Chapter 142 Pathology of Choroidal Melanoma
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Processing of specimens
Gross examination
Different types of tissue obtained include cytologic material, a tumor biopsy (incisional or excisional), or an enucleated globe. Clinical information and the type of fixative are noted. Gross examination depends on the submitted specimen:1–4 For cytologic material, the submitted material (e.g., number of slides, quantity and appearance of fluid) is registered. For tumor biopsies, the number of pieces, size (three dimensions if possible), and descriptive features (e.g., color) are noted. A globe should be evaluated according to the guidelines of the Association of Directors of Anatomic and Surgical Pathology.1–4 After orientation of the globe, careful inspection for extraocular growth is followed by transillumination to localize the tumor and to measure its basal diameter. Vortex veins are submitted in different cassettes labeled respective to their localization. A standard section of the globe incorporates the pupil-optic nerve (p-o) section and the tumor. Detailed macroscopic examination and photodocumentation of the entire globe as well as a thorough description (including evidence of previous treatment) and tumor measurements are recommended.
Staining
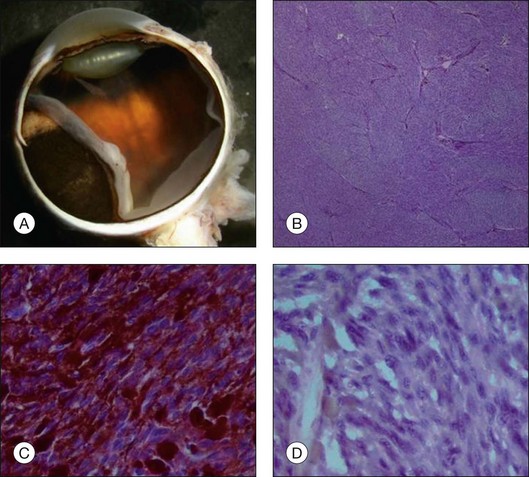
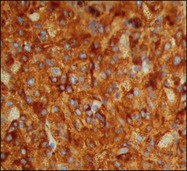
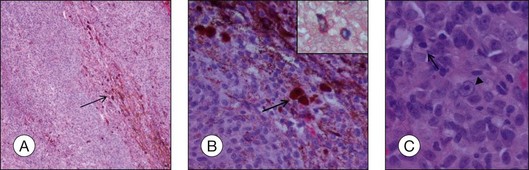
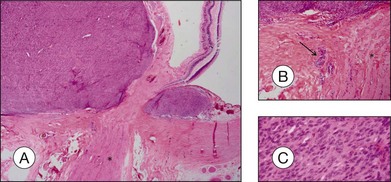
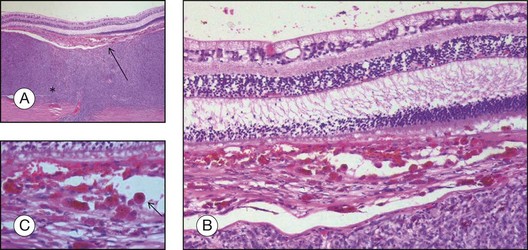
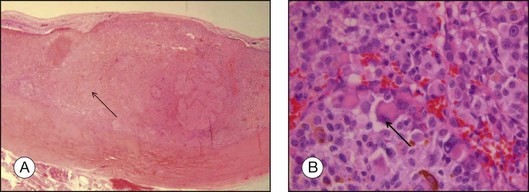
Routine stains for choroidal melanoma comprise hematoxylin and eosin (H&E), periodic acid Schiff (PAS), and bleached H&E stains, to reveal the cytologic features in heavily pigmented tumors (Fig. 142.1). Immunohistochemical stains are not routinely used for the diagnosis of uveal melanoma but may be helpful in selected cases including fine-needle aspiration biopsy (FNAB) samples.
Gross appearance of choroidal melanoma
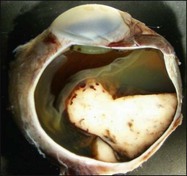
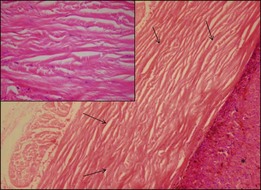
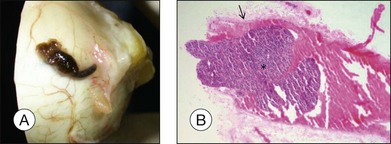
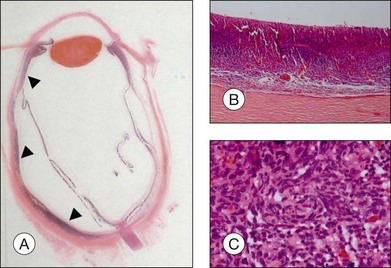
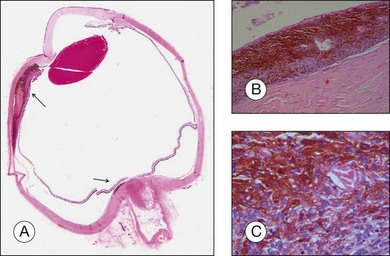
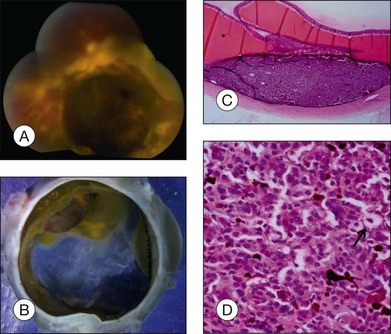
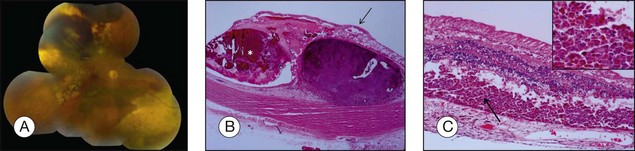
The gross examination of intraocular tumors is the basis for clinicopathologic correlations as it allows for a detailed macroscopic evaluation and measurement of the tumor size including the prognostically relevant largest basal diameter.5,6 The largest basal diameter can be reliably assessed on gross examination, although the tumor size may be underestimated when measured after fixation due to tumor shrinkage.7 Choroidal melanomas exhibit an oval or fusiform shape (Fig. 142.2) when confined by Bruch’s membrane or a collar-button/mushroom configuration (Fig. 142.3) when the tumor has broken through Bruch’s membrane.
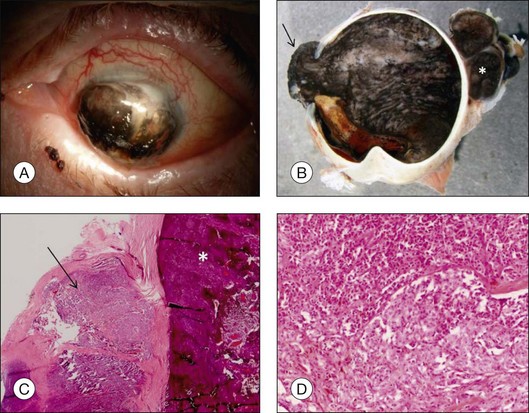
Ocular structures may be invaded by choroidal melanomas. Invasion into the ciliary body may be detected macroscopically, whereas invasion into the sclera, retina, and optic nerve as well as invasion into the vitreous, iris, and anterior chamber is usually detected by microscopic examination. Extraocular extension (Fig. 142.4) usually occurs along vortex veins or emissary canals and, dependent on the extent, may be diagnosed clinically or on gross examination.
The amount of pigmentation of uveal melanomas varies from tumor to tumor or even within a single tumor and may be related to overall survival.5,8 Most tumors exhibit mild to moderate pigmentation, although heavily or non-pigmented (amelanotic) choroidal melanomas (Fig. 142.5) may be observed.
Histopathologic features of tumor cells and their prognostic relevance
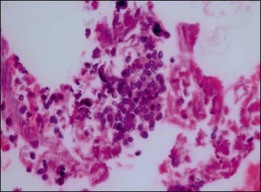
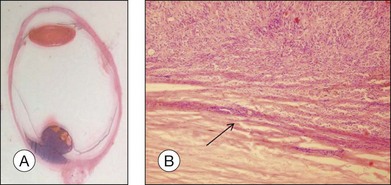
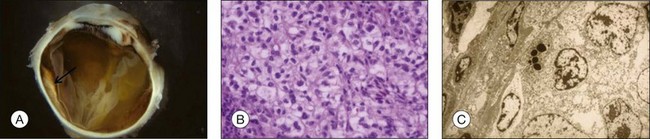
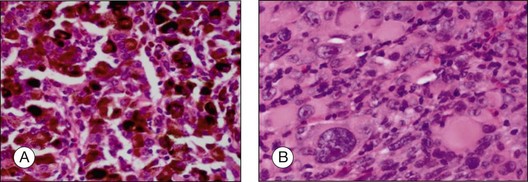
The prognosis of choroidal melanoma is based on clinical, histologic, and genetic parameters (see Chapter 141, Molecular genetics of choroidal melanoma). Some parameters such as ciliary body invasion, extraocular extension and the largest basal diameter are incorporated into the TNM classification.9 Histopathologic examination allows for further characterization of the tumor including its cytologic features. In FNAB specimens, the cell type is more difficult to determine than in enucleated eyes or completely excised tumors due to the scarce amount of tissue and artifacts (Fig. 142.6).
Choroidal melanomas usually arise de novo but may also arise from a nevus or a melanocytoma (magnocellular nevus). In these cases, remnants of the underlying nevus consisting of spindle A cells (spindle cell nevus (Fig. 142.7)) or, extremely rare magnocellular nevus cells (melanocytoma10,11), may be histologically detected.
Cytologic features
First developed by Callender in 1931,12 the so-called “Callender classification” distinguished five types of uveal melanomas depending on the most prominent cell type: spindle cell subtype A; spindle cell subtype B; epithelioid type; fascicular type, and mixed cell type (consisting of mixtures of spindle and epithelioid cells) of uveal melanoma.
Addressing the disagreement about the interpretation and application of Callender’s classification, a “modified Callender classification” was introduced by McLean and associates in 1983, comprising three different types in uveal melanoma based on cell morphology13: spindle cell melanoma (composed of spindle B cells); epithelioid cell melanoma, and melanoma of mixed-cell type. Spindle A cells were no longer regarded as malignant cells and were recognized as benign nevus cells. In addition, a new cell type was introduced, the small epithelioid cell (intermediate cell), that was smaller than the Callender’s epithelioid cell and exhibited features intermediate between spindle B and epithelioid cells.
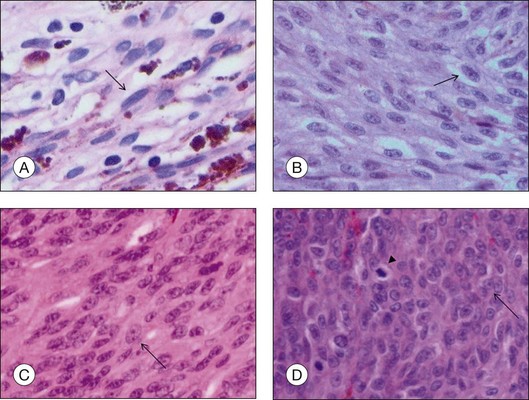
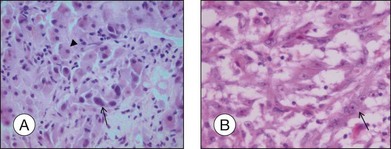
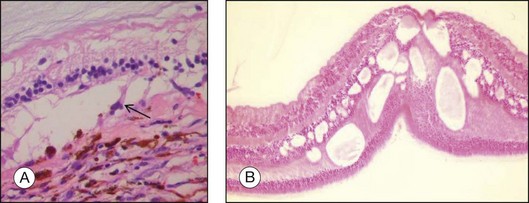
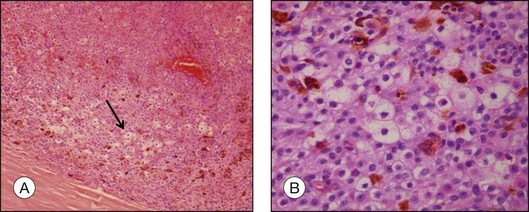
• Spindle A cells are fusiform, cohesive cells with poorly defined cell borders. The nucleus is also spindle-shaped and its central fold can be seen light microscopically as a dark stripe (Fig. 142.8A). This cell type is regarded as a benign nevus cell, but can be observed in uveal melanomas arising from a nevus.
• Spindle B cells are also fusiform and cohesive with poorly defined cell borders but are plumper than spindle A cells. Prominent nucleoli are detected in the spindle-shaped nucleus (Fig. 142.8B). This is the most common cell type in choroidal melanoma.
• Epithelioid cells are non-cohesive cells with defined cell borders. The round, large nucleus contains a prominent nucleolus (Fig. 142.8C). This cell type is associated with a poor outcome.
• Intermediate cells (small epithelioid cells) are a frequent cell type intermediate between spindle B cells and epithelioid cells (Fig. 142.8D),
Although not incorporated into the TNM classification, the cell type of uveal melanomas is also an important prognostic parameter.6 Melanomas solely composed of spindle cells have a more favorable prognosis than mixed-cell type tumors. Epithelioid cell melanomas are associated with monosomy 3 and class 2 molecular profile, a higher metastatic and mortality rate – and thus displays the worst prognosis.6,14,15
Evaluation of the number of mitotic figures per high-power field in the tumor cells (Fig. 142.8D) is part of the routine evaluation of choroidal melanomas. It serves as an indicator for the proliferative activity and is thus a prognostic parameter.6
Morphometry (measurement of the largest nucleoli using a digital filar micrometer) as well as measurements of the nuclear DNA ploidy (DNA content) by image cytometry of Feulgen stained sections, are historical approaches to establish criteria with regard to long-term survival in patients with uveal melanoma.6,16 These tests were replaced by newer approaches such as analysis of the chromosome 3 status15 and gene expression profiling.14
Immunohistochemical features
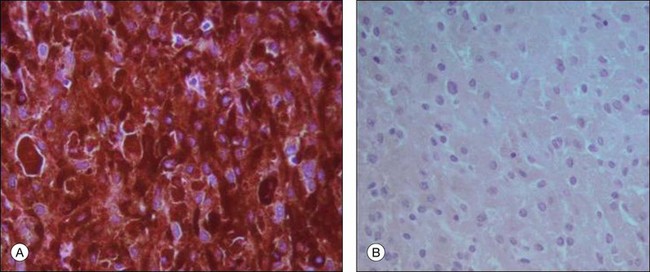
The most important immunohistochemical markers expressed by the tumor cells are HMB45, S100, Melan A, MITF, and tyrosinase.17–21 HMB 45 (Fig. 142.9) and Melan A are melanocytic markers. While Melan A stains melanocytes in general, HMB45 is predominantly expressed in “activated” melanocytes and is therefore more suggestive of malignant melanocytic lesions.21,22 S100 is expressed in different types of cells including melanocytes and very often used in combination with HMB45 as a marker for uveal melanoma.21 Microphthalmia transcription factor (MITF) is essential for the development and survival of melanocytes and therefore expressed in various melanocytic lesions including uveal melanoma.18–20 Tyrosinase is an enzyme that is involved in the metabolism of melanocytes and was recently introduced as a melanoma marker.17 At least two markers (e.g., HMB45 and S100) should be employed for the diagnosis of uveal melanoma if the diagnosis cannot be made on histologic features alone.
Ki67 antigen – a proliferation marker expressed in the nucleus – is suitable to detect the proliferative activity in tumors and has prognostic relevance.23,24 Immunohistochemical stains for Phospho-Histone H3 Ser10 (PHH3) may help to detect mitotic figures in uveal melanomas.25
Electron microscopy
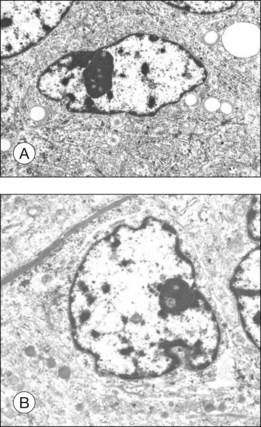
The different cell types in choroidal melanoma such as spindle A, spindle B, and epithelioid cells can also be differentiated by transmission electron microscopy although the ultrastructural differences between the cell types are not clear-cut.26
Spindle A cells appear elongated and show spindle-shaped nuclei with marked indentations and an unsuspicious nucleolus. In the cytoplasm, there are cell organelles present such as mitochondria, a relatively small number of free ribosomes, rough-surfaced endoplasmic reticulum (RER), largely immature melanin granules (premelanosomes), and numerous cytoplasmic filaments.26
The shape of spindle B cells (Fig. 142.10A) is similar to spindle A cells except they have a plumper cell body. The nucleoli of spindle B cells are also larger and show a reticular pattern. While spindle B cells have more RER and free ribosomes in their cytoplasm, the number of cytoplasmic filaments is decreased compared to spindle A cells.26
The largest nucleolus is found in the round to polygonal epithelioid cells (Fig. 142.10B) and exhibits a reticular pattern. The nucleolus of the epithelioid cells can have indentations but they are less prominent than in spindle cells. Many cell organelles, in particular mitochondriae – some of them exhibiting a bizarre form and structure – and free ribosomes, but only a few filaments, are present in the cytoplasm.26
In summary, the ultrastructural features are consistent with the present consensus that the cellular malignancy increases from spindle A (nevus cells) through spindle B to epithelioid types of melanoma cells in conjunction with an increasing number of cell organelles (e.g., mitochondriae, free ribosomes) and increasing size of nucleoli that are regarded as signs of activity.26
Other histopathologic characteristics and their prognostic relevance
Tumor stroma
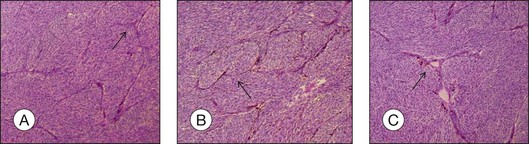
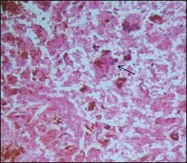
As choroidal melanomas metastasize hematogenously (Fig. 142.11), much attention has been paid to “intratumoral vessels and vascular-like structures” – nine different morphologic patterns can be distinguished: normal, silent, straight, parallel, parallel with crosslinking, arcs, arcs with branching, closed vascular loops (large “vessel” occluded by tumor cells), vascular networks (at least three back-to-back closed vascular loops) (Fig. 142.12).27 These vascular-like structures have been later shown to reflect fibrovascular septae rather than microvasculature leading to the term “vasculogenic mimicry”.28,29 The presence of these extravascular matrix patterns, in particular loops, that are identified by a PAS stain is associated with death from metastatic melanoma.27,30 Microvascular density (MVD) itself has been identified as prognostically relevant,27,31 and the presence of tumor cells in intratumoral blood vessels is also regarded as a factor for unfavorable outcome.32
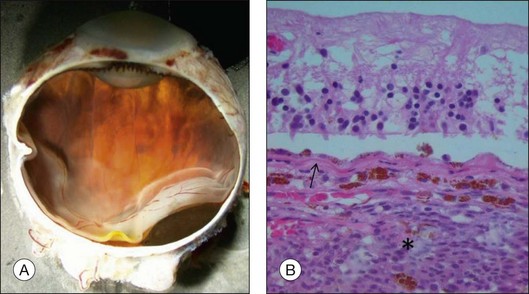
A high number of tumor-infiltrating/associated lymphocytes (TILs/TALs) (Fig. 142.13) is associated with a more aggressive behavior and a higher risk of metastasis than a low number of TILs33 – and so are tumor-infiltrating/associated macrophages (TIMs/TAMs).6,34 In particular, M2 macrophages that exhibit proangiogenic and anti-inflammatory characteristics (in contrast to M1 macrophages with antibacterial and antiangiogenic features) are linked to increased microvascular density, ciliary body involvement, monosomy 3 and thus, a worse prognosis for survival.35,36
The degree of pigmentation varies between different tumors and also within one tumor. Tumors cells as well as pigment-laden macrophages (that are typically larger than tumor cells) contribute to the degree of pigmentation that is roughly classified into amelanotic, mildly, moderately, and heavily pigmented (Figs 142.1, 142.5). In heavily and moderately pigmented tumors, bleaching is mandatory in order to analyze the cytologic features. The degree of pigmentation in comparison with other features only has weak prognostic value.5,6
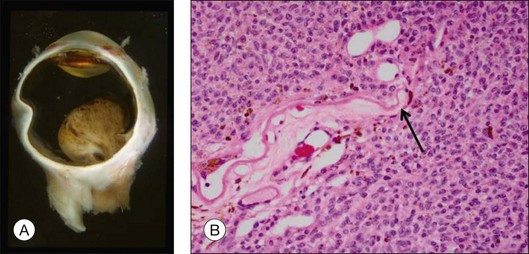
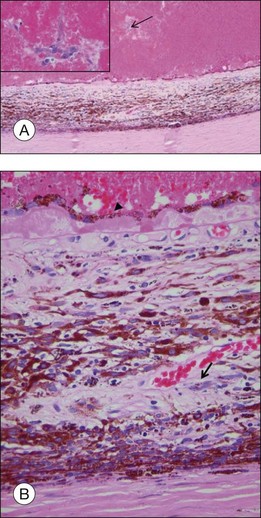
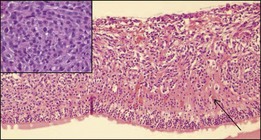
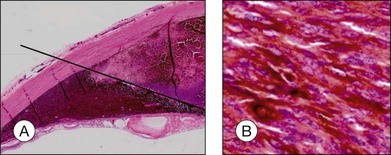
“Melanoma-associated spongiform scleropathy (MASS)” is a degenerative, noninflammatory process in the sclera underlying the tumor that occurs in 38% of enucleated eyes harboring uveal melanoma.37 MASS is associated with age and basal tumor diameter (extent of direct contact between tumor and sclera) but not with long-term survival.38 A high incidence of MASS is found in eyes with scleral invasion and extrascleral extension as altered sclera collagen may allow for tumor invasion. On gross examination, whitish spindle-shaped areas within the sclera adjacent to the tumor may be detected.37 Degraded collagen fibers and glycosaminoglycan accumulation leading to the typical picture of spongiotic areas surrounded by feathery fragmented collagen fibers are microscopically observed (Fig. 142.14).37
Tumor extension
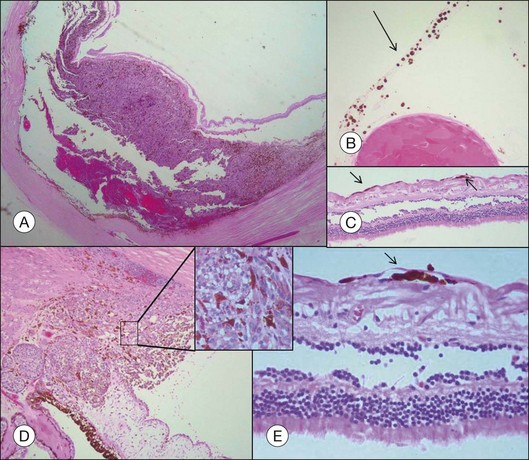
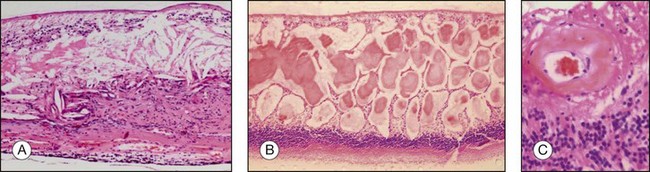
Choroidal melanomas may invade into several ocular tissues: Most uveal melanomas invade into the sclera (Fig. 142.15). Continuous horizontal growth may lead to invasion of the adjacent ciliary body and is associated with a worse prognosis. Choroidal melanomas invading into the ciliary body do not necessarily also invade into the iris and tumor growth is usually restricted by the reticulum of Müller. Once the tumor has broken through Bruch’s membrane it may invade into the retina but rarely gains access to the vitreous cavity with vitreous spread (Fig. 142.16).39 Melanoma cells in the anterior chamber and trabecular meshwork are rarely observed in choroidal tumors that do not continuously invade into the ciliary body and iris (Fig. 142.16). Peripapillary uveal melanomas may invade into the optic nerve head, but rarely extend far retrolaminary (Fig. 142.17).6
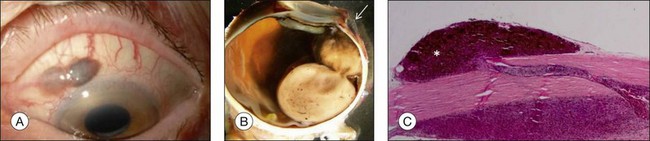
Extraocular extension occurs most frequently along emissary canals which include ciliary nerves and vascular channels, in particular vortex veins, as well as aqueous channels (Fig. 142.18).40 Extension via the optic nerve or through iatrogenic wounds has also been described.40–42 Extensive orbital extension is rarely observed (Fig. 142.19). Histologic examination of step sections of the globe and vortex veins enables pathologists to issue reliable reports that include these prognostically important parameters. Extraocular extension of uveal melanoma is associated with a significant risk for liver metastases and an increased local recurrence rate.43
Degenerative changes
Accompanying degenerative changes may be observed in the retinal pigment epithelium (RPE) and the retina (Fig. 142.20). The RPE may exhibit atrophy or proliferation. Secondary drusen and orange pigment may also be found. The retina overlying the tumor usually displays atrophy with or without edema. Serous retinal detachment may occur at the margin of as well as opposite to the tumor (Fig. 142.20).
Orange pigment histologically corresponds to lipofuscin accumulation in macrophages on the tumor surface (within and under the neurosensory retina) and proliferating RPE cells and occurs secondary to necrotic changes and/or as a sign of metabolic activity (Fig. 142.21).44
Special types of uveal melanoma
Diffuse uveal melanoma
A diffuse growth pattern of uveal melanoma defined as a tumor <5 mm in height that involves at least 25% of the uveal tract,45 is rare as it affects only 3% of all melanoma patients,46 and exhibits horizontal rather than vertical growth (Fig. 142.22). It is associated with a worse prognosis than mound-shaped or fusiform growth patterns.
Multifocal unilateral uveal melanoma
Multifocal unilateral uveal melanomas are extremely rare and only a few cases have been reported (Fig. 142.23).47 Serial sections should be employed in order to exclude local contiguous spread.
Bilateral uveal melanoma
Bilateral uveal melanoma occurs rarely (either simultaneously or lagged) and has to be distinguished from metastasis of uveal melanoma to the fellow eye.48,49
Clear cell differentiation of uveal melanoma
Uveal melanoma may rarely exhibit clear cell differentiation with cytologic features similar to clear cells tumors elsewhere in the body (Fig. 142.24).50 The clear cells are typically oval, contain centrally placed nuclei with malignant features, and their clear cell appearance can be attributed to cytoplasmic glycogen accumulation in melanoma cells. These tumors have to be carefully distinguished from metastatic (renal) clear cell carcinoma to the choroid.
Balloon cell melanoma
Balloon cell melanoma is pathogenetically associated with degradation of melanosomes and exhibit cells with vacuolated, foamy cytoplasm due to degenerative changes with lipid accumulation.51 Uveal melanomas may exhibit varying amounts of balloon cells. A “true” balloon cell melanoma is rarely observed. Balloon cells may also occasionally be observed in eyes treated with brachytherapy or proton beam irradiation (Fig. 142.25).52
Necrotic melanoma
A necrotic melanoma consists of mostly necrotic cells with undetectable cytologic features (Fig. 142.26). This entity can lead to melanomalytic glaucoma, which is a rare kind of secondary open angle glaucoma in uveal melanoma occurring in the setting of spontaneous necrosis of uveal melanoma. Macrophages that phagocytose necrotic melanoma cells (melanomacrophages) accumulate in the trabecular meshwork resulting in an increased intraocular pressure.53
Retinoinvasive melanoma
Retinoinvasive melanomas are an absolute rarity and tend to evolve from a ring melanoma.54 In contrast to other diffuse growing uveal melanomas that only erode the overlying retina, this specific and obviously slow-growing type of uveal melanoma nearly replaces the retina and may infiltrate the optic nerve although it spares the choroid (Fig. 142.27).
Histologic changes after treatment
Brachytherapy
Brachytherapy has been shown by the Collaborative Ocular Melanoma Study (COMS) to be equally effective as enucleation with regard to patient survival for choroidal melanomas ≤16 mm basal diameter and 2.5–10 mm in height.55 Histologic changes after brachytherapy may be studied in eyes that undergo enucleation for treatment failure or therapy-resistant neovascular glaucoma.
Histopathologic findings after brachytherapy (Fig. 142.28) include degenerative changes such as necrosis (sometimes present as hemorrhagic necrosis) with balloon cell or signet ring cell formation, vacuolization (cystic degeneration), lipoidal degeneration as well as fibrosis of the tumor stroma. Viable tumor cells may be found, especially spindle cells, as epithelioid cells are more sensitive to radiation. Mitotic activity is lower than in non-irradiated tumors.56–58 Vascular damage includes hyalinization of vessels walls and vascular obstruction within the tumor and the retina. The retina displays gliosis and atrophy, and an exudative retinal detachment may be present adjacent to the tumor. Subretinal gliosis, RPE irregularities and atrophy, chorioretinal atrophy and scleral scarring/necrosis are also observed within the field of radiation.56–58
Inflammatory cell infiltrates and the accumulation of tumor infiltrating (melano)macrophages are also present56–58 as well as macrophages in extratumoral tissue including the adjacent choroid, sclera, ciliary body, and subretinal space (Fig. 142.29).59 Pigmented macrophage-related episcleral deposits are also frequently found in eyes with uveal melanoma after brachytherapy.59
Inadequate plaque treatment such as an undersized plaque (Fig. 142.30) or plaque tilt as well as radioresistance of the tumor are accounted for treatment failure with subsequent tumor growth.60,61 Histopathologic evaluation may assist with the evaluation of the reason for treatment failure.
Proton beam irradiation
Proton beam irradiation shows histologic findings similar to those described for plaque brachytherapy, including degenerative changes in melanoma cells such as cytoplasmic lipid vacuoles, pyknotic nuclei, and balloon cell formation, areas of necrosis within the tumor, vascular changes, and chronic inflammatory cell infiltrates (Fig. 142.31).52,62
Radiation retinopathy
Radiation retinopathy (Fig. 142.32) occurs in approximately 42% of eyes within 5 years after brachytherapy (or proton beam irradiation) for uveal melanoma and often leads to irreversible visual impairment.63 Radiation retinopathy manifests an acute transudative and slowly progressive occlusive vasculopathy with nonproliferative and/or proliferative retinopathy.64,65 Histologic features are characterized as an obliterative endarteritis with endothelial cell loss and capillary occlusion as the primary vascular event leading to the development of dilated capillary collaterals and microaneurysms due to limited capillary regeneration.64,65 Large telangiectatic vascular channels with fibrin and exudates in their vessel wall are pathognomonic for radiation retinopathy. Because of inner retinal ischemia, the retinal parenchyma appears edematous with associated intraretinal exudates, necrosis, and gliosis. Development of proliferative radiation retinopathy as a response to severe long-standing ischemia is observed in less than 10% of irradiated eyes.66
Transpupillary thermotherapy (TTT)
TTT is used as a primary treatment for choroidal melanoma or as an adjunct to plaque brachytherapy. Histologic findings include necrosis, cytolysis, and occluded tumor blood vessels within the tumor as well as fibrotic and degenerative changes of the retina and RPE in the area of the tumor (Fig. 142.33).67–69 Signs of marked inflammation are usually not observed.69 Scleral damage is usually insignificant and intrascleral tumor cells may be present after treatment and are regarded as a possible source for treatment failure.69 The presence of pigment-laden macrophages in the subretinal space are also observed after TTT.70 Histopathologic effects of TTT also include damage to adjacent structures and are related to both energy level and fundus pigmentation.71
APPENDIX: Histologic differential diagnoses
The histologic differential diagnoses of choroidal melanoma include:
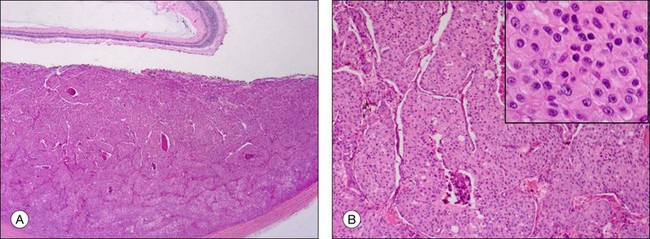
A choroidal nevus represents a benign lesion and consists therefore mainly of spindle A cells with a varying degree of pigmentation (Fig. 142.34). The distinction between a choroidal nevus and a melanoma is more important with regard to an incidental finding in an enucleated or eviscerated eye, as usually only eyes with large choroidal melanomas (that are not clinically confused with a nevus) are enucleated.
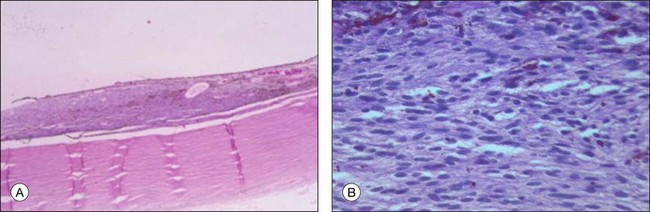
Fig. 142.34 (A) Choroidal nevus (H&E, ×4) consisting predominantly of spindle A cells (B) (H&E, ×40).
Melanocytomas (synonym: magnocellular nevus) are jet-black lesions that predominantly occur on the optic nerve head. They are benign and rarely exhibit malignant transformation.11 In contrast to melanocytoma of the optic disc, the diagnosis of uveal melanocytoma is rarely made on clinical appearance because of the difficulty to distinguish it from malignant melanoma.72 Histology confirms the diagnosis as it reveals a tumor composed of uniform deeply pigmented cells with melanosomes (Fig. 142.35). After bleaching, round or slightly polyhedral plump cells with abundant cytoplasm and small uniform nuclei with inconspicuous nucleoli (type I cells) as well as spindle-shaped, sparsely pigmented cells (type II cells) become visible. Mitotic figures are uncommon.73
Choroidal metastases (Fig. 142.36) exhibit characteristics of the primary tumor (predominantly carcinomas, 82%). Most frequently found are breast (47%) and lung cancer (21%), followed by gastrointestinal tract (4%), kidney (2%), skin (2%), prostate (2%), and other cancers (4%). There is a relatively high rate of unknown primary malignancies after systemic evaluation (17%).74
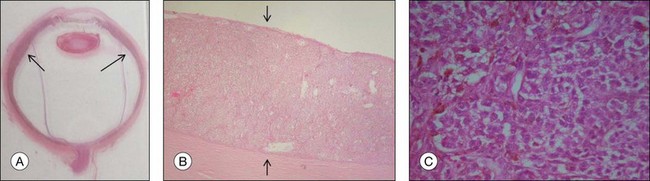
Bilateral diffuse uveal melanocytic proliferation (BDUMP) is a rare paraneoplastic syndrome with a typical clinical presentation including multiple round to oval, slightly elevated choroidal patches and an associated “leopard spot” appearance on fluorescein angiography (FA). Except for one unilateral case,75 BDUMP occurs bilaterally – in contrast to choroidal melanoma. Histologic examination usually reveals a diffuse or poorly defined choroidal infiltrative process with small hypopigmented spindle-shaped benign-appearing melanocytes.76,77 The choriocapillaris is often sparred and the overlying RPE is depigmented (Fig. 142.37).
Acknowledgments
![]() For online Appendix: Histologic Differential Diagnoses visit http://www.expertconsult.com
For online Appendix: Histologic Differential Diagnoses visit http://www.expertconsult.com
![]() For online acknowledgments visit http://www.expertconsult.com
For online acknowledgments visit http://www.expertconsult.com
![]() Bonus images for this chapter can be found online at http://www.expertconsult.com
Bonus images for this chapter can be found online at http://www.expertconsult.com
Fig. 142.34(A) Choroidal nevus (H&E, ×4) consisting predominantly of spindle A cells (B) (H&E, ×40).
Fig. 142.35(A) Magnocellular nevus (melanocytoma) composed of heavily pigmented cells (H&E, ×40). (B) After bleaching, the polygonal cells with their unsuspicious nuclei are visible (bleached H&E stain, ×40).
Fig. 142.36(A) Choroidal mass consistent with a breast carcinoma metastasis composed of tumor cells arranged in a papillomatous configuration (H&E, ×4). (B) Higher magnification shows glandular-like structures (H&E, ×10) and large tumor cells with prominent nucleoli (inset).
Fig. 142.37(A) Overview illustrating a bilateral diffuse uveal melanocytic proliferation (BDUMP) invading the choroid (arrows) (H&E). (B) The lesion effaces the entire choroid (arrows) but spares the choriocapillaris (H&E, ×4). (C) The lesion is composed of hypopigmented bland melanocytes (H&E, ×40). (Courtesy of Curtis E. Margo, MD.)
1 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Mod Pathol. 2003;16:725–730.
2 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Am J Surg Pathol. 2003;27:999–1004.
3 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. The Association of Directors of Anatomic and Surgical Pathology. Hum Pathol. 2003;34:114–118.
4 Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Am J Clin Pathol. 2003;119:179–184.
5 Seddon JM, Albert DM, Lavin PT, et al. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983;101:1894–1899.
6 McLean IW, Saraiva VS, Burnier MN, Jr. Pathological and prognostic features of uveal melanomas. Can J Ophthalmol. 2004;39:343–350.
7 Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study: COMS report no. 21. Arch Ophthalmol. 2003;121:1163–1171.
8 Coleman DJ, Silverman RH, Rondeau MJ, et al. Correlations of acoustic tissue typing of malignant melanoma and histopathologic features as a predictor of death. Am J Ophthalmol. 1990;110:380–388.
9 American Joint Committee on Cancer. Cancer staging manual, 7th ed. New York: Springer; 2010.
10 Shetlar DJ, Folberg R, Gass JD. Choroidal malignant melanoma associated with a melanocytoma. Retina. 1999;19:346–349.
11 Kurli M, Finger PT, Manor T, et al. Finding malignant change in a necrotic choroidal melanocytoma: a clinical challenge. Br J Ophthalmol. 2005;89:921–922.
12 Callender GR. Malignant melanotic tumors of the eye. A study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;36:131–142.
13 McLean IW, Foster WD, Zimmerman LE, et al. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–509.
14 Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209.
15 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
16 Grossniklaus HE, Oakman JH, Cohen C, et al. Histopathology, morphometry, and nuclear DNA content of iris melanocytic lesions. Invest Ophthalmol Vis Sci. 1995;36:745–750.
17 Fernandes BF, Odashiro AN, Saraiva VS, et al. Immunohistochemical expression of melan-A and tyrosinase in uveal melanoma. J Carcinog. 2007;6:6.
18 Iwamoto S, Burrows RC, Kalina RE, et al. Immunophenotypic differences between uveal and cutaneous melanomas. Arch Ophthalmol. 2002;120:466–470.
19 O’Reilly FM, Brat DJ, McAlpine BE, et al. Microphthalmia transcription factor immunohistochemistry: a useful diagnostic marker in the diagnosis and detection of cutaneous melanoma, sentinel lymph node metastases, and extracutaneous melanocytic neoplasms. J Am Acad Dermatol. 2001;45:414–419.
20 Mouriaux F, Vincent S, Kherrouche Z, et al. Microphthalmia transcription factor analysis in posterior uveal melanomas. Exp Eye Res. 2003;76:653–661.
21 Rohrbach JM, Steuhl KP, Thanos S. [Monoclonal antibody HMB-45 in diagnosis of intra-ocular melanoma]. Klin Monbl Augenheilkd. 1991;199:274–277.
22 Burnier MN, Jr., McLean IW, Gamel JW. Immunohistochemical evaluation of uveal melanocytic tumors. Expression of HMB-45, S-100 protein, and neuron-specific enolase. Cancer. 1991;68:809–814.
23 Mooy CM, Luyten GP, de Jong PT, et al. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am J Pathol. 1995;147:1097–1104.
24 Karlsson M, Boeryd B, Carstensen J, et al. Correlations of Ki-67 and PCNA to DNA ploidy, S-phase fraction and survival in uveal melanoma. Eur J Cancer. 1996;32A:357–362.
25 Angi M, Damato B, Kalirai H, et al. Immunohistochemical assessment of mitotic count in uveal melanoma. Acta Ophthalmol. 2011;89:e155–e160.
26 Iwamoto T, Jones IS, Howard GM. Ultrastructural comparison of spindle A, spindle B, and epithelioid-type cells in uveal malignant melanoma. Invest Ophthalmol. 1972;11:873–889.
27 Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398.
28 Foss AJ, Alexander RA, Hungerford JL, et al. Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol. 1997;81:240–248.
29 Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381.
30 Folberg R, Arbieva Z, Moses J, et al. Tumor cell plasticity in uveal melanoma: microenvironment directed dampening of the invasive and metastatic genotype and phenotype accompanies the generation of vasculogenic mimicry patterns. Am J Pathol. 2006;169:1376–1389.
31 Makitie T, Summanen P, Tarkkanen A, et al. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40:2471–2480.
32 Ly LV, Odish OF, Wolff-Rouendaal D, et al. Intravascular presence of tumor cells as prognostic parameter in uveal melanoma: a 35-year survey. Invest Ophthalmol Vis Sci. 2010;51:658–665.
33 de la Cruz PO, Jr., Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65:112–115.
34 Makitie T, Summanen P, Tarkkanen A, et al. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421.
35 Bronkhorst IH, Ly LV, Jordanova ES, et al. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci. 2011;52:643–650.
36 Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510.
37 Alyahya GA. Melanoma associated spongiform scleropathy: characterization, biochemical and immunohistochemical studies. Acta Ophthalmol 2008;86(Thesis 3):1–21.
38 Yanoff M, Sassani JW. Ocular pathology, 6th ed. St Louis: Mosby; 2009.
39 Heindl LM, Mardin CY, Holbach LM, et al. Vitreal seeding from uveal melanoma detected by high-resolution spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127:1062–1064.
40 Coupland SE, Campbell I, Damato B. Routes of extraocular extension of uveal melanoma: risk factors and influence on survival probability. Ophthalmology. 2008;115:1778–1785.
41 Grossniklaus HE, Brown RH, Stulting RD, et al. Iris melanoma seeding through a trabeculectomy site. Arch Ophthalmol. 1990;108:1287–1290.
42 Kavanagh MC, Everman KR, Opremcak EM, et al. Uveal melanoma with massive extrascleral extension via pars plana vitrectomy sites. Ophthal Plast Reconstr Surg. 2008;24:334–336.
43 Affeldt JC, Minckler DS, Azen SP, et al. Prognosis in uveal melanoma with extrascleral extension. Arch Ophthalmol. 1980;98:1975–1979.
44 Font RL, Zimmerman LE, Armaly MF. The nature of the orange pigment over a choroidal melanoma. Histochemical and electron microscopical observations. Arch Ophthalmol. 1974;91:359–362.
45 Char DH. Tumor of the eye and the ocular adnexa. Lewiston: BC Decker Inc; 2001.
46 Shields CL, Shields JA, De Potter P, et al. Diffuse choroidal melanoma. Clinical features predictive of metastasis. Arch Ophthalmol. 1996;114:956–963.
47 Dithmar S, Volcker HE, Grossniklaus HE. Multifocal intraocular malignant melanoma: report of two cases and review of the literature. Ophthalmology. 1999;106:1345–1348.
48 Hadden PW, Damato BE, McKay IC. Bilateral uveal melanoma: a series of four cases. Eye (Lond). 2003;17:613–616.
49 Sturm V, Richard G. [The prevalence of bilateral malignant uveal melanoma]. Klin Monbl Augenheilkd. 2007;224:770–774.
50 Grossniklaus HE, Albert DM, Green WR, et al. Clear cell differentiation in choroidal melanoma. COMS report no. 8. Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:894–898.
51 Khalil MK. Balloon cell malignant melanoma of the choroid: ultrastructural studies. Br J Ophthalmol. 1983;67:579–584.
52 Saornil MA, Egan KM, Gragoudas ES, et al. Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol. 1992;110:1112–1118.
53 McMenamin PG, Lee WR. Ultrastructural pathology of melanomalytic glaucoma. Br J Ophthalmol. 1986;70:895–906.
54 Kivela T, Summanen P. Retinoinvasive malignant melanoma of the uvea. Br J Ophthalmol. 1997;81:691–697.
55 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–1693.
56 Avery RB, Diener-West M, Reynolds SM, et al. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008;126:207–212.
57 Messmer E, Bornfeld N, Foerster M, et al. Histopathologic findings in eyes treated with a ruthenium plaque for uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1992;230:391–396.
58 Schilling H, Sehu KW, Lee WR. A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci. 1997;38:2081–2092.
59 Toivonen P, Kivela T. Pigmented episcleral deposits after brachytherapy of uveal melanoma. Ophthalmology. 2006;113:865–873.
60 Char DH, Crawford JB, Kaleta-Michaels S, et al. Analysis of radiation failure after uveal melanoma brachytherapy. Am J Ophthalmol. 1989;108:712–716.
61 Almony A, Breit S, Zhao H, et al. Tilting of radioactive plaques after initial accurate placement for treatment of uveal melanoma. Arch Ophthalmol. 2008;126:65–70.
62 Seddon JM, Gragoudas ES, Albert DM. Ciliary body and choroidal melanomas treated by proton beam irradiation. Histopathologic study of eyes. Arch Ophthalmol. 1983;101:1402–1408.
63 Gunduz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609–614.
64 Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy–clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond). 1991;5(Pt 2):239–251.
65 Finger PT. Anti-VEGF bevacizumab (Avastin) for radiation optic neuropathy. Am J Ophthalmol. 2007;143:335–338.
66 Bianciotto C, Shields CL, Pirondini C, et al. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117:1005–1012.
67 Singh AD, Eagle RC, Jr., Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121:397–400.
68 Diaz CE, Capone A, Jr., Grossniklaus HE. Clinicopathologic findings in recurrent choroidal melanoma after transpupillary thermotherapy. Ophthalmology. 1998;105:1419–1424.
69 Journee-de Korver JG, Oosterhuis JA, de Wolff-Rouendaal D, et al. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Br J Ophthalmol. 1997;81:234–239.
70 Kiratli H, Bilgic S, Soylemezoglu F, et al. Peripheral subretinal pigment accumulation following transpupillary thermotherapy for choroidal melanoma. Ophthalmic Surg Lasers Imaging. 2008;39:60–62.
71 Connolly BP, Regillo CD, Eagle RC, Jr., et al. The histopathologic effects of transpupillary thermotherapy in human eyes. Ophthalmology. 2003;110:415–420.
1 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Mod Pathol. 2003;16:725–730.
2 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Am J Surg Pathol. 2003;27:999–1004.
3 Folberg R, Salomao D, Grossniklaus HE, et al. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. The Association of Directors of Anatomic and Surgical Pathology. Hum Pathol. 2003;34:114–118.
4 Association of Directors of Anatomic and Surgical Pathology. Recommendations for the reporting of tissues removed as part of the surgical treatment of common malignancies of the eye and its adnexa. Am J Clin Pathol. 2003;119:179–184.
5 Seddon JM, Albert DM, Lavin PT, et al. A prognostic factor study of disease-free interval and survival following enucleation for uveal melanoma. Arch Ophthalmol. 1983;101:1894–1899.
6 McLean IW, Saraiva VS, Burnier MN, Jr. Pathological and prognostic features of uveal melanomas. Can J Ophthalmol. 2004;39:343–350.
7 Comparison of clinical, echographic, and histopathological measurements from eyes with medium-sized choroidal melanoma in the collaborative ocular melanoma study: COMS report no. 21. Arch Ophthalmol. 2003;121:1163–1171.
8 Coleman DJ, Silverman RH, Rondeau MJ, et al. Correlations of acoustic tissue typing of malignant melanoma and histopathologic features as a predictor of death. Am J Ophthalmol. 1990;110:380–388.
9 American Joint Committee on Cancer. Cancer staging manual, 7th ed. New York: Springer; 2010.
10 Shetlar DJ, Folberg R, Gass JD. Choroidal malignant melanoma associated with a melanocytoma. Retina. 1999;19:346–349.
11 Kurli M, Finger PT, Manor T, et al. Finding malignant change in a necrotic choroidal melanocytoma: a clinical challenge. Br J Ophthalmol. 2005;89:921–922.
12 Callender GR. Malignant melanotic tumors of the eye. A study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;36:131–142.
13 McLean IW, Foster WD, Zimmerman LE, et al. Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–509.
14 Onken MD, Worley LA, Ehlers JP, et al. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209.
15 Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347:1222–1225.
16 Grossniklaus HE, Oakman JH, Cohen C, et al. Histopathology, morphometry, and nuclear DNA content of iris melanocytic lesions. Invest Ophthalmol Vis Sci. 1995;36:745–750.
17 Fernandes BF, Odashiro AN, Saraiva VS, et al. Immunohistochemical expression of melan-A and tyrosinase in uveal melanoma. J Carcinog. 2007;6:6.
18 Iwamoto S, Burrows RC, Kalina RE, et al. Immunophenotypic differences between uveal and cutaneous melanomas. Arch Ophthalmol. 2002;120:466–470.
19 O’Reilly FM, Brat DJ, McAlpine BE, et al. Microphthalmia transcription factor immunohistochemistry: a useful diagnostic marker in the diagnosis and detection of cutaneous melanoma, sentinel lymph node metastases, and extracutaneous melanocytic neoplasms. J Am Acad Dermatol. 2001;45:414–419.
20 Mouriaux F, Vincent S, Kherrouche Z, et al. Microphthalmia transcription factor analysis in posterior uveal melanomas. Exp Eye Res. 2003;76:653–661.
21 Rohrbach JM, Steuhl KP, Thanos S. [Monoclonal antibody HMB-45 in diagnosis of intra-ocular melanoma]. Klin Monbl Augenheilkd. 1991;199:274–277.
22 Burnier MN, Jr., McLean IW, Gamel JW. Immunohistochemical evaluation of uveal melanocytic tumors. Expression of HMB-45, S-100 protein, and neuron-specific enolase. Cancer. 1991;68:809–814.
23 Mooy CM, Luyten GP, de Jong PT, et al. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am J Pathol. 1995;147:1097–1104.
24 Karlsson M, Boeryd B, Carstensen J, et al. Correlations of Ki-67 and PCNA to DNA ploidy, S-phase fraction and survival in uveal melanoma. Eur J Cancer. 1996;32A:357–362.
25 Angi M, Damato B, Kalirai H, et al. Immunohistochemical assessment of mitotic count in uveal melanoma. Acta Ophthalmol. 2011;89:e155–e160.
26 Iwamoto T, Jones IS, Howard GM. Ultrastructural comparison of spindle A, spindle B, and epithelioid-type cells in uveal malignant melanoma. Invest Ophthalmol. 1972;11:873–889.
27 Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993;100:1389–1398.
28 Foss AJ, Alexander RA, Hungerford JL, et al. Reassessment of the PAS patterns in uveal melanoma. Br J Ophthalmol. 1997;81:240–248.
29 Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381.
30 Folberg R, Arbieva Z, Moses J, et al. Tumor cell plasticity in uveal melanoma: microenvironment directed dampening of the invasive and metastatic genotype and phenotype accompanies the generation of vasculogenic mimicry patterns. Am J Pathol. 2006;169:1376–1389.
31 Makitie T, Summanen P, Tarkkanen A, et al. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40:2471–2480.
32 Ly LV, Odish OF, Wolff-Rouendaal D, et al. Intravascular presence of tumor cells as prognostic parameter in uveal melanoma: a 35-year survey. Invest Ophthalmol Vis Sci. 2010;51:658–665.
33 de la Cruz PO, Jr., Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990;65:112–115.
34 Makitie T, Summanen P, Tarkkanen A, et al. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1414–1421.
35 Bronkhorst IH, Ly LV, Jordanova ES, et al. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci. 2011;52:643–650.
36 Maat W, Ly LV, Jordanova ES, et al. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510.
37 Alyahya GA. Melanoma associated spongiform scleropathy: characterization, biochemical and immunohistochemical studies. Acta Ophthalmol. 2008;86(Thesis 3):1–21.
38 Yanoff M, Sassani JW. Ocular pathology, 6th ed. St Louis: Mosby; 2009.
39 Heindl LM, Mardin CY, Holbach LM, et al. Vitreal seeding from uveal melanoma detected by high-resolution spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127:1062–1064.
40 Coupland SE, Campbell I, Damato B. Routes of extraocular extension of uveal melanoma: risk factors and influence on survival probability. Ophthalmology. 2008;115:1778–1785.
41 Grossniklaus HE, Brown RH, Stulting RD, et al. Iris melanoma seeding through a trabeculectomy site. Arch Ophthalmol. 1990;108:1287–1290.
42 Kavanagh MC, Everman KR, Opremcak EM, et al. Uveal melanoma with massive extrascleral extension via pars plana vitrectomy sites. Ophthal Plast Reconstr Surg. 2008;24:334–336.
43 Affeldt JC, Minckler DS, Azen SP, et al. Prognosis in uveal melanoma with extrascleral extension. Arch Ophthalmol. 1980;98:1975–1979.
44 Font RL, Zimmerman LE, Armaly MF. The nature of the orange pigment over a choroidal melanoma. Histochemical and electron microscopical observations. Arch Ophthalmol. 1974;91:359–362.
45 Char DH. Tumor of the eye and the ocular adnexa. Lewiston: BC Decker Inc; 2001.
46 Shields CL, Shields JA, De Potter P, et al. Diffuse choroidal melanoma. Clinical features predictive of metastasis. Arch Ophthalmol. 1996;114:956–963.
47 Dithmar S, Volcker HE, Grossniklaus HE. Multifocal intraocular malignant melanoma: report of two cases and review of the literature. Ophthalmology. 1999;106:1345–1348.
48 Hadden PW, Damato BE, McKay IC. Bilateral uveal melanoma: a series of four cases. Eye (Lond). 2003;17:613–616.
49 Sturm V, Richard G. [The prevalence of bilateral malignant uveal melanoma]. Klin Monbl Augenheilkd. 2007;224:770–774.
50 Grossniklaus HE, Albert DM, Green WR, et al. Clear cell differentiation in choroidal melanoma. COMS report no. 8. Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:894–898.
51 Khalil MK. Balloon cell malignant melanoma of the choroid: ultrastructural studies. Br J Ophthalmol. 1983;67:579–584.
52 Saornil MA, Egan KM, Gragoudas ES, et al. Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol. 1992;110:1112–1118.
53 McMenamin PG, Lee WR. Ultrastructural pathology of melanomalytic glaucoma. Br J Ophthalmol. 1986;70:895–906.
54 Kivela T, Summanen P. Retinoinvasive malignant melanoma of the uvea. Br J Ophthalmol. 1997;81:691–697.
55 The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–1693.
56 Avery RB, Diener-West M, Reynolds SM, et al. Histopathologic characteristics of choroidal melanoma in eyes enucleated after iodine 125 brachytherapy in the collaborative ocular melanoma study. Arch Ophthalmol. 2008;126:207–212.
57 Messmer E, Bornfeld N, Foerster M, et al. Histopathologic findings in eyes treated with a ruthenium plaque for uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 1992;230:391–396.
58 Schilling H, Sehu KW, Lee WR. A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci. 1997;38:2081–2092.
59 Toivonen P, Kivela T. Pigmented episcleral deposits after brachytherapy of uveal melanoma. Ophthalmology. 2006;113:865–873.
60 Char DH, Crawford JB, Kaleta-Michaels S, et al. Analysis of radiation failure after uveal melanoma brachytherapy. Am J Ophthalmol. 1989;108:712–716.
61 Almony A, Breit S, Zhao H, et al. Tilting of radioactive plaques after initial accurate placement for treatment of uveal melanoma. Arch Ophthalmol. 2008;126:65–70.
62 Seddon JM, Gragoudas ES, Albert DM. Ciliary body and choroidal melanomas treated by proton beam irradiation. Histopathologic study of eyes. Arch Ophthalmol. 1983;101:1402–1408.
63 Gunduz K, Shields CL, Shields JA, et al. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch Ophthalmol. 1999;117:609–614.
64 Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy–clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond). 1991;5(Pt 2):239–251.
65 Finger PT. Anti-VEGF bevacizumab (Avastin) for radiation optic neuropathy. Am J Ophthalmol. 2007;143:335–338.
66 Bianciotto C, Shields CL, Pirondini C, et al. Proliferative radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology. 2010;117:1005–1012.
67 Singh AD, Eagle RC, Jr., Shields CL, et al. Clinicopathologic reports, case reports, and small case series: enucleation following transpupillary thermotherapy of choroidal melanoma: clinicopathologic correlations. Arch Ophthalmol. 2003;121:397–400.
68 Diaz CE, Capone A, Jr., Grossniklaus HE. Clinicopathologic findings in recurrent choroidal melanoma after transpupillary thermotherapy. Ophthalmology. 1998;105:1419–1424.
69 Journee-de Korver JG, Oosterhuis JA, de Wolff-Rouendaal D, et al. Histopathological findings in human choroidal melanomas after transpupillary thermotherapy. Br J Ophthalmol. 1997;81:234–239.
70 Kiratli H, Bilgic S, Soylemezoglu F, et al. Peripheral subretinal pigment accumulation following transpupillary thermotherapy for choroidal melanoma. Ophthalmic Surg Lasers Imaging. 2008;39:60–62.
71 Connolly BP, Regillo CD, Eagle RC, Jr., et al. The histopathologic effects of transpupillary thermotherapy in human eyes. Ophthalmology. 2003;110:415–420.
72 Robertson DM, Campbell RJ, Salomao DR. Mushroom-shaped choroidal melanocytoma mimicking malignant melanoma. Arch Ophthalmol. 2002;120:82–85.
73 Juarez CP, Tso MO. An ultrastructural study of melanocytomas (magnocellular nevi) of the optic disk and uvea. Am J Ophthalmol. 1980;90:48–62.
74 Shields CL, Shields JA, Gross NE, et al. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104:1265–1276.
75 Reddy S, Finger PT. Unilateral diffuse uveal melanocytic proliferation (DUMP). Br J Ophthalmol. 2007;91:1726–1727.
76 Gass JD, Gieser RG, Wilkinson CP, et al. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol. 1990;108:527–533.
77 Barr CC, Zimmerman LE, Curtin VT, et al. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms. A recently recognized syndrome. Arch Ophthalmol. 1982;100:249–255.