Chapter 27 Patient Selection for Spine Surgery
Between 70% and 85% of all people will have back pain at some time in their lives.1 Lumbar spine disorders are the most common cause of disability in persons younger than 45 years of age.2 More than 500,000 lumbar procedures are performed each year for the treatment of lumbar spine disorders.3 Of those with low back pain (LBP), approximately 151,000 undergo a lumbar fusion each year.4 Given the large number of individuals that experience back pain, one can only surmise that the number of surgeries performed on the lumbar spine will continue to increase. The vast majority of patients with lumbar spine problems do not require surgery. Nonoperative treatment, however, is very expensive, and the data regarding its efficacy demonstrate equivocal results, at best. It is important for the physician to truly appreciate the indications for lumbar spine surgery, and also to become adept at determining the optimal surgical procedure when surgery is indeed indicated. Based on the best evidence available, this chapter addresses patient selection, clinical management results, and surgical outcomes of surgery for LBP.
Patient Evaluation
Individual factors such as work-related injuries and psychosocial support also should be assessed. Low job satisfaction, litigation, and workers’ compensation can be predictive of a poor outcome.5 Trief et al. looked at 160 patients who underwent lumbar spinal fusion. The patients completed preoperative questionnaires regarding their mental health, functional status, workers’ compensation, and job satisfaction. Patients with higher mental component scores reported less back and leg pain.5 In randomized controlled trials (RCTs) from Fairbank et al.6 and Fritzel et al.,7 patients in litigation did worse after spinal fusion than their counterparts who were not in litigation. With these studies, however, both subgroups that underwent surgery (with and without litigation) did better than the matched patients who underwent conservative management. The group of patients with the worst response overall were the patients in litigation who received conservative treatment. This, however, was not statistically significant. In another RCT by Haag et al.,8 several sociologic factors were analyzed 2 years after fusion (workers’ compensation, disability pension, unemployment, sick leave due to back pain, cohabitant/married). Overall, the operative group did better than the conservative treatment group. However, the groups that realized the greatest improvement were patients with a lighter job, not cohabiting/married, and not on sick leave. In a recent literature review, Mroz et al.9 demonstrated that people in litigation usually fare poorly. Nevertheless, they do even worse with conservative treatment. They concluded that socioeconomic factors should not be the sole factor to contraindicate surgical treatment for back pain. Health-related factors such as obesity and smoking also play a role. Recent studies have demonstrated an increase in surgical site infections in morbidly obese patients.10 Elderly obese patients undergoing lumbar surgery report a high rate of dissatisfaction with the surgery outcome compared with nonobese patients.10 Smoking is known to be a predictor of poor surgical outcome in lumbar fusion surgery. Habitual nicotine use is thought to decrease the revascularization of the graft, slowing healing rates and increasing the risk of infection and of pseudarthrosis.11 There are no clear guidelines on preoperative lumbar fusion and cessation of smoking. However, patients should be encouraged to stop smoking as early as possible before undergoing surgery to increase the chance of long-term success.
Chronic LBP may trigger anxiety, depression, and fear, thereby changing the way people perceive pain. The Minnesota Multiphasic Personality Inventory (MMPI) is one of the most widely used personality tests. Patients are asked to answer questions regarding their anxiety and depressive symptoms. The scale attempts to identify patients who are preoccupied with their symptoms, are depressed, or feel a high level of anxiety, because these individuals tend to fare worse.12 These factors are more predictive of a good outcome than physical findings or radiographic measures. Studies have suggested that fear-avoidance beliefs about physical activity and work might form specific cognitions intervening between LBP and disability.13 A Fear-Avoidance Beliefs Questionnaire (FABQ) was developed, based on theories of fear and avoidance behavior and focused specifically on patients’ beliefs about how physical activity and work affected their LBP. FABQ screening could be useful in patient evaluation for lumbar surgery because it could accurately identify subjects with elevated levels of fear.13
After the history is collected, the physical examination should focus on deficits in sensation, muscular weakness, deep tendon reflexes, and any abnormal reflexes such as a Hoffman or Babinski reflex.14 The clinician also should be aware of any suspicious symptoms or signs that are consistent with malingering. These physical examination findings include pain at the top of the tailbone, entire leg pain or numbness, giveaway weakness, persistent pain, intolerance to treatment, and multiple emergency admissions to hospitals with simple backache (Waddell signs). Clinicians should also be wary of patients who present with a gross limp, use of physical supports (e.g., corset, crutches, transcutaneous electrical nerve stimulation [TENS] unit), or any continuous or repetitive movement.15 A well-performed hip examination that includes palpation of the greater trochanter to rule out bursitis as well as rotational maneuvers to rule out primary hip joint pathology is imperative when examining the lumbar spine, as are the shoulder and upper extremity peripheral nerves examination when examining the neck. It is very important to be aware of and not miss the “red flag” signs in a patient with pain of spinal origin. The clinician should consider ordering imaging studies after the first encounter in patients with a history of trauma, night pain, weight loss, cancer, persistent weakness, urinary or fecal incontinence, saddle anesthesia, or constitutional symptoms.16
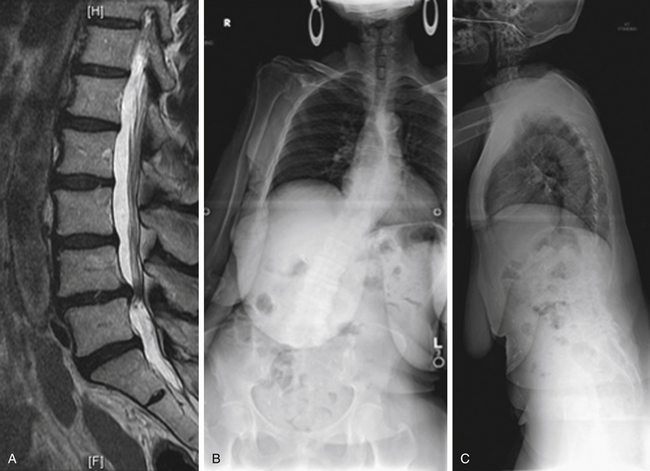
Case Presentation 1: The Importance of Standing Films
The patient presented with a chief complaint of L3, L4, and L5 radiculopathy in the right lower extremity. The MRI examination (Fig. 27-1A) shows stenosis, predominantly foraminal (not shown in this cut). No deformity or instability is observed. On standing radiographs (Figs. 27-1B and C), 12-cm coronal and 17-cm sagittal imbalances are discovered. The imbalances significantly affect the clinical decision-making process.
Low Back Pain: Evidence for Treatment
Low Back Surgery for Adult Low-Grade Spondylolisthesis
Conservative versus Surgical Treatment
Two RCTs,18 (same population, different follow-up) comparing low-grade spondylolisthesis patients with those who presented with either chronic LBP or radiculopathy, or both, treated either with fusion or conservative treatment, found that the surgical group did statistically and clinically better, as assessed by pain scores and the Disability Rating Index (DRI). Even though the long-term follow-up (5 years) showed that some of the initial improvement (1–2 years) had been lost, 76% of the patients in the surgical group still considered their overall outcome as “much better” compared with only 50% in the conservative group. Another prospective RCT found significant difference when treating patients with low-grade spondylolisthesis with an advanced core strengthening program performed by a specialist physical therapist and a control group that performed simple exercises prescribed by their primary physician at 3, 6, and 30 months. The advanced exercise group had better outcomes.19 Sinaki et al. have shown, in a retrospective analysis, significant benefit of flexion exercises in comparison with extension exercises to control the symptoms of low-grade spondylolisthesis.20 Daniel et al. reported very poor results with conservative treatment for spondylosis, including the use of a full-time thoracolumbar orthosis. Twenty-nine of 31 patients failed treatment and progressed to surgery.21
Low-Grade Spondylolisthesis: To Fuse or Not to Fuse? Role of Instrumentation? Best Approach?
When assessing the role of fusion in the management of adult low-grade spondylolisthesis, Herkowitz and Kurz22 performed an RCT comparing decompression alone with decompression and uninstrumented PLF (posterolateral fusion). A mean 3-year follow-up demonstrated better outcomes for leg and back pain for the fusion group (P = .0001). Other authors in prospective nonrandomized23 and retrospective24–26 series also support decompression and fusion over decompression alone when spondylolisthesis is present.
The role of instrumentation in achieving fusion and improving clinical outcomes in low-grade spondylolisthesis was studied by Fischgrund et al.27 in an RCT. Patients were divided into two groups: (1) decompression and noninstrumented PLF and (2) decompression with instrumented PLF (pedicle screws). The fusion rate was 83% versus 45%, respectively, for the instrumented versus noninstrumented groups. Clinical outcomes, however, were similar for both groups (78% vs. 85%, instrumented vs. noninstrumented). Kornblum et al. 28 combined the noninstrumented patients from both the Fischgrund27 and the Herkowitz22 studies to compare 47 patients with either a solid fusion or a nonunion. The solid fusion group had a satisfactory result in 86% of patients, whereas patients in the nonunion group had 56% satisfactory results at 5 to 14 years of follow-up.
Two additional RCTs were performed. In these studies, instrumentation was associated with higher fusion rates and better clinical outcomes for low-grade spondylolisthesis. Zdeblick29 reported a 65% fusion rate and 71% satisfactory clinical outcome in the noninstrumented group, versus a 95% fusion rate and 95% satisfactory clinical outcome in the rigid instrumentation group. Bridwell et al.30 randomized 43 patients into three groups: (1) decompression only, (2) decompression with uninstrumented posterolateral fusion, and (3) decompression with rigid pedicle screw instrumentation posterolateral fusion. The fusion rates were 33% and 87.5% for groups 2 and 3. Clinical improvement was 30%, 33%, and 83.3% for groups 1, 2, and 3, respectively.
Kwon et al.31 reviewed the literature regarding surgical approaches to low-grade spondylolisthesis (ventral/dorsal/360 degrees). In a total of 1100 patients from 34 studies (4 of which were RCTs), the clinical results and fusion rates were better when the combined ventral/dorsal approach was used. When comparing anterior lumbar interbody fusion (ALIF) plus PLF with instrumentation versus ALIF plus instrumentation without PLF, Shofferman et al.32 found that both groups had improvement in function, but no difference in results could be detected.
In 2005, the Scoliosis Research Society released a consensus statement on the treatment of low-grade acquired/isthmic spondylolisthesis,33 making the following points:
• The achievement of a solid fusion is associated with better clinical outcomes.
• The adjunctive use of pedicle screw instrumentation improves fusion rates.
• While instrumentation does not show significant improvement on patient-scored outcome measures, the positive effect on fusion alone warrants its use.
• There is no consensus regarding the approach for fusion—interbody, dorsolateral, or combined.
Lumbar Fusion for Back Pain without Signs of Instability
Pain in the anular region may be triggered by chemical and mechanical sensitization of dorsal anular nociceptors.34 In the 1940s and 1950s, the disc itself was thought to have no nerve supply and, therefore, to have no possibility of generating pain. Subsequent studies have proved this theory wrong. In the normal human disc, sensory nerves extend into the outer third of the anulus. In the degenerated disc, the innervation is deeper and more extensive, with some nerve fibers penetrating into the nucleus pulposus.35 Pain also may be produced by the sensitization and irritation of nerve endings in the end plate.34–38 This model of chemical nociception is supported by numerous studies showing disc immunoreactivity to inflammatory mediators such as substance P and calcitonin as well as elevated levels of prostaglandin E2, interleukin (IL)-2, IL-6, IL-8, phospholipase A2, leukotrienes, thromboxane B2, and tumor necrosis in the degenerated intervertebral disc.39–41 It is now generally accepted that intervertebral discs can be a significant source of back pain. Normal discs resist pain with stimulation because they lack both the chemical sensitization and the mechanical overloading seen in diseased discs.
Since its advent more than 50 years ago, the use of discography has been mired in controversy. Discograms are pain-provoking tests that show radiographic abnormalities of the disc. The procedure is performed with the patient awake in order to assess the level of pain he or she experiences upon injection of normal saline or a water-soluble dye into the intervertebral disc at different segments of the lumbar spine suspected of being abnormal.42 A positive discogram is associated with the elicitation of a concordant pain response coupled with pain-related behavior (e.g., grimacing, guarding, withdrawing, and verbalizing). Provocative discography is no longer used for the routine evaluation of radiculopathy, having been largely replaced by the advent of noninvasive, more sensitive tests such as CT and MRI. The evidence that these modalities are not only safer but also more accurate than plain discography in detecting herniated nuclear material is irrefutable.42
Several authors have attempted to determine the prevalence of discogenic pain in patients suffering from LBP. In one of the most cited studies, Schwarzer et al. found the incidence of IDD (defined in his study by positive discography) to be 39% in 92 patients with chronic LBP.43
The first study to question the validity of discography was published in 1968 by Holt, who found false-positive results in 37% of 30 asymptomatic subjects. All participants were prisoners.44
Some studies have attempted to correlate discography results with surgical findings and outcomes. Colhoun et al. evaluated surgical outcomes in 162 patients who underwent preoperative discography for axial LBP. In the 137 patients whose discography provoked pain, 89% had a favorable outcome at a mean follow-up of 3.6 years. In the 25 patients whose discs showed morphologic abnormalities but no provocation of symptoms, only 52% reported significant benefit.45
Although Colhoun reported successful data, some other studies have not had such positive conclusions. The predictive value of provocative discography on surgical outcome also was assessed in a study by Madan et al. involving 73 patients with chronic LBP. Thirty-two patients underwent spinal fusion based on pain provocation during discography; the remaining 41 patients had surgery without discography. In the discography group, 75.6% of patients had satisfactory outcomes at a minimum 2-year follow-up versus 81.2% in the group who did not have preoperative discography.46 In summary, the lack of strong evidence for the use of discography in fusion surgery to treat degenerative disc disease (DDD) and the methodologic flaws in the existing studies make interpretation of the data exceedingly difficult. Based on the data that are available, the results are mixed as to whether or not preoperative discography improves surgical outcomes in patients with discogenic LBP.47,48
Extensive studies have been performed regarding the use of temporary external fixation before lumbar fusion surgery to help distinguish the area of disease. The aim of externally fixing a lumbar spinal motion segment is to prevent movement, therefore alleviating pain. This may be predictive of subsequent surgical success.49,50 Although some authors have observed improvement in pain51 and found good prediction for satisfactory fusion surgery outcomes with this technique, others found a high rate of complications such as pin site infection, neurologic compromise, and cerebrospinal leakage,52 and found that the technique is a poor predictor of successful fusion surgery.52,53 Currently, this practice has fallen out of favor.
Clinical Results for the Conservative and Surgical Treatment of Mechanical Low Back Pain
The fact that many patients with DDD suffer from other concomitant causes of back pain that may not respond to operative intervention continues to bring significant controversy to the clinical decision-making debate. Although outcome studies for spinal arthrodesis vary widely, it is generally acknowledged to be less beneficial than surgery for radicular pain, with success rates ranging from less than 50% to almost 90%.54 Fritzell et al., in a multicenter RCT, compared conservative treatment to spinal fusion for the treatment of chronic LBP. Forty-six percent of the surgical group reported good to excellent results, while only 18% of patients in the conservative group achieved similar results.7 In another RCT comparing conservative treatment with PLF to treat chronic LBP, Brox et al. report no difference between the two groups.55
In the Pro-Disc total disc replacement IDE RCT (2-year follow-up), Zigler et al. report successful outcomes in 54.9% of patients undergoing ALIF plus PLF, compared with a 69% success rate in patients receiving the disc replacement (P = .03).56 Guyer et al.57 recently published the results of the 5-year follow-up of the U.S. Food and Drug Administration’s IDE trial of the Charité disc. Success results were 57.8% for total disc replacement and 51.2% for the fusion group (ALIF; P = .03). There was no difference between groups in the Oswestry Disability Index (ODI), visual analogue scale, or 36-Item Short Form Health Survey (SF-36) scores. The authors concluded that there was no clinical difference between groups, and that these results were consistent with the 2-year Charité outcomes previously published.58 Greater clinical success (i.e., good results between 70% and 90%) in lumbar fusion surgery for back pain (using different techniques, e.g., uninstrumented and instrumented PLF, ALIF, posterior lumbar interbody fusion, transforaminal lumbar interbody fusion) are found in smaller, less strictly controlled studies. Most of them were case series.59–63
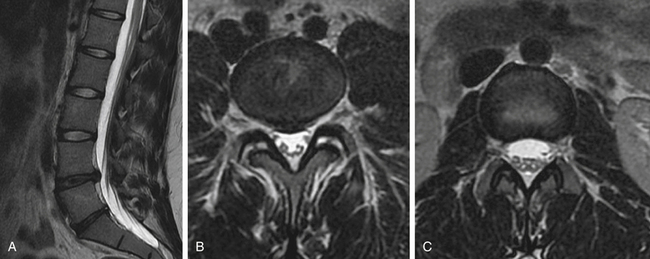
Case Presentation 2: Each Case Is Unique
A 21-year-old female college student presented with a history of LBP for 6 years despite multiple trials of physical therapy and oral medication. Pain was worsened in extension and better in flexion. There was no radiculopathy. Sagittal MRI (Fig. 27-2A) showed degeneration of the L4-5 and L5-S1 discs. Coronal MRI showsed L4-5 bilateral facet joint incompetence with an increased amount of synovial fluid and early degenerative changes (Fig. 27-2B). Fig 27-2C showed normal L3-4 facets for comparison. The patient enjoyed 100% pain relief (at 1 and 4 months) with bilateral L4-5 facet injections with steroids and bupivacaine.
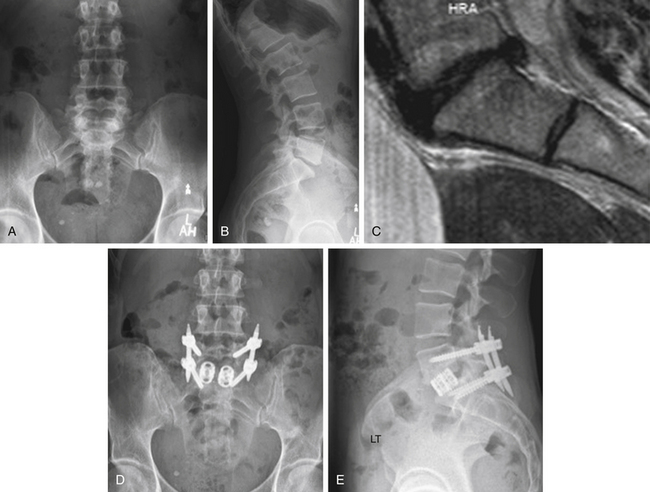
Case Presentation 3: Patient Selection Is Paramount
A 42-year-old female physical therapist and runner (Fig. 27-3) had given up running due to a 2-year history of mechanical LBP. She exhibited no radicular symptoms. Pain had become incapacitating and was interfering not only with running but with daily activities. Preoperative radiographs were obtained (see Figs. 27-3A and B). On MRI (see Fig. 27-3C), the only abnormality was degeneration of the L5-S1 disc. After several months of aggressive medical management and after thorough psychosocial evaluation, the patient underwent an L5-S1 ALIF with percutaneous pedicle screw fixation (Figs. 27-3D and E). She is pain free following surgery. She won a 10K race 6 months postoperatively.
Esses S.I., Botsford D.J., Kostuilk J.P. The role of external skeletal fixation in the assessment of low-back disorders. Spine (Phila Pa 1976). 1989;14:594-601.
Ghogawala Z., Benzel E.C., Amin-Hanjani S., et al. Prospective outcomes evaluation after decompression with and without instrumented fusion for lumbar stenosis and degenerative grade I spondylolisthesis. J Neurosurg Spine. 2004;1:267-272.
Holt E.P. The question of lumbar discography. J Bone Joint Surg [Am]. 1968;50:720-726.
Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg [AM]. 2006;88(Suppl 2):21-24.
LaCaile R., DeBerard M., Masters K. Presurgical biopsychosocial factors predict multidimensional outcomes of interbody lumbar fusion. Spine (Phila Pa 1976). 2005;5:71-78.
Olerud S., Sjostrom L., Karlstrom G., Hamberg M. Spontaneous effect of increased stability of the lower lumbar spine in cases of severe chronic low back pain. The answer of an external transpeduncular fixation test. Clin Orthop Relat Res. 1986;203:67-74.
Waddell G., McCulloch J.A., Kummel E., Venner R.M. Nonorganic physical signs in low-back pain. Spine (Phila Pa 1976). 1980;5:117-125.
1. Chou R., Huffman L. Evaluation and management of low back pain: evidence review. Glenview, IL: American Pain Society; 2009.
2. Chou R., Loeser J., Owens D.K., et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34(10):1066-1077.
3. Katz J.N. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg [Am]. 2006;88(Suppl 2):21-24.
4. Lipson S.J. Spinal fusion surgery – advances and concerns. N Engl J Med. 2004;350(7):643-644.
5. Olsen M., Mayfield J., Lauryssen C. Risk factors for surgical site infection in spinal surgery. J Neurosurg. 2003;98:149-155.
6. Fairbank J., Frost H., Wilson-McDonald J., et al. Randomized controlled trial to compare surgical stabilization of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC stabilization trial. BMJ. 2005;330:1233.
7. Fritzell P., Haag O., Wessberg P., et al. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine (Phila Pa 1976). 2001;26(23):2521-2532.
8. Haag O., Fritzell P., Ekselius L., et al. Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish Lumbar Spine Study. Eur Spine J. 2003;12:22-33.
9. Mroz T., Norvell D., Ecker E., et al. Fusion vs nonoperative management for chronic low back pain. Do sociodemographic factors alter outcomes? Spine (Phila Pa 1976). 2011:S75-S86. 12S
10. Telfein A., Reiter G., Durham S., Murcotte P. Spine surgery in morbidly obese patients. J Neurosurg. 2002;97:20-24.
11. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;9:942-943.
12. LaCaile R., DeBerard M., Masters K. Presurgical biopsychosocial factors predict multidimensional outcomes of interbody lumbar fusion. Spine (Phila Pa 1976). 2005;5:71-78.
13. Waddel G., Newton M., Henderson I., et al. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157-168.
14. Waddell G., Somerville D., Henderson I., Newton M. Objective clinical evaluation of physical impairment in chronic low back pain. Spine (Phila Pa 1976). 1992;17:617-628.
15. Waddell G., McCulloch J.A., Kummel E., Venner R.M. Nonorganic physical signs in low-back pain. Spine (Phila Pa 1976). 1980;5:117-125.
16. Scavone J.G., Latshaw R.F., Rohrer G.V. Use of lumbar spine films. Statistical evaluation at a university teaching hospital. JAMA. 1981;246:1105-1108.
17. Moller H., Hedlund R. Surgery vs. conservative management in adult isthmic spondylolisthesis—a prospective ramdomized study. Part 1. Spine (Phila Pa 1976). 2000;25:1711-1715.
18. Ekman P., Moller H., Hedlund R. The long term effect of PLF in adult isthmic spondylolisthesis: a randomized controlled study. Spine J. 2005;5:36-44.
19. O’Sullivan P.B., Phyty G.D., Twomey L.T., et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiographic diagnosis of spondylosis or spondylolisthesis. Spine (Phila Pa 1976). 1997;22:2959-2967.
20. Sinaki M., Lutness M.P., Isltrup D.M., et al. Lumbar spondylolisthesis: retrospective comparison and three year FU of two conservative treatment programs. Arch Phys Med Rehabil. 1989;70:594-598.
21. Daniel J.N., Polly D.W.Jr., Van Dam B.E. A study of the efficacy of nonoperative treatment of presumed traumatic spondylolisthesis in a young patient population. Mil Med. 1995;160:553-555.
22. Herkowitz H.N., Kurz L.T. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am]. 1991;73:802-808.
23. Ghogawala Z., Benzel E.C., Amin-Hanjani S., et al. Prospective outcomes evaluation after decompression with and without instrumented fusion for lumbar stenosis and degenerative grade I spondylolisthesis. J Neurosurg Spine. 2004;1:267-272.
24. Feffer H.L., Wiesel S.W., Cuckler J.M., et al. Degenerative spondylolisthesis. To fuse or not to fuse. Spine (Phila Pa 1976). 1985;10:287-289.
25. Lombardi J.S., Wiltse L.L., Reynolds J., et al. Treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976). 1985;10:821-827.
26. Yone K., Sakou T., Kawauchi Y., et al. Indication for fusion in lumbar spinal stenosis in elderly patients and its significance. Spine (Phila Pa 1976). 1996;21:242-248.
27. Fischgrund J.S., Mackay M., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976). 1997;22:2807-2812.
28. Kornblum M.B., Fischgrund J.S., Herkowitz H.N., et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long term study comparing fusion and pseudoarthrosis. Spine (Phila Pa 1976). 2004;29:726-733.
29. Zdeblick T.A. A prospective, randomized, study of lumbar fusion: preliminary results. Spine (Phila Pa 1976). 1993;18:983-991.
30. Bridwell K.H., Segewick T.A., O’Brien M.F. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461-472.
31. Kwon B.K., Hilibrand A.S., Malloy K., et al. A critical analysis of the literature regarding surgical approach and outcome for adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech. 2005;18S:S30-S40.
32. Schofferman J., Slosar P., Reynolds J., et al. A prospective ramdomized comparison of 270 degrees fusions and 360 degrees fusions (circumferential fusions). Spine (Phila Pa 1976). 2001;26:E207-E212.
33. Mardjetko S., Albert T., Anderson G., et al. Spine/SRS spondylolisthesis summary statement. Spine (Phila Pa 1976). 2005;30:6S.
34. Wiberg G. Back pain in relation to nerve supply of the intervertebral disc. Acta Orthop Scand. 1947;19:211-221.
35. Ikari C. A study of the mechanism of low back pain. The neurohistological examination of the disease. J Bone Joint Surg [Am]. 1954;36:195.
36. Freemont A.J., Peacock T.E., Goupille P., et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
37. Coppes M.H., Marani E., Thomeer R.T., et al. Innervation of annulus fibrosis in low back pain. Lancet. 1990;336:189-190.
38. Coppes M.H., Marani E., Thomeer R.T., Groen G.J. Innervation of “painful” lumbar discs. Spine (Phila Pa 1976). 1997;22:2342-2349.
39. Kang J.D., Georgescu H.I., McIntyre-Larkin L., et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976). 1996;21:271-277.
40. Burke J.G., Watson R.W., McCormack D., et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg [Br]. 2002;84:196-201.
41. Saal J.S., Franson R.C., Dobrow R., et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine (Phila Pa 1976). 1990;15:674-678.
42. Carragee E.J., Chen Y., Tanner C.M., et al. Provocative discography in patients after limited lumbar discectomy. Spine (Phila Pa 1976). 2000;25:3065-3071.
43. Schwarzer A.C., Aprill C.N., Derby R., et al. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;17:1878-1883.
44. Holt E.P. The question of lumbar discography. J Bone Joint Surg [Am]. 1968;50:720-726.
45. Colhoun E., McCall I.W., Williams L., Cassar Pullicino V.N. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg [Br]. 1988;70:267-271.
46. Madan S., Gundanna M., Harley J.M., et al. Does provocative discography screening of discogenic back pain improve surgical outcomes? J Spinal Disord Tech. 2002;15(3):245-251.
47. Gibson J.N., Waddell G., Grant I.C. Surgery for degenerative lumbar spondylosis. Cochrane Database Syst Rev. 2, 2000. CD001352
48. Deyo R.A., Nachemson A., Mirza S.K. Spinal-fusion surgery—The case for restraint. N Engl J Med. 2004;350:722-726.
49. Esses S.I., Botsford D.J., Kostuilk J.P. The role of external skeletal fixation in the assessment of low-back disorders. Spine (Phila Pa 1976). 1989;14:594-601.
50. Bednar D.A., Raducan V. External spinal skeletal fixation in the management of back pain. Clin Orthop Relat Res. 1996;322:131-139.
51. Olerud S., Sjostrom L., Karlstrom G., Hamberg M. Spontaneous effect of increased stability of the lower lumbar spine in cases of severe chronic low back pain. The answer of an external transpeduncular fixation test. Clin Orthop Relat Res. 1986;203:67-74.
52. Faraj A.A. External fixation in lumbar segmental instability. Acta Orthop Belg. 2003;69:9-12.
53. Bednar D.A. Failure of external spinal skeletal fixation to improve the predictability of lumbar arthrodesis. J Bone Joint Surg [Am]. 2001;83:1656-1659.
54. Junge A., Frohlich M., Ahrens S., et al. Predictors of bad and good outcome of lumbar spine surgery. A prospective clinical study with 2 years’ follow up. Spine (Phila Pa 1976). 1996;21:1056-1064.
55. Brox J.I., Sorensen R., Friis A., et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine (Phila Pa 1976). 2003;28:1913-1921.
56. Zigler J., Delamarter R., Spivak J.M., et al. Results of the prospective, randomized, multicenter, FDA IDE study of lumbar total disc replacement vs circumferential fusion for the treatment of 1 level degenerative disease. Spine (Phila Pa 1976). 2007;32:1155-1162.
57. Guyer R.D., McAfee P.C., Banco R.J., et al. Prospective, randomized, multicenter FDA IDE study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: five year follow up. Spine J. 2009;9:374-386.
58. Blumenthal S., McAfee P.C., Guyer R.D., et al. A prospective, randomized, multicenter FDA IDE study of lumbar total disc arthroplasty with the CHARITE artificial disc vs lumbar fusion. Part I: evaluation of clinical outcomes. Spine (Phila Pa 1976). 2005;30:1565-1575.
59. McCullogh J.A. Uninstrumented PL lumbar fusion for single level isolated disc resorbtion and/or degenerative disc disease. J Spinal Disord. 1999;12:34-39.
60. Lorenz M., Zindrick M., Schwaegler P., et al. A comparison of single level fusions with and without hardware. Spine (Phila Pa 1976) 16(Suppl 8). 1991:S455-S458.
61. Loguidice V.A., Johnson R.G., Guyer R.D., et al. Anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1988;13:366-369.
62. Moore K.B., Pinto M.R., Butler L.M. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine (Phila Pa 1976). 2002;27:1680-1686.
63. Lowe T.G., Tahernia A.D., O’Brien M.F. Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique and 2 year results. J Spinal Disord Tech. 2002;15:31-38.