Chapter 49 Rectal Cancer
Etiology and Epidemiology
The etiology of colorectal cancer appears to be multifactorial and includes both environmental and genetic factors. Approximately 75% of colorectal cancers are sporadic, whereas 15% to 20% develop in those with either a positive family history or a personal history of colorectal cancer or polyps.2 The remaining cases occur in people with genetic predispositions, such as hereditary nonpolyposis colorectal cancer (HNPCC) (4% to 7%) or familial adenomatous polyposis (FAP) (1%), or in people with inflammatory bowel disease, particularly chronic ulcerative colitis (1%).
The fundamental role of environmental factors is supported by observations in migrant populations. Generally, migrants from low-incidence regions in Africa and Asia who relocate to high-incidence regions of North America or Australia assume the incidence of the host country within one generation.3 Specifically, a high-fat, low-fiber diet is associated with the development of colorectal cancer, although it is unclear if it is causative. Conversely, the ingestion of a high-fiber diet is associated with protection against colorectal cancer. Fiber causes the formation of a soft, bulky stool that dilutes out carcinogens; it also decreases colonic transit time, allowing less time for carcinogenic substances to contact the mucosa. The more sedentary lifestyle in Western countries, cigarette smoking, and alcohol consumption also appear to be linked with the risk of colorectal neoplasia.
Most colorectal cancers arise from benign adenomatous polyps lining the wall of the bowel. Those that grow to a large size (>2 cm) and have a villous appearance or contain dysplastic cells are most likely to progress to cancer. This progression from adenoma to carcinoma is associated with an accumulation of genetic alterations, including activation of oncogenes and inactivation of tumor suppressor genes.4 One of the early steps in this process is the interruption of the APC/?β-catenin/TCF-4 pathway, allowing unchecked cellular replication at the crypt surface.5 This can occur in the germline of FAP patients, with the second allele being inactivated somatically, or in sporadic cancers, in which both alleles are somatically inactivated.
There are likely three pathways that lead to colorectal cancer: chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP).6 CIN is the genetic reason for tumor formation in 50% to 70% of colorectal cancers and the mechanism operative in FAP. It is typically coupled with mutations in the KRAS, APC, and TP53 genes, ultimately leading to loss of heterozygosity of TP53 and malignant transformation. MSI is found in more than 90% of HNPCCs that carry a germline inactivation in DNA mismatch repair genes, but also in 15% of sporadic cancers, in which epigenetic hypermethylation silences gene transcription of hMLH1. With MSI, multiple frameshift mutations at microsatellite sequences occur, including those in exon coding sequences of the transforming growth factor-beta receptor II, the proapoptotic BAX gene, and the DNA mismatch repair genes (hMSH3 and hMSH6 genes). The third type, CIMP, is a relatively heterogeneous subgroup and occurs in approximately 10% to 30% of patients, is predominantly associated with KRAS gene mutations but sometimes with BRAF gene mutations, usually lacks chromosomal instability, and has the poorest prognosis of the three types and perhaps a poorer response to chemotherapy. Of the numerous molecular markers examined to date, MSI, 18q, thymidylate synthase, and TP53 overexpression are among those which appear to have the most prognostic significance in colon cancer.7 Although the markers are not currently incorporated into the staging system, they should be noted.
Colorectal cancer is the fourth commonest form of cancer worldwide, with an estimated 800,000 new cases diagnosed each year, accounting for roughly 10% of all cancers.8 Globally, the colorectal cancer incidence per 100,000 persons in 1990 was 19.4 for men and 15.3 for women. High incidence rates are found in North America, Western Europe, and Australia (40 to 45 cases per 100,000 population) and intermediate rates are found in Eastern Europe (26 cases per 100,000 population), with the lowest rates found in Africa (3 to 8 cases per 100,000 population). Approximately two-thirds of cases occur in the colon and one-third in the rectum. In the United States, 39,760 new cases of rectal cancer were estimated to occur in 2011 (22,620 in men, 17,050 in women),1 there is little difference in incidence among whites, African Americans, and Asian Americans.
The occurrence of sporadic colorectal cancer increases continuously above the age of 45 to 50 years for both genders and peaks in the seventh decade. Subgroups of patients, including those with inherited syndromes such as familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC), or hamartomatous polyposis conditions (e.g., Peutz-Jeghers syndrome), can experience colorectal cancer at a much earlier age.9
Prevention and Early Detection
Primary Prevention
Primary prevention involves the identification and elimination of factors that cause or promote colorectal cancer or interfere with the adenoma-to-carcinoma cascade. Dietary and lifestyle approaches employ higher-fiber, lower-fat components and increased physical activity to inhibit the carcinogenic process. Other dietary components, such as selenium, carotenoids, and vitamins A, C, and E, may also have protective effects by scavenging free oxygen radicals. Folic acid, a component of fresh fruits and green vegetables, supplies methyl groups necessary for nucleotide synthesis and gene regulation. Prospective studies generally support an inverse association between folate intake and colorectal cancer risk.10
Chemopreventive strategies are based on population-based studies that strongly support an inverse relationship between the use of nonsteroidal anti-inflammatory drugs, such as aspirin, sulindac, or the new selective COX-2 inhibitors, and the risk of colorectal adenomas or carcinomas.11 COX-2 is overexpressed in more than 50% of adenomas and 80% to 85% of adenocarcinomas. Trials are currently under way that focus on FAP patients, those with resection of early-stage colorectal carcinomas, and those with a history of adenomatous polyps.
Screening for Early Detection
The process of malignant transformation from adenoma to carcinoma takes several years. The goal of screening is to prevent rectal cancer deaths through the detection and treatment of benign or premalignant lesions and curable-stage cancers.12,13 For the average-risk population, screening should begin at age 50 years and should follow one of the following testing options: fecal occult blood testing every year, flexible sigmoidoscopy every 5 years, yearly fecal blood testing and flexible sigmoidoscopy every 5 years, colonoscopy (preferably) every 10 years, or double-contrast barium enema studies every 5 years. If polyps are found, however, colonoscopy should be performed every year until the patient is polyp free.
Two promising but investigational approaches to screening include virtual colonoscopy and molecular stool testing.7,14 Virtual colonoscopy employs virtual reality technology from cross-sectional CT or MRI scans. Molecular stool testing is based on the molecular detection of neoplasm-specific DNA from exfoliated cancer cells.
Biologic Characteristics and Molecular Biology
The most important prognostic factor for overall survival is the pathologic extent of disease as determined by the degree of bowel wall penetration by the tumor, and the presence or absence of lymph node metastases or distant metastases (TNM stage).15 Tumor differentiation is also prognostically important because poorly differentiated adenocarcinomas are associated with lymph node metastases in more than 50% of cases and also correlate with the likelihood of lymphatic and venous invasion.16 An elevated carcinoembryonic antigen (CEA) level at the time of presentation has an adverse impact on survival times independent of tumor stage.17 If the level is higher than 100 ng/mL, the patient has distant metastasis (most likely, to the liver or lung) until proven otherwise. Detection of occult metastases in lymph nodes, blood, or bone marrow by molecular techniques may allow further refinement in pathologic staging. Molecular strategies added to the more traditional histopathologic information may help in the future to identify patients at a lower stage whose tumors are likely to behave like higher-stage lesions, and vice versa. Several biologic markers, including allelic loss of 18q, alteration in KRAS, MSI, thymidylate synthase, thymidine phosphorylase, vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), P21, P27, BCL2, BAX, and TP53, among others, are now being prospectively evaluated in clinical trials to determine their prognostic utility.2 At the present time, however, the TNM staging system is the most reliable.
Pathology and Pathways of Spread
Histopathology
The majority (>90%) of colorectal cancers are adenocarcinomas. Some adenocarcinomas have mucin, which can be extracellular (colloid) or intracellular (signet-ring cell). Colloid cancer, which occurs in 15% to 20% of adenocarcinomas, is not an independent prognostic factor, whereas signet-ring cell carcinoma, which occurs in 1% to 2% of adenocarcinomas, is an independently poor prognostic factor for survival.18,19 Other histologic types are rare and include carcinoid tumors,20 leiomyosarcomas,21,22 lymphomas,23 and squamous cell cancers.24 The grading system used for adenocarcinomas refers to the degree of differentiation. Some institutions use a three-grade system (well, moderate, poor), and others use a four-grade system. Despite substantial interobserver and intraobserver variability in tumor grading, poorly differentiated tumors have consistently been found to be associated with a worse prognosis in multivariate analysis.
Anatomy and Pathways of Spread
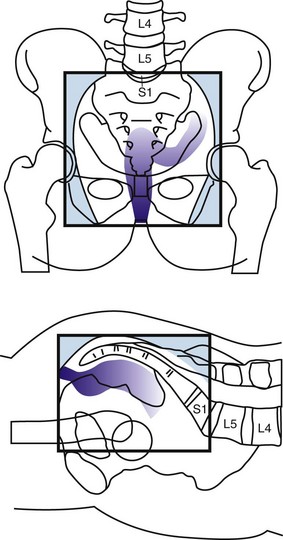
The anterior peritoneal reflexion represents the point at which the rectum exits the peritoneal cavity and becomes a retroperitoneal structure (approximately 12 to 15 cm from the anal verge). Below this level, there is a mesorectal, or circumferential, resection margin all around the rectum. The circumferential resection margin (CRM) is related more to the type of surgical procedure than to an anatomic landmark, however. While distal intramural spread usually extends no more than millimeters beyond the grossly recognizable margin of the tumor, microscopic tumor nodules (satellites) are found in the mesorectum, predominantly in a radial direction but also in a distal one, some centimeters from the lower tumor margin.25 A layer of visceral fascia encloses both the rectum and mesorectum and thus forms a separate compartment within the pelvis. With total mesorectal excision (TME), the entire specimen is removed by sharp dissection along the mesorectal plane. Patients entered into postoperative adjuvant rectal trials in the United States were required to have tumors with the inferior aspect at or below the peritoneal reflection. For entry into preoperative trials, most use tumors with a distance of less than 12 cm from the anal verge for eligibility. The German trial allowed tumors at a distance of as much as 16 cm, however.26
Clinical Manifestations, Patient Evaluation, and Staging
Patient Evaluation
The standard diagnostic algorithm for rectal cancer is seen in Table 49-1. The pretreatment evaluation of a patient with rectal cancer should include a careful history and physical examination. By digital rectal examination (the average finger can reach approximately 7 to 8 cm), tumors can be assessed for size, ulceration, and fixation to surrounding structures. This examination also permits evaluation of sphincter function, which is critical when determining whether a patient is a candidate for a sphincter-sparing procedure. Rigid proctosigmoidoscopy allows direct visualization of the lesion and provides an estimation of the size of the lesion and degree of obstruction. This procedure is used to obtain biopsies and gives an accurate measurement of the distance of the lesion from the anal verge. A complete evaluation of the large bowel, preferably by colonoscopy, should be done to rule out synchronous neoplasms.
| History | Including family history of colorectal cancer or polyps |
| Physical examination | Including assessment of size, minimum diameter of lumen, mobility, distance from anal verge, and cursory evaluation of sphincter function |
| Proctoscopy | Including assessment of mobility, minimum diameter of lumen, and distance from anal verge. Allows biopsy of the primary tumor. |
| Colonoscopy or barium enema | To detect possible synchronous neoplasm |
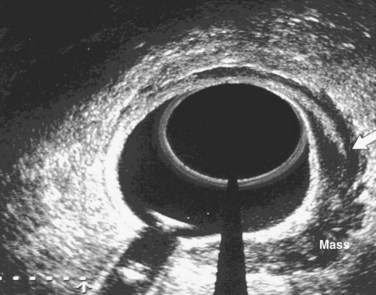
| Endorectal ultrasound | To assess extent of primary tumor, if considering local excision or preoperative therapy |
| Pelvic CT or MRI scanning | To assess extent of primary tumor and lymph node involvement (for accuracy of transrectal ultrasound, CT; MRI for T and N category) (see Table 49-2) |
| Chest radiograph and abdominal ultrasound | To detect possible metastatic disease |
| Abdominal CT/MRI scanning Lung CT scanning |
To further investigate suspicious findings of chest radiographs and abdominal ultrasound |
| PET/CT scanning | To rule out metastatic blood-borne or nodal disease |
| CEA levels | To obtain baseline CEA level (a prognostic factor and important for follow-up) |
| Complete blood count | To check for anemia secondary to bleeding |
CEA, carcinoembryonic antigen; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
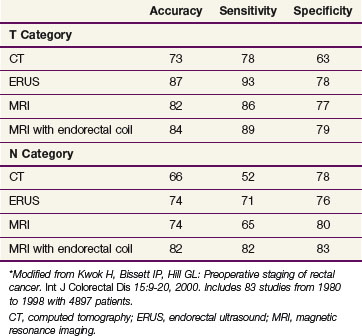
The primary imaging modalities for assessing the extent of the primary tumor are endorectal ultrasound (ERUS), CT, positron emission tomography (PET), and MRI. ERUS and high-resolution pelvic MRI are the most accurate tools in predicting the T stage of rectal cancers (Fig. 49-1 and Table 49-2). With the shift to preoperative therapy, clinical staging to accurately identify both T stage and N stage is critical. Imaging techniques to assess the extent of the primary tumor include CT, MRI, PET with 18F-fluorodeoxyglucose (FDG-PET), and transrectal ultrasound. In the United States, ultrasound plus CT or MRI is commonly used, whereas in many European countries, high-resolution pelvic MRI is preferred. High-resolution MRI also allows for identification of patients likely to have close or positive radial margins if they undergo initial surgery and therefore they are selected to receive preoperative therapy.27 For example, the Mercury trial, based in the United Kingdom, uses MRI to select for the intensity of preoperative therapy.28 In a retrospective analysis of 504 patients reported by Bail and colleagues,29 even following preoperative chemoradiation, those with positive radial margins still had a higher local recurrence rate (35% vs. 11%) and a lower 5-year survival rate (27% vs. 73%) compared with those with negative radial margins.
The overall accuracy of imaging studies in predicting disease extent varies by imaging modality and disease category (T vs. N vs. M). The accuracy for T category is approximately 50% to 90% with transrectal ultrasound30 or high-resolution MRI31 and 50% to 70% with CT or conventional MRI scanning.32 For identification of metastatic disease (M stage), FDG-PET/CT may be more accurate than CT alone.33 The use of PET/CT to restage patients following preoperative chemoradiation remains controversial.34–36
The identification of positive lymph nodes is more difficult. The overall accuracy in detecting positive pelvic lymph nodes with the above techniques is approximately 50% to 75%. The accuracy of MRI is similar to that of CT, but it is improved with the use of external and/or endorectal coils. Both CT and MRI can identify lymph nodes measuring 1 cm or more, although enlarged lymph nodes are not pathognomonic of tumor involvement. The accuracy of MRI is similar to that of CT and may be further enhanced with the use of superparamagnetic iron oxide particles.37 Likewise, the accuracy of ultrasound for the detection of involved perirectal lymph nodes may be augmented if ultrasound is combined with fine-needle aspiration.38 Despite these advances, the ability to accurately predict the pathologic stage following preoperative chemoradiation with MRI,39,40 ultrasound,41,42 FDG-PET,34 or physical examination43 remains suboptimal. The tumor regression grade44,45 may help predict lymph node positivity; patients would have already received preoperative chemoradiation, however.
Screening for distant metastatic disease is commonly performed with CT and/or MRI. The major advantage of a PET/CT scan is to differentiate between recurrent tumor and scar tissue by measuring tissue metabolism of an injected glucose-based substance.46,47 Scar tissue is inactive, whereas tumor tissue is generally hypermetabolic. At the present time, PET/CT is considered investigational for a routine preoperative metastatic workup in a patient who presents with primary disease,33 but its use should be considered strongly for patients with presumed locoregional relapse who are being considered for aggressive treatment approaches with curative intent.33
Imaging after Chemoradiation
Although ERUS, CT, and MRI can be used to assess downstaging of the tumor after neoadjuvant treatment, none is accurate in distinguishing among ypT0, ypT1, ypT2, or ypT3 tumors. Overstaging is common, especially when there is a fibrotic thickening of the rectal wall. A reasonably high level of accuracy has been observed by phased-array MRI for differentiating ypT0 to T2 versus ypT3 disease.48 Both diffusion-weighted MRI and FDG-PET/CT have been used to monitor therapy response and to predict the outcome following preoperative therapy. Of the two, FDG-PET/CT has a larger role. Many studies have reported a significant decrease in standardized uptake values (SUVs) on postirradiation FDG-PET/CT scans in responders when compared with nonresponders, but the clinical value of this information remains to be determined.49
Staging
The first practical staging system was the Dukes’ classification.50 This system classified colorectal tumors from A to C, with stage A indicating penetration into but not through the bowel wall, stage B indicating penetration through the bowel wall, and stage C indicating involvement of lymph nodes, regardless of the extent of bowel wall penetration. The system has since been modified by many authors, including Dukes, to reflect finer levels of penetration and nodal metastases and has been extended to include the colon as well as the rectum.
The Astler-Coller staging system allowed separation of wall penetration and nodal status. The Gunderson-Sosin modification of the Astler-Coller staging system subdivided T3 tumors into those with microscopic (B2m or C2m) or gross (B2m+g or C2m+g) penetration of tumor through the bowel wall.51 It also defined tumors adherent to or invading an adjacent organ or structure as B3 if the nodes were negative and C3 if the nodes were positive. Several studies have analyzed both local failure and survival using the modified Astler-Coller staging system,52 with most confirming the predictive capability of this system.
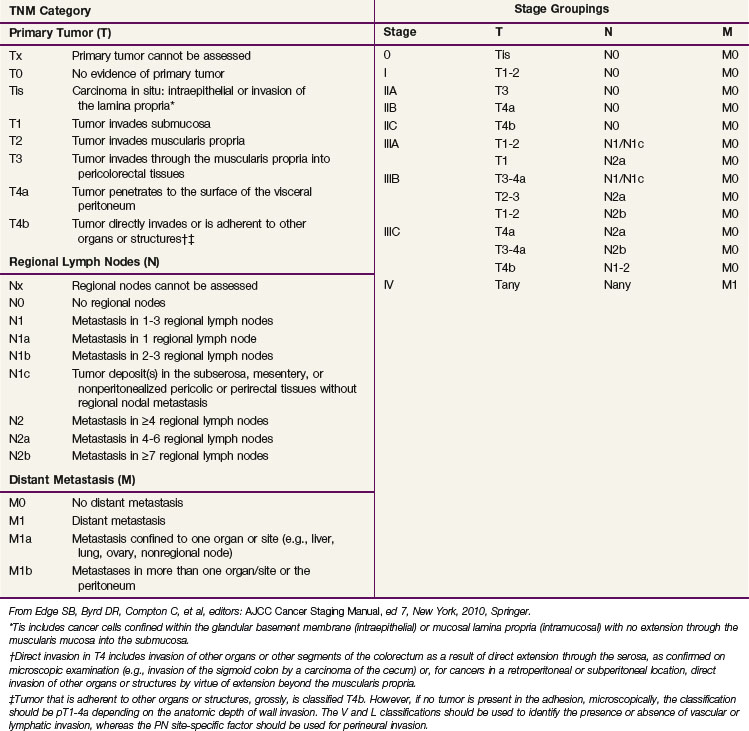
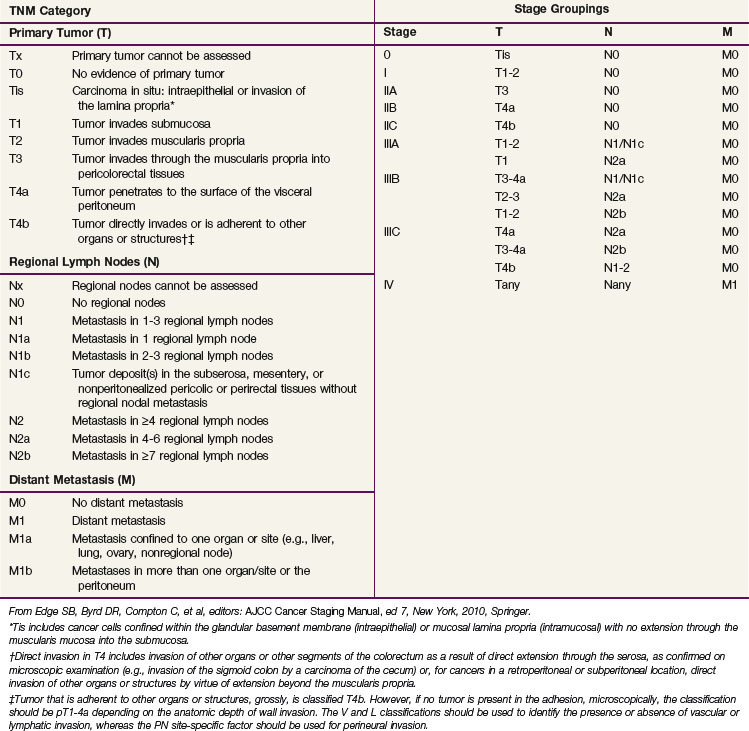
In the sixth edition of the AJCC staging manual, stage II colorectal cancer was subdivided into IIA (T3N0) and IIB (T4N0) and stage III was subdivided into IIIA (T1-2N1M0), IIIB (T3-4N1M0), and IIIC (any TN2M0), based on improved outcomes for patients with IIA versus IIB disease and IIIA versus IIIB disease.53 The prognostic validity of this change was supported by a subsequent pooled analysis of the U.S. GI Intergroup and National Surgical Adjuvant Breast and Bowel Project (NSABP) postoperative trials54 and the retrospective analysis of the American College of Surgeons National Cancer Database (NCDB).55 The placement of all TN2 patients into stage IIIC, however, was not based on in-depth outcomes analyses, because the impact of depth of bowel wall invasion (T category) in N2 patients had not been evaluated in detail.
Before making substantive changes in the AJCC seventh edition staging manual for colon and rectal cancer (Table 49-3), the AJCC Hindgut Task Force sought population-based validation that depth of invasion and nodal status interact to affect survival rates. Surveillance, Epidemiology, and End Results (SEER) survival data were obtained for patients with invasive colon and rectal cancer and evaluable TN category of disease (35,829 rectal and 109,953 colon cancer patients).56 T4N0 cancers were stratified by “tumors that perforate visceral peritoneum” (T4a) versus “tumors that invade or are adherent to adjacent organs or structures” (T4b). N1 and N2 were stratified by number of involved nodes (N+): N1a/N1b (1 vs. 2 or 3 nodes), and N2a/N2b (4 to 6 vs. ≥7 nodes). Rates of 5-year observed and relative survival were obtained for each TN category. The analyses indicated the following information: survival rates are better for T1-2N0 cancers than T3N0 cancers, better for T3N0 cancers than T4N0 cancers, better for T1-2N2 cancers than T3-4N2 cancers, and similar for T4bN1 and T4N2 cancers. In addition, patients with T4a lesions have better survival rates than those with T4b lesions by N category, and the number of positive lymph nodes affects survival for each T category. The SEER population-based colon and rectal cancer analyses supported subdividing T4 (T4a/T4b), N1 (N1a/N1b), and N2 (N2a/N2b) and supported revised substaging of stages II and III (shift of T1-2N2 lesions from IIIC to IIIA/IIIB and T4bN1 lesions from IIIB to IIIC.56,57
There are additional descriptors that do not affect the stage grouping but do indicate cases needing separate analysis. The AJCC TNM staging system should be used routinely for staging and treatment purposes. Although CEA has prognostic importance, it was not added to the staging system.15
The role of the pathologist is critical to proper staging. Following surgery alone, at least 12 pelvic nodes must be examined to obtain an accurate pN stage. Tumors treated with preoperative chemoradiation are downstaged, however, and it is commonly not possible to evaluate 12 lymph nodes for this purpose.58,59
Primary and Adjuvant Therapy for Resectable Disease
Adjuvant Irradiation without Chemotherapy
Postoperative Irradiation
Until 1990, most patients in the United States underwent surgery and, if needed, received postoperative external beam radiation therapy (EBRT). The primary advantage with this approach was pathologic staging and avoiding overtreatment with preoperative therapy. As confirmed by the German CAO/ARO/AIO 94 phase III trial that randomized preoperative versus postoperative chemoradiation, the disadvantage of postoperative therapy is significantly higher rates of local recurrence and acute and chronic toxicity, as well as a significantly lower chance of sphincter preservation.26 Lastly, if the patient has undergone an APR, the radiation field must be extended to include the perineal scar.
Historical nonrandomized data from the Massachusetts General Hospital60 and M.D. Anderson Cancer Center61 reveal crude local failure rates of 4% to 31% in patients with stage pT3-4N0M0 disease and 8% to 53% in patients with stage pT3-4N1-2M0 disease who received 45 to 55 Gy without chemotherapy.
There are five randomized trials examining the use of adjuvant postoperative irradiation without chemotherapy in stage pT3 and/or N1-2 rectal cancer.62,63,64,65,66 None have shown an improvement in overall survival (OS). Two reveal a decrease in local failure rates; NSABP trial R-01 (16% vs. 25%; p = .06) and the Medical Research Council (MRC) trial (21% vs. 34%; p = .001).65
Preoperative Irradiation
Dose escalation has not shown a clear benefit. In the Lyon R96-02 trial, 88 patients with cT2 to T3 rectal cancer received 39 Gy in 13 fractions to the pelvis and were randomized to observation or a boost with contact irradiation to a total dose of 85 Gy.67 A total of 23 patients were selected to receive postoperative chemotherapy. Patients who received the boost had a decrease in the local failure rate (2% vs. 7%) but there was no difference in 2-year disease-free survival (DFS) (92% vs. 88%).
A unique clinical situation in which preoperative radiation therapy alone is recommended is when a patient has a distal uT2N0 tumor and refuses an APR. Although APR is the standard therapy, an alternative treatment is preoperative irradiation followed by low anterior resection (LAR) and, if the pelvic nodes are positive, postoperative chemotherapy. In a series of 27 patients with cT2N0 distal rectal cancer who refused APR and received preoperative EBRT, 78%underwent a sphincter-sparing operation.68 The incidence of 5-year actuarial local recurrence in those undergoing sphincter preservation was 13%; the colostomy-free survival rate, 100%; and the OS, 85%. Overall, 77% of those who underwent a sphincter-sparing procedure had good to excellent bowel function at 24 to 36 months after surgery.
Short-Course Preoperative Irradiation
There are 12 modern randomized trials of preoperative radiation therapy (without chemotherapy).69 All use low to moderate doses of radiation. Most of the trials showed a decrease in local recurrence rates, and in five of the trials, this difference reached statistical significance. Although in some trials a subset analysis revealed a significant improvement in survival rates, the Swedish Rectal Cancer Trial is the only one that reported a survival advantage for the total treatment group. Two meta-analyses report conflicting results. Although both revealed a decrease in local recurrence rates, the analysis by Camma and associates70 reported a survival advantage, whereas the analysis by the Colorectal Cancer Collaborative Group71 did not.
In the Swedish Rectal Cancer Trial, patients with cT1-3 rectal cancer were randomized to receive 25 Gy in 1 week followed by surgery 1 week later versus surgery alone.72 Those who received preoperative irradiation had a significant decrease in local recurrence rates (12% vs. 27%; p <.001) and a correspondingly significant improvement in 5-year OS (58% vs. 48%; p = .004). After 13 years, the OS is still significantly improved (38% vs. 30%; p = .008).73 Of interest, the local recurrence rate in node-positive patients who underwent surgery alone was 46%, illustrating the inferior results of surgery before the adoption of TME.
The Dutch CKVO 95-04 trial randomized 1805 patients with cT1 to T3 disease to TME or intensive short-course preoperative radiation therapy followed by TME.74 Irradiation significantly decreased the local recurrence rate (8% vs. 2%; p < .0001), but there was no difference in the 2-year survival rate (82%). With longer follow-up, the 5-year local failure rate was higher with TME (11%) but was still significantly decreased (to 6%) with preoperative irradiation.75 The acute toxicity rates in the Dutch CKVO 95-04 trial included a 10% rate of neurotoxicity, a 29% rate of perineal wound complications, and a 12% rate of postoperative leaks.76 Of the patients who developed postoperative leaks, 80% required surgery, resulting in death in 11%.
The presence of a positive circumferential margin is an important negative prognostic sign. In the Dutch CKVO trial, 17% had positive circumferential margins. Although they received 50 Gy postoperatively, this did not compensate for positive margins.77 Few centers perform the necessary pathologic examination to detect positive circumferential margins.29,78 MRI can help identify patients who will have positive margins with surgery alone as well as select those who may benefit from preoperative therapy.79–81
Preoperative plus Postoperative Irradiation
This approach, also known as the sandwich technique, includes a short preoperative course of radiation (5 to 15 Gy) followed by surgery, and in patients with pT3-4N1-2 disease, an additional 40 to 45 Gy postoperatively. The Radiation Therapy Oncology Group (RTOG) trial 81-15 randomized 350 patients (87% with rectal cancer) to preoperative irradiation with 5 Gy versus surgery alone.82 Patients with stage pT3 and/or N1-2 disease received a minimum dose of 45 Gy postoperatively. No chemotherapy was delivered. There were no differences in rates of local failure, distant failure, or OS between the arms. A retrospective analysis of 155 patients treated at the Institut Gustave Roussy also revealed no advantage of the sandwich technique compared with preoperative irradiation.83 This approach is no longer used.
Adjuvant Combined-Modality Therapy
There are two conventional treatment approaches for clinically resectable rectal cancers. For patients with cT1-2N0 disease, the initial treatment is surgery, and if the tumor is pT3N0 or TanyN1-2, this is followed by postoperative chemoradiation.84 For patients with cT3-4N0 or TanyN+ lesions, preoperative chemoradiation is given, followed by surgery and postoperative adjuvant chemotherapy.
Postoperative Chemoradiation
The National Cancer Institute (NCI) Consensus Conference concluded in 1990 that chemoradiation was the standard postoperative adjuvant treatment for patients with pT3 and/or N1-2 disease.84 This recommendation was based on phase III trials that compared postoperative chemoradiation arms with control arms of either surgery alone or surgery plus postoperative irradiation (Mayo/NCCTG 79-47-51) and demonstrated improvements in both DFS and OS.85 The standard design in U.S. trials was to deliver six cycles of bolus 5-fluorouracil (5-FU)–based chemotherapy; concurrent irradiation was also given during cycles 3 and 4.
The U.S. GI Intergroup 86-47-51 trial did not demonstrate an incremental benefit to semustine when it was added to postoperative irradiation plus concurrent and maintenance 5-FU.86 However, a 2 × 2 component of the study demonstrated a positive benefit for giving protracted venous infusion (PVI) 5-FU rather than interrupted bolus 5-FU concurrently with pelvic irradiation. Patients randomized to receive concurrent PVI 5-FU (225 mg/m2/day, 7 days/week or until intolerance) had improvements in rates of disease control, DFS (at 4 years, 63% vs. 53%; p = .01), and OS (at 4 years, 70% vs. 60%; p = .005).
The successor INT 0114 four-arm trial randomized patients with pT3-4N0 and/or TanyN+ rectal cancer to postoperative irradiation and bolus 5-FU with or without leucovorin, levamisole, or leucovorin plus levamisole (INT 86-47-51 results were not available before INT0114 study design and completion). There was no significant difference in local control or survival rates among the four arms.87 With longer follow-up, the study also revealed that local control and survival results continued to deteriorate after 5 years. At 7 years, the local failure rate was 17% and the survival rate was 56%, compared with 14% and 64%, respectively, at 5 years. Patients with high-risk (pT3N+ or T4) disease had a lower survival rate compared with lower-risk (pT1-2N+ or T3N0) disease (45% vs. 70%). Further analysis of the INT 0114 trial has revealed that body mass is related to outcome and treatment-related toxicity,88 and both surgeons and hospitals with higher volumes of rectal cancer surgery have improved outcomes compared with those with lower volumes.89
The subsequent INT 0144 postoperative adjuvant rectal trial was designed to follow up on the positive results achieved with concurrent PVI 5-FU during irradiation in trial 86-47-51.90 Patients were randomized to three arms: arm 1, bolus 5-FU/PVI 5-FU + RT/bolus 5-FU (control arm from 86-47-51); arm 2, PVI 5-FU/PVI 5-FU + RT/PVI 5-FU; arm 3, bolus 5-FU–leucovorin(LV)–levamisole/bolus 5-FU–LV-levamisole + RT/bolus 5-FU–LV-levamisole. The concurrent PVI 5-FU arms did not confirm a survival benefit relative to the bolus 5FU/LV/levamisole arm, but arm 2 did report a lower incidence of grade 3+ hematologic toxicity. Based on these results, when 5-FU is used with either preoperative or postoperative chemoradiation, PVI has been the preferable standard.
Do Patients with Pathologic Node-Negative Rectal Cancer Require Pelvic Irradiation?
The 1990 NCI Consensus Conference recommendation for postoperative chemoradiation was published nearly 20 years ago and was based on trials where neither a TME nor an examination of 12 nodes or more was required. Retrospective data suggest that there may be a subset of patients with pT3N0 disease who may not require adjuvant therapy. Nissan and associates91 reported results of 100 patients with uT2-3N0 disease who underwent TME alone and had 12 nodes or more examined.
The sixth edition of the AJCC staging manual subdivided stage III into IIIA (T1-2N1), IIIB (T3-4N1), and IIIC (TanyN2). The prognostic validity of this change was supported by both the rectal pooled analysis of the U.S. GI Intergroup and NSABP postoperative trials54 and the retrospective analysis of the American College of Surgeons National Cancer Database.55 The 5-year survival rates by stages IIIA, B, and C in the pooled analysis were 81%, 57%, and 49% and in the NCDB database were 55%, 35%, and 25%, respectively. These data provided further evidence that patients who undergo TME, have at least 12 nodes examined, and have stage pT3N0 disease may not need the radiation component of chemoradiation, dependent on the adequacy of radial and distal margins of resection. The approximately 3% to 4% benefit in local control with irradiation may not be worth the risks, especially in women of reproductive age. However, patients with pT3N0 tumors with adverse pathologic features, or who undergo resection without TME, or who have fewer than 12 nodes examined should still receive postoperative chemoradiation.
Although the number of nodes is defined in the AJCC staging system, the location of pelvic nodes is not. Leibold and associates59 treated 121 patients with preoperative chemoradiation and found that the incidence of metastatic disease was higher in those patients with positive nodes in the proximal pelvis compared with positive nodes anywhere in the pelvis (46% vs. 32%). Of note, the proximal nodes are above the superior border of the typical radiation field (L5/S1) because they are located along the apical and mid portion of the inferior mesenteric artery.
Preoperative Chemoradiation
Based on the German CAO/ARO/AIO 94 trial, preoperative chemoradiation is standard treatment for patients with cT3-4N0 or TanyN+ disease.26 The disadvantage of preoperative therapy is the possible overtreatment of patients with either early-stage disease (pT1-2N0) or undetected metastatic disease. In the German trial that used CT and transrectal ultrasound, 18% of patients were overstaged. A number of questions and issues exist with regard to patient selection for preoperative chemoradiation and optimization of treatment that will be addressed in the following subsections.
The Need for Chemotherapy with Preoperative Irradiation
Almost every randomized trial for the past two decades has confirmed a 10% to 15% survival benefit of a total of 6 months of adjuvant 5-FU-based chemotherapy for patients with lymph node-positive colon or rectal cancer. Both retrospective92 and randomized trials,93,94 however, question whether chemotherapy improves the results of preoperative irradiation in patients with cT3-4 rectal cancer.
The European Organization for Research and Treatment of Cancer (EORTC) trial 22921 was a four-arm randomized trial of 1011 patients who received preoperative irradiation of 45 Gy with or without a concurrent bolus of 5-FU/leucovorin followed by surgery with or without four cycles of postoperative 5-FU/leucovorin.93 Only 37% had a TME. The EORTC trial revealed a significant decrease in the local failure rate in those patients who receive chemoradiation compared with irradiation (8% to 10% vs. 17%; p <.001) but no difference in the 5-year OS (65%). However, only 43% received 95% or more of the planned postoperative chemotherapy, which may explain the negative results. Furthermore, a subset analysis of the EORTC trial revealed that patients who responded to preoperative chemoradiation had a survival benefit from postoperative chemotherapy.95
A trial by the Fédération Francophone de la Cancérologie Digestive (FFCD 9203) was a two-arm trial of 742 patients randomized to preoperative irradiation of 45 Gy with or without bolus 5-FU/leucovorin.94 All patients were scheduled to receive postoperative chemotherapy, and 73% received such. The FFCD trial reported a similar decrease in local failure rates (8% vs. 17%; p <.05) and a corresponding increase in pathologic complete response (pCR) (11% vs. 4%; p <.05) with preoperative chemoradiation vs. irradiation alone, but no survival benefit (68% vs. 67%).
Because most patients did not receive adequate doses of postoperative chemotherapy in the EORTC trial and the FFCD trial only tested the impact of concurrent chemotherapy with preoperative irradiation, preoperative chemoradiation followed by surgery and 4 months of postoperative adjuvant chemotherapy remains the standard practice in North America. However, there remains considerable controversy in some European countries regarding its use. A recent consensus conference failed to reach a definitive recommendation regarding its use.96
Potential Overtreatment with Preoperative Therapy
In the German CAO/ARO/AIO 94 rectal trial, 18% of patients clinically staged as cT3N0 preoperatively and who underwent initial surgery without preoperative therapy had pT1-2N0 disease.26 Therefore, those patients would have been overtreated if they had received preoperative therapy. Although not ideal, preoperative therapy is still preferred to performing surgery first because even after preoperative chemoradiation (which downstages tumors), Guillem and coauthors97 reported that 22% will have ypN+ disease at the time of surgery. In patients who undergo surgery alone, this number is as high as 40%.98 These patients will then require postoperative chemoradiation, which, compared with preoperative chemoradiation, has inferior rates of local control, higher rates of acute and chronic toxicity, and, if a low anastomosis is performed, inferior functional results.
In the report by Guillem and coauthors,97 the incidence of positive nodes was not dependent on the distance from the anal verge. Of the 103 patients with tumors that were 0 to 5 cm from the anal verge, 23% were ypN+, whereas of the 85 patients with tumors that were 6 to 12 cm from the anal verge, the incidence was 20%. These data suggest that in tumors up to 12 cm from the anal verge, the risk of positive nodes (and likely local recurrence) is similar.
Positive Radial (Circumferential) Margins
Although the distal margin is predictive of both local recurrence and the feasibility of a sphincter-preserving operation, the radial (circumferential) margin also has a substantial impact on the local recurrence rate.99 An analysis of more than 17,500 pathologic specimens by Nagtegaal and associates99 reported inferior survival rates in patients with positive CRMs after neoadjuvant treatment compared with immediate surgery (hazard ratio [HR], 6.3; 95% CI, 3.7 to 16.7 vs. HR, 2; 95% CI, 1.4 to 2.9, respectively).
In the Dutch CKVO trial, 17% of patients had positive circumferential margins. In a subset analysis by Nagtegaal and colleagues,100 patients with positive radial margins who underwent TME alone had a local recurrence rate of 17% after an LAR and 30% after an APR.
Unfortunately, few centers perform the necessary pathologic examination to detect positive circumferential margins.78 High-resolution MRI can help identify patients who will have positive margins.79–81 In a retrospective analysis reported by Bail and colleagues,29 despite receiving preoperative chemoradiation, 504 patients with positive radial margins had a higher local recurrence rate (35% vs. 11%) and a lower 5-year OS (27% vs. 73%) compared with those with negative radial margins.
As reported with preoperative therapy, postoperative treatment has limited ability to control positive radial margins. In the MRC CR-07 trial, patients with positive radial margins who received postoperative chemoradiation had an 11% local recurrence rate.101 Likewise, in a subset analysis of the Dutch CKVO trial, 50 Gy postoperatively did not compensate for positive margins.77
Distance from the Anal Verge
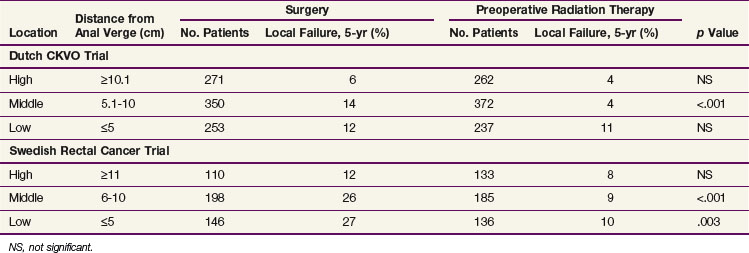
There are no prospective randomized data examining the impact of the distance from the anal verge on local recurrence. The available data are subset analyses from randomized trials that were not stratified by distance. Of the three trials, two used short-course preoperative irradiation and included patients with cT1-2N0 disease (Dutch CKVO75 and the Swedish Rectal Cancer Trial),73 whereas the German trial used chemoradiation and was limited to patients with cT3N+ disease.26,102 The MRC-C07 trial made a similar subset analysis, and the distance from the anal verge was determined by rigid sigmoidoscopy. There were additional variables that could have contributed to the differences in local failure rates. For example, TME (and its associated impact on radial margins) was used in the Dutch CKCO and German trials and not in the Swedish trial. All three trials included patients with tumors more than 12 cm from the anal verge in the “upper or high” category. Since the peritoneal reflection varies from 12 to 16 cm, some patients with tumors above the peritoneal reflection (colon cancer) were included in the three trials. Most investigators now limit preoperative treatment to tumors less than 12 cm from the anal verge.97 Lastly, distance measurements using a flexible proctoscope are less accurate than straight proctoscope measurements. Flexible scopes were used in the Dutch CKVO trial. The German trial used a straight scope. In the Swedish trial, proctoscopic information was not mentioned. However, eligibility was limited to tumors “below the promontory as identified by barium enema.” The Polish trial is not discussed, because all tumors were palpable by digital examination.103
As seen in Table 49-4, by univariate analysis, “high” tumors in both the Dutch CKVO and Swedish trials (defined as ≥10.1 cm and ≥11 cm, respectively) had a lower incidence of local recurrence compared with mid and lower tumors. Short-course irradiation did not significantly decrease local recurrence rates. By multivariate analysis, tumor location was an independent prognostic variable in the Dutch trial. It is interesting to note that irradiation did significantly decrease local recurrence rates for mid tumors in both trials, whereas for lower tumors it was helpful only in the Swedish trial.
In contrast, there was no significant difference between mid and upper tumors in the German trial.104 However, data were not provided. In a recent subset analysis, patients with tumors above 6 cm had a lower local recurrence rate (Rödel, personal communication).
In summary, given the conflicting data combined with the report from Guillem and associates97 confirming that the incidence of positive nodes (ypN0 disease following preoperative chemoradiation) is the same from 0 to 12 cm from the anal verge, treatment decisions based on the current definitions of low versus mid versus high should not be used.
Sphincter Preservation with Preoperative Chemoradiation
An analysis of 1316 patients who received intensive short-course preoperative irradiation revealed that downstaging was most pronounced when the interval between the completion of irradiation and surgery was at least 10 days.105 In the Dutch CKVO 95-04 trial, where the interval was 1 week, there was no downstaging.106
When the goal of preoperative therapy is sphincter preservation, conventional irradiation doses and techniques are recommended. These include multiple-field techniques to a total dose of 45 to 50.4 Gy at 1.8 Gy per fraction. Surgery should be performed 4 to 8 weeks following the completion of irradiation. Unlike the intensive short course of irradiation regimen, this conventional design allows for two important events to occur. First is the recovery from the acute side effects of irradiation, and second is adequate time for tumor downstaging. Data from the Lyon R90-01 trial of preoperative irradiation suggest that an interval of longer than 2 weeks following the completion of irradiation increases the chance of downstaging.107 Most series recommend a 4- to 8-week interval.108–110 Whether increasing the interval between the end of intensive short-course irradiation and surgery to more than 4 weeks will increase downstaging is not known. This question is being addressed in the ongoing Stockholm III trial.
Clinical Experience with Sphincter Preservation
A valid concern of surgeons is that to perform sphincter preservation in those patients who would otherwise require an APR, the distal resection margin may be suboptimal (<1 cm). Can preoperative therapy compensate for this? Retrospective data from the Memorial Sloan-Kettering Cancer Center reveal that with preoperative chemoradiation, the 3-year local control rates were similar regardless of the margins being more than 2 cm, less than 2 cm, more than 1 cm, or less than 1 cm, providing they were negative.78,111 Similar data have been reported from other investigators.112
Sphincter preservation without good function is of questionable benefit. In a series of 73 patients who underwent surgery, Grumann and associates113 reported that the 23 patients who underwent an APR had a more favorable quality of life compared with the 50 who underwent an LAR. Krouse and colleagues114 reported that both men and women with ostomies had significantly worse social well-being compared with those without ostomies, however.
Although preoperative chemoradiation may adversely affect sphincter function,115 the impact is most likely less than postoperative chemoradiation.116 Most phase II trials that examine functional outcome report good to excellent results. Functional results continue to improve up to 1 year after surgery. Functional data from the German trial are pending.
Is Surgery Needed after Preoperative Chemoradiation?
In one series, the value of radical surgery in patients who had a biopsy-proven complete response was questioned.117 The trial included patients with cT1-3 disease, however, and has not been reproduced by other investigators. In series limited to patients with cT3 disease who received preoperative chemoradiation, radical surgery is still necessary to fully evaluate whether a pathologic response has been achieved. Neither post-treatment ultrasound41,42 nor physical examination (which is only 25% accurate)118 is sufficient. The use of PET scanning119,120,34 and diffusion MRI121 as noninvasive measures of response is being investigated, and reported results have been mixed. Although Kalff and associates35 reported that postchemoradiation FDG-PET identification of residual viable tumor in 63 patients had a high positive predictive value (0.94; 95% CI, 85% to 99%), other groups have reported opposite results.
Glynne-Jones and colleagues122 reviewed 218 phase II and 28 phase III trials of preoperative irradiation or chemoradiation and confirmed that a clinical and/or radiologic response does not sufficiently correlate with the pathologic response; they do not recommend a “wait and see” approach to surgery following preoperative therapy.
Randomized Trials of Preoperative Versus Postoperative Chemoradiation
Two randomized trials of preoperative versus postoperative chemoradiation for clinically resectable rectal cancer have been performed, NSABP R0-3123 and the German CAO/ARO/AIO 94 trial.26
The German trial completed the planned accrual of over 800 patients and randomized patients with uT3-4 and/or LN+ rectal cancers less than 16 cm from the anal verge to preoperative chemoradiation (with CI 5-FU during weeks 1 and 5) versus postoperative chemoradiation.26 Patients were stratified by surgeon. Compared with postoperative chemoradiation, patients who received preoperative therapy had a significant decrease in rates of local failure (6% vs. 13%; p = .006), acute toxicity (27% vs. 40%; p = .001), and chronic toxicity (14% vs. 24%; p = .012), and in the 194 patients judged by the surgeon before treatment to require an APR, there was a significant increase in rates of sphincter preservation (39% vs. 20%; p = .004). With a median follow-up of 40 months, there was no difference in 5-year OS (74% vs. 76%). A subsequent analysis revealed that the treatment center, schedule, and gender were independent prognostic factors for local control.124
The NSABP R-03 study accrued only 267 of a planned 900 patients with cT3-4 rectal cancers.123 Patients received induction chemotherapy followed by conventional chemoradiation and were randomized to receive it either preoperatively or postoperatively. TME was not required, and some patients underwent local excision. Patients who received preoperative versus postoperative therapy had a significant improvement in 5-year DFS (65% vs. 53%; p = .011) and a borderline significant improvement in 5-year OS (75% vs. 66%; p = .065). There was no difference in 5-year local recurrence rates (11%). There was a correspondingly higher incidence of grade 4+ toxicity, almost all related to early diarrhea (33% vs. 23%), but the incidence of grade 3+ toxicity was lower (41% vs. 50%). Lastly, based on a prospective office assessment by the operating surgeon, there was no improvement in rates of sphincter preservation (48% vs. 39%). The results do not parallel the German trial, likely because of the fact that only 267 of the 900 planned patients were accrued, thereby limiting the statistical power to detect differences.
Given the improved local control rates, acute and long-term toxicity profiles, and sphincter preservation rates reported in the German trial, patients with cT3-4 rectal cancer who require chemoradiation should receive it preoperatively. In the German trial, 18% of patients who were clinically staged as cT3N0 preoperatively and underwent surgery without preoperative therapy had pT1-2N0 disease. Therefore, those patients would have been overtreated if they had received preoperative therapy. Although not ideal, this is preferred to performing surgery first because 20% to 40% will have LN+ disease at the time of surgery and require postoperative chemoradiation, which has inferior rates of local control, higher rates of acute and chronic toxicity, and, if a low anastomosis is performed, inferior functional results.97
Clearly, the development of more accurate methods to identify LN+ disease, including imaging techniques and/or molecular markers, is essential because more patients are being treated with preoperative chemoradiation.44,125–128
The UK Medical Research Council trial (MRC C07) randomized 1350 patients with clinical stage I to III rectal cancer to preoperative irradiation of 5 Gy in five fractions or selective postoperative chemoradiation (45 Gy with concurrent 5-FU), which was delivered only to patients with a histologic CRM of less than 1 mm (12% of all patients with immediate surgery).130 With a median follow-up of 4 years, patients who received preoperative compared with selective postoperative treatment had significantly lower 3-year local recurrence rates (4.4% vs. 10.6%; p <.0001) and higher 3-year DFS (77.5% vs. 71.5%; p = .013).
Short-Course Preoperative Irradiation Versus Standard-Course Preoperative Chemoradiation
Bujko and colleagues103,129 performed a randomized trial of two preoperative approaches. A total of 316 patients with cT3 rectal cancer were randomized to 5 Gy in five fractions followed by surgery (median, 8 days) versus conventional preoperative chemoradiation (50.4 Gy plus bolus 5-FU/leucovorin daily for 5 days, during weeks 1 and 5) followed by surgery (median, 78 days). All tumors were above the anorectal ring; TME was performed for distal tumors; and there was no irradiation quality control review. The incidence of CRM positivity was lower following chemoradiation compared with irradiation alone (4% vs. 13%; p = .017).
Standard and Novel Combined-Modality Therapy Regimens and Issues
The NCCTG 85-47-51 postoperative adjuvant rectal trial revealed a 10% survival benefit for patients who received concurrent PVI versus bolus 5-FU.86 Therefore, when 5-FU is combined with irradiation, either preoperatively or postoperatively, it should be delivered as a continuous infusion (CI). Although a survival benefit with CI 5-FU was not confirmed in the Intergroup 0144 trial, the CI 5-FU arm was associated with a lower incidence of hematologic toxicity.90,131 Although it is just now being studied in rectal cancer, the combination of CI 5-FU and oxaliplatin (FOLFOX) has replaced CI 5-FU as a standard postoperative chemotherapy treatment based on the efficacy demonstrated in stage III colon cancer patients.132
As an alternative, based on the X-ACT trial, which reported equivalence with the Mayo Clinic regimen in patients with stage II and III colon cancer, it is reasonable to substitute capecitabine for 5-FU.133 However, capecitabine has not been directly compared with CI 5-FU, and this is one of the end points of the NSAPB R-04 rectal trial.
The Cancer and Leukemia Group B (CALGB) 89803 trial illustrated that the survival benefit of irinotecan in patients with metastatic disease does not necessarily translate into the adjuvant setting.134 In contrast, based on the MOSAIC trial, which revealed a survival benefit with FOLFOX for node-positive colon cancer, oxaliplatin-based chemoradiation programs have been investigated. Many phase I and II trials have shown higher pCR rates compared with 5-FU-based chemoradiation regimens.
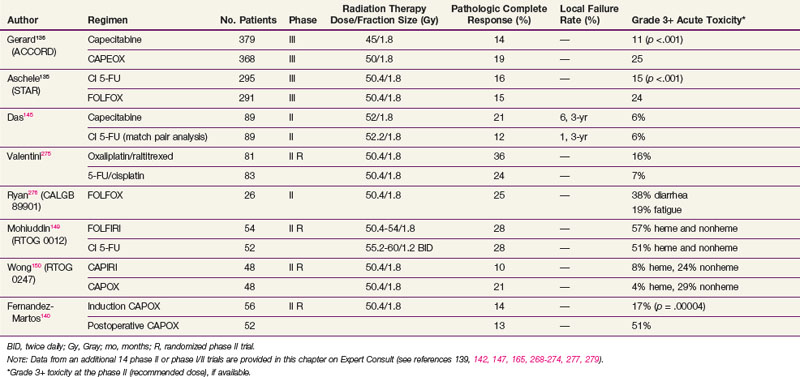
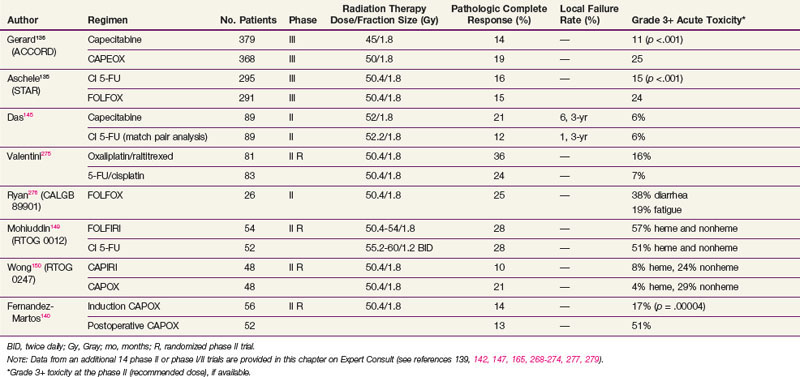
However, two phase III trials have reported significantly higher acute toxicity rates with no benefit in the pCR rate with the addition of oxaliplatin to CI 5-FU-based chemoradiation regimens in patients with cT3-4N+ rectal cancer (see Table 49-5). The STAR-01 trial randomized 747 patients to preoperative chemoradiation with 50.4 Gy plus CI 5-FU with or without oxaliplatin (60 mg/m2 weekly).135 There was a significant increase in grade 3+ toxicity with oxaliplatin (24% vs. 8%; p <.001) with no improvement in the pCR rate (15% vs. 16%). The ACCORD trial randomized 598 patients to preoperative chemoradiation with 50 Gy plus CAPOX versus 45 Gy plus capecitabine.136 There was a similar significant increase in grade 3+ toxicity with oxaliplatin (25% vs. 11%; p <.001) with no improvement in the pCR rate (19% vs. 14%). Although local control and survival outcomes are pending, these early results underscore the importance of phase III data.
Most preoperative chemoradiation regimens have been developed somewhat empirically without clear criteria for timing of the irradiation. For example, in a retrospective analysis, Yu and colleagues137 reported a significantly improved pCR rate when capecitabine was delivered 1 hour before irradiation compared with other intervals (24% vs. 10%; p = .01). Most phase I to II chemoradiation trials do not control for this variable.
Both cytotoxic and targeted chemotherapeutic agents have been incorporated into phase I and II combined-modality programs, most commonly in the preoperative setting. Selected agents (alone or in combination with other agents) include tegafur-uracil (UFT), raltitrexed, oxaliplatin, irinotecan, gefitinib, tegafur-oteracil-gimeracil (S-1), bevacizumab, cetuximab, and capecitabine with pelvic radiation therapy. Selected series are seen in Table 49-5 (expanded version of Table 49-5 available on the Expert Consult website![]() ). Most of the regimens reveal higher pCR rates compared with 5-FU alone (10% in the German trial). For some agents, however, with this increased pCR rate is an associated increase in acute toxicity rates. Phase III trials are needed to determine if these regimens offer a local control or survival advantage compared with 5-FU or capecitabine chemoradiation regimens.
). Most of the regimens reveal higher pCR rates compared with 5-FU alone (10% in the German trial). For some agents, however, with this increased pCR rate is an associated increase in acute toxicity rates. Phase III trials are needed to determine if these regimens offer a local control or survival advantage compared with 5-FU or capecitabine chemoradiation regimens.
Only one randomized phase III trial, performed by the Hellenic Cooperative Oncology Group, compared concurrent postoperative chemoradiation with 5-FU/folinic acid plus irinotecan versus 5-FU/folinic acid alone.138 There were no differences between the arms in rates of 3-year OS, DFS, and local relapse-free survival, whereas the incidence of severe leukopenia was significantly higher in the irinotecan-containing arm.
Chua and colleagues139 have examined the use of induction CAPOX followed by chemoradiation with capecitabine. This approach circumvents the need for the 4 months of postoperative chemotherapy. Their pilot trial of 77 patients reported a 24% pCR rate. Since there is a 6-month interval between diagnosis and surgery, the radiologic response rate was followed by MRI. After induction CAPOX, the overall response rate was 88%, which increased to 97% following the completion of chemoradiation, suggesting that there was no detriment in response rates. Based on these encouraging results, the Spanish GCR-3 randomized phase II trial was developed comparing this approach with conventional preoperative chemoradiation followed by surgery and postoperative chemotherapy.140 A total of 108 patients received preoperative treatment with 50.4 Gy plus CAPOX and were randomized to receive 4 months of CAPOX either by induction or adjuvant (postoperative) administration. Although the pCR rates were not different (14% vs. 13%), the rate of grade 3+ toxicity was lower (17% vs. 51%; p = .00004) and the rate of ability to receive all four chemotherapy cycles was higher (93% vs. 51%; p = .0001) with the induction approach.
Choice of Adjuvant Chemotherapy
In colon cancer, the results of chemotherapy trials in metastatic disease cannot be extrapolated to the adjuvant setting. For example, trials using chemotherapeutic agents such as irinotecan and bevacizumab were positive in the metastatic setting but did not improve survival rates when tested in the adjuvant setting (CALGB 89803134 and NSABP C-08,141 respectively).
FOLFOX has replaced bolus 5-FU/leucovorin as a standard postoperative chemotherapy treatment.87 For patients who receive preoperative chemoradiation and are selected to receive postoperative adjuvant chemotherapy, four cycles of mFOLFOX6 is recommended.
Chemoradiation Using Targeted Therapies
The role of targeted biologic agents such as bevacizumab is the subject of ongoing clinical trials. Preliminary phase I trials from Duke University142 and the M.D. Anderson Cancer Center143 using preoperative chemoradiation with CAPOX plus bevacizumab reveal pCR rates of 18% and 24%, respectively. There is one report of three patients who received irradiation and when receiving bevacizumab 6 to 16 months later developed ischemic bowel complications.144 These data underscore the caution needed when combining new agents with radiation therapy.
Phase I to II trials examining the addition of cetuximab to preoperative chemoradiation have had mixed results. Although the report from Heidelberg with CAPEIRI reported a pCR rate of 25%,145 other trials with 5-FU, capecitabine, or CAPOX have more limited rates of 5% to 12%.146,147 Whether the benefit of patient selection based on wild versus mutated KRAS seen in patients with metastatic disease will be helpful in the adjuvant rectal setting is unknown, but it seems likely that the same biologic mechanisms will hold true.143
In the metastatic setting, the addition of a second targeted therapy (panitumumab) to bevacizumab combined with cytotoxic chemotherapy (either irinotecan or oxaliplatin) had both higher toxicity rates and lower progression-free survival rates.148 Other phase III trials have shown a similar lack of improvement. Therefore chemoradiation regimens with more than one targeted agent have not been developed.
RTOG Phase II Randomized Preoperative Chemoradiation Trials
The RTOG had reported two phase II randomized trials. RTOG 0012 enrolled 106 patients who received preoperative chemoradiation with either CI 5-FU plus twice-a-day irradiation versus FOLFIRI plus conventional daily fractionated irradiation.149 Although the pCR rates were 26%, the grade 3+ toxicity rates were 42% and 55%, respectively. Neither of these preoperative regimens was moved into phase III trials.
In a more recent trial, the RTOG compared preoperative chemoradiation with CAPEIRI versus CAPOX in 101 patients with cT3-4 disease (RTOG 0247).150 Although not statistically significant, patients who received CAPOX had a higher pCR rate (21% vs. 10%) with similar hematologic (4% vs. 8%) and nonhematologic toxicity rates (29% vs. 24%).
Impact of Tumor Response to Preoperative Chemoradiation
Although some series show no correlation,110 most series suggest that there is an improved outcome with increasing pathologic response to preoperative chemoradiation.151,152 A retrospective review of 566 patients who achieved a pCR after receiving a variety of preoperative chemoradiation regimens at multiple European centers was reported by Capirci and associates.153 With a median follow-up of 46 months, the local recurrence rate was only 1.6% and 5-year DFS and OS were 85% and 90%, respectively.
Analysis of biopsies examining selected molecular markers154–156 have had varying success in helping to select patients who may best respond to preoperative therapy. In a recent review, Kuremsky and colleagues157 identified 1204 articles examining a total of 36 molecular biomarkers that may have predictive value. Restricting the analysis to patients treated with preoperative chemoradiation and to gene products examined by five or more studies, only TP53, epidermal growth factor receptor (EGFR), thymidylate synthase, Ki-67, P21, and BAX/BCL-2 met these criteria. Of these, quantitatively evaluated EGFR or EGFR polymorphisms, thymidylate synthase polymorphisms, and P21 have been identified as promising candidates that should be evaluated in larger prospective trials for their ability to guide preoperative therapy. Because the studies are limited retrospective trials and most do not examine multiple markers, the need for adjuvant therapy should still be based solely on T and N category.
Konski and associates158 performed pretreatment and post-treatment 18FDG-PET scans on 53 patients receiving preoperative chemoradiation. By multivariate analysis, the percentage of decrease in SUV was marginally significant in predicting pCR (p = .07).
The pCR rate may provide an alternative endpoint for assessing the efficacy of novel preoperative chemoradiation regimens. Because patients with rectal cancer who receive adjuvant chemoradiation can develop late relapses, however, a minimum follow-up of 7 years is needed.88 Current intergroup rectal trials prospectively collect tissues for these and other markers.
Alternative Methods for Sphincter Preservation
Endocavitary Irradiation
Endocavitary irradiation alone159–161 has been used for early, noninvasive tumors. For more advanced tumors (cT2-3 and/or LN+), it is combined with a temporary iridium-192 (192Ir) implant and/or pelvic irradiation.67,162–164 This technique is also known as the Papillion technique. Before delivery, the anus is dilated and a 4-cm proctoscope is introduced. A low-energy x-ray unit is placed through the scope almost against the tumor. Generally, 50-kV x rays are delivered at 30 Gy per fraction in three or four fractions over 1 month. Patients who develop local failure can successfully undergo surgical salvage.166
Clinical outcomes with endocavitary irradiation alone or combined with EBRT have been reported. At the Mayo Clinic, 29 patients were treated with curative intent with endocavitary irradiation alone to a total dose of up to 155 Gy in one to five fractions. The local control rate was 76% at 10 years, and OS was 65% at 5 years and 42% at 10 years.159 At Washington University, patients received pelvic irradiation (20 Gy in 5 fractions for those with cT1; the remainder received 45 Gy in 25 fractions) followed 6 to 7 weeks later by two endocavitary treatments of 30 Gy each.164 Results by stage were uT1, 100% DFS; uT2, 85% local control rate; and uT3 (patients with uT3 tumors were not optimal candidates for surgery) or tethered uT2, 56% local control rate (67% after salvage surgery). Gerard and associates166 have reported a similar experience in 44 patients. Because the 50-kVp radiation machine is not available, there are limited centers that continue to treat patients with contact irradiation.
Local Excision with or without Radiation Therapy
Local excision has been performed both before and after radiation therapy. The advantage of performing a local excision before irradiation is that pathologic details can be well characterized. Highly selected patients with pT1 tumors without adverse pathologic factors have local failure rates of 5% to 10%. However, once adverse pathologic factors are present (high grade, vascular invasion, or signet-ring cells) or the tumor invades into or through the muscularis propria, the local failure rate is at least 17% and the incidence of positive mesorectal and/or pelvic nodes is at least 10% to 15%.167 Nash and associates168 reported that in 145 patients who underwent radical surgery for cT1N0 disease, 20% were found to have pathologically positive nodes.
There are a variety of surgical approaches, including transanal local excision, posterior proctotomy, and transsphincteric excision. Transanal endoscopic microsurgery (TEM) has emerged as another option for local treatment of rectal cancer, either alone for T1 tumors or combined with irradiation for selected patients with T2-3 disease.169 Regardless of the technique, the excision should be full thickness and nonfragmented and should have negative margins.170
Local Excision Followed by Postoperative Therapy
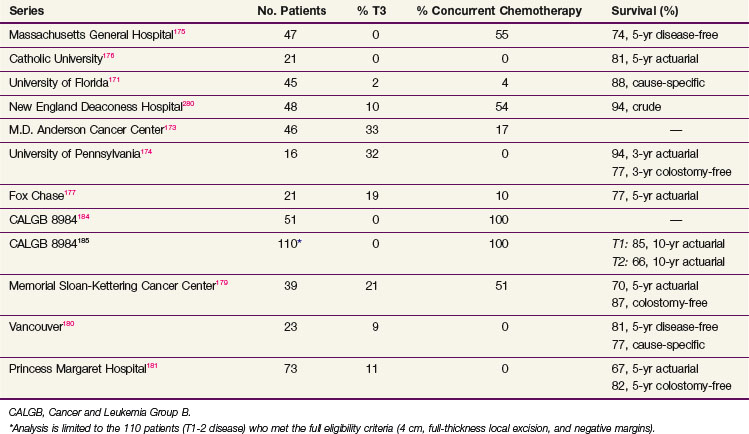
When all series are combined, the average crude local failure rate after local excision followed by postoperative radiation increases with T stage: pT1, 5%; pT2, 14%; and pT3, 22%.171–181 However, when the analysis is limited to the series with a minimum of 4 years of follow-up,175,178,181,182 the incidence of local recurrence for pT2 disease is 14% to 24% (Table 49-6). Therefore patients who are treated with local excision and postoperative adjuvant therapy require close follow-up beyond 5 years. Surgical salvage is possible, with most series reporting that approximately half of the patients who undergo an APR can be cured.183
The CALGB performed a phase II trial of local excision and selective postoperative chemoradiation (CALGB 8894).184 A total of 91% underwent a full-thickness local excision. Patients with pT1 disease were observed, and pT2 patients received postoperative treatment with 54 Gy plus concurrent 5-FU. With a median follow-up of 4 years, the local recurrence rate in 59 patients with pT1 disease was 5% and with pT2 disease was 14%.
A separate analysis of the 110 patients who met the full eligibility criteria in CALGB 8894 was reported by Greenberg and colleagues.185 These were limited to tumors less than 4 cm, full-thickness excision, and negative margins. Patients with pT1Nx tumors underwent local excision alone, and those with pT2Nx received postoperative chemoradiation. With a median follow-up of 7.1 years, the rates of local recurrence and 10-year actuarial survival were T1, 8% and 84%, and T2, 18% and 66%, respectively. The median time to failure was T1, 4 years, and T2, 2 years.
Sphincter Function
Prospective assessment of functional results after local excision and radiation therapy is limited. The groups from the Memorial Sloan-Kettering Cancer Center179 and Gemelli Hospital in Rome176 report rates of 94% and 100%, respectively, for good to excellent function. Using a different scale, investigators from the Fox Chase Cancer Center177 reported an 82% rate of good to excellent function; the University of Pennsylvania174 reported a 92% rate of satisfactory function; and the M.D. Anderson Cancer Center173 reported that all patients were continent. In the preoperative setting, sphincter function was reported as good to excellent in 88% to 91%.186,187
Preoperative Therapy Followed by Local Excision
Experience with preoperative chemoradiation followed by local excision is more limited.186–192 Most series selected patients with cT3 disease who were either medically inoperable or refused radical surgery. Local recurrence rates ranged from 0% to 20% and 5-year OS ranged from 78% to 90%. Borschitz and associates192 reported local recurrence rates by pathologic stage as ypT1, 2%, and ypT2, 6% to 20%. Rates were as high as 43% in ypT3 tumors that did not respond to chemoradiation.
Chemotherapy
There are limited data examining the use of chemotherapy in patients who undergo local excision and preoperative or postoperative irradiation. In most series, concurrent 5-FU was delivered as a radiosensitizer rather than in the adjuvant setting. However, given the positive impact of chemotherapy on local control and survival rates in patients with resectable rectal cancer reported in the randomized postoperative rectal adjuvant trials,131 all patients should receive two cycles of 5-FU–based therapy concurrently with irradiation. For patients with pT2-3 disease where the incidence of pelvic lymph nodes is at least 20%, an additional four cycles of adjuvant chemotherapy for a total of six cycles is recommended.
Locally Advanced Disease and Palliation
Standard Treatment
With the exception of the uncommon suture line-only recurrence, patients with locally unresectable primary or recurrent disease should receive preoperative chemoradiation. Braendengen and colleagues193 randomized 207 patients with locally nonresectable T4 primary rectal carcinomas or local recurrence from rectal carcinomas to chemotherapy (5-FU/leucovorin) concurrent with 50 Gy plus postoperative adjuvant therapy for 16 weeks versus 50 Gy alone. Patients who received chemoradiation had a higher R0 resection rate (84% vs. 68%; p = .009), pCR rate (16% vs. 7%), and 5-year local control rate (82% vs. 67%; p = .03) but a nonsignificant improvement in the OS (66% vs. 53%; p = .09).
Approximately 10% of rectal cancers require extensive surgery such as a pelvic exenteration to obtain negative margins.194 These include tumors invading the prostate, the base of the bladder, or the uterus and vagina where the disease can be resected en bloc with negative margins. Midline posterior tumors adherent to or invading the distal sacrum may be resectable for cure with APR extended to include the sacrum. The 5-year OS range between 33% and 50%, with significant rates of morbidity and mortality up to 6%.195 Improvements in perioperative care, patient selection, and surgical technique, such as vascularized tissue flaps to facilitate the healing of pelvic and perineal wounds, have improved the results.196
Extended surgery to obtain negative margins is still recommended even if there is a favorable response after preoperative therapy. Given the limitation of the total EBRT dose that can be delivered to the bulky tumor in the pelvis195 and the frequent problem of local recurrence, the surgeon should be aggressive.197
Tethered cancers have the most favorable outcome of all cT4 cancers. In a separate report from the Massachusetts General Hospital, the results of 28 patients with tethered rectal cancers treated with preoperative irradiation were presented.198 Although a complete resection with negative margins was possible in 93%, the local failure rate was 24%. Tobin and colleagues199 report a local failure rate of 14% and 5-year OS of 68% in 49 patients with tethered cancers treated with preoperative irradiation. The preoperative chemoradiation series do not report the results of cT4 tethered cancers separately.
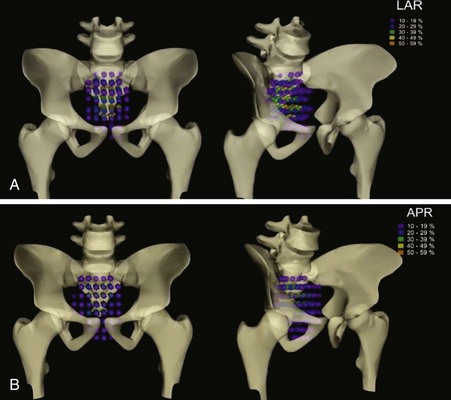
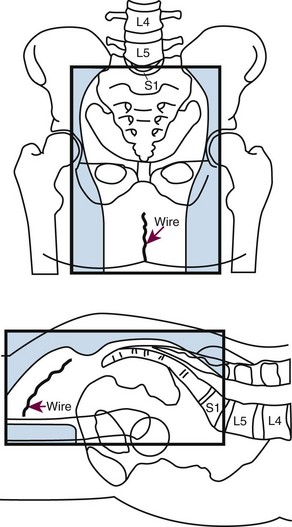
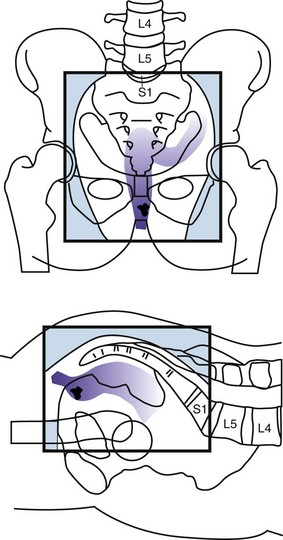
Intraoperative Irradiation (IORT)
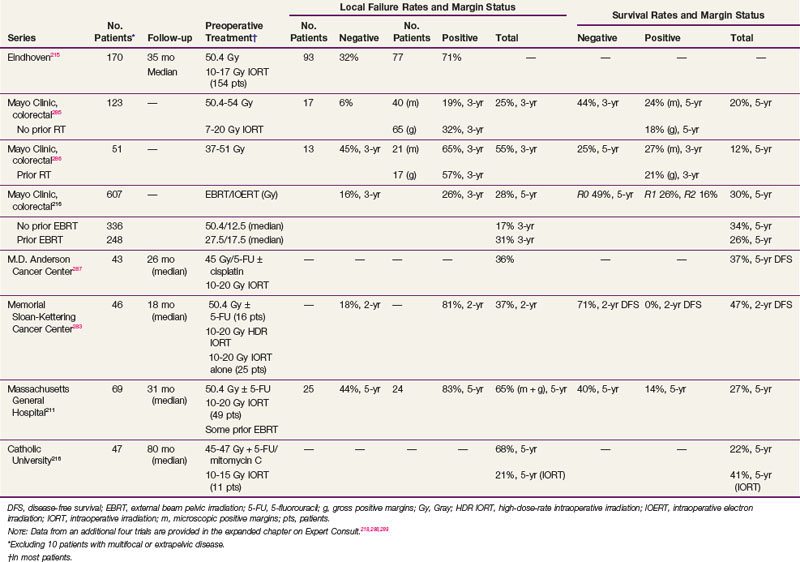
The primary advantage of IORT is that radiation can be delivered at the time of surgery to the site with the highest risk of local failure (the tumor bed) while decreasing the dose to the surrounding normal tissues. IORT can be delivered by two techniques: electron beam or brachytherapy. Brachytherapy is most commonly delivered by the high-dose-rate technique, and the dose rate is similar to that used for electron beam IORT.200,201,202 The results (and recommended dose) of IORT depend on whether the patient has primary unresectable or recurrent disease and on whether the margins of resection are negative or there is microscopic or gross residual disease. In general, series have used 10- to 20-Gy IORT depending on the volume of residual disease. For example, at the Mayo Clinic, patients with locally unresectable primary cancers receive 7.5 to 10 Gy after R0 resection with narrow margins, 10 to 12.5 Gy after R1 resection, and 15 to 20 Gy after R2 resection.203
The incidence of IORT-related toxicity increases with IORT doses of 15 to 20 Gy. In a series from the Netherlands, 79 patients surveyed reported fatigue (44%), perineal pain (42%), sciatic pain (21%), walking difficulties (36%), and voiding dysfunction (42%).204 In addition, functional impairment consisted of requiring help with basic activities (15%), sexual inactivity (56%), the loss of former lifestyle (44%), and the loss of professional occupation (40%). The University of Navarra reported peripheral neuropathy up to 5 years after IORT.205 These consequences must be weighed against the chance of cure if the patient is treated and the disability eventually caused by uncontrolled tumor progression if the patient is not treated.
The long-term morbidity in the Mayo Clinic IORT series of 146 patients with locally unresectable colorectal cancers was 53% and included neuropathy (in 28 of 146 patients, or 19%), small bowel obstruction (14%), and ureteral obstruction (12%).203 Severe toxicity (grade 3 or 4) was evident in 32 of 146 patients (22%), with small bowel obstruction in 14 patients (9.5%) and severe neuropathy in only 3 (2%).
Although sphincter preservation is a major goal for patients with primary resectable disease, this should be a lesser goal for patients with locally recurrent cancers. In view of the extensive surgery required for patients with local recurrence and the likelihood of high-dose EBRT (prior adjuvant EBRT, repeat low-dose preoperative EBRT), patients may have a better functional outcome with a permanent colostomy than with coloanal anastomosis or a very low anterior resection.206
Primary Unresectable Disease
Results of selected series with or without IORT are seen in Table 49-7. The results of the series from Rotterdam,284 Madrid,207 and Eindhoven139 include patients with both cT3 and cT4 disease and do not report the data separately. However, the Rotterdam and Eindhoven series examined outcome and did not find a significant difference. The overall incidence of local failure is approximately 10% to 15%, and central failure in the IORT field is uncommon (i.e., 2% in the Mayo Clinic IORT series of 146 patients with locally unresectable colorectal cancer).203
TABLE 49-7 Primary Locally Advanced/Unresectable Rectal Cancer with or without Intraoperative Radiation Therapy (IORT): Selected Series
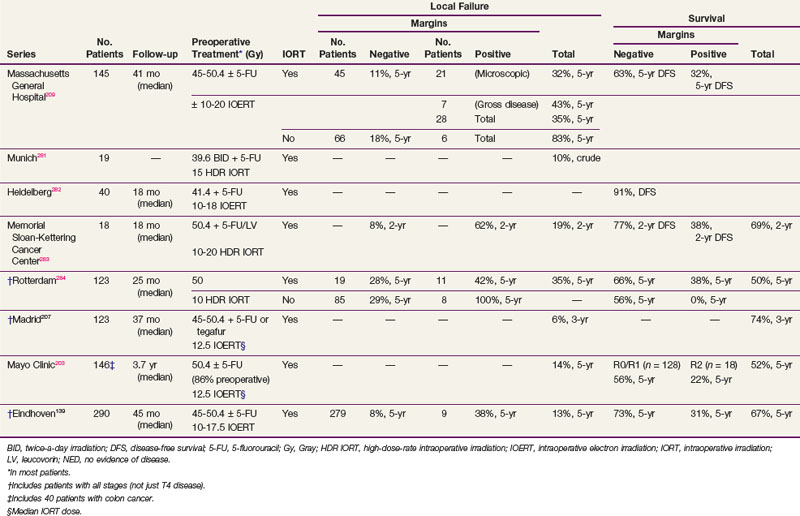
The most favorable results are obtained in patients with gross total resection and negative margins (R0 resection) or microscopically positive margins (R1 resection). The 5-year local recurrence and OS in the Eindhoven IORT series were R0, 8% and 73%, and R1-2, 38% and 31%, respectively. Valentini and colleagues208 treated 100 patients with primary, clinical, T4M0, extraperitoneal rectal cancer with preoperative chemoradiation with or without IORT. The R0 resection rate was 78%. The 5-year local control rates in R0 patients were 90% and 100% in the IORT group. The 5-year OS was 59%, and was better after an R0 versus an R1-2 resection (68% vs. 22%). Although the numbers are small, in the Rotterdam series, the 5-year local recurrence rate was lower in the 11 patients with positive margins who received IORT compared with the 8 who did not (58% vs. 100%; p = .016).
In the Mayo Clinic IORT series of 146 colorectal patients, R0-1 resection was accomplished in 128 patients and R2 resection in 18. Those with IORT after R0-1 resection had better 5-year OS than those with R2 resection (56% vs. 22%; p = .0006).203
In the Massachusetts General Hospital (MGH) series of 145 rectal patients,209 local failure in patients with negative margins decreased from 18% without IORT to 11% with IORT. In patients with positive margins, local failure was 83% without IORT versus 43% with IORT for gross residual disease and 32% with IORT for microscopic residual disease. For all patients in the series (with or without IORT), the 5-year DFS was 63% for patients with negative margins and 32% for patients with positive margins. These results underscore the importance of delivering preoperative therapy to help achieve negative margins. If negative margins cannot be obtained, then microscopic residual disease is preferable to gross residual disease. Reports from other centers (see Table 49-7) are similar.
At the MGH, of the 95 patients with T4 disease who received preoperative irradiation and underwent complete resection, 40 patients had an IORT boost and 55 did not, because it was not indicated secondary to either a favorable response or it was not technically feasible.210,211 Regardless of the response to preoperative therapy, higher local failure rates were seen in patients not receiving IORT (responders, 0% vs. 16%; nonresponders, 12% vs. 27%) These data suggest that IORT should be delivered independently of the extent of tumor downstaging.
Locally Recurrent Disease
At the University of Wurzburg, sites of failure were analyzed in 155 patients.212 The incidence of failure sites was similar for APR versus LAR: local plus nodal disease, 61% versus 66%; isolated lymph node disease, 4% versus 5%; internal iliac and presacral node disease, 47% versus 59%; and external iliac disease, 7% versus 2%. Local recurrence was most commonly seen in the presacral pelvis and in patients who underwent an LAR; the anastomosis was involved in 93%.
Recurrences can be heterogeneous, and the pattern of extension is more infiltrative within the operative bed compared with primary rectal cancers. Localized pelvic recurrences may be classified according to the tumor location within the pelvis. At the Mayo Clinic, 106 patients with local recurrence, treated by subtotal resection alone or plus IORT (42 patients) and/or EBRT, were stratified during the surgical procedure according to no pelvic infiltration of the tumor (F0) or infiltration of the tumor to one pelvic site (F1), two pelvic sites (F2), or more than two pelvic sites (F3).213 This classification system significantly correlated with survival. At the Catholic University of the Sacred Heart, Rome, 47 patients with locally recurrent, nonmetastatic rectal carcinoma were treated by preoperative chemoradiation plus IORT and were classified by CT scan according to the Mayo Clinic system.214 A further (F4) class was added when tumor infiltrated small bowel or bone structures. The classification system significantly predicted R0 resectability (p = .01) and survival (p = .008).
Results in selected series with or without IORT are seen in Table 49-8. As seen in patients treated for primary unresectable disease, the margin status and delivery or nondelivery of IORT have an impact on outcome for patients with locally recurrent disease.
In the MGH IORT series of 49 patients, the overall 5-year local control rate was 35% and was higher with negative versus positive margins (56% vs. 13%).211 The 5-year OS for the total group was 27% and was higher in patients with negative versus positive margins (40% vs. 12%).
Kusters and colleagues215 from Eindhoven treated 170 patients with recurrent rectal cancer with preoperative irradiation with or without chemotherapy followed by surgery. The local recurrence rate in patients with negative margins was 32% and with positive margins was 71%. The 154 patients selected to receive IORT had a local recurrence rate of 47%. Consistent with other trials, patients with posterior (presacral) recurrences were more likely to have positive margins than those with anastomotic recurrences (72% vs. 23%) and the 5-year OS was only 19%.
The most recent Mayo Clinic Rochester analysis included 607 recurrent colorectal cancer patients receiving IOERT as a component of treatment (colon, 180 patients; rectum, 427 patients).216 Survival at 5 years was 30% for the total group of patients. On multivariate analysis, complete resection, no prior chemotherapy, and treatment after 1996 were associated with improved survival rates. In patients with an R0 resection (227 patients) versus an R1 resection (224 patients) or R2 resection (156 patients), 5-year OS were 46%, 27%, and 16% (p <.0001, multivariate analysis), respectively.
Investigators at the M.D. Anderson Cancer Center and in Oslo reported no benefit of IORT for patients with positive margins.217,218 In the report from Oslo, 107 patients with isolated pelvic recurrence received 46 to 50 Gy preoperatively followed by resection alone or plus IORT.218 Regardless of the volume of residual disease, there was no significant difference in local recurrence or survival rates whether or not patients received IORT.
Reirradiation Followed by Surgery
Few studies have analyzed the role of radiation retreatment in pelvic recurrence. Data from Mohiuddin and colleagues219 suggest that reirradiation with doses of 30 Gy (and if the small bowel can be excluded from the irradiation field, 40 Gy) can be used for limited volumes. A total of 103 patients with recurrent disease underwent reirradiation with concurrent 5-FU-based chemotherapy. The initial radiation dose to the pelvis ranged from 30 to 74 Gy, with a median dose of 50.4 Gy. Irradiation techniques consisted of two lateral fields with or without a posterior pelvic field to include recurrent tumor with a margin of 2 to 4 cm. Doses ranged from 15 Gy to 49.2 Gy (median, 34.8 Gy). After reirradiation, 34 underwent surgical resection for residual disease. For the total group, median survival was 26 months and 5-year OS was 19%. Patients who underwent resection had significantly higher median survival times (44 months vs. 14 months) and 5-year OS (22% vs. 15%) (p = .001). Late complications were seen in 22 patients and were unrelated to radiation dose.
A multicenter Italian trial of 59 patients with recurrent disease who had received less than 55 Gy were retreated preoperatively with concurrent CI 5-FU plus 30 Gy (1.2 Gy twice daily) to the gross tumor volume (GTV) plus a 4-cm margin.220 A boost was delivered, with the same fractionation schedule, to the GTV plus a 2-cm margin (10.8 Gy). Grade 3+ toxicity was 5% for acute toxicity and 12% for late toxicity. The prior median radiation dose was 50.4 Gy. The pCR rate was 9%, and 83% of those with pelvic pain before treatment had a symptomatic response. With a median follow-up of 36 months, the rate of local failure was 48%, median survival was 42 months, and 5-year OS was 39% (R0, 67% vs. R1-2, 22%). Multivariate analysis confirmed the impact of a longer DFS on local control (p = .016) and DFS (p = .002). Patients who underwent an R0 resection had improved local control and DFS (p = .016).
In the recent Mayo Clinic IORT analysis of 607 patients with locally recurrent colorectal cancer, 248 had received prior EBRT.216 Additional EBRT was given in 228 patients (median, 27.5 Gy; range, 5 to 39.6 Gy). Prior in-field EBRT versus none was associated with an increased risk of local relapse (3-year rate, 31% vs. 17%; p <.0001) and trends for decreased 5-year OS that did not reach significance (28% vs. 34%; p = .07). Mayo Clinic investigators prefer to give preoperative EBRT (25 to 30 Gy in 1.8-Gy fractions) plus concurrent CI 5-FU or oral capecitabine before resection and IOERT in this group of patients.
Irradiation Alone for Locally Unresectable or Recurrent Disease
Some investigators have advocated the use of radiation therapy alone, most commonly in patients who are medically inoperable or refuse surgery.162,221,222 A variety of techniques have been used, including various combinations of EBRT, brachytherapy, and intracavitary irradiation. In most series, patients received pelvic radiation therapy followed by a boost with EBRT and/or brachytherapy.
Brierley and colleagues223 from the Princess Margaret Hospital reported the results of EBRT alone (40 to 60 Gy) in patients who refused surgery or had unresectable or medically inoperable disease. The 5-year OS was 27% for all patients; if the primary tumor was mobile, the rate was 47%; if partially fixed, 27%; and if fixed, 4%. These data suggest that patients with mobile or partially fixed rectal cancers who are medically inoperable should be treated aggressively with pelvic irradiation as a component of their therapy.
Gerard and associates222 reported the combination of EBRT, intracavitary therapy, and brachytherapy in 63 patients with uT2-3 tumors. For patients with uT3 disease, the 5-year local failure and survival rates were 20% and 35%, respectively.
Pelvic irradiation offers effective palliation. In a subset of 80 patients with metastatic disease who received pelvic irradiation, Crane and coauthors224 reported that 94% had complete resolution of pelvic symptoms and the 2-year pelvic symptom-free control rate was 82%. The Princess Margaret Hospital series reported similar palliative benefits.223 In the subset of 84 patients who received more than 45 Gy, the following presenting symptoms were palliated by 6 to 8 weeks following the completion of radiation therapy: pain, 89%; bleeding, 79%; neurologic symptoms, 52%; mass effect, 71%; discharge, 50%; urologic symptoms, 22%; and other symptoms, 42%.230 In the Thomas Jefferson University series, complete plus partial symptomatic relief was achieved in the following categories: pain (65% + 28%), bleeding (100%), and mass effect (24% + 64%), respectively.219 The duration of palliation was 8 to 10 months. In patients who are unable to receive irradiation, laser treatment or stenting225 offers some palliative benefit.
Treatment of Patients with Metastatic Disease
With the development of more effective systemic chemotherapy, the median survival of patients with metastatic colorectal cancer now approaches 2 years.226 Aggressive techniques to address liver metastasis with surgery and nonsurgical ablative techniques such as radiofrequency ablation, cryosurgery, microspheres, and EBRT have further improved median survival.227 More innovative radiation techniques such as stereotactic body radiotherapy of liver metastases are under investigation.228 With these advances, the historical notion that chemoradiation is not necessary because patients die before developing a local recurrence is being challenged. In the setting of disease presentation with distant metastasis, there is no standard of care and treatment should be decided on a case-by-case basis with the help of a multidisciplinary tumor board. In general, patients who respond to chemotherapy and have a risk of developing local recurrence should be considered for treatment consolidation with chemoradiation.
Irradiation Techniques and tolerance
Patterns of Relapse that Define Radiation Portals
The relative frequency and sites of pelvic failures were delineated by the seminal work of Gunderson and Sosin.51 In this reoperative series of 75 patients (initial APR), locoregional failures occurred in the soft tissue of the pelvis or the anastomotic site in 69%, pelvic lymph nodes in 42%, and perineum in 25%. A more contemporary series of 269 patients studied by Hruby and colleagues229 confirmed that the majority of local failures occurred in the posterior central pelvis (47%) or at the anastomosis (21%), while anterior recurrences (11%) were mainly restricted to T4 tumors. Perineal recurrences occurred in 16% of patients who underwent APR. Figure 49-2 depicts sites and frequency of recurrences in a German series of 123 patients diagnosed with rectal cancer recurrences between 1998 and 2001.230
Irradiation Fields
The whole-pelvic radiation field should adequately cover the primary tumor and tumor bed as well as the primary nodes at risk. General guidelines for the design of pelvic radiation therapy fields are listed in Box 49-1. The intent of the boost is to treat the primary tumor and tumor bed and not to include the nodes. The exact size is determined by the size and location of the primary tumor. Whole-pelvic and boost fields are usually treated with a three-field (PA and laterals) or four-field technique. Field shaping by blocks is used to spare additional small intestine anteriorly and superiorly, the posterior muscle and soft tissue behind the sacrum, and the region inferior to the symphysis pubis. Specific examples of field arrangements with conventional techniques for a variety of presentations are seen in Figures 49-3 to 49-5.
Box 49-1 General Guidelines for Pelvic Radiation Therapy
Whole Pelvic Field
AP/PA
Lateral Fields
Three-Dimensional Treatment Planning and IMRT
Innovative techniques using three-dimensional (3D) treatment planning are being investigated. A major contribution of 3D treatment planning is the ability to plan and localize the target and normal tissues at all levels of the treatment volume and to obtain dose-volume histogram data. Examples of 3D and intensity-modulated radiation therapy (IMRT) treatment plans are seen in Figures 49-6 and 49-7, respectively.
Using a 3D planning system, Koelbl and associates231 found that in patients receiving postoperative EBRT, the use of the prone position plus a belly board decreased the small bowel volume treated versus the supine position. Guidelines for the definition and delineation of the clinical target volumes (CTVs) are now available from a number of investigators.232,233 Three-dimensional planned EBRT is desirable for patients who undergo reirradiation in order to limit the dose to previously irradiated critical structures.
The clinical utility of routine 3D and IMRT treatment planning techniques is being investigated.234,235 An analysis of 3D treatment planning techniques at the Massachusetts General Hospital suggests that the volume of small bowel in the radiation field is decreased with protons as compared with photons.236 IMRT treatment planning techniques can also decrease the volume of small bowel in the field when compared with 3D conformal techniques.237 The clinical benefit of IMRT compared with 3D or conventional treatment delivery remains to be determined, however.235
Irradiation Fractionation, Modality, and Dose
Hyperfractionated radiation has been examined in phase I and II trials.238 In general, the pCR rates may be improved but at the expense of increased acute toxicity. Coucke and colleagues239 treated 250 assessable patients with cT3-4 and/or N+ disease with 41.6 Gy at 1.6 Gy twice daily and reported a 92% actuarial 5-year local control rate but a survival rate of only 60%. The rate of acute grade 3+ toxicity was 42% in the 5-FU plus twice-a-day radiation arm of RTOG R-0012 (1.2 Gy to 45.6 Gy, with a boost of 9.6 to 14.4 Gy). Hyperfractionated and accelerated fractionated radiation, especially in combination with chemotherapy, remains investigational.
Other investigational approaches such as neutrons,240 carbon ions,241 and hyperthermia242–244 have been examined. None have shown a clear advantage compared with conventional pelvic EBRT.
A meta-analysis concluded that biologically effective preoperative doses above 30 Gy resulted in a statistically significant reduction in locoregional recurrence rates.71 With conventional fractionation, the doses necessary to treat microscopic disease with a high level of local control are in the range of 45 to 50.4 Gy in 5 to 6 weeks.245 A retrospective comparison of 143 patients treated in three phase II trials of chemoradiation with 40 Gy, 46 Gy, and 50 Gy revealed a significant improvement in 2-year OS for those receiving 46 and 50 Gy (94% and 92%, respectively) versus 40 Gy (72%; p = .03).246
A boost of 5.4 Gy to the primary tumor or tumor bed may be delivered if the small bowel is excluded from the high-dose field. However, it is not clear that doses higher than 50.4 Gy improve local control rates. Higher preoperative doses up to 60 Gy are associated with increased pCR rates; however, they may also significantly increase acute and long-term morbidity rates. The RTOG R-0012 phase II randomized trial compared twice-daily preoperative chemoradiation of up to 60 Gy (1.2 Gy to 45.6 Gy, with a boost of 9.6 to 14.4 Gy) with conventional fractionation (1.8 Gy to 45 Gy, with a boost of 5.4 to 9 Gy) plus 5-FU/irinotecan.149 Both regimens resulted in a 28% pCR rate but were also associated with a more than 40% rate of grade 3 to 4 acute toxicity.
In the postoperative setting, if there is incomplete resection (R1 or R2 resection), radiation doses of more than 60 Gy are required. EBRT is limited by normal tissue tolerance, and results for patients with residual disease who received postoperative EBRT alone are disappointing.77,247 As previously discussed, IORT may help to overcome this problem by direct visualization and irradiation of the persistent tumor.
Methods to Reduce Acute and Chronic Toxicity
Complications of pelvic radiation therapy are a function of the volume of the radiation field, overall treatment time, fraction size, radiation energy, total dose, technique, and sequence of radiotherapy.248 Acute side effects such as diarrhea and increased bowel frequency (small bowel), acute proctitis (large bowel), and dysuria are common during treatment.249 These conditions are usually transient and resolve within a few weeks following the completion of irradiation. The symptoms appear to be a function of the dose rate and fraction size rather than the total dose. The mechanism is primarily the depletion of actively dividing cells in what is otherwise a stable cell renewal system. In the small bowel, loss of the mucosal cells results in malabsorption of various substances, including fat, carbohydrate, protein, and bile salts. Examination during treatment frequently reveals an inflamed, edematous, and friable rectal mucosa. The bowel mucosa usually recovers completely in 1 to 3 months following irradiation. Management usually involves the use of antispasmodic and/or anticholinergic medications.
Small bowel-related complications are proportional to the volume of small bowel in the radiation field.250 In patients receiving combined irradiation and chemotherapy, the volume of small bowel in the radiation field limited the ability to escalate the dose of 5-FU.248
Delayed complications occur less frequently but are substantially more serious. The initial symptoms commonly occur 6 to 18 months following completion of irradiation. Complications may include persistent diarrhea and increased bowel frequency, proctitis and strictures at the anastomotic site, small bowel obstruction, perineal/scrotal tenderness, delayed perineal wound healing, urinary incontinence, and bladder atrophy and bleeding. Injury to the vascular and supporting stromal tissues is the presumed pathophysiology. Analysis of pooled patients from 1599 patients treated in Swedish rectal cancer trials revealed a 1.5% increase in in-field secondary tumors for those treated with irradiation compared with those not receiving irradiation.251 Irradiation still had a positive effect on local control rates, however.
The most common delayed severe complications result from small bowel damage and include small bowel enteritis, adhesions, and small bowel obstruction requiring surgical intervention. The incidence of small bowel obstruction requiring surgery following postoperative pelvic irradiation for rectal cancer is 4% to 12% in historical series. In the Massachusetts General Hospital series, the incidence of small bowel obstruction with conventional postoperative radiation therapy was 6% as compared with 5% with surgery alone.60 It was 2% in the preoperative chemoradiation arm of the German CAO/ARO/AIO-94 trial.26
Even with appropriate doses and techniques of irradiation, approximately 1% of patients will have significant long-term toxicity of pelvic organs. Radiotherapeutic and surgical techniques as well as general methods to decrease treatment-related toxicity, especially small bowel complications, are seen in Box 49-2. Active inflammatory bowel disease is a relative contraindication to pelvic irradiation, although there are some reports of such patients who have tolerated EBRT.252,253 Pelvic fractures following pelvic irradiation are rare.254,255 Testosterone levels are decreased when the testicles are near or in the radiation field.256,257 Radiation, alone or in combination with surgery can have a negative impact on sexual function.258,259,260 In the Dutch CKVO trial, patients experienced new or worsening sexual dysfunction following treatment (men, 76%, and women, 62%).261 By multivariate analysis, independent factors included irradiation in men and the psychological presence of a stoma in both men and women. The authors concluded that despite the additional effect of irradiation, sexual dysfunction was mainly caused by surgery.
Box 49-2 Techniques and Methods to Decrease Toxicity
Sequencing of Radiotherapy and Surgery
Preoperative radiotherapy causes less acute and chronic toxicity compared with postoperative treatment. This is likely because of the fact that small bowel in an unviolated abdomen will be mobile and less likely to be within a pelvic radiation portal; the irradiated volume does not require coverage of the perineum following an APR. In the German CAO/ARO/AIO-94 trial, grade 3 to 4 gastrointestinal toxicity was significantly reduced with the preoperative approach (acute toxicity, 12% vs. 18%; p = .04; and long-term toxicity, 9% vs. 15%; p = .07).26 Strictures at the anastomotic site were reduced (4% vs. 12%; p = .003).
Randomized trials have investigated the use of sucralfate enemas to decrease acute radiation proctitis, olsalazine and mesalazine to decrease acute enteritis, and butyric acid to decrease chronic radiation proctitis.262 All of these trials have been negative. The radioprotector WR-2721 did not reduce toxicity in early trials, but there is a suggestion of a benefit in a more recent study.263 Rectally administered amifostine is well tolerated. However, its efficacy remains to be determined.264 Other trials of amifostine have not shown clear benefits.265
Treatment Algorithms, Controversies, Problems, Challenges, and Future Possibilities
Treatment Algorithms
Treatment algorithms can be based on evidence or consensus, or both. Although purely evidence-based algorithms are the most rigorous, there are a variety of clinical settings where prospective trials do not specifically address a treatment controversy. Therefore multidisciplinary panels that use evidence from randomized trials as well as lower-level sources such as clinical experience reports offer a more practical approach. The National Comprehensive Cancer Network (NCCN) has published clinical practice guidelines for rectal cancer based on multidisciplinary panels that use evidence from randomized trials as well as lower-level sources such as clinical experience reports266 (see web-only Fig. 49-1 on the Expert Consult website![]() ).
).
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines for rectal cancer. Current guidelines can be found at www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
Controversies and Future Directions
Recent data from phase III trials are helping to clarify some of the issues about both the benefit and type of concurrent/maintenance chemotherapy in chemoradiation regimens.292–297 In NSABP RO4, chemoradiation regimens using PVI 5-FU and capecitabine had similar pCR rates and tolerance.292 In three separate phase III trials, the addition of oxaliplatin to either PVI 5F-U or capecitabine did not improve pCR but added toxicity. Accordingly, the current standard is not to include oxaliplatin in preoperative chemoradiation regimens, pending longer term outcomes of local control and survival.292–294 Additional text is found on the Expert Consult website![]() with regard to the benefit of concurrent chemoradiation, sequencing with surgery, and the impact of type of concurrent chemotherapy on outcomes.
with regard to the benefit of concurrent chemoradiation, sequencing with surgery, and the impact of type of concurrent chemotherapy on outcomes.
Preoperative Chemoradiation: Update of Controversies
Chemoradiation versus Radiation Alone: Sequencing
The benefits of concurrent chemoradiation and the issue of sequencing with surgery have been addressed in updates of prior phase III trials. The EORTC and FFCD trials of preoperative radiation versus chemoradiation have been updated with a median follow-up of 5.6 years. A pooled analysis of the two trials confirmed that patients who received preoperative chemoradiation versus radiation alone had a significant decrease in local recurrence (11% vs. 15%, p = .0001) but no difference in 5-year OS (66%).292 With regard to sequencing, the results of the German CAO/ARO/AIO 94 trial were updated with a median follow-up of 11.2 years. At 10 years the local control benefit of preoperative versus postoperative therapy was sustained (6% vs 10%) with no difference in OS (60%).293
Chemoradiation: Type of Chemotherapy
The NSABP R-04 trial compared preoperative chemoradiation with CI 5-FU versus capecitabine (with or without oxaliplatin). Compared with CI 5-FU, capecitabine had similar rates of pCR (22% vs. 19%), sphincter-sparing surgery (63% vs. 61%), and grade 3+ diarrhea (11%).294 Therefore, chemoradiation regimens using CI 5-FU or capecitabine have similar response rates.
Four randomized trials have examined the impact of addition of oxaliplatin to 5-FU– or capecitabine-based CMT on response rates and acute toxicity in patients with cT3-4N+ rectal cancer. The STAR-01 trial randomized 747 patients to preoperative chemoradiation with 50.4 Gy/28 fractions + CI 5-FU with or without oxaliplatin.295 There was a significant increase in grade 3+ toxicity with oxaliplatin (24% vs. 8%, p <.001), but no improvement in the pCR rate (15% vs. 16%).
In the ACCORD trial, 598 patients were randomized to preoperative chemoradiation with 50 Gy plus capecitabine + oxaliplatin (CAPOX) versus 45 Gy plus capecitibine.296 There was a similar significant increase in grade 3+ toxicity with oxaliplatin (25% vs. 11%, p <.001) with no improvement in the pCR rate (19% vs. 14%).
The NSABP R-04 trial was a four-arm trial (2 × 2 comparison) of CI 5-FU– vs. capecitabine-based preoperative chemoradiation (50.4 Gy/28 fractions) with or without oxaliplatin.294 A total of 1606 patients with cT3 and/or N+ disease were randomized. The addition of oxaliplatin (to either 5-FU or capecitabine) was associated with a significantly higher incidence of grade 3+ diarrhea (15% vs. 7%, p = .0001) with no improvement in the incidence of pCR (21% vs. 19%) or sphincter-sparing surgery (60% vs. 64%).
The German CAO/ARO/AIO-04 randomized 1265 patients with cT3-4 and/or N+ disease to the preoperative arm of CAO/ARO/AIO-94 (50.4 Gy/28 fractions + 5-FU) versus 50.4 Gy/5-FU+ oxaliplatin (50 mg/m2 weekly).297 In contrast with the STAR-01, ACCORD, and NSABP R-04 trials, patients who received oxaliplatin-based CMT had a significant improvement in pCR (17% vs. 13%, p = .045) with no corresponding increase in acute grade 3+ toxicity (23% vs. 22%). The results of a similar trial (PETACC-6) are pending.
25 Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;1:996-999.
26 Sauer R, Becker H, Hohenberger P, et al. Preoperative chemoradiotherapy as compared with postoperative chemoradiotherapy for locally advanced rectal cancer. N Engl J Med. 2004;351:11-20.
43 Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate endpoint. J Clin Oncol. 2005;23:3475-3479.
45 Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2006;23:8688-8696.
51 Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278-1292.
54 Gunderson LL, Sargent D, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer. A pooled analysis. J Clin Oncol. 2004;22:1785-1796.
57 AJCC Hindgut Taskforce. Colon and rectum. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC cancer staging manual. ed 7. New York: Springer; 2010:143-164.
63 Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465-1472.
66 Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer. Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21-29.
71 Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer. A systematic overview of 22 randomised trials involving 8507 patients. Lancet. 2001;358:1291-1304.
72 Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987.
74 Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646.
75 Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years. Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701.
84 National Institutes of Health Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. J Am Med Assoc. 1990;264:1444-1450.
85 Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715.
86 O’Connell MJ, Martenson JA, Weiand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-507.
87 Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer. Analysis of stage, sex, and local control—final report of Intergroup 0114. J Clin Oncol. 2002;20:1744-1750.
90 Smalley SR, Benedetti JK, Williamson SK, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer. GI INT 0144. J Clin Oncol. 2006;24:3542-3547.
93 Bosset JF, Collette L, Bardet E, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123.
94 Gerard JP, Conroy T, Bonnetain F. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers. Results of FFCD 9203. J Clin Oncol. 2006;28:4620-4625.
95 Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy. Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organization for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386.
97 Guillem JG, Diaz-Gonzalez J, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368-373.
99 Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312.
103 Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223.
106 Marijnen CAM, Nagtegaal ID, Kranenbarg EK, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976-1984.
111 Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined modality therapy. Ann Surg Oncol. 2003;10:80-85.
113 Grumann MM, Noack EM, Hoffman IA, et al. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149-156.
122 Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer. Is a “wait and see” policy justified? Dis Colon Rectum. 2008;51:10-20.
123 Roh MS, Colangelo LH, O’Connell MJ, et al. Pre-operative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum (NSABP-R-03). J Clin Oncol. 2009;27:5124-5130.
130 Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016). A multicenter, randomised trial. Lancet. 2009;373:811-820.
135 Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU) based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer. Pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial. J Clin Oncol. 2009;27:18s. suppl; abstract CRA4008
136 Gerard JP, Azria D, Gourgou-Bourgade S, et al. Randomized multicenter phase III trial comparing two neoadjuvant chemoradiotherapy (CT-RT) regimens (RT45-Cap versus RT50-Capox) in patients with locally advanced rectal cancer (LARC). Results of the ACCORD 12/0405 PRODIGE 2. J Clin Oncol. 2009;27:18s. suppl; abstract LBA4007
139 Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer. A phase 2 trial. Lancet Oncol. 2010;11:241-248.
157 Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688.
166 Gerard JP, Ortholan C, Benezery K, et al. Contact x-ray therapy for rectal cancer. Experience in Centre Antoine-Lacassagne, Nice, 2002-2006. Int J Radiat Oncol Biol Phys. 2008;72:665-670.
185 Greenberg JA, Shibata D, Herndon JE, et al. Local excision of distal rectal cancer. An update of Cancer and Leukemia Group B 8984. Dis Colon Rectum. 2008;51:1185-1194.
202 Nuyttens JJ, Kolkman-Deurloo IKK, Vermess M, et al. High dose intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:106-112.
207 Diaz-Gonzalez J, Calvo FA, Cortes J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys. 2006;64:1122-1128.
211 Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83-87.
216 Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143-150. Epub 2010 Apr 13
223 Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:255-259.
234 Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer. Intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer. 2007;6:348-356.
248 Minsky BD, Conti JA, Huang Y, et al. The relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol. 1995;13:1409-1416.
259 Marijnen CAM, van de Velde CJH, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer. Report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847-1858.
262 Haddock MG, Sloan JA, Bollinger JW, et al. Patient assessment of bowel function during and after pelvic radiotherapy. Results of a prospective phase III North Central Cancer Treatment Group clinical trial. J Clin Oncol. 2007;25:1255-1259.
1 Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
2 Learn PA, Kahlenberg MS. Hereditary colorectal cancer syndromes and the role of the surgical oncologist. Surg Clin North Am. 2009;18:121-144.
3 Whittemore AS, Wu-Williams AH, Lee M, et al. Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Inst. 1991;82:915-926.
4 Leslie A, Carey FA, Pratt NR, et al. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845-860.
5 Powell SM, Zilz N, Beazer-Barclay Y. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235-237.
6 Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23:11-27.
7 Huerta S. Recent advances in the molecular diagnosis and prognosis of colorectal cancer. Expert Rev Mol Diagn. 2009;8:277-288.
8 Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33-64.
9 Diep CB, Thorstensen L, Meling GI, et al. Genetic tumor markers with prognostic impact in Dukes’ stages B and C colorectal cancer patients. J Clin Oncol. 2003;21:820-829.
10 Lashner BA, Provencher KS, Seidner DL. The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology. 1997;112:29-32.
11 Williams CS, Mann M, DuBois RN. The role of cyclo-oxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916.
12 Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening. Clinical guidelines and rationale. Gastroenterology. 1997;112:594-642.
13 Benson ABIII, Desch CE, Flynn PJ, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586-3588.
14 Yee J. CT colonography. Techniques and applications. Radiol Clin North Am. 2009;47:133-145.
15 Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer prognostic factors consensus conference. Colorectal Working Group. Cancer. 2000;88:1739-1757.
16 Brodsky JT, Richard GK, Cohen AM, et al. Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer. 1992;69:322-326.
17 Meling GI, Rognum TO, Clausen OP. Serum carcinoembryonic antigen in relation to survival, DNA ploidy pattern, and recurrent disease in 406 colorectal carcinoma patients. Gastroenterology. 1992;27:1061-1068.
18 Minsky BD. Clinicopathologic impact of colloid in colorectal carcinoma. Dis Colon Rectum. 1990;33:714-719.
19 Chen JS, Hsieh PS, Tang R, et al. Clinical significance of signet ring cell carcinoma. Int J Colorectal Dis. 2004;19:102-107.
20 Fahy B, Tang LH, Klimstra D, et al. Carcinoid of the rectum risk stratification (CaRRS) strategy for preoperative outcome assessment. Ann Surg Oncol. 2007;14:396-404.
21 Hatch KF, Blanchard DK, Hatch GF, et al. Tumors of the rectum and anal canal. World J Surg. 2000;24:437-443.
22 Grann A, Paty PB, Cohen AM, et al. Sphincter preservation of leiomyosarcoma of the rectum and anus with local excision and brachytherapy. Dis Colon Rectum. 1999;42:1296-1299.
23 Fan CW, Changchien CR, Wang JY, et al. Primary colorectal lymphoma. Dis Colon Rectum. 2000;43:1277-1282.
24 Nahas CSR, Shia J, Joseph R, et al. Squamous cell carcinoma of the rectum. A rare but curable tumor. Dis Colon Rectum. 2007;50:1-8.
25 Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumor spread and surgical excision. Lancet. 1986;1:996-999.
26 Sauer R, Becker H, Hohenberger P, et al. Preoperative chemoradiotherapy as compared with postoperative chemoradiotherapy for locally advanced rectal cancer. N Engl J Med. 2004;351:11-20.
27 Salerno GV, Daniels IR, Moran BJ, et al. Magnetic resonance imaging prediction of an involved surgical resection margin in low rectal cancer. Dis Colon Rectum. 2009;52:632-639.
28 Mercury Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer. Prospective observational study. Br Med J. 2006;333:779-782.
29 Bail SH, Kim NK, Lee YC, et al. Prognostic significance of circumferential resection margin following total mesorectal excision and adjuvant chemoradiotherapy in patients with rectal cancer. Ann Surg Oncol. 2007;14:462-469.
30 Barbaro B, Valentini V, Coco C, et al. Tumor vascularity evaluated by transrectal color Doppler US in predicting therapy outcome for low-lying rectal cancer. Int J Radiat Oncol Biol Phys. 2005;63:1304-1308.
31 Valentini V, DePaoli A, Gambacorta MA, et al. Chemoradiation with infusional 5-FU and ZD1839 (Gefitinib-Iressa) in patients with locally advanced rectal cancer. A phase II trial. Int J Radiat Oncol Biol Phys. 2006;66:s168.
32 Kim NK, Kim MJ, Park JK, et al. Preoperative staging of rectal cancer with MRI. Accuracy and clinical usefulness. Ann Surg Oncol. 2000;7:732-737.
33 Nahas CSR, Akhurst T, Yeung H, et al. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol. 2008;15:704-711.
34 Kristiansen C, Loft A, Berthelsen AK, et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21-25.
35 Kalff V, Ware R, Heriot A, et al. Radiation changes do not interfere with postchemoradiation restaging of patients with rectal cancer by FDG PET/CT before curative surgical therapy. Int J Radiat Oncol Biol Phys. 2009;74:60-66.
36 Dietz DW, Dehdashti F, Grigsby PW, et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma. A pilot study. Dis Colon Rectum. 2008;51:1641-1648.
37 Harisinghani MG, Barentsz J, Hahn PF. Noninvasive detection of clinically occult lymph-node metastasis in prostate cancer. N Engl J Med. 2003;348:2491-2499.
38 Shami VM, Parmer KS, Waxman I. Clinical impact of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in the management of rectal carcinoma. Dis Colon Rectum. 2004;47:59-65.
39 Kim YH, Kim DY, Kim TH, et al. Usefulness of magnetic resonance volumetric evaluation in predicting response to preoperative concurrent chemoradiotherapy in patients with resectable rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:761-768.
40 Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48:23-28.
41 Gavioli M, Bagni A, Piccagli I, et al. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer. Dis Colon Rectum. 2000;43:1075-1083.
42 Barbaro B, Schulsinger A, Valentini V, et al. The accuracy of transrectal ultrasound in predicting the pathological stage of low-lying rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:1043-1047.
43 Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate endpoint. J Clin Oncol. 2005;23:3475-3479.
44 Vecchio FM, Valentini V, Minsky B, et al. The relationship of pathologic tumor regression grade (TRG) and outcome after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760.
45 Rodel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2006;23:8688-8696.
46 Whiteford MH, Whiteford HM, Yee LF, et al. Usefulness of FDG-PET scan in the assessment of suspected metastatic or recurrent adenocarcinoma of the colon and rectum. Dis Colon Rectum. 2000;53:759-770.
47 Desai DC, Zervos EE, Arnold MW, et al. Positron emission tomography affects surgical management in recurrent colorectal cancer patients. Ann Surg Oncol. 2003;10:59-64.
48 Barbaro B, Fiorucci C, Tebala C, et al. Locally advanced rectal cancer. MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730-739.
49 Calvo FA, Domper M, Matute R, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58:528-535.
50 Dukes CE. The classification of cancer of the rectum. J Pathol. 1932;35:323-332.
51 Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer. 1974;34:1278-1292.
52 Minsky BD, Mies C, Recht A, et al. Resectable adenocarcinoma of the rectosigmoid and rectum. 1. Patterns of failure and survival. Cancer. 1988;61:1408-1416.
53 AJCC Colorectal Taskforce. Colon and rectum. In: Green FL, Page DL, Fleming ID, et al, editors. AJCC cancer staging manual. ed 6. New York: Springer; 2002:113-124.
54 Gunderson LL, Sargent D, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer. A pooled analysis. J Clin Oncol. 2004;22:1785-1796.
55 Green FL, Stewart AK, Norton HJ. New tumor-node-metastasis staging system for node-positive (stage III) rectal cancer. An analysis. J Clin Oncol. 2004;22:1778-1784.
56 Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256-263.
57 AJCC Hindgut Taskforce. Colon and rectum. In: Edge SB, Byrd DR, Compton CC, et al, editors. AJCC cancer staging manual. ed 7. New York: Springer; 2010:143-164.
58 Baxter N, Morris AM, Rothenberger DA, et al. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation. A population based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426-431.
59 Leibold T, Shia J, Ruo L, et al. Prognostic implications of the distribution of lymph node metastasizing rectal cancer after neoadjuvant chemotherapy. J Clin Oncol. 2008;26:2106-2111.
60 Willett CG, Tepper JE, Kaufman DS, et al. Adjuvant postoperative radiation therapy for rectal adenocarcinoma. Am J Clin Oncol. 1992;15:371-375.
61 Romsdahl MM, Withers HR. Radiotherapy combined with curative surgery. Its use as therapy for carcinomas of the sigmoid colon and rectum. Arch Surg. 1978;ll3:446-453.
62 Balslev I, Pedersen M, Teglbjaerg PS, et al. Postoperative radiotherapy in Dukes’ B and C carcinoma of the rectum and rectosigmoid. A randomized multicenter study. Cancer. 1986;58:22-28.
63 Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465-1472.
64 Arnaud JP, Nordlinger B, Bosset JF, et al. Radical surgery and postoperative radiotherapy as combined treatment in rectal cancer. Final results of a phase III study of the European Organization for Research and Treatment of Cancer. Br J Surg. 1997;84:352-357.
65 Medical Research Council Rectal Cancer Working Party. Randomized trial of surgery alone versus surgery followed by post-operative radiotherapy for mobile cancer of the rectum. Lancet. 1996;348:1610-1615.
66 Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer. Results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21-29.
67 Gerard JP, Chapet O, Nemoz C, et al. Improved sphincter preservation in low rectal cancer with high dose preoperative radiotherapy. The Lyon R96-02 randomized trial. J Clin Oncol. 2004;22:2404-2409.
68 Rengan R, Paty P, Wong WD, et al. Distal cT2N0 rectal cancer. Is there an alternative to abdominoperineal resection? J Clin Oncol. 2005;23:4905-4912.
69 Skibber JM, Hoff PM, Minsky BD. Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia: Williams and Wilkins; 2001:1271-1318.
70 Camma C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. J Am Med Assoc. 2000;284:1008-1015.
71 Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer. A systematic overview of 22 randomised trials involving 8507 patients. Lancet. 2001;358:1291-1304.
72 Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987.
73 Birgisson H, Pahlman L, Glimelius B. Adverse effects of preoperative radiation therapy for rectal cancer. Long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23:8697-8705.
74 Kapiteijn E, Marijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646.
75 Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years. Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701.
76 Marijnen CAM, Kapiteijn E, van de Velde CJH, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer. Report of a multicenter randomized trial. J Clin Oncol. 2002;20:817-825.
77 Marijnen CAM, Nagtegaal ID, Kapiteijn E, et al. Radiotherapy does not compensate for positive resection margins in rectal cancer patients. Report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1311-1320.
78 Guillem JG, Chessin DB, Shia J, et al. A prospective pathologic analysis using whole mount sections of rectal cancer following preoperative combined modality therapy. Implications for sphincter preservation. Ann Surg. 2007;245:88-93.
79 Branagan G, Chave H, Fuller C, et al. Can magnetic resonance imaging predict circumferential margins and TNM stage in rectal cancer? Dis Colon Rectum. 2004;47:1317-1322.
80 Burton S, Brown G, Daniels I, et al. MRI identified prognostic features of tumors in distal sigmoid, rectosigmoid, and upper rectum. Treatment with radiotherapy and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:445-451.
81 Rutten H, Sebag-Montefiore D, Glynne-Jones R, et al. Capecitabine, oxaliplatin, radiotherapy, and excision (CORE) in patients with MRI-defined locally advanced rectal adenocarcinoma. Results of an international multicenter phase II study. J Clin Oncol. 24(18s), 2006. June 20 Suppl; abstract 3528
82 Falk PM, Gupta NC, Thorson AG, et al. Positron emission tomography for preoperative staging of colorectal carcinoma. Dis Colon Rectum. 1994;37:153-156.
83 Lusinchi A, Wibault P, Lasser P, et al. Abdominoperineal resection combined with pre- and postoperative radiation therapy in the treatment of low-lying rectal carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:59-65.
84 National Institutes of Health Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. J Am Med Assoc. 1990;264:1444-1450.
85 Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709-715.
86 O’Connell MJ, Martenson JA, Weiand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-507.
87 Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer. Analysis of stage, sex, and local control—final report of Intergroup 0114. J Clin Oncol. 2002;20:1744-1750.
88 Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer. Findings from Intergroup trial 0114. J Clin Oncol. 2004;22:648-657.
89 Meyerhardt JA, Tepper JE, Neidzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high risk curatively resected rectal cancer. Findings from the Intergroup 0114 study. J Clin Oncol. 2004;22:166-174.
90 Smalley SR, Benedetti JK, Williamson SK, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer. GI INT 0144. J Clin Oncol. 2006;24:3542-3547.
91 Nissan A, Stojadinovic A, Shia J, et al. Predictors of recurrence in patients with T2 and early T3, N0 adenocarcinoma of the rectum treated with surgery alone. J Clin Oncol. 2006;24:4078-4084.
92 Chan AK, Wong A, Jenken DA, et al. Is postoperative adjuvant chemotherapy necessary in locally advanced rectal cancers after preoperative chemoradiation? Int J Radiat Oncol Biol Phys. 2004;60:s297.
93 Bosset JF, Collette L, Bardet E, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123.
94 Gerard JP, Conroy T, Bonnetain F. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers. Results of FFCD 9203. J Clin Oncol. 2006;28:4620-4625.
95 Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy. Does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organization for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386.
96 Valentini V, Aristei C, Glimelius B, et al. Multidisciplinary rectal cancer management. 2nd European Rectal Cancer Consensus Conference. Radiother Oncol. 2009;92:148-163.
97 Guillem JG, Diaz-Gonzalez J, Minsky BD, et al. cT3N0 rectal cancer. Potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368-373.
98 Mendenhall WM, Bland KI, Rout WR, et al. Clinically resectable adenocarcinoma of the rectum treated with preoperative irradiation and surgery. Dis Colon Rectum. 1988;31:287-290.
99 Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26:303-312.
100 Nagtegaal ID, van de Velde CJH, Marijnen CAM, et al. Low rectal cancer. A call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264.
101 Sebag-Montefiore D, Steele R, Quirke P, et al. Routine short course pre-operative radiotherapy or selective post-op chemoradiotherapy for resectable rectal cancer? Preliminary results of the MRC CR07 randomised trial. J Clin Oncol. 24(18s), 2006. June 20 Suppl; abstract 3511
102 Sauer R, Rodel C. Author reply. N Engl J Med. 2005;352:509-511.
103 Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223.
104 Sauer R, Rödel C. The author’s reply. N Engl J Med. 2005;352:510-511.
105 Graf W, Dahlberg M, Osman MM, et al. Short-term preoperative radiotherapy results in down-staging of rectal cancer. A study of 1316 patients. Radiother Oncol. 1997;43:133-137.
106 Marijnen CAM, Nagtegaal ID, Kranenbarg EK, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976-1984.
107 Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer. The Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396-2402.
108 Dolinsky CM, Mahmoud NN, Mick R, et al. Effect of time interval between surgery and preoperative chemoradiotherapy with 5-fluorouracil or 5-fluorouracil and oxaliplatin on outcomes in rectal cancer. J Surg Oncol. 2007;96:207-212.
109 Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279-286.
110 Stein DE, Mahmoud NN, Anne PR, et al. Longer time interval between completion of neoadjuvant chemoradiation and surgical resection does not improve downstaging of rectal carcinoma. Dis Colon Rectum. 2003;46:448-453.
111 Moore HG, Riedel E, Minsky BD, et al. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined modality therapy. Ann Surg Oncol. 2003;10:80-85.
112 Kuvshinoff B, Maghfoor I, Miedema B, et al. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas. Are <1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163-169.
113 Grumann MM, Noack EM, Hoffman IA, et al. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149-156.
114 Krouse RS, Herrinton LJ, Grant M, et al. Health-related quality of life among long-term rectal cancer survivors with an ostomy. Manifestations by sex. J Clin Oncol. 2009;28:4664-4670.
115 Temple LKF, Wong WD, Minsky B. The impact of radiation on functional outcomes in patients with rectal cancer and sphincter preservation. Semin Radiat Oncol. 2003;13:469-477.
116 Kollmorgen CF, Meagher AP, Pemberton JH, et al. The long term effect of adjuvant postoperative chemoradiotherapy for rectal cancer on bowel function. Ann Surg. 1994;220:676-682.
117 Habr-Gama A, Santinho B, de Souza PM, Ribeiro U, et al. Low rectal cancer. Impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087-1096.
118 Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer. An analysis of 488 patients. J Am Coll Surg. 2002;194:131-136.
119 Guillem JG, Moore HG, Akhurst T, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation. A means for determining long-term outcomes of rectal cancer. J Am Coll Surg. 2004;199:1-7.
120 Heriot AG, Hicks RJ, Drummond EGP, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451-458.
121 Dzik-Jurask A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307-308.
122 Glynne-Jones R, Wallace M, Livingstone JI, et al. Complete clinical response after preoperative chemoradiation in rectal cancer. Is a “wait and see” policy justified? Dis Colon Rectum. 2008;51:10-20.
123 Roh MS, Colangelo LH, O’Connell MJ, et al. Pre-operative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum (NSABP-R-03). J Clin Oncol. 2009;27:5124-5130.
124 Fietkau R, Rodel C, Hohenberger W, et al. Rectal cancer delivery of radiotherapy in adequate time and with adequate dose is influenced by treatment center, treatment schedule, and gender and is prognostic parameter for local control. Results of study CAO/ARO/AIO-94. Int J Radiat Oncol Biol Phys. 2007;67:1008-1019.
125 Beets-Tan RGH, Beets GL. Rectal cancer. How accurate can imaging predict the T stage and the circumferential resection margin? Int J Colorectal Dis. 2003;18:385-391.
126 Kim JH, Beets GL, Kim MJ, et al. High-resolution MR imaging for nodal staging in rectal cancer. Are there any criteria in addition to the size? Eur J Radiol. 2004;52:78-83.
127 Roedel C, Fietkau R, Raab R, et al. Tumor regression grading as a prognostic factor in patients with locally advanced rectal cancer treated with preoperative radiochemotherapy. Int J Radiat Oncol Biol Phys. 2004;60:s140.
128 Read TE, Andujar JE, Caushaj FP, et al. Neoadjuvant therapy for rectal cancer. Histologic response of the primary tumor predicts nodal status. Dis Colon Rectum. 2004;47:825-831.
129 Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer. Report of a randomized trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15-24.
130 Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016). A multicenter, randomised trial. Lancet. 2009;373:811-820.
131 Reference deleted in proofs.
132 Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351.
133 Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704.
134 Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan, fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer. Results of CALGB 89803. J Clin Oncol. 2007;25:3456-3461.
135 Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU) based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer. Pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial. J Clin Oncol. 27(18s), 2009. Suppl; abstract CRA4008
136 Gerard JP, Azria D, Gourgou-Bourgade S, et al. Randomized multicenter phase III trial comparing two neoadjuvant chemoradiotherapy (CT-RT) regimens (RT45-Cap versus RT50-Capox) in patients with locally advanced rectal cancer (LARC). Results of the ACCORD 12/0405 PRODIGE 2. J Clin Oncol. 2009;27:18s. Suppl; abstract LBA4007
137 Yu CS, Kim TW, Kim JH, et al. Optimal time interval between capecitabine intake and radiotherapy in preoperative chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2007;67:1020-1026.
138 Kalafonos HP, Bamias A, Koutras A, et al. A randomised phase III trial of adjuvant radio-chemotherapy comparing irinotecan, 5FU and leucovorin to 5FU and leucovorin in patients with rectal cancer. A Hellenic Cooperative Oncology Group Study. Eur J Cancer. 2008;44:1693-1700.
139 Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer. A phase 2 trial. Lancet Oncol. 2010;11:241-248.
140 Fernandez-Martos C, Aparicio J, Salud A, et al. Multicenter randomized phase II study of chemoradiation (CRT) followed by surgery (S) and chemotherapy (CT) versus induction CT followed by CRT and S in high-risk rectal cancer. GCR-3 final efficacy and safety results. J Clin Oncol. 27(15s), 2009. Suppl; abstract 4103
141 Wolmark N, Yothers G, O’Connell MJ, et al. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon. Results of NSABP protocol C-08. J Clin Oncol. 27(18s), 2009. June 20 Suppl; abstract LBA4
142 Willett CG, Duda D, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer. A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020-3026.
143 van Cutsem E, Lang I, D’haens G, et al. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab. The CRYSTAL experience. J Clin Oncol. 26, 2008. May Suppl; abstract 2
144 Lordick F, Geinitz H, Theisen J, et al. Increased risk of ischemic bowel complications during treatment with bevacizumab after pelvic irradiation. Report of three cases. Int J Radiat Oncol Biol Phys. 2006;64:1295-1298.
145 Das P, Lin EH, Bhatia S, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer. A matched-pair analysis. Int J Radiat Oncol Biol Phys. 2006;66:1378-1383.
146 Chung KY, Minsky B, Schrag D, et al. Phase I trial of preoperative cetuximab with concurrent continuous infusion 5-fluorouracil and pelvic radiation in patients with local-regionally advanced rectal cancer. Proc Am Soc Clin Oncol. 2006;24:18s. abstract 3560
147 Rodel C, Arnold D, Hipp M, et al. Phase I-II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:1081-1086.
148 Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIb trial of chemotherapy, bevacizumab, and panitumamab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680.
149 Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Radiation Therapy Oncology Group trial 0012. J Clin Oncol. 2006;24:650-655.
150 Wong SJ, Winter K, Meropol NJ, et al. RTOG 0247. A randomized phase II study of neoadjuvant capecitabine and irinotecan versus capecitabine and oxaliplatin with concurrent radiation therapy for locally advanced rectal cancer. J Clin Oncol. 2008. May 23 Suppl; abstract 4021
151 Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664-674.
152 Guillem JG, Chessin DB, Cohen AM, et al. Long term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829-838.
153 Capirci C, Valentini V, Cionini L, et al. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer. Long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107.
154 Bertolini F, Bengala C, Losi L, et al. Prognostic and predictive value of baseline and post treatment molecular marker expression in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1455-1461.
155 Unsal D, Under A, Akyuerk N, et al. Matrix Metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:196-203.
156 Johnston PG. Prognostic markers of local relapse in rectal cancer. Are we any further forward? J Clin Oncol. 2006;24:4049-4050.
157 Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74:673-688.
158 Konski A, Li T, Sigurdson E, et al. Use of molecular imaging to predict clinical outcome in patients with rectal cancer after preoperative chemotherapy and radiation. Int J Radiat Oncol Biol Phys. 2009;74:55-59.
159 Lavertu S, Schild SE, Gunderson LL, et al. Endocavitary radiation therapy for rectal adenocarcinoma. 10 year results. Am J Clin Oncol. 2003;26:508-512.
160 Rauch P, Bey P, Peiffert D, et al. Factors affecting local control and survival after treatment of carcinoma of the rectum by endocavitary radiation. A retrospective study of 97 cases. Int J Radiat Oncol Biol Phys. 2001;49:117-124.
161 Gerard JP, Romestang P, Ardiet JM, et al. Endocavitary radiation therapy. Semin Radiat Oncol. 1998;8:13-23.
162 Coatmeur O, Truc G, Barillot I, et al. Treatment of T1-T2 rectal tumors by contact therapy and interstitial brachytherapy. Radiother Oncol. 2004;70:177-182.
163 Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy. Results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys. 2001;51:363-370.
164 Myerson RJ. Conservative alternatives to radical surgery for favorable rectal cancers. Ann Ital Chir. 2001;72:605-609.
165 Hofheinz RD, von Gerstenberg-Helldorf B, Wenz F, et al. Phase I trial of capecitabine and weekly irinotecan in combination with radiotherapy for neoadjuvant therapy of rectal cancer. J Clin Oncol. 2005;23:1350-1357.
166 Gerard JP, Ortholan C, Benezery K, et al. Contact x-ray therapy for rectal cancer. Experience in Centre Antoine-Lacassagne, Nice, 2002-2006. Int J Radiat Oncol Biol Phys. 2008;72:665-670.
167 Minsky BD, Mies C, Rich TA, et al. Lymphatic vessel invasion is an independent prognostic factor for survival in colorectal cancer. Int J Radiat Oncol Biol Phys. 1989;17:311-318.
168 Nash GM, Weiser MR, Guillem JG, et al. Long term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52:577-582.
169 Lezoche E, Guerrieri M, Paganini AM, et al. Long term results in patients with T2-3 distal rectal cancer undergoing radiotherapy before transanal endoscopic microsurgery. Br J Surg. 2005;92:1546-1552.
170 Willett CG. Local excision followed by postoperative radiation therapy. Semin Radiat Oncol. 1998;8:24-29.
171 Mendenhall WM, Rout WR, Vauthey JN, et al. Conservative treatment of rectal adenocarcinoma with endocavitary irradiation or wide local excision and post-operative irradiation. J Clin Oncol. 1997;15:3241-3248.
172 Bleday R, Breen E, Jessup JM, et al. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum. 1997;40:388-392.
173 Ota DM, Skibber J, Rich TA. M.D. Anderson Cancer Center experience with local excision and multimodality therapy for rectal cancer. Surg Oncol Clin North Am. 1992;1:147-152.
174 Rosenthal SA, Yeung RS, Weese JL, et al. Conservative management of extensive low-lying rectal carcinomas with transanal local excision and combined preoperative and postoperative radiation therapy. Report of a Phase I/II trial. Cancer. 1992;69:335-341.
175 Chakravarti A, Compton CC, Shellito PC, et al. Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg. 1999;230:49-54.
176 Valentini V, Morganti AG, De Santis M, et al. Local excision and external beam radiotherapy in early rectal cancer. Int J Radiat Oncol Biol Phys. 1996;35:759-764.
177 Fortunato L, Ahmad NR, Yeung RS, et al. Long-term follow-up of local excision and radiation therapy for invasive rectal cancer. Dis Colon Rectum. 1995;38:1193-1199.
178 Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum. Long-term results of Radiation Therapy Oncology Group protocol 89-02. Int J Radiat Oncol Biol Phys. 2000;46:313-322.
179 Wagman RT, Minsky BD. Conservative management of rectal cancer with local excision and adjuvant therapy. Oncology. 2001;15:513-524.
180 Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg. 1998;175:360-363.
181 Benson R, Wong CS, Cummings BJ, et al. Local excision and postoperative radiotherapy for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2001;50:1309-1316.
182 Butch RJ, Stark DD, Wittenberg J, et al. Staging rectal cancer by MR and CT. Am J Radiol. 1986;146:1155-1160.
183 Weiser MR, Landmann RG, Wong WD, et al. Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum. 2005;48:1169.
184 Steele GJr, Tepper J, Herndon J, et al. Failure and salvage after sphincter-sparing treatment for distal rectal adenocarcinoma—A CALGB Coordinated Intergroup study. Proc Am Soc Clin Oncol. 1999;18:235a.
185 Greenberg JA, Shibata D, Herndon JE, et al. Local excision of distal rectal cancer. An update of Cancer and Leukemia Group B 8984. Dis Colon Rectum. 2008;51:1185-1194.
186 Schell SR, Zlotecki RA, Mendenhall WM, et al. Transanal excision of locally advanced rectal cancers downstaged using neoadjuvant chemoradiotherapy. J Am Coll Surg. 2002;194:584-591.
187 Ahmad NR, Nagle DA. Preoperative radiation therapy followed by local excision. Semin Radiat Oncol. 1998;8:36-38.
188 Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg. 2001;234:352-358.
189 Lezoche E, Guerrieri M, Paganini AM, et al. Long-term results of patients with pT2 rectal cancer treated with radiotherapy and transanal endoscopic microsurgical excision. World J Surg. 2002;26:1170-1174.
190 Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys. 2004;60:1098-1105.
191 Ruo L, Guillem JG, Minsky BD, et al. Preoperative radiation with or without chemotherapy and full-thickness transanal excision for selected T2 and T3 distal rectal cancers. Int J Colorectal Dis. 2002;17:54-58.
192 Borschitz T, Wachtlin D, Mohler M, et al. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720.
193 Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;22:3687-3694.
194 Jimenez A, Shoup M, Cohen AM, et al. Contemporary outcomes of total pelvic exenteration in the treatment of colorectal carcinoma. Dis Colon Rectum. 2004;46:1619-1625.
195 Law WL, Chu KW, Choi HK. Total pelvic exenteration for locally advanced rectal cancer. J Am Coll Surg. 2004;190:78-83.
196 Goldberg JM, Piver MS, Hempling RE, et al. Improvements in pelvic exenteration. Factors responsible for reducing morbidity and mortality. Ann Surg Oncol. 1998;5:399-406.
197 Reerink O, Verschueren RCJ, Szabo BG, et al. A favourable pathological stage after neoadjuvant radiochemotherapy in patients with initially irresectable rectal cancer correlates with a favorable prognosis. Eur J Cancer. 2003;39:192-195.
198 Willett CG, Shellito PC, Rodkey GV, et al. Preoperative irradiation for tethered rectal carcinoma. Radiother Oncol. 1991;21:141-142.
199 Tobin RL, Mohiuddin M, Marks G. Preoperative irradiation for cancer of the rectum with extrarectal fixation. Int J Radiat Oncol Biol Phys. 1991;21:1127-1132.
200 Strassmann G, Walter S, Kolotas C, et al. Reconstruction and navigation system for intraoperative brachytherapy using the flab technique for colorectal tumor bed irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1323-1329.
201 Alekitar KM, Zelefsky MJ, Paty PB, et al. High dose rate intraoperative brachytherapy for recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2000;48:219-226.
202 Nuyttens JJ, Kolkman-Deurloo IKK, Vermess M, et al. High dose intraoperative radiotherapy for close or positive margins in patients with locally advanced or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:106-112.
203 Mathis KL, Nelson H, Pemberton JH, et al. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg. 2009;248:592-598.
204 Mannaerts GHH, Rutten HJT, Martijn H, et al. Effects on functional outcome after IORT-containing multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2002;54:1082-1088.
205 Azinovic I, Calvo FA, Puebla F, et al. Long-term normal tissue effects of intraoperative electron radiation therapy (IOERT.: Late sequelae, tumor recurrence, and second malignancies. Int J Radiat Oncol Biol Phys. 2001;49:597-604.
206 Shibata D, Guillem JG, Lanouette NM, et al. Functional and quality of life outcomes in patients with rectal cancer after combined modality therapy, intraoperative radiation therapy, and sphincter preservation. Dis Colon Rectum. 2000;43:752-758.
207 Diaz-Gonzalez J, Calvo FA, Cortes J, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys. 2006;64:1122-1128.
208 Valentini V, Coco C, Rizzo G, et al. Outcomes of clinical T4M0 extra-peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery. A prospective evaluation of a single institutional experience. Surgery. 2009;145:486-494.
209 Nakfoor BM, Willett CG, Shellito PC, et al. The impact of 5-fluorouracil and intraoperative electron beam radiation therapy on the outcome of patients with locally advanced primary rectal and rectosigmoid cancer. Ann Surg. 1998;228:194-200.
210 Willett CG, Nakfoor BM, Daley W, et al. Pathologic downstaging does not guide the need for IORT in primary locally advanced rectal cancer. Front Radiat Ther Oncol. 1997;31:245-256.
211 Lindel K, Willett CG, Shellito PC, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol. 2001;58:83-87.
212 Bagatzounis A, Kolbl O, Muller G, et al. The locoregional recurrence of rectal carcinoma. A computed tomographic analysis and a target volume concept for adjuvant radiotherapy. Strahlenther Onkol. 1997;173:68-75.
213 Suzuki K, Gunderson LL, Devine RM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer. 1995;75:939-952.
214 Valentini V, Morganti AG, De Franco A, et al. Chemoradiation with or without intraoperative radiation therapy in patients with locally recurrent rectal carcinoma. Prognostic factors and long term outcome. Cancer. 1999;86:2612-2624.
215 Kusters M, Dresen RC, Martijn H, et al. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:1444-1449.
216 Haddock MG, Miller RC, Nelson H, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys. 2011;79:143-150. Epub 2010 Apr 13
217 Sanfilippo NJ, Crane CH, Skibber J, et al. T4 rectal cancer treated with preoperative chemoradiation to the posterior pelvis followed by multivisceral resection. Patterns of failure and limitations of treatment. Int J Radiat Oncol Biol Phys. 2001;51:176-183.
218 Wiig JN, Tveit KM, Poulsen JP, et al. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative radiotherapy (IORT) be of additional benefit? Radiother Oncol. 2002;62:207-213.
219 Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer. 2002;95:1144-1150.
220 Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis. A multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129-1139.
221 Horiot JC, Roth SL, Calais G, et al. The Dijon clinical staging system for early rectal carcinomas amenable to intracavitary treatment techniques. Radiother Oncol. 1990;18:329-337.
222 Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2002;54:142-149.
223 Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:255-259.
224 Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys. 2001;49:107-116.
225 Liberman H, Adams DR, Blatchford GJ, et al. Clinical use of the self-expanding metallic stent in the management of colorectal cancer. Am J Surg. 2001;180:407-412.
226 Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer. Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872-877.
227 Timmerman RD, Bizekis CS, Pass HI, et al. Local surgical, ablative, and radiation treatment of metastasis. CA Cancer J Clin. 2009;59:145-170.
228 Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastasis. J Clin Oncol. 2009;27:1585-1591.
229 Hruby G, Barton M, Miles S. Sites of local recurrence after surgery, with or without chemotherapy, for rectal cancer. Implications for radiotherapy field design. Int J Radiat Oncol Biol Phys. 2003;55:138-143.
230 Hocht S, Hammad R, Thiel H. A multicenter analysis of 123 patients with recurrent rectal cancer within the pelvis. Front Radiat Ther Oncol. 2004;38:41-51.
231 Koelbl O, Vordermark D, Flentje M. The relationship between belly board position and patient anatomy and its influence on dose-volume histogram of small bowel for postoperative radiotherapy of rectal cancer. Radiother Oncol. 2003;67:345-349.
232 Roels S, Duthoy W, Haustermans K, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129-1142.
233 Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer. A radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824-830.
234 Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer. Intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer. 2007;6:348-356.
235 Aristu JJ, Arbea L, Rodriguez J, et al. Phase I-II trial of concurrent capecitabine and oxaliplatin with preoperative intensity modulated radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2008;71:748-755.
236 Tatsuzaki H, Urie MM, Willett CG. 3-D comparative study of proton vs. x-ray radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys. 1991;22:369-374.
237 Callister MD, Ezzell GA, Gunderson LL. IMRT reduces the dose to small bowel and other pelvic organs in the preoperative treatment of rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:s290.
238 Ceelen W, Boterberg T, Pattyn P, et al. Neoadjuvant chemoradiation versus hyperfractionated accelerated radiotherapy in locally advanced rectal cancer. Ann Surg Oncol. 2007;14:424-431.
239 Coucke PA, Notter M, Stamm B, et al. Preoperative hyperfractionated accelerated radiotherapy (HART) in locally advanced rectal cancer (LARC) immediately followed by surgery. A prospective phase II trial. Radiother Oncol. 2006;79:52-58.
240 Duncan W, Arnott SJ, Jack WJL, et al. Results of two randomized trials of neutron therapy in rectal adenocarcinoma. Radiother Oncol. 1987;8:191-198.
241 Yamada S, Kamada T, Yasuda S, et al. Phase I/II trial of carbon-ion therapy for patients with locally recurrent rectal cancer. Proc Am Soc Clin Oncol. 2005;22:280s.
242 Anscher MS, Lee C, Hurwitz HI, et al. A pilot study of preoperative continuous infusion 5-fluorouracil, external microwave hyperthermia, and external beam radiotherapy for treatment of locally advanced, unresectable, or recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:719-724.
243 Rau B, Wust P, Tilly W, et al. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer. Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys. 2000;48:381-391.
244 van der Zee J, Gonzalez DG, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors. A prospective, randomised, multicenter trial. Lancet. 2000;355:1119-1125.
245 Withers HR, Peters LJ, Taylor JM. Dose-response relationship for radiation therapy of subclinical disease. Int J Radiat Oncol Biol Phys. 1995;31:353-359.
246 Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer. Effect of dose escalation on pathologic complete response, local recurrence-free survival, disease free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709-716.
247 De Neve W, Martijn H, Lybeert MM, et al. Incompletely resected rectum, rectosigmoid, or sigmoid carcinoma. Results of postoperative radiotherapy and prognostic factors. Int J Radiat Oncol Biol Phys. 1991;21:1297-1302.
248 Minsky BD, Conti JA, Huang Y, et al. The relationship of acute gastrointestinal toxicity and the volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol. 1995;13:1409-1416.
249 Miller RC, Sargent DJ, Martenson JA, et al. Acute diarrhea during adjuvant therapy for rectal cancer. A detailed analysis from a randomized Intergroup trial. Int J Radiat Oncol Biol Phys. 2002;54:409-413.
250 Robertson JM, Lockman D, Yan D, et al. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2008;70:413-418.
251 Birgisson H, Pahlman L, Gunnarsson U, et al. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23:6126-6131.
252 Willett CG, Ooi CJ, Zeitman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995-998.
253 Lawrie WT, Song DS, Abrams RA, et al. Acute and late radiotherapy toxicity in patients with inflammatory bowel disease. Int J Radiat Oncol Biol Phys. 2000;48:226.
254 Tai P, Hammond A, Dyk JV, et al. Pelvic fractures following irradiation of endometrial and vaginal cancers—a case series and review of literature. Radiother Oncol. 2000;56:23-28.
255 Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. J Am Med Assoc. 2005;294:2587-2593.
256 Yoon FH, Perera F, Fisher B, et al. Alterations in hormone levels after adjuvant chemoradiation in male rectal cancer patients. Int J Radiat Oncol Biol Phys. 2009;74:1186-1190.
257 Dueland S, Guren MG, Olsen DR, et al. Radiation therapy induced changes in male sex hormone levels in rectal cancer patients. Radiother Oncol. 2003;68:249-253.
258 Heriot AG, Tekkis PP, Fazio VW, et al. Adjuvant radiotherapy is associated with increased sexual dysfunction in male patients undergoing resection for rectal cancer. A predictive model. Ann Surg. 2005;242:502-511.
259 Marijnen CAM, van de Velde CJH, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer. Report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847-1858.
260 Pietrzak L, Bujko K, Nowacki MP, et al. Quality of life, anorectal, and sexual functions after preoperative radiotherapy for rectal cancer. Report of a randomised trial. Radiother Oncol. 2007;84:217-225.
261 Lange MM, Marijnen CAM, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer. 2009;45:1578-1588.
262 Haddock MG, Sloan JA, Bollinger JW, et al. Patient assessment of bowel function during and after pelvic radiotherapy. Results of a prospective phase III North Central Cancer Treatment Group clinical trial. J Clin Oncol. 2007;25:1255-1259.
263 Antonadou D, Athanassiou H, Sarris N, et al. Final results of a randomized phase III trial of chemoradiation treatment + amifostine in patients with colorectal cancer. Clinical Radiation Oncology Hellenic Group. Int J Radiat Oncol Biol Phys. 2004;60:s140-s141.
264 Ben-Joseph E, Han S, Tobi M, et al. A pilot study of topical intrarectal application of amifostine for prevention of late radiation rectal injury. Int J Radiat Oncol Biol Phys. 2002;53:1160-1164.
265 Kouloulias VE, Kouvaris JR, Pissakas G, et al. Phase II multicenter randomized study of amifostine for prevention of acute rectal toxicity. Topical intrarectal versus subcutaneous application. Int J Radiat Oncol Biol Phys. 2005;62:486-493.
266 Engstrom PF, Benson ABIII, Chen YJ, et al. Rectal cancer. Clinical practice guidelines in oncology. J Natl Comp Cancer Network. 2005;3:492-508.
267 Kwok H, Bissett IP, Hill GL. Preoperative staging of rectal cancer. Int J Colorectal Dis. 2000;15:9-20.
268 Krishnan S, Janjan NA, Hoff PM, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:762-771.
269 Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2008;76:824-830.
270 Czito B, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer. Phase I trial results. J Clin Oncol. 2006;24:656-662.
271 Hospers GAP, Punt CJA, Tesselaar ME, et al. Preoperative chemoradiotherapy with capecitabine and oxaliplatin in locally advanced rectal cancer. A phase I-II multicenter study of the Dutch Colorectal Cancer Group. Ann Surg Oncol. 2007;14:2773-2779.
272 Fakih MG, Rajput A, Yang GY, et al. A phase I study of weekly intravenous oxaliplatin in combination with oral daily capecitabine and radiation therapy in the neoadjuvant treatment of rectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;65:1462-1470.
273 Machiels JP, Duck L, Honhon B, et al. Phase II study of preoperative oxaliplatin, capecitabine, and external beam radiotherapy in patients with rectal cancer. The RadiOxCape study. Ann Oncol. 2005;16:1898-1905.
274 Avallone A, Delrio P, Guida C, et al. Biweekly oxaliplatin, raltitrexed, 5-fluorouracil and folinic acid combination chemotherapy during preoperative radiation therapy for locally advanced rectal cancer. A phase I-II study. Br J Cancer. 2006;94:1809-1815.
275 Valentini V, Coco C, Minsky BD, et al. Randomized multicenter phase IIB study of preoperative chemoradiotherapy in T3 mid-distal recta cancer. Raltitrexed + oxaliplatin + radiotherapy versus cisplatin + 5-fluorouracil radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:403-412.
276 Ryan DP, Niedzwiecki D, Hollis D, et al. Phase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer. Cancer and Leukemia Group B 89901. J Clin Oncol. 2006;24:2557-2562.
277 Bertolini F, Chiara S, Bengala C, et al. Neoadjuvant treatment with single-agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy. A phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;73:466-472.
278 Navarro M, Dotor E, Rivera F, et al. A phase II study of preoperative radiotherapy and concomitant weekly irinotecan in combination with protracted venous infusion 5-fluorouracil for resectable locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2006;66:201-205.
279 Allal A, Bieri S, Gervaz P, et al. Preoperative concomitant hyperfractionated radiotherapy and gemcitabine for locally advanced rectal cancers. A phase I-II trial. Cancer J. 2005;11:133-139.
280 Ohno S, Tomoda M, Tomisaki S, et al. Improved surgical results after combining preoperative hyperthermia with chemotherapy and radiotherapy for patients with carcinoma of the rectum. Dis Colon Rectum. 1997;40:401-406.
281 Huber FT, Stepan R, Zimmermann F, et al. Locally advanced rectal cancer. Resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy. Dis Colon Rectum. 1996;39:774-779.
282 Kallinowski F, Eble MJ, Buhr HJ, et al. Intraoperative radiotherapy for primary and recurrent rectal cancers. Eur J Surg Oncol. 1995;21:191-194.
283 Harrison LB, Minsky BD, Enker WE, et al. High dose rate intraoperative radiation therapy (HDR-IORT) as part of the management strategy for locally advanced primary and recurrent rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:325-330.
284 Ferenschild FT, Vermaas M, Nuyttens JJ, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum. 2006;49:1257-1265.
285 Gunderson LL, Nelson H, Martenson JA, et al. Intraoperative electron and external beam irradiation with or without 5-Fluorouracil and maximum surgical resection for previously unirradiated, locally recurrent colorectal cancer. Dis Colon Rectum. 1996;39:1379-1395.
286 Haddock MG, Nelson H, Donohue JH, et al. Intraoperative electron radiotherapy as a component of salvage therapy for patients with colorectal cancer and advanced nodal metastasis. Int J Radiat Oncol Biol Phys. 2003;56:966-973.
287 Lowy AM, Rich TA, Skibber JM, et al. Preoperative infusional chemoradiation, selective intraoperative radiation, and resection for locally advanced pelvic recurrence of colorectal adenocarcinoma. Ann Surg. 1996;223:177-185.
288 Bussieres E, Gilly FN, Rouanet P, et al. Recurrences of rectal cancers. Results of a multimodal approach with intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1995;34:49-56.
289 Eble MJ, Lehnert T, Treiber M, et al. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol. 1998;49:169-174.
290 Bosset JF, Horiot JC, Hamers HP, et al. Postoperative pelvic radiotherapy with or without elective irradiation of para-aortic nodes and liver in rectal cancer patients. A controlled clinical trial of the EORTC Radiotherapy Group. Radiother Oncol. 2001;61:7-13.
291 Taylor N, Crane C, Skibber J, et al. Elective groin irradiation is not indicated for patients with adenocarcinoma of the rectum extending to the anal canal. Int J Radiat Oncol Biol Phys. 2001;51:741-747.
292 Bonnetain F, Bosset JF, Gerard JP, et al. An analysis of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer on survival in a pooled analysis of EORTC 22921 and FFCD 9203 trials: Surrogacy in question? Proc ASCO. 2011;29:222s. (abstr)
293 Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Proc ASCO. 2011;29:225s. (abstr)
294 Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. Proc ASCO. 2011;29:221s. (abstr)
295 Aschele C, Pinto C, Cordio S, et al. Preoperative fluorouracil (FU) based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: pathologic response analysis of the Studio Terapia Adjuvante Retto (STAR)-01 randomized phase III trial. Proc ASCO. 2009;27:804s. (abstr)
296 Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III ACCORD 12/0405 PRODIGE 2. J Clin Oncol. 2010;28:1638-1644.
297 Roedel C, Becker H, Fietkau R, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: First results of the German CAO/ARO/AIO-04 randomized phase III trial. Proc ASCO. 2011;29:222s. (abstr)