CHAPTER 37 Pathophysiology of Subdural Hematomas
Chronic subdural hematoma (CSDH) is frequently encountered in neurosurgical practice and occurs at a rate of 1 to 2 per 100,000 per year. Nonetheless, there has been ongoing debate over the fundamental pathophysiologic mechanisms of the development, evolution, and recurrence of CSDH. Virchow1 in 1857 first described pachymeningitis haemorrhagica and ascribed the condition to dural inflammation; however, by the early 20th century, the traumatic nature of CSDH was established and widely accepted. During the subsequent century, through ultrastructural analysis of subdural membranes and various components of CSDH fluid, a complex pathophysiology has evolved.
Pathophysiology of the Development of Chronic Subdural Hematomas
Grossly, CSDHs may vary in color from clear yellow to dark purple and in consistency from thin liquid to semisolid. A thin, often translucent inner membrane and a thicker outer membrane often encapsulate the hematoma.2,3 The contents of the hematoma and the histology of the outer membrane have been the focus of investigation on the mechanisms by which CSDHs develop and expand.2–6
In 1932, Gardner first proposed the osmotic gradient theory as the predominant pathophysiology of CSDH. He postulated that the increased protein content in CSDH fluid causes ingress of fluid as a result of increased oncotic pressure.7 Although CSDH fluid contains high levels of protein and lipid, Weir showed CSDH fluid to be isosmotic to both blood and cerebrospinal fluid.8 Instead of an osmotic force pulling plain water into the CSDH, microscopic examination of fluid from CSDHs of any age reveals fresh erythrocytes, thus indicating that clinically silent rehemorrhage or progressive leakage of fresh blood into the CSDH is ongoing.9,10
The probable source of rebleeding is the CSDH membranes. These membranes consist of blood vessels, eosinophils, smooth muscle cells, fibroblasts, and myofibroblasts supported by a matrix of collagen and elastin.4,5,11 The membranes arise from cleavage of the dural border layer, which results in dural border cells on both sides of the hematoma cavity. Some elements of both the inner and outer membranes resemble this dural border layer; however, the presence of blood vessels and eosinophils and attenuation of the remaining cells set a CSDH membrane apart.4
Blood vessels are absent in the normal dura-arachnoid interface. Neovasculature is abundant, but just in the outer CSDH membrane. Abnormal dilated sinusoids measuring as large as 1000 µm, with an incomplete basement membrane and attenuated endothelial cells, share the outer membrane with rapidly growing microcapillaries. Both vessel types are composed of endothelial cells with irregular surface because of numerous pseudopod-like structures extending into the vascular lumen.6 Erythrocytes and platelets in various stages of degeneration are frequently found deposited in the perivascular space.4,6 These sinusoids contain gap junctions as large as 8 µm, sufficient to allow leakage of plasma and even red blood cells into the hematoma cavity.12
Inflammatory mediators present in CSDH fluid may potentiate chronic rebleeding of the fragile neovasculature. Kallikrein, bradykinin, and platelet-activating factor (PAF) have all been identified at significant levels in CSDH fluid. These inflammatory mediators stimulate vasodilation, increase vascular permeability, prolong the clotting time, and release tissue plasminogen activator (t-PA) from endothelial cells.13–15 Other work has focused on disturbances of the prostaglandin system (local and potentially systemic) as possible components in the pathophysiology.16
Eosinophil degranulation in the outer membrane may be the source of the fibrinolytic factors and inflammatory mediators causing local coagulopathy and cell destruction in the CSDH. An abundance of eosinophils in the outer membrane has been noted for decades.17,18
Evolution of Chronic Subdural Hematomas
As early as 1826, Bayle proposed that repeated bleeding episodes cause the ongoing expansion of CSDHs.1 Decades of clinical experience and modern technologies—including computed tomography, magnetic resonance imaging, advanced microscopy, molecular biology techniques, and other innovations—have further supported his theory. In an early computed tomographic study, Bergström and colleagues19 showed that acute subdural hematomas undergo a predictable loss of attenuation over time, but that some CSDHs return to high attenuation. They postulated that this change reflects an instance of sudden or chronic rebleeding as predicted by Bayle.19
As discussed previously, the presence of defective neovasculature certainly potentiates the process of rebleeding and absorption of plasma proteins into the subdural space, but this does not fully explain the growth of CSDHs. Normal hemostatic mechanisms should halt the process long before the CSDH reaches a clinically significant size.15,19,20 Instead, considerable evidence supports the presence of a localized coagulopathy within the CSDH.
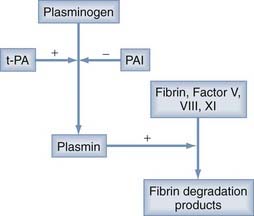
Labadie and Glover21 analyzed fluid from two recurrent CSDHs. They found accelerated clot formation by the partial thromboplastin time and normal formation by the prothrombin time; however, the clots formed were structurally defective in comparison with controls. They postulated that the clots within CSDHs degrade rapidly, a supposition supported by high levels of fibrin degradation products (FDPs) and low mean plasminogen in their fluid samples.21 FDPs inhibit coagulation, platelet aggregation, and fibrin polymerization and promote the activity of t-PA.22
Subsequent studies have identified lower levels of all coagulation factors in CSDH fluid than in plasma. Factors II,V, VII, VIII, and X are disproportionately depleted.23 These findings reflect a phase of accelerated fibrinolytic activity after the rapid and defective clot formation. The end result is a milieu of anticoagulant proteins (chiefly FDPs) and depleted coagulation factors.2,21–24
The source of this accelerated fibrinolysis may be t-PA. t-PA transforms plasminogen into plasmin, which, in turn, degrades fibrin to fibrin split products (Fig. 37-1). Investigations by Ito and associates25 found a threefold increase in t-PA in the outer membranes of CSDHs as opposed to the dura. Weir and Gordon24 corroborated Ito and colleagues’ work by showing increased t-PA and decreased α2-antiplasmin (an inhibitor of plasminogen activation) in CSDH fluid.
Other authors have suggested that the PAF derived from lysis of red blood cells may stimulate the synthesis and release of t-PA, as well as induce chemotaxis of inflammatory cells to the CSDH membranes. They found increased levels of PAF in CSDH fluid and elevated plasma levels of PAF in patients with CSDH versus healthy volunteers. The latter observation may suggest a systemic predilection to the development of CSDH.15
In measurements of CSDH fluid from 23 patients, Lim and coworkers22 found a correlation between the ratio of t-PA–to–plasminogen activator inhibitor (PAI) and hematoma size. These authors postulated that PAI may interrupt the hyperfibrinolysis to slow the growth of CSDHs or promote gradual spontaneous resorption.22
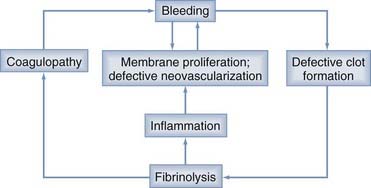
Localized coagulopathy and defective clot formation within the CSDH completes the cycle of bleeding, membrane formation, defective vascularization, fibrinolysis, coagulopathy, and rebleeding (Fig. 37-2).3,22
Treatment of Chronic Subdural Hematomas
Surgical Therapy
Effective treatment of CSDH does not seem to require treating the underlying pathophysiology. In 1925, Putnam and Cushing26 published an extensive work on CSDHs and advocated craniotomy with complete removal of the outer membrane and hematoma contents as the procedure of choice.26 As noted earlier, the pathophysiology of CSDH development and evolution would appear to support such an approach; however, clinical experience has been to the contrary.
Svien and Gelety27 reported in 1964 that CSDH patients treated by bur hole drainage had better outcomes and lower reoperation rates than did those undergoing craniotomy.27 Tabaddor and Shulmon28 corroborated this finding with a 1977 study comparing twist drill craniostomy, bur holes, and craniotomy. The craniotomy group had the highest mortality rate and poorest outcomes.28
More recent debate has focused on the value of irrigating the CSDH cavity. It has been proposed that washing out the coagulopathic and hyperfibrinolytic milieu of the CSDH fluid would minimize recurrence; however, at least two recent studies failed to corroborate a benefit of irrigation. Suzuki and associates29 found closed system drainage without irrigation to be as effective as closed system drainage with irrigation.29 Smely and coauthors30 reported that twist drill drainage without irrigation was superior to bur hole drainage with irrigation, although other studies have shown contrary results. Thus, clinical experience would suggest that many aspects of the pathophysiology of CSDH are self-limited and need not be addressed at surgery.3
Medical Therapy
Medical therapy, including corticosteroids, osmotic agents, and angiotensin-converting enzyme (ACE) inhibitors, may interrupt the pathophysiology of CSDH. Eosinophils (present in the outer membranes of many CSDH specimens) have been invoked as driving the hyperfibrinolysis, vasodilation, and vascular permeability of CSDH. Corticosteroid therapy decreases leukocyte chemotaxis, inhibits degranulation, and has been shown to inhibit neomembrane formation and prevent clot enlargement in one experimental model of CSDH.31 The membranes of patients undergoing osmotic therapy have been demonstrated on ultrastructural analysis to lack new capillary growth and have decreased vascular permeability, thus suggesting potential clinical therapeutic use of these agents.32 In this regard, some clinical evidence supports the potential efficacy of these treatments. Bender and Christoff33 reported a series of 97 CSDH patients with symptoms ranging from headache to stupor managed primarily with bed rest, mannitol and, later in the study, corticosteroids. In 75 cases the CSDH resolved, and only 2 of these patients had residual deficits. Twenty-two of the original 97 patients failed medical management and required surgery. The authors noted more rapid neurologic improvement after introducing corticosteroids to the treatment regimen, thereby allowing shorter hospitalization.33
Neovascularization is a key component of membrane formation and probably the primary source of renewed hemorrhage. Recent evidence suggests that ACE inhibitor therapy may interrupt this pathophysiology by systemically inhibiting vascular endothelial growth factor. A recent retrospective review of 438 CSDH patients found a lower percentage of hematomas in patients regularly taking ACE inhibitors than in age-matched controls, thus suggesting that ACE inhibitor therapy prevents the evolution of CSDH. They also found a statistically significant decrease in the postoperative recurrence rate (5% with ACE inhibition versus 18% without).34 It remains to be seen whether ACE inhibitor use initiated at the time of diagnosis of CSDH has similar clinical benefit.
Recurrence
Reported recurrence rates of CSDH range from 2% to 37%, largely because the pathophysiologic mechanisms have not been fully elucidated. Theories range from recurrent outer membrane bleeding, to brain stiffness preventing reexpansion, to altered cerebral blood flow and its effects on reexpansion. These theories remain the subject of debate because, as noted, removal of the outer membrane is not necessary to achieve lasting CSDH resolution, and the patient population most subject to the development of CSDH tend to have atrophic, stiff brains with preexisting derangements in blood flow.35–39
Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31:73-79.
Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45:393-397.
Hirashima Y, Endo S, Kato R, et al. Platelet-activating factor (PAF) and the development of chronic subdural haematoma. Acta Neurochir. 1994;129:20-25.
Hirashima Y, Nagahori T, Nishijima M, et al. [Analysis of plasma and hematoma lipids related to choline glycerophospholipid in patients with chronic subdural hematoma.]. Neurol Med Chir (Tokyo). 1994;34:131-135.
Ito H, Komai T, Yamamoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978;48:197-200.
Ito H, Yamamoto S, Komai T, et al. Role of local hyper-fibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976;45:26-31.
Killeffer JA, Killeffer FA, Schochet SS. The outer neomembrane of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:407-412.
Labadie EL, Glover D. Local alterations of hemostatic-fibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975;25:669-675.
Müller W, Firsching R. Significance of eosinophilic granulocytes in chronic subdural hematomas. Neurosurg Rev. 1990;13:305-308.
Putnam TJ, Cushing H. Chronic subdural hematoma: its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Arch Surg. 1925;11:329-393.
Stoodley M, Weir B. Contents of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:425-434.
Svien HJ, Gelety JE. On the surgical management of encapsulated subdural hematoma: a comparison of the results of membranectomy and simple evacuation. J Neurosurg. 1964;21:172-177.
Weigel R, Hohenstein A, Schlickum L, et al. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61:788-793.
Weir B. The osmolality of subdural hematoma fluid. J Neurosurg. 1971;34:528-533.
Weir BK, Gordon P. Factors affecting coagulation, fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983;58:242-245.
Wilberger J. Pathophysiology of evolution and recurrence of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:435-438.
Yamashima T, Shimoji T, Komai T, et al. [Growing mechanism of chronic subdural hematoma: light and electron microscopic study on outer membranes of chronic subdural hematoma (authors’ transl).]. Neurol Med Chir (Tokyo). 1978;18:734-752.
Yamashima T, Yamamoto S. How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery. 1984;15:672-678.
1 Virchow R. Das heamotom der dura mater. Verhandlungender Phys Med Gesellesch. 1857;24:134-142.
2 Stoodley M, Weir B. Contents of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:425-434.
3 Wilberger J. Pathophysiology of evolution and recurrence of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:435-438.
4 Killeffer JA, Killeffer FA, Schochet S. The outer neomembrane of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:407-412.
5 Yamashima T. The inner membrane of chronic subdural hematomas: pathology and pathophysiology. Neurosurg Clin N Am. 2000;11:413-424.
6 Yamashima T, Yamamoto S. How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery. 1984;15:672-678.
7 Gardner W. Traumatic subdural hematoma with particular reference to the latent interval. Arch Neurol Psychol. 1932;27:846-858.
8 Weir B. The osmolality of subdural hematoma fluid. J Neurosurg. 1971;34:528-533.
9 Ito H, Yamamoto S, Komai T, et al. Role of local hyper-fibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 1976;45:26-31.
10 Kao M. Sedimentation level in chronic subdural hematoma visible on computerized tomography. J Neurosurg. 1983;58:246-251.
11 Yoshihiko T, Akira O, Fumihide Y, et al. [Ultrastructure of collagen fibers in the outer membrane of recurrent chronic subdural hematoma.]. Neurol Med Chir (Tokyo). 1996;36:627-631.
12 Yamashima T, Shimoji T, Komai T, et al. [Growing mechanism of chronic subdural hematoma; light and electron microscopic study on outer membranes of chronic subdural hematoma (authors’ transl).]. Neurol Med Chir (Tokyo). 1978;18:734-752.
13 Fujisawa H, Ito H, Kashiwagi S, et al. Kallikrein-kinin system in chronic subdural haematomas: its roles in vascular permeability and regulation of fibrinolysis and coagulation. J Neurol Neurosurg Psychiatry. 1995;59:388-394.
14 Hirashima Y, Endo S, Kato R, et al. Platelet-activating factor (PAF) and the development of chronic subdural haematoma. Acta Neurochir. 1994;129:20-25.
15 Hirashima Y, Nagahori T, Nishijima M, et al. [Analysis of plasma and hematoma lipids related to choline glycerophospholipid in patients with chronic subdural hematoma.]. Neurol Med Chir (Tokyo). 1994;34:131-135.
16 Matsumori K, Yoshioka M. [Kinetics of prostaglandin and its significance in chronic subdural hematoma.]. Neurol Med Chir (Tokyo). 1987;27:498-504.
17 Friede R. Incidence and distribution of neomembranes of dura mater. J Neurol Neursurg Psychiatry. 1971;34:439-446.
18 Müller W, Firsching R. Significance of eosinophilic granulocytes in chronic subdural hematomas. Neurosurg Rev. 1998;13:305-308.
19 Bergström H, Ericson K, Levander B, et al. CT of cranial subdural and epidural hematomas: variation of attenuation related to time and clinical events such as rebleeding. J Comput Assist Tomogr. 1977;1:445-449.
20 Elie M, Primeau F, Cole MG. Chronic subdural hematoma in the elderly: a case report. J Geriatr Psychiatry Neurol. 1996;9:100-101.
21 Labadie EL, Glover D. Local alterations of hemostatic-fibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975;25:669-675.
22 Lim DJ, Chung YG, Park YK, et al. Relationship between tissue plasminogen activator, plasminogen activator inhibitor and CT image in chronic subdural hematoma. J Korean Med Sci. 1995;10:373-378.
23 Kawakimi Y, Tanimoto T, Shimamura Y. [Coagulopathy in chronic subdural hematoma.]. Neurol Med Chir (Tokyo). 1991;31:32-36.
24 Weir BK, Gordon P. Factors affecting coagulation, fibrinolysis in chronic subdural fluid collections. J Neurosurg. 1983;58:242-245.
25 Ito H, Komai T, Yamamoto S. Fibrinolytic enzyme in the lining walls of chronic subdural hematoma. J Neurosurg. 1978;48:197-200.
26 Putnam TJ, Cushing H. Chronic subdural hematoma: its pathology, its relation to pachymeningitis hemorrhagica and its surgical treatment. Arch Surg. 1925;11:329-393.
27 Svien HJ, Gelety JE. On the surgical management of encapsulated subdural hematoma: a comparison of the results of membranectomy and simple evacuation. J Neurosurg. 1964;21:172-177.
28 Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220-226.
29 Suzuki K, Sugita K, Akai T, et al. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50:231-234.
30 Smely C, Madlinger A, Scheremet R. Chronic subdural hematoma—a comparison of two different treatment modalities. Acta Neurochir. 1997;139:818-825.
31 Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45:393-397.
32 Suzuki J, Takaku A. Nonsurgical treatment of chronic subdural hematoma. J Neurosurg. 1970;33:548-553.
33 Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31:73-79.
34 Weigel R, Hohenstein A, Schlickum L, et al. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61:788-793.
35 Fukuhara T, Gotoh M, Asari S, et al. The relationship between brain surface elastance and brain reexpansion after evacuation of chronic subdural hematoma. Surg Neurol. 1996;45:570-574.
36 Ishikawa T, Kawamura S, Hadeshi H, et al. Cerebral blood flow and oxygen metabolism in hemiparetic patients with chronic subdural hematoma. Quantitative evaluation using positron emission tomography. Surg Neurol. 1995;43:130-136.
37 Kuwabara H. Regional cerebral blood flow and metabolism in chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:499-502.
38 Tanaka A, Nakayama Y, Yoshinaga S. Cerebral blood flow and intracranial pressure in chronic subdural hematomas. Surg Neurol. 1997;47:346-351.
39 Tanaka A, Yoshinaga S, Kimura M. Xenon-enhanced computed tomographic measurement of cerebral blood flow in patients with chronic subdural hematomas. Neurosurgery. 1990;27:554-561.