43 Pancreatic Disorders
• Pancreatitis is an inflammatory condition of the pancreas that results from premature activation of pancreatic enzymes and autodigestion of the gland.
• Gallstones and alcohol use are the most common causes of acute pancreatitis.
• Elevations in serum pancreatic enzyme values help confirm the diagnosis in suspected cases.
• Computed tomography scans are not usually indicated on admission unless needed to exclude other serious causes of abdominal pain.
• The spectrum of illness ranges from mild (edematous) to severe (necrotizing) disease.
• Most patients with acute pancreatitis require hospitalization for supportive care and have a benign course.
• Necrotizing pancreatitis carries significant rates of morbidity and mortality, especially if infection is present.
Epidemiology
More than 200,000 patients with acute pancreatitis are admitted to U.S. hospitals each year.1 Eighty percent of these patients suffer from mild disease and demonstrate an overall mortality rate of just 1%. Approximately 20% of patients have severe necrotizing pancreatitis, however, which has a mortality rate of up to 25%.2,3 The estimated incidence of pancreatitis in the United States is 79.8 per 100,000.4 Men are affected more commonly than women, and the condition develops in most patients between 40 and 60 years of age.5
Pathophysiology
Acute pancreatitis is most often caused by gallstones or alcohol use. Gallstone pancreatitis occurs secondary to obstruction of the common bile or pancreatic duct. Alcohol or its metabolic by-products are thought to act as a direct toxin to the pancreas, and the effects are usually dose dependent. Other less common causes are hyperlipidemia, hypercalcemia, medications, toxins, trauma, surgery, sphincter of Oddi dysfunction, invasive diagnostic procedures (endoscopic retrograde cholangiopancreatography), and hereditary causes. Approximately 20% of cases are idiopathic, with occult microlithiasis thought to be the underlying cause in half of them.6 Acute pancreatitis can be classified histologically as edematous or necrotizing, which corresponds to clinically mild or severe disease, respectively. Risk factors for severe disease include older age (>55 years), obesity (body mass index > 30 kg/m2), and pleural effusions or infiltrates (or both).7 Pancreatic necrosis (nonviable tissue) is associated with significant morbidity and mortality, especially if infection is present. Complications from pancreatitis include pseudocyst and abscess formation.
Standard definitions and terminology for acute pancreatitis (Table 43.1) have been proposed to establish an exact vocabulary among institutions and within the literature.8
| Acute pancreatitis | Acute inflammation of the pancreas |
| Mild acute pancreatitis | Minimal organ dysfunction responsive to fluid administration |
| Severe acute pancreatitis | One of the following: local complications (pancreatic necrosis, pancreatic pseudocyst, pancreatic abscess), organ failure, ≥3 Ranson criteria, APACHE II score ≥ 8 |
| Acute fluid collections | Fluid collection in or near the pancreas, without a defined wall, occurring early in the course of disease |
| Acute pseudocyst | Fluid collection containing pancreatic secretions, with a defined wall |
| Pancreatic necrosis | Nonviable pancreatic tissue diagnosed by contrast-enhanced computed tomography |
| Pancreatic abscess | Collection of purulent material in or near the pancreas |
APACHE II, Acute Physiology and Chronic Health Evaluation, version 2.
Diagnosis
Diagnosis of acute pancreatitis requires two of the following three features: (1) characteristic abdominal pain, (2) elevated serum pancreatic enzymes, and (3) characteristic findings on computed tomography (CT).7
Diagnostic Testing
Laboratory Tests
No biochemical marker is considered the “gold standard” for diagnosis or assessment of the severity of acute pancreatitis.9 Serum amylase and lipase measurements remain important diagnostic tests for acute pancreatitis. Other useful prognostic tests are a complete blood count; measurements of blood urea nitrogen and serum electrolyte, creatinine, glucose, and triglyceride levels; and liver function tests.
Total serum amylase has a reported sensitivity of 83% and specificity of 88% for acute pancreatitis.10 Amylase levels rise within 6 to 12 hours of onset and usually remain elevated for 3 to 5 days. A normal amylase value would generally exclude the diagnosis of acute pancreatitis except in cases involving hyperlipidemia, acute exacerbations of chronic pancreatitis, or markedly delayed manifestations (in which case amylase levels may have normalized). Acute pancreatitis should not be excluded on the basis of a normal or mildly elevated amylase value when clinical suspicion of this diagnosis is high. Serum amylase values cannot be used to estimate the severity or determine the cause of acute pancreatitis. Nonpancreatic causes of elevated serum amylase levels are listed in Box 43.1.
Box 43.1 Nonpancreatic Causes of Elevations in Serum Amylase
The serum lipase level is more sensitive (92%) and specific (96%) than total amylase for acute pancreatitis.10 Lipase has greater sensitivity in patients with acute alcoholic pancreatitis. It is useful in delayed clinical manifestations because the serum lipase value stays elevated longer than the serum amylase value does. However, serum lipase is not as specific for acute pancreatitis as once thought. The value is elevated in as many disorders as the amylase value (Box 43.2). As with serum amylase values, serum lipase values cannot be used to estimate the severity or determine the cause of acute pancreatitis.
A uniform threshold has not been established for serum amylase or lipase for the diagnosis of acute pancreatitis, but most of the published literature has reported thresholds of two to four or more times the upper limit of normal. However, the diagnosis of acute pancreatitis should not rely solely on elevations in serum enzymes above the arbitrary limit of normal laboratory levels but instead should be made on the basis of the onset of clinical symptoms because the sensitivities of these enzyme values change with the timing of their measurements.10
The urinary dipstick test for detecting pancreatic amylase in urine has demonstrated a sensitivity of 97%. This test for amylase may therefore be a useful point-of-care screening test for acute pancreatitis in the ED but is currently not in widespread use.11
C-reactive protein (CRP) is the best available serum marker for assessing the severity of acute pancreatitis.4 A threshold level greater than 150 mg/L is now accepted as a proven predictor of severity. However, CRP levels must be monitored after hospital admission because it takes 48 to 72 hours for the value to peak, thus making it less useful in the ED.
Hemoconcentration secondary to volume depletion has been identified as an early marker of pancreatic necrosis. In one study, an admission hematocrit value of 47% or higher and failure of the value to decrease at 24 hours after admission were associated with the development of pancreatic necrosis.12
An increase in serum alanine transaminase levels to higher than 150 IU/L is suggestive of gallstone pancreatitis.13 A lower value does not exclude the diagnosis, however.
Measurement of isoamylases, immunoreactive trypsinogen, macroamylases, or elastase has no role in the routine management of acute pancreatitis in the ED.14
Imaging
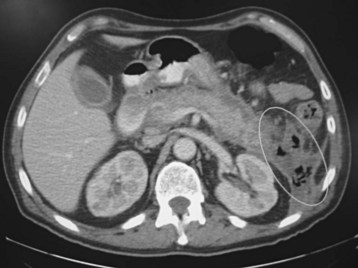
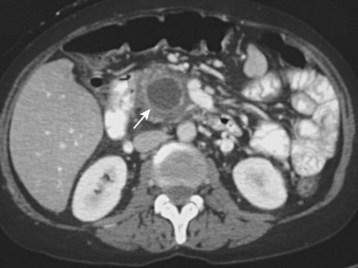
CT of the abdomen can aid in the diagnosis of acute pancreatitis and its complications, as well as assess the severity of disease. Most ED patients with acute pancreatitis do not require a CT scan at admission unless it is needed to exclude other serious causes of acute abdominal pain, such as a perforated ulcer. Contrast-enhanced CT of the abdomen is considered the best available test for the noninvasive diagnosis of pancreatic necrosis.15 Necrosis may develop several days after admission and should be suspected in patients with clinical evidence of increased severity. Affected portions of the necrotic pancreas do not show normal contrast enhancement on CT (Fig. 43.1). Non–contrast-enhanced CT will not aid in the diagnosis of pancreatic necrosis but may be considered in patients with renal insufficiency to provide other useful information. CT can diagnose complications such as pseudocysts (Fig. 43.2), phlegmon, and abscesses.
Magnetic resonance imaging (MRI) has been used in the diagnosis and management of acute pancreatitis. This modality is superior to CT in its categorization of fluid collections, necrosis, abscess, hemorrhage, and pseudocysts.16 Disadvantages include the lack of availability of MRI when urgently needed and the difficulty of caring for critically ill patients undergoing MRI.
Criteria for Determining Severity
The two most important markers of severity in acute pancreatitis are the presence of organ failure because of the systemic release of local inflammatory mediators (i.e., shock, pulmonary insufficiency, renal failure, gastrointestinal bleeding) and pancreatic necrosis.7
Contrast-enhanced CT of the abdomen is considered the best available test for determining the severity of disease, particularly after 2 to 3 days of illness.17 A CT Severity Index Score can be calculated on the basis of CT findings, with a score of 6 or higher indicating severe disease (Table 43.2).18
Table 43.2 Computed Tomography Severity Index Score for Pancreatitis*
| GRADE† | CT FINDINGS | SCORE |
|---|---|---|
| A | Normal pancreas | 0 |
| B | Focal or diffuse enlargement of the pancreas, contour irregularities, heterogeneous attenuation, no peripancreatic inflammation | 1 |
| C | Grade B plus peripancreatic inflammation | 2 |
| D | Grade C plus a single fluid collection | 3 |
| E | Grade C plus multiple fluid collections or gas | 4 |
| PERCENT NECROSIS PRESENT ON CT | SCORE |
|---|---|
| 0 | 0 |
| <33 | 2 |
| 33-50 | 4 |
| >50 | 6 |
* Severity Index Score = Grade score + Percent necrosis score. Maximum score = 10; severe disease = 6 or higher.
† Severity of the acute inflammatory process.
Though not as accurate as the CT system, scoring systems that include clinical criteria may also be used to estimate severity but have a limited role in the ED because of either timing or the complexity of the data elements. The most commonly used clinical scores for determining the severity of acute pancreatitis are the Ranson criteria (Box 43.3) and the second version of the Acute Physiology and Chronic Health Evaluation (APACHE II).
The Ranson score is based on 11 prognostic signs.19 Five of the signs are measured on admission, and six are measured 48 hours later, thus making it less useful in the ED setting. Mortality estimates are based on the number of signs present. Mortality is less than 0.9% when three or fewer signs are present; it is 100% when more than six signs are noted.
The APACHE II score is based on 12 physiologic variables, age, and previous health.17 The APACHE II score is probably the best clinical predictor of the severity of acute pancreatitis.20 A severity score can be assessed on admission and recalculated daily. (A calculator is available at www.sfar.org/scores2/apache22.html.) An APACHE II score higher than 8 indicates severe disease. However, the complexity of the APACHE II score limits its usefulness in the ED setting. An APACHE III score, developed to improve accuracy, does not appear to be more useful than APACHE II in differentiating mild from severe disease.21
The “harmless acute pancreatitis score” takes a completely new approach and attempts to predict mild disease instead of severe pancreatitis. This simple clinical algorithm assesses three variables (no rebound tenderness or guarding, normal hematocrit, normal creatinine) to identify patients who have a benign course 98% of the time. This information may quickly aid the physician in determining the appropriate level of care for admission if warranted.22
Treatment
No specific treatment is available for pancreatitis other than supportive care. Supplemental oxygen, aggressive intravenous hydration, pain management, and monitoring are the mainstays of treatment. Supplemental oxygen is vital for treating and preventing hypoxemia. Aggressive intravenous hydration consisting of fluid boluses to establish hemodynamic stability followed by appropriate maintenance fluids is of critical importance in treating and preventing hypovolemia. Hypovolemia occurs secondary to vomiting, decreased oral intake, diaphoresis, third-space losses, and inflammatory-mediated increased vascular permeability. Hypovolemia compromises the pancreatic microcirculation and can contribute to the development of pancreatic necrosis. Nasogastric tubes are not indicated in all patients but may be helpful in those with significant vomiting or in whom enteral feeding is indicated. Anticholinergic agents, once given to decrease gastric secretions, are no longer recommended. Endoscopic retrograde cholangiopancreatography is usually indicated within 24 to 48 hours of arrival in patients with severe pancreatitis secondary to gallstones (Box 43-3).
![]() See Box 43-3, Ranson Criteria and Score, online at www.expertconsult.com.
See Box 43-3, Ranson Criteria and Score, online at www.expertconsult.com.
Narcotic analgesia is often needed to control the pain of acute pancreatitis. Controversy exists over which narcotic to use. Morphine has historically been avoided because of the potential to cause spasm of the sphincter of Oddi and subsequent worsening of symptoms.23 Meperidine is often used in place of morphine, but no conclusive evidence supports the use of meperidine rather than other narcotics.24 In fact, meperidine has several disadvantages, including the potential formation of neurotoxic metabolites, muscle fibrosis if given intramuscularly, and a short duration of action. Administration of adequate doses of narcotic analgesia is probably more important than the particular narcotic used.
Management of necrotizing pancreatitis usually involves admission to a higher level of care (step-down unit or intensive care unit) because of the associated increased morbidity and mortality. The use of prophylactic antibiotics to prevent infection in patients with pancreatic necrosis is no longer recommended.7 Antibiotics are indicated for septic patients while an investigation for the source of infection is undertaken. Carbapenems are acceptable empiric choices.25 Infected necrotizing pancreatitis is considered uniformly fatal without intervention and should be suspected in patients with clinical evidence of sepsis. Urgent surgical consultation should be obtained if infection is suspected because aggressive surgical débridement (necrosectomy) may be necessary.9 CT-guided fine-needle aspiration may be necessary to establish the diagnosis in suspicious cases. Drainage options for patients with pancreatic necrosis who will not undergo surgery (poor surgical candidates or patients whose infection is well contained) are expanding and include both percutaneous therapy (interventional radiology) and endoscopic therapy. Treatment of sterile necrosis remains supportive.
Pancreas Transplant Complications
Acute rejection can occur immediately or at any point during the course of the patient’s life. Patients who have undergone pancreatic transplantation are maintained on immunosuppressive agents and thus are at risk for a variety of infectious complications. Vascular thrombosis of the pancreatic portal vein is a very early complication typically seen within 24 to 48 hours of transplantation and is thought to be due to the relatively low-flow state of the pancreatic graft. Transplantation pancreatitis occurs to some degree in all patients postoperatively within 48 to 96 hours and is usually transient and mild.26
![]() Priority Actions
Priority Actions
1. Provide aggressive intravenous hydration to treat or prevent hypovolemia.
2. Provide appropriate parental narcotics for pain control.
3. Consider computed tomography if concerned about other possible serious causes of abdominal pain, such as a perforated ulcer.
4. Consider admission to the intensive care unit for patients with organ failure.
5. Start broad-spectrum antibiotics for clinical evidence of sepsis.
Tips and Tricks
The diagnosis of acute pancreatitis should not rely solely on elevations of serum enzymes above the arbitrary limits of normal laboratory levels; instead, it should be based on the onset of clinical symptoms because the sensitivities of these enzyme values change with the timing of their measurements.10
1 Russo MW, Wei JT, Thiny MT, et al. Digestive and liver disease statistics. Gastroenterology. 2004;126:1448–1453.
2 Uhl W, Warshaw A, Imrie C, et al. IAP guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565–573.
3 Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Int J Pancreatol. 1999;25:195–210.
4 Go VLW. Etiology and epidemiology of pancreatitis in the United States. In: Bradley EL, III. Acute pancreatitis: diagnosis and therapy. New York: Raven Press; 1994:235–239.
5 Lankisch PG, Burchard-Reckert S, Petersen M, et al. Morbidity and mortality in 602 patients with acute pancreatitis seen between the years 1980–94. Z Gastroenterol. 1996;34:371–377.
6 Ros E, Navarro S, Bru C, et al. Occult microlithiasis in “idiopathic” acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701–1709.
7 Banks PA, Freeman M. L, for the Practice Parameters Committee of the American College of Gastroenterolgy. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400.
8 Bradley EL, III. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, 11–13 September 1992, Atlanta, GA. Arch Surg. 1993;128:586–590.
9 Baron HB, Morgan DE. Acute necrotizing pancreatitis. N Engl J Med. 1999;340:1412–1417.
10 Dominguez-Munoz JE. Diagnosis of acute pancreatitis: any news or still amylase? In: Buchler M, Uhl E, Friess H, et al. Acute pancreatitis: novel concepts in biology and therapy. London: Blackwell Science; 1999:171–180.
11 Hedstrom J, Svens E, Kenkimaki P. Evaluation of new urinary amylase test strip in the diagnosis of acute pancreatitis. Scand J Clin Lab Invest. 1998;58:611–616.
12 Baillargeon JD, Ramagopal V, Tenner SM, et al. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93:2130–2134.
13 Tenner S, Dubner H, Steinberg W. Predicting gallstone pancreatitis with laboratory parameters: a meta-analysis. Am J Gastroenterol. 1994;89:1863–1866.
14 Thomson HJ, Obekpa PO, Smith AN, et al. Diagnosis of acute pancreatitis: a proposed sequence of biochemical investigation. Scand J Gastroenterol. 1987;22:719–724.
15 Balthazar EJ, Freeny PC, Van Sonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297–306.
16 Arvanitakis M, Delhaye M, Maertelaere V, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715–723.
17 Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829.
18 Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331–336.
19 Ranson JH, Pasternack BS. Statistical methods for quantifying the severity of clinical acute pancreatitis. J Surg Res. 1977;22:79–91.
20 Larvin M, McMahon MJ. APACHE-II score for assessment and monitoring of acute pancreatitis. Lancet. 1989;2:201–205.
21 Williams M, Simms HH. Prognostic usefulness of scoring systems in critically ill patients with severe acute pancreatitis. Crit Care Med. 1999;27:901–907.
22 Lankisch PG, Weber-Dany B, Hebel K, et al. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of non-severe disease. Clin Gastroenterol Hepatol. 2009;7:702–705.
23 Isenhour HL, Mueller BA. Selection of narcotic analgesics for pain associated with pancreatitis. Am J Health Syst Pharm. 1998;55:480–486.
24 Voorthuizen T, Helmers JH, Tjoeng MM, et al. Meperidine outdated as analgesic in acute pancreatitis. Ned Tijdschr Geneeskd. 2000;144:656–658.
25 Heinrich S, Schafer M, Rousson V, et al. Evidenced-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154–168.
26 Kaufman DB. Pancreas transplantation. eMedicine, accessed 1/13/2011. Available at http://emedicine.medscape.com/article/429408-followup