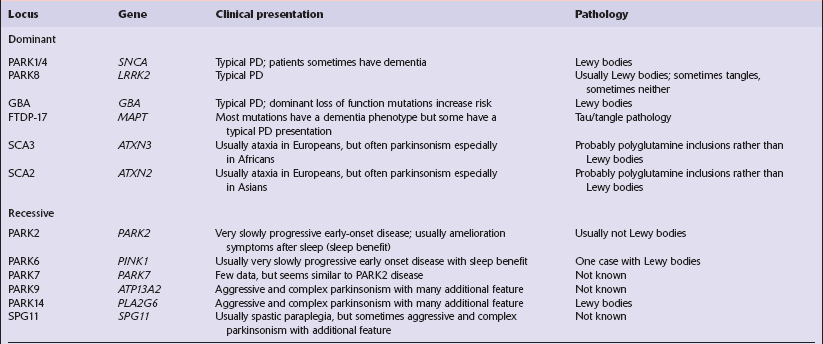
Parkinsonism and akinetic–rigid disorders
PARKINSON’S DISEASE (PD)
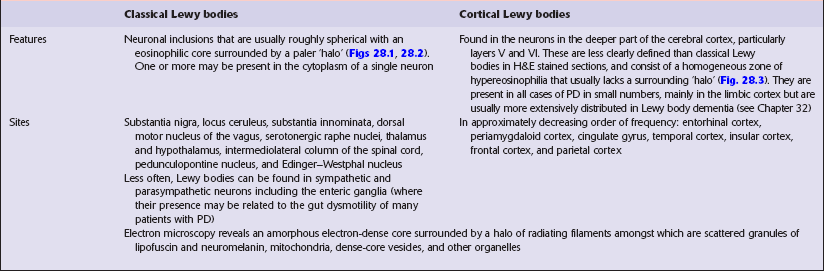
LEWY BODIES
The pathologic hallmark of Parkinson’s disease is the presence of neuronal inclusions called Lewy bodies. There are two main types, termed ‘classical’ and ‘cortical’ (Table 28.2), which are found in different locations. The presence of Lewy bodies defines several conditions, termed Lewy body disorders (see Tables 28.3 and 28.4).
Table 28.3
Distribution of Lewy bodies in different disorders and their clinical-pathologic correlation
| Disorder | Main site of Lewy body pathology | Clinical correlate |
| Parkinson’s disease (PD) | Substantia nigra | Akinetic–rigid syndrome |
| Parkinson’s disease with dementia (PDD) | Substantia nigra, cerebral cortex | Dementia occurs ≥1 year after a clinical diagnosis of PD |
| Dementia with Lewy bodies (DLB) | Cerebral cortex, substantia nigra | Dementia with akinetic–rigid syndrome. Dementia occurs within a year of onset of parkinsonian features |
| Autonomic failure | Sympathetic neurons in spinal cord | Autonomic failure |
| Lewy body dysphagia | Dorsal vagal nucleus | Dysphagia |
Table 28.4
Anatomical regions susceptible to Lewy pathology, according to two major classification schemes
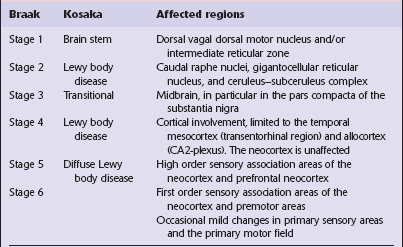
Braak et al. (2003); Kosaka et al. (1988).
MACROSCOPIC APPEARANCES
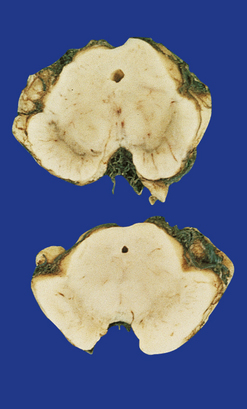
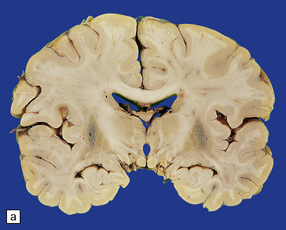
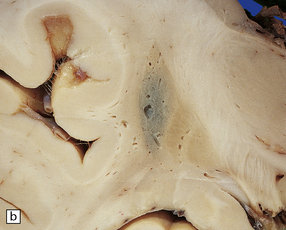
Sections through the midbrain and pons reveal loss of pigment from the substantia nigra and locus ceruleus (Fig. 28.1 – note that pallor of the substantia nigra is normal in childhood and adolescence, the slate-gray color being acquired during early adulthood). The globus pallidus, putamen, and caudate nucleus appear normal.
MICROSCOPIC APPEARANCES
The substantia nigra and other pigmented brain stem nuclei show:
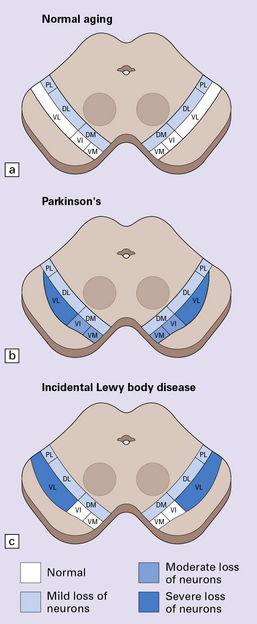
28.2 Cell loss from the substantia nigra is not random, but occurs in a region-specific manner.
The pars compacta of the substantia nigra can be divided into ventral and dorsal tiers, which project to different brain areas, and each tier can be further subdivided into regions (medial to lateral). (a) In normal aging, the estimated rate of cell loss from the dorsal tier of the substantia nigra is 7% per decade, leading to 40–50% cell loss by 65 years of age. (b) In PD, cell loss is greatest in the ventrolateral tier (VL). Typically, 70–90% have been lost by the time a patient dies. The ventromedial tier (VM) is next most affected. Cell loss from the dorsal tier is not significantly different from that in normal aging. (c) It has been suggested that symptoms of PD occur only after 50% of ventral tier neurons have been lost. This is preceded by a subclinical phase that can be regarded as incidental Lewy body disease, in which there is less pronounced cell loss, largely confined to the ventrolateral tier (VL). The age-specific prevalence of Lewy bodies rises from 3.8% to 12.8% between the 6th and 9th decades.
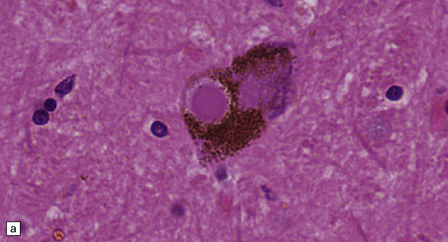
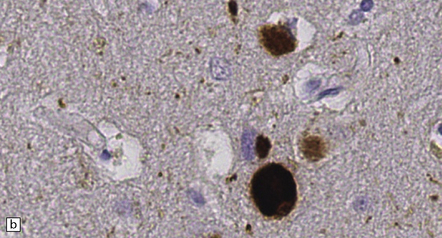
 accumulation of neuromelanin in macrophages (Figs 28.6, 28.7)
accumulation of neuromelanin in macrophages (Figs 28.6, 28.7)
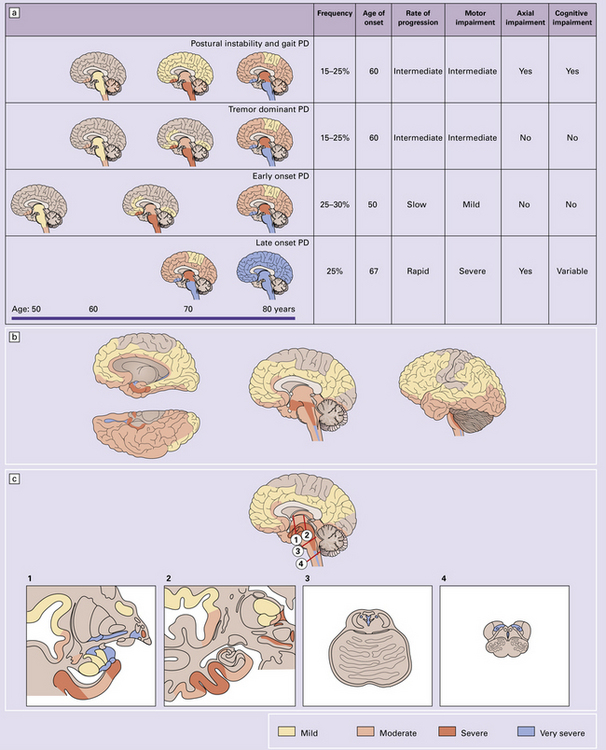
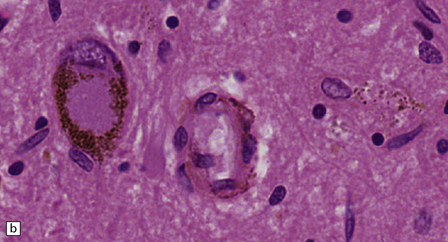
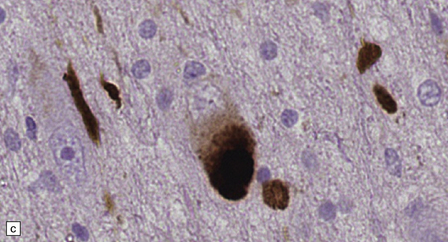
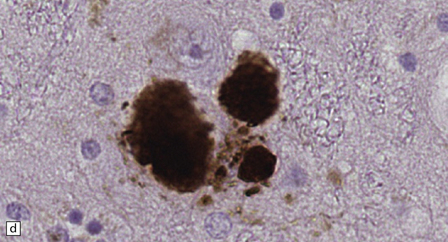
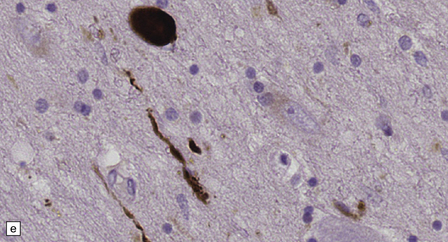
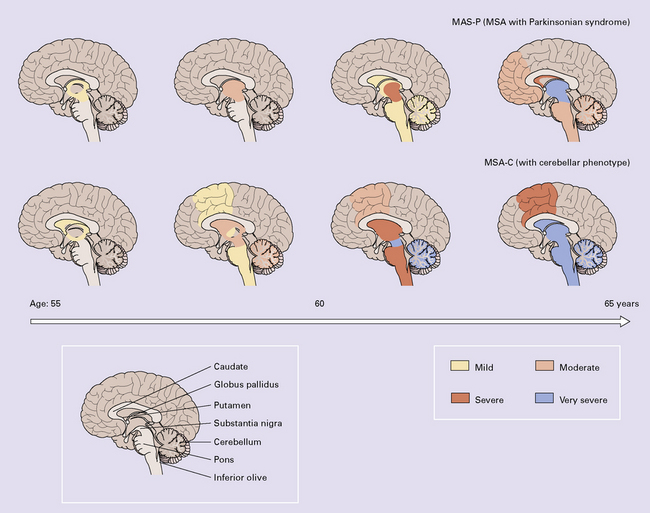
28.3 α-Synuclein pathology in Parkinson’s disease. (a)
Frequency and clinical features of the four major types phenotypes of PD. Brain schematics show increasing severity with age (blue bar). (b,c) Anatomic distribution of α-synuclein pathology. (Adapted from Halliday GM et al. 2011. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122(2):187–204 and Braak H et al. 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211.)
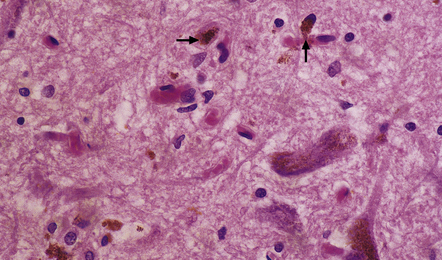
28.6 Phagocytosis of neuromelanin.
Neuromelanin from neurons that have degenerated is taken up into macrophages (arrows).
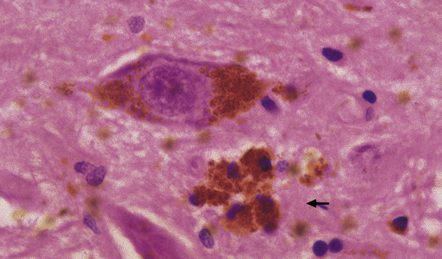
28.7 Phagocytosis of neuromelanin.
A cluster of pigment-laden macrophages (arrow) marks the site of degeneration of a nigral neuron.
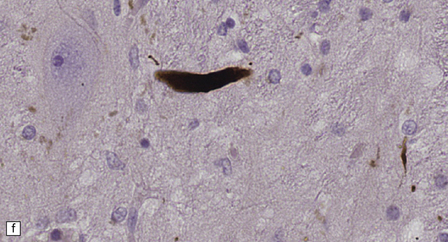
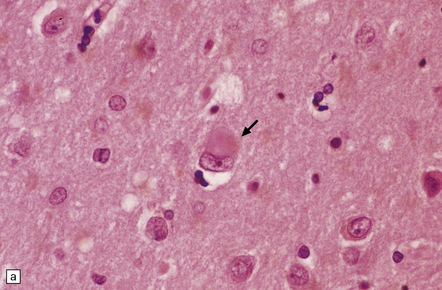
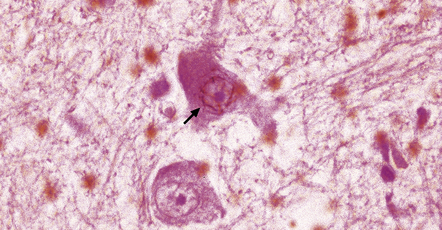
 Lewy bodies and pale bodies (see Fig. 28.5) in some remaining neurons
Lewy bodies and pale bodies (see Fig. 28.5) in some remaining neurons
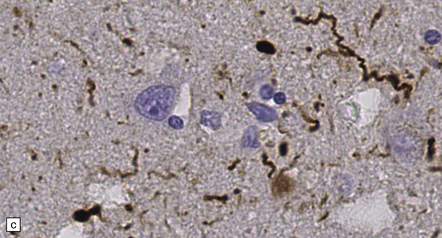
A distinctive form of neuritic degeneration (Lewy neurites), demonstrable by immunostaining for α-synuclein or ubiquitin, but not by silver impregnation, occurs in Lewy body diseases, including PD (see Figs 28.4, 28.5). The Lewy neurites may be detected in the substantia nigra, CA2/3 region of the hippocampus, dorsal motor nucleus of the vagus, nucleus basalis of Meynert, and amygdala.
DRUG AND TOXIN-RELATED PARKINSONISM
Other causes of drug- and toxin-related parkinsonism are described in Chapter 25.
PROGRESSIVE SUPRANUCLEAR PALSY (PSP) (STEELE–RICHARDSON–OLSZEWSKI SYNDROME)
The cause of PSP is not known, but the disease is strongly associated with the H1 haplotype of MAPT, the tau gene (this haplotype is also associated with CBD, see below). In the brain of patients with PSP, as in CBD (and also argyrophilic grain disease, see Chapter 31), four-repeat tau predominates, i.e. tau that is synthesized from transcripts that include exon 10 and therefore encode four microtubule-binding domains rather than three. Approximately 1–8% of patients diagnosed clinically as having PD have PSP.
MACROSCOPIC APPEARANCES
There is loss of pigment from the substantia nigra and locus ceruleus (Fig. 28.8) and, occasionally, atrophy of the midbrain, pontine tegmentum, and globus pallidus.
MICROSCOPIC APPEARANCES
Certain abnormalities are common to several regions of the CNS (Fig. 28.9):
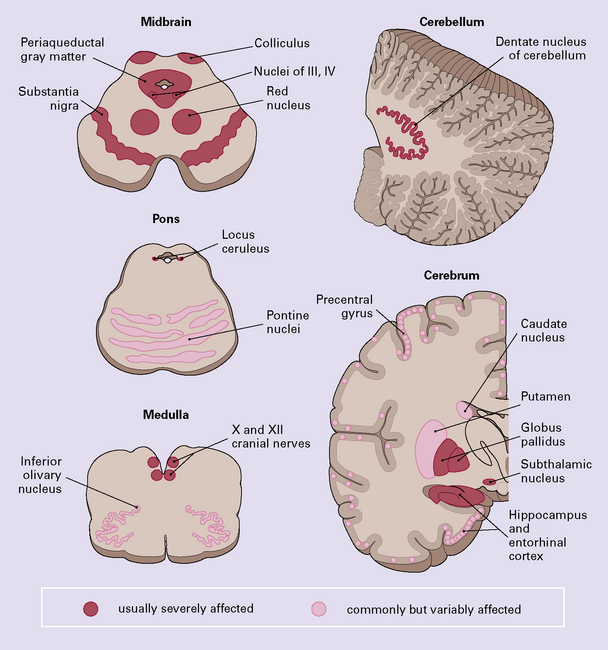
28.9 Pathologic changes in PSP.
The areas affected in PSP can be divided into those that are consistently and severely affected, and those that are less consistently affected.
 Neuronal accumulation of abnormal tau protein (Fig. 28.10), either diffusely distributed and detectable only immunohistochemically, or aggregated into neurofibrillary tangles, many of which are also demonstrable by silver impregnation. The tangles stain poorly for ubiquitin.
Neuronal accumulation of abnormal tau protein (Fig. 28.10), either diffusely distributed and detectable only immunohistochemically, or aggregated into neurofibrillary tangles, many of which are also demonstrable by silver impregnation. The tangles stain poorly for ubiquitin.
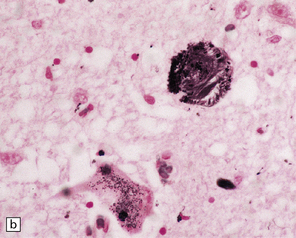
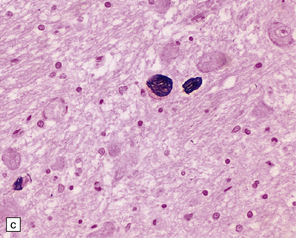
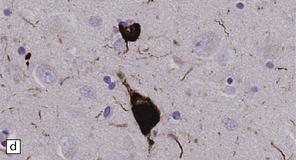
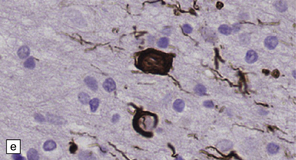
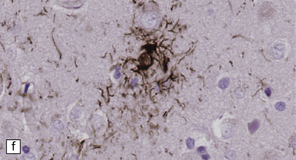
28.10 Types of tau-positive inclusion in PSP as demonstrated by labeling with the AT8 antibody.
(a) Typical basophilic, rounded or globose tangle in the substantia nigra. (b) Gallyas silver impregnation is a sensitive method of detecting the tangles, as in this section of substantia nigra. (c) Tangles in the pontine nuclei. (d) Cortical neurons containing tau-positive tangles. Scattered neurites are also labeled. (e) Oligodendroglial tau inclusions (coiled bodies and interfascicular threads) in the subcortical white matter. (f) Tufted astrocytes in the cortex. This form of astrocytic tau is specific for PSP and is distinct from the astrocytic plaques of CBD.
 Glial accumulation of tau protein. Tufted astrocytes seen in gray matter are especially characteristic of PSP (Fig. 28.10f).
Glial accumulation of tau protein. Tufted astrocytes seen in gray matter are especially characteristic of PSP (Fig. 28.10f).
The findings vary in different regions of the CNS:
 In the cerebral cortex there are commonly neuronal tangles, tau-immunoreactive glia, and neuropil threads, particularly in the precentral gyrus, entorhinal cortex, and hippocampus. An occasional neuron in the cerebral cortex and basal ganglia may appear swollen and achromasic. An abundance of swollen neurons suggests corticobasal degeneration (CBD).
In the cerebral cortex there are commonly neuronal tangles, tau-immunoreactive glia, and neuropil threads, particularly in the precentral gyrus, entorhinal cortex, and hippocampus. An occasional neuron in the cerebral cortex and basal ganglia may appear swollen and achromasic. An abundance of swollen neurons suggests corticobasal degeneration (CBD).
 In the substantia nigra, neuronal loss may be severe, especially ventromedially. Other findings include basophilic globose neuronal tangles, astrocytic gliosis, conspicuous neuropil threads, and tau-immunoreactive glia.
In the substantia nigra, neuronal loss may be severe, especially ventromedially. Other findings include basophilic globose neuronal tangles, astrocytic gliosis, conspicuous neuropil threads, and tau-immunoreactive glia.
 The pontine nuclei, cerebellar dentate nucleus, striatum, globus pallidus, red nucleus, subthalamic nucleus, and brain stem nuclei contain neuronal tangles, conspicuous neuropil threads, astrocytosis, and tau-immunoreactive glia.
The pontine nuclei, cerebellar dentate nucleus, striatum, globus pallidus, red nucleus, subthalamic nucleus, and brain stem nuclei contain neuronal tangles, conspicuous neuropil threads, astrocytosis, and tau-immunoreactive glia.
 The cerebellar dentate nucleus may show grumose degeneration (i.e. accumulation of granular eosinophilic material, composed of swollen degenerating Purkinje cell axon terminals, around the neurons).
The cerebellar dentate nucleus may show grumose degeneration (i.e. accumulation of granular eosinophilic material, composed of swollen degenerating Purkinje cell axon terminals, around the neurons).
 The inferior olives often contain small numbers of neuronal tangles. Neuronal hypertrophy and vacuolation may occur in the olives owing to degeneration of neurons in the cerebellar dentate nuclei or central tegmental tracts.
The inferior olives often contain small numbers of neuronal tangles. Neuronal hypertrophy and vacuolation may occur in the olives owing to degeneration of neurons in the cerebellar dentate nuclei or central tegmental tracts.
 The spinal cord may contain tau-immunoreactive neuronal tangles, neuropil threads and glia, particularly in the dorsal horns.
The spinal cord may contain tau-immunoreactive neuronal tangles, neuropil threads and glia, particularly in the dorsal horns.
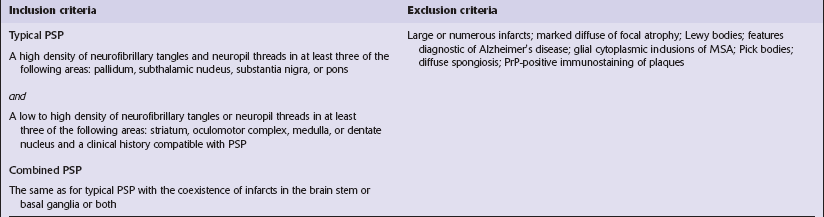
Criteria for pathologic diagnosis of PSP have been proposed (Table 28.5).
Table 28.5
POSTENCEPHALITIC PARKINSONISM
MACROSCOPIC AND MICROSCOPIC APPEARANCES
Histologically, the substantia nigra is gliotic and severely depleted of neurons (Fig. 28.11), the locus ceruleus moderately so. A mononuclear inflammatory infiltrate is typically present, but this tends to be very sparse in long-surviving patients. Neurofibrillary tangles composed of abnormal tau protein and biochemically and ultrastructurally identical to those in Alzheimer’s disease (see Chapter 31), are present in the remaining neurons in the substantia nigra, and also in the locus ceruleus, hippocampus, and the entorhinal, temporal, frontal, parietal, and insular cortex. There are tau-immunoreactive glial cells in the affected regions.
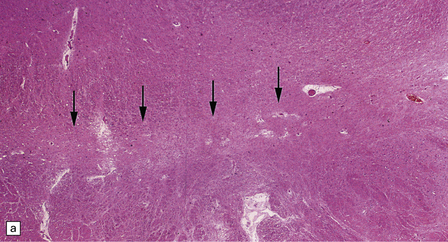
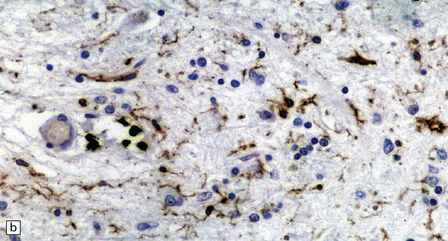
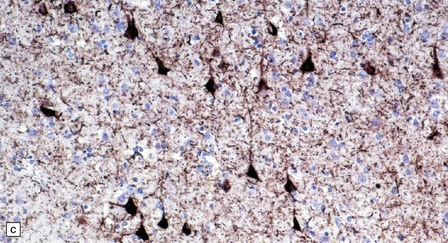
28.11 Postencephalitic parkinsonism.
(a) The substantia nigra (arrows) is severely depleted of neurons. (b) A higher-magnification view of the substantia nigra, showing sparse perivascular lymphocytes, and scattered lymphocytes and microglia within the parenchyma. (c) The medial temporal cortex contains numerous tangle-bearing neurons and neuropil threads that can be immunostained for tau with monoclonal antibody AT8.
MULTIPLE SYSTEM ATROPHY (MSA)
MACROSCOPIC APPEARANCES
Depending on the systems involved, there may be:
 atrophy of the cerebellum, middle cerebellar peduncles, and pons (Fig. 28.13)
atrophy of the cerebellum, middle cerebellar peduncles, and pons (Fig. 28.13)
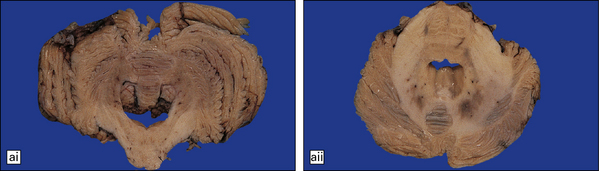
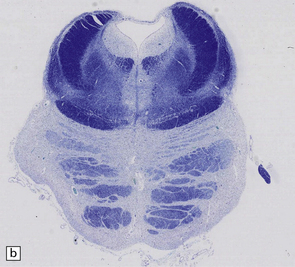
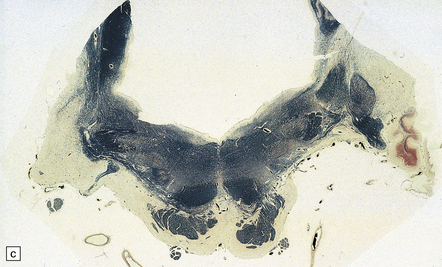
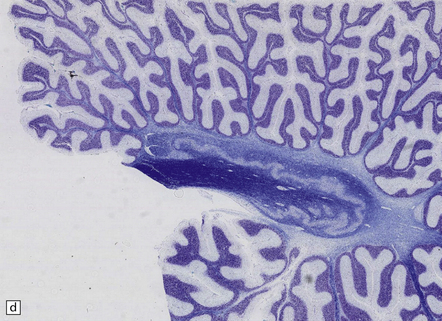
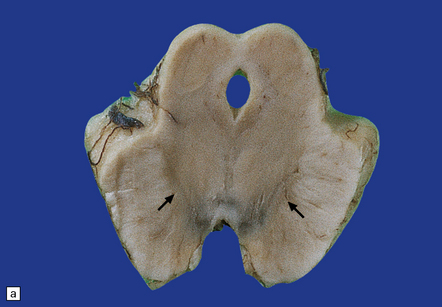
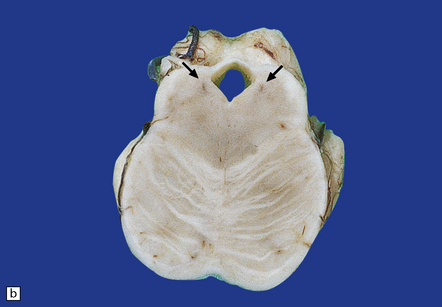
28.13 MSA-OPCA pattern.
Horizontal sections through the pons and medulla of a patient with OPCA pattern of MSA (ai) and a normal control (aii). In OPCA there may be marked atrophy of the cerebellum, middle cerebellar peduncles, and base of the pons, the last two features giving the pons a ‘beaked’ appearance. (b) Marked atrophy of the base of the pons with loss of transverse pontine fibers. The superior cerebellar peduncles are preserved. (c) Loss of pontocerebellar fibers, but preservation of the pontine tegmentum and superior cerebellar peduncles. (d) Atrophy of the olivary nucleus sin MSA. At higher magnification (e) the neuronal loss and a gliosis become visible.
 pallor of the substantia nigra and locus ceruleus (Fig. 28.14)
pallor of the substantia nigra and locus ceruleus (Fig. 28.14)
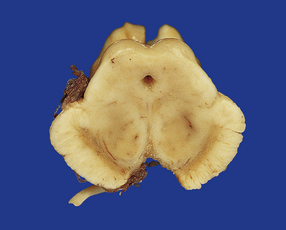
28.14 Striatonigral-type MSA.
Pallor of the substantia nigra in MSA, reflecting the loss of pigmented neurons. The loss of nigral neurons contributes in part to the extrapyramidal features of MSA.
 atrophy and gray-brown discoloration of the putamen (Fig. 28.15).
atrophy and gray-brown discoloration of the putamen (Fig. 28.15).
MICROSCOPIC APPEARANCES
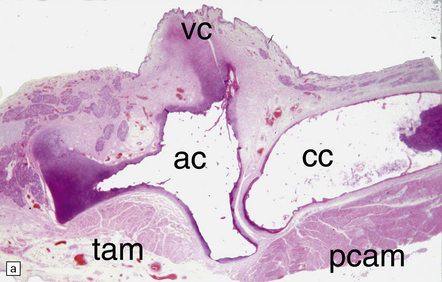
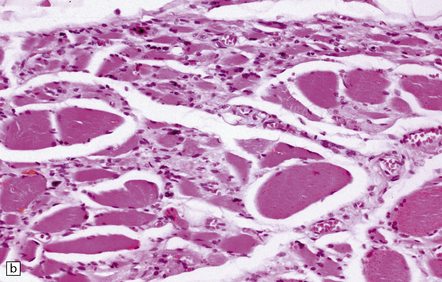
Glial cytoplasmic inclusions (Fig. 28.16)
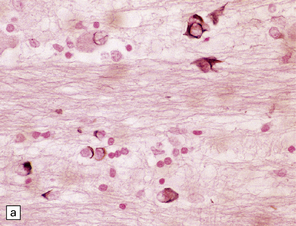
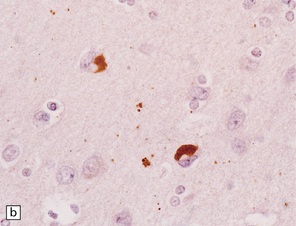
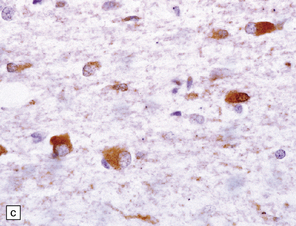
28.16 Glial cytoplasmic inclusions in MSA.
(a) Flame- or sickle-shaped glial cytoplasmic inclusions. (b) The glial cytoplasmic inclusions are immunoreactive for α-synuclein. (c) Glial cytoplasmic inclusions immunoreactive for ubiquitin.
 Present in high density in MSA.
Present in high density in MSA.
 Affect oligodendroglia in gray and white matter.
Affect oligodendroglia in gray and white matter.
 Demonstrable by silver impregnation (Gallyas is particularly sensitive).
Demonstrable by silver impregnation (Gallyas is particularly sensitive).
 Usually flame- or sickle-shaped.
Usually flame- or sickle-shaped.
 Also immunoreactive for tau protein (with phosphorylation-independent antibodies), ubiquitin, tubulin, and αB-crystallin.
Also immunoreactive for tau protein (with phosphorylation-independent antibodies), ubiquitin, tubulin, and αB-crystallin.
 Appear as randomly arranged 20–40 nm tubules or filaments associated with granular material on ultrastructural examination.
Appear as randomly arranged 20–40 nm tubules or filaments associated with granular material on ultrastructural examination.
Neuronal cytoplasmic inclusions
 Rounded filamentous structures, predominantly seen in the pontine nuclei, putamen, subthalamic nucleus, amygdala, hippocampus, dentate fascia, substantia nigra, inferior olivary nucleus, and the brain stem reticular formation.
Rounded filamentous structures, predominantly seen in the pontine nuclei, putamen, subthalamic nucleus, amygdala, hippocampus, dentate fascia, substantia nigra, inferior olivary nucleus, and the brain stem reticular formation.
 Demonstrable by silver impregnation (Gallyas is particularly sensitive).
Demonstrable by silver impregnation (Gallyas is particularly sensitive).
 Immunoreactive for α-synuclein and ubiquitin.
Immunoreactive for α-synuclein and ubiquitin.
 Revealed on ultrastructural examination as a meshwork of 18–28 nm filaments associated with granular material.
Revealed on ultrastructural examination as a meshwork of 18–28 nm filaments associated with granular material.
 Present in large numbers in the basis pontis and putamen only.
Present in large numbers in the basis pontis and putamen only.
Nuclear inclusions
 Sparse in most cases, these inclusions are seen predominantly in the basis pontis and putamen.
Sparse in most cases, these inclusions are seen predominantly in the basis pontis and putamen.
 Demonstrable in neuronal and glial nuclei by Gallyas silver impregnation as well as α-synuclein immunohistochemistry (Fig. 28.17).
Demonstrable in neuronal and glial nuclei by Gallyas silver impregnation as well as α-synuclein immunohistochemistry (Fig. 28.17).
 Evident as a web of fine fibrils beneath the nuclear membrane in neurons (Fig. 28.18) and as small rods in glia.
Evident as a web of fine fibrils beneath the nuclear membrane in neurons (Fig. 28.18) and as small rods in glia.
CORTICOBASAL DEGENERATION (CBD)
CBD is one of the tauopathies. Other terms for the same disorder include: corticodentatonigral degeneration with neuronal achromasia, cortical–basal ganglionic degeneration, and corticonigral degeneration. Along with other tau disorders it is now recognized that some cases are associated with mutations in the tau gene. However, most are sporadic, although as in PSP there is a strong genetic association with the H1 tau gene haplotype. In the brain in corticobasal degeneration, four-repeat tau predominates, i.e. tau synthesized from transcripts that contain exon 10 and therefore include four microtubule-binding domains rather than three. This is as occurs in progressive supranuclear palsy and also argyrophilic grain disease (see Chapter 31).
MICROSCOPIC APPEARANCES
In affected cortical areas
 Neuronal loss and astrogliosis, leading to thinning of the cortical ribbon (Fig. 28.19).
Neuronal loss and astrogliosis, leading to thinning of the cortical ribbon (Fig. 28.19).
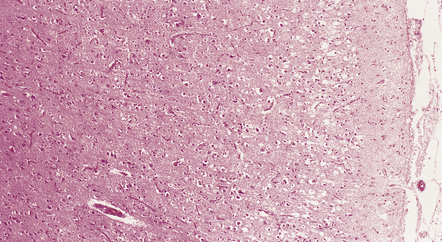
28.19 CBD showing cortical microvacuolation.
The microvacuolation is initially restricted to the superficial cortical layers. Later it affects all layers and is associated with marked astrocytic gliosis.
 Swollen or ballooned neurons (BN) are scattered in third, fifth, and sixth cortical layers (Fig. 28.20). BN are eosinophilic or amphophilic and are often vacuolated. They may be weakly argyrophilic, and show loss of Nissl substance (best demonstrated by staining with cresyl violet). BN occasionally contain granulovacuolar bodies.
Swollen or ballooned neurons (BN) are scattered in third, fifth, and sixth cortical layers (Fig. 28.20). BN are eosinophilic or amphophilic and are often vacuolated. They may be weakly argyrophilic, and show loss of Nissl substance (best demonstrated by staining with cresyl violet). BN occasionally contain granulovacuolar bodies.
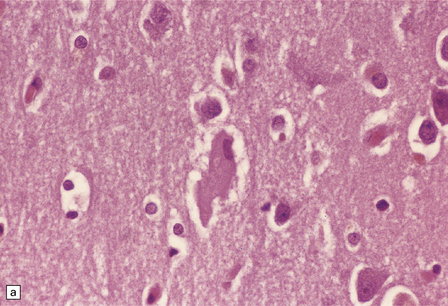
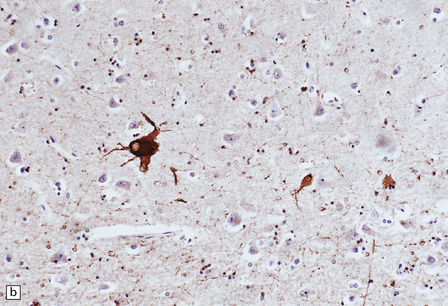
28.20 Swollen cortical neurons (also termed achromasic or ballooned neurons) are a distinctive finding in CBD.
(a) They are easily detected in hematoxylin and eosin-stained sections and are most frequent in layers III, IV, and VI, in the peri-Rolandic, posterior frontal, and parietal cortices. In advanced cases such swollen neurons may be seen in the insular and frontal cortex and the tip of the temporal lobe. (b) Cortex stained to show αB-crystallin. The swollen cells strongly express phosphorylated neurofilament proteins and αB-crystallin. Here immunostaining of αB-crystallin emphasizes the distortion of the axons and dendrites of the swollen neurons.
 BN are immunoreactive for phosphorylated neurofilament proteins, αB-crystallin, heat shock protein 27, and sometimes ubiquitin. They do not contain Alzheimer-type tangles but often show diffuse tau positivity, particularly at the periphery of the cytoplasm (Fig. 28.21).
BN are immunoreactive for phosphorylated neurofilament proteins, αB-crystallin, heat shock protein 27, and sometimes ubiquitin. They do not contain Alzheimer-type tangles but often show diffuse tau positivity, particularly at the periphery of the cytoplasm (Fig. 28.21).
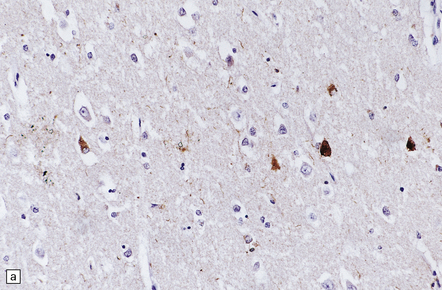
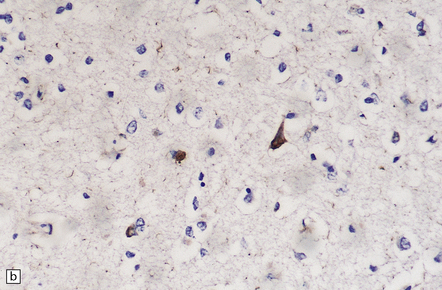
28.21 CBD cortical inclusion bodies.
(a,b) These are sections from superficial cortex immunostained to show tau-proteins. The tau-immunoreactive globular or angular inclusions (which are not ubiquitinated) are present in small numbers of neurons in the superficial cortex. The inclusions occur in residual neurons in areas of superficial microvacuolation, but may not be detectable in severely gliotic cortex.
 Superficial or laminar spongiosis or microvacuolation; in some cases all cortical layers are affected.
Superficial or laminar spongiosis or microvacuolation; in some cases all cortical layers are affected.
 Astrocytosis that is most prominent in superficial cortical layers and at the gray-white matter junction.
Astrocytosis that is most prominent in superficial cortical layers and at the gray-white matter junction.
Patterns of tau immunoreactivity
 Diffuse or granular cytoplasmic immunoreactivity typical of so-called ‘pre-tangles’ (this is the pattern in most of the tau-positive neurons).
Diffuse or granular cytoplasmic immunoreactivity typical of so-called ‘pre-tangles’ (this is the pattern in most of the tau-positive neurons).
 Densely-packed small tau-positive inclusions reminiscent of Pick bodies or small NFT.
Densely-packed small tau-positive inclusions reminiscent of Pick bodies or small NFT.
 Dispersed or skein-like cytoplasmic filamentous staining.
Dispersed or skein-like cytoplasmic filamentous staining.
 Globose neurofibrillary tangles in brain stem monoaminergic nuclei, such as the locus ceruleus and substantia nigra (similar to the globose neurofibrillary tangles seen in these structures in AD and PSP).
Globose neurofibrillary tangles in brain stem monoaminergic nuclei, such as the locus ceruleus and substantia nigra (similar to the globose neurofibrillary tangles seen in these structures in AD and PSP).
 Many fine, tau-immunopositive cell processes within the neuropil (it is incorrect to use the term neuropil threads in the context of CBD, as the processes are astrocytic) (Fig. 28.22).
Many fine, tau-immunopositive cell processes within the neuropil (it is incorrect to use the term neuropil threads in the context of CBD, as the processes are astrocytic) (Fig. 28.22).
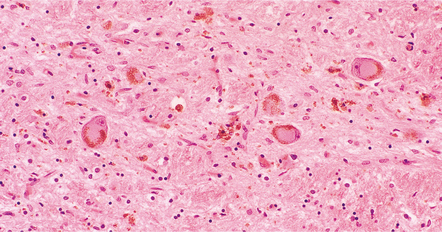
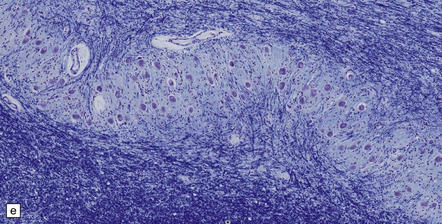
28.22 Nigral neuronal loss and astrocytic gliosis in CBD.
Argyrophilic filamentous inclusions termed ‘corticobasal inclusions’ are present in residual nigral neurons. In H&E-stained sections the inclusions are moderately to weakly basophilic and displace the neuromelanin. Similar inclusions may be seen in the locus ceruleus. The inclusions are immunoreactive for tau-protein. Ultrastructurally, some consist of paired helical filaments, while others are composed of straight tubules and resemble the tangles of PSP.
 Tau-positive argyrophilic inclusions in oligodendroglia (termed oligodendroglial microtubular masses, or coiled bodies); these tau-positive oligodendroglial inclusion differ from the flame-shaped α-synuclein-positive oligodendroglial inclusions in MSA (Fig. 28.23).
Tau-positive argyrophilic inclusions in oligodendroglia (termed oligodendroglial microtubular masses, or coiled bodies); these tau-positive oligodendroglial inclusion differ from the flame-shaped α-synuclein-positive oligodendroglial inclusions in MSA (Fig. 28.23).
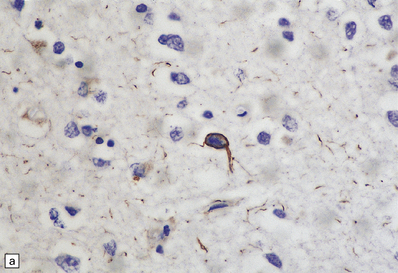
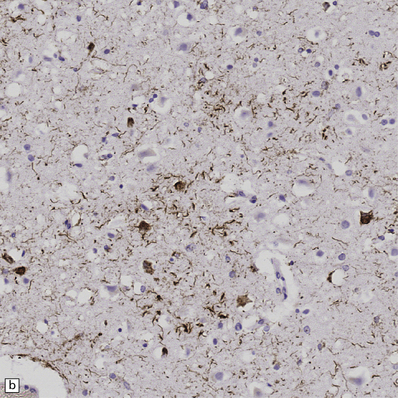
28.23 Tau-immunoreactive glial inclusions in CBD.
(a) Glial inclusions which are immunoreactive for tau protein are present in large numbers in CBD. Most appear as coils and grains as shown here. These can also be detected by Gallyas silver impregnation. (b) Some glial tau-reactive inclusions resemble those seen in MSA but are present in low density and do not stain with α-synuclein.
ARTERIOSCLEROTIC PSEUDOPARKINSONISM
MACROSCOPIC AND MICROSCOPIC APPEARANCES
Pathologically, there are usually lacunar infarcts in the basal ganglia (Fig. 28.24) or ischemic lesions in the white matter of the frontal lobes (Fig. 28.25). Infarction of the substantia nigra is a very rare cause of parkinsonism.
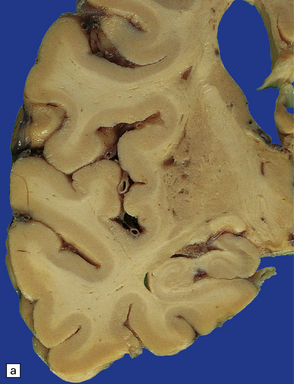
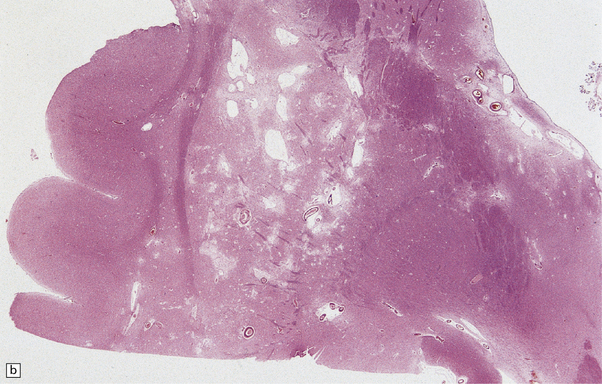
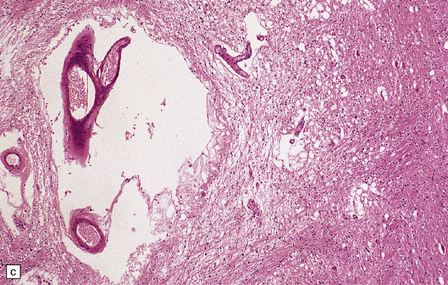
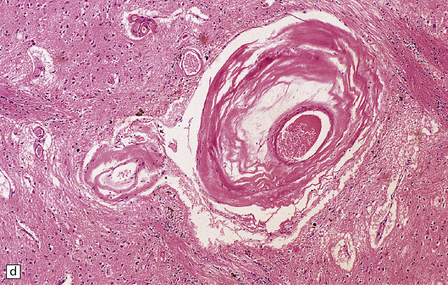
28.24 Arteriosclerotic pseudoparkinsonism.
(a) Multiple lacunar infarcts in the putamen. (b) Histology of basal ganglia shows dilated perivascular spaces with rarefaction of parenchyma. (c) Astrocytic gliosis is present around the dilated perivascular spaces. (d) Vessels in the basal ganglia are markedly arteriosclerotic.
GUAM PARKINSONISM-DEMENTIA
MACROSCOPIC AND MICROSCOPIC APPEARANCES
The brain usually shows generalized atrophy, with pallor of the substantia nigra and locus ceruleus.
Histologically (Fig. 28.26), neuronal loss and astrocytic gliosis are associated with neurofibrillary tangles in:
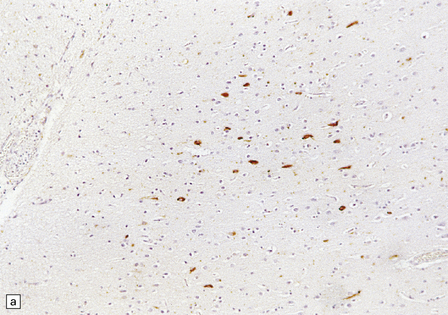

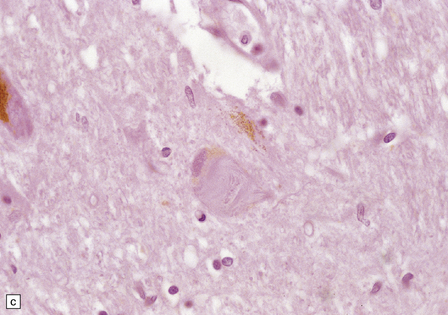
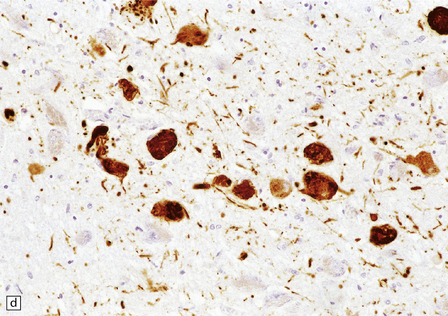
28.26 Guam parkinsonism–dementia.
(a) Tau-immunoreactive neurofibrillary tangles in the superficial laminae of the neocortex. (b) Silver impregnation of two adjacent gyri reveals numerous tangles in one and relatively few in the other. (c) Tangle in a remaining neuron in the substantia nigra. (d) Numerous tau-immunoreactive tangles and neurites in the oculomotor nucleus.
REFERENCES
Braak, H., Del Tredici, K., Rüb, U., et al. Del Tredici, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211.
Dickson, D.W., Braak, H., Duda, J.E., et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157.
Goker-Alpan, O., Schiffmann, R., LaMarca, M.E., et al. Parkinsonism among Gaucher disease carriers. J Med Genet.. 2004;41(12):937–940.
Halliday, G.M., Holton, J.L., Revesz, T., et al. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122(2):187–204.
Hardy, J. Genetic analysis of pathways to Parkinson disease. Neuron. 2010;68(2):201–206.
Hardy, J., Lewis, P., Revesz, T., et al. The genetics of Parkinson’s syndromes: a critical review. Curr Opin Genet Dev. 2009;19(3):254–265.
Houlden, H., Baker, M., Morris, H.R., et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56(12):1702–1706.
Jellinger, K.A. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–12.
Kosaka, K., Tsuchiya, K., Yoshimura, M. Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol. 1988;7:299–305.
Lees, A.J., Hardy, J., Revesz, T. Parkinson’s disease. Lancet. 2009;373:2055–2066.
Polymeropoulos, M.H. Genetics of Parkinson’s disease. Ann N Y Acad Sci. 2000;920:28–32.
Spillantini, M.G., Schmidt, M.L., Lee, V.M., et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840.
Yoshino, H., Tomiyama, H., Tachibana, N., et al. Phenotypic spectrum of patients with PLA2G6 mutation and PARK14-linked parkinsonism. Neurology. 2010;75(15):1356–1361.
Progressive supranuclear palsy
Houlden, H., Baker, M., Morris, H.R., et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology. 2001;56(12):1702–1706.
Komori, T. Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 1999;9(4):663–679.
Liu, W.K., Le, T.V., Adamson, J., et al. Relationship of the extended tau haplotype to tau biochemistry and neuropathology in progressive supranuclear palsy. Ann Neurol. 2001;50(4):494–502.
Morris, H.R., Gibb, G., Katzenschlager, R., et al. Pathological, clinical and genetic heterogeneity in progressive supranuclear palsy. Brain. 2002;125(Pt 5):969–975.
Wakabayashi, K., Takahashi, H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004;24(1):79–86.
Geddes, J.F., Hughes, A.J., Lees, A.J., et al. Pathological overlap in cases of parkinsonism associated with neurofibrillary tangles. A study of recent cases of postencephalitic parkinsonism and comparison with progressive supranuclear palsy and Guamanian parkinsonism-dementia complex. Brain. 1993;116(Part 1):281–302.
Gilman, S., Wenning, G.K., Low, P.A., et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676.
Halliday, G.M., Holton, J.L., Revesz, T., et al. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122(2):187–204.
Jellinger, K.A. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–12.
Trojanowski, J.Q., Revesz, T. Neuropathology Working Group on MSA. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33(6):615–620.
Ahmed, Z., Doherty, K.M., Silveira-Moriyama, L., et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011;122(4):415–428.
Dickson, D.W., Bergeron, C., Chin, S.S., et al. Office of Rare Diseases’ neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61(11):935–946.
Forman, M.S., Schmidt, M.L., Kasturi, S., et al. Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol. 2002;160(5):1725–1731.
Oyanagi, K., Makifuchi, T., Ohtoh, T., et al. Amyotrophic lateral sclerosis of Guam: the nature of the neuropathological findings. Acta Neuropathol. 1994;88(5):405–412.
Oyanagi, K., Tsuchiya, K., Yamazaki, M., et al. Substantia nigra in progressive supranuclear palsy, corticobasal degeneration, and parkinsonism-dementia complex of Guam: specific pathological features. J Neuropathol Exp Neurol. 2001;60(4):393–402.
Plato, C.C., Galasko, D., Garruto, R.M., et al. ALS and PDC of Guam: forty-year follow-up. Neurology. 2002;58(5):765–773.