Chapter 24 Paraplegia and Spinal Cord Syndromes
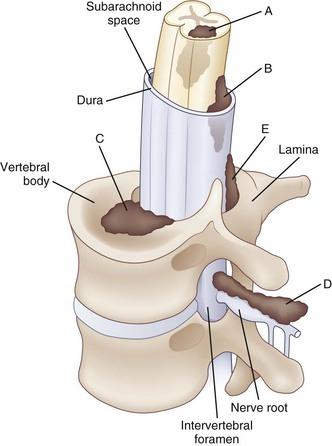
Paraplegia may result from a variety of systemic and primary CNS medical conditions, as well as trauma at all segmental levels of the spinal cord (Box 24.1). A spinal cord syndrome may develop from extramedullary and intramedullary pathological processes (Fig. 24.1). Initial symptoms may be gradual in onset and progressive, including pain, dysesthesia, or subtle upper-or lower-extremity weakness. In other cases, such as an inflammatory myelitis, acute onset of severe motor, sensory, and autonomic deficits may develop without premonitory symptoms. Trauma from a cervical flexion-extension injury, for example, may produce a central cord injury of the lower cervical spinal cord with incomplete quadriparesis, whereas a complete transection injury at the lower thoracic spinal cord from a fall may result in complete paraplegia. Thus, both the rostrocaudal segmental level of disease involvement or trauma and completeness of the lesion in the transverse plane anticipate the person’s impairments and disability. Details about the relationships between specific spinal cord segments and sensory dermatomes are reviewed in Chapter 28, and the segmental innervation of specific muscle groups are reviewed in Chapters 29 and 30. The sensorimotor clinical examination allows localization of the lesion (Fig. 24.2).
Box 24.1 Differential Diagnosis of Diseases Affecting the Spinal Cord
Compressive Lesions
Noncompressive Myelopathies
Demyelinating (e.g., MS, ADEM)
Viral myelitis (e.g., varicella-zoster, AIDS–related myelopathy, human T-lymphotropic virus type I infection)
Vitamin B12 deficiency and other nutritional deficiencies
Ischemia and hemorrhage from vascular malformations
Spirochetal diseases (syphilis and Lyme disease)
Toxic myelopathies (e.g., radiation-induced)
When examining a patient who presents with paraplegia, a careful neurological examination is critical for planning additional diagnostic workup and care. Identifying distinct spinal cord syndromes and determining the likely location of the underlying pathological process will guide subsequent imaging and electrodiagnostic studies. Structural information about the integrity of the spine may be obtained from radiographic plain films and computed tomography (CT) for bone pathology. Magnetic resonance imaging (MRI) and myelography are best to reveal cord pathology. A review of imaging of the spine is provided in Chapter 33A.
Acute and long-term care of patients is influenced by the clinical presentation, severity of neurological deficits, underlying pathology, and prognosis for gains over time. Patients presenting with an acute spinal cord syndrome after trauma show both early (days to 3 months) and late (up to 2 years) changes in their motor and sensory deficits (Fawcett et al., 2007). Both neurological improvements and clinical worsening may occur. When some sparing of sensation and movement is present in the first 72 hours after trauma, the prognosis for walking is rather good. Indeed, up to 90% of patients with a cervical central cord injury who have modest sensation and movement below the level of injury by 4 weeks after trauma will become functional ambulators (Dobkin et al., 2006). Thus, serial and careful neurological examinations are important to monitor the injury-related deficits, especially in the first weeks after onset. Rehabilitation of patients with paraplegia follows after the acute medical needs have been addressed. The aim is to promote as much functional independence as possible with and without assistive devices, decrease the risk of complications, and reintegrate the patient into home and community. Neurological rehabilitation for paraparesis after spinal cord syndromes is reviewed in Chapter 48.
Common Spinal Cord Syndromes
Spinal Shock
Spinal shock refers to the period of depressed spinal reflexes caudal to an acute spinal cord injury; it is followed by emergence of pathological reflexes and return of cutaneous and muscle stretch reflexes (Ditunno et al., 2004). During the acute phase of spinal shock, paralysis of muscles, sensory impairment, and loss of autonomic function are present below the level of injury. Commonly, the first reflex to appear after a clinically complete spinal cord injury is the delayed plantar response. It is induced by stroking the plantar surface of the foot with a blunt instrument upward from the heel, along the lateral surface, and across the metatarsal heads. A delayed response consists of plantar flexion of the great toe and other toes, often detectable within the first hours after injury. When the delayed plantar response persists for more than 1 week, it is often associated with a severe spinal cord injury and a less favorable prognostic outlook for a significant functional recovery. The return of reflexes over days and weeks after the initial period of spinal shock appears to follow a general pattern, with cutaneous reflexes recovering before muscle stretch reflexes. Specifically, the bulbocavernosus and cremasteric reflexes commonly return before the ankle jerk, Babinski sign, and knee jerk.
Incomplete Lesions of the Spinal Cord
Central Cord Syndrome
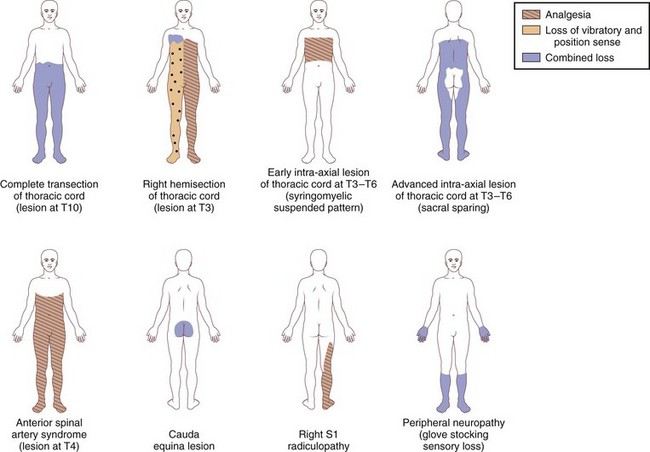
Traumatic central cord syndrome is commonly characterized by the triad of (1) motor impairment that is disproportionately more severe in the upper than the lower extremities, (2) bladder dysfunction that usually includes urinary retention, and (3) sensory dysfunction of varying degrees. An international consensus group recently suggested that an upper- and lower-extremity difference of at least 10 motor score points, based on the Medical Research Council scale, can be considered as a quantitative addition to the commonly used qualitative criteria for making the diagnosis (van Middendorp et al., 2010). An additional clinical feature of the traumatic central cord syndrome is a dissociated sensory loss for pain and temperature, whereas vibration and position sense remain preserved. This sensory presentation may be explained by a direct injury to intramedullary decussating fibers, which normally would ascend contralaterally as part of the spinothalamic tract. As a result, a capelike sensory deficit may be encountered in patients with a cervical level injury, but sensation within more caudal dermatomes would generally be spared (Fig. 24.3).
Characteristic Clinical Features of Lesions at Different Levels
Thoracic Levels
Traumatic spinal cord injury at the thoracic level usually produces a complete lesion. The segmental level of injury is best determined by a careful sensory examination of dermatomes (Maynard et al., 1997). Useful clinical landmarks are the nipple line for the T4 dermatome and the umbilicus for the T10 dermatome. Pain may follow a radicular pattern around the chest or abdomen, corresponding to the segmental levels of injury. Sensory testing of pin, temperature, pressure, and light touch appreciation may determine the most caudal dermatome of normal sensation, as well as a zone of partial preservation. The sensory testing should include evaluation of dermatomes of the left and right side of the body, with comparisons of homologous levels. In addition to a combination of at-level pain, sensory deficits, and muscular weakness, autonomic dysfunction may develop from long-tract involvement and include urinary retention, bladder-sphincter dyssynergia, and bladder hyperreflexia.
Conus Medullaris and Cauda Equina
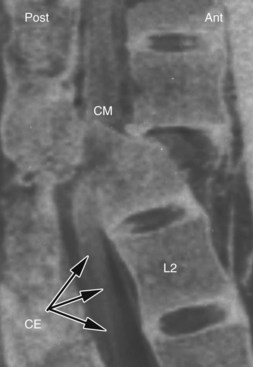
The conus medullaris of the spinal cord terminates approximately at the level of the L1 vertebra, although the precise location of the tip of the conus may show marked variability among subjects. This anatomical aspect of the spinal cord is important because spine trauma commonly takes place at the thoracolumbar junction, and the extent of such injuries is highly variable (Kingwell et al., 2008). Traumatic injuries to the conus medullaris usually result in weakness or paralysis of the lower extremities, absence of lower-extremity reflexes, and saddle anesthesia (Fig. 24.4). However, some patients with conus medullaris injuries exhibit a mixed upper and lower motoneuron syndrome. In contrast, a cauda equina injury that lesions lumbosacral roots below the level of the conus medullaris is a pure lower motoneuron syndrome. Cauda equina injuries present with lower-extremity weakness, areflexia and decreased muscle tone, and variable sensory deficits. At least a third of these patients suffer considerable central pain. Affected limb and pelvic floor muscles develop flaccid weakness, and electromyography shows denervation after either a conus medullaris or cauda equina injury, especially following anatomically complete lesions.
Both conus medullaris and cauda equina injuries are associated with bladder, bowel, and sexual dysfunction (Pavlakis et al., 1983). Urodynamic evaluations typically demonstrate detrusor areflexia, and a rectal exam identifies a flaccid anal sphincter. In addition, the bulbocavernosus reflex is typically absent or diminished, and reflexogenic erection in males is commonly lost.
Pain and Autonomic Dysfunction
In addition to motor and sensory impairments, pain is frequently associated with spinal cord injuries, along with autonomic impairments affecting bladder, bowel, sexual, and respiratory functions. The type and severity of autonomic dysfunction may vary and depend on location and severity of spinal cord injury. International spinal cord injury societies recently provided a practical system to document remaining autonomic function after a spinal cord injury (Alexander et al., 2009). These efforts are likely to increase our ability to communicate the effects of spinal cord injury on, for instance, cardiovascular, bladder, bowel, and sexual functions.
Pain after Spinal Cord Injury
Pain often accompanies injury to the spinal cord. Regardless of segmental level or completeness of injury, most patients with a traumatic spinal cord injury develop a clinically significant pain syndrome at some post-lesion time point (Waxman and Hains, 2006). Neuropathic pain after spinal cord injury may affect different locations. At-level pain affects the portions of the body that are innervated by the injured spinal cord segments. Below-level pain is located in body segments receiving innervation from the spinal cord caudal to the lesioned segments. Above-level pain is less common compared to the other two forms of postinjury pain but, when present, affects body segments innervated by spinal cord segments rostral to the site of spinal cord injury. Musculoskeletal sources of pain and pain with overuse of joints and soft tissues also are frequent but remedial sources of pain.
Autonomic Dysreflexia
Injuries to the spinal cord that result in paraplegia from a lesion above T6 may also impair autonomic control and result in episodes of severe hypertension or hypotension (Blackmer, 2003). Autonomic dysreflexia represents an acute syndrome characterized by excessive and uncontrolled sympathetic output from the spinal cord. As a result, the blood pressure is suddenly and markedly elevated. Associated symptoms include headache; malaise; blurring of vision; flushed, sweaty skin above the level of injury; and pale, cool skin below it. An episode of autonomic dysreflexia can be triggered by any noxious stimulation below the segmental level of injury. Common triggers include bladder distension, constipation, rectal fissures, joint injury, and urinary tract infection. Autonomic dysreflexia may present soon after the initial injury but more commonly becomes symptomatic several months after the spinal cord injury. Prevention is the best approach. Treatment of acute symptoms targets removal of noxious stimuli and cautious lowering of the blood pressure.
Bladder Dysfunction
Normal bladder and bowel control depend on segmental reflexes involving both autonomic and somatic motor neurons, as well as descending and ascending tracts of the spinal cord (Fowler et al., 2008). As a result, bladder and bowel function may be impaired after an injury to any segmental level of the spinal cord. Different clinical syndromes develop depending on whether the injury or disease process affects the sacral spinal cord directly or higher segmental levels. Traumatic spinal cord injuries with paraplegia taking place above the T12 vertebra will interrupt spinal cord long-tract connections between supraspinal micturition centers in the brainstem and cerebral cortex and the sacral spinal cord. An upper motoneuron syndrome follows, with detrusor-sphincter dyssynergia caused by impaired coordination of autonomic and somatic motor control of the bladder detrusor and external urethral sphincter, respectively. Incomplete bladder emptying results. In addition, the upper motoneuron syndrome also includes detrusor hyperreflexia with increased pressure within the bladder. In contrast, injury to the T12 vertebra and below results in a direct lesion to the sacral spinal cord and associated nerve roots. A direct lesion to preganglionic parasympathetic neurons and somatic motoneurons of the Onuf nucleus located within the S2-S4 spinal cord segments results in denervation of pelvic targets. Injuries to both the conus medullaris and cauda equina present as a lower motoneuron syndrome characterized by weak or flaccid detrusor function. Urinary retention follows, with risk of overflow incontinence. The goal for all bladder care is to avoid retrograde urine flow, urinary tract infections, and renal failure. Management of both upper and lower motoneuron bladder impairment commonly includes clean intermittent bladder catheterizations. Chapter 38 discusses evaluation and treatment.
Alexander M.S., Biering-Sorensen F., Bodner D., et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord. 2009;47:36-43.
Blackmer J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ. 2003;169:931-935.
Ditunno J.F., Little J.W., Tessler A., et al. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42:383-395.
Dobkin B., Apple D., Barbeau H., et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI: a multicenter randomized clinical trial. Neurology. 2006;66:484-493.
Fawcett J.W., Curt A., Steeves J.D., et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190-205.
Fowler C.J., Griffiths D, de Groat W.C. The neural control of micturition. Nat Rev Neurosci. 2008;9:453-466.
Kingwell S.P., Curt A, Dvorak M.F. Factors affecting neurological outcome in traumatic conus medullaris and cauda equina injuries. Neurosurg Focus. 2008;25:E7.
Maynard F.M., Bracken M.B., Creasy G, et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266-274.
Pavlakis A.J., Siroky M.B., Goldstein I, et al. Neurourologic findings in conus medullaris and cauda equina injury. Arch Neurol. 1983;40:570-573.
van Middendorp J.J., Pouw M.H., Hayes K.C., et al. Diagnostic criteria traumatic central cord syndrome. Part 2: A questionnaire survey among spine specialists. Spinal Cord. 2010;48:657-663.
Waxman S.G., Hains B.C. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207-215.