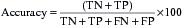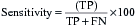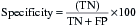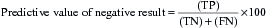Chapter 4 Diagnostic pathology in clinical practice
Laboratory techniques play an important part in the diagnosis and treatment of disease in patients. Many of the tests performed in pathology laboratories are diagnostic, quantitative measurements or prognostic, but these are complemented by expert advice on the interpretation of the results. Microbiologists are also involved in formulating policies designed to prevent spread of infection in hospitals; haematologists have clinical responsibilities for treating patients with haematological malignancies and other disorders.
In this chapter the general principles of diagnostic tests, quantitative measurements and prognostic tests are given and these are then related to the specific roles of clinical chemistry, cytogenetics, cytopathology, haematology, histopathology, immunology, microbiology and autopsies.
TYPES OF LABORATORY TESTS
Diagnostic tests
Diagnostic tests are those that are made on a sample from a patient, the result allocating the case to a diagnostic grouping; an example would be a needle core biopsy of a lesion of the breast which is sent for histopathological examination and classified into a benign or malignant (i.e. cancer) category. Quantitative measurements, such as haemoglobin concentration or arterial blood oxygen tension, may be used in the clinician’s diagnostic process but they do not by themselves assign a patient to a particular diagnostic category. A diagnostic test may be based on:
The ideal diagnostic test would produce complete separation between two diagnostic categories; usually, however, there is some overlap. This problem can be illustrated by taking as an example a screening test for colorectal carcinoma which makes measurements on a sample of faeces (many attempts have been made to devise such a test using measurements of blood contained in the faeces and other parameters). An ideal diagnostic test would produce complete separation of patients with and without colorectal carcinoma (Fig. 4.1). The majority of real diagnostic tests do not provide complete separation between diagnostic categories and there is overlap (Fig. 4.2).
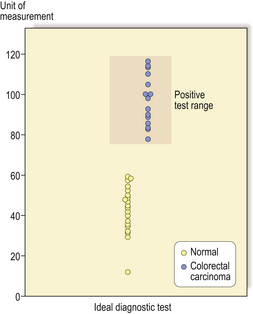
Fig. 4.1 Distribution graph for an ideal diagnostic test. There is complete separation of the population into those with colorectal carcinoma (shaded area) and those without. In this example a measurement of above 70 units would indicate that the subject had colorectal carcinoma and a measurement below 60 units would indicate that the subject did not have colorectal carcinoma.
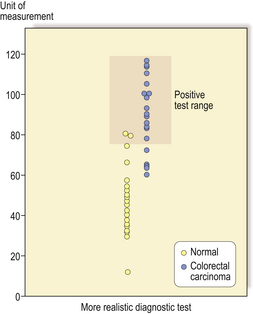
Fig. 4.2 Distribution graph of a more realistic diagnostic test. In this example there is a range of values between 60 and 80 units where there are subjects with and without colorectal carcinoma.
The effectiveness of a diagnostic test can be expressed using a number of different parameters:
Table 4.1 True and false test results in needle core biopsy of the breast (NCB)
| Test result from NCB | ||
|---|---|---|
| Actual outcome | Benign | Malignant |
| Benign | True negative | False positive |
| Malignant | False negative | True positive |
These can be combined into the following measures:
The desired values of these for a particular test will vary according to the action taken on the result. A malignant NCB result can result in a surgeon excising the breast (mastectomy), so the specificity and predictive value of a positive result must be as close to 100% as possible. In contrast, if a disease has a relatively safe, non-toxic treatment (such as a course of antibiotics) but the consequences of not detecting the disease can be fatal (e.g. bacterial meningitis), the sensitivity and predictive value of a negative result should be as high as possible. In most situations there is a direct ‘trade-off’ between sensitivity and specificity and a suitable threshold has to be set that will give the best overall performance (Fig. 4.3).
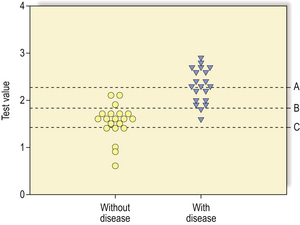
Fig. 4.3 A graph showing the effect of moving the threshold value for a test on its sensitivity and specificity. If the threshold is set at A then there are no false positives so the specificity is 100% but the sensitivity is low at about 60%. If the threshold is moved down to C there are no false negatives so the sensitivity is 100% but the rise in false positives has led to a reduction in the specificity to about 30%. At threshold B the test gives the greatest overall accuracy with three false positives and two false negatives.
In many medical situations a continuous biological spectrum is arbitrarily divided into a number of discrete categories which will always lead to some apparent misclassification but is necessary to give information on which clinicians can base their management decisions (e.g. division of intra-epithelial neoplasia of the uterine cervix into three categories, see Ch.19).
A laboratory’s performance in diagnostic tests should be monitored by a formal audit process and by use of appropriate positive and negative controls in tests.
Quantitative measurements
Many tests in pathology do not categorise results into discrete groups but give a quantitative result which is interpreted in relation to a ‘normal’ range of values. Examples of such tests include measurement of haemoglobin concentration, electrolyte concentrations, and blood oxygen and carbon dioxide levels.
The measures of performance for such tests differ from diagnostic grouping tests. In quantitative tests the accuracy of the measurement (how close the measured value is to the ‘true’ value determined by a more accurate or absolute method) and the reproducibility of the measurement (what variation there is when measuring the same sample many times) are important parameters. These can be assessed by using reference samples with ‘known’ values and putting these through the measurement system at regular intervals; most laboratories will have their own reference samples which are used frequently (internal quality assurance), and graphs of single measurement and running mean values will be used to ensure that the test is performing within expected limits and not showing ‘drift’ away from the central expected value (Fig. 4.4). Many countries also have external quality assurance schemes where reference samples are sent to all participating laboratories to ensure acceptable analytical performance.
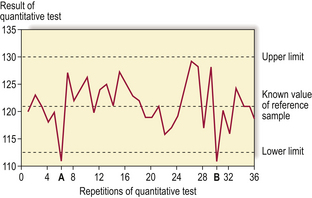
Fig. 4.4 Internal quality assurance graph for a quantitative pathological test. A reference sample is used for each test; tests A and B lie outside the acceptable range and the process of the test would have to be investigated for sources of error (e.g. out of date reagents, contamination, etc.).
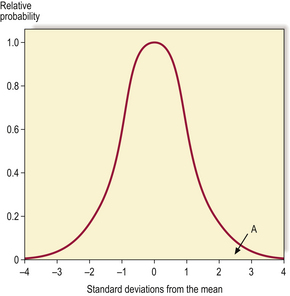
When a laboratory gives a quantitative result for a parameter that is under physiological control, a reference range is often given to facilitate interpretation of the result. If a parameter shows normal (Gaussian) distribution in the local population, the ‘normal’ range is often given as two standard deviations below the mean to two standard deviations above the mean. If a value lies outside this range then it lies outside 95% of the results for that population (Fig. 4.5) and may be regarded as abnormal, but 2.5% of the healthy population will have values lying outside the range at either end. Thus, all the details of the individual case must be considered, including other measurements, as a number of results at the top end of the ‘normal’ range could be more significant than a single result just above the ‘normal’ range. If the distribution is not Gaussian it may require normalisation by transformation, or non-parametric methods must be used.
Prognostic tests
In many tumours, assignment to a diagnostic category (e.g. adenoma or carcinoma) gives an indication of the prognosis for the individual patient, but within such groupings (e.g. colorectal carcinoma) there may be wide variation in the biological behaviour of the tumour. In order to plan appropriate treatment and to be able to give useful information and counselling to individual patients, many prognostic pathological tests have been developed.
In tumour pathology one of the most predictive prognostic tests is staging of the tumour (extent of spread), which is always assessed in the histopathological examination of specimens. One of the best examples of this is Dukes’ staging of colorectal carcinoma (Ch. 11 and Ch. 15). The histological type of tumour has important prognostic implications, particularly in some organs; subjects with papillary thyroid carcinoma have a life expectancy that is the same as for the rest of the general population without the tumour, whereas subjects with anaplastic thyroid carcinoma have a median survival of a few months. The grade of the tumour, an assessment of its degree of differentiation and proliferative activity, also has predictive value; well-differentiated tumours (closely resembling parent tissue) with few mitoses have a better prognosis.
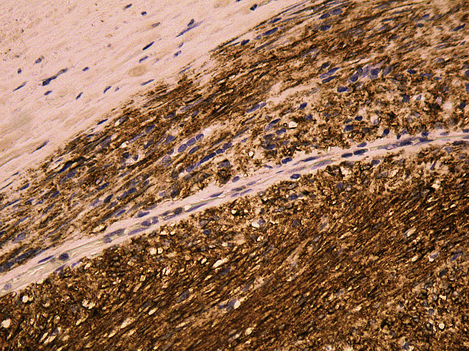
In tumours that produce substances that enter the blood or urine (e.g. alpha-fetoprotein produced by testicular teratomas, see Ch. 20), measurement of the levels of these at the time of diagnosis may be predictive of prognosis (and can be used in follow-up). As more becomes known of the molecular abnormalities of tumours, the possibilities for specific molecular tests that will have prognostic value increase, but the translation of an apparently significant research result into a routinely used prognostic test is not straightforward. When evaluating any new prognostic test the significance for the individual patient has to be considered; a test that shows a statistically significant difference between two large groups of patients may not assign individual cases to a prognostic category with a sufficient degree of certainty to be useful in management decisions or patient information. One recently developed test that has found usage is the detection of expression of the transmembrane receptor tyrosine kinase KIT, which is defined by the CD117 antigen and is the product of the c-kitproto-oncogene in stromal tumours of the gastrointestinal tract. This can be detected by immunohistochemistry (Fig. 4.6), which, if positive, predicts that the patient’s tumour will respond to treatment with a specific tyrosine kinase inhibitor, imatinib mesylate.
SPECIALISED TESTS
 Clinical chemistry: measurement and interpretation of substances in blood, other body fluids and tissues
Clinical chemistry: measurement and interpretation of substances in blood, other body fluids and tissues
Clinical chemistry
Methods in clinical chemistry detect and measure subcellular substances—usually in the blood but also in other bodily fluids and tissue:
The range of molecules measured is constantly expanding, ranging through electrolytes (such as sodium and potassium), larger inorganic molecules (urea), proteins (including many enzymes) and exogenous molecules (such as carbon monoxide and drugs):
Since many of the tests in clinical chemistry are quantitative, the laboratories have extensive programmes of internal and external quality control, and laboratory reports quote reference ranges. For many tests, ranges appropriate for the age and sex of the patient may be quoted.
As with all pathological tests the clinician with direct responsibility for the patient must decide whether a particular test is an appropriate investigation and what sample is most appropriate for that test. These considerations are especially important in clinical chemistry where large automated machines can measure a wide range of substances on a single sample and, if not used selectively, may generate non-essential data which may be difficult to interpret and lead to unnecessary further investigations.
The type of sample and the circumstances in which it is taken are also important. It is outside the scope of this chapter to give specific recommendations for individual tests but examples of inappropriate samples would be blood taken for glucose analysis shortly after a large carbohydrate-rich meal, blood taken for electrolyte analysis from a vein in an arm receiving an intravenous infusion, and blood taken for a digoxin level immediately after a dose of the drug.
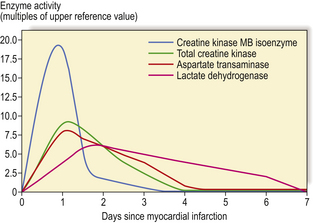
The interpretation of results also requires knowledge about the substances being assayed, and the advice of a specialist clinical chemist is often useful. An example of this is the use of cardiac enzymes measured to determine whether a myocardial infarct has occurred. The enzymes lactate dehydrogenase, aspartate transaminase and creatine phosphokinase normally reside intracellularly in muscle cells; if muscle is damaged, the enzymes gain entry to the blood and elevated levels may be detected. The interpretation of these assays requires knowledge about the time course of the enzyme release and the possible sites of enzyme release. The enzymes are not released immediately when the myocytes become hypoxic because the cell membranes take some time to break down; Figure 4.7 shows typical curves of the enzymes in blood after a myocardial infarct; it can also be seen from this graph that total creatine kinase and aspartate transaminase reach their peaks earlier than lactate dehydrogenase. The interpretation of the enzyme results will thus require knowledge of these properties and an estimate of when the ischaemic myocardial event is likely to have occurred in the patient. Cardiac muscle is not the only tissue to contain these enzymes; they are also present in skeletal muscle, but different forms of the enzymes (isoenzymes) are present in the different sites. If an assay is used that measures the total amount of these enzymes, damage to skeletal muscle would produce elevations. Thus, if a patient had been found collapsed at home and had been lying on the floor, measurement of the isoenzymes, such as creatine kinase MB, or muscle proteins would be required to ascertain whether an ischaemic myocardial event had precipitated the collapse. Similar interpretative considerations apply to all tests in clinical chemistry.
Cytogenetics and molecular pathology
Cytogenetics and molecular pathology are playing an increasingly important role in clinical pathology (Ch. 3) with more discrete genetic abnormalities being identified in specific tumours (Ch. 11). Laboratory techniques may look at the number and form of chromosomes, the karyotype, or at more specific areas of DNA within chromosomes. The techniques for investigating individual genetic abnormalities are described in Chapter 3.
The karyotype can be examined using a sample of peripheral blood. Phytohaemagglutinin is added to the blood, which stimulates the T-lymphocytes to divide; colchicine is then added to arrest the dividing cells in metaphase when the chromosomes will be most easily visible. The chromosomes may be stained by several methods but the most common is the Giemsa method which produces alternate light and dark bands when the preparation is viewed by light microscopy (G-banding); the patterns of banding allow identification of each chromosome and visualisation of missing or additional material of about 4000 kilobases or more. Abnormalities may be divided into:
The number of chromosomal abnormalities associated with specific tumours is growing rapidly; currently there are over 30 human tumour types associated with non-random chromosomal abnormalities. One of the chromosomal abnormalities with the strongest association with a malignancy is the Philadelphia chromosome in chronic myeloid leukaemia. This abnormality is a reciprocal translocation between chromosomes 9 and 22 resulting in the translocation of the abl oncogene to a breakpoint cluster region, which results in a hybrid gene producing a novel protein that may be responsible for the neoplastic transformation (Ch. 23). Another chromosomal abnormality strongly associated with a specific tumour is the 13q14 microdeletion seen in retinoblastoma.
The karyotyping of chromosomes is a relatively coarse method of detecting genetic abnormalities. A more specific technique is fluorescent in situ hybridisation (FISH) which is used to detect and localise the presence or absence of specific DNA sequences on chromosomes. The technique uses fluorescent probes that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy can then be used to localise the fluorescent probe in relation to the tissue being examined. A common use of FISH is to detect amplification of the HER2 gene in breast cancers, which then indicates that trastuzumab (Herceptin) therapy will be effective against that tumour.
As more of these abnormalities are found it becomes increasingly important to send tumour samples for cytogenetic analysis as a diagnostic/prognostic procedure. Cytogenetic analysis requires fresh tissue that has been placed in an appropriate transport medium and that must be transported rapidly to the cytogenetics laboratory. It is important to send appropriate samples, which might include ‘normal’ background tissue as well as tumour; the most appropriate staff to do this might be the histopathologists if they receive the specimen fresh before immersion in a fixative solution.
Cytopathology
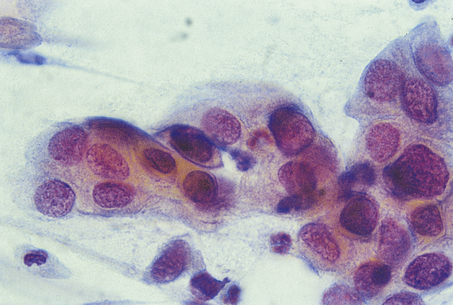
Cytopathology specimens consist of single cells or clumps of cells that are dissociated from their surrounding tissues (Fig. 4.8). The technique is used mainly for the investigation and diagnosis of malignancy. The cells are distributed on glass slides, either by the person who takes the sample smearing them directly onto the slide at the time the sample is taken or by centrifugation methods in the laboratory. The slides are stained by an appropriate method, which is most often the Papanicolaou technique, and examined by light microscopy. Since the cells are dissociated from their surrounding tissue, some features that are used in histopathological diagnosis, such as invasion and other architectural abnormalities, are not available for assessment. The main features used in cytopathological diagnosis are:
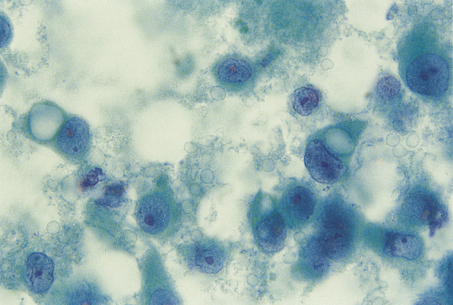
Fig. 4.8 Cytological preparation of a fine needle aspirate of a breast carcinoma. The specimen consists of dissociated cells with no surrounding tissue.
Cells may be collected for cytological examination from epithelium shed or scraped from a body surface (exfoliative cytology) or by aspirating cells through a fine-bore needle into a syringe (aspiration cytology). Many cytopathological specimens are taken to assess dysplasia or malignancy in tissues but infective pathologies may also be diagnosed by this method; for example, Pneumocystis jiroveci pneumonia in immunosuppressed patients may be detected by cytological examination of alveolar washings.
Cancer screening
Cervix
One of the most frequent uses of cytopathological techniques is in the detection and assessment of dysplasia and neoplasia in the uterine cervix (Ch. 19). The surface of the cervix is relatively accessible by speculum examination and cells are scraped from the surface at the junction between the squamous and glandular epithelium (the transformation zone) using a spatula. The cells are either spread directly onto a glass slide and fixed or put into a liquid transport medium and sent to the laboratory where the cells can be centrifuged onto a slide (liquid-based cytology which gives specimens that are easier to interpret). The cells are stained using the Papanicolaou technique. Cells from areas of dysplasia or neoplasia are recognised by their abnormal nuclear (dyskaryotic) features and the degree of abnormality is graded in a range from mild to severe. Mild abnormalities represent early dysplastic or reactive changes in the cervical epithelium, which may regress, so the usual management for those women is surveillance by further smears. More severe changes (Fig. 4.9) represent marked dysplasia or carcinoma; women whose smears show such changes are referred to gynaecologists for further assessment and probable surgical treatment. In many countries cervical cytology is performed as a screening programme; the aim is to take samples at regular intervals from all women who are at risk of developing cervical cancer (which is most women with a uterus who have had sexual intercourse) and to detect early abnormalities which can be treated before invasive carcinoma has developed. The method of cytopathological examination of cells from the uterine cervix is effective in detecting the abnormalities but most cervical screening programmes have not been totally effective because a significant proportion of women have failed to attend for screening.
Haematology
Haematology covers diseases of the blood; the pathology of these is described in Chapter 23. The work of haematologists is usually divided into three areas:
The diagnosis of haematological disorders is based on clinical history and examination, measurement of parameters in the blood, microscopic examination of blood films and often microscopic examination of bone marrow aspirates and trephine samples.
Automated machines measure many parameters in a sample of blood; the most common are:
Such machines can produce a plethora of data and the same problems of interpretation may occur as described in the section on clinical chemistry above, but in haematology many of the parameters (e.g. haemoglobin, red cell count and mean cell volume) are linked and need to be examined together when making a diagnosis. Other measurements, such as of serum ferritin or cyanocobalamin (vitamin B12), may need to be made to confirm the diagnosis.
Examination of the blood film can reveal abnormalities of red blood cell shape and size (e.g. anisocytosis, poikilocytosis, macrocytosis—see Ch. 23) and abnormal white blood cells such as blast cells in leukaemia. Some features, such as rouleaux formation by red blood cells, may suggest abnormalities in the non-cellular components of blood (in this case possible overproduction of antibodies or immunoglobulin).
Bone marrow examination
Samples of the bone marrow may be taken by insertion of a relatively large-bore needle into a site, such as the iliac bone, and aspiration by a syringe. At the same time a tissue sample of marrow can be sampled with a trephine needle. A smear of aspirated cells, stained by the Giemsa method, allows identification of cells, and their relative proportions may be quantified. This is an integral part of the diagnosis of leukaemia and assessment of its response to treatment (Ch. 23). Trephine samples of bone marrow retain the architecture of the tissue and allow assessment of the overall cellularity, amount of reticulin and site of different cell types; such samples are essential in diseases that produce fibrosis of the bone marrow, such as myelofibrosis or metastatic prostatic carcinoma, as aspirates will usually produce a very low cellular yield.
Blood transfusion
The primary purpose of blood transfusion is the supply of a product for the treatment of patients. The blood products that can be supplied include:
Primary concerns in the operation of a blood transfusion laboratory will include an error-free system of cross-matching (as a mismatched transfusion may prove fatal), safeguards against transmission of microbiological agents (such as human immunodeficiency virus (HIV), hepatitis B and C) by transfusion, and balancing supply and demand of the products.
Histopathology
Histopathology involves the macroscopic examination of tissue with selection of tissue samples for light microscopic examination. Histopathology is usually the primary mode of diagnosis for tumours and also gives prognostic information by grading and staging of surgical resection specimens. Diagnosis of infective and inflammatory conditions can also be made as, for instance, in the detection of Helicobacter pylori in gastric biopsies or the diagnosis of inflammatory conditions of the skin.
Most diagnostic histopathology is performed on haematoxylin and eosin (H&E)-stained sections of paraffin wax-embedded tissue. The tissue removed by surgical excision or biopsy is placed in a solution of fixative (most commonly formaldehyde) and transported to the histopathology laboratory. On receipt it is examined by the laboratory staff; a macroscopic description is given and tissue is selected for light microscopic examination. Larger specimens, where most of the tissue will not be examined by light microscopy, are assessed and sampled by medically trained staff who are familiar with a wide range of macroscopic appearances and have a detailed knowledge of anatomy. The samples taken will vary but in a resection specimen would include samples of:
The samples of tissue are processed by machine into paraffin wax, a process involving progressive dehydration through increasingly pure solutions of alcohol that is usually carried out overnight. The wax-embedded tissue samples are then mounted on a microtome and sections 5–7μm in thickness are cut, mounted on glass slides and stained. These slides are interpreted by expert pathologists and reports are issued to the clinicians who sent the specimens. The reports are tailored to the type of specimen and the clinical details given on the request form. If a tumour is being examined the report will include the type of tumour, its grade (well, moderately or poorly differentiated), its stage (how far it has spread locally, whether any vascular invasion is detected and whether any sampled lymph nodes contain tumour) and comments on the surrounding tissue (e.g. whether there is dysplasia in background epithelium).
Although H&E is the most commonly used stain, there are other stains that may be used to investigate specific features of the tissue. Many of these are standard tinctorial procedures (Table 4.2).
Table 4.2 Commonly used stains in histopathology
| Stain | Use |
|---|---|
| Haematoxylin and eosin (H&E) | Routine stain for histological sections |
| Masson’s trichrome | Fibrous tissue |
| Perls’ | Haemosiderin |
| Masson–Fontana | Melanin |
| Modified Giemsa | Helicobacter |
| Ziehl–Neelsen | Acid-fast bacilli |
| Gram | Bacteria |
| Periodic acid–Schiff (PAS) | Glycogen, fungi |
| Grocott’s silver stain | Fungi |
| Alcian blue | Acidic mucin |
| Periodic acid–Schiff with diastase | Neutral mucin |
Immunohistochemistry
An increasingly commonly used technique is immunohistochemistry. In this method antibodies are used that have been raised artificially to specific substances of interest (e.g. low molecular weight cytokeratins in a suspected epithelial tumour) and these bind to the specific substances if they are present in the tissue section. The bound antibody is then visualised using one of a variety of methods, such as antibodies against the initial antibody and a dye complex such as diaminobenzidine. Immunohistochemistry is useful in:
In situ hybridisation (ISH)
DNA probes can be constructed that will bind to specific DNA or messenger RNA (mRNA) in tissue sections. The DNA probes are single-stranded sequences of DNA from tens to thousands of kilobases long and are labelled with radioisotopes, or now more commonly biotin or digoxigenin, to visualise the site of hybridisation using a colorimetric or fluorescent agent (the same method as used in cytogenetics). The DNA in the tissue section is made into a single-stranded form, by conditions such as strong alkalis, and the probe will bind to complementary sequences in the target DNA or mRNA. This technique is useful for detecting infectious agents in tissue sections, such as cytomegalovirus or Epstein–Barr virus. It can also be used to detect production (rather than simply storage) of proteins in cells by detection of the mRNA for the specific protein.
An excellent example of how all these histopathology techniques are integral to patient management is the current treatment of breast cancer. Breast cancer may be detected by mammographic screening, or a woman may present with a self-discovered lump, but the diagnosis is made by histological examination of a sample of the lesion—most commonly by needle core biopsy. The most usual treatment of breast cancer is primary surgical excision, with sampling of the axillary lymph nodes to detect metastases. These specimens are sent to the histopathology laboratory where examination produces a large amount of information that is vital for further management. Examination of H&E-stained sections will give the histological type of the breast cancer, its histological grade, the size of the cancer and whether the axillary lymph nodes contain metastases. This information makes a reasonably reliable prediction of the biological behaviour of the breast cancer. A small, low-grade tumour with no lymph nodes metastases is unlikely to have metastasised at the time of surgical resection and the side-effects of adjuvant systemic chemotherapy will probably outweigh the possible benefits (i.e. the ablation of metastases that have not yet been detected). A large, high-grade tumour that has already metastasised to the axillary lymph nodes has a high risk of spread to other parts of the body, and the benefits of adjuvant systemic chemotherapy in eradicating or reducing the size and number of these is likely to be greater than the side-effects of this treatment. There are now a range of immunohistochemical tests that are performed on breast cancers and are useful in planning therapy for individual patients (Fig. 4.10). If the tumour expresses oestrogen and progesterone receptors then anti-oestrogen drugs, such as tamoxifen, will reduce the risk of recurrence or metastases. If the tumour has amplification of the human epidermal growth factor receptor-2 (HER2) gene (seen by overexpression of the Her2 protein on immunohistochemistry or by FISH for the HER2 DNA sequence), then adjuvant trastuzumab (Herceptin) therapy will markedly reduce the risk of death. A basal phenotype of breast cancer has recently been defined by its expression of basal cytokeratins (and absence of oestrogen receptor expression and lack of HER2 amplification) that has a poor prognosis but does respond to a specific chemotherapy regimen. It is likely that more of these markers will be developed for a wider range of tumours, heralding an era of individualised therapy for cancer patients.
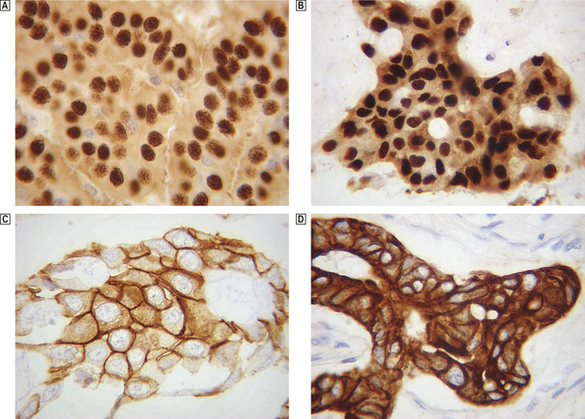
Fig. 4.10 Immunohistochemical staining of breast cancers for specific proteins.  A breast cancer showing strong nuclear positivity for oestrogen receptor (dark brown staining nuclei).
A breast cancer showing strong nuclear positivity for oestrogen receptor (dark brown staining nuclei).  A breast cancer showing strong nuclear positivity for progesterone receptor (dark brown staining nuclei).
A breast cancer showing strong nuclear positivity for progesterone receptor (dark brown staining nuclei).  A breast cancer showing strong membranous staining for the HER2 protein which indicates amplification of the HER2 gene.
A breast cancer showing strong membranous staining for the HER2 protein which indicates amplification of the HER2 gene.  A breast cancer showing strong staining for an antibody directed against a basal cytokeratin suggesting that this tumour is showing a basal phenotype.
A breast cancer showing strong staining for an antibody directed against a basal cytokeratin suggesting that this tumour is showing a basal phenotype.
Electron microscopy
Electron microscopy may be used to visualise subcellular detail in tissue samples. In the past this technique was used for detecting features of differentiation in tumours (such as melanosomes in malignant melanomas) but immunohistochemistry has largely replaced this function. Electron microscopy is still used in the classification of glomerulonephritis, where the site and nature of immune complexes in the glomerular basement membrane may be visualised (Ch. 21).
Immunology
Immunology is concerned with the immune response, both antibody and cell mediated, in health and disease. The range of antibodies and cellular features that can be detected and measured has increased so much in recent years that many centres have a separate immunology department to deal with these. The various tests may be divided into those measuring antibodies and those measuring cells.
Immunoglobulins and antibodies
The overall levels of antibodies of certain classes can be measured but this is of little diagnostic use except in generalised immunodeficiencies such as hypogammaglobulinaemia. Detection or measurement of antibodies directed against specific antigens is important in the diagnosis and assessment of autoimmune diseases. Samples of a patient’s serum are placed on tissue sections and any bound antibody can be visualised by applying further antibodies against human immunoglobulin (or a specific subclass) to which is attached an immunofluorescent dye. Auto-antibodies detected in this way include antinuclear antibodies found in systemic lupus erythematosus. To detect auto-antibodies bound to the patient’s own tissues a sample of tissue is taken from the patient (this might be skin or a renal biopsy), antibodies against human immunoglobulins are applied to the biopsy and any bound antibody is visualised by immunofluorescent or other techniques. This technique is used in the assessment of glomerulonephritis (Ch. 21) and bullous skin disorders (pemphigus, pemphigoid, dermatitis herpetiformis, etc., see Ch. 24).
Lymphocytes
There are now antibodies to the specific antigens of most subsets of lymphocytes, such as T-cells or B-cells, T-suppressor cells, T-helper cells, etc., and in conjunction with other techniques (such as fluorescence-activated cell sorting—FACS) the number of lymphocytes in each subclass can be measured. These measurements can give important information about a patient’s immune status. In acquired immune deficiency syndrome (AIDS) there is selective destruction of T-helper cells by HIV so that a reduction in the T-helper cell:T-suppressor cell ratio in HIV-positive subjects can indicate the onset of AIDS (Ch. 9). In organ transplantation the detection of acute cellular rejection is important if appropriate immunosuppressive therapy is to be given in time to prevent loss of the graft. Rejection is primarily detected by histological examination of a biopsy of the graft (e.g. kidney) but measurement of the T-cell helper:suppressor ratio provides useful additional information and, with more specific subtyping of lymphocytes, such tests may eventually replace graft biopsy.
Microbiology
Microbiology involves the detection and identification of micro-organisms, including viruses, bacteria, fungi, protozoa and helminths. These may be detected by direct examination of a sample from a patient or by culture of such a sample to increase the number of organisms before using a detection method. Evidence of infection can also be inferred from serological tests for an antibody response to the organism. The susceptibility of cultured organisms to therapeutic agents, such as antibiotics, will also be assessed and microbiologists have wider responsibilities for general control of infection in hospitals and the community.
Direct detection methods in microbiology include:
These methods give rapid results, which can be very useful to clinicians. Examples of direct detection include the identification of Pneumocystis jiroveci in bronchoalveolar washings from immunosuppressed patients (such as those with AIDS), immunofluorescent detection of Cryptosporidium in faeces, and immunofluorescent detection of respiratory syncytial virus in nasopharyngeal aspirates.
Viruses
Viruses are obligate intracellular parasites and so can be grown only in a cellular culture, such as ‘immortal’ cells derived from tumours or cultures with a finite life-span derived from embryonic tissues. The presence of a virus may be detected by the presence of a cytopathic effect, by haemadsorption/haemagglutination or by the direct methods described above. The identity of the virus is confirmed by neutralisation of the cytopathic effect or haemadsorption/haemagglutination by antibodies raised against specific viruses. Serological tests are often used to diagnose viral infection: such tests involve the measurement of antibodies against specific viruses using a detection system such as ELISA, radio-immunoassay, immunofluorescence or complement fixation tests. A detectable level of virus-specific IgM or a four-fold rise in the titre of other classes of virus-specific antibody is an indication of recent infection with that virus.
Bacteria
Bacteria may be cultivated in cell-free media. For most purposes the medium used is solid rather than liquid (‘broth’). Most solid culture media are based on agar, to which blood or other nutrients are added. Where it is wished to identify a specific pathogen existing in the presence of other bacteria, substances may be incorporated that will inhibit the growth of these other bacteria while not affecting the specific pathogen being sought (‘selective media’). For any given type of specimen a range of media is chosen that will support the growth of all pathogens relevant to the clinical condition. Cultures are then incubated at appropriate temperatures and atmospheric conditions (i.e. aerobic and anaerobic). Most bacteria will grow within a few days and can then be identified by:
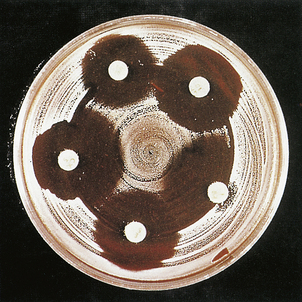
Some bacteria require specialised media and prolonged incubation in order to produce detectable colonies (for example Mycobacterium tuberculosis may need up to 8 weeks’ incubation on Löwenstein–Jensen medium). The susceptibility of bacteria to antibiotics may be determined by various methods, most commonly by observing inhibition of bacterial growth around antibiotic-impregnated filter-paper discs placed on culture plates prior to incubation (Fig. 4.11). Microbiologists should provide advice on the empirical choice of antibiotics in cases where treatment may need to begin before the results of susceptibility tests are available.
Fungi
Fungi are grown on simple media (such as glucose peptone agar or blood agar with antibiotics to inhibit bacterial overgrowth) in aerobic conditions. Cultured fungi are identified by the method of spore production (asexual and sexual), morphology of the colony, morphology of vegetative and aerial hyphae, biochemical reactions and antigenic structure.
Parasites
Diseases caused by parasites are major problems in many countries, particularly those with tropical climates in which the vectors (e.g. insects) thrive. Parasites may be identified in, for example, tissue samples or faeces by their often distinctive morphology.
Precautions
When requesting microbiological tests it is especially important to send suitable specimens. Such samples should come from the likely site of infection, should not contain contaminants, should not contain substances likely to inhibit growth (such as antibiotics), should be put into a suitable container (which may contain a transport medium) and should be transported rapidly to the microbiology laboratory. If septicaemia is suspected but no focus of infection has been identified, multiple samples, including blood and urine, should be sent before systemic antibiotic therapy is started. The risk to staff looking after patients with microbiological infections, or handling specimens from them, is roughly classified according to the degree of hazard (Table 4.3). Most infective agents are included in category 2 (according to the scheme used in the UK). If a patient potentially has a category 3 pathogen then all samples should be marked as such because laboratories receiving these samples will have to take special precautions in handling them (this includes samples sent for non-microbiological investigations).
Table 4.3 Categories of risk (in the UK) for infectious organisms∗
| Category | Risk | Examples |
|---|---|---|
| 1 | An organism that is most unlikely to cause human disease | Algae |
| 2 | An organism that may cause human disease and may be a hazard to those handling it, but is unlikely to spread to the community and effective prophylaxis or treatment is usually available | Staphylococcus aureus, Escherichia coli |
| 3 | An organism that may cause severe human disease and present a serious hazard to those handling it. It may present a risk of spread to the community but there is usually effective prophylaxis available | Hepatitis B virus, Mycobacterium tuberculosis, Salmonella typhi |
| 4 | An organism that causes severe human disease and is a serious hazard to those handling it. It may present a high risk of spread to the community and there is usually no effective prophylaxis or treatment | Lassa fever virus, Marburg virus |
∗ Adapted from: Categorisation of pathogens according to hazard and categories of containment 1990, 2nd edn. ACDP, HMSO
Hospital-acquired infections
Hospitals contain many patients with microbiological infections and there is considerable potential for spread to other patients. All hospitals should have agreed procedures for preventing the spread of infection, including adequate sterilisation and disinfection, and isolation or barrier nursing when required. Such policies will have been formulated in consultation with the microbiologists of the hospital. The microbiological laboratory will be in a position to detect outbreaks of particular infections if there is suitable monitoring of laboratory results. An increasing problem in hospitals is the emergence of bacteria that are resistant to antibiotics, and this can be limited by the development of protocols for antibiotic usage.
AUTOPSIES
In most countries autopsies fall into two main categories:
Medicolegal autopsies
Medicolegal autopsies are performed to determine the cause of death and to collect evidence that may be used in the prosecution of those alleged to be responsible for the death. In many cases of murder the cause of death (e.g. bullet wounds or stab wounds) is obvious and most of the work of the pathologist is the collection of evidence, such as trace evidence confirming contact between the deceased and the person accused of the murder (e.g. blood stains, tissue beneath the deceased’s fingernails, semen in body orifices) or evidence to link a specific weapon with the deceased’s wounds (e.g. retrieval of bullets from wounds).
Clinical autopsies
Non-medicolegal (clinical) autopsies performed on patients who die in hospital may appear to be diagnostic tests that have been performed too late, but much useful information can be gathered from these procedures. Many studies have shown that the certified cause of death given by the clinicians with primary responsibility for the patient shows a discrepancy with the cause identified at autopsy, to the extent of being in a different organ system in about 30% of cases. The hospital autopsy is therefore very useful in providing more accurate data about the cause of death; this is important for clinical audit, for education of clinicians, and for national allocation of health resources if the cause of death is used as an index of the prevalence of disease (which it is in many countries, including the UK). The hospital autopsy is also useful in defining the extent of disease and response to treatment. If a patient has had a malignant tumour, such as malignant melanoma, that has spread to other sites in the body and that patient has then received systemic treatment, it is important to have the most accurate data available about the organs to which the tumour had spread and whether the therapy had any apparent effect on the tumour. Modern methods of in vivo imaging, such as computed tomography and magnetic resonance imaging, may provide some of these data but, if the patient dies, an autopsy is a simple and cost-effective method of gathering accurate data.
The rate of autopsies on patients dying in hospital has shown a decline in most countries over the past decade; this will inevitably lead to loss of much useful information about human disease.
Autopsy techniques
Performing an autopsy is a relatively cheap, low technology procedure that has not changed much since the pioneering work of Virchow in the 19th century. A midline incision from the neck to the symphysis pubis is made and the thoracic and abdominal organs are removed. The scalp is reflected from the skull and the cranium is opened to remove the brain. All the organs are dissected in detail by a medically trained pathologist and the macroscopic appearances and weights are recorded; samples may be taken for microscopic examination, clinical chemistry analysis or microbiological culture. Return of the organs to the body cavities and reconstruction of the body produces an acceptable cosmetic result so that relatives can view the body after autopsy. More limited examination of the body can still generate useful information and so the examination may be limited (by the deceased’s relatives’ wishes) to certain areas of the body. This may allow an autopsy examination to be performed where permission would otherwise be refused (e.g. exclusion of examination of the cranial cavity in a patient who had received chemotherapy and had no hair, making any scalp incision clearly visible). The ultimate limited autopsy is the needle autopsy where percutaneous samples of organs are taken for histological examination using a needle core biopsy needle or fine needle aspiration techniques; such a technique is useful to assess liver disease in cases of hepatitis B or C where risk of infection may preclude a full autopsy.
Generic aspects of pathology in clinical practice
Plebani M. Errors in laboratory medicine and patient safety: the road ahead. Clinical Chemistry and Laboratory Medicine. 2007;45:700-707.
Clinical chemistry
Clinical chemistryMarshall W.J., Bangert S.K.. Clinical chemistry, 5th edn. Oxford: Mosby, 2004.
Cytogenetics and molecular pathology
Shaffer L.G., Bejjani B.A.. Medical applications of array CGH and the transformation of clinical cytogenetics. Cytogenetic and Genome Research. 2006;115:303-309.
Sreekantaiah C. FISH panels for hematologic malignancies. Cytogenetic and Genome Research. 2007;118:284-296.
Cytopathology
Jenkins D. Histopathology and cytopathology of cervical cancer. Disease Markers. 2007;23:199-212.
Haematology
Hoffbrand A .V., Pettit J., Moss P.. Essential haematology, 5th edn. Oxford: Blackwell Publishing, 2006.
Histopathology
Bao T., Prowell T., Stearns V.. Chemoprevention of breast cancer: tamoxifen, raloxifene, and beyond. American Journal of Therapeutics. 2006;13:337-348.
Boughey J .C., Buzdar A.U., Hunt K.K.. Recent advances in the hormonal treatment of breast cancer. Current Problems in Surgery. 2008;45:13-55.
Gusterson B.A., Ross D.T., Heath V .J., Stein T.. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Research. 2005;7:143-148.
Hudis C .A.. Trastuzumab—mechanism of action and use in clinical practice. New England Journal of Medicine. 2007;357:39-51.
Immunology
Delves P .J., Martin S., Burton D., Roitt I.. Roitt’s essential immunology, 11th edn. Oxford: Blackwell Publishing, 2006.
Microbiology
Goering R., Dockrell H., Zuckerman M., Wakelin D., Roitt I.. Mims’ medical microbiology, 4th edn. Oxford: Mosby, 2007.
Autopsies
Burton J.L., Rutty G.N.. The hospital autopsy, 2nd edn. Oxford: Oxford University Press, 2001.