Chapter 72 Pediatric Hodgkin’s Lymphoma
Given the high cure rates in treating children with Hodgkin’s lymphoma, the ongoing challenge has been the development of less toxic therapy. In this regard, the progress in Hodgkin’s lymphoma management in children has often presaged that in adult patients. Although many similarities between pediatric and adult Hodgkin’s lymphoma exist, there are a number of distinctions beginning with a bimodal incidence with one peak within the pediatric population. Differences related to age include gender ratio, the most common histologic subtype, the underlying biologic characteristics (e.g., the role of Epstein-Barr virus [EBV]), and the potential for cure.1 Pediatric Hodgkin’s lymphoma has an excellent prognosis, with higher survival rates compared with those of adults even when the therapeutic approaches are similar.1,2 Given the high cure rates, Hodgkin’s lymphoma, particularly in children, has provided much of the knowledge base about late toxicities of radiotherapy and cytotoxic chemotherapy. Because of the increased vulnerability of children to the adverse effects of therapy, the management of pediatric Hodgkin’s lymphoma has led the way in the evolution of treatment strategies that consider both the toxicity and efficacy of therapy.
Historically, the desire to avoid the musculoskeletal hypoplasia that occurred following high-dose extended-field radiotherapy (EFRT) and the leukemogenesis and infertility associated with certain alkylating agents led to a more rational plan for combined-modality therapy. Subsequent observations of cardiovascular dysfunction and the increased risk of secondary cancers have played additional roles in modifying therapeutic approaches. The first generation of combined-modality therapy regimens used cycles of chemotherapy to replace a portion of the radiotherapy in laparotomy-staged children.3,4,5,6–8 Second-generation regimens used combinations containing doxorubicin to replace or reduce offending alklylating agents. Concurrent with advances in diagnostic imaging, investigators eventually abandoned surgical staging after demonstrating the efficacy of the combined-modality treatment approach.
In time, risk-adapted trials evolved that prescribed fewer cycles of multiagent chemotherapy and lower radiation doses and treatment volumes for patients with favorable clinical presentations. The definition of risk groups for disease stratification can vary in different trials and has changed with therapeutic advances. In some trials, gender-related predispositions also influence the treatment algorithm. For example, following cumulative doses of alkylating agent chemotherapy used in primary treatment regimens, boys exhibit a greater sensitivity to gonadal toxicity compared with girls, who generally maintain ovarian function unless chemotherapy is combined with abdominopelvic radiotherapy.9 Conversely, young women treated with thoracic radiotherapy during puberty have a markedly increased risk of a breast cancer that is not observed in their male counterparts, but current approaches with more restricted radiotherapy volumes are decreasing this risk.10–12
Because of the spectrum of prognostic factors in pediatric Hodgkin’s lymphoma, and the unique developmental and gender-related predispositions to therapy effects, no single treatment method is ideal for all patients. Contemporary treatment for children and adolescents with Hodgkin’s lymphoma uses a risk-adapted approach that considers presenting risk features at diagnosis. Therapy duration and intensity are selected to maintain long-term remission with minimal treatment-related morbidity. Moreover, the response to initial chemotherapy is itself an important prognostic factor.7,13,14 In turn, investigation of response-based therapy in which the rapidity of a complete response to chemotherapy may trigger either a reduction or augmentation of therapy is under cautious investigation in clinical trials.
Consequently, the evolution of therapy for pediatric Hodgkin’s lymphoma has served as a model for other cancers. Pediatric Hodgkin’s lymphoma management has also been early to adopt improved risk stratification and, more recently, response-based strategies that titrate the aggressiveness of therapy in order to improve the therapeutic ratio. Ongoing investigations aim to identify subgroups of Hodgkin’s lymphoma that may be treated with reduced volume and dose of irradiation or with chemotherapy alone. The concerns about radiotherapy, however, are to a certain extent the legacy of the significant late complications (secondary malignant tumors, cardiopulmonary dysfunction, and musculoskeletal hypoplasia) observed following archaic extended-field high-dose radiotherapy techniques that have limited bearing on modern clinical practice. Individual trials have generally shown a small improvement in disease-free survival rates from the use of adjuvant radiotherapy in Hodgkin’s lymphoma. It is only from a meta-analysis of the broad array of both adult and childhood Hodgkin’s lymphoma trials that one can glean a suggestion that adjuvant radiotherapy may provide a small survival advantage, at least in early-stage patients.15 The late effects beyond a decade or two of follow-up with the approach of primary chemotherapy and low-dose, involved-field radiation therapy (IFRT) (combined-modality therapy) are under investigation, however.
Etiology and Epidemiology
Pediatric Hodgkin’s lymphoma exhibits distinctive epidemiologic features. The childhood form, which presents in patients younger than 15 years of age, is associated with a marked male predominance, increasing family size, and decreasing socioeconomic status.16,17 In developed countries, Hodgkin’s lymphoma is rarely diagnosed in children younger than 5 years of age. The young adult form, which presents in patients ages 15 to 34 years, is associated with a higher socioeconomic status in industrialized countries. Overall, the incidence is highest in developed countries (North America and Europe) and very rare in Asian populations; in childhood, however, some developing regions have relatively higher incidences.18 In adolescents, the incidence between males and females is roughly equal, and most older adolescent patients are white. The risk for young adult Hodgkin’s lymphoma decreases significantly with increased sibship size and birth order.19,20 Specifically, the risk of Hodgkin’s lymphoma in young adults is lower in individuals with multiple older, but not younger, siblings. Histologic subtypes also vary by age at presentation. The mixed cellularity subtype is more common in childhood Hodgkin’s lymphoma, whereas the nodular sclerosing subtype is more frequently observed in affluent societies.
Pediatric Hodgkin’s lymphoma exhibits epidemiologic features similar to those seen with paralytic poliomyelitis. Delayed exposure to an infectious agent might increase the risk of the young adult form of Hodgkin’s lymphoma, whereas early and intense exposure to an infectious agent might increase the risk for the childhood form of Hodgkin’s lymphoma.19 However, data also indicate an association between nursery school or daycare attendance and a reduced risk of Hodgkin’s lymphoma among young adults, supporting a model in which childhood exposure to common infections promotes maturation of cellular immunity.21 The presence of high-antibody titers to EBV, in situ hybridization evidence of EBV genomes in Reed-Sternberg cells, and EBV early RNA1 and 2 (EBER1 and EBER2) sequences provide evidence that enhanced activation of EBV may play a role in the development of Hodgkin’s lymphoma.22,23 The incidence of EBV-associated Hodgkin’s lymphoma varies by age, gender, ethnicity, histologic subtype, and regional economic level.24,25 More specifically, an association with EBV is greater in populations of lower socioeconomic status, cases of mixed-cellularity Hodgkin’s lymphoma, and cases occurring in children or the elderly.22
Pathology and Pathways of Spread
The pathologic features of Hodgkin’s lymphoma are similar in adults and children; however, the distribution of the histologic subtypes defined by the World Health Organization may vary by age at presentation.26,27 Nodular lymphocyte-predominant Hodgkin’s lymphoma (NLPHL) makes up almost 10% of pediatric cases. This histologic subtype usually presents as clinically localized disease and is more common among male and younger patients. In contrast to classical Hodgkin’s lymphoma, which is CD15 and CD30 positive, NLPHL is an indolent B cell neoplasm admixed with lacunar or Reed-Sternberg-like giant cells that is CD20 positive and usually CD15 and CD30 negative.
Nodular sclerosing Hodgkin’s lymphoma represents the most common histologic subtype in pediatric cases under the rubric of classical Hodgkin’s lymphoma, affecting approximately 70% of adolescents and children.28 Nodular sclerosing Hodgkin’s lymphoma most commonly involves the lower cervical, supraclavicular, and mediastinal lymph nodes. The bulky growth of some involved nodal regions (particularly in the mediastinum) may be associated with persistent radiographic abnormalities even when the patient has fully responded to therapy. The other classical form, mixed-cellularity Hodgkin’s lymphoma, is observed in approximately 15% of patients, is more common in children aged 10 years or younger, and more frequently presents as advanced disease with extranodal involvement.28 Lymphocyte-depleted Hodgkin’s lymphoma is rare in children but relatively more common in patients infected with human immunodeficiency virus (HIV).29 Lymphocyte-depleted disease in HIV-positive patients is often associated with EBV. Lymphocyte-rich classical Hodgkin’s lymphoma (LRHL) makes up approximately 5% of all Hodgkin’s lymphoma cases and closely overlaps with the nodular lymphocyte-predominant subtype in presenting clinical features and prognosis.30 The median age at presentation for LRHL (32 years) is, however, higher than for NLPHL, and there is a slightly higher incidence of mediastinal involvement and stage III disease at presentation.31
Clinical Manifestations, Patient Evaluation, and Staging
Clinical Manifestations
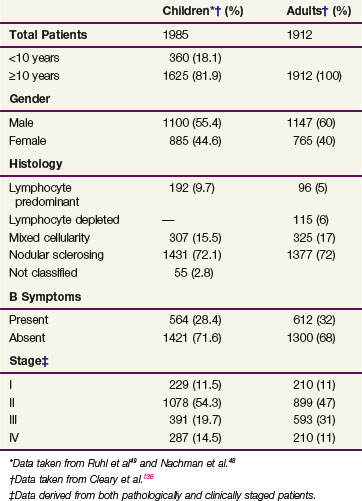
Pediatric patients most commonly present with painless cervical or supraclavicular lymphadenopathy. Mediastinal lymphadenopathy occurs in up to 66% of patients and may be associated with a nonproductive cough or other symptoms of tracheal or bronchial compression. Axillary or inguinal lymphadenopathy is less frequently seen as the first presenting sign. Primary infradiaphragmatic disease is rare in pediatric patients and occurs in fewer than 5% of cases. Splenic involvement occurs in 30% to 40% of pediatric patients with Hodgkin’s lymphoma, whereas hepatic involvement is exceedingly rare. The pulmonary parenchyma, chest wall, pleura, and pericardium are the most commonly involved extranodal sites of disease. Bone marrow involvement at the time of initial presentation of Hodgkin’s lymphoma is also uncommon in children. Approximately 65% of children have stage I and II disease and 35% have stage III and IV disease (Table 72-1).
TABLE 72-1 Pediatric Hodgkins Lymphoma: Demographic and Clinical Characteristics at Presentation
| Children*† (%) | Adults† (%) | |
|---|---|---|
| Total Patients | 1985 | 1912 |
| <10 years | 360 (18.1) | |
| ≥10 years | 1625 (81.9) | 1912 (100) |
| Gender | ||
| Male | 1100 (55.4) | 1147 (60) |
| Female | 885 (44.6) | 765 (40) |
| Histology | ||
| Lymphocyte predominant | 192 (9.7) | 96 (5) |
| Lymphocyte depleted | — | 115 (6) |
| Mixed cellularity | 307 (15.5) | 325 (17) |
| Nodular sclerosing | 1431 (72.1) | 1377 (72) |
| Not classified | 55 (2.8) | |
| B Symptoms | ||
| Present | 564 (28.4) | 612 (32) |
| Absent | 1421 (71.6) | 1300 (68) |
| Stage‡ | ||
| I | 229 (11.5) | 210 (11) |
| II | 1078 (54.3) | 899 (47) |
| III | 391 (19.7) | 593 (31) |
| IV | 287 (14.5) | 210 (11) |
* Data taken from Ruhl et al49 and Nachman et al.48
† Data taken from Cleary et al.136
‡ Data derived from both pathologically and clinically staged patients.
Nonspecific systemic symptoms are often associated with lymphadenopathy and may include fatigue, anorexia, mild weight loss, and pruritus. The prognostically significant constitutional or B symptoms that are included in the staging assignment are unexplained fever with temperatures taken orally that are higher than 38° C, unexplained weight loss of 10% within 6 months preceding diagnosis, and drenching night sweats. B symptoms occur in approximately 33% of patients (see Table 72-1). Laboratory changes observed at presentation are nonspecific but may provide clues about the extent of disease. Hematologic abnormalities may include anemia, neutrophilic leukocytosis, lymphopenia, eosinophilia, and monocytosis. Anemia may be associated with the presence of advanced disease and may result from impaired mobilization of iron stores or, less commonly, from hemolysis. Several autoimmune disorders have been reported in patients with Hodgkin’s lymphoma, including nephrotic syndrome, autoimmune hemolytic anemia, autoimmune neutropenia, and immune thrombocytopenia.32 These conditions typically remit as the lymphoma is responding to therapy. Several acute phase reactants, including erythrocyte sedimentation rate and serum copper, ferritin, and C-reactive protein levels, may be elevated at diagnosis and useful in follow-up evaluations.
Patient Evaluation
Functional nuclear imaging studies are appropriately used in patients with Hodgkin’s lymphoma as a diagnostic and monitoring modality. PET scanning uses uptake of the radioactive glucose analog 18fluoro-2-deoxyglucose (FDG) as a correlate of tumor activity. PET scanning is now widely available and is a standard part of the staging workup and assessment of response to therapy. Fused PET/CT offers the advantage of integrating functional and anatomic tumor characteristics. Residual or persistent gallium or FDG avidity is useful in predicting prognosis and the need for additional therapy in post-treatment evaluation.33,34,35,36,37 Moreover, PET may be useful in evaluating abnormalities that become clinically manifest or appear on imaging in order to assess recurrence.38 The utility of PET for follow-up is being studied because reports suggest low rates of diagnosing relapsed disease and problems with a high degree of false-positive findings.39,40,41
Because extranodal disease involving the bones and bone marrow is relatively uncommon in children, these staging evaluations can be omitted in patients presenting with localized and asymptomatic disease. Bone pain should be evaluated with plain radiographs. PET scanning is supplanting technetium-99 (99Tc) bone scans, which historically have been performed for an elevated serum alkaline-phosphatase concentration beyond that expected for age, or extranodal disease identified by other staging evaluations. A bone marrow biopsy should be performed in any patient with clinical stage III or IV disease or B symptoms. Because the pattern of infiltration in the bone marrow may be diffuse or focal and is often accompanied by reversible marrow fibrosis, a bone marrow aspirate alone is inadequate to assess the marrow for disease.
Staging
Physical examination and diagnostic imaging evaluations are used to designate a clinical stage according to the Ann Arbor staging system.42 In the past, pathologic staging, based on the findings of a staging laparotomy, including splenectomy, was routinely used to assess infradiaphragmatic disease. The increasing use of systemic therapy in children and the development of more accurate diagnostic imaging modalities led to the routine use of clinical staging and abandonment of surgical staging except to assess equivocal findings. Currently, surgical staging—most typically, nodal sampling without splenectomy—is pursued only if the anticipated findings will significantly alter the treatment plan.
Primary Therapy
Risk-Adapted Treatment Approach
Contemporary treatment for children and adolescents with Hodgkin’s lymphoma involves a risk-adapted approach based on the patient’s presenting features at diagnosis.* Factors included in the risk assessment vary across studies, but they most often include the presence of B symptoms, mediastinal and peripheral lymph node bulk, extranodal extension of disease to contiguous structures, number of involved nodal regions, Ann Arbor stage, and gender. A favorable clinical presentation is typically characterized as localized (stage I/II) nodal involvement in the absence of B symptoms and bulky disease. Although the historical definition of mediastinal bulk was based on a ratio greater than one-third between the transverse dimension of the mediastinal mass to the intrathoracic cavity on an upright chest radiograph, some trials have moved to use a simple size criteria on cross-sectional imaging as used for peripheral lymph node bulk. That definition, however, is highly variable across studies, ranging from 4 to 10 cm as a minimal threshold. Moreover, there is additional subjectivity in the definition of bulk when there are multiple matted or adjacent nodes, contributing to confusion on such risk stratification in practice. Fewer than three or four involved nodal regions are considered favorable.
In some risk-adapted treatment protocols, patients with localized disease presenting with unfavorable features are designated intermediate in risk and treated similarly to those with advanced-stage disease, whereas in others a therapy intermediate in intensity is prescribed. The criteria for unfavorable clinical presentations have also differed among studies, but the criteria most often used are B symptoms, bulky lymphadenopathy, hilar lymphadenopathy, more than three to four involved nodal regions, extranodal extension to contiguous structures, and advanced-stage (IIIB to IV) disease. The results of contemporary trials indicate that children and adolescents with early-stage or favorable presentations of Hodgkin’s lymphoma are excellent candidates for reduced therapy.44,47,48,49 Ongoing trials are evaluating whether intensification of therapy improves outcomes in patients with intermediate-risk and high-risk presentations.
Although not widely used to guide therapy assignment in pediatric trials, other factors such as gender, age at diagnosis, and histologic findings are considered in individual patients. The trials organized by the German-Austrian Pediatric Oncology Group (GPOH) and one trial by the Children’s Cancer Group (now integrated in the Children’s Oncology Group—COG) have been unique in their aims to prospectively evaluate gender-based therapy.49,51 Long-term follow-up of the GPOH 90 and 95 studies demonstrate that the substitution of etoposide for procarbazine in the vincristine, prednisone, procarbazine, and doxorubicin (OPPA) regimen does not compromise disease-free survival rates and provides less potential risk for gonadal toxicity.45,49 Although age at presentation has not been used as a criterion to assign therapy in prospective trials, reports describing outcomes after treatment with chemotherapy alone stress the benefits of this approach in younger children at higher risk of radiation-related toxicity.46,48
Lastly, retrospective reports of excellent outcome in patients with completely resected nodular lymphocyte-predominant disease have motivated prospective studies prescribing observation alone.52,53 At the same time, early-stage nodular lymphocyte-predominant Hodgkin’s lymphoma responds well to chemotherapy alone, IFRT alone, or combined-modality therapy. As an example of the later, VAMP (vinblastine, Adriamycin [doxorubicin], methotrexate, and prednisone) plus low-dose IFRT (LDRT) in a series of 33 patients had a 100% 10-year survival rate and EFS rate.54 Because data supporting the effectiveness of IFRT alone are based on full-dose RT (e.g., ≥30 Gy), it is not considered appropriate for children who are not fully grown.
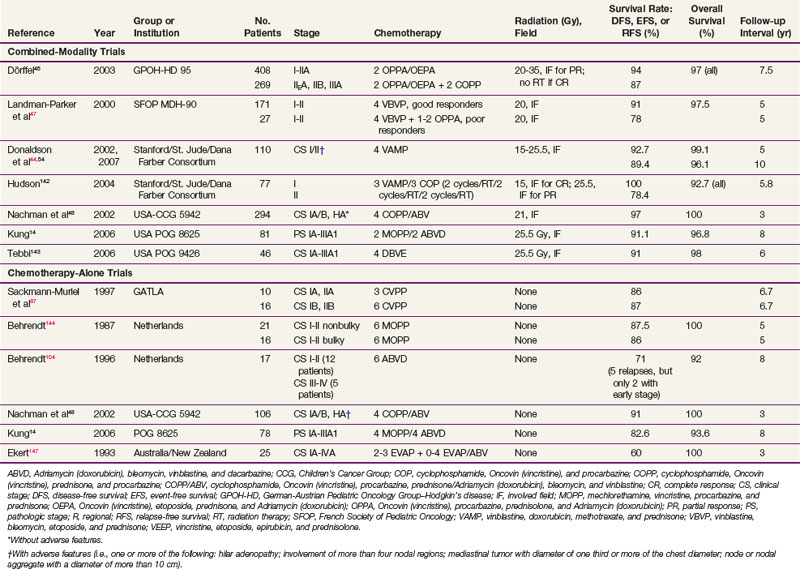
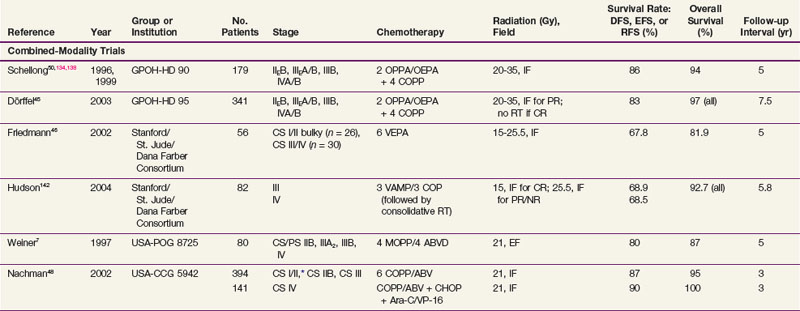
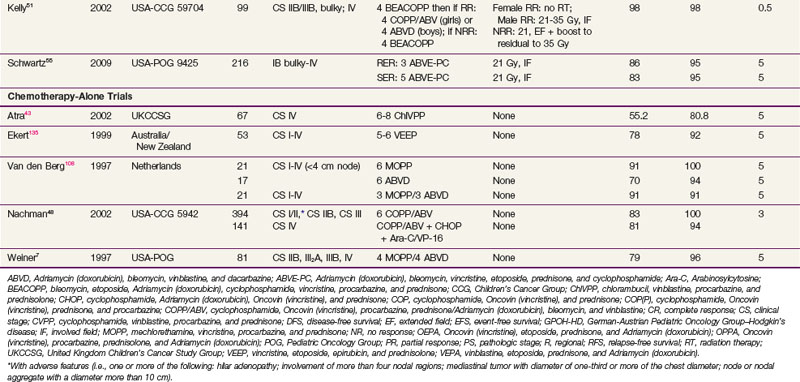
A summary of trials in children with early-stage and advanced-stage Hodgkin’s lymphoma is provided in Tables 72-2 and 72-3 (a more complete tabulation is available in the Expert Consult online version of this chapter![]() ).
).
Response-Based Therapy
The response to chemotherapy, either early in its course or at its completion, is known to be an important prognostic factor in Hodgkin’s lymphoma.13 This knowledge has led to a hypothesis that modifications in therapy may be based on the initial response to chemotherapy in a similar fashion to the management of acute lymphoblastic leukemia. Based on rapidity of a complete response to chemotherapy, a reduction or augmentation of therapy may be possible, a concept under cautious investigation in clinical trials. Response-based approaches titrate the overall duration of chemotherapy and/or the need for radiotherapy by assessing the early response to chemotherapy. Two Pediatric Oncology Group trials (POG 8725 and 8625) comparing chemotherapy alone versus chemoradiotherapy supported this idea that a rapid early response (RER) to chemotherapy reflects the chemosensitivity of a patient’s Hodgkin’s lymphoma and is a predictor of good long-term control.7,14 The implication is that treatment can be reduced in intensity or duration for those with RER in order to mitigate toxicity, or increased for those with a slow early response (SER) in order to improve disease control.
The augmentation in therapy for SER can be an increased radiotherapy dose or additional chemotherapy, or both. The French Society of Pediatric Oncology MDH90 treated 202 children with stage I or II Hodgkin’s lymphoma with four cycles of vinblastine, bleomycin, etoposide, and prednisone (VBVP). Good responders received 20 Gy IFRT alone, while poor responders were given an additional one to two cycles of OPPA and then either 20 Gy IFRT (good responders at second evaluation) or 40 Gy IFRT (poor responders). The 5-year overall survival (OS) and EFS rates were 97.5% and 91.1%, respectively.47 In the German trial, GPOH HD-95, early-stage patients who had a complete response to chemotherapy (two cycles of OPPA for girls or two cycles of OEPA for boys) did not receive adjuvant radiotherapy. OPPA/OEPA chemotherapy alone produced a 5-year disease-free survival rate of 88%, which was not significantly different from that observed in patients who received radiotherapy (92%).45 In this trial, higher-risk patients who received radiotherapy were prescribed radiation doses of up to 35 Gy if a complete response was not achieved. The use of higher-dose radiotherapy with simple anterior-posterior beams is certainly known to cause undesirable musculoskeletal toxicities and the potential for increased cardiopulmonary injury and secondary cancers, such that augmentation of chemotherapy or more conformal radiotherapy may be better approaches for nonresponders. POG 9425 administered three versus five cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) chemotherapy and 21-Gy regional radiotherapy for rapid and slow responders, respectively.55 The 2-year EFS rate was 88.2%, with no statistical difference between early and slow responders. These studies suggest that interim or early response-adapted therapy may be useful in identifying patients with favorable disease, who can be treated with lower radiotherapy doses or abbreviated chemotherapy regimens.
Although most completed trials have used CT, with or without gallium or PET to determine response, it is becoming increasingly clear that PET scanning after the initial (one or two) chemotherapy cycles will better identify good-prognosis patients and facilitate treatment intensification.13,36,56,57 Whether radiotherapy can be omitted among patients with a negative PET scan after chemotherapy is unknown and should be considered investigational at the present. The corollary problem is how to define a response by PET criteria because low levels of residual FDG activity after therapy are common. Another unresolved problem is the interpretation of splenic or hepatic involvement by Hodgkin’s lymphoma given the physiologic uptake of FDG in these organs. A consensus panel has promulgated PET-based response criteria known as the International Harmonization Project.58,59
Chemotherapy Regimens and Radiation Therapy
MOPP and Derivative Chemotherapy
The prototype alkylator combination that provided the first effective systemic therapy for Hodgkin’s lymphoma was nitrogen mustard, vincristine, procarbazine and prednisone (MOPP).60 Follow-up studies of MOPP-treated survivors confirmed that secondary acute myeloid leukemia (s-AML) and infertility resulted from the alkylating agents in the regimen and exhibited a dose-dependent relationship.61 Subsequently, investigators developed a variety of MOPP-derivative regimens in an effort to reduce the risk of secondary leukemogenesis and gonadal toxicity.
The risk of secondary leukemia following alkylating agent chemotherapy peaks in frequency in the first 5 to 10 years after treatment and plateaus to less than 2% after 10 years from diagnosis.62 Older age at treatment, history of splenectomy, presentation with advanced disease, treatment with high cumulative doses of alkylating agents, and history of relapse have been reported to predispose to this complication.* Some alkylating agents are more potent leukemogens than others; the 15-year cumulative incidence of s-AML is 4% to 8% after MOPP-based therapy compared with less than 1% with cyclophosphamide, Oncovin (vincristine), procarbazine, and prednisone (COPP-based therapy) that substitutes cyclophosphamide for nitrogen mustard.68 Pediatric protocols that limit the total dose of alkylating agents or substitute other less leukemogenic drugs, such as cyclophosphamide, for mechlorethamine, have been associated with very low incidence rates of s-AML.68
Gonadal injury is common in pediatric patients treated with MOPP and its derivative combinations. Azoospermia is typically irreversible in men treated with six or more cycles of MOPP-like therapy.61,69 However, germ cell function may be preserved if treatment is limited to no more than three cycles of alkylator therapy.70 In contrast, most young women will maintain or resume menses after a temporary period of amenorrhea following treatment including alkylating agents.69 Ovarian transposition and shielding reduces the incidence of gonadal injury in young women requiring pelvic radiation, but these patients will experience a higher risk of premature menopause.71 The radiation oncologist should also be aware of the age dependency in the risk for ovarian failure.72 Younger females tolerate a modestly higher radiation dose related to the gradual decline in oocyte numbers associated with aging.
ABVD and Derivative Chemotherapy
The Adriamycin (doxorubicin), bleomycin, vinblastine, and dacarbazine (ABVD) combination provided a systemic therapy that produced superior disease-free survival rates compared with MOPP and was not associated with an excess risk of secondary leukemia or infertility.4,73 However, follow-up of patients treated with the ABVD regimen established its association with cardiopulmonary toxicity that was enhanced with the addition of thoracic irradiation.4 Many ABVD derivatives soon followed, aiming to reduce the risk of these sequelae.
Anthracycline agents are an important component of chemotherapy regimens for children with Hodgkin’s lymphoma because of their significant lympholytic effects. In adults, the cumulative incidence of cardiomyopathy increases significantly after anthracycline exceeds 550 mg/m2; children are at increased risk of cardiac dysfunction at lower cumulative doses.74–77 Other risk factors for anthracycline toxicity identified in studies of childhood leukemia survivors include younger age at treatment (especially, <5 years old) and female gender. Combination treatment with chest irradiation or other cardiotoxic agents such as amsacrine or cyclophosphamide may also enhance the risk of cardiac dysfunction.76,78,79–81 Because treatment protocols for pediatric Hodgkin’s lymphoma frequently include chest irradiation or other chemotherapeutic agents with potential cardiotoxicity, most regimens limit cumulative doses of anthracycline agents to below 250 mg/m2, particularly for patients with favorable-risk disease presentations.
Bleomycin in the ABVD regimen increases the risk of pulmonary toxicity that is most commonly manifested as pulmonary fibrosis and chronic pneumonitis.82 Thoracic irradiation may augment this risk. Patients at highest risk of pulmonary complications are those treated with cumulative bleomycin doses exceeding 400 U/m2. Contemporary protocols use bleomycin doses in the range of 60 to 100 U/m2; these cumulative exposures are usually associated with asymptomatic pulmonary restriction and diffusion deficits in long-term survivors, some of which will improve over time.83,84 Serial monitoring of pulmonary function during therapy and withholding of bleomycin in patients exhibiting significant declines in pulmonary function (≥20% from baseline) may reduce the risk of further pulmonary injury and does not appear to compromise disease control.85
Chemotherapy Combinations Including Etoposide
Etoposide has been increasingly incorporated into treatment regimens for pediatric Hodgkin’s lymphoma. This agent is used in risk-adapted regimens for favorable patients as an effective alternative to alkylating agents in an effort to reduce gonadal toxicity.* Etoposide has been added to alkylating and anthracycline chemotherapy in regimens for advanced and unfavorable patients to enhance treatment response. For example, the previously mentioned dose-intensive ABVE-PC regimen used in recent cooperative groups in the United States yields excellent survival outcomes in intermediate-risk to high-risk patients.55 Balanced with this survival advantage is an excess risk of s-AML seen with topoisomerase II inhibitors such as etoposide and doxorubicin that differs in epidemiology and biology from alkylator-related s-AML.88 Secondary AML that occurs in association with topoisomerase II inhibitors is characterized by a brief time of onset from primary diagnosis, absence of a preceding myelodysplastic phase, monoblastic and myelomonoblastic histologic findings, and translocations involving the MLL gene at chromosome band 11q23. Studies of childhood leukemia patients suggest that intermittent weekly or twice-weekly dosing schedules may result in transforming mutations of myeloid progenitor cells by epipodophyllotoxins.89 A relationship between leukemogenic activity and cumulative epipodophyllotoxin dose could not be established in an evaluation of s-AML cases developing in patients treated with multiagent chemotherapy regimens including epipodophyllotoxins, alkylating agents, doxorubicin, and dactinomycin for pediatric solid tumors.88 However, the risk of s-AML following treatment with regimens that restricted etoposide doses to 5 g/m2 or less did not exceed that associated with other agents used in solid tumor regimens.88
Pertinent to this discussion is the recent report of an increased incidence of secondary malignant tumors observed in children treated with doxorubicin, bleomycin, vincristine, and etoposide (ABVE) or dose-intensified ABVE with prednisone and cyclophosphamide and the cardiopulmonary protectant dexrazoxane.90 In this Pediatric Oncology Group trial, patients were randomly assigned to receive dexrazoxane to evaluate its potential to decrease cardiopulmonary toxicity. Unexpectedly, 10 patients developed a secondary malignancy with AML/myelodysplastic syndrome (MDS) accounting for 8 of the 10 cases. Six of the eight cases of AML/MDS and two solid tumors occurred in children randomized to receive dexrazoxane. The authors speculated that the use of multiple topoisomerase II inhibitors (dexrazoxane, etoposide, and doxorubicin) may have potentiated carcinogenesis. Collectively, most data support the relative safety of using limited doses of etoposide, but reports of s-AML in patients receiving etoposide for treatment of favorable pediatric Hodgkin’s lymphoma raise concerns about whether this agent should be avoided in favorable disease presentations.47
irradiation technique
Historically, the results of treatment with radiation therapy alone were superior at institutions that treated many Hodgkin’s lymphoma patients. Although different institutions and radiation oncologists may use slightly different treatment techniques, underlying principles and, in fact, most of the technical details remain constant.91 Because most children are treated on institutional (or multi-institutional) studies, radiation oncologists should confirm all aspects of the diagnostic workup and staging; they must also understand study requirements to deliver appropriate radiation therapy. Supportive of these premises, an up-front centralized review of patients entered into the GPOH-HD 90 study altered the treatment approach in a large number of children.92 Similarly, prior POG trials observed inferior outcomes related to major deviations in radiation protocol compliance. These data support the need for prospective central review of radiotherapy in current COG Hodgkin’s lymphoma protocols.
The incidence of late effects resulting from radiotherapy for Hodgkin’s lymphoma—particularly secondary malignant tumors—is to a large degree historic since the effects resulted from extended-field, high-dose radiotherapeutic approaches not currently appropriate, both in terms of treatment volume and dose. For example, the Late Effects Study Group analyzed patients treated from 1955 to 1986, before the widespread use of customized lung shielding, megavoltage linear accelerators, or CT-based, imaged-guided radiation therapy planning.64 Both the radiation therapy doses and volumes were larger than those currently used and cannot be extrapolated to modern therapy. Moreover, several studies reveal a marked decrease in the risk for secondary breast cancer when radiation therapy volumes and doses are reduced.12,93,94 An important confounding factor leading to reduction in breast cancer risk related to radiation therapy occurs from alkylating agent chemotherapy or pelvic radiotherapy, ostensibly due to ovarian failure and accompanying loss of hormonal influence on the neoplastic process. Nevertheless, the transition from EFRT to IFRT significantly reduced the dose to breast and lung tissue95 and has been predicted to result in a substantial reduction in secondary cancer risk.11 Thyroid cancer may be one exception to the general concept that higher radiation doses correlate with a higher risk for secondary cancers.9 An update to the Late Effects Study Group has shown a nonlinear relationship of dose and cancer incidence with a maximum risk associated with radiation doses in the range of 15 to 25 Gy and decreasing risk with doses greater than 29 Gy.96 What effects radiation-induced hypothyroidism and thyroid replacement therapy have on this dose-response relationship is unclear.
Volume
Most children with Hodgkin’s lymphoma are treated with combined chemotherapy and low-dose IFRT. Meticulously and judiciously designed fields are necessary for maximum success in terms of both disease control and minimal tissue damage. The definitions of such fields depend on the anatomy of the region in terms of lymph node distribution, patterns of disease extension into regional areas, and consideration for match-line problems should disease recur. Involved fields typically should include not just the identifiably abnormal lymph nodes but the entire lymph node region containing the involved nodes (Table 72-4).
| Involved Node(s) | Radiation Field |
|---|---|
| Cervical | Neck and infraclavicular/supraclavicular* |
| Supraclavicular | Neck and infraclavicular/supraclavicular ± axilla |
| Axilla | Axilla ± infraclavicular/supraclavicular |
| Mediastinum | Mediastinum, hila, infraclavicular/supraclavicular*† |
| Hila | Hila, mediastinum |
| Spleen | Spleen ± para-aortics |
| Para-aortics | Para-aortics ± spleen |
| Iliac | Iliacs, inguinal, femoral |
| Inguinal | External iliac, inguinal, femoral |
| Femoral | External iliac, inguinal, femoral |
* Upper cervical region is not treated if supraclavicular involvement is extension of the mediastinal disease.
† Prechemotherapy volume is treated except for lateral borders of the mediastinal field, which is based on postchemotherapy volume.
Radiation therapy for unfavorable and advanced Hodgkin’s lymphoma is variable and protocol dependent. Although IFRT remains the standard when patients are treated with combined-modality therapy, restricting radiation therapy to areas of initial bulky disease (generally defined as 5 to 6 cm or more at presentation) or postchemotherapy residual disease (generally defined as 2 to 2.5 cm or more, or residual PET avidity) is under investigation. Also under investigation is the use of involved-nodal radiation therapy (INRT) where the initial nodes grossly involved by lymphoma are targeted with a small margin rather than treating an entire anatomic compartment. The rationale for this relates to both the anatomic definitions of lymph node regions and the patterns of recurrence. Clinical definitions of lymph node regions are historic and based on anatomic or bony landmarks without the benefit of CT scanning to identify the location of nodes. The European Organization for Research and Treatment of Cancer–Groupe d’Études des Lymphomes de l’Adulte (EORTC-GELA) has recently introduced the concept of INRT.97,98 This uses all available clinical information, including prechemotherapy and postchemotherapy imaging with CT and FDG/PET scans, to define the treatment field according to the prechemotherapy extent of disease. The concept is based on evidence that disease recurs most often in initially involved lymph nodes, 99,100 suggesting that chemotherapy is adequate to treat disease contained within radiologically normal lymph nodes, while RT is needed only to treat sites of macroscopic enlargement. In a study of early stage Hodgkin’s lymphoma patients, PET identified 36% of involved lymph nodes that were radiographically normal on CT scanning. Therefore, evaluation with FDG-PET scanning before chemotherapy may help delineate the complete extent of disease.97 Initial clinical data with the use of INRT are emerging. Campbell and colleagues101 reviewed clinical outcomes of patients with limited-stage Hodgkin’s lymphoma treated with EFRT, IFRT, and INRT of 5 cm or less. No marginal recurrences or locoregional failures occurred with INRT.101
Dose
In the setting of combined therapy, the intensity of the chemotherapy is important to consider in the choice of the radiation therapy dose and volume. However, doses of 15 to 25 Gy are typical with shrinking fields and boosts individualized (see Tables 72-2 and 72-3). Doses of more than 25 Gy are uncommon in the pediatric setting. Of interest are the results of the GPOH HD-90 trial, in which radiation therapy was administered in combination with vincristine, prednisone, procarbazine, and doxorubicin (OPPA) or OEPA, with or without COPP. The radiation doses were 20 to 25 Gy, with a local boost of 5 to 10 Gy for insufficient remission following chemotherapy. Tumor burden, indicated by bulky disease or number of involved nodes, proved not to be prognostically significant, likely because of the high doses used for bulky disease.102 Most current treatment approaches for children would not include radiation doses of this magnitude.
Treatment Approaches
Chemotherapy Alone versus Chemotherapy Combined with Radiation Therapy
Non-cross-resistant combination chemotherapy alone is well established as an effective modality for the treatment of pediatric Hodgkin’s lymphoma.* This treatment approach eliminates the risk of radiation-induced complications. However, the higher-cumulative doses of anthracyclines, alkylating agents, and bleomycin chemotherapy increase the risk of cardiopulmonary toxicity, infertility, and leukemogenesis, and may be less effective than combined-modality therapy for treatment of bulky nodal disease. Chemotherapy alone trials have been limited by their small numbers of patients, nonrandom treatment assignments, lack of long-term follow-up related to disease control, and late-treatment sequelae. Despite these deficiencies, identification of clinical factors predicting an optimal outcome following treatment with chemotherapy alone remains an important objective of many ongoing trials because of the desire to avoid late radiation therapy sequelae.
Investigators from North American pediatric cooperative groups have undertaken three longitudinal controlled trials to evaluate chemotherapy alone versus combined-modality therapy.6,7,48 The first two trials failed to show a statistically significant advantage in EFS or OS rates with the addition of radiation therapy to non-cross-resistant chemotherapy. The CCG compared 12 cycles of alternating MOPP/ABVD with 6 cycles of ABVD plus low-dose (21 Gy) radiation.6 Rates of EFS and OS suggested a survival advantage for the combined-modality group (90% for 4-year EFS rate) over the chemotherapy alone group (84% for 4-year EFS rate), but this difference was statistically insignificant. In a POG trial, adding low-dose radiation to four cycles of alternating MOPP and ABVD chemotherapy did not improve disease-free or OS rates.7 However, statistical and quality assurance issues produced problems with interpretation of these data. Moreover, the findings of both studies are irrelevant to present-day practice because the treatments included the more leukemogenic MOPP-based therapy and an excessive number of treatment cycles.
Using a more contemporary regimen, the CCG compared chemotherapy alone with the COPP/ABV hybrid to combined-modality therapy including low-dose IFRT.48 Clinical risk features, including the presence of B symptoms, hilar lymphadenopathy, mediastinal and peripheral lymph node bulk, and the number of involved nodal regions, determined treatment assignment. Patients who achieved a complete response to chemotherapy were eligible to be randomized to receive low-dose IFRT or no further therapy. The trial was prematurely terminated because patients treated with chemotherapy alone had a significantly higher number of relapses. The difference in outcome was most marked for stage IV patients in whom combined-modality therapy produced a 3-year 90% EFS rate compared with 81% in those randomized to receive chemotherapy alone.
The current standard of care is to include IFRT across all stages of pediatric Hodgkin’s lymphoma. The results of chemotherapy alone investigations, however, document excellent efficacy of this treatment approach, particularly in children with early-stage favorable Hodgkin’s lymphoma. Circumstances where radiotherapy may have particular toxicity, as in the very young child, may warrant chemotherapy alone. The incremental value of adjuvant radiotherapy is small, but its importance lies in the avoidance of relapse therapy. Balanced against this is the incremental toxicity, which is difficult to quantify because the modern techniques have insufficient long-term follow-up. At the same time, there is an appreciation that radiation volume reductions should be an area of active investigation to reduce side effects, but only to the extent that the risk of relapse is not enhanced.97,98,99,100
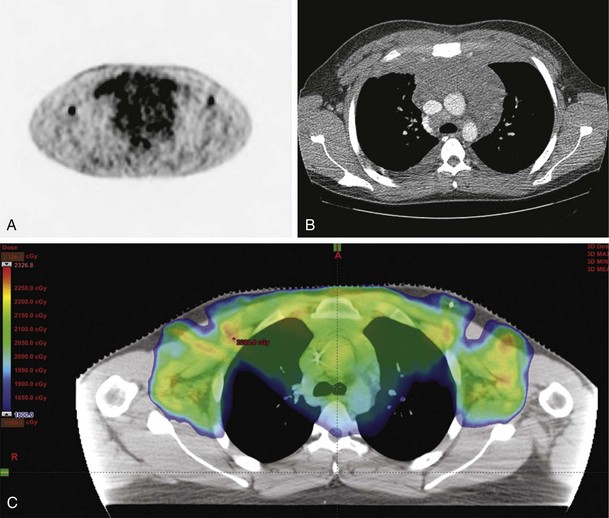
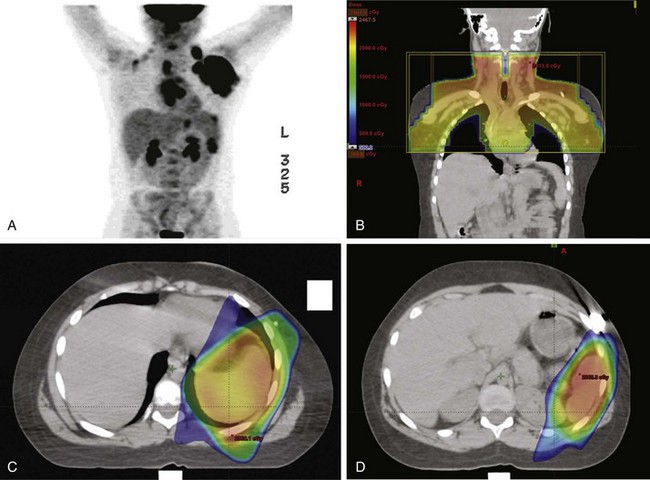
By the same token, conformal techniques such as intensity-modulated radiotherapy (IMRT) or proton radiotherapy are also under investigation in Hodgkin’s lymphoma.* Figures 72-1 and 72-2 are illustrative examples of the use of IMRT to ensure coverage of involved regions. In the first case, the mediastinal disease has extensive anterior chest wall extension, whereas the second case depicts splenic IMRT with AP-PA mantle fields. In these examples, radiation exposure to normal tissues (lung, heart, kidney) was minimized to reduce toxicity, not readily accomplished by standard AP-PA beam arrangements.
An argument often cited for abandoning radiotherapy in Hodgkin’s lymphoma is the lack of any survival advantage for radiotherapy in the vast majority of studies. A recent meta-analysis of adult and pediatric trials, however, suggests that favorable-risk patients may in fact have a small survival advantage without excessive risk for secondary malignant tumors.15 Nevertheless, a valid objective of ongoing studies is to identify patient subgroups where radiotherapy may have no value. The response-based paradigm may yield important observations of when chemotherapy alone approaches are preferred. PET imaging is proving particularly useful for response assessment and radiation therapy planning.116 One note of caution was observed in an adult Italian randomized Hodgkin’s lymphoma trial, however: When IFRT was omitted in patients with bulky disease who had had a complete response to chemotherapy based on PET, there was a 10% reduction in the EFS rate.117
Treatment of Relapsed and Refractory Disease
The generally excellent outcome in pediatric Hodgkin’s lymphoma has limited opportunities to evaluate salvage therapy programs. Most relapses in patients with Hodgkin’s lymphoma occur within the first 3 years, but some patients may relapse as long as 10 years after the initial diagnosis.118 Treatment and prognosis after relapse depend on the primary therapy and duration of remission (time of relapse). Re-treatment with conventional multiagent chemotherapy and radiation therapy may salvage 40% to 50% of children relapsing after a sustained remission (≥1 year); but adverse effects of treatment, including secondary malignant tumors, may reduce the ultimate survival times.119–121
Patients who develop refractory disease within 1 year after completing therapy respond poorly to conventional salvage therapy, as do patients with multiple relapses. These high-risk patients have a better chance of achieving a durable remission if they are consolidated with myeloablative therapy followed by hematopoietic cell transplantation (HCT). OS in children and adolescents with relapsed Hodgkin’s lymphoma treated with this approach range from 30% to 60%. Because of the higher transplant-related mortality associated with allogeneic transplantation, autologous HCT is preferred for patients with relapsed Hodgkin’s lymphoma. However, recent investigations of reduced-intensity allogeneic transplantation have demonstrated acceptable rates of transplant-related mortality.122–124 Nonmyeloablative conditioning regimens most often use fludarabine or low-dose total-body irradiation to provide a nontoxic immunosuppression and establish a graft-versus-lymphoma effect. Information about treatment outcomes using this approach is limited to reports of small, heterogeneous, adult-patient cohorts treated with a variety of conditioning regimens. Longer follow-up is needed to establish the efficacy of this approach.
IFRT to sites of recurrent disease should be considered in the setting of HCT. In a Stanford report, patients with stages I to III disease at relapse who received autologous bone marrow transplantation and IFRT had a 3-year rate of freedom from relapse of 100% and an OS of 85%, compared with 67% and 60%, respectively, for patients not receiving IFRT.125 For patients not previously irradiated, IFRT was associated with an improved freedom from relapse rate of 85% and OS of 93%, in contrast to 57% and 55%, respectively, for those previously irradiated. Morbidity was similar to those not irradiated, although radiation therapy may have contributed to the peritransplant death of two patients.
Other reports on the use of consolidative radiotherapy suggest a benefit and low to moderate morbidity, although these are mostly adult data that suffer from inherent selection and publication biases.126,127–129,130,131 Central issues relating to the use of IFRT are the dose, target volume, and timing with respect to the transplant. Radiation therapy doses are generally 15 to 25 Gy, in 1.5- to 2-Gy fractions. This variation relates to potential normal tissue toxicity as well as the consideration for higher radiation doses in patients with an identifiable tumor that demonstrates radiation responsiveness. Radiation therapy volume can vary and include treatment to all sites of initial disease, recurrent disease, persistent disease following salvage chemotherapy, persistent disease following the preparative regimen for transplant, or all nodal sites. Unless protocol-specific therapy is directed, individual considerations for such decision making are necessary at this time. IFRT can be administered prior to the high-dose chemotherapy program to place patients in a minimal disease state. Alternatively, radiation therapy can be administered after the high-dose chemotherapy program to decrease the overall potential for disease progression and avoid radiotherapy-related peritransplant morbidity such as esophagitis, pneumonitis, cardiomyopathy, and veno-occlusive disease. Possible disadvantages of this approach include the loss of the pretransplant cytoreductive effect and the theoretical carcinogenic effect of radiation therapy on the newly proliferating hematopoietic system.
Treatment Algorithms
Table 72-5 summarizes recommendations for risk-adapted treatment approaches. Because patients with localized favorable disease presentations can achieve long-term disease-free survival using regimens that do not contain alkylators, ABVD or derivative chemotherapy is preferred for this group.44,47,132,133 Alkylating agents or etoposide can be added to the regimen, however, without compromising disease outcome if the investigator prefers to restrict anthracycline chemotherapy exposure to preserve cardiac function.48,49,134 Combined-modality treatment approaches, including low-dose IFRT, have produced excellent results in children with favorable localized disease and can reduce cumulative chemotherapy doses.
TABLE 72-5 Recommendations for Treatment Approach in Pediatric Hodgkin’s Lymphoma
| Clinical Presentation | Stage | Recommended Treatment Approach |
|---|---|---|
| Early | Recommended Therapy | |
| Fewer than three or four nodal regions; no B symptoms, bulk, or extranodal extension from contiguous nodal disease | IA, IIA | Two to four cycles non–cross-resistant chemotherapy without alkylators plus low-dose IFRT (15-25 Gy) |
| In Clinical Trial Setting Only | ||
| Two to four cycles of chemotherapy alone if there is negative PET2; or such chemotherapy followed by INRT or RT volume limited to initial bulky sites or residual sites of disease (15-25 Gy) | ||
| Intermediate | Recommended Therapy | |
| Three or four or more nodal regions; bulky lymphadenopathy (mediastinal ratio ≥33%; peripheral lymph node mass ≥6-10 cm) | IA, IIA, IIB* IIIA |
Four to six cycles (three to five compacted, dose-intensive cycles) non–cross-resistant chemotherapy plus low-dose IFRT (15-25 Gy) |
| In Clinical Trial Setting Only | ||
| Four to six cycles (three to five compacted, dose-intensive cycles) non–cross-resistant chemotherapy alone if there is negative PET2; or such chemotherapy followed by INRT or RT volume limited to initial bulky sites or residual sites of disease (15-25 Gy) | ||
| Advanced | Recommended Therapy | |
| Stage II patients with fever or weight loss; any patient with advanced stage | IIB* IIIB IV | Six cycles (four to six compacted, dose-intensive cycles) of non–cross-resistant chemotherapy plus low-dose IFRT (15-25 Gy) |
| In Clinical Trial Setting Only | ||
| Six cycles (four to six compacted, dose-intensive cycles) of non–cross-resistant chemotherapy plus low-dose IFRT (15-25 Gy) restricted to (1) sites of initial bulky disease, (2) PET2 positive disease, or (3) residual masses |
IFRT, involved-field radiotherapy; INRT, involved-nodal radiotherapy; PET2, PET scan after second cycle of chemotherapy used as indicator of rapid early response.
* Stage IIB patients have been variably treated as intermediate- or unfavorable-risk patients. Some studies use associated factors, such as weight loss, bulky disease, or extranodal extension, for further risk stratification.
Combinations derived from both ABVD and MOPP with the addition of etoposide still provide the most effective chemotherapy strategies for children and adolescents with intermediate-risk or high-risk disease presentations.48,49 In most regimens reporting good long-term outcomes, low-dose radiation therapy is administered following chemotherapy to areas of bulky disease or nodes (usually, the regions harboring the involved nodes) present at diagnosis. In contrast to treatment regimens used for adults with advanced or unfavorable Hodgkin’s lymphoma that commonly prescribe six cycles of ABVD, an alkylating agent regimen is most often alternated with ABVD or similar hybrid therapy in an effort to reduce potential cardiopulmonary toxicity. Attempts to completely omit alkylating agents in these high-risk groups have resulted in unsatisfactory outcomes, as have protocols prescribing ABVD or derivative chemotherapy alone.46,135 Abbreviated, dose-dense regimens that induce rapid tumor response may permit reduction of cumulative chemotherapy doses below threshold levels associated with significant long-term toxicity.55
1 Punnett A, Tsang RW, Hodgson DC. Hodgkin lymphoma across the age spectrum. Epidemiology, therapy, and late effects. Semin Radiat Oncol. 2010;20:30-44.
4 Fryer CJ, Hutchinson RJ, Krailo M, et al. Efficacy and toxicity of 12 courses of ABVD chemotherapy followed by low-dose regional radiation in advanced Hodgkin’s disease in children. A report from the Children’s Cancer Study Group. J Clin Oncol. 1990;8:1971-1980.
6 Hutchinson RJ, Fryer CJ, Davis PC, et al. MOPP or radiation in addition to ABVD in the treatment of pathologically staged advanced Hodgkin’s disease in children. Results of the Children’s Cancer Group Phase III Trial. J Clin Oncol. 1998;16:897-906.
7 Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients. A Pediatric Oncology Group study. J Clin Oncol. 1997;15:2769-2779.
8 Weiner MA, Leventhal BG, Marcus R, et al. Intensive chemotherapy and low-dose radiotherapy for the treatment of advanced-stage Hodgkin’s disease in pediatric patients. A Pediatric Oncology Group study. J Clin Oncol. 1991;9:1591-1598.
10 Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-475.
11 Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110:2576-2586.
12 De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma. Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-4246.
13 Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52-59.
14 Kung FH, Schwartz CL, Ferree CR, et al. POG 8625. A randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin disease. A report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2006;28:362-368.
15 Franklin J, Pluetschow A, Specht L. Chemotherapy, Radiotherapy and Combined Modality for Hodgkin’s Disease, with Emphasis on Second Cancer Risk (Review). The Cochrane Collaboration, New York: John Wiley & Sons; 2009.
33 Friedberg JW, Fischman A, Neuberg D, et al. FDG-PET is superior to gallium scintigraphy in staging and more sensitive in the follow-up of patients with de novo Hodgkin lymphoma. A blinded comparison. Leuk Lymphoma. 2004;45:85-92.
36 Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma. A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746-3752.
37 Sher DJ, Mauch PM, Van Den Abbeele A, et al. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma. The importance of involved-field radiotherapy. Ann Oncol. 2009;20:1848-1853.
38 Zinzani PL, Stefoni V, Tani M, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol. 2009;27:1781-1787.
39 Mocikova H, Obrtlikova P, Vackova B, et al. Positron emission tomography at the end of first-line therapy and during follow-up in patients with Hodgkin lymphoma. A retrospective study. Ann Oncol. 2010;21(6):1222-1227.
45 Dorffel W, Luders H, Ruhl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin’s disease in children and adolescents. Analysis and outlook. Klin Padiatr. 2003;215:139-145.
46 Friedmann AM, Hudson MM, Weinstein HJ, et al. Treatment of unfavorable childhood Hodgkin’s disease with VEPA and low-dose, involved-field radiation. J Clin Oncol. 2002;20:3088-3094.
48 Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765-3771.
49 Ruhl U, Albrecht M, Dieckmann K, et al. Response-adapted radiotherapy in the treatment of pediatric Hodgkin’s disease. An interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys. 2001;51:1209-1218.
50 Schellong G, Potter R, Bramswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease. The German-Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin’s Disease Study Group. J Clin Oncol. 1999;17:3736-3744.
51 Kelly KM, Hutchinson RJ, Sposto R, et al. Feasibility of upfront dose-intensive chemotherapy in children with advanced-stage Hodgkin’s lymphoma. Preliminary results from the Children’s Cancer Group Study CCG-59704. Ann Oncol. 2002;13(Suppl 1):107-111.
52 Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al. Lymphocyte-predominant Hodgkin’s lymphoma in children. Therapeutic abstention after initial lymph node resection—a study of the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:2948-2952.
53 Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma. Experience from the European network group on pediatric Hodgkin lymphoma. Cancer. 2007;110:179-185.
54 Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol. 2007;25:332-337.
55 Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma. The results of P9425. Blood. 2009;114:2051-2059.
58 Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586.
59 Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma. Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571-578.
62 Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745-751.
64 Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease. Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386-4394.
65 Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort. The relation to age at treatment. J Clin Oncol. 2000;18:498-509.
72 Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304-1312.
78 Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55-75.
93 Constine LS, Tarbell N, Hudson MM, et al. Subsequent malignancies in children treated for Hodgkin’s disease. Associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72:24-33.
94 Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901-3907.
96 Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer, Findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356-2362.
98 Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes. To be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008;88:202-210.
99 Shahidi M, Kamangari N, Ashley S, et al. Site of relapse after chemotherapy alone for stage I and II Hodgkin’s disease. Radiother Oncol. 2006;78:1-5.
101 Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma. A question of field size. J Clin Oncol. 2008;26:5170-5174.
102 Dieckmann K, Potter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin’s disease and require higher radiation doses? Results from the German-Austrian Pediatric Multicenter Trial DAL-HD-90. Int J Radiat Oncol Biol Phys. 2003;56:644-652.
110 Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients. Conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173-1180.
111 Ghalibafian M, Beaudre A, Girinsky T. Heart and coronary artery protection in patients with mediastinal Hodgkin lymphoma treated with intensity-modulated radiotherapy. Dose constraints to virtual volumes or to organs at risk? Radiother Oncol. 2008;87:82-88.
116 Korholz D, Kluge R, Wickmann L, et al. Importance of F18-fluorodeoxy-D-2-glucose positron emission tomography (FDG-PET) for staging and therapy control of Hodgkin’s lymphoma in childhood and adolescence—consequences for the GPOH-HD 2003 protocol. Onkologie. 2003;26:489-493.
117 Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-1727.
118 Wasilewski-Masker K, Liu Q, Yasui Y, et al. Late recurrence in pediatric cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1709-1720.
126 Constine LS, Rapoport AP. Hodgkin’s disease, bone marrow transplantation, and involved field radiation therapy. Coming full circle from 1902 to 1996. Int J Radiat Oncol Biol Phys. 1996;36:253-255.
130 Yahalom J. Management of relapsed and refractory Hodgkin’s disease. Semin Radiat Oncol. 1996;6:210-224.
142 Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol. 2004;22:4541-4550.
143 Tebbi CK, Mendenhall N, London WB, et al. Treatment of stage I, IIA, IIIA1 pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation. A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer. 2006;46:198-202.
1 Punnett A, Tsang RW, Hodgson DC. Hodgkin lymphoma across the age spectrum. Epidemiology, therapy, and late effects. Semin Radiat Oncol. 2010;20:30-44.
2 Eichenauer DA, Bredenfeld H, Haverkamp H, et al. Hodgkin’s lymphoma in adolescents treated with adult protocols. A report from the German Hodgkin Study Group. J Clin Oncol. 2009;27:6079-6085.
3 Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin’s disease. J Clin Oncol. 1987;5:742-749.
4 Fryer CJ, Hutchinson RJ, Krailo M, et al. Efficacy and toxicity of 12 courses of ABVD chemotherapy followed by low-dose regional radiation in advanced Hodgkin’s disease in children. A report from the Children’s Cancer Study Group. J Clin Oncol. 1990;8:1971-1980.
5 Gehan EA, Sullivan MP, Fuller LM, et al. The intergroup Hodgkin’s disease in children. A study of stages I and II. Cancer. 1990;65:1429-1437.
6 Hutchinson RJ, Fryer CJ, Davis PC, et al. MOPP or radiation in addition to ABVD in the treatment of pathologically staged advanced Hodgkin’s disease in children. Results of the Children’s Cancer Group Phase III Trial. J Clin Oncol. 1998;16:897-906.
7 Weiner MA, Leventhal B, Brecher ML, et al. Randomized study of intensive MOPP-ABVD with or without low-dose total-nodal radiation therapy in the treatment of stages IIB, IIIA2, IIIB, and IV Hodgkin’s disease in pediatric patients. A Pediatric Oncology Group study. J Clin Oncol. 1997;15:2769-2779.
8 Weiner MA, Leventhal BG, Marcus R, et al. Intensive chemotherapy and low-dose radiotherapy for the treatment of advanced-stage Hodgkin’s disease in pediatric patients. A Pediatric Oncology Group study. J Clin Oncol. 1991;9:1591-1598.
9 Bramswig JH, Heimes U, Heiermann E, et al. The effects of different cumulative doses of chemotherapy on testicular function. Results in 75 patients treated for Hodgkin’s disease during childhood or adolescence. Cancer. 1990;65:1298-1302.
10 Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-475.
11 Hodgson DC, Koh ES, Tran TH, et al. Individualized estimates of second cancer risks after contemporary radiation therapy for Hodgkin lymphoma. Cancer. 2007;110:2576-2586.
12 De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma. Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239-4246.
13 Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52-59.
14 Kung FH, Schwartz CL, Ferree CR, et al. POG 8625. A randomized trial comparing chemotherapy with chemoradiotherapy for children and adolescents with Stages I, IIA, IIIA1 Hodgkin disease. A report from the Children’s Oncology Group. J Pediatr Hematol Oncol. 2006;28:362-368.
15 Franklin J, Pluetschow A, Specht L. Chemotherapy, radiotherapy and combined modality for Hodgkin’s disease, with emphasis on second cancer risk (Review). The Cochrane Collaboration, New York: John Wiley & Sons; 2009.
16 Grufferman S, Delzell E. Epidemiology of Hodgkin’s disease. Epidemiol Rev. 1984;6:76-106.
17 Spitz MR, Sider JG, Johnson CC, et al. Ethnic patterns of Hodgkin’s disease incidence among children and adolescents in the United States, 1973-82. J Natl Cancer Inst. 1986;76:235-239.
18 Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
19 Chang ET, Montgomery SM, Richiardi L, et al. Number of siblings and risk of Hodgkin’s lymphoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1236-1243.
20 Westergaard T, Melbye M, Pedersen JB, et al. Birth order, sibship size and risk of Hodgkin’s disease in children and young adults. A population-based study of 31 million person-years. Int J Cancer. 1997;72:977-981.
21 Chang ET, Zheng T, Weir EG, et al. Childhood social environment and Hodgkin’s lymphoma. New findings from a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1361-1370.
22 Weiss LM, Movahed LA, Warnke RA, et al. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502-506.
23 Wu TC, Mann RB, Charache P, et al. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin’s disease. Int J Cancer. 1990;46:801-804.
24 Glaser SL, Lin RJ, Stewart SL, et al. Epstein-Barr virus-associated Hodgkin’s disease. Epidemiologic characteristics in international data. Int J Cancer. 1997;70:375-382.
25 Weinreb M, Day PJ, Niggli F, et al. The role of Epstein-Barr virus in Hodgkin’s disease from different geographical areas. Arch Dis Child. 1996;74:27-31.
26 Stein H, Delsol G, Pileri S. Hogkin lymphoma. In: Jaffe ES, Harris NL, Stein H, et al, editors. World Health Organization Classification of Tumors. Tumors of Hematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001:237-253.
27 Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, ed 4. Lyon, France: International Agency for Research on Cancer; 2008.
28 Donaldson SS, Hudson M, Oberlin O. Pediatric Hodgkin’s Disease. In: Mauch PM, Armitage JO, Diehl V, editors. Hodgkin’s Disease. Philadelphia: Lippincott Williams & Wilkins; 1999:531-605.
29 Uccini S, Monardo F, Stoppacciaro A, et al. High frequency of Epstein-Barr virus genome detection in Hodgkin’s disease of HIV-positive patients. Int J Cancer. 1990;46:581-585.
30 Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease. Histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96:1889-1899.
31 Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease. Report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17:776-783.
32 Cavalli F. Rare syndromes in Hodgkin’s disease. Ann Oncol. 1998;9(Suppl 5):S109-S113.
33 Friedberg JW, Fischman A, Neuberg D, et al. FDG-PET is superior to gallium scintigraphy in staging and more sensitive in the follow-up of patients with de novo Hodgkin lymphoma. A blinded comparison. Leuk Lymphoma. 2004;45:85-92.
34 Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429-433.
35 Weiner M, Leventhal B, Cantor A, et al. Gallium-67 scans as an adjunct to computed tomography scans for the assessment of a residual mediastinal mass in pediatric patients with Hodgkin’s disease. A Pediatric Oncology Group study. Cancer. 1991;68:2478-2480.
36 Gallamini A, Hutchings M, Rigacci L, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma. A report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746-3752.
37 Sher DJ, Mauch PM, Van Den Abbeele A, et al. Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma. The importance of involved-field radiotherapy. Ann Oncol. 2009;20:1848-1853.
38 Zinzani PL, Stefoni V, Tani M, et al. Role of [18F]fluorodeoxyglucose positron emission tomography scan in the follow-up of lymphoma. J Clin Oncol. 2009;27:1781-1787.
39 Mocikova H, Obrtlikova P, Vackova B, et al. Positron emission tomography at the end of first-line therapy and during follow-up in patients with Hodgkin lymphoma. A retrospective study. Ann Oncol. 2010;21(6):1222-1227.
40 Zuckerman D, Lacasce A, Jacobsen E, et al. High false positive rate with the use of CT and FDG-PET in post-remission surveillance for Hodgkin lymphoma. ASH Annual Meeting Abstracts. 2007;110:2327.
41 Maeda LS, Horning SJ, Iagaru AH, et al. Role of FDG-PET/CT surveillance for patients with classical Hodgkin’s disease in first complete response. The Stanford University experience. ASH Annual Meeting Abstracts. 2009;114:1563.
42 Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860-1861.
43 Atra A, Higgs E, Capra M, et al. ChlVPP chemotherapy in children with stage IV Hodgkin’s disease. Results of the UKCCSG HD 8201 and HD 9201 studies. Br J Haematol. 2002;119:647-651.
44 Donaldson SS, Hudson MM, Lamborn KR, et al. VAMP and low-dose, involved-field radiation for children and adolescents with favorable, early-stage Hodgkin’s disease. Results of a prospective clinical trial. J Clin Oncol. 2002;20:3081-3087.
45 Dorffel W, Luders H, Ruhl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin’s disease in children and adolescents. Analysis and outlook. Klin Padiatr. 2003;215:139-145.
46 Friedmann AM, Hudson MM, Weinstein HJ, et al. Treatment of unfavorable childhood Hodgkin’s disease with VEPA and low-dose, involved-field radiation. J Clin Oncol. 2002;20:3088-3094.
47 Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin’s disease. Response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol. 2000;18:1500-1507.
48 Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765-3771.
49 Ruhl U, Albrecht M, Dieckmann K, et al. Response-adapted radiotherapy in the treatment of pediatric Hodgkin’s disease. An interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys. 2001;51:1209-1218.
50 Schellong G, Potter R, Bramswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin’s disease. The German-Austrian multicenter trial DAL-HD-90. The German-Austrian Pediatric Hodgkin’s Disease Study Group. J Clin Oncol. 1999;17:3736-3744.
51 Kelly KM, Hutchinson RJ, Sposto R, et al. Feasibility of upfront dose-intensive chemotherapy in children with advanced-stage Hodgkin’s lymphoma. Preliminary results from the Children’s Cancer Group Study CCG-59704. Ann Oncol. 2002;13(Suppl 1):107-111.
52 Pellegrino B, Terrier-Lacombe MJ, Oberlin O, et al. Lymphocyte-predominant Hodgkin’s lymphoma in children. Therapeutic abstention after initial lymph node resection—a study of the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:2948-2952.
53 Mauz-Korholz C, Gorde-Grosjean S, Hasenclever D, et al. Resection alone in 58 children with limited stage, lymphocyte-predominant Hodgkin lymphoma. Experience from the European network group on pediatric Hodgkin lymphoma. Cancer. 2007;110:179-185.
54 Donaldson SS, Link MP, Weinstein HJ, et al. Final results of a prospective clinical trial with VAMP and low-dose involved-field radiation for children with low-risk Hodgkin’s disease. J Clin Oncol. 2007;25:332-337.
55 Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma. The results of P9425. Blood. 2009;114:2051-2059.
56 Jhanwar YS, Straus DJ. The role of PET in lymphoma. J Nucl Med. 2006;47:1326-1334.
57 Reinhardt MJ, Herkel C, Altehoefer C, et al. Computed tomography and 18F-FDG positron emission tomography for therapy control of Hodgkin’s and non-Hodgkin’s lymphoma patients. When do we really need FDG-PET? Ann Oncol. 2005;16:1524-1529.
58 Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586.
59 Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma. Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571-578.
60 DeVita VTJr, Canellos GP, Moxley JH3rd. A decade of combination chemotherapy of advanced Hodgkin’s disease. Cancer. 1972;30:1495-1504.
61 Longo DL, Young RC, Wesley M, et al. Twenty years of MOPP therapy for Hodgkin’s disease. J Clin Oncol. 1986;4:1295-1306.
62 Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745-751.
63 Sankila R, Garwicz S, Olsen JH, et al. Risk of subsequent malignant neoplasms among 1,641 Hodgkin’s disease patients diagnosed in childhood and adolescence. A population-based cohort study in the five Nordic countries. Association of the Nordic Cancer Registries and the Nordic Society of Pediatric Hematology and Oncology. J Clin Oncol. 1996;14:1442-1446.
64 Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease. Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386-4394.
65 Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin’s disease in a collaborative British cohort. The relation to age at treatment. J Clin Oncol. 2000;18:498-509.
66 van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin’s disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487-497.
67 Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435-2443.
68 Schellong G, Riepenhausen M, Creutzig U, et al. Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin’s disease. German-Austrian Pediatric Hodgkin’s Disease Group. J Clin Oncol. 1997;15:2247-2253.
69 Horning SJ, Hoppe RT, Kaplan HS, et al. Female reproductive potential after treatment for Hodgkin’s disease. N Engl J Med. 1981;304:1377-1382.
70 da Cunha MF, Meistrich ML, Fuller LM, et al. Recovery of spermatogenesis after treatment for Hodgkin’s disease. Limiting dose of MOPP chemotherapy. J Clin Oncol. 1984;2:571-577.
71 Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer. A report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890-896.
72 Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304-1312.
73 Bonadonna G, Valagussa P, Santoro A. Alternating non-cross-resistant combination chemotherapy or MOPP in stage IV Hodgkin’s disease. A report of 8-year results. Ann Intern Med. 1986;104:739-746.
74 Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28:2-7.
75 Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808-815.
76 Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738-1743.
77 van Dalen EC, Caron HN, Kremer LCM. Prevention of anthracycline-induced cardiotoxicity in children. The evidence. Eur J Cancer. 2007;43:1134-1140.
78 Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55-75.
79 Green DM, Gingell RL, Pearce J, et al. The effect of mediastinal irradiation on cardiac function of patients treated during childhood and adolescence for Hodgkin’s disease. J Clin Oncol. 1987;5:239-245.
80 Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11:1208-1215.
81 Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer. The Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544-1552.
82 Kreisman H, Wolkove N. Pulmonary toxicity of antineoplastic therapy. Semin Oncol. 1992;19:508-520.
83 Marina NM, Greenwald CA, Fairclough DL, et al. Serial pulmonary function studies in children treated for newly diagnosed Hodgkin’s disease with mantle radiotherapy plus cycles of cyclophosphamide, vincristine, and procarbazine alternating with cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine. Cancer. 1995;75:1706-1711.
84 Mefferd JM, Donaldson SS, Link MP. Pediatric Hodgkin’s disease. Pulmonary, cardiac, and thyroid function following combined modality therapy. Int J Radiat Oncol Biol Phys. 1989;16:679-685.
85 Hudson MM, Greenwald C, Thompson E, et al. Efficacy and toxicity of multiagent chemotherapy and low-dose involved-field radiotherapy in children and adolescents with Hodgkin’s disease. J Clin Oncol. 1993;11:100-108.
86 Shankar AG, Ashley S, Atra A, et al. A limited role for VEEP (vincristine, etoposide, epirubicin, prednisolone) chemotherapy in childhood Hodgkin’s disease. Eur J Cancer. 1998;34:2058-2063.
87 Sackmann-Muriel F, Zubizarreta P, Gallo G, et al. Hodgkin disease in children. Results of a prospective randomized trial in a single institution in Argentina. Med Pediatr Oncol. 1997;29:544-552.
88 Smith MA, Rubinstein L, Anderson JR, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol. 1999;17:569-577.
89 Pui CH, Ribeiro RC, Hancock ML, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med. 1991;325:1682-1687.
90 Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493-500.
91 Kaplan HS. Hodgkin’s Disease. Cambridge, Massachusetts: Harvard University Press; 1980.
92 Dieckmann K, Potter R, Wagner W, et al. Up-front centralized data review and individualized treatment proposals in a multicenter pediatric Hodgkin’s disease trial with 71 participating hospitals. The experience of the German-Austrian pediatric multicenter trial DAL-HD-90. Radiother Oncol. 2002;62:191-200.
93 Constine LS, Tarbell N, Hudson MM, et al. Subsequent malignancies in children treated for Hodgkin’s disease. Associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72:24-33.
94 Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901-3907.
95 Koh ES. A dosimetric study of mantle versus involved-field radiotherapy for Hodgkin’s lymphoma. Implications for second cancer risk and cardiac toxicity. Int J Radiat Oncol Biol Phys. 2005;63:S422-S423.
96 Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer. Findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356-2362.
97 Girinsky T, Ghalibafian M. Radiotherapy of Hodgkin lymphoma. Indications, new fields, and techniques. Semin Radiat Oncol. 2007;17:206-222.
98 Girinsky T, Specht L, Ghalibafian M, et al. The conundrum of Hodgkin lymphoma nodes. To be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol. 2008;88:202-210.
99 Shahidi M, Kamangari N, Ashley S, et al. Site of relapse after chemotherapy alone for stage I and II Hodgkin’s disease. Radiother Oncol. 2006;78:1-5.
100 Dhakal S. Patterns and timing of initial relapse in patients with Hodgkin’s and non-Hodgkin’s lymphoma. Blood. 2006;108:1049a-1050a.
101 Campbell BA, Voss N, Pickles T, et al. Involved-nodal radiation therapy as a component of combination therapy for limited-stage Hodgkin’s lymphoma. A question of field size. J Clin Oncol. 2008;26:5170-5174.
102 Dieckmann K, Potter R, Hofmann J, et al. Does bulky disease at diagnosis influence outcome in childhood Hodgkin’s disease and require higher radiation doses? Results from the German-Austrian Pediatric Multicenter Trial DAL-HD-90. Int J Radiat Oncol Biol Phys. 2003;56:644-652.
103 Baez F, Ocampo E, Conter V, et al. Treatment of childhood Hodgkin’s disease with COPP or COPP-ABV (hybrid) without radiotherapy in Nicaragua. Ann Oncol. 1997;8:247-250.
104 Behrendt H, Brinkhuis M, Van Leeuwen EF. Treatment of childhood Hodgkin’s disease with ABVD without radiotherapy. Med Pediatr Oncol. 1996;26:244-248.
105 Ekert H. Treatment of childhood Hodgkin’s disease. J Clin Oncol. 1991;9:528-529.
106 Ekert H, Waters KD, Smith PJ, et al. Treatment with MOPP or ChlVPP chemotherapy only for all stages of childhood Hodgkin’s disease. J Clin Oncol. 1988;6:1845-1850.
107 Lobo-Sanahuja F, Garcia I, Barrantes JC, et al. Pediatric Hodgkin’s disease in Costa Rica. Twelve years’ experience of primary treatment by chemotherapy alone, without staging laparotomy. Med Pediatr Oncol. 1994;22:398-403.
108 van den Berg H, Stuve W, Behrendt H. Treatment of Hodgkin’s disease in children with alternating mechlorethamine, vincristine, procarbazine, and prednisone (MOPP) and adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) courses without radiotherapy. Med Pediatr Oncol. 1997;29:23-27.
109 Goodman KA, Toner S, Hunt M, et al. Intensity-modulated radiotherapy for lymphoma involving the mediastinum. Int J Radiat Oncol BiolPhys. 2005;62:198-206.
110 Chera BS, Rodriguez C, Morris CG, et al. Dosimetric comparison of three different involved nodal irradiation techniques for stage II Hodgkin’s lymphoma patients. Conventional radiotherapy, intensity-modulated radiotherapy, and three-dimensional proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1173-1180.
111 Ghalibafian M, Beaudre A, Girinsky T. Heart and coronary artery protection in patients with mediastinal Hodgkin lymphoma treated with intensity-modulated radiotherapy. Dose constraints to virtual volumes or to organs at risk? Radiother Oncol. 2008;87:82-88.
112 Girinsky T, Pichenot C, Beaudre A, et al. Is intensity-modulated radiotherapy better than conventional radiation treatment and three-dimensional conformal radiotherapy for mediastinal masses in patients with Hodgkin’s disease, and is there a role for beam orientation optimization and dose constraints assigned to virtual volumes? Int J Radiat Oncol Biol Phys. 2006;64:218-226.
113 Plowman PN, Cooke K, Walsh N. Indications for tomotherapy/intensity-modulated radiation therapy in paediatric radiotherapy. Extracranial disease. Br J Radiol. 2008;81:872-880.
114 Weber DC, Peguret N, Dipasquale G, et al. Involved-node and involved-field volumetric modulated arc vs. fixed beam intensity-modulated radiotherapy for female patients with early-stage supra-diaphragmatic Hodgkin lymphoma. A comparative planning study. Int J Radiat Oncol Biol Phys. 2009;75:1578-1586.
115 Yahalom J. Transformation in the use of radiation therapy of Hodgkin lymphoma. New concepts and indications lead to modern field design and are assisted by PET imaging and intensity modulated radiation therapy (IMRT). Eur J Haematol. 2005;66(Suppl):90-97.
116 Korholz D, Kluge R, Wickmann L, et al. Importance of F18-fluorodeoxy-D-2-glucose positron emission tomography (FDG-PET) for staging and therapy control of Hodgkin’s lymphoma in childhood and adolescence—consequences for the GPOH-HD 2003 protocol. Onkologie. 2003;26:489-493.
117 Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48:1721-1727.
118 Wasilewski-Masker K, Liu Q, Yasui Y, et al. Late recurrence in pediatric cancer. A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:1709-1720.
119 Baker KS, Gordon BG, Gross TG, et al. Autologous hematopoietic stem-cell transplantation for relapsed or refractory Hodgkin’s disease in children and adolescents. J Clin Oncol. 1999;17:825-831.
120 Lieskovsky YE, Donaldson SS, Torres MA, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin’s disease. Results and prognostic indices. J Clin Oncol. 2004;22:4532-4540.
121 Williams CD, Goldstone AH, Pearce R, et al. Autologous bone marrow transplantation for pediatric Hodgkin’s disease. A case-matched comparison with adult patients by the European Bone Marrow Transplant Group Lymphoma Registry. J Clin Oncol. 1993;11:2243-2249.
122 Carella AM, Cavaliere M, Lerma E, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. J Clin Oncol. 2000;18:3918-3924.
123 Castagna L, Sarina B, Todisco E, et al. Allogeneic stem cell transplantation compared with chemotherapy for poor-risk Hodgkin lymphoma. Biol Blood Marrow Transplant. 2009;15:432-438.
124 Robinson SP, Sureda A, Canals C, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma. Identification of prognostic factors predicting outcome. Haematologica. 2009;94:230-238.
125 Poen JC, Hoppe RT, Horning SJ. High-dose therapy and autologous bone marrow transplantation for relapsed/refractory Hodgkin’s disease. The impact of involved field radiotherapy on patterns of failure and survival. Int J Radiat Oncol Biol Phys. 1996;36:3-12.
126 Constine LS, Rapoport AP. Hodgkin’s disease, bone marrow transplantation, and involved field radiation therapy. Coming full circle from 1902 to 1996. Int J Radiat Oncol Biol Phys. 1996;36:253-255.
127 Mundt AJ, Sibley G, Williams S, et al. Patterns of failure following high-dose chemotherapy and autologous bone marrow transplantation with involved field radiotherapy for relapsed/refractory Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1995;33:261-270.
128 Rapoport AP, Rowe JM, Kouides PA, et al. One hundred autotransplants for relapsed or refractory Hodgkin’s disease and lymphoma. Value of pretransplant disease status for predicting outcome. J Clin Oncol. 1993;11:2351-2361.
129 Roach M3rd, Brophy N, Cox R, et al. Prognostic factors for patients relapsing after radiotherapy for early-stage Hodgkin’s disease. J Clin Oncol. 1990;8:623-629.
130 Yahalom J. Management of relapsed and refractory Hodgkin’s disease. Semin Radiat Oncol. 1996;6:210-224.
131 Dawson LA, Saito NG, Ratanatharathorn V, et al. Phase I study of involved-field radiotherapy preceding autologous stem cell transplantation for patients with high-risk lymphoma or Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2004;59:208-218.
132 Oberlin O, Leverger G, Pacquement H, et al. Low-dose radiation therapy and reduced chemotherapy in childhood Hodgkin’s disease. The experience of the French Society of Pediatric Oncology. J Clin Oncol. 1992;10:1602-1608.
133 Vecchi V, Pileri S, Burnelli R, et al. Treatment of pediatric Hodgkin disease tailored to stage, mediastinal mass, and age. An Italian (AIEOP) multicenter study on 215 patients. Cancer. 1993;72:2049-2057.
134 Schellong G. Treatment of children and adolescents with Hodgkin’s disease. The experience of the German-Austrian Paediatric Study Group. Baillieres Clin Haematol. 1996;9:619-634.
135 Ekert H, Toogood I, Downie P, et al. High incidence of treatment failure with vincristine, etoposide, epirubicin, and prednisolone chemotherapy with successful salvage in childhood Hodgkin disease. Med Pediatr Oncol. 1999;32:255-258.
136 Cleary SF, Link MP, Donaldson SS. Hodgkin’s disease in the very young. Int J Radiat Oncol Biol Phys. 1994;28:77-83.
137 Schellong G, Bramswig JH, Hornig-Franz I. Treatment of children with Hodgkin’s disease—results of the German Pediatric Oncology Group. Ann Oncol. 1992;3(Suppl 4):73-76.
138 Schellong G. The balance between cure and late effects in childhood Hodgkin’s lymphoma. The experience of the German-Austrian Study-Group since 1978. German-Austrian Pediatric Hodgkin’s Disease Study Group. Ann Oncol. 1996;7(Suppl 4):67-72.
139 Shankar AG, Ashley S, Radford M, et al. Does histology influence outcome in childhood Hodgkin’s disease? Results from the United Kingdom Children’s Cancer Study Group. J Clin Oncol. 1997;15:2622-2630.
140 Cramer P, Andrieu JM. Hodgkin’s disease in childhood and adolescence. Results of chemotherapy-radiotherapy in clinical stages IA-IIB. J Clin Oncol. 1985;3:1495-1502.
141 Hunger SP, Link MP, Donaldson SS. ABVD/MOPP and low-dose involved-field radiotherapy in pediatric Hodgkin’s disease. The Stanford experience. J Clin Oncol. 1994;12:2160-2166.
142 Hudson MM, Krasin M, Link MP, et al. Risk-adapted, combined-modality therapy with VAMP/COP and response-based, involved-field radiation for unfavorable pediatric Hodgkin’s disease. J Clin Oncol. 2004;22:4541-4550.
143 Tebbi CK, Mendenhall N, London WB, et al. Treatment of stage I, IIA, IIIA1 pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation. A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer. 2006;46:198-202.
144 Behrendt H, Van Bunningen BN, Van Leeuwen EF. Treatment of Hodgkin’s disease in children with or without radiotherapy. Cancer. 1987;59:1870-1873.
145 Sripada PV, Tenali SG, Vasudevan M, et al. Hybrid (COPP/ABV) therapy in childhood Hodgkin’s disease. A study of 53 cases during 1989-1993 at the Cancer Institute, Madras. Pediatr Hematol Oncol. 1995;12:333-341.
146 Olweny CL, Katongole-Mbidde E, Kiire C, et al. Childhood Hodgkin’s disease in Uganda. A ten year experience. Cancer. 1978;42:787-792.
147 Ekert H, Fok T, Dalla-Pozza L, et al. A pilot study of EVAP/ABV chemotherapy in 25 newly diagnosed children with Hodgkin’s disease. Br J Cancer. 1993;67:159-162.
148 Mauch PM, Weinstein H, Botnick L, et al. An evaluation of long-term survival and treatment complications in children with Hodgkin’s disease. Cancer. 1983;51:925-932.
149 Jenkin D, Doyle J, Berry M, et al. Hodgkin’s disease in children. Treatment with MOPP and low-dose, extended field irradiation without laparotomy. Late results and toxicity. Med Pediatr Oncol. 1990;18:265-272.
150 Hutchinson RJ, Krailo M, Fryer C. Prognostic factor analysis in advanced Hodgkin’s disease (stages III and IV). Results of the CCG 521 trial. Med Pediatr Oncol. 61, 1993.