CHAPTER 55 Pelvic organ prolapse
Prevalence
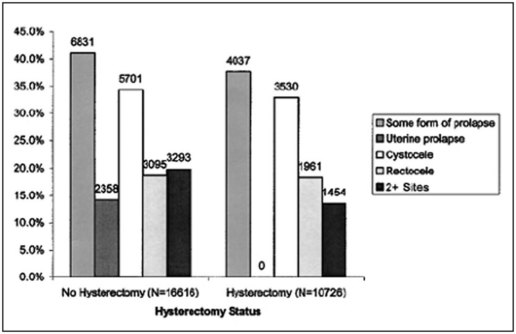
Pelvic organ prolapse is very common. In the Women’s Health Initiative (WHI), 41% of 27,342 women aged 50–79 years showed some degree of POP (Hendrix et al 2002, Figure 55.1). Indeed, with both life expectancy and rates of obesity increasing, the prevalence of POP is likely to rise. However, the majority of such women are symptom free and much of this prolapse is not considered clinically significant. Large cross-sectional studies have demonstrated rates of symptomatic POP of 3–11% (Tegerstedt et al 2005, Nygaard et al 2008, Fritel et al 2009). Women whose prolapse extends beyond the hymenal remnants are more likely to be symptomatic (Swift et al 2003). Although POP is rarely life threatening, it has a substantial impact on quality of life, such that 6.7% of women in the USA will undergo a surgical procedure for prolapse by 80 years of age (Olsen et al 1997).
Aetiology
The aetiology of POP is poorly understood, which is remarkable given the magnitude of the problem. Although multifactorial in nature, vaginal birth has emerged as the principal risk factor (Mant et al 1997). Avulsion injury to the levator ani during childbirth and, less commonly, pudendal neuropathy and fascial damage appear to be the most common causes (Dietz 2008a, Figure 55.2). Unsurprisingly, therefore, increasing parity, fetal macrosomia and perineal trauma increase the odds of subsequent POP, although the much-quoted link to instrumental vaginal delivery is not borne out in all studies (Uma et al 2005, Tegerstedt et al 2006).
Nulliparous women may also develop prolapse, and various non-obstetric aetiological factors have been identified (Miedel et al 2009). A number of reports, including the WHI, have demonstrated increasing rates of POP with advancing age (Hendrix et al 2002, Handa et al 2004), although a simple linear relationship is not universally accepted (Dietz 2008b). Some studies have shown an impact of ethnicity on POP, with lower rates of POP among Black women and higher rates among Hispanic women compared with White women (Hendrix et al 2002, Rortveit et al 2007). An association with body mass index (BMI) is generally agreed (Whitcomb et al 2009), and is of particular concern given the global rise in obesity. In addition, twin studies have demonstrated a clear genetic component to the disorder (Altman et al 2008), supported by stronger family histories among affected women (McLennan et al 2008).
The evidence directly linking hysterectomy and POP is not compelling and is potentially confounded by the indication for the original hysterectomy. Among 384 women undergoing surgery for POP/incontinence, 60% had a previous hysterectomy and/or pelvic floor repair, suggesting a potential aetiological role (Olsen et al 1997). Despite this, the cumulative probability of requiring a POP repair 20 years following hysterectomy for non-prolapse is only 0–2%, raising the possibility that it is the history of prolapse and not the hysterectomy per se which is the dominant risk factor (Blandon et al 2007). Finally, conditions associated with increased intra-abdominal pressure (constipation, cough, heavy lifting) are generally considered to be risk factors for prolapse (Rortveit et al 2007).
Classification
Management of POP is determined primarily by the findings on physical examination; thus, a standardized and reproducible classification system is crucial. Initially, POP is classified by vaginal compartment into (Figure 55.3):
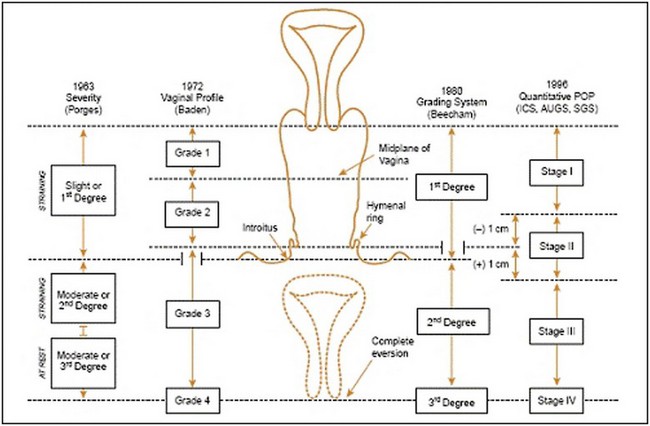
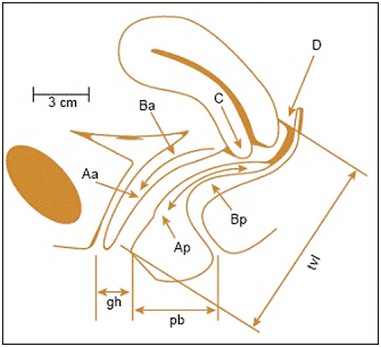
The anterior compartment is the most common site of POP, although in most cases, there will be evidence of prolapse in more than a single compartment (Hendrix et al 2002). In addition to classifying POP by anatomical compartment, an assessment of prolapse severity (stage) is undertaken. A variety of systems to stage POP have been described (Figure 55.4). However, in 1996, the Pelvic Organ Prolapse Quantification (POP-Q) system was developed by consensus of international expert groups (Bump et al 1996). It is a more objective, site-specific system for staging pelvic support with proven inter- and intraobserver reliability (Hall et al 1996). The POP-Q system references nine vaginal points (two anterior, two posterior, two superior, two frontal and total vaginal length) to the plane of the hymen during Valsalva strain to create a ‘topographic’ map of the vagina (Figure 55.5). These anatomical points are measured following demonstration of the maximum extent of the prolapse to determine the stage. Typically, the nine-point findings are translated into an ordinal staging system (stage 0–IV) to aid comparative analyses and facilitate more practical communication (Figure 55.4).
Pelvic Anatomy
On each side of the pelvis, a dense layer of connective tissue, the endopelvic fascia, envelops the uterus and cervix and attaches to the pelvic sidewall. As well as providing support, the endopelvic fascia also serves as a neurovascular conduit and is composed of collagen, smooth muscle, blood vessels and nerves. In certain areas, thickened condensations of this fascia form pelvic ‘ligaments’ which add support. The clinically important ligaments include the uterosacral ligaments (extending laterally from the posterior cervix to the anterior sacrum), the cardinal ligaments (transverse cervical/Mackenrodt’s ligaments, extending from the lateral cervix and upper vagina to the pelvic sidewall) and the round ligament (extending from the uterine cornu to the labia majora as the superior part of the broad ligament) (Raizada and Mittal 2008).
DeLancey introduced the concept of three levels of connective tissue support in the anterior pelvis. Level I (apical vaginal) support is via the uterosacral/cardinal ligament complex, and damage at this level results in uterine/vaginal vault prolapse (DeLancey 1992). Level II (midvaginal) support is achieved via the endopelvic fascia and its lateral insertion into the white line. Level III (distal vaginal) support incorporates the endopelvic fascia anteriorly and the perineal body posteriorly. Anterior and posterior compartment prolapse result from damage to level II/III support, respectively. Although DeLancey’s system creates artificial divides in what is essentially a continuous connective tissue structure, it does aid understanding of how loss of support correlates with clinical findings, and is widely reproduced in the gynaecological literature.
Clinical Presentation
Patients who seek medical care have a wide range of symptoms (Table 55.1). Women frequently present with a sensation of ‘something coming down’ or of ‘something falling out of the vagina’. Other women may see or feel a vaginal bulge or they may complain of less specific herniation symptoms, such as pelvic pressure or heaviness. As might be expected, a visible vaginal bulge correlates better with objective evidence of POP on examination than the more general complaint of pelvic heaviness or discomfort (Miedel et al 2008).
Table 55.1 Common symptoms associated with pelvic organ prolapse
| Vaginal symptoms |
Urinary symptoms
Among 237 women with symptomatic POP, 87% reported frequency/urgency, 73% reported urinary incontinence and 50–60% reported symptoms of voiding dysfunction (Ellerkmann et al 2001). A complex relationship exists between POP and stress urinary incontinence, and may result from shared risk factors rather than a direct causal link (Smith and Appell 2005). In practice, many women present with both cystocele and stress incontinence, and loss of the anterior vaginal wall support is thought to contribute to urethral hypermobility and subsequent stress incontinence (DeLancey 2002). However, the relationship is not linear and some studies have demonstrated lower rates of stress leakage in more advanced POP (Yalcin et al 2001, Burrows et al 2004). This is likely due to a direct compressive effect or a urethral kinking mechanism, and is important given the risk of hidden or ‘occult’ stress incontinence manifesting once the prolapse is reduced (see ‘Investigation’ section). Interestingly, posterior vaginal wall prolapse has recently been linked to stress incontinence (Miedel et al 2008). More advanced POP can produce obstructive voiding symptoms or, rarely, urinary retention. The need to manually reduce a bulge to void is progressively linked to more severe POP (Burrows et al 2004, Miedel et al 2008). The relationship between POP and overactive bladder symptoms has not been studied as extensively. Analysis of 330 women undergoing surgery for POP or incontinence found that urgency/urge incontinence occurred more often in women with less advanced prolapse (Burrows et al 2004). Other studies, however, have shown a positive association between urge incontinence and both anterior and posterior compartment prolapse (Miedel et al 2008). This is supported by short-term improvements in overactive bladder symptoms following prolapse repair (Nguyen and Bhatia 2001, Digesu et al 2007).
Bowel symptoms
Bowel symptoms and difficulty with defaecation are common symptoms among women with POP. These include straining, sensation of incomplete emptying and the need to manually assist defaecation (digital pressure to the vagina or perineum). Among 280 women with symptomatic POP, almost three-quarters (73%) reported at least one bowel symptom (Miedel et al 2008). The most consistently reported prevalence of faecal incontinence associated with POP ranges from 14% to 19% (Jackson et al 1997, Burrows et al 2004, Jelovsek et al 2005, Miedel et al 2008). The extent of POP does not appear to predict bowel symptoms (Jelovsek et al 2005), although the need to manually assist defaecation has been associated with more severe prolapse (Burrows et al 2004). Much has been written about the relationship between posterior compartment prolapse and constipation, and it has been suggested that constipation contributes to the development of rectocele and vice versa. However, the exact nature of the association remains uncertain. It is notable that several studies have failed to identify a relationship between constipation and POP (Samuelsson et al 1999, Jelovsek et al 2005).
Sexual dysfunction
Sexual dysfunction is very common in women attending urogynaecology clinics (Pauls et al 2006), although it may not be volunteered due to patient embarrassment or poor history taking. Sexual satisfaction is a complex issue and studies examining the association between POP and sexual dysfunction have yielded conflicting results. Although high rates of satisfaction have been reported in women with POP (Barber et al 2002, Burrows et al 2004), other data have shown POP to have an adverse effect on sexual function (Rogers et al 2001, Novi et al 2005). It appears that women with POP are more likely to complain of problems with sexual function than those with urinary incontinence (Barber et al 2002). Weber found no differences in measures of sexual function in women with POP and incontinence compared with continent women without prolapse (Weber et al 1995). Furthermore, the benefits of pelvic floor surgical repair on sexual function have not been consistent (Thakar 2009).
Prediction of symptoms
Attempts have been made to correlate the severity (stage) of POP with the likely development of symptoms. Consistently strong correlations between increasing severity of POP and the development of symptoms have not been demonstrated (Burrows et al 2004). Many studies indicate a lower rate of stress incontinence in more severe anterior compartment prolapse. Miedel et al (2008) showed that most urinary and bowel symptoms associated with POP lack stage dependency, although there was correlation between a vaginal bulge and more advanced prolapse in all compartments. However, a large prospective study of 477 patients suggested that women whose prolapse extends beyond the hymenal remnants are more likely to be symptomatic (Swift et al 2003).
Physical examination
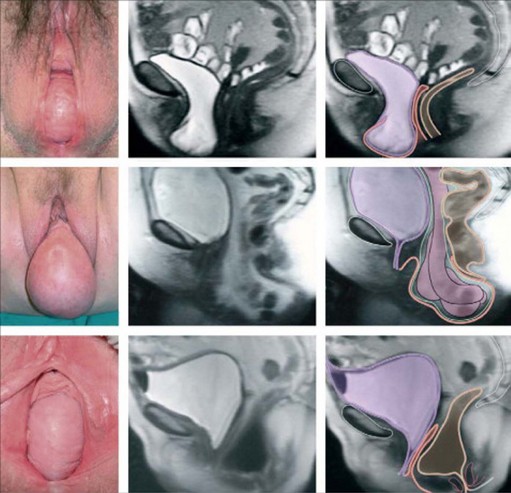
All women who present with symptoms consistent with POP should undergo a physical examination, including pelvic examination. Although some authorities advocate the need for physical examination in the standing position for all patients, use of the dorsal lithotomy position with Valsalva has been shown to be as good (Swift and Herring 1998). In practice, many clinicians examine women lying supine during resting and straining, and it is reasonable to reserve the standing position for women who feel that their prolapse is not being seen at its worst extent in that position (American College of Obstetricians and Gynecologists 2007). Conventionally, prolapse is examined while asking the woman to perform a Valsalva or cough stress test so that the maximum extent of the prolapse can be appreciated. A Sim’s speculum (or the posterior blade of a bivalve speculum) is inserted into the vagina to retract the posterior wall and allow visualization of the anterior wall; the extent of any prolapse is noted while the woman strains. The process is repeated for the posterior wall with the speculum retracting anteriorly. Sometimes, an anterior wall prolapse may be better appreciated with the woman in the left lateral position and her right hip flexed. Finally, the cervix or vaginal vault is assessed either through visualization or with gentle traction from a ring forceps, although, in practice, many women find the latter uncomfortable. Areas of bleeding/ulceration, which can be seen in prolapse extending past the introitus in particular, should be noted (Figure 55.6). Women with recurrent prolapse should have a meticulous examination to assess previous surgical repair and plan future management. Staging of the prolapse should be recorded for all compartments using the practitioner’s chosen method, although the POP-Q system is the internationally accepted standard (Figure 55.5, see ‘Classification’ section).
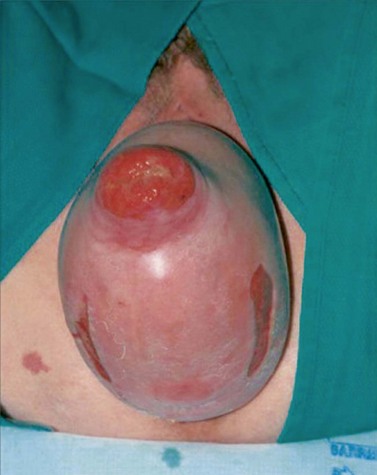
Figure 55.6 Complete uterovaginal prolapse. Note the linear erosions on the posterior vaginal mucosa.
Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369:1027–38.
In addition to staging POP, a number of other features of the physical examination are noteworthy. Abdominal palpation and a bimanual pelvic examination should be performed in these patients as, occasionally, a large pelvic mass, such as a fibroid uterus or ovarian mass, may be detected. Although routine rectal examination for all patients is unnecessary, it can be useful in defining a posterior compartment prolapse, particularly where there is suspicion of an enterocele (which can be felt between a thumb placed in the vagina and a finger placed in the rectum). In addition, it can be useful to look for stress urinary incontinence on provocation by asking the patient to cough, assuming the bladder has not been emptied recently. Furthermore, in cases of advanced anterior compartment prolapse in women who do not complain of urinary leakage, there is concern that prolapse reduction may unmask ‘occult’ stress incontinence. Therefore, a cough test following temporary reduction of the prolapse into a more anatomical position (manually or with a cotton swab or ring forceps) may be useful (Fatton 2009). In a large randomized trial, reduction using a cotton swab most closely reproduced postoperative status following sacrocolpopexy compared with other reduction techniques (Visco et al 2008). Finally, digital assessment of the strength of the patient’s pelvic floor muscle contraction is beneficial in women with concomitant stress urinary incontinence.
Investigation
The most contentious issue in the investigation of POP is the need for preoperative urodynamic testing. Women with POP without stress leakage have a 10–50% chance of developing de-novo (‘occult’) stress incontinence post repair (Fatton 2009). An anti-incontinence procedure may be performed concomitantly but will likely overtreat a proportion of women, and increases postoperative voiding problems and overactive bladder symptoms (de Tayrac et al 2004). Alternatively, a two-stage strategy can be adopted, where an interval continence procedure is reserved for patients with troubling stress urinary incontinence following prolapse repair. The use of preoperative urodynamics to better stratify patients into those who may or may not benefit from concomitant continence surgery has been suggested. However, there is little evidence that preoperative urodynamics improves the outcome of prolapse surgery (Roovers and Oelke 2007). The CARE (Colpopexy and Urinary Reduction Efforts) trial did show that women with POP and preoperative urodynamic stress incontinence during reduction testing had a higher rate of stress leak following abdominal sacrocolpopexy without the continence procedure. It is notable that even with optimal prolapse reduction during preoperative urodynamics (by cotton swab), one-third of women who were dry before surgery developed postoperative stress incontinence (Visco et al 2008).
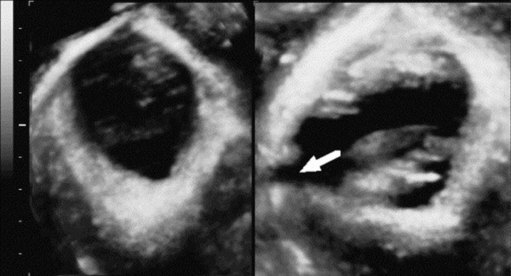
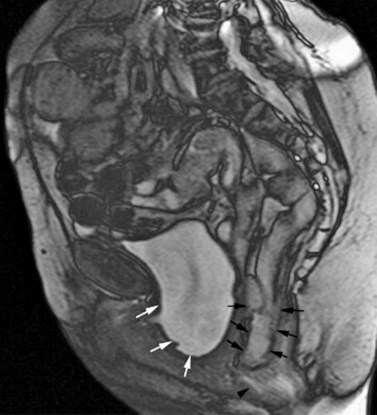
Various radiographic modalities have been proposed to further define the extent of POP, although none are in mainstream clinical use. Dynamic magnetic resonance imaging (MRI) is the most commonly described technique (Law and Fielding 2008, Taylor 2009). Dynamic MRI offers excellent anatomical detail and allows visualization of all three compartments together (Figure 55.7), but is expensive and dispute remains over the appropriate radiological landmarks for staging POP (Broekhuis et al 2009). Transperineal ultrasound (TPUS), and increasingly three- and four-dimensional TPUS, have also been used in staging POP radiologically (Dietz 2004). Recently, moderate-to-good correlation was demonstrated between three-dimensional TPUS and defaecography (traditionally the gold standard investigation) in the assessment of posterior compartment prolapse (Steensma et al 2009). However, TPUS does not appear to be superior to clinical assessment of POP (Kluivers et al 2008). Finally, anorectal physiology studies (including anal manometry and pudendal nerve latency) and endoanal ultrasound should be considered in women with symptoms of bowel dysfunction, particularly faecal incontinence.
Preventative Strategies/Natural History
POP and surgical prolapse repair place a significant demand on healthcare resources. Therefore, strategies to minimize POP and reduce the resultant disease burden are vital (Subramanian et al 2009).
Lifestyle modification
Four-year prospective results from one of the WHI study centres have confirmed that progression of POP over time is not inevitable; in some cases, POP can regress. Specifically, among 259 non-hysterectomized postmenopausal women undergoing serial POP-Q assessments, the maximum vaginal descent increased by ≥2 cm in 11% and regressed by this amount in 2.7% (Bradley et al 2007). POP progression was more likely in obese women, of higher parity (particularly for five children or more) and with more severe baseline degrees of prolapse. Progression was less likely in smokers, although this may be related to a lower BMI in women who smoked rather than a genuine protective effect. Another large secondary analysis from the WHI study confirmed a higher rate of progression in women with raised BMI, but found that weight loss was not associated with regression of POP, suggesting irreversible pelvic floor changes secondary to obesity (Kudish 2009). Therefore, although weight loss forms part of the standard advice given to overweight women with POP, highlighting the importance of avoiding weight gain in women of normal BMI may be more valuable. Pelvic floor muscle (Kegel) exercises are frequently recommended for women with POP. Although they are an effective therapy in stress urinary incontinence (which may coexist), pelvic floor exercises are of no proven benefit in the prevention of POP, although studies are lacking (Bø 2006).
Obstetric risk
Given the clear aetiological link between vaginal childbirth and POP, the role of caesarean delivery as a strategy to prevent POP is debated (Richter 2006, Weber 2007). The relative contributions made by the pregnancy, labour and mode of delivery itself are difficult to tease out. Based on POP-Q assessments on 94 primigravidae at 36 weeks of gestation and again at 6 weeks post partum, intrapartum caesarean section performed during the first stage of labour has not been shown to protect against POP compared with women undergoing vaginal delivery (Sze et al 2002). A similar study of 135 primigravidae involved POP-Q assessments on women from each trimester and post partum (O’Boyle et al 2005). POP was significantly more common in both the third trimester and post partum compared with the first trimester. Forty percent of women examined in the third trimester had stage II POP or worse. Among postpartum women, there was a higher incidence of stage II POP in the vaginal birth group compared with those who delivered by intrapartum caesarean. Although this finding contrasts with that of Sze et al (2002), the role of elective caesarean delivery was not examined and both studies were underpowered to determine the impact of mode of delivery on POP. A survey of 4458 women showed POP to be three times more common among vaginally parous women compared with nulliparous women [adjusted odds ratio (AOR) 3.2]. A history of caesarean delivery alone was protective (AOR 1.6 compared with nulliparous). However, elective prelabour caesarean conferred a higher benefit than caesarean in labour, suggesting that intrapartum events and not simply mode of delivery contribute to POP (Lukacz et al 2006).
Hysterectomy
The precise incidence of posthysterectomy POP is difficult to determine. A recent case–control study demonstrated a cumulative incidence of vaginal vault prolapse repair of 0.5% at 3–23 year follow-up (Dällenbach et al 2008). Overall, rates of pelvic floor repair are higher in women whose original hysterectomy was performed for prolapse compared with other clinical indications (Mant et al 1997, Blandon et al 2007). Numerous surgical strategies to prevent posthysterectomy vault prolapse have been described. The McCall and modified McCall culdoplasty techniques involve various means of anchoring the uterosacral pedicles to the vaginal vault during vaginal hysterectomy. Many studies have reported success rates in excess of 90% in preventing enterocele/vault prolapse at 12–108 months (Chene et al 2008). These techniques are superior to the Moschcowitz procedure in this regard (Cruikshank and Kovac 1999). Some authorities recommend prophylactic sacrospinous ligament suspension at the time of vaginal hysterectomy if the vault descends to the introitus during closure (Royal College of Obstetricians and Gynaecologists 2007), but there is no evidence to support this practice.
Treatment
Non-surgical treatment
Pelvic floor muscle training
As has been noted above, the effectiveness of pelvic floor exercises in the treatment of POP remains unproven. Although pelvic floor muscle training is unlikely to cause harm and may benefit stress urinary incontinence, there are associated cost implications. An international, multicentre, randomized controlled trial to assess the role of physiotherapy in prolapse is currently recruiting (http://www.charttrials.abdn.ac.uk/poppy/index.php). A pilot study of 47 women with stage I or II POP showed improvement in both subjective and objective measures of POP in women treated with pelvic floor muscle training and lifestyle advice compared with lifestyle advice alone (Hagen et al 2009).
Weight loss
A large population-based study of almost 5500 Swedish women showed that women who are overweight (BMI 26–30) are twice as likely to complain of symptomatic POP as women of normal weight (BMI 20–24, Miedel et al 2009). Weight loss should be encouraged in the management of POP for all women as it does not cause harm, reduces perioperative complication rates if surgery is later contemplated, and is of proven benefit in treating stress urinary incontinence, which often coexists (Subak et al 2009).
Vaginal pessaries
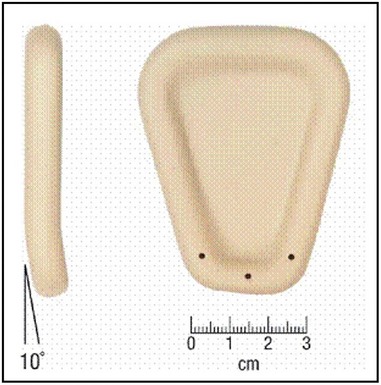
Vaginal pessaries represent the only proven non-surgical option for women who require treatment for POP. Although all women can be offered a trial of pessary use, they are particularly useful for women who decline surgery or are medically unsuitable for surgery, during pregnancy, and as an interim measure prior to surgery or for those who desire future childbearing. Pessary materials have evolved and are now made predominantly from non-reactive silicone, which is non-allergenic, durable and autoclavable (Shah et al 2006). A wide range of styles and shapes is available, and the ring pessary, which permits sexual intercourse, is the most widely used (Cundiff et al 2000, Figure 55.8). Some styles are also useful in the treatment of urinary incontinence. Pessaries are valid treatment options for advanced POP, although sturdier, ‘space-filling’ designs, such as the Gelhorn pessary, may be required (Powers et al 2006). In advanced POP, double pessaries are occasionally used (Singh and Reid 2001).
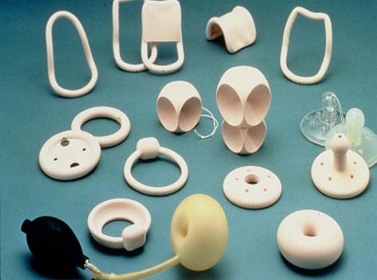
Figure 55.8 Different types of vaginal support pessaries.
Elneil S (2009) Complex pelvic floor failure and associated problems. Best Practice & Research Clinical Gastroenterology 23: 555–573.
Fitting a pessary is based on a clinical assessment of vaginal length, introital size and severity of POP, and is often a process of trial and error. A pessary which is too small will not be retained, whereas one that is too large will be uncomfortable. The aim should be to place the largest size of which the patient is unaware. Most women will require trials of more than one option (Hanson et al 2006), and it is good practice to schedule a return appointment to the clinic 1–2 weeks following the initial fitting to assess discomfort and the development of vaginal bleeding or de-novo incontinence. Results from multiple studies suggest that pessaries can be successfully fitted in approximately two-thirds of candidates. Reported predictors of an unsuccessful fitting include prior prolapse surgery or hysterectomy, younger age, higher parity and obesity (Mutone et al 2005, Nguyen and Jones 2005). Local oestrogen cream improves the success rate, even for patients taking systemic hormone replacement, and should be offered to all women (Hanson et al 2006). Women in whom pessaries are successfully fitted usually require assessment, vaginal examination and pessary removal/cleaning/reinsertion every 4–6 months, although there is no clear consensus on the most appropriate follow-up protocol. For those who can be taught self-removal, less frequent visits are reasonable. Prolonged use can lead to vaginal erosion with subsequent granulation formation. This can cause vaginal discharge and bleeding, which are common reasons for discontinuation. However, serious complications such as fistula, and urinary and bowel obstruction are rare and are usually limited to pessaries that have been neglected (Arias et al 2008).
Surgery
Women with symptomatic POP who decline or fail pessary management require surgical intervention. The surgical procedures and approaches available to women are rapidly evolving, due primarily to concerns about the longevity of more traditional prolapse repairs. The aims of surgical intervention are to restore anatomy, relieve symptoms, and preserve or restore urinary, bowel and sexual function. Among US women undergoing prolapse surgery, 20% will have a concomitant anti-incontinence procedure (Brown et al 2002).
In patients considering surgical management of POP, a fastidious preoperative work up is essential as a number of these patients have significant comorbidities. During surgery, the use of Allen legs in the vaginal and laparoscopic procedures will reduce iatrogenic injury to the patient and allow safer manipulation of the legs. The use of self-retaining retractors, such as the ReeTrakt (Insightra Medical, Irvine, CA, USA), will aid vaginal surgery significantly (Figure 55.9). Following surgery, attention should be paid to thromboprophylaxis and prophylactic antibiotic coverage. Appropriate dietary advice, fluid intake and, if necessary, stool softeners should be given to avoid the woman straining at stool in the immediate postoperative period.
Options for surgical repair of POP can be classified by compartment and further subdivided into vaginal and abdominal approaches. However, it is important to remember that prolapse of a single compartment is rare, and failure to repair all defects may result in higher recurrence rates. Level I evidence supports a superior restoration of anatomy via the abdominal route, which may be offset partly by higher short-term morbidity associated with a more invasive approach (Maher et al 2007, Brubaker et al 2009). Comparison of different surgical techniques for POP is made difficult by the lack of consensus on outcome measures, which include subjective cure by symptoms, anatomical cure by examination and/or the need for reoperation for recurrent POP.
Surgery for apical prolapse
The apex is the cornerstone of good pelvic organ support, and if the uterus or vaginal vault is poorly supported, the posterior and, in particular, the anterior vaginal wall are exposed to intra-abdominal forces (Lowder et al 2008). Optimal surgical repair of advanced anterior compartment prolapse will often necessitate an apical support procedure (Rooney et al 2006). Abdominal sacrocolpopexy and vaginal sacrospinous ligament suspension are the two most common procedures performed for apical prolapse. Abdominal sacrocolpopexy involves suspending the vaginal apex to the anterior longitudinal ligament of the sacrum using an intervening material, typically a synthetic mesh. Traditionally, this is performed via a Pfannenstiel incision, and reported anatomical success rates range from 74% to 100% (Hilger et al 2003, Nygaard et al 2004). Laparoscopic sacrocolpopexy aims to minimize the morbidity while retaining the benefits associated with abdominal sacrocolpopexy (Figure 55.10). Although no randomized trials comparing these two approaches with sacrocolpopexy have been published, medium-term observational data from more than 1000 patients have shown a 94% satisfaction rate and 6% need for reoperation (Ganatra et al 2009).
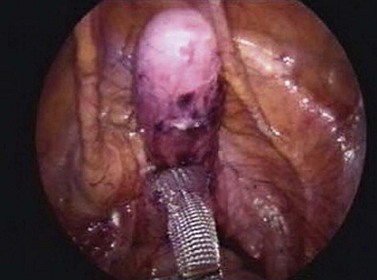
Figure 55.10 Mesh applied to the perineal body and on to part of the vaginal vault during a laparoscopic sacrocolpopexy procedure.
Elneil S (2009) Complex pelvic floor failure and associated problems. Best Practice & Research Clinical Gastroenterology 23: 555–573.
Sacrospinous ligament suspension (sacrospinous colpopexy) suspends the vaginal apex to the lateral aspect of the sacrospinous ligament using permanent suture material. Typically, the sacrospinous ligament suspension uses an extraperitoneal approach to the right sacrospinous ligament, although bilateral fixation may be performed. Reported anatomical cure rates following sacrospinous fixation range from 63% to 97% (Sze and Karram 1997, Maher et al 2004). Meta-analysis comparing abdominal sacrocolpopexy with sacrospinous ligament suspension found lower rates of recurrent vault prolapse [relative risk (RR) 0.23] and dyspareunia (RR 0.39), but a longer operating time (on average 21 min), increased cost and increased risk of mesh erosion in women undergoing abdominal sacrocolpopexy (Maher et al 2007). Mesh erosion complicates 2–11% of sacrocolpopexies (Nygaard et al 2004), with higher rates among smokers and, in some studies, women undergoing concomitant hysterectomy (Cundiff et al 2008). It may be reasonable to offer younger women the higher success rate with sacrocolpopexy; in older, frailer patients, the reduced morbidity and quicker return to daily activities associated with sacrospinous ligament suspension may be more appropriate. In addition to abdominal sacrocolpopexy and sacrospinous ligament suspension, a number of other procedures for apical prolapse have been described. Iliococcygeus suspension involves suspension of the vaginal vault to the iliococcygeus fascia over the levator plate. Theoretically, this is meant to reduce anterior wall recurrence and pudendal neurovascular injury compared with sacrospinous ligament suspension, although data are sparse and limited to case series (Meeks et al 1994, Maher et al 2001). Uterosacral ligament suspension is also performed vaginally and, although originally described 80 years ago (Miller 1927), attention has once again started to focus on the uterosacral ligament as a potential site for apical suspension (Shull et al 2000, Silva et al 2006), although ureteric injury remains a concern and randomized trials are needed.
Surgery for anterior compartment prolapse
The traditional procedure for anterior compartment prolapse has been the anterior colporrhaphy (Kelly 1913). Many variations have been described, involving midline plication of the vaginal muscularis and adventitia followed by excision and/or plication of redundant vaginal wall mucosa. Case series have reported success rates of 80–100% for anterior colporrhaphy; however, prospective randomized trials have shown the success of anterior colporrhaphy alone to be only 37–57% (Sand et al 2001, Weber et al 2001). It is likely that such variable rates of success result from the heterogeneous nature of anterior compartment prolapse and different surgical techniques. In addition to midline defects of the endopelvic fascia, lateral or paravaginal defects resulting from detachment of the lateral vaginal wall from the arcus tendineus fascia pelvis (‘white line’) can also give rise to anterior support abnormalities. This concept of ‘site-specific’ anterior vaginal wall defects was introduced by George White 100 years ago (White 1909), and subsequently repopularized by Cullen Richardson in the 1970s (Richardson et al 1976). Originally, this procedure was described as a treatment for stress urinary incontinence, but significant benefits for anterior wall support were demonstrated. Paravaginal repair can be approached both transabdominally and transvaginally, which largely depends on surgeon preference as no direct comparison has been performed. The procedure involves reapproximating the white line with the thickened lateral portion of the fascia overlying the obturator internus muscle. Similar success rates have been reported from both abdominal paravaginal repair (76–97%) and vaginal paravaginal repair (67–100%), although the transvaginal approach is technically more difficult (Brubaker et al 2009). Currently published data on laparoscopic paravaginal repair are sparse; of the studies reported, many include a concomitant Burch colposuspension (Diwadkar et al 2008). In one prospective study, the objective cure rate of laparoscopic paravaginal repair at 14 months was 74% (Behnia-Willison et al 2007).
Given the relatively high failure rate for anterior colporrhaphy and following on from the successful use of grafts in abdominal hernia repair and tension-free vaginal tape, there has been a recent trend towards the use of graft materials in prolapse surgery to augment more traditional repairs (Sung et al 2008, Jakus et al 2008). However, the overall advantage of mesh and other graft materials has not been definitively proven, as the potential benefit in reducing failure rates must be balanced against the risk of mesh-related complications. Indeed, the appropriate use of reconstructive materials in prolapse surgery has become one of the most debated issues in contemporary gynaecological practice, with most authorities acknowledging the risk of adverse events and the lack of data on long-term functional outcomes (Murphy 2008, Brubaker et al 2009). Grafts may be biological (allograft, autograft or xenograft) or synthetic in nature. Synthetic materials are further subclassified as absorbable or non-absorbable and by pore size [macroporous (>75 µm) or microporous (<75 µm)]. A meta-analysis of ten randomized controlled trials found that at 1 year post-operatively, women who had anterior repair with graft use had a lower rate of recurrence than women undergoing standard ‘native tissue’ repair (odds ratios 0.56 and 0.44 for biological and absorbable synthetic mesh, respectively) (Foon et al 2008).
The risk of mesh-related complications — predominantly mesh erosion, infection, mesh shrinkage and fibrosis — remains a concern (Bako and Dhar 2009, Figure 55.11). Additionally, since 2004, trocar-guided transvaginal surgical mesh ‘kits’ have gained rapid widespread acceptance due to the suggestion that reconstructive materials in prolapse surgery require ‘anchoring’ outside of the affected tissues (Elmér et al 2009). A variety of armed mesh kits are currently available, including the Perigee/Apogee (American Medical Systems), the Prolift (Johnson & Johnson) and the Posterior Intravaginal Slingplasty (Tyco Healthcare).
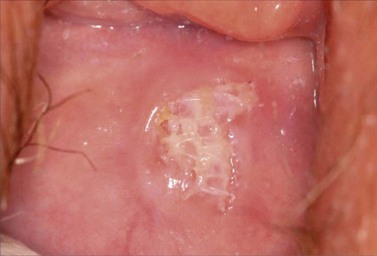
Figure 55.11 Mesh erosion seen on posterior vaginal wall.
Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369:1027–38.
However, randomized data to support the use of mesh kits are sparse. Furthermore, in addition to the risk of ‘mesh-related’ complications noted above, these kits also have the added risk of ‘trocar-related’ complications, including bladder/rectal perforation and vascular injury (Feiner et al 2009). A different procedure, using a trocarless mesh and a silicone vaginal device to support the graft during tissue in-growth, has been described (Carey et al 2008, Figure 55.12). The same authors recently reported a modification of this technique, which uses a silicone device and inflatable balloon to support the mesh repair (Gynecare Prosima, Ethicon, Somerville, NJ, USA; Figure 55.13). A recent multicentre study described an 88% success rate at 1 year for women with stage II and III prolapse following this procedure. The vaginal support device was shown to be well tolerated (Slack et al 2009).
Surgery for posterior compartment prolapse
The standard surgical procedure for posterior compartment prolapse is the posterior colporrhaphy, involving midline plication of the rectovaginal fascia. The traditional description of this procedure also involves midline plication of the levator ani muscles (Francis and Jeffcoate 1961), although this can result in high rates of postoperative dyspareunia among sexually active women. Analogous to the anterior compartment, site-specific repair of posterior defects has also been described (Richardson 1993), whereby rectovaginal defects are identified by a rectal finger. A prospective randomized trial found higher anatomical cures at 1 year in the colporrhaphy group (86%) compared with the site-specific repair group (78%) (Paraiso et al 2006). In addition, modification of the standard sacrocolpopexy to include extension of the posterior mesh to the rectovaginal septum or perineal body can also benefit posterior prolapse.
Although gynaecologists conventionally repair posterior compartment prolapse transvaginally in the lithotomy position, the colorectal surgeon typically performs a transanal repair. Level I evidence to support the superiority of the transvaginal repair over the transanal approach has been published (Nieminen et al 2004). There is currently no evidence to support the routine use of graft augmentation in the surgical repair of posterior compartment prolapse (Brubaker et al 2009).
Laparoscopic/robotic pelvic floor repair
A variety of laparoscopic reconstructive procedures have been described which aim to improve anatomical detail, minimize surgical morbidity and reduce hospital stay, while retaining the advantages of the abdominal operation. Paravaginal repair, sacrocolpopexy, enterocele repair and uterosacral ligament suspension have all been described laparoscopcially (Paraiso et al 2006, Behnia-Willison et al 2007). However, in many cases, direct comparison with the gold standard has not been performed, and the efficacy of a standard surgical technique should not be compromised simply so that the procedure can be completed laparoscopically.
To date, robotic surgery has been most widely accepted in urological oncology. Recently, a limited number of publications have described the use of robotic systems in achieving sacrocolpopexy, with 95% success at 24 months, albeit with small numbers (Elliott et al 2006, Akl et al 2009). Robotic-assisted laparoscopic hysterectomy has also been reported (Reynolds and Advincula 2006). It is unlikely, however, that robotics will significantly impact pelvic reconstructive surgery, at least in the short term.
Obliterative surgical procedures
In addition to the reconstructive surgical procedures outlined above, women who do not wish to preserve coital function may be suitable for a vaginal obliterative procedure. Colpocleisis is a relatively straightforward surgery associated with high cure rates and fast recovery times (Hullfish et al 2007). It may be performed in women with or without a uterus, and involves excision of rectangular pieces of vaginal epithelium anteriorly and posteriorly, vaginal inversion and closure with sutures, thus obliterating the vaginal space. Among appropriately selected women, studies have demonstrated low rates of regret for loss of sexual function (Wheeler et al 2005). There is a risk of stress urinary incontinence developing after colpocleisis, and a concomitant midurethral tape may be appropriate in selected cases.
Conclusions
KEY POINTS
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 85. Pelvic Organ Prolapse. ACOG; 2007.
Altman D, Forsman M, Falconer C, Lichtenstein P. Genetic influence on stress urinary incontinence and pelvic organ prolapse. European Urology. 2008;54:918-922.
Akl MN, Long JB, Giles DL, et al. Robotic-assisted sacrocolpopexy: technique and learning curve. Surgical Endoscopy. 2009;23:2390-2394.
Arias BE, Ridgeway B, Barber MD. Complications of neglected vaginal pessaries: case presentation and literature review. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1173-1178.
Bako A, Dhar R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20:103-111.
Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology. 2002;99:281-289.
Behnia-Willison F, Seman EI, Cook JR, O’Shea RT, Keirse MJ. Laparoscopic paravaginal repair of anterior compartment prolapse. Journal of Minimally Invasive Gynecology. 2007;14:475-480.
Blandon RE, Bharucha AE, Melton LJ3rd, et al. Incidence of pelvic floor repair after hysterectomy: a population-based cohort study. American Journal of Obstetrics and Gynecology. 2007;197:664.e1-664.e7.
Bø K. Can pelvic floor muscle training prevent and treat pelvic organ prolapse? Acta Obstetricia et Gynecologica Scandinavica. 2006;85:263-268.
Bradley CS, Zimmerman MB, Qi Y, Nygaard IE. Natural history of pelvic organ prolapse in postmenopausal women. Obstetrics and Gynecology. 2007;109:848-854.
Broekhuis SR, Fütterer JJ, Barentsz JO, Vierhout ME, Kluivers KB. A systematic review of clinical studies on dynamic magnetic resonance imaging of pelvic organ prolapse: the use of reference lines and anatomical landmarks. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20:721-729.
Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. American Journal of Obstetrics and Gynecology. 2002;186:712-716.
Brubaker L, Glazener C, Jacquetin B, et al. Surgery for pelvic organ prolapse. Paris. Abrams P, Cardozo L, Koury S, et al, editors. 4th International Consultation on Incontinence. 2009, 1273-1320.
Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. American Journal of Obstetrics and Gynecology. 1996;175:10-17.
Burrows LJ, Meyn LA, Walters MD, Weber AM. Pelvic symptoms in women with pelvic organ prolapse. Obstetrics and Gynecology. 2004;104:982-988.
Carey M, Slack M, Higgs P, Wynn-Williams M, Cornish A. Vaginal surgery for pelvic organ prolapse using mesh and a vaginal support device. BJOG: an International Journal of Obstetrics and Gynaecology. 2008;115:391-397.
Chene G, Tardieu AS, Savary D, et al. Anatomical and functional results of McCall culdoplasty in the prevention of enteroceles and vaginal vault prolapse after vaginal hysterectomy. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1007-1011.
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. American Journal of Obstetrics and Gynecology. 1999;180:859-865.
Cundiff GW, Varner E, Visco AG, et al. Risk factors for mesh/suture erosion following sacral colpopexy. American Journal of Obstetrics and Gynecology. 2008;199:688.e1-688.e5.
Cundiff GW, Weidner AC, Visco AG, Bump RC, Addison WA. A survey of pessary use by members of the American Urogynecologic Society. Obstetrics and Gynecology. 2000;95:931-935.
Dällenbach P, Kaelin-Gambirasio I, Jacob S, Dubuisson JB, Boulvain M. Incidence rate and risk factors for vaginal vault prolapse repair after hysterectomy. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1623-1629.
DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. American Journal of Obstetrics and Gynecology. 1992;166:1717-1724.
DeLancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. American Journal of Obstetrics and Gynecology. 2002;187:93-98.
de Tayrac R, Gervaise A, Chauveaud-Lambling A, Fernandez H. Combined genital prolapse repair reinforced with a polypropylene mesh and tension-free vaginal tape in women with genital prolapse and stress urinary incontinence: a retrospective case–control study with short-term follow-up. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:950-954.
Dietz HP. Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound in Obstetrics and Gynecology. 2004;23:615-625.
Dietz HP. Childbirth and pelvic floor trauma. Best Practice and Research Clinical Obstetrics and Gynaecology. 2005;19:913-924.
Dietz HP. The aetiology of prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1323-1329.
Dietz HP. Prolapse worsens with age, doesn’t it? Australian and New Zealand Journal of Obstetrics and Gynaecology. 2008;48:587-591.
Digesu GA, Salvatore S, Chaliha C, Athanasiou S, Milani R, Khullar V. Do overactive bladder symptoms improve after repair of anterior vaginal wall prolapse? International Urogynecology Journal and Pelvic Floor Dysfunction. 2007;18:1439-1443.
Diwadkar GB, Chen CC, Paraiso MF. An update on the laparoscopic approach to urogynecology and pelvic reconstructive procedures. Current Opinion in Obstetrics and Gynecology. 2008;20:496-500.
Ellerkmann RM, Cundiff GW, Melick CF, Nihira MA, Leffler K, Bent AE. Correlation of symptoms with location and severity of pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2001;185:1332-1337.
Elliott DS, Krambeck AE, Chow GK. Long-term results of robotic assisted laparoscopic sacrocolpopexy for the treatment of high grade vaginal vault prolapse. Journal of Urology. 2006;176:655-659.
Elmér C, Altman D, Engh ME, Axelsen S, Väyrynen T, Falconer C. Trocar-guided transvaginal mesh repair of pelvic organ prolapse. Obstetrics and Gynecology. 2009;113:117-126.
Elneil S. Complex pelvic floor failure and associated problems. Best Practice and Research Clinical Gastroenterology. 2009;23:555-573.
Fatton B. Is there any evidence to advocate SUI prevention in continent women undergoing prolapse repair? An overview. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20:235-245.
Feiner B, Jelovsek JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse of the vaginal apex: a systematic review. BJOG: an International Journal of Obstetrics and Gynaecology. 2009;116:15-24.
Foon R, Toozs-Hobson P, Latthe PM. Adjuvant materials in anterior vaginal wall prolapse surgery: a systematic review of effectiveness and complications. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1697-1706.
Francis WJ, Jeffcoate TN. Dyspareunia following vaginal operations. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1961;68:1-10.
Fritel X, Varnoux N, Zins M, Breart G, Ringa V. Symptomatic pelvic organ prolapse at midlife, quality of life, and risk factors. Obstetrics and Gynecology. 2009;113:609-616.
Ganatra AM, Rozet F, Sanchez-Salas R, et al. The current status of laparoscopic sacrocolpopexy: a review. European Urology. 2009;55:1089-1130.
Hagen S, Stark D, Glazener C, Sinclair L, Ramsay I. A randomized controlled trial of pelvic floor muscle training for stages I and II pelvic organ prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20:45-51.
Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. American Journal of Obstetrics and Gynecology. 1996;175:1467-1470.
Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. American Journal of Obstetrics and Gynecology. 2004;190:27-32.
Hanson LA, Schulz JA, Flood CG, Cooley B, Tam F. Vaginal pessaries in managing women with pelvic organ prolapse and urinary incontinence: patient characteristics and factors contributing to success. International Urogynecology Journal and Pelvic Floor Dysfunction. 2006;1:155-159.
Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women’s Health Initiative: gravity and gravidity. American Journal of Obstetrics and Gynecology. 2002;186:1160-1166.
Hilger WS, Poulson M, Norton PA. Long-term results of abdominal sacrocolpopexy. American Journal of Obstetrics and Gynecology. 2003;189:1606-1610.
Hullfish KL, Bovbjerg VE, Steers WD. Colpocleisis for pelvic organ prolapse: patient goals, quality of life, and satisfaction. Obstetrics and Gynecology. 2007;110:341-345.
Jackson SL, Weber AM, Hull TL, Mitchinson AR, Walters MD. Fecal incontinence in women with urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology. 1997;89:423-427.
Jakus SM, Shapiro A, Hall CD. Biologic and synthetic graft use in pelvic surgery: a review. Obstetrical and Gynecological Survey. 2008;63:253-266.
Jelovsek JE, Barber MD, Paraiso MF, Walters MD. Functional bowel and anorectal disorders in patients with pelvic organ prolapse and incontinence. American Journal of Obstetrics and Gynecology. 2005;193:2105-2111.
Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. The Lancet. 2007;369:1027-1038.
Kelly HA. Incontinence of urine in women. Urologic and Cutaneous Review. 1913;17:291-293.
Kluivers KB, Hendriks JC, Shek C, Dietz HP. Pelvic organ prolapse symptoms in relation to POPQ, ordinal stages and ultrasound prolapse assessment. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1299-1302.
Kudish BI, Iglesia CB, Sokol RJ, et al. Effect of weight change on natural history of pelvic organ prolapse. Obstetrics and Gynecology. 2009;113:81-88.
Law YM, Fielding JR. MRI of pelvic floor dysfunction: review. AJR American Journal of Roentgenology. 2008;191:S45-S53.
Lowder JL, Park AJ, Ellison R, et al. The role of apical vaginal support in the appearance of anterior and posterior vaginal prolapse. Obstetrics and Gynecology. 2008;111:152-157.
Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstetrics and Gynecology. 2006;107:1253-1260.
Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM. Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstetrics and Gynecology. 2001;98:40-44.
Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. American Journal of Obstetrics and Gynecology. 2004;190:20-26.
Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S 2007 Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 18: CD004014.
Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. BJOG: British Journal of Obstetrics and Gynaecology. 1997;104:579-585.
McLennan MT, Harris JK, Kariuki B, Meyer S. Family history as a risk factor for pelvic organ prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:1063-1069.
Meeks GR, Washburne JF, McGehee RP, Wiser WL. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. American Journal of Obstetrics and Gynecology. 1994;171:1444-1452.
Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Symptoms and pelvic support defects in specific compartments. Obstetrics and Gynecology. 2008;112:851-858.
Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstetrics and Gynecology. 2009;113:1089-1097.
Miller N. A new method of correcting complete inversion of the vagina. Surgery, Gynecology and Obstetrics. 1927;44:550-554.
Mouritsen L. Classification and evaluation of prolapse. Best Practice and Research Clinical Obstetrics and Gynaecology. 2005;19:895-911.
Murphy M. Clinical practice guidelines on vaginal graft use from the society of gynecologic surgeons. Obstetrics and Gynecology. 2008;112:1123-1130.
Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2005;193:89-94.
Nguyen JK, Bhatia NN. Resolution of motor urge incontinence after surgical repair of pelvic organ prolapse. Journal of Urology. 2001;166:2263-2266.
Nguyen JN, Jones CR. Pessary treatment of pelvic relaxation: factors affecting successful fitting and continued use. Journal of Wound Ostomy and Continence Nursing. 2005;32:255-261.
Nieminen K, Hiltunen KM, Laitinen J, Oksala J, Heinonen PK. Transanal or vaginal approach to rectocele repair: a prospective, randomized pilot study. Diseases of the Colon and Rectum. 2004;47:1636-1642.
Novi JM, Jeronis S, Morgan MA, Arya LA. Sexual function in women with pelvic organ prolapse compared to women without pelvic organ prolapse. Journal of Urology. 2005;173:1669-1672.
Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstetrics and Gynecology. 2004;104:805-823.
Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. Journal of the American Medical Association. 2008;300:1311-1316.
O’Boyle AL, O’Boyle JD, Calhoun B, Davis GD. Pelvic organ support in pregnancy and postpartum. International Urogynecology Journal and Pelvic Floor Dysfunction. 2005;16:69-72.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstetrics and Gynecology. 1997;89:501-506.
Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. American Journal of Obstetrics and Gynecology. 2006;195:1762-1771.
Pauls RN, Segal JL, Andre Silva W, Kleeman SD, Karram MM. Sexual function in patients presenting to a urogynecology practice. International Urogynecology Journal and Pelvic Floor Dysfunction. 2006;17:576-580.
Powers K, Lazarou G, Wang A, et al. Pessary use in advanced pelvic organ prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2006;17:160-164.
Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterology Clinics of North America. 2008;37:493-509.
Reynolds RK, Advincula AP. Robot-assisted laparoscopic hysterectomy: technique and initial experience. American Journal of Surgery. 2006;191:555-560.
Richardson AC. The rectovaginal septum revisited: its relationship to rectocele and its importance in rectocele repair. Clinical Obstetrics and Gynecology. 1993;36:976-983.
Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. American Journal of Obstetrics and Gynecology. 1976;126:568-573.
Richter HE. Cesarean delivery on maternal request versus planned vaginal delivery: impact on development of pelvic organ prolapse. Seminars in Perinatology. 2006;30:272-275.
Rogers GR, Villarreal A, Kammerer-Doak D, Qualls C. Sexual function in women with and without urinary incontinence and/or pelvic organ prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2001;12:361-365.
Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. American Journal of Obstetrics and Gynecology. 2006;195:1837-1840.
Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. International Urogynecology Journal and Pelvic Floor Dysfunction. 2007;18:455-460.
Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstetrics and Gynecology. 2007;109:1396-1403.
Royal College of Obstetricians and Gynaecologists. Green-Top Guideline No. 46. The Management of Post-hysterectomy Vaginal Vault Prolapse. London: RCOG; 2007.
Samuelsson EC, Arne Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. American Journal of Obstetrics and Gynecology. 1999;180:299-305.
Sand PK, Koduri S, Lobel RW, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. American Journal of Obstetrics and Gynecology. 2001;184:1357-1362.
Shah SM, Sultan AH, Thakar R. The history and evolution of pessaries for pelvic organ prolapse. International Urogynecology Journal and Pelvic Floor Dysfunction. 2006;17:170-175.
Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. American Journal of Obstetrics and Gynecology. 2000;183:1365-1373.
Singh K, Reid WM. Non-surgical treatment of uterovaginal prolapse using double vaginal rings. BJOG: an International Journal of Obstetrics and Gynaecology. 2001;108:112-113.
Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstetrics and Gynecology. 2006;108:255-263.
Slack M, Zyczynski H, Reisenauer C, et al. A new operation for vaginal prolapse repair using mesh and a vaginal support device: 1 year anatomic and functional results of an international multicentre study. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20(Suppl):S157-S158.
Smith PP, Appell RA. Pelvic organ prolapse and the lower urinary tract: the relationship of vaginal prolapse to stress urinary incontinence. Current Urology Reports. 2005;6:340-347.
Steensma AB, Oom DM, Burger CW, Rudolph Schouten W. Assessment of posterior compartment prolapse; a comparison of evacuation proctography and 3D transperineal ultrasound. Colorectal Disease. 2009;12:533-539.
Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. New England Journal of Medicine. 2009;360:481-490.
Subramanian D, Szwarcensztein K, Mauskopf JA, Slack MC. Rate, type, and cost of pelvic organ prolapse surgery in Germany, France, and England. European Journal of Obstetrics, Gynecology and Reproductive Biology. 2009;144:177-181.
Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstetrics and Gynecology. 2008;112:1131-1142.
Swift SE, Herring M. Comparison of pelvic organ prolapse in the dorsal lithotomy compared with the standing position. Obstetrics and Gynecology. 1998;91:961-964.
Swift SE, Tate SB, Nicholas J. Correlation of symptoms with degree of pelvic organ support in a general population of women: what is pelvic organ prolapse? American Journal of Obstetrics and Gynecology. 2003;189:372-377.
Sze EH, Karram MM. Transvaginal repair of vault prolapse: a review. Obstetrics and Gynecology. 1997;89:466-467.
Sze EH, Sherard GB3rd, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstetrics and Gynecology. 2002;100:981-986.
Taylor SA. Imaging pelvic floor dysfunction. Best Practice and Research Clinical Gastroenterology. 2009;23:487-503.
Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. International Urogynecology Journal and Pelvic Floor Dysfunction. 2005;16:497-503.
Tegerstedt G, Miedel A, Maehle-Schmidt M, Nyrén O, Hammarström M. Obstetric risk factors for symptomatic prolapse: a population-based approach. American Journal of Obstetrics and Gynecology. 2006;194:75-81.
Thakar R. Review of current status of female sexual dysfunction evaluation in urogynecology. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20(Suppl 1):S27-S31.
Uma R, Libby G, Murphy DJ. Obstetric management of a woman’s first delivery and the implications for pelvic floor surgery in later life. BJOG: an International Journal of Obstetrics and Gynaecology. 2005;112:1043-1046.
Visco AG, Brubaker L, Nygaard I, et al. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. International Urogynecology Journal and Pelvic Floor Dysfunction. 2008;19:607-614.
Weber AM. Elective cesarean delivery: the pelvic perspective. Clinical Obstetrics and Gynecology. 2007;50:510-517.
Weber AM, Walters MD, Schover LR, Mitchinson A. Vaginal anatomy and sexual function. Obstetrics and Gynecology. 1995;86:946-949.
Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. American Journal of Obstetrics and Gynecology. 2001;185:1299-1304.
Wheeler TL2nd, Richter HE, Burgio KL, et al. Regret, satisfaction, and symptom improvement: analysis of the impact of partial colpocleisis for the management of severe pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2005;193:2067-2070.
Whitcomb EL, Lukacz ES, Lawrence JM, Nager CW, Luber KM. Prevalence and degree of bother from pelvic floor disorders in obese women. International Urogynecology Journal and Pelvic Floor Dysfunction. 2009;20:289-294.
White GR. Cystocele: a radical cure by suturing lateral sulci of vagina to white line of pelvic fascia. Journal of the American Medical Association. 1909;21:1707-1710.
Yalcin OT, Yildirim A, Hassa H. The effects of severe cystocele on urogynecologic symptoms and findings. Acta Obstetricia et Gynecologica Scandinavica. 2001;80:423-427.