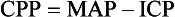Pediatric Trauma
Perspective
Injuries to children (<18 years old) account for more than 8 million visits to emergency departments in the United States each year. More than half of all deaths in this age group are the direct result of injury, with more than 10,000 traumatic deaths annually.1 Motor vehicle collisions (MVCs) account for more than half of all pediatric traumatic deaths.1 In the United States, estimates of mortality for children hospitalized after injury are uniformly low; however, most fatalities occur in the field before arrival at a health care facility, which contributes to an underestimation of the magnitude of overall mortality figures.
Multiple injuries are common in pediatric trauma patients, and the emergency physician should evaluate all organ systems in any injured child when the mechanism of injury is concerning. The most common single organ system injury associated with death in injured children is head trauma, but the great majority of pediatric trauma deaths involve multisystem injury.2
Accidental falls predominate nonfatal injuries in all pediatric age groups. Other nonfatal injuries vary by age. Young children (<4 years of age) experience higher rates of animal bites and burns. School-age children (5 to 9 years old) are more likely to experience bicycle and pedestrian injuries. Older children (>9 years) have high incidences of both fatal and nonfatal motor vehicle–related trauma, and higher incidences of suicide and self-inflicted harm. Homicide by firearm shows a fourfold and tenfold increase when the 5- to 9-year age group is compared with the 10- to 14- and 15- to 24-year age groups, respectively. Homicide by firearm is nearly 31 times more common in the 15- to 24-year age group when compared with the 10- to 14-year age group.1,2
Throughout the United States the number of children who are victims of violent acts has increased. Some children’s hospitals report that 7% of all pediatric injuries seen in emergency departments and 25 to 35% of all pediatric trauma deaths are caused by nonaccidental trauma.3
Principles of Disease
Between pediatric and adult patients, there are major anatomic and physiologic differences that play a significant role in the evaluation and management of a pediatric trauma patient (Box 38-1). Any given force is more widely distributed through the body of a child than the body of an adult, making multiple injuries significantly more likely to occur in children. The proportionately large surface area of infants and children relative to weight predisposes them to greater amounts of heat loss as a result of evaporation. During resuscitation, even mild to moderate hypothermia has direct negative effects on cardiac function, inotropy, left-ventricular contractility, catecholamine responsiveness, platelet function, renal and hepatic drug clearance, and metabolic acidemia. Therefore maintenance requirements for free water, electrolytes, and minerals are proportionally greater compared with those for adults. Oxygen extraction and consumption as well as glucose utilization are much higher per kilogram in infants and small children than in adults. These factors contribute to a significantly higher energy and caloric requirement for an injured child compared with an injured adult. A child’s physiologic response to injury is different from an adult’s response, depending on the age and maturation of the child and the severity of the injury. Children have a great capacity to maintain blood pressure despite significant acute blood losses constituting 25 to 30% of total blood volume.4 A child’s cardiac output is primarily determined by the heart rate and systemic vascular resistance. Changes in inotropy play a relatively minor role in children after trauma, compared with adults. Compensated shock should be considered and promptly addressed when a child’s heart rate is elevated, especially if the capillary refill time is delayed. Changes in heart rate, blood pressure, and extremity perfusion commonly precede cardiorespiratory failure and should not be overlooked.
Clinical Features
Initial Assessment Priorities and Primary Survey
A—Airway and Cervical Spine Stabilization
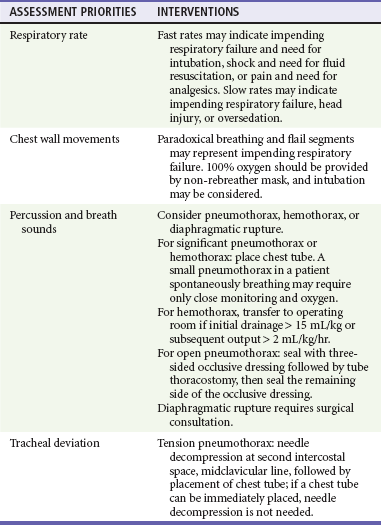
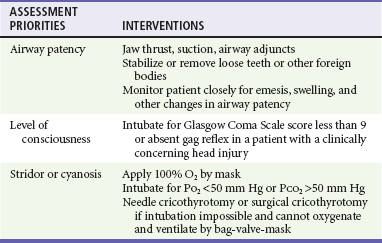
Table 38-1 describes anatomic considerations that have implications in the management of the pediatric airway. The physician assesses for possible airway obstruction or inability of the child to maintain his or her own airway. While the neck is being stabilized, the airway can be opened with a jaw-thrust maneuver. Maxillofacial trauma, loose teeth, blood, swelling, or vomitus may obstruct the airway, and efforts should be made toward clearing the oropharynx of debris. Gurgling or stridor may indicate upper airway obstruction. The physician must know normal pediatric oral anatomy and tooth development to recognize the possibility of missing primary or secondary teeth. Efforts to perform cricoid pressure, or ligatures such as ties on gowns, can easily occlude the infant’s or child’s airway with as little as 0.2 pounds of direct force.5 Table 38-2 describes priorities in the assessment of the pediatric airway.
Table 38-2
Airway: Assessment and Treatment
O2, oxygen; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen.
Intubation of pediatric patients involves special considerations (see Table 38-1). In general, the orotracheal approach is recommended. In children, nasotracheal intubation can be complicated by the acute angle of the posterior pharynx, the potential for bleeding, and infection (sinusitis). Furthermore, nasotracheal intubation can cause increased intracranial pressure (ICP). In children younger than age 8, the cricoid ring is the narrowest portion of the airway. The cricoid ring may form a physiologic cuff on endotracheal tubes (ETT). However, the use of a cuffed tube allows for greater airway protection and may be considered in the injured child. Appropriate ETT size can be estimated through use of a length-based resuscitation tape or by the formulas in Box 38-2.
B—Breathing and Ventilation
For assessment of “ventilation,” pulse oximetry is useful; however, pulse oximetry measures adequacy of oxygenation only. The measurement of exhaled carbon dioxide (CO2) is useful to confirm ETT position. Historically, a colorimetric semiquantitative device has been used to detect the presence of exhaled CO2 in patients with perfusion. Continuous end-tidal CO2 capnography provides far more information and continues to be underused.6 In a patient with adequate perfusion, in addition to serving as an initial qualitative device to confirm successful intubation of the trachea, it may also provide an early warning of unintended extubation, tube kinking or partial occlusion, or ventilator malfunction. Continuous end-tidal CO2 capnography also characterizes the response to therapeutic maneuvers instantaneously, provides a quantitative tool to manage the ventilatory aspects of respiration, and may provide prognostic information when used in patients with cardiac arrest. It can also be used to measure the effectiveness of cardiopulmonary resuscitation (CPR). The lack of appropriate CO2 detection when the tube is in proper position often indicates poor perfusion. The use of end-tidal CO2 capnography allows better ventilatory management during head injury resuscitation, and its values can be confirmed with a single venous or arterial blood gas measurement. This can assist greatly with continuing ventilatory management without the need for recurrent blood draws and the inherent delays and discomfort of acquiring blood gases (assuming stable pulmonary function). Table 38-3 describes priorities in the assessment of breathing in pediatric trauma patients.
C—Circulation and Hemorrhage Control
Shock is not defined by any specific blood pressure but is, instead, a state in which the body is unable to maintain adequate tissue perfusion. Maintenance of systolic blood pressure does not ensure that the patient is not in shock. The pediatric vasculature has the ability to constrict and increase systemic vascular resistance in an attempt to maintain perfusion. Signs of poor perfusion (cool distal extremities, decreases in peripheral versus central pulse quality, and delayed capillary refill time) are signs of pediatric shock, even when blood pressure is maintained at normal levels. Palpable pulses are detectable at a systolic blood pressure greater than 80 mm Hg in children over approximately 10 years of age; however, pulses may be felt at even lower pressures in infants and younger children. Normal capillary refill time is less than 2 seconds; however, many variables affect this clinical finding. Alteration in a child’s response to the environment or interaction with caregivers may indicate respiratory failure or shock. External hemorrhage should be sought and controlled with direct pressure. The assessment of circulation in pediatric trauma patients is described in Box 38-3.
D—Disability Assessment (Thorough Neurologic Examination)
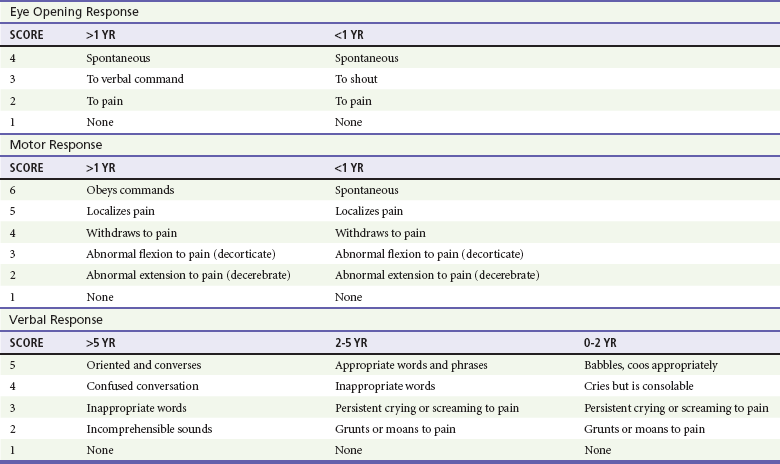
For assessment of patient disability, a rapid neurologic and mental status evaluation is needed. The assessment of disability in pediatric trauma patients is described in Box 38-4. The AVPU system (Box 38-5) and the modified pediatric GCS (Table 38-4) can also be useful to the clinician.
E—Exposure and Thorough Examination
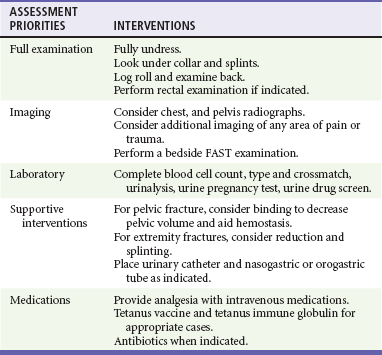
Fully undressing the patient to assess for hidden trauma is essential in injured children. Maintenance of normothermia is paramount in the undressed infant and toddler because metabolic needs are greatly increased by hypothermia. In addition to increased ambient temperature, additional warming methods such as warmed humidified oxygen, warmed fluids, warmed blood, head wraps, and convective warmers or radiant heat sources should be used as soon as possible. Preventing and treating hypothermia is not a matter of comfort for traumatized infants and children but, instead, one of survival. The exposure phase of the survey is often a good time to concurrently begin imaging and further diagnostic testing (Table 38-5).
F—FAST and Family
The focused assessment with sonography in trauma (FAST) can be a very useful examination in injured children.7 Bedside ultrasound evaluates for traumatic free fluid in the peritoneum (hepatorenal, perisplenic, and retrovesicular views) and pericardial space. In hemodynamically unstable children, a FAST may point to hemorrhage in the abdomen or the pericardial space and the need for intervention. In hemodynamically stable children, the FAST examination may indicate the need for computed tomography (CT) imaging, closer observation, repeat abdominal examinations, or repeat ultrasound examinations.
Secondary Survey
After completion of the primary survey and requisite procedures, the secondary survey is performed. The secondary survey is an organized, complete assessment to detect additional injury not found on the primary survey. A more complete and detailed history is obtained at this time. Features of the history that need to be obtained can be remembered by the mnemonic AMPLE (Box 38-6). Ongoing assessment of the patient occurs after the secondary survey, and key points are summarized in Box 38-7.
Management and Diagnostic Strategies
All pediatric patients who have sustained major trauma should be placed on a cardiac monitor; receive supplemental oxygen; and have constant reassessment of vital signs, oximetry, and end-tidal CO2 monitoring. Vascular access is best obtained by accessing the upper extremity for the establishment of two large-bore intravenous lines. In the absence of available upper extremity peripheral sites, lower extremity sites can be used. Many clinicians favor the femoral vein as a safe site for insertion of a central line by use of a guidewire technique. A guide to suggested sizing of femoral catheters is shown in Box 38-2.
Most hypovolemic pediatric trauma patients respond to 20-mL/kg boluses of isotonic crystalloid solutions. If 40 mL/kg has not reversed systemic signs of hypoperfusion, an additional 20-mL/kg bolus of crystalloid may be given, but the infusion of packed red blood cells at 10 mL/kg should be considered. In patients in decompensated hemorrhagic shock or cardiopulmonary failure secondary to severe anemia, crystalloid and blood products may be prudently administered simultaneously. With massive transfusion (>1 blood volume = approximately 80 mL/kg), it is important to add additional blood products to correct coagulopathy. Some experts now recommend (based predominantly on adult studies) that blood and fresh frozen plasma (FFP) be given in a near 1 : 1 ratio if massive transfusion is expected. Other experts believe a ratio closer to 2.5 : 1 may suffice and may decrease the risk of multiorgan failure. In general, FFP should be administered at 15 to 25 mL/kg. Platelet transfusion dosage can be very confusing. Practically all platelet units currently used are apheretic platelets from a single donor. Each apheretic unit roughly equates to six of the older concentrate units (a “six-pack” of platelets). The usual dose in trauma is 10 mL/kg; however, the response may be quite variable (i.e., it can vary by more than a factor of two) owing partly to the heterogeneity of the concentration of platelets between apheretic units. A general goal in trauma patients is to raise the platelet count above 50 × 109/L. The platelet count should be rechecked at 1 and 24 hours after transfusion, or more often if the patient has ongoing difficulties with hemostasis or need for recurrent transfusion of red blood cells. The primary goal of giving cryoprecipitate is to increase the fibrinogen to levels of 1 to 1.5 g/dL, especially after central nervous system trauma. Although dependent on the fibrinogen concentration in the individual cryoprecipitate bags, the dose is typically 0.1 to 0.2 bags/kg. Each bag of cryoprecipitate contains approximately 150 mg of fibrinogen and 80 units of factor VIII.8
Physical Examination
A rectal examination is not required in all cases of pediatric trauma and should be performed only when its result has a reasonable chance of meaningfully changing the patient’s treatment.9,10 A rectal examination may provide information on sphincter tone in possible spinal injury and the presence of blood in penetrating trauma. Unfortunately, the rectal examination lacks sensitivity. Its findings, when negative, are often misleading, and additional workup should be considered.
Reexamination of trauma patients throughout their time in the emergency department is of utmost importance to ensure that their condition has not changed, that their pain is controlled, and that no injuries are overlooked. Up to 70% of injuries with delayed diagnosis in pediatric trauma are orthopedic in nature.11
Diagnostic Evaluation
In patients with hypovolemic shock, the hemoglobin alone is unreliable because equilibration will not have occurred at the time of presentation to the emergency department.12 Serial hemoglobin measurements may be useful to assess the possibility of ongoing bleeding.13
Radiology
Chest and pelvic radiographs can assess for causes of respiratory failure, sites of blood loss, and causes of shock. In stable, alert children without distracting injuries, the pelvic film may be eliminated if no suggestion of sacral or pelvic fracture is found on thorough clinical examination. The following seven criteria are required to rule out any relevant pelvic fracture: patient age older than 3 years, no impairment of consciousness, no other major distracting injury, no complaint of pelvic pain, no signs of fracture on inspection, no pain on iliac or pubic symphysis compression, and no pain on hip rotation or flexion.14–16 In patients with remarkable sacral tenderness and negative plain radiographs, a CT scan should be strongly considered. Sacral fractures can be difficult to discern reliably on plain films.
Specific Disorders and Injuries
Perspective
Each year, more than 500,000 children (ages 0 to 14 years) visit emergency departments in the United States after head injury.17 Falls account for 50.2% of pediatric head injuries. On an age-related basis, infants and toddlers are more prone to falls from their own height, school-age children are involved in sports injuries and MVCs, and children of all ages are subject to the sequelae of abuse. Although MVCs account for only 6.8% of pediatric head injuries, they represent more than 30% of fatal head injuries.17
Clinical Features
The prognostic significance of vomiting after pediatric head trauma is unclear. There is no adequate study defining an acceptable time frame in which vomiting after head injury is benign in nature. Vomiting appears to be more strongly correlated to personal or familial tendency to vomit than to intracranial injury; however, recurrent vomiting is commonly seen in patients with significant head injury and is often considered in the decision to obtain a CT study.18
The development of seizures after head trauma has been well studied.19 A brief seizure that occurs immediately after an insult (with rapid return to normal level of consciousness) is commonly called an impact seizure. This type of seizure is not usually associated with intracranial parenchymal injury. A CT scan is not necessary if the only concern is the impact seizure; the decision to scan should take into account the mechanism of injury and current neurologic status of the child. An isolated impact seizure does not require anticonvulsant therapy. Seizures that occur later (more than 20 minutes after the insult) portend the greater possibility of traumatic brain injury and the development of seizures at a later date. A CT scan is indicated for these later post-traumatic seizures. These patients may benefit from treatment with anticonvulsants, benzodiazepines for sedation if intubated, or both as the seizure threshold is generally lower in children. Having one later seizure (nonimpact) raises the risk of subsequent additional seizure, and seizure activity raises ICP while often decreasing oxygenation and ventilation. Children who experience later seizures often require neurosurgical evaluation.
Several methods are available for evaluating the mental status of head-injured patients, including the AVPU system and the GCS. A commonly used modification of the GCS for children is shown in Table 38-4. Although the pediatric GCS is widely used, none of the pediatric modifications of the GCS has the inter-rater reliability or predictive validity of the adult GCS. Even children with low initial GCS scores can have favorable outcomes and neurologic status. The important message is that no matter what the patient’s neurologic presentation, all efforts should be initiated to ensure survival and maintain stable neurologic status in the emergency department.
Examining a brain-injured child involves mental status testing, cranial nerve testing, motor testing, sensory testing, and memory testing. The evaluation of cranial nerve function is essentially no different from that in an adult. The most important aspect of motor and cranial nerve evaluation involves ruling out the presence of increased ICP. Common symptoms and signs of increased ICP in infants and children should be sought (Boxes 38-8 and 38-9).
Specific Injuries
Concussion.: Strictly speaking, a concussion is defined as a brain insult with transient alteration of consciousness. Patients who sustain concussive insults may have anorexia, amnesia, vomiting, unsteadiness, or pallor after the insult. Simple concussions are those in which symptoms resolve within 10 days of the injury, and complex concussions have symptoms lasting longer than 10 days. All children with concussive symptoms should rest until the symptoms are resolved then return slowly to their baseline activities. Children with complex concussions should have further medical examination by their primary care provider or formal neuropsychological testing before returning to baseline activities or sport.
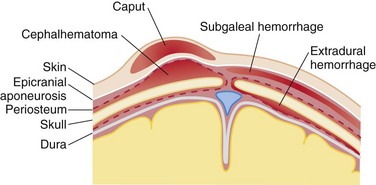
Scalp Injuries.: Minor injury to the scalp of infants and children involves the development of three common injury complexes.20–23 For these injury complexes to be better understood, the layers of the SCALP (skin, connective tissue, aponeurosis, loose areolar tissue, and periosteum) should be considered (Fig. 38-1). Caput succedaneum refers to injury with a hematoma in the connective tissue layer. This is freely mobile and crosses suture lines. A subgaleal hematoma refers to a hematoma that is subgaleal within the loose areolar tissue above the periosteum. Lastly, cephalohematoma refers to a collection of blood under the periosteum. Because the periosteum adheres tightly to the various suture lines, the cephalohematoma does not cross them. Bleeding from scalp wounds is often profuse and can lead to hemodynamic embarrassment in infants and small children if not quickly controlled. Although children may develop shock from a scalp injury, it is prudent to search for other causes of shock while this bleeding is being controlled.
Skull Fractures.: In children, skull fractures occur in many different configurations. Simple linear fractures, the most common type of skull fracture, rarely require therapy and often are associated with good outcomes. Factors associated with poor outcomes include the presence of a fracture overlying a vascular channel, a depressed fracture, a diastatic fracture, or a fracture that extends over the area of the middle meningeal artery. Diastatic fractures, or defects extending through suture lines, are different from simple linear fractures in that leptomeningeal cysts (“growing fractures”) may develop at these sites. Fractures of the basilar portions of the occipital, temporal, sphenoid, or ethmoid bones commonly occur in children. The presence of cerebrospinal fluid rhinorrhea and otorrhea has been associated with these injuries. Signs of basilar skull fractures in children are similar to signs in adults and include the presence of periorbital subcutaneous hematoma (raccoon eyes), and posterior auricular ecchymosis (Battle’s sign).
Cerebral Contusions.: Cerebral contusions are often the result of coup and contrecoup forces at work. Cerebral contusions may not be associated with any loss of consciousness at the time of insult. Patients often have associated symptoms, such as altered level of consciousness, severe headache, vomiting, or focal deficits on neurologic assessment. Contusions are clearly demonstrable on CT.
Epidural Hematoma.: Traditional teaching regarding the development of epidural hematomas involves the typical triad of head injury followed by a lucid interval, followed by rapid deterioration as intracranial hemorrhage worsens. In contrast to epidural hematomas in adults, pediatric epidural hematomas may be the result of venous bleeding, which predisposes them to a delay in the development of symptoms. After head trauma, guardians are informed of the delayed signs and symptoms that should prompt immediate reassessment. In any event, epidural hematomas are associated with a high incidence of overlying skull fractures (60-95% of cases). Patients with small fracture-related epidural hematomas localized only to the site of the inner table fracture should be monitored closely in the hospital but often do not require surgical intervention.
Subdural Hematoma.: Special attention should be directed toward infants and toddlers to rule out the presence of subdural hematomas. This clinical scenario is most often secondary to rupture of bridging veins and is only occasionally associated with the presence of overlying fractures (<30%). Subdural hematomas most commonly occur in patients younger than 2 years of age. Chronic subdural hematomas are most often encountered in patients who have been subjected to what has been termed the “shaken baby syndrome.” This clinical complex involves forcible shaking of the child with accelerating and decelerating forces affecting the cranial vault.24 This syndrome is most often a result of nonaccidental trauma, and 22% of abused children have central nervous system injuries. These patients have nonspecific findings, such as vomiting, failure to thrive, change in level of consciousness, or seizures. Retinal hemorrhages are present in the majority of cases, and all head injury patients should undergo careful funduscopic examinations to rule out the presence of these nearly pathognomonic findings. Definitive examinations should be performed by an ophthalmologist after pupil dilation to characterize the specific type of retinal hemorrhage. Those that are diffuse, occur in multiple retinal layers, or extend to the periphery are more likely secondary to abuse.25 Retinal hemorrhages are not observed in children with mild to moderate trauma from other causes and are not associated with a prior history of CPR; the presence of retinal hemorrhages suggests nonaccidental trauma. Similarly, subdural hematomas at multiple sites, over areas other than the convexities, in the posterior fossa, or in the posterior interhemispheric fissure should strongly suggest the possibility of nonaccidental trauma.26,27 Left to their own development, the worst cases may manifest with signs of increased ICP. Coagulation studies, platelet count, and platelet function assays should be performed in these cases. When indicated, metabolic tests for glutaric aciduria should also be considered.
Diagnostic Strategies and Management
As a basic rule, serial examinations are the most reliable indicators of clinical deterioration.28 The presence of focality is a reliable indicator of a localized insult, whereas the absence of focality may be misleading. The signs of increased ICP usually develop late in the course of the process in infants. As in an adult, papilledema may require days to develop. The classic Cushing’s response (bradycardia and hypertension) does not always occur in children, but when it occurs, it is often an ominous sign. If ICP elevation is suspected, emergency intervention and neurosurgical consultation are initiated immediately (Table 38-6).
Table 38-6
Emergent Management of Increased Intracranial Pressure
| THERAPY | DOSE | MECHANISM OF ACTION |
| Head elevation (30 degrees) | Lowers intracranial venous pressure. | |
| Head in midline | Prevents jugular vein compression. | |
| Hyperventilation | Maintenance PaCO2 38-42 mm Hg If acute increase in ICP then reduce PaCO2 to 30-35 mm Hg |
Promptly but temporarily decreases cerebral blood volume and thus intracranial pressure. Recommended only for short-term treatment of acute ICP elevation. |
| Hyperosmolar agents | ||
| Mannitol | 0.25-0.5 g/kg IV | Both agents effect rapid osmotic diuresis. Diuresis may decrease BP and CPP. Mannitol should be given through filter. HTS may require a central line. Effect from osmotic and rheologic effects. Avoid dehydration. |
| Hypertonic saline (HTS) | 0.1-1 mL/kg of 3% Titrate to effect |
|
| Pentobarbital | 5-10 mg/kg over 30 minutes, then 5 mg/kg/hr for 3 hours, then 1 mg/kg/hr Rarely indicated or started in emergency department |
Thought to lower cerebral metabolism; also may have some effect on free radical formation. Other barbiturates (phenobarbital) have also been used. May decrease BP and CPP. |
| Decompressive craniotomy | Allows more space for swelling and decreases ICP. | |
| Mild hypothermia (35° C) | Thought to decrease cerebral blood flow and metabolic rate. Can cause cardiac dysrhythmias. Is currently under investigation. |
|
| Maintain euvolemia | Clinically or invasive monitoring | Maintenance of mean arterial pressure. |
| Pressors if needed to maintain CBF | Depends on agent used | Maintain CBF and CPP by increasing MAP. |
| Neuromuscular blockade | Depends on agent used | Helps maintain lower ICP. |
| Sedation | Depends on agent used | Do not assume patient is completely incapable of response to noxious stimuli or situation. |
| Prevent fever | Acetaminophen 15 mg/kg OG | Fever raises ICP and metabolic demand. |
| Treat seizure aggressively | Depends on agent used | Prophylactic treatment controversial. Treatment of seizure is not controversial and is aggressive to prevent increased ICP, hypoxia, hyperpyrexia, and hypercarbia. |
The contents of the skull are composed of essentially three compartments: brain, cerebrospinal fluid, and blood. The volume of the skull is fixed. Although not a perfect model, the Monroe-Kellie doctrine suggests the effects that changes in each compartment may have on the others. For example, in the presence of an intracerebral hemorrhage of significant volume, either cerebral spinal fluid or brain must leave the cranial vault. Similarly, if the brain swells, cerebral spinal fluid, blood, or both must leave the cranial vault. When this balance is disrupted and the autoregulatory system’s capacity to adapt is exceeded, the ICP rapidly increases. ICP can quickly reach a level that is not conducive to localized brain survival or continued blood flow to the brain. If the condition is left untreated, herniation may occur. An ICP more than 20 to 25 mm Hg should be treated, but the absolute value less than this that should trigger treatment is unclear. From the standpoint of global cerebral perfusion, CPP is equated with MAP minus ICP. However, this model does not allow the accurate prediction of CPP at the specific site of injury or within the ischemic penumbra. Measurement of oxygen extraction (through use of modifications of the Fick principle) and outcome studies have played a role in the following recommendations. In general, it is best to keep the CPP above 50 to 65 mm Hg in children and above 70 mm Hg in adults. There appears to be an age continuum with regard to necessary CPP. Hackbarth and co-workers demonstrated that the single greatest prognostic sign of outcome from traumatic brain injury in children is the ability to maintain a CPP greater than 50 mm Hg.28 Many have adopted this as the minimum acceptable CPP.
Most clinicians favor early and controlled intubation in pediatric patients with GCS scores that are deteriorating or are less than 9. However, in the out-of-hospital phase of care, or if the physician is not knowledgeable and experienced in pediatric rapid sequence induction, BMV ventilation should be strongly considered during short transports and until additional, more experienced support personnel are available.29,30 An OG tube should be considered if BMV ventilation is used, to decrease the chance of emesis and to prevent respiratory embarrassment from gastric distention with air. Isolated head injury is uncommon; a careful search for other injuries should be made via meticulous and repetitive examinations as well as indicated laboratory and imaging tests.
The use of anticonvulsants after moderate to severe head injury in children is controversial. Early prophylaxis does not decrease the incidence of late seizures and is not recommended for this purpose.31 Clearly, the effects on temperature, intracranial oxygenation, and cerebral perfusion during an early seizure after trauma are discordant with the management principles of acute brain injury. In addition, early seizures often disrupt the evaluation and management of the patient’s head injury and other trauma. However, the evidence for phenytoin effectiveness in preventing early seizures after trauma is weak. It has now been demonstrated that in moderate to severe head injury the incidence of early seizure was much lower than expected. Phenytoin did not substantially lower this risk in a study by Young.31 Others have suggested that topiramate or levetiracetam may be more effective with decreased risks of side effects, but research in this area is ongoing.32,33 It may be prudent to treat seizures aggressively if they occur and to consider use of sedative medications with anticonvulsant properties, such as benzodiazepines, reserving the use of prophylactic anticonvulsants for the highest-risk patients in consultation with the neurosurgical service.
Herniation syndromes in children are similar to those in adults. Uncal herniation is suggested early on by the presence of a unilaterally dilated pupil (compression of ipsilateral third nerve parasympathetic fibers), contralateral hemiplegia (caused by ipsilateral cerebral peduncle compression against the tentorium), and spontaneous hyperventilation. With progression, the ipsilateral eye may be noted to be looking downward and outward secondary to the loss of third nerve motor function but continued fourth and sixth cranial nerve function. Often, bilateral third nerve compression occurs very early, leading to bilateral “blown” pupils. In Kernohan’s phenomenon, the temporal lobe compresses the contralateral cerebral peduncle against the tentorium, leading to ipsilateral paresis, making localization of the lesion challenging without neuroimaging. Small pupils, sluggish pupils, decorticate posturing, and Cheyne-Stokes respirations characterize early central diencephalic herniation. If this progresses and extends to the pons or medulla, the patient will have fixed and dilated pupils, flaccid muscle tone, and slow or apneustic breathing or frank apnea and cardiorespiratory arrest. Management of suspected acute herniation begins with immediate controlled hyperventilation.33 Clinical endpoints of hyperventilation are improved patient status or constriction of dilated pupils. End-tidal CO2 capnography is used with arterial or venous blood gas correlation to assess adequacy of hyperventilation with a target partial pressure of carbon dioxide (PCO2) of 30 to 35 mm Hg. Excessive hyperventilation can result in excessive cerebral vasoconstriction and secondary brain injury; ventilation should be started at an age-appropriate rate, and then the rate should be increased until pupillary function returns. Subsequent management of herniation includes hyperosmolar agents, followed by other specific interventions in the intensive care unit (ICU).34,35
Radiology
Skull Radiographs.: Most clinicians agree that firm indications for skull radiographs alone include the skeletal survey involved with the evaluation of child abuse, establishment of a functioning ventricular peritoneal shunt, some penetrating wounds of the scalp, or the suspicion of foreign bodies underlying scalp lacerations. In children requiring neuroimaging because of concern for intracranial injury, a noncontrast CT scan is the recommended test because plain skull radiography lacks sufficient sensitivity to be used as a screening tool.
Computed Tomography of the Head.: There has been a considerable amount of research on the indications and relative value of CT scanning in pediatric head-injured patients. Multiple decision rules have been proposed and continue to be evaluated; however, the need for further research remains. Two variables have been highly associated with the presence of intracranial hemorrhage: the presenting neurologic status and the presence of multiple systemic injuries. It has been suggested that children with isolated head injury with any loss of consciousness can be discharged without a CT scan after careful examination alone, if they are older than 2 years and are neurologically normal. Other authorities contradict those suggestions, establishing a clear association with parenchymal injury and loss of consciousness.36
Other than the controversial issue of loss of consciousness, recommendations for CT scanning include the presence of neurologic deficits, GCS scores of less than 15, and injury patterns that are the result of major forcible insults. Studies have shown various combinations of characteristics that make significant intracranial injury very unlikely but have provided less guidance in selection of which patients actually need a head CT scan (high negative predictive value but low positive predictive value).37 Dunning and coauthors’ meta-analysis showed a statistically significant correlation of intracranial hemorrhage with focality (relative risk [RR] = 9.4), skull fracture (RR = 6.1), altered level of consciousness (RR = 2.23), and GCS scores less than 15 (RR = 5.51).38 Children younger than 1 year are a special challenge to the clinician because their neurologic milestones are more difficult to evaluate. Within this age group, any loss of consciousness, protracted vomiting, irritability, poor feeding, or suspicion of abuse should trigger strong consideration for CT scanning. The value of brief loss of consciousness and the determination of the need for CT in children older than 1 year are less clear, but loss of consciousness lasting longer than a minute is considered an indication for neuroimaging by many practitioners. The presence of a significant cephalohematoma has also been highly correlated with underlying parenchymal brain injury and should also be an indication for neuroimaging.
Spinal Injury
In the United States, more than 1400 children sustain spinal injury annually.39 Although cervical spinal injury is rare, representing only 1 to 2% of pediatric traumatic injuries, higher cord level injuries are more common in children than adults and can lead to devastating outcomes. Cervical injury patterns vary with the age of the patient. Fractures below the C3 level account for only 30% of spinal lesions in children younger than 8 years, which differs dramatically from the patterns seen in adults and older children. Likewise, SCI without obvious radiographic abnormality (SCIWORA) has been found in 25 to 50% of spinal cord injuries in this same age group.40 SCIWORA may be a misnomer in the era of magnetic resonance imaging (MRI). Intraneural or extraneural findings are usually seen immediately on MRI but may be delayed, necessitating immobilization and a follow-up MRI to prevent late or recurrent injury. Length of immobilization is controversial, but it may be up to 12 weeks. Whenever a spinal injury is noted or suspected, careful attention is paid to the entire spine as multilevel injuries are common.41
Principles of Disease
Anatomic features of the cervical spine approach adult patterns between the ages of 8 and 10 years (Box 38-10). However, injury patterns identical to those of adults often do not fully manifest until 15 years of age. The pediatric spine has greater elasticity of the supporting ligamentous structures than the adult spine. The joint capsules of the child have greater elastic properties, and the cartilaginous structures are less calcified than in adults. In the spine, there is a relatively horizontal orientation of the facet joints and uncinate processes, and the anterior surfaces of the vertebral bodies have a more wedge-shaped appearance. Compared with the adult, the child has relatively underdeveloped neck musculature and a head that is disproportionately large and heavy compared with the body. Both of these differences lead to an “anatomic fulcrum of the spine” in children that is at the level of the C2 and C3 vertebrae versus the lower cervical vertebrae as found in adults. These combined anatomic features lead to higher cervical cord injuries and an increased incidence of SCIWORA in children.42
Radiology
Some experts believe that children with neck pain, involvement in an MVC, or any suspicion of cervical injury should receive radiographic evaluation because these factors may be very sensitive in identifying cervical spine injuries in this patient population. Other experts support the use of the National Emergency X-Radiography Utilization Study (NEXUS) criteria to determine who needs cervical radiographs. These criteria were derived from a study of 3065 children younger than 18 years; however, only 4 of 30 cervical spine fractures were in children younger than 9 years, and none of the 88 children younger than 2 years had cervical fractures.43,44 No pediatric cases of SCIWORA were found, and the data showed that 45.9% of cervical spine injuries in the NEXUS study cohort were between the levels of C5 and C7. This may reflect the fact that 2160 of the pediatric patients were 8 to 17 years old. The sensitivity for detection of cervical fractures was reported as 100% (95% confidence interval 87.8-100%); however, less than 1% of children in the study had an injury, making the 100% negative predictive value less meaningful and the sensitivity (at least in young infants) difficult to rely on.43 The majority of injured patients were older than age 9 years and had characteristics more similar to adults than infants.44 Because of the limitations of the NEXUS criteria as they pertain to children, a low threshold for imaging is maintained in children with mechanisms worrisome for cervical injury. A report of discomfort, any distracting injury, or even transient neurologic symptoms should be considered an indication for radiologic evaluation.
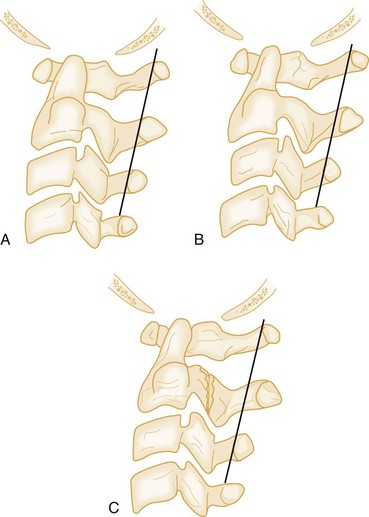
Cervical spine CT should be considered as a first-line imaging modality in children receiving a head CT scan for a head injury, and in children with a high clinical pretest likelihood for cervical fracture. Although the negative predictive value of plain radiographs in low-risk populations seems high, the sensitivity of plain films to detect fractures is far less impressive. When obtained, plain radiographic evaluation should routinely consist of three views: a cross-table lateral, an anteroposterior, and an open-mouth odontoid view. The sensitivity of the three-view cervical spine series is highly variable. Interpretation of plain cervical spine radiographs in children may be especially challenging because of the anatomic changes that occur with growth (see Box 38-10). In addition, pseudosubluxation of C2 on C3 is common on nonextended cervical spine radiographs in children up to adolescence, occurring in approximately 40% of patients.42 The emergency physician distinguishes between pseudosubluxation and true subluxation on nonextended cervical spine radiographs through use of the posterior cervical line and the relationship of the spinolaminar line (also called the line of Swischuk) to the anterior cortical margin of the spinous process at C2. A line is drawn from the anterior cortical margin of the spinous process of C1 down through the anterior cortical margin of C3. If this line at C2 crosses the anterior cortical margin of the spinous process at C2 or is anterior to it by less than 2 mm, no anterior cervical soft tissue swelling is seen, and no fractures are visualized, the patient likely has pseudosubluxation versus true subluxation at that level (Fig. 38-2). Pseudosubluxation may be seen less commonly at C3-C4 as well. Exceptions to this guideline do occur, and the clinical scenario is taken into account before it is applied.
The pretest likelihood of fracture is considered when decisions are being made regarding the removal of cervical immobilization in children with apparently normal imaging. Patients with continued neck pain despite negative radiographs or CT may require MRI evaluation.45 Rare cases may necessitate evaluation by neurosurgery under fluoroscopy. The use of flexion-extension views is rarely indicated or helpful.
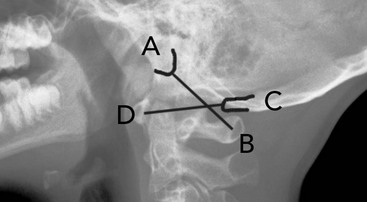
Classically, young children have been considered at greater risk for upper cervical spine injury. Unfortunately, many occipital cervical junction injuries are immediately fatal. However, survival is possible in some cases.46 Early detection and immobilization is crucial. Occipital cervical junction injuries should be suspected in any child pedestrian versus vehicle accident, especially if the child has a laceration under the chin from a forward fall. In many fatal cases, distraction and displacement are obvious. However, in nonfatal cases, they can be subtle. A Power’s ratio greater than 1 indicates an atlanto-occipital dislocation until proven otherwise (normal, approximately 0.77). Power’s ratio is shown in Figure 38-3. It is calculated as the ratio of the distance from the basion to the anterior cortex of the posterior arch of the atlas divided by the distance from the opisthion to the posterior cortex of the anterior arch of the atlas. An additional method to suggest this injury is to draw a vertical line from the posterior border of the odontoid and then measure the distance from this line to the basion. If this distance is greater than 12 mm, then atlanto-occipital separation should be suspected.
In children with upper cervical spine tenderness, it is prudent to consider a fracture of the synchondrosis between the odontoid and C2. This can be difficult to diagnose on plain radiographs, but it is often recognized as a subtle anterior tilt to the odontoid on C2. A CT scan with sagittal reconstructions will clarify this entity and in many cases should be considered as a first-line imaging modality.47,48
Cardiothoracic Injury
Most serious chest injuries in children (>80%) are caused by blunt trauma and result from MVCs, pedestrian accidents, and falls.49 Isolated chest injury is a relatively infrequent occurrence considering the typical mechanisms of blunt trauma in the pediatric patient. Pediatric trauma patients with thoracic injury have a twentyfold increase in mortality over pediatric trauma patients without thoracic trauma.49,50 Sequelae of blunt injury include rib fractures, pulmonary contusion, pneumothorax, hemothorax, myocardial injury, pericardial injury, and vascular injury.
Children subjected to penetrating trauma, in contrast to the injuries associated with blunt trauma, often die from the primary insult. Penetrating trauma accounts for 5 to 15% of thoracic insults in children.49 Nationwide misuse of firearms has resulted in an increasing incidence of penetrating trauma, often with children as victims. The vast majority of these cases are related to the criminal use of handguns; however, improper storage and poor parental supervision lead to devastating consequences in a relatively small, but nevertheless preventable, number of cases each year. Families of children with emotional difficulties or depression should consider removing guns from their homes because of their lethality when used as instruments of suicide. All patients with self-inflicted penetrating trauma should also be assessed for ingested toxins.
Specific clinical patterns should alert the clinician to the potential for concurrent abdominal and thoracic injury. Any patient with penetrating trauma at or below the level of the nipples falls into this category.51 Apparent isolated thoracic trauma does not exclude abdominal injury.
Specific Disorders
Pneumothorax.: The development of a traumatic pneumothorax is commonly associated with significant pulmonary injury. In contrast to spontaneous pneumothoraces, these insults do not resolve spontaneously and often are associated with the presence of a hemothorax. Signs and symptoms include external evidence of chest trauma, such as abrasion, contusion, or ecchymoses; tachypnea; respiratory distress; hypoxemia; and chest pain. Decreased breath sounds may not be appreciated in children with pneumothoraces because of the wide transmission of breath sounds in the chest and upper abdomen. It is critical to listen to the chest from the axilla in children. This location helps with lateralization to distinguish decreased breath sounds on one side compared with the other and, after intubation, to assess for proper ETT position.
Management of a hemopneumothorax includes the placement of a large-caliber chest tube far enough posteriorly, near the mid-axillary line, to prevent encroaching on more anterior soft tissue that will later become part of the breast. Chest tube size for hemopneumothorax management can be found in Box 38-2 or can be found on a length-based resuscitation tape. A chest tube should be considered for any patient with a pneumothorax who will be undergoing mechanical ventilation. In the most conservative of scenarios, such as small (<20%) simple pneumothoraces without tension in a child who will not be mechanically ventilated, the child may be observed carefully for extended periods with 100% oxygen supplementation for nitrogen washout. Reassessment can be accomplished by repeat chest radiographs at selected intervals, or a pigtail catheter can be placed percutaneously with a modified Seldinger technique.
Open Pneumothorax.: An open pneumothorax exists when the chest wall is injured sufficiently to allow bidirectional flow of air through the wound. The patient is unable to expand the lung because of equalization of pressures between the atmosphere and the chest cavity. Ventilation and oxygenation are severely impaired.
Tension Pneumothorax.: Pulmonary air leaks that occur in a one-way valve arrangement favor the development of a tension pneumothorax. Increasing amounts of free air within the pleural cavity cause the mediastinal structures to shift toward the opposite side, compromising cardiac output. The final common pathway involves hypoxia, hypotension, and refractory shock. Most patients with tension pneumothoraces have severe respiratory distress, decreased breath sounds (often bilaterally), and a shift in the point of maximal cardiac impulse. In the worst scenario, there is mediastinal shift, contralateral tracheal deviation, and distention of the neck veins with decreased venous return to the thorax. In pediatric patients, signs of tension pneumothorax are often subtle. A short neck and increased soft tissue may make detection of tracheal deviation difficult. Pediatric patients with tension pneumothorax may have only subtle signs or only tachycardia, shock, and respiratory distress. The emergency physician should consider the diagnosis of tension pneumothorax and, if it is detected or strongly suspected, should treat the patient immediately with decompression. Respiratory embarrassment, hypotension, and circulatory collapse will occur without adequate decompression.
Hemothorax.: Significant bleeding may occur when injury is directed toward the intercostal vessels, internal mammary vessels, or lung parenchyma. Without an upright chest radiograph, it is difficult to quantify the degree of bleeding on plain films. The only sign of hemothorax on a supine radiograph may be a slightly less radiolucent appearance on the affected side of the chest. Development of a massive hemothorax is rare in children and is associated most often with severe impact, such as that seen in high-velocity MVCs, falls from extreme heights, or the use of high-powered firearms. These injuries are evaluated and treated quickly. Clinically, patients have decreased breath sounds and dullness to percussion on the affected side. A pneumothorax may coexist with a hemothorax. The pediatric patient may demonstrate early or late signs of hypovolemic shock.
Indications for thoracotomy in an operating room include evacuated blood volumes exceeding 15 mL of blood per kilogram immediately after the placement of the chest tube, persistent blood loss (e.g., exceeding 2-4 mL/kg/hr over 3 hours), or continued air leak. Emergency department thoracotomy is reserved for patients with thoracic trauma who deteriorate to cardiopulmonary failure despite maximal resuscitation in the out-of-hospital setting or emergency department. Guidelines for emergency pediatric thoracotomy are often institution specific. There are few published data on pediatric emergency department thoracotomy, and guidelines are often based on adult data. For both children and adults, more liberal use in penetrating injury and limited use in blunt trauma are recommended. In patients with penetrating chest trauma and CPR for less than 15 minutes, a left anterior thoracotomy may be warranted, whereas patients with blunt trauma should undergo rapid assessment by ultrasound for tamponade. If tamponade is present and CPR has been performed for less than 10 minutes, then a left anterior thoracotomy may be indicated.52 Suggested contraindications to emergency department resuscitative thoracotomy after out-of-hospital CPR include (1) blunt trauma with CPR for longer than 10 minutes with asystole and no signs of life on presentation without ultrasound evidence of cardiac tamponade and (2) penetrating trauma with CPR for greater than 15 minutes and asystole with no signs of life on arrival without ultrasound evidence of cardiac tamponade.
Pulmonary Contusion.: Penetrating and blunt thoracic trauma may result in the development of a pulmonary contusion. The compliance of the rib cage in children renders them susceptible to the development of pulmonary contusion even in the absence of external signs of chest trauma. Injury to capillary membranes allows blood to collect within the interstitial spaces, resulting in hypoxia and respiratory distress. If bleeding is severe enough, oxygenation and ventilation are impaired. Initial chest radiographs may not show the classic findings of pulmonary consolidation. In addition, in the early stages of injury, blood gases may be normal.
Traumatic Diaphragmatic Hernia.: Children involved in MVCs who are wearing lap belts are predisposed to the development of diaphragmatic herniation.50,53 Mechanisms of injury involve sudden increases in intra-abdominal pressure. Patients initially are in stable condition, with the degree of respiratory distress directly proportional to the amount of abdominal contents that protrude into the pulmonary space. The presence of bruising from lap belt–only compression should alert the clinician to the possibility of diaphragmatic hernia and other intra-abdominal injuries (small bowel injury) and the possibility of associated thoracolumbar spinal insults such as Chance fractures. Most commonly, the herniation occurs on the left side because the liver prevents herniation of bowel on the right.53
Cardiac and Vascular Injuries.: Injuries to the heart and large vessels are uncommon in children.54 In cardiac and vascular injuries, an electrocardiogram may show tachycardia with low voltage (pericardial tamponade), findings consistent with acute myocardial injury (ST segment elevation), or a variety of other nonspecific abnormalities.
Commotio Cordis.: Commotio cordis is a disorder described in pediatric patients that results from sudden impact to the anterior chest wall (e.g., when a child is struck in the chest with a thrown or hit baseball), which causes cessation of normal cardiac function. The patient may have an immediate dysrhythmia or ventricular fibrillation that is refractory to resuscitation efforts. Significant morbidity and mortality are associated with this disorder, and although most patients recover completely, some require extended treatment with antiarrhythmic agents, cardiac pacemaker placement, inotropic agents, or intra-aortic balloon pump. In patients with prolonged cardiac instability, cardiogenic shock and death are common despite maximal therapeutic intervention.
Abdominal Injury
Blunt trauma related to MVCs causes more than 50% of abdominal injuries in children and is the most lethal. “Lap belt” injury, including small bowel injury and Chance fractures, may occur in restrained children involved in MVCs.55–57 Another common cause of abdominal injury involves bicycle crashes. Handlebars are a serious cause of injury and subsequent hospitalization for the pediatric population. Often the effects of bicycle injuries may not be seen on initial presentation, with the mean elapsed time to onset of symptoms being nearly 24 hours after injury. All children with epigastric pain after blunt trauma, especially when concentrated force has been applied in this area, should be considered to have duodenal hematoma until proven otherwise. Pancreatic injury, including transection, should be strongly considered as well.
Clinical Features
Signs and symptoms of abdominal injury in children include tachypnea from impaired diaphragmatic excursion, abdominal tenderness, ecchymoses, and signs of shock. Restrained children involved in MVCs with abdominal bruising are much more likely to have an intra-abdominal injury than those without bruising.57 Abdominal distention is a common nonspecific finding that is often the result of air swallowing subsequent to a painful event. Children with hepatic and splenic injuries may have trouble localizing their pain. Kehr’s sign (left shoulder pain with spleen injury) may be the only indication of an intra-abdominal injury. Any abdominal tenderness on examination should prompt further evaluation of the abdomen. Vomiting can be associated with duodenal hematoma or traumatic pancreatic injury but is usually a late sign. Signs of small bowel injury may be delayed and noted clinically only with serial examinations. Pelvic bone stability should be assessed in cases of abdominal trauma, and a genital examination searching for signs of injury should be performed. Rectal examination is insensitive and nonspecific when used as a general screening test for all patients after serious trauma. Patients with suspected injury should receive further evaluation even if rectal examination findings are unremarkable.
Diagnostic Strategies and Management
In patients with suspected abdominal injury or with mechanisms of possible injury, management and resuscitation must be rapid. Because of fear and pain, children can compound the difficulties in the management of serious penetrating or blunt abdominal trauma. Children tend to distend the stomach greatly with ingested air, which can decrease the diaphragmatic excursion. This can compromise respiratory efforts, and early decompression with an NG or OG tube should be considered. In children who have undergone major trauma and have a stable pelvis without risk of urethral trauma, a urinary catheter should be considered for decompression of the bladder, evaluation for the presence of urinary retention, examination for the presence of blood in the urine, and measurement of urine output. The bladder should be decompressed before any invasive evaluation of the abdomen to prevent accidental laceration during the procedure. Urinary catheter size estimates are shown in Box 38-2.
Indications for laparotomy are listed in Box 38-11. Whatever the surgical preference within a health care facility, it is important to establish a protocol for approaching these challenging patients. Patients who remain hypotensive after adequate crystalloid infusion, have active arterial bleeding on CT scan, or have consistent decreases in their hemoglobin level are likely candidates for early invasive intervention. Exploratory laparoscopy or laparotomy is often required, but patients with a known source of bleeding may be appropriate candidates for arterial embolization in an angiography suite.
Spleen Injury.: Injuries to the spleen are the most common injuries in pediatric abdominal trauma. Children with injuries from MVCs, sudden deceleration injuries, and contact sports–related injuries may sustain splenic trauma. Typical findings include left upper quadrant abdominal pain radiating to the left shoulder. The abdominal examination may show evidence of peritoneal irritation in the left upper quadrant of the abdomen. Patients may be hemodynamically stable or, after significant splenic rupture or laceration, may be persistently hypotensive or in fulminant cardiovascular collapse. Stable patients may undergo CT for radiologic evaluation. Most often with minor splenic trauma, bleeding is controlled spontaneously without operative intervention; however, all patients with a splenic injury should be evaluated by a surgeon. In patients with a contained splenic subcapsular hematoma, extracapsular bleeding may occur days after capsular rupture. Patients with splenic injury should be admitted to the hospital for close observation and repeated examinations. Because of the desire for splenic salvage to maintain immunocompetency, an injured spleen is often left in place as long as the patient can be resuscitated adequately with crystalloid and blood products.
Liver Injury.: The liver is the second most commonly injured solid organ in the pediatric patient with abdominal trauma. However, it is the most common cause of lethal hemorrhage, with a mortality of 10 to 20% in severe liver injury. Mechanisms of injury causing splenic injury also may cause liver trauma. Tenderness on palpation of the right upper quadrant of the abdomen and the complaint of abdominal pain in this region or in the right shoulder are signs of possible liver injury. Patients managed conservatively often do well; however, patients who are initially treated conservatively but then go on to require delayed laparotomy often have significant morbidity and mortality. Close observation in the hospital, serial abdominal examinations, and serial hemoglobin measurements are recommended.
Renal Injury.: The kidney is less susceptible to trauma from forces applied to the anterior abdomen, but it is often injured in the pediatric patient with multiple injuries.58 Because this organ is retroperitoneal, signs and symptoms of kidney injury are often less obvious and more diffuse than signs and symptoms of other abdominal organ injuries. Often, dull back pain, ecchymosis in the costovertebral region, and hematuria are the only clues to renal injury.59 Renal ultrasound and CT may be used in a stable patient to assess the degree of renal involvement. Other organs, such as the pancreas and gastrointestinal tract, are less frequently injured in pediatric patients.
Penetrating Injury.: Penetrating wounds to the abdomen usually require rapid evaluation by a surgeon and, in some cases, operative intervention. With hemodynamic instability, or peritonitis, urgent laparotomy is indicated. In the hemodynamically stable patient, further workup with a CT scan, local wound exploration, diagnostic laparoscopy, and observation may be warranted.
Diagnostic peritoneal tap (DPT) and diagnostic peritoneal lavage (DPL) have been largely supplanted by other diagnostic modalities in modern practice, such as CT and diagnostic laparoscopy.60 However, they may be necessary in resource-poor areas and in large-scale disasters. The aspiration of 10 mL of blood or fecal or vegetable matter from the abdomen with DPT would typically indicate intraperitoneal hemorrhage, bowel injury, or both, and the likely need for laparotomy. DPL is performed by placing 15 mL of warm sodium chloride per kilogram in the peritoneal space and then removing the fluid by gravity. In blunt trauma, a positive DPL is defined as a finding of more than 100,000 red blood cells per milliliter, more than 500 white blood cells per milliliter, or gram-negative bacteria or vegetative material (stool) seen on microscopy. DPL does not evaluate for retroperitoneal bleeding. The threshold values must be lowered for penetrating trauma. In general, DPLs are not performed on stable patients because CT scanning can be done quickly and give the clinician far more information, especially about intraparenchymal injury and retroperitoneal injury.
Radiology.: Pediatric patients frequently sustain injury to the spleen, liver, kidneys, and gastrointestinal tract. CT of the abdomen can provide high sensitivity and specificity for identification of these injuries while being relatively noninvasive.61,62 Abdominal CT has a high negative predictive value.61,62 Oral contrast does not add to the accuracy of CT for trauma; thus one can avoid oral contrast–related delays in evaluation, difficulty with administration, and risk of aspiration.
References
1. Centers for Disease Control and Prevention. Web-Based Injury Statistics Query and Reporting System (WISQARS). www.cdc.gov/injury/wisqars/index.html.
2. Sharma, O, Oswanski, M, Stringfellow, K, Raj, S. Pediatric blunt trauma: A retrospective analysis in a Level I trauma center. Am Surg. 2006;72:538–543.
3. Roaten, J, et al. Nonaccidental trauma is a major cause of morbidity and mortality among patients at a regional level 1 pediatric trauma center. J Pediatr Surg. 2006;41:2013–2015.
4. Partrick, D, Bensard, D, Janik, J, Karrer, F. Is hypotension a reliable indicator of blood loss from traumatic injury in children? Am J Surg. 2002;184:555–559.
5. Milkovich, SM, Owens, J, Stool, D, Chen, X, Beran, M. Accidental childhood strangulation by human hair. J Pediatr Otorhinolaryngol. 2005;69:1621.
6. Langhan, M, Chen, L. Current utilization of continuous end-tidal carbon dioxide monitoring in pediatric emergency departments. Pediatr Emerg Care. 2008;24:211–213.
7. Murphy, R, Ghosh, A. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. The accuracy of abdominal ultrasound in paediatric trauma. Emerg Med J. 2001;18:208–209.
8. Dehmer, J, Adamson, W. Massive transfusion and blood product use in the pediatric trauma patient. Semin Pediatr Surg. 2010;19:286–291.
9. Ball, C, et al. Traumatic urethral injuries: Does the digital rectal examination really help us? Injury. 2009;40:984–986.
10. Shlamovitz, G, et al. Lack of evidence to support routine digital rectal examination in pediatric trauma patients. Pediatr Emerg Care. 2007;23:537.
11. Willner, E, Jackson, H, Nager, AL. Delayed diagnosis of injuries in pediatric trauma: The role of radiographic ordering practices. Am J Emerg Med. 2012;30:115–123.
12. Capraro, A, Mooney, D, Waltzman, M. The use of routine laboratory studies as screening tools in pediatric abdominal trauma. Pediatr Emerg Care. 2006;22:480–484.
13. Zehtabchi, S, Sinert, R, Goldman, M, Kapitanyan, R, Ballas, J. Diagnostic performance of serial haematocrit measurements in identifying major injury in adult trauma patients. Injury. 2006;37:46–52.
14. Sauerland, S, et al. The reliability of clinical examination in detecting pelvic fractures in blunt trauma patients: A meta analysis. Arch Orthop Trauma Surg. 2003;124:123–128.
15. Junkins, E, Furnival, R, Bolte, R. The clinical presentation of pediatric pelvic fractures. Pediatr Emerg Care. 2001;17:15–18.
16. Junkins, E, Nelson, D, Carroll, K. A prospective evaluation of the clinical presentation of pediatric pelvic fractures. J Trauma. 2001;51:64–68.
17. Faul, M, Xu, L, Wald, MM, Coronado, VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta, Ga: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010.
18. Da Dalt, L, et al. Characteristics of children with vomiting after minor head trauma: A case control study. J Pediatr. 2007;150:274–278.
19. Holmes, J, Palchak, M, Conklin, M, Kuppermann, N. Do children require hospitalization after immediate posttraumatic seizures? Ann Emerg Med. 2004;43:706–710.
20. Yoshikawa, H. Benign setting sun phenomenon in full term infants. J Child Neurol. 2003;18:424–425.
21. Sorantin, E, Brader, P, Thimary, F. Neonatal trauma. Eur J Radiol. 2006;60:199–207.
22. Amar, A, Aryan, H, Meltzer, H, Levy, M. Neonatal subgaleal hematoma causing brain compression: Report of two cases and review of the literature. Neurosurgery. 2003;52:1470–1474.
23. Kilani, R, Wetmore, J. Neonatal subgaleal hematoma: Presentation and outcome—radiological findings and factors associated with mortality. Am J Perinatol. 2006;23:41–48.
24. Oehmihen, M, Meissner, C, Saternus, KS. Fall or shaken: Traumatic brain injury in children caused by falls or abuse at home—a review on biomechanics and diagnosis. Neuropediatrics. 2005;36:240–245.
25. Togioka, B, et al. Retinal hemorrhages and shaken baby syndrome: An evidence-based review. J Emerg Med. 2009;37:98–106.
26. Stoodley, N. Controversies in non-accidental head injury in infants. Br J Radiol. 2006;79:550–559.
27. Gerber, P. Nonaccidental head trauma in infants. Childs Nerv Syst. 2007;23:499–507.
28. Hackbarth, R, et al. Survival and functional outcome in pediatric traumatic brain injury: A retrospective review and analysis of predictive factors. Crit Care Med. 2002;30:1630–1635.
29. Gausche, M, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurologic outcome. JAMA. 2000;283:783–790.
30. Gerritse, B, Draaisma, J, Schalkwijk, A, van Grunsven, P, Scheffer, G. Should EMS-paramedics perform paediatric tracheal intubation in the field? Resuscitation. 2008;79:225–229.
31. Young, K, et al. A randomized double blinded placebo controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med. 2004;43:435–436.
32. Giza, C, Mink, R, Madikians, A. Pediatric traumatic brain injury: Not just little adults. Crit Care. 2007;13:143–182.
33. Szaflarski, J, Sangha, K, Lindsell, C, Shutter, L. Prospective, randomized, single-blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care. 2010;12:165–172.
34. Adelson, P, Bratton, S, Carney, N. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003;4(3 Suppl):S1.
35. Scaife, E. Traumatic brain injury: Preferred methods and targets for resuscitation. Curr Opin Pediatr. 2010;22:339–345.
36. Davis, R. The use of cranial CT scans in the triage of pediatric patients with mild head injuries. Pediatrics. 1995;95:345–349.
37. Güzel, A, et al. Indications for brain computed tomography and hospital admission in pediatric patients with minor head injury: How much can we rely upon clinical findings? Pediatr Neurosurg. 2009;45:262–270.
38. Dunning, J, et al. A meta analysis of variables that predict significant intracranial injury in minor head trauma. Arch Dis Child. 2004;89:653–659.
39. Vitale, MG, Goss, JM, Matsumoto, H, Roye, DP, Jr. Epidemiology of pediatric spinal cord injury in the United States: Years 1997 and 2000. J Pediatr Orthop. 2006;26:745–749.
40. Trigylidas, T, Yuh, S, Vassilyadi, M, Matzinger, M, Mikrogianakis, A. Spinal cord injuries without radiographic abnormality at two pediatric trauma centers in Ontario. Pediatr Neurosurg. 2010;46:283–289.
41. Mortazavi, M, et al. Pediatric multilevel spine injuries: An institutional experience. Childs Nerv Syst. 2011;27:1095–1100.
42. Gore, P, Chang, S, Theodore, N. Cervical spine injuries in children: Attention to radiographic differences and stability compared to those in the adult patient. Semin Pediatr Neurol. 2009;16:42–58.
43. Viccellio, P, et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2008;108:E20.
44. Sun, B, Hoffman, J, Mower, W. Evaluation of a modified prediction instrument to identify significant pediatric intracranial injury after blunt head trauma. Ann Emerg Med. 2007;49:325–332.
45. Eubanks, J, Gilmore, A, Bess, S, Cooperman, D. Clearing the pediatric cervical spine following injury. J Am Acad Orthop Surg. 2006;14:552–564.
46. Salinsky, J, Scuderi, G, Crawford, A. Occipito-atlanto-axial dissociation in a child with preservation of life: A case report and review of the literature. Pediatr Neurosurg. 2007;43:137–141.
47. Hutchings, L, Willett, K. Cervical spine clearance in pediatric trauma: A review of current literature. J Trauma. 2009;67:687–691.
48. Silva, C, et al. Do additional views improve the diagnostic performance of cervical spine radiography in pediatric trauma? AJR Am J Roentgenol. 2010;194:500–508.
49. Sartorelli, K, Vane, D. The diagnosis and management of children with blunt injury of the chest. Semin Pediatr Surg. 2004;13:98–105.
50. Woosley, C, Mayes, T. The pediatric patient and thoracic trauma. Semin Thorac Cardiovasc Surg. 2008;20:58–63.
51. Balci, A, et al. Blunt thoracic trauma in children: Review of 137 cases. Eur J Cardiothorac Surg. 2004;26:387–392.
52. Moore, E, et al. Defining the limits of resuscitative emergency department thoracotomy: A contemporary Western Trauma Association perspective. J Trauma. 2011;70:334–339.
53. Turhan, K, et al. Traumatic diaphragmatic rupture: Look to see. Eur J Cardiothorac Surg. 2008;33:1082–1085.
54. Tiao, G, Griffith, P, Szmuszkovicz, J, Hossein Mahour, G. Cardiac and great vessel injuries in children after blunt trauma: An institutional review. J Pediatr Surg. 2000;35:1656–1660.
55. Achildi, O, Betz, R, Grewal, H. Lapbelt injuries and the seatbelt syndrome in pediatric spinal cord injury. J Spinal Cord Med. 2007;30(Suppl 1):S21–S24.
56. Santschi, M, Echavé, V, Laflamme, S, McFadden, N, Cyr, C. Seat-belt injuries in children involved in motor vehicle crashes. Can J Surg. 2005;48:373–376.
57. Lutz, N, et al. Incidence and clinical significance of abdominal wall bruising in restrained children involved in motor vehicle crashes. J Pediatr Surg. 2006;39:972–975.
58. Tsui, A, Lazarus, J, Sebastian van As, AB. Non-operative management of renal trauma in very young children: Experiences from a dedicated South African paediatric trauma unit. Injury. 2011.
59. Abou-Jaoude, WA, Sugarman, JM, Fallat, ME, Casale, AJ. Indicators of genitourinary tract injury or anomaly in cases of pediatric blunt trauma. J Pediatr Surg. 1996;31:86–89.
60. Como, J, et al. Practice Management Guidelines for Nonoperative Management of Penetrating Abdominal Trauma. Chicago: Eastern Association for the Surgery of Trauma (EAST); 2007.
61. Hom, J. The risk of intra-abdominal injuries in pediatric patients with stable blunt abdominal trauma and negative abdominal computer tomography. Acad Emerg Med. 2010;17:469–475.
62. Awashti, S, et al. Is hospital admission and observation required after a normal abdominal computed tomography scan in children with blunt abdominal trauma. Acad Emerg Med. 2008;15:895–899.


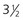 kg+ = 3-3.5 mm
kg+ = 3-3.5 mm