Hydrocarbons
Perspective
Human exposure to hydrocarbons (HCs) is a common problem. In 2010, U.S. poison centers reported 43,000 exposures to HCs, with the majority of cases managed in an outpatient setting.1 Exposure to HCs of patients who present to the emergency department (ED) can generally be classified into four types. The first is the accidental ingestion involving children younger than 5 years. This is the most common scenario causing fatality, and it usually involves significant pulmonary injury. Second is the intentional inhalational abuse of volatile HCs. Recreational abuse has been a medical problem since solvent inhalation became popular during the late 1800s. Fatalities in this group will typically occur within distinct demographic groups (Native Americans, homosexual men, and teenagers).1–3 Third is the accidental inhalational or dermal exposure to HCs in the household or workplace setting. The fourth type is massive oral ingestion of HCs in a suicide attempt.
Principles of Disease
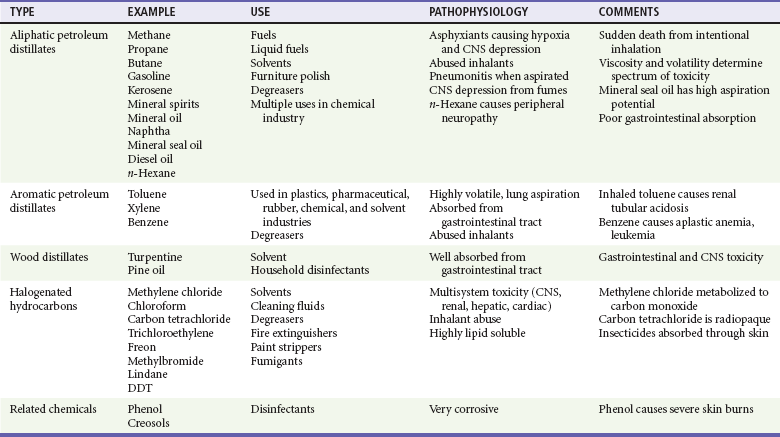
HCs are a diverse group of organic compounds that contain hydrogen and carbon (Table 158-1). Most HCs (e.g., gasoline) are byproducts of crude oil and are therefore called petroleum distillates. Some products, such as turpentine, are derived from pine oil, not petroleum. HCs can also be classified by their structure. The two main categories are straight chain HCs (aliphatic, such as propane) and those containing a benzene ring structure (aromatic, such as toluene). HCs can also have multiple nonorganic side chains. For example, halogenated HCs usually will have one or more bromide, chloride, fluoride, or iodide moieties (e.g., carbon tetrachloride). Finally, HCs are used as the solvent base for many toxic chemicals, such as insecticides and metals, that in turn can cause a separate distinct syndrome of poisoning. Although the range of toxicity of HCs can vary widely, the majority of human exposures are confined to petroleum distillates.
Pathophysiology
Acute HC toxicity usually affects three main target organs: the lungs, the heart, and the central nervous system (CNS). Although certain HCs can enter the body through the skin or gastrointestinal tract, HCs cause the most damage through the lungs. Despite the fact that there are thousands of different types of HCs, their potential for acute toxicity depends on four characteristics4,5:
1. Viscosity is the capacity to resist flow or change. Low viscosity allows a substance to spread rapidly, and low-viscosity HCs spread easily into the airway and lungs. Viscosity is measured in Saybolt seconds universal (SSU), and substances with an SSU of less than 60 have the highest potential risk of aspiration. Lubricants and mineral oil have high viscosity and low toxicity, whereas furniture polish has low viscosity and high pulmonary toxicity.
2. Volatility is the ease for a liquid to turn into a gas. High volatility often has a detectable odor. HCs with high volatility can displace alveolar oxygen and cause hypoxia. Butane and propane are types of HCs with high volatility.
3. Surface tension is the capacity for a substance to collect on a liquid surface. Low surface tension enables a substance (e.g., turpentine) to disperse easily.
4. Chemical side chains often increase potential toxicity. These toxic side chains include metals (e.g., arsenic), halogens (e.g., carbon tetrachloride), and aromatic structures (e.g., toluene).
Pulmonary Pathophysiology
The primary target organ for toxicity is the lung. Fatalities after ingestion usually occur with an accompanying aspiration. A small amount of HC in the trachea can be devastating, whereas a much larger amount of the same compound in the stomach remains benign.1,5,6
HCs affect the lungs through several mechanisms. HCs are usually poorly water soluble and penetrate into the lower airways, producing bronchospasm and an inflammatory response. Second, volatized HCs can displace oxygen in the alveolar space, causing hypoxia. Third, HCs can cause direct injury to pulmonary alveoli and capillaries, producing distinct uniform lesions. Autopsy findings of these lesions include hyperemia, diffuse hemorrhagic exudative alveolitis with granulocytic infiltration, and microabscesses. Finally, HCs can inhibit surfactant function, leading to alveolar instability and collapse. These mechanisms lead to alveolar dysfunction, ventilation-perfusion mismatch, hypoxemia, and respiratory failure.5,6
Central Nervous System Pathophysiology
Certain HCs cause CNS depression (i.e., toluene, benzene, gasoline, butane, and chlorinated HCs). After respiratory exposure, most HCs passively diffuse through the pulmonary alveolus and are highly absorbed in blood and tissues. These HCs can cause euphoria, disinhibition, confusion, and obtundation. With an isolated single exposure, these effects usually have a rapid onset of intoxication and rapid recovery. For these reasons, substance abusers seek these HCs for recreational use. Inhalation of these substances avoids hepatic first-pass metabolism and generates high concentrations in the CNS. Chronic use of inhaled HCs can cause severe abnormalities in nervous system function, which include peripheral neuropathy, cerebellar degeneration, neuropsychiatric disorders, chronic encephalopathy, and dementia. More than 50% of patients who abuse toluene for more than 10 years will have cerebral cortical atrophy with histologic changes that include loss of neurons, diffuse gliosis, and axonal degeneration.3,7
Cardiac Pathophysiology
HCs can precipitate sudden death, especially after intentional inhalation. These compounds are thought to produce myocardial sensitization of endogenous and exogenous catecholamines, which then precipitates ventricular dysrhythmias and myocardial dysfunction. This is particularly true for halogenated and aromatic HCs (e.g., difluoroethane found in computer keyboard cleaning canisters).2,8
Other Pathophysiology
Various HCs have been reported to be toxic to other organ systems. Certain recognized syndromes include toluene-induced renal tubular acidosis, benzene-induced bone marrow toxicity and leukemia, methylene chloride–induced carbon monoxide poisoning, and chlorinated HC–induced centrilobular hepatic necrosis and renal failure. Direct skin exposure of certain HCs can cause extensive chemical burns.5 HCs are often used as solvents for other chemicals that may have their own significant inherent toxicity.
Clinical Features
After oral ingestion, severe poisoning is related to aspiration, which is manifested with early respiratory symptoms, including cyanosis, coughing, grunting (small children), noisy respirations, or repeated bouts of vomiting. A patient may initially have mild symptoms and then develop tachypnea, dyspnea, bronchospasm, wheezing, rales, and fever during several hours.9–12 A change in mental status can be a manifestation of hypoxia or hypercapnia, but it is also a direct effect of HCs. In extreme cases, patients may have frank respiratory failure. Various additives or solutes can produce symptoms independently (e.g., seizures from camphorated HCs or cyanosis from nitrite-induced methemoglobinemia). Pesticides are often dissolved in an HC base. With pesticide exposures, it can be difficult to distinguish acute respiratory distress syndrome induced by HC aspiration from pulmonary edema induced by organophosphate exposures (see Chapter 163). Ingestion or injection of HC as a mechanism for deliberate self-harm is exceedingly rare.
The second scenario for HC toxicity is with solvent abuse. Solvent abuse is associated with various paraphernalia, such as plastic bags used for “bagging” (a method of pouring HCs in a bag or container and then inhaling deeply) and HC-soaked cloth used for “huffing” (a method in which abusers inhale through a saturated cloth). Patients often have the distinctive odor associated with organic HCs. A characteristic rash may be present over the mouth and nose (“glue-sniffer’s rash”) (Fig. 158-1). These patients can also present to the ED with CNS intoxication with euphoria, agitation, hallucinations, confusion, or bizarre behavior. This may progress to CNS depression and seizures. In the extreme case, these patients will be in cardiac arrest. This typically involves an individual who has inhaled solvents, performed some type of physical activity, such as running, and then suddenly collapsed. This is thought to be due to cardiac sensitization by endogenous catecholamine and the ensuing development of dysrhythmias.2–6 Drug abusers who chronically inhale HCs may be brought to medical attention not specifically for treatment of their drug abuse but rather for behavioral problems or nonspecific medical symptoms caused by their abuse. The long-term chronic abuser may clinically appear similar to the long-term “skid row” alcoholic, with peripheral neuropathy, cerebellar degeneration, and encephalopathy.7,13,14
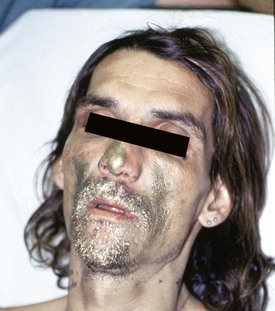
Figure 158-1 Presentation of paint sniffer (“huffer”) with paint around the face and sedation. (Courtesy Chris Tomaszewski, MD.)
A third common scenario is the accidental dermal or inhaled (nonaspiration) respiratory exposure to HCs in the workplace or home. Such exposures are rarely life-threatening. Most cases do not present for medical care or are handled primarily through lay discussion with consultants at local poison control centers.1 The few patients who present to the ED typically will be asymptomatic or have transient nonspecific symptoms, such as headache, dizziness, or nausea. Those with significant respiratory exposure may have persistent pulmonary complaints and physical findings such as coughing, wheezing, and cyanosis. Patients with significant acute dermal exposures may have pain and evidence of chemical burns consisting of erythema, swelling, blistering, and dermal destruction.
Diagnostic Strategies
History and examination should focus on possible aspiration. These symptoms include cough, difficulty breathing, and shortness of breath. Signs of a significant exposure include tachypnea, tachycardia, wheezing, and hypoxemia. Patients with a significant HC exposure should have a chest radiograph taken. Radiographic changes can occur within 30 minutes of ingestion and may identify pathologic processes not recognized by auscultation in more than 50% of cases.5 Hypoxemia noted on pulse oximetry or arterial blood gas measurement provides supportive evidence for aspiration.
Management
C camphor, which can cause seizures and status epilepticus
H halogenated HCs, which can cause dysrhythmias and hepatotoxicity
A aromatic HCs, which can cause bone marrow suppression and cancer
M metals (e.g., arsenic, mercury, and lead)
P pesticides, which can cause cholinergic crises, seizures, and respiratory depression
Because HC toxicity can cause rapid decompensation of a patient’s pulmonary, cardiac, and CNS functions, all patients should be in a well-observed area with cardiac monitoring and pulse oximetry. In severe cases, early intubation for airway control and positive end-expiratory pressure has been advocated to minimize aspiration risks and to counteract HC-induced alveoli collapse. However, no studies have proved this to be more beneficial than standard respiratory care. High-frequency jet ventilation, surfactant therapy, and extracorporeal membrane oxygenation have been attempted as treatments of children with respiratory failure secondary to aspiration,1,5,15 but no studies have been done to demonstrate benefit. Corticosteroids and antibiotics have not been shown to be beneficial in HC aspiration, but the differentiation between bacterial and chemical pneumonia may be difficult. More than 50% of children with significant HC poisoning will have a fever and leukocytosis.5 Theoretically, exogenous catecholamine might cause dysrhythmias in HC-sensitized myocardium, so most toxicologists recommend avoidance of cardiac-active catecholamines, such as epinephrine.
Disposition
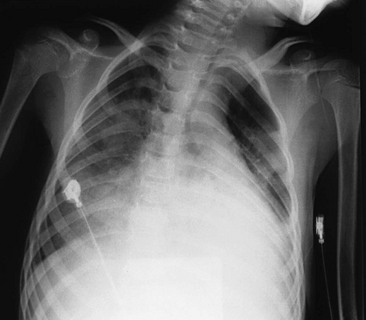
Patients with exposures to known, relatively benign HCs should have a 4- to 6-hour period of observation. If no signs of pulmonary or systemic toxicity develop during the observation period, the patient is discharged to home, with advice for follow-up evaluation if symptoms develop on a delayed basis. If pulmonary symptoms develop, a chest radiograph will show developing pneumonitis (Fig. 158-2), requiring hospital admission for prolonged observation.
References
1. Bronstein, AC, et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th Annual Report. Clin Toxicol (Phila). 2010;48:979–1178.
2. Kurtzman, TL, Otsuka, KN, Wahl, RA. Inhalant abuse by adolescents. J Adolesc Health. 2001;28:170–180.
3. Schaumburg, HH. Toluene. In Spencer PS, Schaumburg HH, eds.: Experimental and Clinical Neurotoxicology, 2nd ed, New York: Oxford University Press, 2000.
4. Cobaugh, DJ, Seger, DL, Krenzelok, EP. Hydrocarbon toxicity: An analysis of AAPCC TESS data. Przegl Lek. 2007;64:194.
5. Gummin, DD, Hryhorczuk, DO. Hydrocarbons. In Goldfrank L, ed.: Goldfrank’s Toxicologic Emergencies, 8th ed, New York: McGraw-Hill, 2006.
6. Holubek, WJ. Solvents. In: Biller J, ed. The Interface of Neurology and Internal Medicine. Philadelphia: Lippincott Williams & Wilkins, 2008.
7. Win-Shwe, T, Fujimaki, H. Neurotoxicology of toluene. Toxicol Lett. 2010;198:93–99.
8. Avella, J, Wilson, JC, Lehrer, M. Fatal cardiac arrhythmia after repeated exposure to 1,1-difluoroethane (DFE). Am J Forensic Med Pathol. 2006;27:58.
9. Bond, GR, et al. A clinical decision rule for triage of children under 5 years of age with hydrocarbon (kerosene) aspiration in developing countries. Clin Toxicol (Phila). 2008;46:222.
10. Jayashree, M, Singhi, S, Gupta, A. Predictors of outcome in children with hydrocarbon poisoning receiving intensive care. Indian Pediatr. 2006;43:715.
11. Lifshitz, M, Sofer, S, Gorodischer, R. Hydrocarbon poisoning in children: A 5-year retrospective study. Wilderness Environ Med. 2003;14:78.
12. Shotar, AM. Kerosene poisoning in childhood: A 6-year prospective study at the Princess Rahmat Teaching Hospital. Neuroendocrinol Lett. 2005;26:835.
13. Borne, J, Riascos, R, Cuellar, H, Vargas, D, Rojas, R. Neuroimaging in drug and substance abuse part II: Opioids and solvents. Top Magn Reson Imaging. 2005;16:239.
14. Costa, LG, Guizzetti, M, Burry, M, Oberdoerster, J. Developmental neurotoxicity: Do similar phenotypes indicate a common mode of action? A comparison of fetal alcohol syndrome, toluene embryopathy and maternal phenylketonuria. Toxicol Lett. 2002;127:197.
15. Horoz, OO, Yildizdas, D, Yimaz, HL. Surfactant therapy in acute respiratory distress syndrome due to hydrocarbon aspiration. Singapore Med J. 2009;50:e130–e132.