, the adverse event profile associated with the use of biologics for these techniques, and, most important, long-term clinical outcomes. Last, it is also critical to emphasize that the majority of the MIS techniques require the use of ionizing radiation for intraoperative navigation (i.e., fluoroscopy, intraoperative computed tomography). All surgeons must keep in mind that the adverse effects of radiation are cumulative and that each surgeon must therefore monitor his or her annual exposure. Overall, while percutaneous and MIS approaches to decompression and fusion of the thoracolumbar spine have shown early clinical promise, the outcomes of these procedures must be better characterized and documented for the field to reach its maximum potential.
Foley K.T., Gupta S.K. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97:7-12.
Jang J.S., Lee S.H. Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine. 2005;3:218-223.
Kawaguchi Y., Matsui H., Tsuji H. Back muscle injury after posterior lumbar spine surgery: a histologic and enzymatic analysis. Spine (Phila Pa 1976). 1996;21:941-944.
Khoo L.T., Palmer S., Laich D.T., et al. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51:S166-S171.
Mayer H.M. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1997;22:691-699. discussion 700
Ozgur B.M., Aryan H.E., Pimenta L., et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
Schwender J.D., Holly L.T., Rouben D.P., et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1-S6.
1. Maroon J.C., Onik G., Vidovich D.V. Percutaneous discectomy for lumbar disc herniation. Neurosurg Clin North Am. 1993;4:125-134.
2. Khoo L.T., Palmer S., Laich D.T., et al. Minimally invasive percutaneous posterior lumbar interbody fusion. Neurosurgery. 2002;51:S166-S171.
3. Eck J.C., Hodges S., Humphreys S.C. Minimally invasive lumbar spinal fusion. J Am Acad Orthop Surg. 2007;15:321-329.
4. Harris E.B., Massey P., Lawrence J., et al. Percutaneous techniques for minimally invasive posterior lumbar fusion. Neurosurg Focus. 2008;25:E12.
5. Foley K.T., Gupta S.K. Percutaneous pedicle screw fixation of the lumbar spine: preliminary clinical results. J Neurosurg. 2002;97:7-12.
6. Park Y., Ha J.W. Comparison of one-level posterior lumbar interbody fusion performed with a minimally invasive approach or a traditional open approach. Spine (Phila Pa 1976). 2007;32:537-543.
7. Pechlivanis I., Kiriyanthan G., Engelhardt M., et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine (Phila Pa 1976). 2009;34:392-398.
8. Schwender J.D., Holly L.T., Rouben D.P., et al. Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech. 2005;18(Suppl):S1-S6.
9. Jang J.S., Lee S.H. Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine. 2005;3:218-223.
10. Peng C.W., Yue W.M., Poh S.Y., et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976). 2009;34:1385-1389.
11. Bergey D.L., Villavicencio A.T., Goldstein T., et al. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976). 2004;29:1681-1688.
12. Shen F.H., Samartzis D., Khanna A.J., et al. Minimally invasive techniques for lumbar interbody fusions. Orthop Clin North Am. 2007;38:373-386. abstract vi
13. Benglis D.M., Vanni S., Levi A.D. An anatomical study of the lumbosacral plexus as related to the minimally invasive transpsoas approach to the lumbar spine. J Neurosurg Spine. 2009;10:139-144.
14. Regev G.J., Chen L., Dhawan M., et al. Morphometric analysis of the ventral nerve roots and retroperitoneal vessels with respect to the minimally invasive lateral approach in normal and deformed spines. Spine (Phila Pa 1976). 2009;34:1330-1335.
15. Ozgur B.M., Aryan H.E., Pimenta L., et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
16. Osman S.G., Marsolais E.B. Endoscopic transiliac approach to L5-S1 disc and foramen. A cadaver study. Spine (Phila Pa 1976). 1997;22:1259-1263.
17. Rodgers W.B., Cox C.S., Gerber E.J. Early complications of extreme lateral interbody fusion in the obese. J Spinal Disord Tech. 2010;23:393-397.
18. McAfee P.C., Regan J.R., Zdeblick T., et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery: a prospective multicenter study comprising the first 100 consecutive cases. Spine (Phila Pa 1976). 1995;20:1624-1632.
19. Escobar E., Transfeldt E., Garvey T., et al. Video-assisted versus open anterior lumbar spine fusion surgery: a comparison of four techniques and complications in 135 patients. Spine (Phila Pa 1976). 2003;28:729-732.
20. Mayer H.M. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1997;22:691-699. discussion 700
21. Saraph V., Lerch C., Walochnik N., et al. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13:425-431.
22. Kaiser M.G., Haid R.W.Jr., Subach B.R., et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery. 2002;51:97-103. discussion 103–105
23. Mathews H.H. InFUSE™ Bone Graft/LT-CAGE™ Lumbar Tapered Fusion Device IDE Clinical Results G960065. fda.gov/ohrms/DOCKETS/ac/02/slides/3828s1_05_Matthews_draft/index.htm. Available at
24. Deen H.G., Fenton D.S., Lamer T.J. Minimally invasive procedures for disorders of the lumbar spine. Mayo Clin Proc. 2003;78:1249-1256.
25. Armin S.S., Holly L.T., Khoo L.T. Minimally invasive decompression for lumbar stenosis and disc herniation. Neurosurg Focus. 2008;25:E11.
26. Verhoof O.J., Bron J.L., Wapstra F.H., et al. High failure rate of the interspinous distraction device (X-stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188-192.
27. Sobottke R., Schluter-Brust K., Kaulhausen T., et al. Interspinous implants (X stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J. 2009;18:1494-1503.
28. Rahman M., Summers L.E., Richter B., et al. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51:100-105.
29. Pao J.L., Chen W.C., Chen P.Q. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18:672-678.
30. Hatta Y., Shiraishi T., Sakamoto A., et al. Muscle-preserving interlaminar decompression for the lumbar spine: a minimally invasive new procedure for lumbar spinal canal stenosis. Spine (Phila Pa 1976). 2009;34:E276-E280.
31. Yagi M., Okada E., Ninomiya K., et al. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10:293-299.
32. Hamasaki T., Tanaka N., Kim J., et al. Biomechanical assessment of minimally invasive decompression for lumbar spinal canal stenosis: a cadaver study. J Spinal Disord Tech. 2009;22:486-491.
33. Horowitz M.B., Moossy J.J., Julian T., et al. Thoracic discectomy using video assisted thoracoscopy. Spine (Phila Pa 1976). 1994;19:1082-1086.
34. Jho H.D. Endoscopic microscopic transpedicular thoracic discectomy: technical note. Neurosurg Focus. 1998;4:E7.
35. Rosenthal D., Rosenthal R., de Simone A. Removal of a protruded thoracic disc using microsurgical endoscopy: a new technique. Spine (Phila Pa 1976). 1994;19:1087-1091.
36. Smith J.S., Ogden A.T., Fessler R.G. Minimally invasive posterior thoracic fusion. Neurosurg Focus. 2008;25:E9.
37. Ringel F., Stoffel M., Stuer C., et al. Minimally invasive transmuscular pedicle screw fixation of the thoracic and lumbar spine. Neurosurgery. 2006;59:ONS361-ONS366. discussion ONS366–ONS367
38. Haufe S.M., Baker R.A., Pyne M.L. Endoscopic thoracic laminoforaminoplasty for the treatment of thoracic radiculopathy: report of 12 cases. Int J Med Sci. 2009;6:224-226.
39. Kawaguchi Y., Matsui H., Gejo R., et al. Preventive measures of back muscle injury after posterior lumbar spine surgery in rats. Spine (Phila Pa 1976). 1998;23:2282-2287. discussion 2288
40. Styf J.R., Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine (Phila Pa 1976). 1998;23:354-358.
41. Weber B.R., Grob D., Dvorak J., et al. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine (Phila Pa 1976). 1997;22:1765-1772.
42. Kawaguchi Y., Yabuki S., Styf J., et al. Back muscle injury after posterior lumbar spine surgery: topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine (Phila Pa 1976). 1996;21:2683-2688.
43. Kawaguchi Y., Matsui H., Tsuji H. Back muscle injury after posterior lumbar spine surgery: a histologic and enzymatic analysis. Spine (Phila Pa 1976). 1996;21:941-944.
44. Kawaguchi Y., Matsui H., Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 2: Histologic and histochemical analyses in humans. Spine (Phila Pa 1976). 1994;19:2598-2602.
45. Rantanen J., Hurme M., Falck B., et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine (Phila Pa 1976). 1993;18:568-574.
46. Sihvonen T., Herno A., Paljarvi L., et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine (Phila Pa 1976). 1993;18:575-581.
47. Mayer T.G., Vanharanta H., Gatchel R.J., et al. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine (Phila Pa 1976). 1989;14:33-36.