CHAPTER 62 Pelvic inflammatory disease
Introduction
The World Health Organization (WHO) guide to essential practice, ‘Integrating STI/RTI Care for Reproductive Health: Sexually Transmitted and Other Reproductive Tract Infections’, defined reproductive tract infections (RTIs) as a broad term that includes sexually transmitted infections (STIs) as well as other infections that are not sexually transmitted (World Health Organization 2005). Not all STIs are RTIs, a reference to the location of the infection, and not all RTIs are STIs. In most instances, STIs have much more severe health consequences than other RTIs, and the terms ‘STI’ and ‘RTI’ are often used together to highlight the importance of STIs within RTIs.
This chapter will address PID as an upper genital tract infection that may be caused by organisms normally present in the reproductive tract (endogenous), or introduced from the outside during sexual contact (STI) or medical procedures (iatrogenic), and will outline its consequences and management as well as wider global prevention. Readers are also referred to Chapter 63 on non-HIV STIs.
Epidemiology
Epidemiological studies of PID are adversely affected by diagnostic and reporting uncertainties. The symptoms are often subtle and mild, and it is common that many cases of PID are missed because women or healthcare professionals fail to recognize mild or non-specific symptoms and their implications. As there are no simple, safe and specific diagnostic tests for PID, Simms et al (2003) proposed that it was time to rethink its diagnosis.
To complicate matters, adolescent and young adult women are reported to have anxiety surrounding pelvic examination (Millstein et al 1984), and in another study, over half of teens screened for STIs by urine testing and pelvic examination preferred the urine screening (Serlin et al 2002) for evaluating genitourinary (GU) symptoms without undergoing a speculum examination. Such new approaches may encourage young women who fear or otherwise avoid pelvic examinations to receive more timely evaluation, and could also result in access to diagnostic services in settings that do not currently provide them, such as school-based health centres, community centres and detention facilities, enabling clinicians to diagnose and treat many high-risk youth whose infections might otherwise go undetected (Burstein et al 1998, Pack et al 2000).
Estimates of prevalence
Adolescent and young adult women account for nearly half of over 1 million cases of PID reported annually in the USA (Washington and Katz 1991). Chlamydia infection is the most common bacterial STI in the USA, with more than 2.8 million new cases estimated to occur each year (Weinstock et al 2004); this is the most common cause of PID. During 2007, approximately 1.1 million cases of chlamydia were reported to the Centers for Disease Control and Prevention (CDC); more than half of these were in females aged 15–25 years (Centers for Disease Control and Prevention 2009a).The annual incidence and prevalence of STIs is difficult to calculate. The incidence of STIs may be estimated on the bases of nationally notifiable diseases, national surveys, WHO reports and medical literature in this area. There is almost certainly under reporting due to the frequently asymptomatic nature of the disease, and the true annual incidence and prevalence are very likely to be much higher. A recent trend in developed countries highlights a shift in the microbial aetiology of PID, with an increasing role of chlamydia and a decreased role of gonococcal infection (van der Heyden et al 2000). Similarly, PID is now a disease that is mainly diagnosed in primary care or other similar outpatient environments. Since the advent of sensitive molecular amplification tests that permit non-invasive diagnosis of chlamydia, epidemiological evidence suggests that the proportion of chlamydial PID is increasing compared with other causative agents (Hughes et al 2001). There is also evidence that a significant number of chlamydial infections are asymptomatic, which allows silent cross-infection, and if untreated remains infectious in male and female hosts for months. Joyner et al (2002) demonstrated the persistence of chlamydia in 87% of men and women. WHO estimated 89 million new cases of genital chlamydial infections worldwide in 1995 and 92 million in 1999 (Peeling et al 1998, World Health Organization 2001).
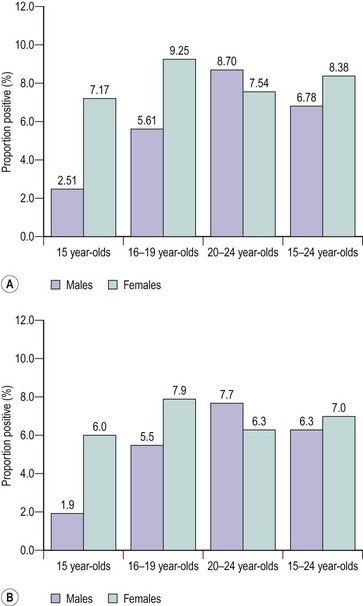
In the UK, STI surveillance systems are based on data from GU medicine clinics and laboratory reports submitted to the Health Protection Agency Centre for Infections. However, this does not take into account the substantial number of relatively asymptomatic STIs that are diagnosed and treated in primary care settings. Simms et al (2006) examined the strengths and weaknesses of the Royal College of General Practitioners’ (RCOG) Weekly Returns Service to determine the incidence of a range of STIs and associated clinical conditions. In their study, data were collated from 78 sentinel general practices in England and Wales between 1994 and 2001, covering a population of approximately 600,000. The authors reported that candidiasis was the most common condition reported in both men and women, followed by PID in women. The mean annual incidences per 100,000 women between 15 and 44 years of age were as follows: PID, 1787; vaginal discharge, 285; non-specific urethritis, 8.2; candidiasis, 4218; Trichomonas vaginalis, 28; genital herpes, 127; and genital warts, 184. Interestingly, the incidence of candidiasis and PID declined over this period in all groups, while the incidence of vaginal discharge doubled in 15–24 year olds over the same period. This decline in the incidence of PID is in marked contrast with the 148% increase in the diagnosis of chlamydial infections made in GU medicine clinics in the same period. The increase in diagnosis may have been brought about by an increase in case ascertainment, the increased availability of diagnostic facilities and an increase in disease incidence, while the decrease in PID probably reflects improved diagnosis and management of the syndrome. The aim of the National Chlamydia Screening Programme (NCSP) since its introduction in England in 2003 has been to control the infection through early detection and treatment of asymptomatic individuals. More than one and a half million (1,673,276) tests were performed on 15–24 year olds by the NCSP between 2003 and the end of July 2009, and a further 376,966 non-NCSP, non-GU medicine tests were reported. Interestingly, the test-positive rate has declined steadily for both men and women since the programme started. The average test-positive rates for screened women and men were 8.38% and 6.78% for 2008–2009, and 7% and 6.3% for the first quarter of 2009–2010, respectively. In both durations, the highest rates were found in women aged 16–19 years and men aged 20–24 years (Figure 62.1). Other risk groups were those reporting behavioural risk factors and certain ethnic groups (National Chlamydia Screening Programme 2009).
Pathogenesis and Pathogens
The main causative agent of PID in the UK is Chlamydia trachomatis. Other less common causes include gonorrhoea, mycoplasma, gardnerella and Gram-negative rods (British Association for Sexual Health and HIV 2005). Trichomonas vaginalis appears to have little, if any, role in the aetiology of PID. Bacterial vaginosis has been associated with PID, and the presence of bacterial vaginosis and leukocytes on vaginal slides is associated with a five-fold risk of PID (von Knorring and Wilson 2007). Other pathogens that should be considered, especially in immigrant women, include tuberculosis and schistosoma. Pelvic and genital tuberculosis is a bloodborne infection. Schistosoma infection should be considered in cases of granulomatous salpingitis (Kameh et al 2004). Actinomycosis is another causative agent of PID, and is often associated with intrauterine contraceptive device (IUCD) use.
Chlamydia
Chlamydia is a Gram-negative obligate intracellular parasite that can cause a variety of disease manifestations in humans. Chlamydia can be defined into different species depending upon its biochemical properties, although molecular analysis has led to further reclassification. In humans, serovars A–C cause conjunctivitis and resultant trachoma, whilst serovars D–K cause urethritis, cervicitis, ophthalmia noenatorum and neonatal pneumonia. LGV serovars produce a specific STI called lymphogranuloma venereum. Chlamydia has many features that are similar to a bacterium. It possesses a cell wall, hence it is sensitive towards penicillin, has the capacity to reproduce RNA and DNA, and is treatable with antibiotics. Unlike bacteria but like viruses, it must infect a host cell to reproduce. These characteristics initially made research difficult, but the application of genetic amplification tests resolved this problem. Chlamydia has a great affinity to adhere to the columnar epithelial cells of the endocervical canal, and also the epithelial cells of the endometrium and fallopian tube. Chlamydia is highly infectious in its extracellular state and consists of particles 0.2–0.4 µm in diameter, known as ‘elementary bodies’. These elementary bodies enter the cell via phagocytosis, but have the capacity to avoid immunological destruction by inhibiting fusion with lysosomes. Over the following 24 h, the elementary bodies expand into larger reticulate bodies. These bodies rapidly divide by a process of binary fission to create several intracellular inclusion bodies, each of which is crammed with thousands of new reticulate bodies. These reticulate bodies condense to form new elementary bodies to complete the replication cycle, and upon lysis of the infected cell, thousands of new highly infectious elementary bodies are released. Host cell destruction is, of course, a byproduct of the replication process, but this is not enough tissue damage to explain the significant upper genital tract damage that occurs with chlamydial infection. This is likely to be due to the host humoral and cell-mediated immune response to infection, and would explain the differences in the effect of the host genetics upon the degree of clinical infection and the long-term sequelae. It is interesting to note that primary infection of chlamydia in monkeys in self-limiting but repeat exposure results in tubal damage, supporting a delayed immune response (Agrawal et al 2007).
Source of entry
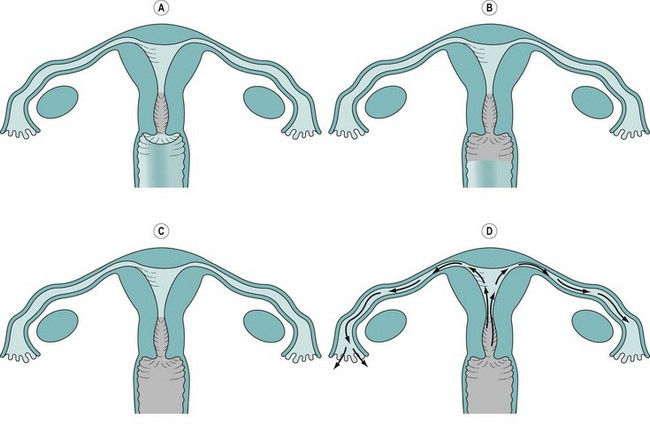
The majority of acute PID are ascending infections through the genital tract to the endocervix, which can act as a reservoir and spread to the endometrium and the peritoneal cavity via the fallopian tubes (Figure 62.2). There is evidence to suggest that the actual degree of symptoms and long-term complications of such infection are greatly dependent upon the type of infection and the host’s immune response. Once infection has gained access to the peritoneal cavity, it can cause a significant inflammatory response including the formation of pus and resultant abscess formation, especially the destructive tubo-ovarian abscess. In addition, pathogens can cause periappendicitis, perisplenitis and perihepatitis. A well-known but inadequately studied phenomenon is the perihepatic adhesions of Fitz–Hughes–Curtis syndrome. It is believed that chlamydia and gonococcus act as primary pathogens disrupting the normal protective barriers of infection, which then allows the clinical infection of other secondary pathogens including endogenous microbes, causing a polyinfection, frequently with anaerobic involvement.
Diagnosis
Symptoms and signs
Even the most experienced reflective clinician may face a dilemma in making a diagnosis of PID, or feel concerned about over- or underdiagnosing PID, primarily due to the lack of good evidence-based diagnostic criteria compounded by its polymicrobial aetiology. Common PID symptoms are outlined in Box 62.1.
The majority of women present with some but not all of these symptoms, which are not pathognomonic and account for the significant differences in determining PID rates among clinicians (Doxanakis et al 2008) and may result in failed treatments. In order to formulate an evidence-based approach to the diagnosis, several authors have attempted to generate a diagnostic scoring system to accept or refute the diagnosis of PID. A frequently used system is that published by Jacobson and Westrom (1969), whereby a diagnosis is made if acute lower abdominal/pelvic pain is accompanied by any two of the following features: abnormal vaginal bleeding, fever, vomiting, menstrual abnormalities, urinary symptoms, proctitis symptoms, marked pelvic tenderness, palpable mass or swelling, or erythrocyte sedimentation rate (ESR) >15 mm/h.
Although this is similar to the current guidelines of the RCOG, it remains a major concern that women with confirmed damaged fallopian tubes have no previous history of PID, mainly due to asymptomatic or atypical disease not being recognized (Sellors et al 1988). Munday’s (2000) comprehensive review of the literature reported that many of the studies undertaken to estimate the accuracy of PID symptoms as a ‘diagnostic test’ had significant methodological flaws. Several used laparoscopy as a gold standard, which unfortunately may not be true, as it was reported to be of limited value with only 64% of suspected PID cases in one series being confirmed at laparoscopy (Sellors et al 1991).
Simms et al (2003) analysed the signs and symptoms of potential PID to generate likelihood ratios, and reported that abnormal vaginal bleeding, fever, vomiting, menstrual irregularity, ongoing bleeding, symptoms of urethritis, marked tenderness of pelvic organs on bimanual palpation, adnexal mass and ESR >15 mm in the first hour did not have significantly high sensitivity or specificity to be clinically valuable, and concluded that there was insufficient evidence to support existing diagnostic criteria for PID.
Current consensus suggests that the positive predictive value of a clinical diagnosis of PID is between 65% and 90% when compared with laparoscopic findings, but this greatly depends upon the population studied (British Association for Sexual Health and HIV 2004). Adnexal tenderness has a particularly high sensitivity of 95%, but a very poor specificity of 22% (Peipert et al 2001).
The CDC factsheet (Centers for Disease Control and Prevention 2009b) indicates that the diagnosis is usually based on clinical findings, and if symptoms are present, a physical examination should be performed and further tests should be undertaken to identify the infection-causing organism. In view of the significant effects and long-term sequelae of untreated PID, it is wise to adopt a low threshold for treatment (Barrett and Taylor 2005) and to initiate early empirical treatment, although there is concern that this may lead to unnecessary treatment of women and there is no evidence to support or refute this strategy (Ross 2001a). With this in mind, clinicians should consider other risk assessments or investigations to improve diagnostic accuracy.
Risk assessment
Risk factors for STIs and PID are the same. In a longitudinal study, it was found that 39% of women with laparoscopically diagnosed PID were found to be infected with chlamydia and 14% with gonorrhoea (Bevan et al 1995). It is important to take a comprehensive sexual history from a patient with suspected PID. Risk factors include multiple sexual partners, lack of barrier contraception, young age and lower socioeconomic group. There is good evidence that anything that disrupts the cervical mucous barrier is a risk factor for PID. This includes uterine instrumentation, termination of pregnancy and insertion of an IUCD. There is significant evidence to support the fact that there are different levels of host susceptibility towards the development of PID, whereby polymorphism of mannose-binding lectin, an important component of the innate immune system and protector against chlamydial infection, may affect the extent of tubal damage in cases of chlamydial infection (Sziller et al 2008).
Evidence-Based Management
Investigations
Specific tests
Several tests may be employed for the diagnosis of PID, including microbiological swabs (endocervical, urinary, high and low vaginal swabs), inflammatory markers (C-reactive protein, ESR and differential white cell counts) and biophysical tests [transvaginal ultrasound scanning, Doppler scanning and magnetic resonance imaging (MRI) of the pelvis]. Microbiological tests remain the principal approach for the diagnosis of gonococcal and chlamydial infections, although the PID rate caused by the former is decreasing. Chlamydial tests have included cell culture, antibody tests and, more recently, nucleic acid amplification tests (NAAT), which allow greater accuracy at a relatively low cost. A recent meta-analysis suggested that pooled sensitivities for ligase chain reaction, polymerase chain reaction, gene probe and enzyme immunoassay of urine specimens were 96.5%, 85.6%, 92% and 38%, respectively, while on cervical swabs, the corresponding sensitivities were for PCR, gene probe and EIA 88.6%, 84% and 65%, respectively (Watson et al 2002). With any test, it is important to remember that the absence of lower genital tract infection does not exclude PID (Royal College of Obstetricians and Gynaecologists 2009).
Biochemical tests
Currently, it is recommended to perform endocervical swabs for chlamydia and gonorrhoea in suspected cases of PID (British Association for Sexual Health and HIV 2005). For chlamydia, NAAT performed on first-catch urine samples or vulvovaginal swabs are acceptable substitutes for endocervical swabs. Endocervical swabs for gonorrhoea should be sent in transport media and must arrive at the laboratory within 24 h. A positive NAAT for gonorrhoea should be confirmed by a positive culture test (British Association for Sexual Health and HIV 2005). Positive chlamydia and gonorrhoea tests (adjusted odds ratio 4.3, 95% confidence interval 2.89–2.63) are strongly associated with endometritis (Peipert et al 2001). Women with PID should be offered screening for other STIs (British Association for Sexual Health and HIV 2005).
Imaging tests
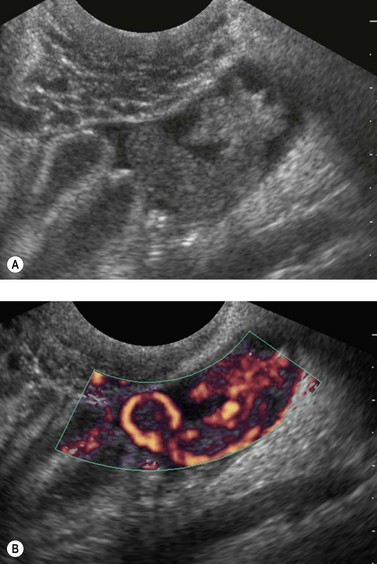
Ultrasound scanning, especially transvaginal scan (TVS) (Figure 62.3), is particularly helpful in the diagnosis of tubo-ovarian abscess, where thick-walled, pus-filled, inflammed tubes may be demonstrated, and also in diagnosing other pathologies (e.g. ovarian cyst torsion, cyst accidents). TVS may also be beneficial in the diagnosis of acute appendicitis. Ultrasound in the acute diagnostic setting has not been subjected to the rigours of a clinical trial. Preliminary data on its use as a follow-up tool reported that of 86 women followed-up 3 months after confirmed acute PID, five developed a hydrosalpinx (Taipale et al 2003). TVS has become a first-line investigative tool in emergency gynaecology, and at least 10% of all referrals for TVS are for the investigation of acute pelvic pain. TVS allows rapid assessment of pelvic anatomy with greater accuracy and diagnostic detail compared with that of a bimanual examination. Abdominal ultrasound can be used in cases of suspected appendicitis. Several other ultrasound features have been noted in cases of PID, including disruption of the normal pelvic anatomy, tenderness during TVS or the presence of free fluid in the pouch of Douglas. In a small study, the frequency of the ultrasound features of acute and chronic PID were assessed (Timor-Tritsch et al 1998). In the 14 patients with acute PID, a thickening of the fallopian wall was noted in all women, 12 demonstrated the ‘cog wheel’ sign and none of the patients demonstrated the ‘beads on a string’ sign. This was in marked contrast to women with chronic PID, where TVS could exclude other pathologies as well as distinguish between acute and chronic PID.
The addition of Doppler to grey-scale TVS may improve the sensitivity and specificity. Molander et al (2001) reported that an increase in the thickness of the fallopian tube walls was present in women with or without acute PID, but the application of Doppler ultrasound demonstrated the hyperaemia associated with acute PID. Pulsatility indices were significantly lower in women with acute PID compared with a control group. The use of power Doppler as a diagnostic tool remains under investigation, and cannot yet be recommended to current practice. The application of percutaneous drainage with ultrasound imaging has been reported, but to date there have been no randomized controlled studies comparing percutaneous drainage with formal laparoscopic draining.
The evidence for the use of CT and MRI in suspected cases of acute PID is extremely limited, and their use is compounded by additional logistical problems and cost. CT imaging in PID may demonstrate thickening of the uterosacral ligaments, and the normally distinct pelvic floor fascial planes become obscured (Sam et al 2002). In more advanced disease, reactive inflammatory changes including large bowel ileus, hydroureter and even right upper quadrant inflammation consistent with Fitz–Hughes–Curtis syndrome may be noted. There is no comparative trial of CT in the diagnosis of PID. MRI has been compared with TVS and was reported to have greater sensitivity and specificity, but was limited by cost and accessibility (Tukeva et al 1999).
Laparoscopy
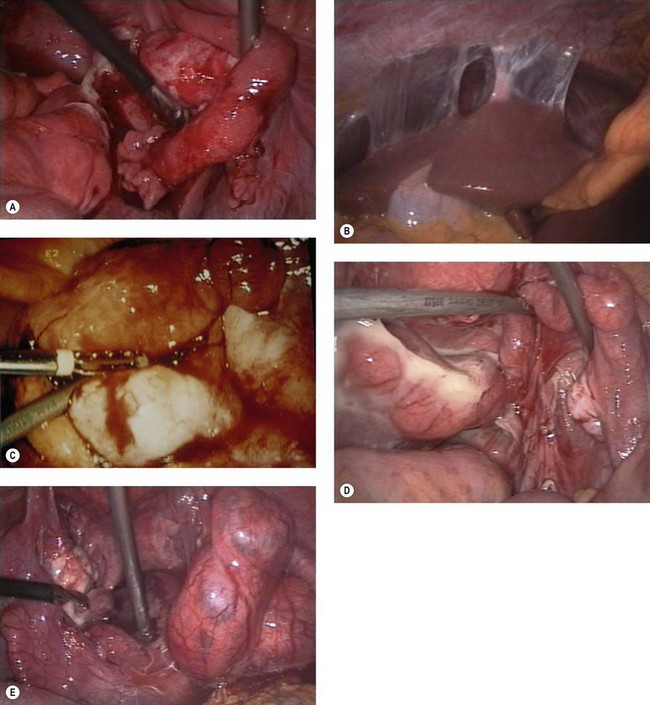
Diagnostic laparoscopy is often described as the diagnostic gold standard for PID; however, a negative laparoscopy does not exclude PID, and equally there is no evidence on the correlation between acute laparoscopic findings and the long-term sequelae of the disease. Its sensitivity is greatly dependent upon the diagnostic criteria used, and should be considered if there is a diagnostic dilemma or the presence of tubo-ovarian abscess is suspected. Features of PID seen at laparoscopy are shown in Figure 62.4.
Treatment
Outpatient care
Women with suspected mild-to-moderate PID can be effectively managed as an outpatient or within primary care provided that an ectopic pregnancy has been ruled out (Royal College of Obstetricians and Gynaecologists 2009). The Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized controlled trial (n = 831) reported that outpatient treatment of women with mild-to-moderate PID is as effective as inpatient treatment (Ness et al 2002). Short-term clinical and microbiological improvements were similar between the two groups, and after a mean follow-up period of 35 months, pregnancy rates were nearly equal (42.0% and 41.7%, respectively). There were also no differences in the time to pregnancy, chronic pelvic pain, ectopic pregnancy and recurrent PID rates between the two groups.
Analgesia
Analgesia should be started with ibuprofen or paracetamol; both should be combined if pain relief is unsatisfactory, or one could consider adding codeine. If this is still unsatisfactory, it may be necessary to revise the diagnosis and treat as an inpatient (Ross 2001b). Drug interactions should be considered in women with medical problems such as epilepsy to avoid worsening of the primary illness.
Antibiotics
Box 62.2 outlines the appropriate antibiotic options for outpatient treatment of mild-to-moderate PID. It is important to inform the patient verbally and in writing that if she is using the combined contraceptive pill, patch or vaginal ring, additional conception is required for the duration of the antibiotic treatment and also for 7 days afterwards. It is also important to enquire about alcohol use before prescribing metronidazole. Patients should be informed that there is a potential risk of flushing, nausea, headache and dizziness if alcohol is taken with metronidazole. The incidence of this reaction is unknown, but it has been reported to occur between 0% and 100% (Baxter 2006). If the patient is unable to tolerate metronidazole, it may be stopped in cases of mild-to-moderate PID as the evidence to support its addition is extremely limited (Royal College of Obstetricians and Gynaecologists 2009).
Box 62.2 Outpatient treatment for mild-to-moderate PID
Regimens containing ofloxacin or azithromycin are not recommended for cases with a high potential for gonococcal infection, as there appears to be increased prevalence of gonococcal resistance in the UK. A large multinational randomized double-blinded study compared the treatment of PID with moxifloxacin alone or with a combination of ofloxacin and metronidazole in women with no evidence of tubo-ovarian abscesses. This study concluded that both treatment options had similar efficacy, but moxifloxacin had fewer side-effects. However, moxifloxacin-resistant gonococci appear to exist (Ross et al 2006). In animal studies, ofloxacin may have associated side-effects and is not currently recommended to patients under 18 years of age (Royal College of Obstetricians and Gynaecologists 2009). Moxiflaxacin is not licensed for the treatment of PID, and has been associated with an increased risk of life-threatening liver reaction (Medicines and Healthcare Products Regulatory Agency 2008).
There is better evidence to support the use of cefoxitin for the treatment of PID. However, it is often not widely available in the UK, and cefriaxone is a suitable third-generation cephalosporin substitute. Patients treated with doxycycline should be advised to avoid sunlamps or direct sunlight as there is a risk of photosensitivity reactions. A commonly used regimen for PID is still metronidazole and doxycycline, although there is no good evidence to support this, with a cure rate as low as 55% (Piyadigamage and Wilson 2005). In a systematic review of 39 studies, two trials evaluated treatment with metronidazole and doxycycline, and reported a clinical cure rate of 75% and a microbiological cure rate of 71% (Walker et al 1999). There is no evidence to support the use of a single dose of azithromycin alone, where better compliance might be an advantage (Bevan et al 2003).
PID in pregnancy
In early or potential early pregnancy, paracetamol is believed to be a safe analgesic, although the evidence for ibuprofen is weaker. The available evidence from the National Teratology Information Service has not noted any increase in congenital malformations before 30 weeks of gestation (National Teratology Information Service 2004). Current RCOG guidelines suggest that administering antibiotics in very early pregnancy is very unlikely to cause any fetal risks as significant drug toxicity would cause failed implantation. PID is very rare in later gestations. Tetracycline compounds should be avoided in pregnancy as they cause brown discoloration of the bone and teeth in fetuses exposed after 15 weeks of gestation, but this side-effect is not encountered with accidental exposure at earlier gestations (Schaefer et al 2007). Currently, the RCOG recommends treatment with cefotaxime, azithromycin and metronidazole for 14 days. No risk was associated with the use of metronidazole in pregnancy (National Teratology Information Service 2004), and no adverse effects were noted from the use of ceftriaxone on fetal development in animal studies. Similarly, azithromycin has very limited published data, but there does not seem to be an increase rate of fetal malformations following in-utero exposure.
PID and HIV
In women with human immunodeficiency virus (HIV), treatment should be the same as for women without HIV (British Association for Sexual Health and HIV 2005). However, in clinically immunocompromised women or when there is failure to respond to standard treatment, advice from a HIV specialist team is recommended.
Compliance and partner screening
Women should be advised and counselled carefully about the implications of PID, the importance of compliance with treatment regimens, avoiding sexual intercourse until the patient and her partner have completed the course of treatment to reduce the risk of reinfection, the advantages of screening for other STIs and the need for contact tracing. The patient may have to use barrier methods if intercourse is unavoidable (Royal College of Obstetricians and Gynaecologists 2009). Sexual partners within the preceding 6 months should be offered referral to a GU medicine clinic or be seen in primary care. The partner(s) should be tested for chlamydia, but empirical treatment would be recommended if this was not available. Gonorrhoea testing is only offered if the woman’s or another partner’s swab results are positive for gonococcus (Royal College of Obstetricians and Gynaecologists 2009).
Long-Term Societal and Global Health Impact
As indicated earlier, it is estimated that 8–10% of chlamydia infections and 8–20% of gonorrhoea infections progress to PID. In developing and low-resource communities, it is estimated that PID-related gynaecology admissions amount to 17–40% of all admissions in Africa, and 15–37% of all admissions in South-East Asia. After unsafe abortion, it is estimated that 10–23% of women with chlamydia and 15% of women with gonorrhoea will develop PID. This accounts for 7–29% of maternal deaths in developing regions. Equally, postpartum infection, which is rare with normal delivery if nothing is introduced into the vagina during labour, is up to 10 times more common in developing countries, accounting for up to 30% of maternal deaths (World Health Organization 2005).
A large (n = 2501) prospective cohort study between 1960 and 1984 reported an increase in infertility after PID (Westrom et al 1992). Sixteen percent of women with abnormal findings at laparoscopy (patients) and 2.7% of women with normal laparoscopy (control) failed to conceive. There was a higher rate of confirmed tubal factor in the women with abnormal findings at laparoscopy (10.8% and 0%, respectively). It was also noted that the incidence of tubal factor infertility increased with the number and severity of PID episodes. It was estimated that tubal factor infertility varied between 37% in developed countries and 85% in developing countries, which was very likely due to the effects of PID. Others have also reported a correlation between the severity of PID and the probability of infertility (Lepine et al 1998). After one episode of PID, the risk of infertility was estimated to be 15–25%, increasing to 50–60% after the third episode, and even higher rates where antibiotic treatment is not available. With this in mind, it is not surprising that there is a 10-fold increase in the incidence of ectopic pregnancy (Westrom and Mardh 1983). In Africa, ectopic pregnancies occur in up to 32 per 1000 live births (World Health Organization 2005).
Thailand demonstrated the success of prevention by greatly reducing the prevalence and transmission of common STIs/RTIs through addressing social and structural challenges. The incidence of the most common STIs was reduced by over 90% (Figure 62.5) through strong government commitment, the use of targeted strategies to reach the population where most STI transmission was taking place, better STI treatment and promoting increased use of condoms among commercial sex workers (World Health Organization 2005).
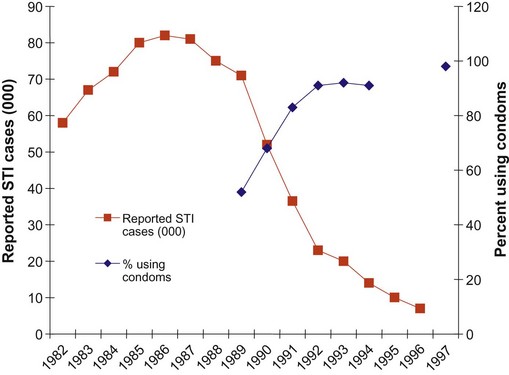
Figure 62.5 Reported cases of sexually transmitted infection (STI) and clients using condoms in Thailand 1982–1997.
Adapted from: Sentinal Serosurveillance, Division of Epidemiology, Ministry of Health, Thailand.
Chronic pelvic pain is common after acute PID and is often difficult to manage satisfactorily. Women with chronic pelvic pain after PID have a lower quality of life, may suffer work-related sequaele, are likely to experience adverse financial implications for treatment (Rein and Gift 2004), and frequently require operative interventions. It has been noted that hysterectomy and removal of adnexa is more common in women with a previous history of PID.
Prevention
Prevention of sexually transmitted infections
Screening programmes and identifying populations at risk
A screening programme is a control and prevention project usually targeted at the highest risk group in order to maximize benefit and be cost-effective. In 2003, two pilot chlamydia screening programmes in England demonstrated that opportunistic screening using urine samples is practical and acceptable, and that the frequency of chlamydia infection in women using these services (including non-GU medicine services) was substantial (Pimenta et al 2003a,b). Subsequently, the Chlamydia Screening Studies (ClaSS) project (Low et al 2007), which screened 19,773 women and men aged 16–39 years selected from general practice lists, used urine and (for women) vulvovaginal swab specimens self-collected at home and sent to a laboratory for chlamydia testing. This study demonstrated that chlamydia infection in the general population was highest in those under 25 years of age, with similar results in men and women. Furthermore, self-collected urine specimens and vulvovaginal swabs were found to be suitable for diagnostic testing using NAATs (Low et al 2007).
Evidence for the effectiveness of chlamydia screening emanates from randomized controlled trials that have demonstrated reductions in the risk of PID among women screened for chlamydia. In the USA, Scholes et al (1996) reported that, at 12 months, women screened for chlamydia had a reduced risk of PID (8/10,000 woman-months) compared with the usual care arm (18/10,000 woman-months), resulting in a relative risk reduction of 56% among those screened. In Denmark, Ostergaard et al (2000) showed that, at 12 months, women offered home-sample screening for chlamydia had reduced prevalence of infection and diagnoses of PID (2.9%) compared with women offered clinic services (6.6%), and 2.1% of the home group were treated for PID compared with 4.2% of the control group. Additionally, it has been reported that the incidence of PID and ectopic pregnancy has declined in a number of countries conducting chlamydia screening, including Sweden, the USA and Canada (Hillis et al 1995, Kamwendo et al 1996, Egger et al 1998, Sutton et al 2005, Rekart et al 2009). Turner et al (2006), using mathematical modelling, estimated that annual screening of 30% of men and women under 25 years of age with 20% partner notification would reduce the population prevalence of chlamydia by 29% after 1 year, 68% after 5 years and 82% after 10 years. If screening coverage was increased to 50%, the estimated reductions in prevalence would be 40% after 1 year, 79% after 5 years and 89% after 10 years, with an even greater decline in prevalence if partner notification rates were higher. Such mathematical modelling estimates are not without limitations, and several important parameters such as the prevalence of undiagnosed/untreated chlamydia, patterns of sexual contacts and chlamydia transmission, and the rate of progression to PID may affect the ultimate effectiveness of screening (US Preventive Services Task Force 2007). In a meta-analysis, Low et al (2009) reported that register-based chlamydia screening of high-risk women and female and male high school students reduced the incidence of PID in women at 1 year, and that opportunistic screening in women undergoing surgical termination of pregnancy reduced postabortion rates of PID compared with no screening. Low et al (2009) concluded that there was no evidence to support opportunistic screening in the general population under 25 years of age. Evidence suggests that a screening programme may be cost-effective at prevalences of 3.1–10% (Honey et al 2002), and that as there is high prevalence of asymptomatic infection in men, efforts to screen them for chlamydia should be strengthened.
Prevention of endogenous infections
KEY POINTS
Agrawal T, Vats V, Salhan S, Mittal A. Mucosal and peripheral immune responses to chlamydial heat shock proteins in women infected with Chlamydia trachomatis. Clinical and Experimental Immunology. 2007;148:461-468.
Barrett S, Taylor C. A review on pelvic inflammatory disease. International Journal of STD and AIDS. 2005;16:715-720.
British Association for Sexual Health and HIV. Recommendations from the Bacterial Special Interest Group of BASHH: testing for sexually transmitted infections in primary care settings. London: BASHH; 2004.
British Association for Sexual Health and HIV. UK National Guideline on the Management of Pelvic Inflammatory Disease. London: Clinical Effectiveness Group (Association for Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases); 2005.
Baxter K. Stockley’s Drug Interactions: a Source Book of Interactions, their Mechanisms, Clinical Importance and Management, 7th edn, London: Pharmaceutical Press; 2006:39-151.
Bevan CD, Johal BJ, Mumtaz G, et al. Clinical, laparoscopic and microbiological findings in acute salpingitis: a report on a United Kingdom cohort. British Journal of Obstetrics and Gynaecology. 1995;102:407-414.
Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. Journal of International Medical Research. 2003;31:45-54.
Burstein G, Gaydos CA, Diener-West M, et al. Incident Chlamydia trachomatis infections among inner-city adolescent females. Journal of the American Medical Association. 1998;280:521-526.
Centers for Disease Control and Prevention. Guidelines for treatment of sexually transmitted diseases. MMWR Recommendations and Reports. 1998;47:1-111.
Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance. Atlanta, GA: US Department of Health and Human Services; 2009. Available at www.cdc.gov/std/stats07/toc.htm
Centers for Disease Control and Prevention. Pelvic Inflammatory Disease — the Facts. CDC Publication No. 99–8827, 2009.
Daling JR, Chow WH, Weiss NS, et al. Ectopic pregnancy in relation to previous induced abortion. Journal of the American Medical Association. 1985;15:1005-1008.
Doxanakis A, Hayes RD, Chen MY, et al. Missing pelvic inflammatory disease? Substantial differences in the rate at which doctors diagnose PID. Sexually Transmitted Infections. 2008;84:518-523.
Egger M, Low N, Smith GD, et al. Screening for chlamydial infections and the risk of ectopic pregnancy in a county in Sweden: ecological analysis. BMJ (Clinical Research Ed.). 1998;316:1776-1780.
Hillis SD, Nakashima A, Amsterdam L, et al. The impact of a comprehensive chlamydia prevention program in Wisconsin. Family Planning Perspective. 1995;27:108-111.
Honey E, Augood C, Templeton A, et al. Cost effectiveness of screening for Chlamydia trachomatis: a review of published studies. Sexually Transmitted Infections. 2002;78:406-412.
Hughes G, Brady AR, Catchpole MA, et al. Characteristics of those who repeatedly acquire sexually transmitted infections: a retrospective cohort study of attendees at three urban sexually transmitted disease clinics in England. Sexually Transmitted Diseases. 2001;28:379-386.
Jacobson L, Westrom L. Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. American Journal of Obstetrics and Gynecology. 1969;105:1088-1098.
Joyner JL, Douglas JM, Foster M, Judson FN. Persistence of Chlamydia trachomatis infection detected by polymerase chain reaction in untreated patients. Sexually Transmitted Diseases. 2002;29:196-200.
Kameh D, Smith A, Brock MS. Female genital schistosomiasis: case report and review of the literature. Southern Medical Journal. 2004;97:525-527.
Kamwendo F, Forslin L, Bodin L, Danielsson D. Decreasing incidences of gonorrhea- and chlamydia-associated acute pelvic inflammatory disease. A 25-year study from an urban area of central Sweden. Sexually Transmitted Diseases. 1996;23:384-391.
Lepine LA, Hillis SD, Marchbanks PA, et al. Severity of pelvic inflammatory disease as a predictor of the probability of live birth. American Journal of Obstetrics and Gynecology. 1998;178:977-981.
Low N, McCarthy A, Macleod J, et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technology Assessment. 2007;11:1-165.
Low N, Bender N, Nartey L, et al. Effectiveness of chlamydia screening: systematic review. International Journal of Epidemiology. 2009;38:435-448.
McCormack WM. Pelvic inflammatory disease. New England Journal of Medicine. 1994;330:115-119.
Medicines and Healthcare Products Regulatory Agency. Moxifloxacin: restricted use. Drug Safety Update. 2008;2:8.
Millstein SG, Adler NE, Irwin CE. Sources of anxiety about pelvic examinations among adolescent females. Journal of Adolescent Health Care. 1984;5:105-111.
Molander P, Sjöberg J, Paavonen J, Cacciatore B. Transvaginal power Doppler findings in laparoscopically proven acute pelvic inflammatory disease. Ultrasound in Obstetrics and Gynecology. 2001;17:233-238.
Munday PE. Pelvic inflammatory disease—an evidence based approach to diagnosis. Journal of Infection. 2000;40:31-41.
National Chlamydia Screening Programme. www.chlamydiascreening.nhs.uk/ps/rd/lit.html, 2009. Data accessed on NCSP website (accessed 10/08/2009)
National Teratology Information Service. Use of Ibuprofen in Pregnancy. NTIS Regional Drug and Therapeutics Centre, Newcastle upon Tyne, UK: TOXBASE; 2004.
Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. American Journal of Obstetrics and Gynecology. 2002;186:929-937.
Ostergaard L, Andersen B, Møller JK, Olesen F. Home sampling versus conventional swab sampling for screening of Chlamydia trachomatis in women: a cluster-randomized 1-year follow-up study. Clinical Infectious Diseases. 2000;31:951-957.
Pack RP, DiClemente RJ, Hook EW, Oh MK. High prevalence of asymptomatic STDs in incarcerated minority male youth: a case for screening. Sexually Transmitted Diseases. 2000;27:175-177.
Peeling RW, Toye B, Jessamine P, Gemmill I. Pooling of urine specimens for PCR testing: a cost saving strategy for Chlamydia trachomatis control programmes. Sexually Transmitted Infections. 1998;74:66-70.
Peipert JF, Ness RB, Blume J, et al. Pelvic Inflammatory Disease Evaluation and Clinical Health Study Investigators. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. American Journal of Obstetrics and Gynecology. 2001;184:856-863.
Pimenta JM, Catchpole M, Rogers PA, et al. Opportunistic screening for genital chlamydial infection. I: Acceptability of urine testing in primary and secondary healthcare settings. Sexually Transmitted Infections. 2003;79:16-21.
Pimenta JM, Catchpole M, Rogers PA, et al. Opportunistic screening for genital chlamydial infection. II: Prevalence among healthcare attenders, outcome, and evaluation of positive cases. Sexually Transmitted Infections. 2003;79:22-27.
Piyadigamage A, Wilson J. Improvement in the clinical cure rate of outpatient management of pelvic inflammatory disease following a change in therapy. Sexually Transmitted Infections. 2005;81:233-235.
Rein DB, Gift TL. A refined estimate of the lifetime costs of pelvic inflammatory disease. Sexually Transmitted Diseases. 2004;31:325.
Rekart M, Gilbert M, Chang M, et al. Documenting the success of chlamydia control in British Columbia (BC). Abstract P4.64. 18th ISSTDR Conference, London, UK, 2009.
Ross JD. European Branch of the International Union against Sexually Transmitted Infections and the European Office of the World Health Organization. European guideline for the management of pelvic inflammatory disease and perihepatitis. International Journal of STD and AIDS. 2001;21:84-87.
Ross J. Pelvic inflammatory disease. Extracts from clinical evidence. BMJ (Clinical Research Ed.). 2001;17:658-659.
Ross JD, Cronje HS, Paszkowski T, et al. Moxifloxacin versus ofloxacin plus metronidazole in uncomplicated pelvic inflammatory disease: results of a multicentre, double blind, randomised trial. Sexually Transmitted Infections. 2006;82:446-451.
Royal College of Obstetricians and Gynaecologists. Pelvic Inflammatory Disease. Green Top Guideline No. 32. London: RCOG Press; 2009.
Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22:1327-1334.
Schaefer C, Peters P, Miller RK, editors. Drugs during Pregnancy and Lactation: Treatment Options and Risk Assessment, 2nd edn, Oxford: Academic Press, 2007.
Scholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. New England Journal of Medicine. 1996;334:1362-1366.
Sellors JW, Mahony JB, Chernesky MA, Rath DJ. Tubal factor infertility: an association with prior chlamydial infection and asymptomatic salpingitis. Fertility and Sterility. 1988;49:451-457.
Sellors J, Mahony J, Goldsmith C, et al. The accuracy of clinical findings and laparoscopy in pelvic inflammatory disease. American Journal of Obstetrics and Gynecology. 1991;164:113-120.
Serlin M, Shafer MA, Tebb K, et al. What sexually transmitted disease screening method does the adolescent prefer? Archives of Pediatric & Adolescent Medicine. 2002;156:588-591.
Simms I, Warburton F, Weström L. Diagnosis of pelvic inflammatory disease: time for a rethink. Sexually Transmitted Infections. 2003;79:491-494.
Simms I, Fleming DM, Lowndes CM, et al. Surveillance of sexually transmitted diseases in general practice: a description of trends in the Royal College of General Practitioners Weekly Returns Service between 1994 and 2001. International Journal of STD and AIDS. 2006;17:693-698.
Sutton MY, Sternberg M, Zaidi A, et al. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sexually Transmitted Diseases. 2005;32:778-784.
Sziller I, Fedorcsák P, Csapó Z, et al. Circulating antibodies to a conserved epitope of the Chlamydia trachomatis 60-kDa heat shock protein is associated with decreased spontaneous fertility rate in ectopic pregnant women treated by salpingectomy. American Journal of Reproductive Immunology. 2008;59:99-104.
Taipale P, Tarjanne H, Ylostalo P. Transvaginal sonography in suspected pelvic inflammatory disease. Ultrasound in Obstetrics and Gynecology. 2003;6:430-434.
Timor-Tritsch IE, Lerner JP, Monteagudo A, et al. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound in Obstetrics and Gynecology. 1998;12:56-66.
Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210:209-216.
Turner KM, Adams EJ, Lamontagne DS. Modelling the effectiveness of chlamydia screening in England. Sexually Transmitted Infections. 2006;82:496-502.
US Preventive Services Task Force. Screening for chlamydial infection: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2007;147:128-134.
van der Heyden JHA, Catchpole MA, Paget WJ, Stroobant A. Trends in gonorrhoea in nine western European countries, 1991–1996. European Study Group. Sexually Transmitted Infections. 2000;76:110-116.
von Knorring N, Wilson J. Sorting out pelvic inflammatory disease. Trends in Urology, Gynaecology and Sexual Health. 2007;12:31-36.
Walker CK, Workowski KA, Washington AE, et al. Anaerobes in pelvic inflammatory disease: implications for the Centers for Disease Control and Prevention’s guidelines for treatment of sexually transmitted diseases. Clinical Infectious Diseases. 1999;28:S29-S36.
Washington AE, Katz P. Cost of and payment source for pelvic inflammatory disease: trends and projections, 1983 through 2000. Journal of the American Medical Association. 1991;266:2565-2569.
Watson EJ, Templeton A, Russell I, et al. The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systematic review. Journal of Medical Microbiology. 2002;51:1021-1031.
Weinstock H, Berman S, Cates WJr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspectives in Sexual and Reproductive Health. 2004;36:6-10.
Westrom LR. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. American Journal of Obstetrics and Gynecology. 1980;138:880-892.
Westrom L, Mardh PA. Chlamydial salpingitis. British Medical Bulletin. 1983;39:145-150.
Westrom LR, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sexually Transmitted Diseases. 1992;19:185-192.
World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infection. Geneva: WHO; 2001.
World Health Organization. Integrating STI/RTI Care for Reproductive Health: Sexually Transmitted and Other Reproductive Tract Infections: a Guide to Essential Practice. Geneva: WHO; 2005.