Pasteurella and Similar Organisms
1. Describe the general characteristics of Pasteurella spp. and the additional organisms included in this chapter.
2. Describe the epidemiology associated with human infections caused by Pasteurella spp. and similar organisms, including the normal habitat and route of transmission.
3. Compare the Gram-stain appearance of the organisms included in this chapter.
4. Explain the limitations of antimicrobial susceptibility testing with respect to Pasteurella spp. and similar organisms.
5. Identify limitations associated with identification of Pasteurella spp. and similar organisms.
Epidemiology, Spectrum of Disease, and Antimicrobial Therapy
Most of the organisms presented in this chapter constitute portions of both domestic and wild animal flora and are transmitted to humans during close animal contact, including bites. For most of these species, virulence factors are not recognized. As a result, the organisms may be considered opportunistic pathogens that require mechanical disruption of host anatomic barriers (i.e., bite-induced wounds; Table 30-1). Of the organisms listed in Table 30-2, P. multocida subsp. multocida is most commonly encountered in clinical specimens. Reported virulence factors for this subspecies include lipopolysaccharide, cytotoxin, six serotypes of the antiphagocytic capsule, surface adhesins, and iron-acquisition proteins. Other manifestations of infection by P. multocida subsp. multocida can include respiratory disease and systemic disease such as endocarditis and septicemia. Liver cirrhosis is viewed as a risk factor for systemic disease. Other Pasteurella spp. can be agents of systemic infection (P. pneumotropica) and genital tract-associated disease (P. bettyae).
TABLE 30-1
Epidemiology of Selected Pasteurella spp. and Similar Organisms
| Organism | Habitat (Reservoir) | Mode of Transmission |
| P. multocida, other Pasteurella spp. | Commensal found in nasopharynx and gastrointestinal tract of wild and domestic animals; potential upper respiratory commensal in humans having extensive occupational exposure to animals | Bite or scratch from variety of veterinary hosts (usually feline or canine); infections may be associated with non-bite exposure to animals; less commonly, infections may occur without history of animal exposure |
| S. indologenes | Unknown; rarely encountered in clinical specimens but may be part of human flora | Unknown |
TABLE 30-2
Pathogenesis and Spectrum of Disease of Selected Pasteurella spp. and Similar Organisms
| Organism | Virulence Factors | Spectrum of Disease and Infections |
| P. bettyae | Unknown | Genital tract infection; neonatal infection |
| P. multocida subsp. multocida | Endotoxin, cytotoxin, surface adhesins, capsule associated with P. multocida | Focal soft tissue infection; chronic respiratory infection, usually in patients with preexisting chronic lung disease and heavy exposure to animals; systemic disease (hematogenous dissemination) such as meningitis, endocarditis, osteomyelitis, dialysis-associated peritonitis, septicemia |
| P. multocida subsp. septica | Unknown | Focal soft tissue infection |
| P. pneumotropica | Unknown | Rare systemic infection |
| S. indologenes | Unknown | Rare ocular infection |
An unusual feature of the organisms considered in this chapter is that most are susceptible to penicillin. Although most other clinically relevant Gram-negative bacilli are intrinsically resistant to penicillin, it is the drug of choice for infections involving P. multocida and several other species listed in Table 30-3. The general therapeutic effectiveness of penicillin and the lack of resistance to this agent among Pasteurella spp. suggest that in vitro susceptibility testing is typically not indicated. This is especially true with isolates emanating from bite wounds. Moreover, bite wounds can be complicated by polymicrobial infection. In this case, the empiric therapy directed toward multiple agents is generally also effective against Pasteurella spp. As a result, antimicrobial susceptibility testing for Pasteurella spp. may have greater utility for isolates recovered from sterile sources (blood, deep tissue) and from respiratory specimens obtained from immunocompromised patients.
TABLE 30-3
Antimicrobial Therapy and Susceptibility Testing for Pasteurella spp. and Similar Organisms
| Organism | Therapeutic Options | Potential Resistance to Therapeutic Options | Validated Testing Methods |
| Pasteurella spp. | Penicillin, ampicillin, amoxicillin are recommended agents; doxycycline, amoxicillin-clavulanate are alternative agents; ceftriaxone, fluoroquinolones may be effective | Clindamycin, cephalexin, nafcillin, erythromycin (deduced from susceptibility testing) | CLSI document M45-A2 |
| S. indologenes | Not well characterized; purported susceptibility to penicillins, chloramphenicol, tetracycline | Unknown | Not available |

Laboratory Diagnosis
Specimen Collection and Transport
No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport.
Cultivation
Media of Choice
Colonial Appearance
Table 30-4 describes the colonial appearance and other distinguishing characteristics (e.g., hemolysis and odor) of these genera on blood agar.
TABLE 30-4
| Organism | Appearance |
| M. haemolytica* | Convex, smooth, grayish, beta-hemolytic (feature may be lost on subculture) |
| P. aerogenes* | Convex, smooth, translucent, nonhemolytic† |
| P. bettyae‡ | Convex, smooth, nonhemolytic |
| P. caballi | Convex, smooth, nonhemolytic |
| P. canis | Convex, smooth, nonhemolytic |
| P. dagmatis | Convex, smooth, nonhemolytic |
| P. multocida | Convex, smooth, gray, nonhemolytic; rough and mucoid variants can occur; may have a musty or mushroom odor |
| P. pneumotropica* | Smooth, convex, nonhemolytic |
| P. stomatis | Smooth, convex, nonhemolytic |
| S. indologenes | Resembles Kingella spp. (see Chapter 31); may spread or pit the surface of blood agar |
*Breakthrough growth may occur on MacConkey agar; will appear as lactose fermenter.
†After 48 hours, colonies may be surrounded by a narrow green to brown halo.
‡Breakthrough growth may occur on MacConkey agar; will appear as non-lactose fermenter.
Approach to Identification
The accuracy of commercial biochemical identification systems has been called into question for the definitive identification of Pasteurella spp. and similar organisms. Table 30-5 summarizes conventional biochemical tests that can assist in the presumptive differentiation or species confirmation of organisms discussed in this chapter. These organisms closely resemble those described in Chapter 31. Therefore, data discussed in both Chapters 30 and 31 can be considered when evaluating an isolate in the clinical laboratory. A more complete conventional biochemical battery, offered as part of a reference laboratory workup, may be required for definitive identification of the isolates. Alternatively, past attempts to definitively identify Pasteurella spp. on the basis of cellular fatty acid analysis have been replaced by 16S rDNA gene sequencing and sodA gene sequencing. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry may provide future utility.
TABLE 30-5
Key Biochemical Characteristics of Selected Pasteurella spp. and Similar Organisms
| Organism | Phenotype | |||||||
| Indole | Urea | Nitrate Reduction | Catalase | ODC† | Mannitol | Sucrose | Maltose | |
| M. haemolytica | − | − | + | + | − | (+) | + | + |
| P. aerogenes | − | (+) | (+) | + | v | − | + | + |
| P. bettyae | (+) | − | (+) | − | − | − | − | − |
| P. caballi | − | − | (+) | − | (+) | (+) | (+) | (+) |
| P. canis | + | − | + | + | (+) | − | (+) | − |
| P. dagmatis | (+) | (+) | (+) | + | − | − | + | (+) |
| P. multocida | (+) | − | (+) | + | (+) | + | + | − |
| P. pneumotropica | (+) | (+)* | (+) | + | (+) | − | + | + |
| P. stomatis | (+) | − | + | + | − | − | (+) | − |
| S. indologenes | (+) | − | − | v | − | − | (+) | (+) |
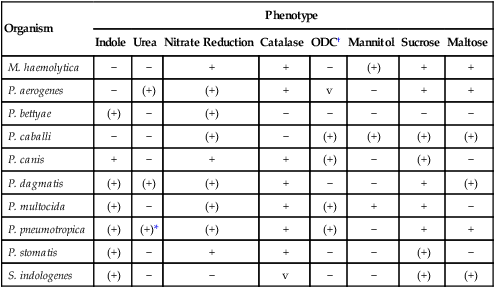
+, >90% of strains positive; (+), >90% of strains positive but reaction may be delayed (i.e., 2 to 7 days); −, >90% of strains negative; v, variable.
*May require a drop of rabbit serum on the slant or a heavy inoculum.
Data compiled from Angen O, Mutters R, Caugant DA, et al: Taxonomic relationships of the [Pasteurella] haemolytica complex as evaluated by DNA-DNA hybridization and 16S rRNA sequencing with proposal of Mannheimia haemolytica gen. nov., comb. nov., Mannheimia granulomatis comb. nov., Mannheimia glucosida sp. nov., Mannheimia ruminalis sp. nov. and Mannheimia varigena sp. nov., Int J Syst Bacteriol 49:67, 1999; Versalovic J, Carroll KC, Funke G, et al, editors: Manual of clinical microbiology, ed 10, Washington, DC, 2011, ASM Press; and Weyant RS, Moss CW, Weaver RE, et al, editors: Identification of unusual pathogenic gram-negative aerobic and facultatively anaerobic bacteria, ed 2, Baltimore, 1996, Williams & Wilkins.
Comments Regarding Specific Organisms
M. haemolytica may be differentiated from members of the Pasteurella genus by its inability to produce indole or ferment mannose. S. indologenes can be separated from Pasteurella spp. with a negative nitrate test and is further delineated from Kingella spp. (discussed in Chapter 31) by indole production and sucrose fermentation.







