Chapter 46 Pancreatic Cancer
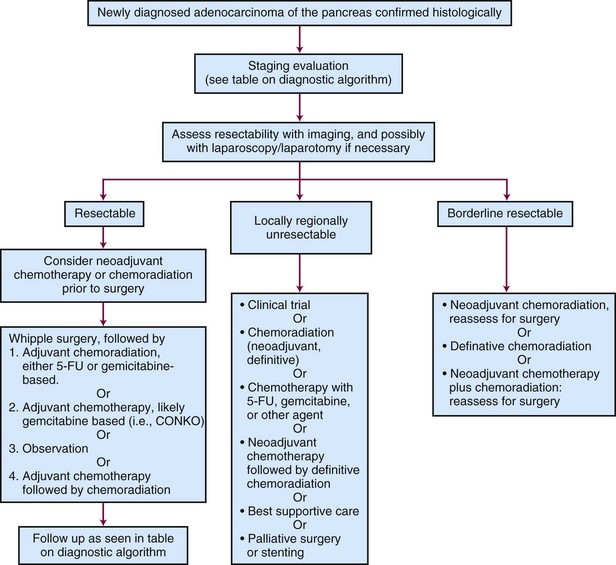
Managing pancreatic adenocarcinoma presents significant challenges. The natural history of the disease is characterized by spread to regional lymphatics, liver, and peritoneal surfaces; and most patients have either clinical or subclinical dissemination to these sites at diagnosis. At presentation, the outcome appears to be determined primarily by the extent of evident disease, resectability, and performance status. Patients with resectable tumors have historically been thought to represent 15% to 20% of those with pancreatic cancer. However, recent Surveillance, Epidemiology, and End Results (SEER) data analyses suggest that in the United States the frequency of potentially resectable disease may be closer to 10% to 12%.1
Most (75% to 80%) pancreatic adenocarcinomas present in the head, neck, and/or uncinate process (periampullary region), and results from these presentations provide the dataset from which clinical management decisions are most commonly made. The dataset for management decisions regarding presentations in the distal pancreatic body and tail is much less robust. The anatomic relationships of the pancreas are illustrated in Figure 46-1.
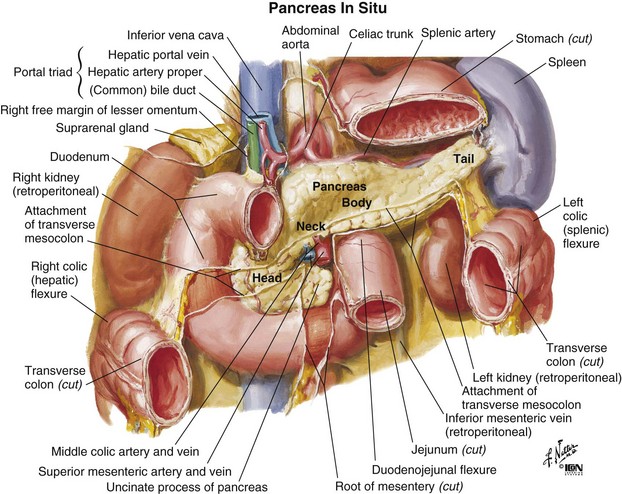
Figure 46-1 Anatomy of the pancreas.
From Netter FH: Atlas of Human Anatomy. West Caldwell, NJ, 1992, CIBA-GEIGY Corporation, plate 279.
Epidemiology And Etiology
Epidemiology
In 2010 there were an estimated 43,140 new cases of pancreatic carcinoma in the United States, with slightly more cases in women than in men.2 In the same year the number of deaths due to carcinoma of the pancreas was estimated to be 36,800. It was anticipated that carcinoma of the pancreas would be the 10th most frequently diagnosed new cancer in men and women, but the 4th leading cause of cancer death (after lung, prostate, and colorectal cancer in men and lung, breast, and colorectal cancer in women). Internationally, incidence rates are highest in Western and industrialized countries.
Most patients with pancreatic cancer present with advanced disease. Incidence rates increase with age, and most cases occur in patients older than 60 years of age. Approximately 68% of cases will be diagnosed in patients 65 years of age or older. In the absence of overt metastatic disease, patients undergoing pancreatic resection have a clear survival advantage as compared with patients not undergoing resection. The 1-, 2-, and 3-year overall survival (OS) rates for patients undergoing resection as compared with those not undergoing resection are 48% versus 23%, 24% versus 9%, and 17% versus 6%, respectively.3
Etiology
Pancreatic cancer is clearly associated with cigarette smoking, diet (higher risk with higher percentages of fat and meat in the diet), obesity,4 chronic diabetes mellitus, and chronic pancreatitis. Of these, the strongest association is with cigarette smoking. There is also an occupational association with the manufacture of 2-naphthylamine and benzidine and with those working with gasoline derivatives.5 Although coffee consumption has sometimes been implicated in the occurrence of carcinoma of the pancreas, a review failed to confirm this association.6
There is an increased risk of developing pancreatic cancer, as well as other intra-abdominal malignancies, after radiation therapy (RT) to the abdomen for management of testicular malignancy, either seminoma or nonseminoma (the observed to expected frequency is 2.30).7 The risk is noted as early as 10 years after management and continues to increase with time.
An important development in our understanding of the etiology of pancreatic cancer is the recognition of familial pancreatic cancer.8 Familial pancreatic cancer may occur in the absence of a recognizable clinical syndrome, and as many as 10% of pancreatic cancers may be familial. Data from the National Familial Pancreas Tumor Registry have shown that the risk of cancer is 18-fold greater in first-degree relatives of familial pancreatic cancer cases (at least two first-degree relatives with pancreatic cancer in the family) than it is in first-degree relatives of sporadic pancreatic cancer cases (families in which there has been only one member with pancreatic cancer).9 In addition, there are associations with other known syndromes such as the familial breast, ovarian, pancreas cancer syndrome associated with BRCA2, the familial pancreas cancer syndrome associated with CDKN2A abnormalities, the Peutz-Jeghers polyposis gastrointestinal malignancy syndrome associated with mismatched repair genes, LKB1/STK11, and the hereditary nonpolyposis colon cancer (HNPCC) syndrome. Current understanding of familial pancreatic cancer has been reviewed by Shi and associates10 and is regularly updated at the webpage of the Johns Hopkins National Familial Pancreatic Tumor registry (pathology.jhu.edu/pancreas/PartNFPTR.php).11
Prevention And Early Detection
Although the just described associations might suggest the identification of a patient population suitable for screening, so far no simple, cost-effective test for the disease has been successful in establishing early diagnosis even in relatively high-risk groups.12 Potential imaging modalities for screening and early detection include computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS). For early detection, EUS may be the imaging modality of choice because it detects smaller pancreatic lesions than those detected with thin-section, dual-phase spiral CT. The accuracy of diagnosis of pancreatic cancer in patients with pancreatic masses suspected of having cancer is close to 100% for EUS and about 92% for dual-phase CT.13 When combined with fine-needle aspiration (FNA), EUS can provide a cytologic diagnosis of lesions as small as 2 to 5 mm not visualized by CT, ultrasonography, or MRI. Endoscopic retrograde cholangiopancreatography (ERCP) is less likely to detect small tumors, is a relatively more invasive screening test owing to the risk of developing pancreatitis (5% to 10%), and may be useful for detailed characterization of ductal abnormalities. Unfortunately, recent efforts at applying these methodologies appear to have been unrewarding, suggesting that, at present, screening even in identified, high-risk populations may not be appropriate.14–15 However, it remains possible that screening of individuals from the highest-risk kindreds (those with three or more first-degree relatives with pancreatic cancer), based on experience at the Johns Hopkins Hospital,16,17 might be useful.
Presently, there is no routinely available blood test that may be used as a screening tool for pancreatic cancer. Although serum carbohydrate antigen 19-9 (CA19-9) is a tumor-associated antigen that is frequently elevated in patients with pancreatic adenocarcinoma,18 it may also be elevated in biliary tract malignancies, some colon cancers, and non–cancer-related conditions such as pancreatitis, cholangitis, poorly controlled diabetes, and biliary obstruction due to benign causes (stones, strictures). Moreover, patients genetically lacking Lewis antigens may be incapable of producing CA 19-9. Consequently, CA 19-9 is better used to help monitor therapeutic response when elevated secondary to tumor in patients with histologically proven pancreatic cancer.19,20,21
Biomarkers for early detection targeting are in development and include DNA-, RNA-, and protein-based approaches. DNA-based techniques aim to detect cancer-specific DNA alterations and methylation changes.22 Analysis of pancreatic juice or fine-needle aspirates may identify genes overexpressed at the RNA level in pancreatic cancers compared with normal pancreas.23 Protein-based markers may also be overexpressed in patients with pancreatic cancer compared with patients with other pancreatic diseases.24
Biologic Characteristics And Molecular Biology
A number of studies have looked at DNA content and proliferative index in patients with pancreatic cancer. Bottger and associates25 studied DNA content, tumor size, lymph node status, tumor stage, nuclear grade, and type of resection on outcomes in a series of 41 patients with adenocarcinoma of the pancreas. They observed that in univariate analysis these factors had a significant (p <.05) association with outcome. However, in multivariate analysis the most important predictors of outcome were the operative procedure (whether the tumor was resected to histologically negative margins) and the DNA content (whether the patient’s tumor was tetraploid, having unfavorable prognostic implications). Similarly, Jorba and colleagues26 found that among 73 patients undergoing pancreaticoduodenectomy for periampullary malignancies, 74% were diploid and 26% were aneuploid. In univariate analysis there was a strong correlation between diploid status and long-term survival, with diploid cancer patients having a median survival of 30.1 months and aneuploid cancer patients having a median survival of 16 months. However, when tumor site (duodenum and ampulla vs. bile duct and pancreas) and tumor size were taken into consideration, the effect of ploidy no longer produced a significant difference.
As summarized by Hruban and colleagues,27 cancer of the pancreas can now be viewed as a disease of acquired and inherited mutations involving a number of cancer-related genes. There are three types of such genes: tumor suppressor genes, oncogenes, and DNA mismatch repair genes. Tumor suppressor genes function to restrain cell proliferation. Oncogenes possess transforming properties when activated by mutation or amputation. DNA mismatch repair genes encode for proteins that correct errors that normally occur during DNA replication. All three types of potentially cancer-causing genes have been implicated in pancreatic cancer.
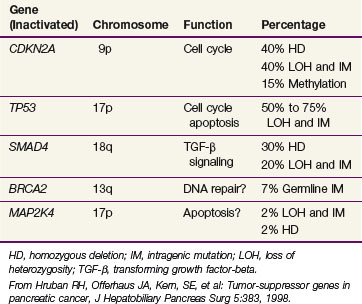
A number of suppressor genes have been found to play a role in the development of pancreatic cancer. Table 46-1 provides a summary of the tumor suppressor genes in pancreatic cancer.27 The CDKN2A tumor suppressor gene on chromosome 9p is inactivated in approximately 95% of pancreatic cancers. The mechanism by which CDKN2A inactivation promotes cell cycle progression is shown in
web-only Figure 46-1 on the Expert Consult website![]()
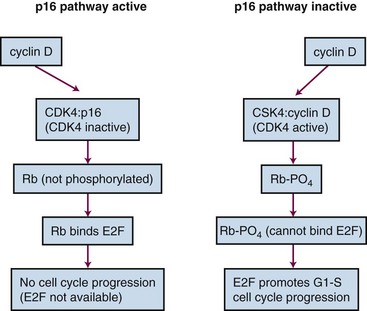
Web-Only Figure 46-1 Inactivation of CDKN2A promotes cell cycle progression.
From Tascilar M, Skinner HG, Rosty C, et al: The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res 7:4115, 2001.
The TP53 gene located on chromosome 17p is inactivated in 50% to 70% of pancreatic cancers. The SMAD4 gene on chromosome 18q is inactivated in approximately 50% of pancreatic cancers. It has been found to be of prognostic significance, with SMAD4-positive patients having improved survival over SMAD4-negative patients. Moreover, SMAD4 positivity retains its prognostic significance in multivariate analysis adjusting for tumor size, lymph node involvement, margin status, and histologic differentiation.28 The BRCA2 gene is inactivated in 70% of pancreatic cancers. BRCA2 is noteworthy because it represents mutation in the inherited germline in some cases. Additionally, BRCA2 has been confirmed as the most common inherited genetic alteration associated with pancreatic cancer.29
Finally, abnormalities in DNA mismatch repair genes have been noted. A specific molecular phenotype called microsatellite instability, also identified in the HNPCC syndrome of colon cancer, has been found in 4% of pancreatic cancers and is associated with a unique morphologic appearance, the presence of wild-type (normal) KRAS, diploidy, and improved prognosis.30
These molecular abnormalities have several applications. They may be used to define the precursors of infiltrating adenocarcinoma of the pancreas or help characterize histologically ambiguous lesions. Moreover, they have the potential to form the basis for new screening tests for pancreatic neoplasia and, subsequently, may serve as important epidemiologic tools.27
Just as specific genetic abnormalities have been associated with pancreatic cancer, karyotyping studies by Griffin and colleagues31,32 have shown consistent losses of chromosomes 18, 13, 12, 17, and 6. This correlates well with the loss of the SMAD4 suppressor gene, which is known to reside on chromosome 18, and the loss of the tumor suppressor gene TP53, which is known to reside on chromosome 17.
In recognition of an evolving understanding of the role of genetics and familial clustering of pancreatic adenocarcinoma, the Johns Hopkins Familial Pancreatic Tumor Registry was established in 1994 and has produced a number of important observations regarding the risk of pancreatic cancer in families with two or more first-degree relatives and genetic patterns in these families. The most recent update of the Johns Hopkins registry was in 2009, and the website can be viewed online (see earlier discussion). First-degree relatives from kindreds in which there are two first-degree relatives with pancreatic cancer may have as much as a 9-fold higher risk of developing pancreatic cancer than a member of the general population. If there are three or more first-degree relatives, this risk increases up to 32-fold. The risk relates to blood-line relatives and does not include spouses. There are now multiple registries for familial pancreatic cancer, and there has been a suggestion for developing an international registry to combine these efforts.33
Pathology And Pathways Of Spread
Pathology
Infiltrating ductal adenocarcinomas are the most common histologic type of malignant tumors of the exocrine pancreas, as shown in Figure 46-2. These malignant epithelial neoplasms show glandular or ductal differentiation, with most arising in the periampullary region of the pancreas.34 Although ductal adenocarcinoma is the most common primary malignancy of the pancreas, accounting for approximately 75% of all primary nonendocrine cancers in this organ, there are a number of other important epithelial malignancies arising in the pancreas that should be recognized because of their variant presentations and prognostic implications.35 Recent reports have nicely summarized the progression of pancreatic intraepithelial neoplasia to invasive ductal carcinoma and also described the molecular and prognostic correlates associated with less common pancreatic tumors.36,37
Acinar cell carcinoma is also a rare histology in which 20% of cases may be associated with subcutaneous fat necrosis, a rash similar to erythema nodosum, peripheral eosinophilia, and polyarthralgias. These associated phenomena are a result of release of large amounts of lipase by the tumor.38 These tumors typically present with large size and average dimension of 11 cm and occur in the head, body, and tail of the pancreas with frequencies of 60%, 30%, and 10%, respectively. Survival is not significantly superior to that seen with standard adenocarcinoma of the pancreas. Those patients presenting with large amounts of lipase production may have a less than average prognosis.
The primary cystic, nonendocrine epithelial tumors are another type of pancreatic neoplasm. Only 5% to 15% of pancreatic cystic lesions are actually neoplastic, with the remaining non-neoplastic lesions including pseudocysts, congenital cysts, and retention cysts. The cystic neoplasms of the pancreas include serous and mucinous neoplasms. Most serous cystic neoplasms are serous cystadenomas, also known as microcystic adenomas and glycogen-rich cystadenomas. They occur more commonly in women, with average age at presentation in the seventh decade.39 These lesions may be large, and patients with von Hippel-Lindau syndrome (familial cancer syndrome associated with clear cell renal cancer, cerebellar and spinal hemangioblastomas, pancreatic islet cell cancers, and other tumors) may be predisposed to develop pancreatic serous cystadenomas. Most serous cystic neoplasms of the pancreas are benign.
In contrast, mucinous cystic neoplasms are more frequently malignant. These are also more common in women and are typically diagnosed in the fifth decade of life. Histologically, these lesions contain a mucin-positive, cloudy fluid and do not communicate with the main pancreatic duct.40 Microscopic analysis reveals three types of mucinous cystic neoplasms: mucinous cystadenomas, borderline mucinous cystic neoplasms, and mucinous cystadenocarcinoma. It is generally thought that mucinous cystadenomas and borderline cystic neoplasms have the potential for progressing to overt malignancy and should be completely resected. Interestingly, mucinous cystadenocarcinomas are associated with a better prognosis than typical adenocarcinoma of the pancreas, with roughly 50% of patients surviving 5 years.
Intraductal papillary mucinous neoplasms communicate with the main pancreatic duct system and are typically diagnosed in the seventh decade. They are associated with a good prognosis compared with more typical pancreatic malignancies.41 A more aggressive variant is the papillary mucinous carcinoma in which the histology displays significant cytologic and architectural atypia and/or overt invasion.
It has been well known that a precursor histology of intraepithelial neoplasia (IN) can be described in sites such as colon, cervix, and prostate. The precursor histology for pancreatic adenocarcinoma (PanIN) has also been well described.42 These lesions have been named PanIN-1A and 1B, PanIN-2, and PanIN-3. They are often seen in association with invasive adenocarcinoma and have occasionally been observed to progress into invasive adenocarcinoma.
Pathways of Spread
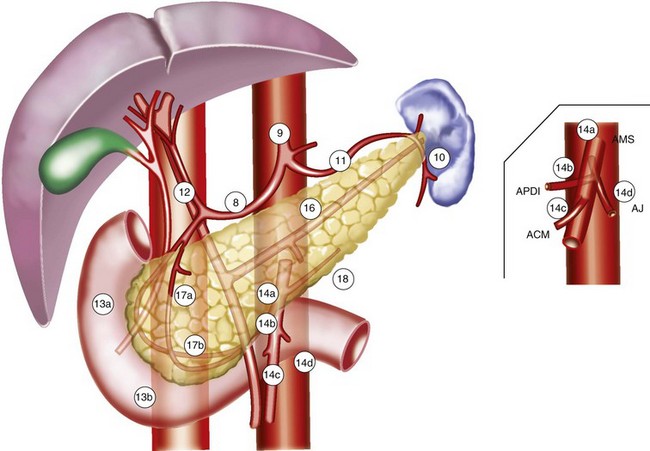
The pancreas is a retroperitoneal organ that lies in close anatomic relationship to the stomach, duodenum, jejunum, kidneys, spleen, celiac trunk, superior mesenteric artery and vein, common bile duct, and portal vein (see Fig. 46-1). The main pancreatic duct is known as the duct of Wirsung, and the accessory pancreatic duct is known as the duct of Santorini. At the time of diagnosis, more than 85% of pancreas tumors will have extended into adjacent organs, lymph nodes, fat, or soft tissue, allowing early metastasis to regional and distant lymph nodes.43
Patterns of involvement in the regional lymphatics have long been well recognized. Frequently involved nodal sites include the posterior pancreaticoduodenum, superior pancreatic head, inferior pancreatic head, superior pancreatic body, and porta hepatis.44,45 There is also a relationship between para-aortic lymph node involvement and involvement of posterior pancreaticoduodenal nodes by direct interlymphatic communication46 (Fig. 46-3). Venous drainage of the pancreas is to the liver via the portal vein.
Patterns of failure after resection have been described, with local recurrence, peritoneal relapse, and hepatic metastases occurring in 50%, 42%, and 62% of patients, respectively.47,48–50 Although it is likely that the peripancreatic burden of disease has multiple direct lymphatic pathways to hepatic tissues, hepatic involvement has been taken as a sign of systemic metastasis. The lung is the most frequently involved extra-abdominal organ.43
Recent reports confirm that these patterns of failure have not changed appreciably over time and also emphasize the effect of the retroperitoneal margin, poor differentiation, and lymph node involvement in determining relapse risk, especially locally.51,52 In addition, rapid autopsy results from Johns Hopkins Hospital studies indicate that local recurrence may be associated with alterations in SMAD4 and that local recurrence may be the cause of death in up to 30% of patients with overt metastatic disease.53
Clinical Manifestations, Patient Evaluation, And Staging
Initial laboratory results may show elevated serum bilirubin, alkaline phosphatase, and gamma glutamyl transpeptidase levels. In addition, the hepatic aminotransferases may be increased. Although CA19-9 has not proved useful as a marker for screening of pancreatic cancer, it has been used as a test to assess prognosis and/or follow patients postoperatively as well as to guide prognosis and response to other treatment when known to be previously elevated in association with a proven pancreatic malignancy.54,55 The value of CA19-9 was confirmed prospectively in analysis of data from the Radiation Therapy Oncology Group (RTOG) 97-04 trial, which showed on multivariate analyses that the postoperative CA19-9 level is a highly significant predictor of overall survival in patients with resected pancreatic cancer.21 Some early data suggest that monitoring levels of KRAS after treatment may possibly give clues as to tumor response in patients treated with gefitinib and chemoradiation, but this has not been proved.56
Patient Evaluation
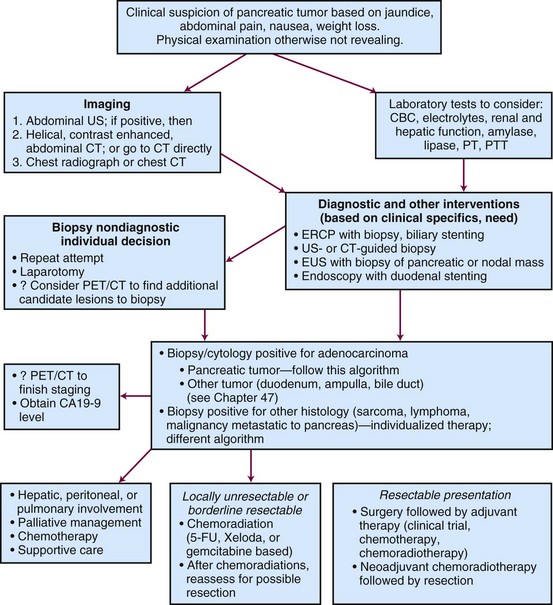
Assessment is guided by the patient’s presenting symptoms, findings on history and physical examination, and initial laboratory results (Fig. 46-4). Ultrasonography, CT, and ERCP are often included in the evaluation of patients with obstructive jaundice. With current spiral CT techniques combined with enhancement after oral and intravenous administration of contrast, pancreatic masses, pancreatic duct dilatation, local invasion, liver metastases, and vascular invasion of the great vessels are readily identified. EUS has improved staging of pancreatic cancer. It is especially effective in detecting vascular invasion of pancreatic cancer when CT is equivocal, which is imperative in determining resectability.57 ERCP is frequently used to obtain biopsy specimens, decompress the biliary tree, and localize the source of obstruction. Biliary obstruction can also be relieved by percutaneous and transhepatic cholangiography. Visceral angiography is selectively used based on concern regarding vascular invasion leading to unresectability. PET may be useful in identifying otherwise occult metastases. A study of 82 patients staged with either CT alone or PET/CT showed that the addition of PET increased sensitivity for the detection of distant metastases and would have changed management in 11% of the patients.58 Laparoscopy is used in some institutions as a prelude to exploration and has been found to increase the detection of intraperitoneal metastases in patients with locally advanced pancreatic cancer. It appears that a tissue diagnosis by FNA before laparotomy is not necessary.59
Staging
The seventh edition of the American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer is shown in Table 46-2. There were no changes between the AJCC sixth and seventh editions, except that the seventh edition now includes neuroendocrine tumors. Treatment and prognosis are defined by resectability (T < T4) and presence of distant metastatic disease, which most commonly involves the liver, peritoneum, or both.60
TABLE 46-2 American Joint Committee on Cancer TNM Staging for Pancreas Cancer
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to the pancreas ≤2 cm in greatest dimension |
| T2 | Tumor limited to the pancreas >2 cm in greatest dimension |
| T3 | Tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery |
| T4 | Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor) |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
From Edge SB, Byrd DR, Compton C, et al, editors: AJCC Cancer Staging Manual, ed 7, New York, 2010, Springer.
Pancreatic tumors staged as T1, T2, or T3 are generally considered to be resectable. Unresectable pancreatic tumors are those in which the tumor cannot be separated from the adjacent large arterial structures (celiac axis or superior mesenteric artery). The extent of resection also carries prognostic significance. A patient who has undergone an R0 complete resection with negative margins will have a better prognosis compared with a patient who has undergone an R1 or R2 resection with microscopic (R) or grossly positive margins (R2). Furthermore, recent data suggest that margins greater than 1.5 mm are needed to optimize locoregional control, and the extent of clear margins in R0 resections may potentially be used to estimate risk of locoregional failure.61 Borderline resectable tumors, which are at high risk for a margin-positive resection, are discussed later in this chapter.
Primary Therapy
Surgery
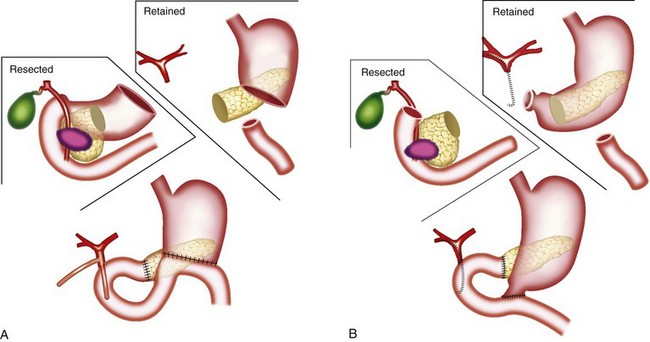
The classic operation for resection of a carcinoma of the head of the pancreas is a pancreaticoduodenectomy, also known as a Whipple procedure. In this operation the gallbladder, common bile duct, second through fourth portions of the duodenum, and the head of the pancreas are resected along with the postpyloric duodenum (pylorus-preserving pancreaticoduodenectomy) or the resection is continued proximally to include the distal stomach (classic pancreaticoduodenectomy). The regional lymph nodes are also resected. It appears well established that the pylorus-preserving pancreaticoduodenectomy improves gastrointestinal function without compromise of oncologic management.62–64 Figure 46-5 illustrates these two variants of surgical resection.65 Within the past two decades, the mortality of this operation has declined from approximately 20% to between 1% and 3% in major centers.66–68 (A video of this operation may be observed at www.orlive.com/umm/videos/whipple-procedure-for-pancreatic-cancer.)
web-only Figure 46-2, available on the Expert Consult website![]()
.69 As illustrated, gastrointestinal reconstruction after pancreaticoduodenectomy requires enteric, biliary, and pancreatic anastomoses. Although postoperative mortality is less than 3% at high-volume centers, the morbidity of pancreaticoduodenectomy remains high, with pancreatic leak and infection, delayed gastric emptying, and pancreatic fistula among the most common complications.70 Based on large-volume, single-institution experience the dramatic improvements in postoperative morbidity and mortality reported in the 1990s have not shown further improvement.71
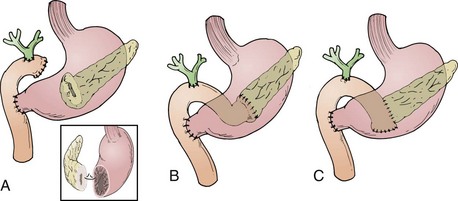
Web-Only Figure 46-2 A, Pancreaticogastrostomy. B, End-to-end pancreaticojejunostomy. C, End-to-side pancreaticojejunostomy. The inset details the location of the posterior gastrostomy.
From Yeo CJ, Cameron JL, Maher MM, et al: A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg 222:580, 1995.
Prognostic Factors Associated with Results of Surgical Resection
Michelassi and colleagues72 reported on their experience with 647 consecutive patients between 1946 and 1987 with tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct, including patients with resectable and nonresectable disease. The resectability rate varied from 16.5% for pancreatic adenocarcinoma to 89.3% for ampullary tumors. Among these 647 patients, there were 133 resections. Their data clearly show the importance of resection for any type of tumor presentation in the periampullary area with respect to 5-year survival. Evaluating only those patients with adenocarcinoma who underwent a curative intent resection and survived the perioperative period (n = 97), the following factors did not affect survival: tumor size, tumor differentiation, the presence of lymphovascular invasion, capillary invasion, perineural microinvasion, lymph node status, or type of procedure (pancreaticoduodenectomy vs. total pancreatectomy). The authors analyzed relapse data and found that based on autopsy experience 29.4% of patients died with local recurrence alone, 23.5% died with distant metastasis alone, and 47.1% had both local and distant relapses.
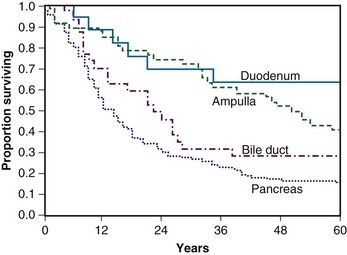
Yeo and colleagues73 have published two important papers that summarize and describe the anticipated patient experience after surgery for periampullary malignancy. In their series of 650 consecutive pancreaticoduodenectomies in the 1990s, there were 443 patients who underwent resection for periampullary malignancy. Their data convincingly show the improved survival seen for resected duodenal and ampullary tumor as opposed to resected pancreatic and distal common bile duct tumors. Moreover, in multivariate analysis, factors associated with improved, postresection survival are the absence of intraoperative blood loss greater than 700 mL, tumor site (with duodenum and ampullary being better than bile duct and pancreas), tumor diameter of less than 3 cm as opposed to 3 cm or more, histologically negative margins, histologically negative nodes, and having a well-differentiated tumor as opposed to a moderately or poorly differentiated tumor. Reoperation for complication was also significantly associated with decreased survival. Yeo and associates74 further analyzed factors associated with long-term survival in 242 patients who underwent surgery between April 1990 and May 1992. They found that negative nodal involvement, tumor differentiation, and resected margin status were all important prognostic factors for periampullary tumors. A more recent update from the same institution has also added extent of lymph node involvement to these previously identified risk factors.75
The prognostic importance of histologic differentiation, nodal involvement, and tumor size (for patients with pancreatic adenocarcinoma) have also been demonstrated by Geer and Brennan.76 The difference in survival at 36 months for well-differentiated tumors versus poorly differentiated tumors is roughly 50% and 10%, respectively. Less extreme but still highly significant differences are seen when comparing node-negative survivors versus node-positive survivors. At 36 months the survivals were approximately 35% and 10%, respectively. In tumors smaller than or larger than 2.5 cm, the survival differences were 40% and 20%, respectively. This group has developed a nomogram based on data from 555 patients to predict outcome at 1, 2, or 3 years.77 The factors correlating with survival in their multivariate analysis are shown in Table 46-3. This nomogram can be accessed at the web page of the Memorial Sloan-Kettering Cancer Center (www.mskcc.org).
TABLE 46-3 Factors Correlating with Survival in Multivariate Analysis
| Factor | p Value |
|---|---|
| Maximum tumor dimension | .001 |
| Number of nodes positive | .001 |
| Splenectomy required | .001 |
| Differentiation | .002 |
| Tumor located in head versus other pancreatic site | .006 |
| Posterior margin status | .049 |
Data from Brennan MF, Kattan M, Klimstra D, Conlon K: Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas, Ann Surg 240: 293, 2004
Although size has prognostic importance in pancreatic cancer, small size does not necessarily equate with a low-risk lesion, as demonstrated by Manabe and colleagues in 1988.78 This observation has subsequently been confirmed in more contemporaneous series.79,80
Patterns of Failure After Surgical Resection
Tepper and colleagues47 reported on 145 patients managed at the Massachusetts General Hospital from 1963 to 1973. Of these patients, only 31 underwent radical surgery, with 5 operative deaths, leaving 26 patients for further analysis. Among these 26 patients, 50% had either pathologically proven local recurrence or clinical evidence of local recurrence at the time of death from disease. In 5 patients, local status was unknown at the time of death. Four patients died of distant metastases without evidence of local recurrence.
Griffin and associates48 did a more extensive analysis of patterns of failure after curative resection for pancreatic carcinoma. This analysis was on 36 patients undergoing resection with curative intent at the University of Kansas between 1977 and 1987. The 2- and 5-year survivals among these patients were 32% and 17%, respectively. The median survival was 11.5 months. Of all the patients who had failure, 100% had a component of failure within the intra-abdominal cavity. Seventy-three percent had a component of local failure, 42% had a component of peritoneal failure, and 62% had a component of hepatic failure. Extra-abdominal metastases were documented in only 27% of patients and never as the sole site of disease.
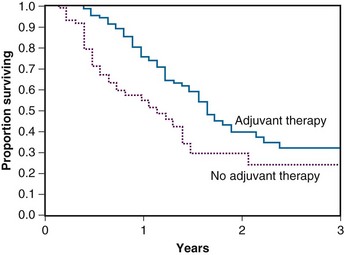
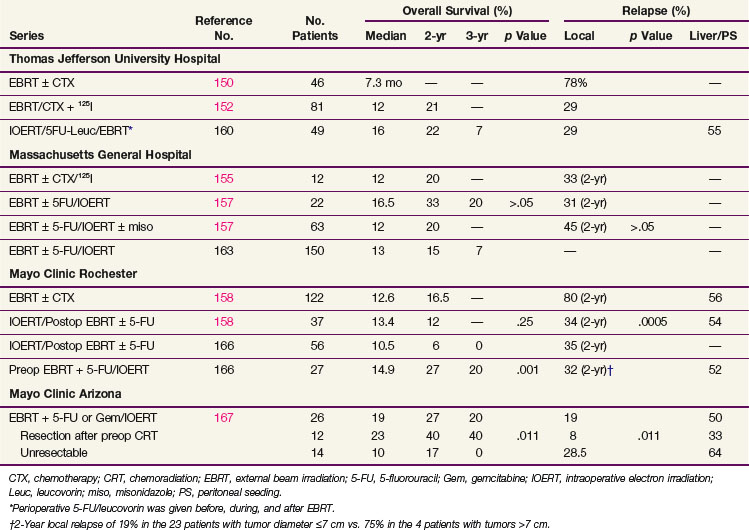
Foo and colleagues81 described the outcome of 29 patients treated with RT after curative surgery for pancreatic cancer at the Mayo Clinic between 1974 and 1986. Their data suggested a significant increase in survival when adjuvant chemoradiation was combined with surgery compared with surgery alone. Eighty-three percent of patients eventually experienced relapse. Among patients receiving chemotherapy and RT, the chemotherapy was 5-fluorouracil (5-FU) and the RT usually consisted of doses between 45 and 54 Gy. In contrast to the studies by Tepper and Griffin and their colleagues,47,48 as a result of adjuvant chemoradiation there was only a 7% rate of local failure. Liver failure and peritoneal seeding remained high at 43%, with 61% of patients having a component of either liver or peritoneal relapse.
Kayahara and associates49 reported on 45 patients undergoing curative resection of carcinoma of the head of pancreas between 1974 and 1991. Of these patients, 30 eventually experienced relapse. Documented at autopsy, patterns of failure included local recurrence in 80%, hepatic metastases in 66%, peritoneal dissemination in 53%, and lymph node relapse in 47%.
Finally, Willett and colleagues82 analyzed patterns of failure after pancreaticoduodenectomy for periampullary carcinoma in 41 patients (surgery alone in 29 patients; adjuvant postoperative RT in 12 patients). They observed excellent local control of 88% with T stages of in-situ disease, T1, or T2 stage compared with 44% for T3 or T4 local stages. Patients with lymph node positivity had a local control rate of 47%, whereas patients with negative lymph nodes had a local control rate of 87%. Moderately differentiated tumors had a local control rate of 81% as compared with patients with poorly differentiated tumors with a 0% 5-year local control rate (3 patients). In the 17 surgery-alone patients who had high-risk features, as defined by the authors (tumor invading the pancreas, poorly differentiated, involved nodes, and positive resection margins), the 5-year local control and OS rates were 50% and 38%, respectively. For the 12 high-risk patients who received postoperative irradiation alone or plus concurrent 5-FU there was a trend toward improvement in 5-year local control (83% vs. 50%) and 5-year OS (51% vs. 38%) rates, but this did not reach statistical significance in view of small patient numbers and the rate of distant metastasis.
Adjuvant Chemotherapy and Irradiation after Resection, Randomized Data
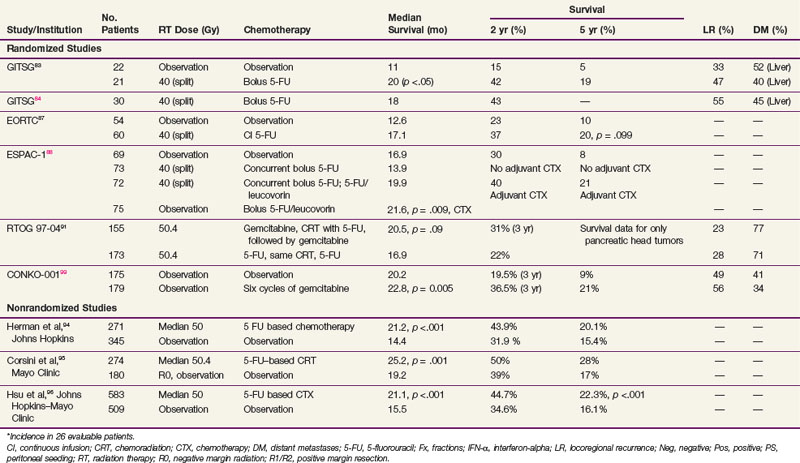
In 1974, the Gastrointestinal Tumor Study Group (GITSG) undertook a prospective, randomized trial of adjuvant 5-FU–based chemoradiation compared with no adjuvant treatment.83,84 This was a well-designed trial that attempted to recruit patients with histologically negative margins of resection between 4 and 10 weeks postoperatively. However, only 49 patients were randomized into the study over an 8-year interval; moreover, 5 withdrew without being treated. The external beam irradiation (EBRT) was given as two courses of 20 Gy in 10 fractions, each with a planned 2-week rest. Treatment fields did not exceed 20 × 20 cm and were given anterior-posterior/posterior-anterior. Shaping of fields was optional, and 5-FU chemotherapy was given on the first 3 days of each half of the RT at a dose of 500 mg/m2 as an IV bolus. Thirty-five percent of patients had tumor confined to the pancreas, 37% had contiguous invasion resected to clear margins, and 28% had nodal involvement.
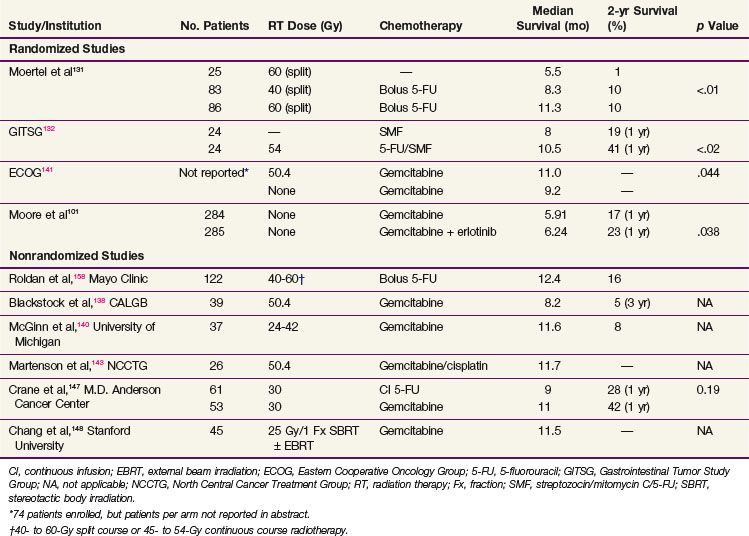
Results showed that patients treated with combined chemoradiation had improved survival (p = .03) compared with patients treated with surgery alone, with a median survival in the treatment group of 20 months versus 11 months in the control arm (Table 46-4). Two-year OS was 42% versus 15%, respectively, and 5-year OS was 19% versus 5%. Prognostic factors including age, sex, type of resection, degree of cellular differentiation, initial performance status, location of tumor, and extent of tumor were evaluated. Performance status and extent of tumor were the only independently significant prognostic variables. The study was terminated prematurely because of poor accrual in spite of the long period of time during which it was open.
TABLE 46-4 Adjuvant Radiation Therapy and Chemotherapy for Resected Ductal Adenocarcinoma of the Pancreas, Selected Series
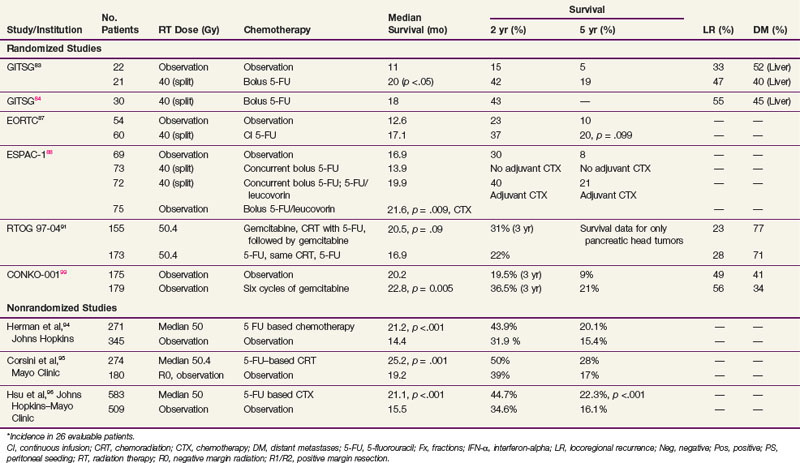
In an effort to address concerns due to poor accrual, the GITSG registered an additional 30 patients in a nonrandomized fashion to receive the same adjuvant therapy. Results in these 30 patients recapitulated the results of the treatment arm in the previous randomized study.84 It was of interest to note that, although the treatment produced relatively little toxicity, the local recurrence rate was between 30% and 50% in all patients regardless of whether they received adjuvant treatment and the incidence of hepatic metastases was between 40% and 50%. These results, although strongly suggesting a treatment effect, imply that the dose of radiation, coupled with the 2-week break, was too low to deal with the regional burden of disease and the efficacy of 5-FU alone was inadequate to sterilize disease outside the treatment fields for most patients.
The European Organization for Research and Treatment of Cancer (EORTC) performed a multi-institutional, randomized trial in which 218 patients with periampullary cancers, including pancreatic adenocarcinoma, were randomized to receive surgery alone or surgery plus postoperative 5-FU–based chemoradiation.85 The radiation dose and fractionation were the same as in the GITSG trial (40 Gy in split-course fashion over 6 weeks). There were two differences in the delivery of 5-FU compared with the GITSG study. Concurrent 5-FU during RT was administered by continuous intravenous infusion, rather than bolus, during the first 3 days of each treatment sequence. Furthermore, no 5-FU was given after the concurrent chemoradiation. In the subset of 114 patients with pancreatic adenocarcinoma, patients in the chemoradiation arm (n = 60) had a median survival of 17.1 months versus 12.6 months in the pancreatectomy alone arm (n = 54). The 2-year OS for the two trial arms were 37% versus 23%, respectively, and 5-year OS was 20% versus 10% (p = .099). There were no differences in locoregional recurrence rates, which were high with both surgery alone (37 of 103, 36% of patients at risk) and postoperative chemoradiation (34 of 104, 33% of patients at risk). The EORTC concluded that there was no demonstrable benefit from postoperative chemoradiation for the total group of patients but that survival trends favored adjuvant postoperative chemoradiation for the 114 patients with pancreatic cancer.
However, several aspects of the EORTC trial design, analysis, and interpretation warrant comment. Patients with T3 lesions were excluded, and the pathologic basis for distinguishing between and including pancreatic and nonpancreatic periampullary lesions (which have different natural histories and prognoses than do pancreatic adenocarcinomas) was not made clear. Additionally, the adequacy of the retroperitoneal margin was not independently assessed, perhaps influencing the frequency of locoregional recurrence. Moreover, 20% of patients assigned to the chemoradiation trial arm never received that treatment (because of patient refusal, poor postoperative performance status, disease progression, etc.) but they were analyzed by intent-to-treat principles. Although this approach is statistically correct, one needs to consider that the chemoradiation group includes a sizable minority of patients who did not receive postoperative chemoradiation. Furthermore, unlike the GITSG trial, no chemotherapy was given after chemoradiation. Moreover, many have criticized the use of a two-sided log-rank test, and demonstrated that if a one-sided test had been used, the survival benefit of chemoradiation would have reached significance.86 Finally, the study lacked sufficient patient numbers to exclude a clinically significant 10% to 15% benefit from the addition of adjuvant chemoradiation to surgery. Thus some investigators consider the EORTC trial to be an underpowered study that provides additional circumstantial evidence to support a role for postoperative chemoradiation. However, the trial was updated in 2007 and continued to show no statistical survival advantage after a median follow-up of 11.7 years.87 Although this trial is used as evidence against chemoradiation, it has several methodologic flaws, as just listed.
An important trial for radiation oncologists to be aware of is the ESPAC-1 study,88 which had a complex design (three separate but concurrent phase III trials) and was initiated to evaluate the independent effects of adjuvant chemotherapy and chemoradiation in 541 patients with grossly resected pancreas cancer. This included a subset of 285 patients who underwent randomization with a 2 × 2 factorial design as follows: (1) observation; (2) concomitant chemoradiation alone (20 Gy in 10 fractions over 2 weeks with 500 mg/m2 5-FU IV bolus during the first 3 days of RT and then repeated after a planned 2-week break) followed by no additional chemotherapy; (3) chemotherapy alone (leucovorin 20 mg/m2 bolus followed by 5-FU 425 mg/m2 administered for 5 consecutive days repeated every 28 days for six cycles); and (4) chemoradiation followed by six cycles of adjuvant 5-FU/leucovorin. Investigators were also allowed to randomize patients into two separate but concurrent trials testing one of the main treatment comparisons (adjuvant chemotherapy vs. none: 188 patients; adjuvant concurrent chemoradiation vs. none: 68 patients). Background therapy was allowed before patients were entered into the latter two trials, and RT was performed according to the standard of the individual institutions in all three trials. In the initial analysis, a survival advantage for adjuvant chemotherapy was achieved only by merging data from the three separate randomized trials (median survival of 19.7 months in the 238 patients randomized to receive adjuvant chemotherapy vs. median survival rate of 14 months in the 235 patients randomized to no adjuvant chemotherapy, p = .005).89 The latest analysis of the ESPAC-1 trial analyzed only the patients randomized to the 2 × 2 factorial design (n = 289; no background therapy allowed). Patients randomized to receive adjuvant chemotherapy (two of the four arms, n = 147) had a survival advantage (2-year, 40% vs. 30%; 5-year, 21% vs. 8%; p = .009).88 Local recurrence was a component of relapse in 99 patients (34% of the 289 patients at risk, 63% of patients with relapse).
As noted in 2001 by Abrams and associates,90 major concerns regarding the design and execution of this trial include more than one randomization scheme (three separate but concurrent phase III trials), patients were allowed to receive “background” therapy in addition to protocol therapy in two of the three trials, patients did not have their disease restaged after resection and before adjuvant therapy, and there were no quality assurance guidelines for the delivery of radiation (i.e., no section in the protocol on appropriate field design, no central audit of radiation fields), which is contrary to the routine in North American cooperative group trials. ESPAC-1 was a trial of varying patient populations, and the nature of the differences in the patients cannot be known. Thus the validity of this analysis should be regarded with concern.
RTOG 97-04, a randomized phase III trial,91 evaluated whether gemcitabine before and after 5-FU–based chemoradiation would provide superior outcome to 5-FU before and after 5-FU–based chemoradiation. In this study, 518 patients with resected pancreatic cancer were treated with postoperative 5-FU–based chemoradiation (50.4 Gy in 28 fractions over 5.5 weeks plus concurrent protracted venous infusion of 5-FU). Patients were randomized to receive either 5-FU (250 mg/m2/day) given as continuous infusion over 3 to 4 weeks or gemcitabine (1000 mg/m2/wk) given for 3 of 4 weeks. Chemotherapy was given for a total of three cycles: one before chemoradiation and two cycles after chemoradiation. On subset analysis of patients with pancreatic head tumors, median survival and 3-year OS were 20.5 vs. 16.9 months and 31% versus 22% for the gemcitabine and 5-FU trial arms, respectively. These results neared statistical significance overall (p = .09) and reached significance on multivariate analysis (p = .05). Although the grade 4 neutropenia rate was higher in the gemcitabine trial arm (14% vs. 1%), there was no difference in febrile neutropenia or in the ability to complete chemotherapy or RT.91
Retrospective and Prospective Adjuvant Chemoradiation Studies, Nonrandomized
The retrospective data of Whittington and colleagues92 lent credence to the need for combined modality adjuvant therapy. They reported on a series of 72 patients with pancreatic cancer managed surgically with curative intent between 1981 and 1989 at the Hospital of the University of Pennsylvania in three sequential treatment groups. The first group received surgery alone, while the second group received postoperative RT, with some of these patients receiving bolus 5-FU on the first 3 days of RT and at the beginning of the fifth week. The last 20 patients reported received RT with 96-hour infusions of 5-FU on the first and fifth weeks of treatment. They observed a decrease in local recurrence from 85% in the patients treated with surgery only to 25% in the patients treated with irradiation and infusional 5-FU (see Table 46-4). Inferior survival was noted with positive surgical margins. Among patients with negative resection margins, the 2-year survival was 41% for surgery alone, 33% for surgery plus RT with or without bolus 5-FU, and 59% for surgery plus RT with infusional 5-FU. More impressively, the 3-year survival was 22% for surgery alone, 11% for surgery plus RT with or without bolus 5-FU, and 47% for patients receiving postoperative RT with infusional 5-FU. Their work also emphasized the importance of nutritional support and the value of CT-based treatment planning.
The Johns Hopkins Hospital published results of a single-institution prospective but nonrandomized trial that was designed to evaluate survival benefit in patients with pancreatic cancer after surgical resection.93 This report, involving 174 patients, demonstrated that patients receiving GITSG-style chemoradiation with maintenance 5-FU truncated at 6 months (rather than 2 years) or a more intensive regimen (involving higher doses of irradiation as well as hepatic irradiation administered without interruption and with continuous infusion 5-FU chemotherapy augmented with leucovorin) did better than patients receiving no postsurgical therapy. The median survival for the more standard regimen was 21 months, with 1- and 2-year survivals of 80% and 44% (see Table 46-4). For the intensive regimen, the median survival was 17.5 months with 1- and 2-year survivals at 70% and 22%. For the control arm, the median survival was 13.5 months with survival at 1 and 2 years of 54% and 30% (Fig. 46-6). The intensive therapy had no survival advantage compared with the standard therapy group, but there was a statistically significant difference between the standard arm versus control (p <.0002). Multivariate analysis confirmed that prognostic factors for disease relapse included margin and lymph node status, tumor size
(see web-only Fig. 46-3 on the Expert Consult website![]() ), and degree of differentiation.
), and degree of differentiation.
Investigators at Johns Hopkins Hospital have updated their prospectively collected cohort of 616 patients who underwent resection of proximal pancreatic cancer between 1993 and 2005.94 The patients receiving chemoradiation were significantly younger and less likely to present with comorbid disease, but they were more likely to have positive margins. Chemoradiation patients had improved median, 2-year, and 5-year OS compared with patients with no chemoradiation (median 21.2 vs. 14.4 months, 2-year OS 44% vs. 20%, 5-year OS 32% vs. 15%; p <.001). Importantly, this survival advantage persisted even after controlling for age, medical comorbidities, and surgical complications.94
A similar retrospective analysis from Mayo Clinic Rochester of 472 patients with R0 resection treated over three decades demonstrated comparable findings.95 Despite having significantly more adverse prognostic factors, patients undergoing adjuvant postoperative chemoradiation had improved median, 2-year, and 5-year OS than patients having surgery alone (median 2.1 vs. 1.6 years; 2-year OS 50% vs. 29%, 5-year OS 28% vs. 17%, p = .001).
Subsequently a Johns Hopkins Hospital–Mayo Clinic collaborative analysis was performed in patients who had pancreaticoduodenectomy for pancreatic adenocarcinoma at Johns Hopkins Hospital and Mayo Clinic Rochester.96 The study consisted of 1092 patients treated with curative intent (n = 618 JHH, n = 474 Mayo), with 583 receiving adjuvant 5-FU–based chemoradiation (median RT dose 50.4 Gy). Propensity score and matched-pair analyses were performed to account for biases associated with nonrandom allocation of patients to adjuvant chemoradiation or surgery alone. Overall survival was significantly longer among patients who received adjuvant chemoradiation versus pancreaticoduodenectomy alone (median 21.1 vs. 15.5 months, p <.001; 2-year OS 44.7% vs. 34.6%, 5-year OS 22.3% vs. 16.1%, p <.001). In propensity score analysis, adjuvant CRT improved survival compared with surgery alone for all patients (relative risk [RR] = 0.67, p <.001) and stratified by age, margin, node, and T stage (RR = 0.57 to 0.75, p <.05), after adjusting for covariates. Matched-pair analysis demonstrated overall survival was longer with adjuvant chemoradiation versus pancreaticoduodenectomy alone (median 21.9 vs. 14.3 months, 2-year OS 45.5% vs. 31.4%, 5-year OS 25.4% vs. 12.2%, p <.001).96
Adjuvant Chemotherapy
First, a randomized trial in patients with symptomatic advanced pancreatic cancer (to be discussed later) showed improvement in symptoms and longer median survival in patients treated with gemcitabine versus those treated with 5-FU.97 As is often the case, success in the advanced setting led to trials exploring the role of gemcitabine in the adjuvant setting. In the CONKO-001 trial conducted in Europe, patients with postoperative CA19-9 values less than or equal to 2.5 times the upper limit of normal and with R0 or R1 resections for pancreatic cancer were randomized to six cycles of gemcitabine (1000 mg/m2 weekly, 3 of every 4 weeks) versus observation. At first publication, the use of gemcitabine significantly improved disease-free survival from 6.9 to 13.9 months (p <.001) and trended toward an improvement in overall survival.98 An update with longer follow-up showed significant improvement in median survival (22.8 vs. 20.2 months, p = .005), as well as improvements in 5-year overall survival (21.0% vs. 9.0%).99 These important results have solidified adjuvant gemcitabine as an important part of adjuvant treatment of pancreatic cancer. However, the lack of a chemoradiation trial arm means the value of RT (either concurrent or sequential) must still be elucidated. In addition the applicability of these results to patients with higher postoperative CA19-9 levels is uncertain.
There has been strong interest in finding a second drug to add to gemcitabine to enhance the benefit of chemotherapy. Unfortunately, in the context of metastatic and locally advanced disease, multiple “doublet” trials (gemcitabine + X) have not demonstrated superiority to gemcitabine alone when “X” has been 5-FU, cisplatin, oxaliplatin, irinotecan, exactecan, and capecitabine. A statistically significant, small improvement in median survival and 1-year survival has been seen with the combination of gemcitabine and erlotinib as compared with gemcitabine alone.100,101
Although gemcitabine is commonly held to be more effective in the adjuvant setting than 5-FU alone based on its superiority in the advanced stage setting, the largest trial comparing adjuvant 5-FU/leucovorin to adjuvant gemcitabine in resected pancreatic cancer shows no benefit. ESPAC-3 was a multicenter trial that randomized 1088 patients after R0 or R1 resections to either 5-FU/leucovorin or gemcitabine for 6 months.102 In this trial there was no difference in median survival for the 5-FU/leucovorin or gemcitabine groups (23.0 vs. 23.6 months, respectively, p = .39). There was also no significant difference in effect of treatment across subgroups according to extent of resection (R0 or R1).
Newer Chemoradiation Regimens
Interferon-Based Chemoradiation
The Virginia Mason Medical Center published their experience of 33 patients with resected pancreatic adenocarcinoma who received combined RT and chemotherapy.103 EBRT was given to a dose of 45 to 54 Gy with standard fractionation and chemotherapy (5-FU 200 mg/m2/day as continuous infusion, weekly cisplatin 30 mg/m2 IV bolus, and interferon-alpha 3 million units SQ every other day) started with RT. After combined modality chemoradiation, chemotherapy alone was administered (5-FU 200 mg/m2/day as continuous infusion) in two 6-week courses during weeks 9 to 14 and 17 to 22. Of note, 13 of 17 patients receiving interferon-based CRT had positive lymph nodes compared with only 7 of 16 patients randomized to the GITSG-based CRT. There were significant grade 3/4 gastrointestinal toxicities, including vomiting, mucositis, diarrhea, and gastrointestinal bleeding in the interferon-based chemotherapy group, requiring hospitalization in 35% of patients. However, the majority of patients were still able to receive more than 80% of the planned therapy. The median survival and 2-year overall survival were statistically superior in the patients treated with interferon-based chemoradiation than versus GITSG-based chemoradiation (24 vs. 18.5 months and 84% vs. 54%, p = .04). A follow-up study by the Virginia Mason Medical Center group included 53 patients with resected pancreas cancer treated with similar interferon-based chemoradiation. The clinical efficacy of this regimen remains encouraging, with median survival of 46 months and 2-year OS of 53%104 (see Table 46-4).
The American College of Surgeons Oncology Group (ACOSOG) initiated a multi-institutional phase II study of this interferon-based chemoradiation regimen in patients with pancreatic adenocarcinoma who had undergone resection. Patients received postoperative RT to 50.4 Gy with 1.8-Gy fractions given concurrently with 5-FU (175 mg/m2 continuous infusion days 1 to 38), cisplatin (30 mg/m2 IV weekly), and interferon-alpha (3 million units SQ every other day). This was followed by continuous infusion 5-FU for two cycles. The radiation fields in this study were designed to omit preoperative tumor bed coverage and included just the regions at highest risk for local recurrence, namely, the surgical margins, celiac axis, and superior mesenteric artery/uncinate regions. Some clinicians fear that omission of the preoperative tumor bed may decrease tumor control, because it is thought to be at high risk in many patients due to narrow vascular margins. Initial results showed promising 2-year OS and median survival (55% and 27.1 months), similar to those found in the initial publication by Picozzi.105 The study was closed early owing to greater than grade 3 acute toxicity rates that were higher than the original limits set. However, the greater than grade 3 acute toxicity rate was only slightly higher than that observed in the gemcitabine arm of RTOG 97-04. These results were also confirmed at Barnes Hospital and at Washington University in St. Louis (2-year OS 56% and median survival of 25 months).106
Gemcitabine-Based Chemoradiation
With the success of gemcitabine alone in the adjuvant setting, researchers have begun to explore gemcitabine-based chemoradiation. The motivation for these adjuvant studies comes both from laboratory observations demonstrating the radiosensitizing properties of gemcitabine in pancreatic carcinoma cells107 and from studies that have established that concurrent gemcitabine-based chemoradiation may be given in advanced pancreatic cancer with acceptable toxicity.108–110
Experience with gemcitabine-based chemoradiation in the adjuvant setting is increasing. The optimal method of combining irradiation with gemcitabine is still being studied, with investigators publishing the use of full-dose gemcitabine, twice-weekly low-dose gemcitabine, and reduced-dose weekly gemcitabine. Researchers from the University of Michigan111 performed a phase I dose escalation study with full-dose gemcitabine (1000 mg/m2) after curative intent surgery. To give full doses of this radiosensitizing drug with RT, treatment volumes were constrained by avoiding elective nodal areas. They found a maximal tolerated dose of 39 Gy given with 2.6-Gy fractions. Blackstock and colleagues112 used twice-weekly low-dose gemcitabine concurrent with upper abdominal RT including elective nodes to 50.4 Gy after resection. This regimen was well tolerated and shows promising activity compared with historic controls. Other series113 have demonstrated the feasibility of reduced-dose weekly gemcitabine (around 300 mg/m2) concurrent with RT in the adjuvant setting. Importantly, all of these experiences with adjuvant gemcitabine-based chemoradiation have shown feasibility with acceptable toxicity profiles and favorable results when compared with prior studies. Future study is needed to see if certain subsets are more likely to benefit from adjuvant chemoradiation as opposed to gemcitabine alone as used in CONKO-001.
The EORTC 40013 trial compared gemcitabine alone (as in CONKO-001) or followed by gemcitabine-based chemoradiation. In this phase II trial, 90 patients were randomized to receive either four cycles of gemcitabine (1000 mg/m2) alone or two cycles of gemcitabine at 1000 mg/m2, followed by weekly gemcitabine 300 mg/m2 with concurrent irradiation to 50.4 Gy in 28 fractions. The primary endpoints were completion of treatment and tolerability. The secondary endpoints were disease-free survival (DFS) and OS. Rates of distant progression were similar between the two trial arms, but the rate of first local recurrence was lower in the chemoradiation arm (11% vs. 24%, p = .16).114 The authors concluded that their trial demonstrated the feasibility and tolerability of gemcitabine-based chemoradiation and that a larger trial was necessary to assess the true benefit of gemcitabine chemoradiation over gemcitabine alone.
Present Status of Adjuvant Therapy for Pancreatic Cancer
A great deal remains unresolved regarding adjuvant therapy for pancreatic cancer after resection with curative intent. Clearly, surgery with curative intent does not accomplish cure with sufficient frequency to be satisfactory for patients, their families, or their physicians. The most convincing level I data currently available are from the CONKO-001 trial, which has clearly established a role for gemcitabine as an adjuvant after surgery. The CONKO-001 trial does not address whether RT has a role to play in this context. Each currently published phase III trial that has included RT is either subject to serious criticism (i.e., GITSG, EORTC, ESPAC-1) or has not directly addressed the question of what the role of RT ought to be in this context (i.e., RTOG 97-04). Current practice and current guidelines reflect this unresolved state of affairs. National Comprehensive Cancer Network guidelines for pancreatic cancer (available online at www.nccn.org) include chemotherapy (gemcitabine, capecitabine, 5-FU) or chemotherapy followed by chemoradiation. However, the highest recommendation goes to a clinical trial.
Designing randomized, phase III adjuvant therapy trials for pancreatic cancer that include RT has proven to be incredibly difficult. Large randomized trials require both consensus and equipoise around the question(s) asked. In addition, it is clear that there are potentially confounding variables other than the therapies in an adjuvant trial in this context. These potentially include the surgery done, numerous identified pathologic variables, and possibly the extent to which RT is designed and delivered as requested by protocol specification.115 Finally, the anticipated difference in therapeutic benefit must be sufficiently large to allow the power calculation to result in realistic and achievable accrual requirements.
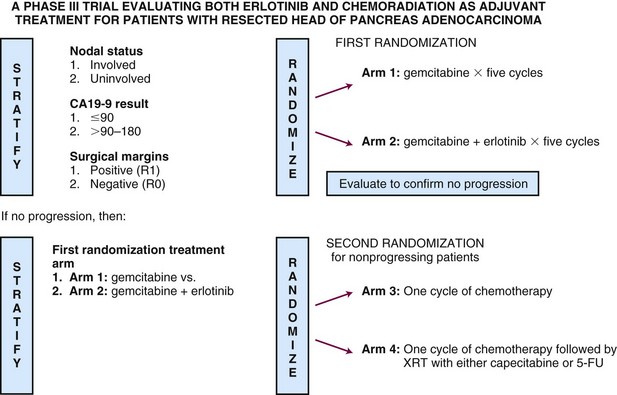
In late 2009, the RTOG in cooperation with the Southwest Oncology Group (SWOG) and the EORTC opened RTOG 08-48, a randomized phase III trial designed to ask two questions. The first is whether gemcitabine plus erlotinib produces superior survival results to gemcitabine alone. The second is whether the addition of RT in combination with 5-FU or capecitabine enhances survival for patients remaining disease free after chemotherapy. The trial stratifies for known prognostic variables, includes a surgical quality assurance component as well as real-time, online quality assurance to ensure RT guideline adherence, and will require the enrollment of more than 900 patients over 4 years to answer these two important questions.116 For trial schema, please see Figure 46-7.
Neoadjuvant Strategies
In the mid to late 1980s, a number of institutions began considering the use of RT or chemotherapy as a planned, preoperative approach (neoadjuvant) for patients with apparently resectable, biopsy-proven adenocarcinoma of the pancreas. Theoretical advantages of this sequencing include superior radiobiologic effect with improved blood flow, avoiding omission of chemoradiation secondary to postoperative recovery, and allowing patients destined to fail treatment early systemically to declare themselves to avert futile surgery. In the early to mid 1990s, these results began to mature and a number of authors have described results in this context. Ishikawa and colleagues117 reported on 54 consecutive patients, with tumors judged to be resectable, who either went directly to surgery (n = 31) or received 50 Gy EBRT to the region of the pancreatic head (n = 23). They found no difference in resectability, 3-year OS, or 5-year OS. There was a slight advantage to 1-year OS for patients treated with preoperative RT. There was a decrease in the incidence of regional relapse during the first 1.5 postoperative years for patients undergoing preoperative RT.
Investigators at the Fox Chase Cancer Center in Philadelphia have also published a series of papers regarding neoadjuvant chemoradiation for periampullary malignancy, most recently in 2004. Their approach originally consisted of 50.4 Gy conventionally fractionated with 4-day infusions of 5-FU at 1000 mg/m2/day and bolus infusion of mitomycin C, 10 mg/m2 on the second day. In a report published in 1993, they described their initial experience with 31 patients, of whom 24 had carcinoma of the head of the pancreas, 2 had carcinoma of the body of the pancreas, and 5 had carcinoma of the duodenum. They found the toxicity to be acceptable, with one death from biliary sepsis and a resectability rate of 38% for pancreatic carcinoma.118 At first update, this group reported on 34 patients with localized pancreatic cancer receiving the same treatment as already described. Twenty-five of these patients underwent laparotomy, and 14 were deemed to have unresectable disease owing to the extent of the primary tumor, hepatic metastases, or peritoneal dissemination. Therefore only 11 underwent surgery with curative intent, with 1 patient dying in the perioperative period. With a median follow-up of 33 months, the median survival was 45 months and median DFS was 27 months in patients who underwent potentially curative resection. In 1998, these workers reported on a comparison of their preoperative studies to 23 patients who were accrued sequentially after closure of their preoperative protocol.119 These patients were treated with postoperative chemoradiation, using the same irradiation and chemotherapy dosing and scheduling as the preoperative regimen. These researchers found that there was no difference in intraoperative management or length of postoperative stay. With preoperative therapy, there were fewer involved nodes and more patients with negative resection margins. After a median follow-up of 44 months, median survival was 20 months versus 25 months in the preoperative group compared with the postoperative group, respectively. Local failure either alone or as a component of overall failure was the same in both groups at 16%. It was concluded that both treatments were similar with respect to toxicity and benefit, but 22% of patients did not receive their intended postoperative therapy. Most recently, in 2004, this group updated their results from 1986 through 2003, an experience including 152 patients, with more than 60 more recent patients treated with gemcitabine-based preoperative treatment. Overall, in their retrospective analysis they did not find improvement in outcomes with the use of gemcitabine instead of 5-FU and they did not find improved median overall survival or disease-free survival in patients treated preoperatively versus those treated postoperatively. However, the patients treated preoperatively tended to have more advanced tumors than those treated postoperatively and to be more marginal surgical candidates to begin with, so the similar overall survival and disease-free survival may still favor the use of preoperative treatment in certain subsets of patients.120 Of note, the Eastern Cooperative Oncology Group (ECOG) experience with 41 patients treated with a similar approach as the Fox Chase Cancer Center were not encouraging, with only 24 of 53 patients undergoing curative resection. Among these patients who underwent resection, median survival was 15.7 months.121
Investigators have also used RT concurrent with gemcitabine neoadjuvantly for resectable pancreatic cancer. Talamonti and associates122 reported on 20 patients with potentially resectable pancreatic cancer treated neoadjuvantly with full-dose gemcitabine (1000 mg/m2 IV) concurrent with RT during the second cycle (36 Gy in 2.4-Gy fractions). Because of the high doses of concurrent gemcitabine, RT was given without prophylactic nodal irradiation. Of the 20 patients, 95% completed treatment without interruption. Radiographically, 80% of patients had stable disease, 15% had partial response, and 5% had progressive disease. Seventeen (85%) patients underwent curative resection with a 94% rate of R0 resection and a 65% rate of negative lymph nodes. Preliminary survival data with median follow-up of 18 months were promising in resected patients (median survival 26 months, 2-year OS 61%), with 41% of the patients alive with no evidence of disease. In 2008, researchers at the M.D. Anderson Cancer Center also reported on a slightly different gemcitabine-based preoperative chemoradiation, with 7 weekly doses (400 mg/m2) concurrent with RT to 30 Gy in 10 fractions, including prophylactic nodal irradiation.123 Of the 86 patients enrolled, 13 did not undergo surgery because of progressive disease or worsening performance status and 9 who did have surgery did not undergo resection because of extrapancreatic disease. Overall, 64 of 86 patients underwent resection (74%), and these patients had excellent survival numbers (median survival, 34 months; 5-year OS, 36%). Promising early survival results were also found by Small and associates124 in patients with initially resectable pancreatic cancer treated with three cycles of full-dose gemcitabine concurrent with irradiation to 36 Gy in 2.4-Gy fractions (without prophylactic nodal radiation).
Neoadjuvant Therapy for Borderline Resectable Disease
Much of the recent data on neoadjuvant therapy has focused on tumors defined as borderline resectable. This definition is controversial, but a working definition includes tumors at high risk postoperatively for having microscopic residual disease, especially at the uncinate or superior mesenteric artery margins. Positive margins after surgery have been associated with significantly worse survival, very similar to that of patients with inoperable disease, as discussed earlier.125 To achieve a higher probability of margin-negative (R0) resection in this patient subset, neoadjuvant therapy has been incorporated into some treatment paradigms.
Researchers at M.D. Anderson Cancer Center have proposed objective CT-based anatomic criteria to classify borderline resectable disease; the adoption of such a standardized definition is vital to studying this group of patients (Table 46-5). A large series of their patients with borderline-resectable disease treated with neoadjuvant therapy was published in 2008 and reported that 56% of surgery specimens had less than 50% viable tumor cells.126 Ammori and associates127 also found a high degree of therapy-related changes in more than 60% of patients treated neoadjuvantly. The M.D. Anderson Cancer Center series found that 41% of patients treated with neoadjuvant therapy ultimately underwent resection with grossly negative margins, and 94% of these were confirmed with negative margins on pathology. Median survival was 40 months for the patients undergoing resection, compared with 13 months to those not receiving surgery (p <.001).126 Similar results from the Fox Chase Cancer Center showed that patients who underwent neoadjuvant therapy followed by surgery had an 85% rate of margin-negative resections.128 These data suggest that neoadjuvant therapy for borderline-resectable pancreatic cancer may improve the likelihood of an R0 resection, but randomized trials are needed to formally test this approach.
TABLE 46-5 Commonly Used Guidelines for Resectability in Pancreatic Cancer
| Borderline Resectable | Unresectable |
|---|---|
GDA, gastroduodenal artery; IVC, inferior vena cava; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Locally Advanced (Unresectable) Disease And Palliation
Palliative Surgery
Classically, a discussion of palliative surgery for periampullary carcinoma would imply hepaticojejunostomy, gastrojejunostomy, or both, to relieve biliary obstruction and gastric outlet obstruction. As summarized by Lillemoe and colleagues,129 this type of operation can be performed with an in-hospital mortality rate of 2.5%, postoperative non–life-threatening morbidity of 37%, and a mean survival of 7.7 months. Several groups have looked at the role of palliative pancreaticoduodenectomy, that is, pancreaticoduodenectomy performed despite the surgeons’ intraoperative realization that the final margins of resection are likely to be visibly positive. Lillemoe and colleagues129 looked at this issue in 64 consecutive patients undergoing surgery between 1986 and 1994 and compared the outcomes to 62 consecutive patients undergoing standard surgical palliation between 1986 and 1991. None of these patients had evidence of hepatic, serosal, or peritoneal dissemination. Patients undergoing palliative pancreaticoduodenectomy fared significantly better than patients undergoing palliative bypass (p <.02) with a 24-month survival of approximately 30% in the pancreaticoduodenectomy patients versus less than 10% in the palliative bypass patients. However, the patients undergoing palliative pancreaticoduodenectomy who received postoperative chemoradiation survived significantly longer than patients undergoing the same operation without adjuvant chemoradiation. Similarly, patients undergoing bypass therapy who received postoperative RT survived significantly longer than patients undergoing bypass therapy without chemoradiation. Similar results have been described by Ouchi and associates.130
Chemoradiation for Locoregional Unresectable Disease
For locoregionally unresectable disease, the GITSG has demonstrated that split-course RT to a total dose of 40 to 60 Gy with concurrent 5-FU bolus was superior to RT alone at a dose of 60 Gy (median survival rate from diagnosis at exploratory laparotomy of 5.5 months [60 Gy alone] vs. 8.3 months [40 Gy + 5-FU] and 11.3 months [60 Gy + 5-FU])131 (Table 46-6). Subsequently, the GITSG demonstrated that combination chemotherapy with an SMF regimen (streptozocin, mitomycin C, 5-FU) produced a significantly inferior survival result for patients with unresectable disease than 5-FU chemoradiation to 54 Gy followed by SMF chemotherapy (median survival 42 vs. 32 weeks; 1-year OS 41% vs. 19%, 2-year OS 18% vs. 0%; advantage to chemoradiation vs. chemotherapy alone at all time points)132 (see Table 46-6).
The ability to give combined chemoradiation, with either continuous or split-course EBRT, to doses between 40 and 60 Gy combined with 5-FU, 5-FU/leucovorin, 5-FU/mitomycin C, and 5-FU/cisplatin, with definite but acceptable toxicity has also been confirmed.133–135,136 Conversion to operability was noted to occur rarely (10%), with pain relief occurring in the majority of patients. Treatment toxicity is related to treatment volume, total EBRT dose, EBRT fraction size, and the use or absence of a planned split, as well as to the intensity of the chemotherapy dosing. In patients with adequate nutrition and with hepatic and gastric obstruction absent or surgically relieved, toxicities, such as nausea and mucositis, are generally readily manageable.
As previously discussed, there has been considerable interest in combining EBRT with gemcitabine owing to its potent radiosensitizing properties. Blackstock and colleagues137 examined, in a phase I study, gemcitabine (starting at 20 mg/m2) twice weekly in combination with EBRT (total dose 50.4 Gy in 1.8-Gy fractions) in 19 patients with locally advanced pancreatic adenocarcinoma. Thrombocytopenia, neutropenia, and nausea/vomiting were dose-limiting toxicities. Of the 15 patients assessable for response, partial responses were identified in 3. A dose of 40 mg/m2 twice weekly in combination with RT to a total dose of 50.4 Gy was subsequently examined by the Cancer and Leukemia Group B (CALGB)138 in a phase II study of 39 patients with locally advanced pancreatic cancer. After chemoradiation, patients without disease progression received gemcitabine alone 1000 mg/m2 weekly × 3 every 4 weeks for five additional cycles. Grade 3/4 hematologic toxicity was significant and identified in 69% of patients. In addition, grade 3/4 gastrointestinal toxicity was identified in 41% of patients. The median survival was 8.2 months (Table 46-6).
The M.D. Anderson Cancer Center has published a corollary phase I study of 18 patients with locally advanced disease using rapid fractionation EBRT. Patients received dose-escalating gemcitabine from 350 mg/m2 to 500 mg/m2 weekly × 7 with concurrent rapid fractionation 3000 cGy EBRT during the first 2 weeks of therapy.139 Hematologic and nonhematologic toxicities were significant in all three patient cohorts. There were eight responses (four minor and four partial). One of two patients who subsequently underwent surgical exploration had a curative resection. The recommended phase II testing dose of gemcitabine was 350 mg/m2.
The University of Michigan140 has described an alternative approach using standard doses of gemcitabine at 1000 mg/m2 weekly × 3 every 4 weeks and administering EBRT as dose escalation beginning at 24 Gy (1.6 Gy in 15 fractions) in 37 patients with locally advanced disease. The majority of patients received additional chemotherapy after chemoradiation at the discretion of the treating physician. Seventy-five percent of patients received at least 85% of planned gemcitabine. Two of six assessable patients experienced dose-limiting toxicity at the final planned radiation dose of 42 Gy in 2.8-Gy fractions. An additional two patients developed late gastrointestinal toxicities at this dose level. Six patients were documented to have a partial response with a complete radiographic response in two patients. In addition, four patients with documented stable disease at the time of initial post-therapy evaluation experienced objective responses (two partial and two complete responses) with additional chemotherapy. Definitive resection was achieved in one of three patients who underwent surgical exploration. With a median follow-up of 22 months, median survival for the entire group was 11.6 months (see Table 46-6).
In a subsequent phase II multi-institutional trial by Small and colleagues, patients were treated with three cycles of full-dose gemcitabine, with concurrent EBRT during the second cycle to 36 Gy in 2.4-Gy fractions. Of the 14 patients with unresectable disease at presentation, 1 ultimately underwent resection and 1-year OS was 47%. This report was one of the first multi-institutional trials showing the safety and efficacy of full-dose gemcitabine with full-dose irradiation in patients with unresectable pancreatic cancer.124
An ECOG phase III trial attempted to assess the benefit of RT combined with gemcitabine in the unresectable pancreatic cancer population but was closed early because of poor accrual (only 74 patients). Although only published in abstract form, adding 50.4 Gy of RT to weekly gemcitabine significantly improved median survival compared with weekly gemcitabine alone (11.0 vs. 9.2 months, p = .044). However, grade IV toxicity was also significantly higher in the chemoradiation trial arm.141
(See additional details at the Expert Consult website. ![]() )
)
The ECOG published a phase I study of 7 patients with locally advanced disease using combination chemotherapy consisting of EBRT to a maximum 59.4 Gy in 1.8-Gy fractions. 5-FU (200 mg/m2/day as continuous infusion [CI] throughout RT) was administered with weekly gemcitabine dose escalation beginning at 100 mg/m2. The study was closed early due to dose-limiting toxicities, even with lowered doses of gemcitabine.142
Gemcitabine has also been combined with cisplatin and RT in published phase I trials, following up on promising preclinical synergistic data. A study based at the Mayo Clinic gave twice-weekly gemcitabine and cisplatin for 3 weeks during EBRT (50.4 Gy in 28 fractions) (see Table 46-6). Dose-limiting toxicities consisted of grade IV nausea and vomiting. The recommended phase II dose was gemcitabine 30 mg/m2 and cisplatin 10 mg/m2.143 Another trial used strictly time-scheduled gemcitabine (dosing on days 2, 5, 26, and 33 after a weekly regimen was too toxic) and cisplatin (days 1 to 5 and 29 to 33) combined with RT, with a recommended phase II dose of 20 mg/m2 for cisplatin and 300 mg/m2 for gemcitabine.144 The response to chemoradiation allowed 10 of 30 patient with initially unresectable disease to undergo surgery, with an R0 resection in nine cases and a complete response in two cases.
Because the ESPAC-3 study showed no difference in OS or DFS in patients treated adjuvantly with 5-FU/leucovorin versus gemcitabine, the question of which agent to use in the locally advanced setting is debatable. The M.D. Anderson Cancer Center145 retrospectively examined its database of 114 patients with locally advanced disease treated with combination EBRT (rapid-fractionation 30 Gy in 10 fractions) with either concurrent continuous infusion 5-FU 200 to 300 mg/m2 (61 patients) or gemcitabine 250 to 500 mg/m2 weekly for 7 weeks (53 patients). Patients receiving gemcitabine developed significantly higher incidences of severe acute toxicity, defined as toxicity requiring a hospital stay of more than 5 days, mucosal ulceration with bleeding, more than three-dose deletions of gemcitabine, or toxicity resulting in surgical intervention or death compared with those patients receiving 5-FU (23% vs. 2%, p <.0001). Five of 53 patients treated with gemcitabine-based chemoradiation subsequently underwent surgical resection compared with 1 of 61 patients treated with 5-FU–based chemoradiation. However, with short median follow-up, median survival was similar (11 vs. 9 months, p = .19) (see Table 46-6).
Another agent being tested in unresectable pancreatic cancer is bevacizumab (Avastin), an anti–vascular endothelial growth factor (VEGF) inhibitor. It is hypothesized that anti-VEGF inhibitors enhance radiation sensitivity because VEGF, which is induced by RT, appears to be cytoprotective.146 Early reports combining bevacizumab with RT in unresectable pancreatic cancer showed an alarming rate of duodenal bleeding at the primary site, but this was thought to be because tumors involving the duodenum at presentation were included.147 RTOG 04-11, which tested RT concurrent with bevacizumab and capecitabine followed by gemcitabine and bevacizumab in unresectable pancreatic cancer, excluded tumors with duodenal invasion. None of the 82 patients tested with the regimen experienced duodenal bleeding. Although the survival was not improved compared with historic controls, this phase II trial showed the tolerability of bevacizumab concurrent with RT for locally advanced pancreatic cancer.147
Finally, a recent area of study incorporates single-fraction stereotactic body radiation therapy (SBRT) for locally unresectable pancreatic cancer. A report from Stanford University in 2009148 describes treating gross tumor plus a margin of 2 to 3 mm to 25 Gy in a single fraction for a heterogeneous population of patients (81% locally advanced, 19% metastatic). Most patients (96%) were treated sequentially with a gemcitabine-based chemotherapy regimen. With a median follow-up of 6 months, the overall rate of freedom from local progression at 6 and 12 months was 91% and 84%, respectively. The 12-month actuarial rate of grade 2 or greater late toxicity (mostly intestinal ulceration) was 25%, and the median survival from time of SBRT for the locally advanced patients was only 6.4 months. Although this early experience with SBRT shows potential, the survival achieved from diagnosis (11.5 months) was similar to both historical and contemporary treatment and the retrospective assessment of toxicity may underestimate the true rate of serious side effects.149 Further study is required to evaluate this technique.
Chemotherapy
Because the benefit of chemoradiation is relatively modest, some oncologists recommend chemotherapy alone for locally advanced disease. Gemcitabine is the most commonly used agent, extrapolating from the metastatic disease setting. This is based on the randomized trial by Burris and colleagues,97 in which 26% of the study subjects had locally advanced disease, and showed that gemcitabine ameliorated symptoms and modestly improved survival compared with 5-FU. The results for patients with locally advanced disease were not reported separately. As discussed earlier, an ECOG phase III trial (E4201) comparing gemcitabine/irradiation versus gemcitabine alone opened in April 2003 to examine this issue and closed prematurely for inadequate accrual in December 2005 with 74 patients enrolled.141 Results were analyzed and presented in abstract form in 2008, showing that patients in the chemoradiation trial arm had a median survival of 11.0 months compared with 9.2 months with gemcitabine alone (p = .044, two-sided log-rank).
As mentioned in the prior adjuvant section, there has been considerable interest in finding a second drug to add to gemcitabine to enhance chemotherapy benefit, with little success. Agents that have been tested in the metastatic or locally advanced setting include 5-FU, cisplatin, oxaliplatin, irinotecan, exactecan, and capecitabine. Unfortunately, these trials have not shown survival advantages, with the exception being a statistically significant, small improvement in median survival and 1-year OS seen with the combination of gemcitabine and erlotinib as compared with gemcitabine alone.100,101
Erlotinib added to gemcitabine was tested in advanced pancreatic cancer by Moore and associates101 in a large randomized phase III trial of 569 patients of whom about one fourth (24%) had locally advanced disease. Subgroup analysis did not suggest a greater effect in patients with locally advanced disease than in those with metastatic disease. With the use of an intent-to-treat analysis, survival was improved with the addition of erlotinib, with a hazard ratio of .82 (median survival 6.24 vs. 5.91 months; p = .038). Progression-free survival and 1-year OS were also significantly improved with the addition of erlotinib. Significantly more patients with erlotinib had adverse reactions, but the majority were grade 1 and 2. Further study with this agent coupled with gemcitabine is recommended to assess its value.
Intraoperative Irradiation or Brachytherapy
Rationale
For unresectable lesions, as discussed previously, the use of EBRT plus concurrent 5-FU or gemcitabine-based chemotherapy results in a doubling of median survival when compared with surgical bypass or stents alone (3 to 6 months median survival vs. 9 to 13 months) and an increase in 2-year survival from 0% to 5% to 10% to 20%. However, 5-year survival is rare and local control is low. In a series from Thomas Jefferson University Hospital, with EBRT doses of 60 to 70 Gy in 1.8- to 2.0-Gy fractions over 7 to 8 weeks, local control was achieved in less than 20% of patients treated with EBRT alone.150,151 With EBRT plus chemotherapy, local control was achieved in approximately 30% of patients.
Outcomes: Brachytherapy or IORT ± EBRT
The combination of EBRT plus brachytherapy or intraoperative electron irradiation (IOERT) has resulted in an improvement in local control in series from Thomas Jefferson University Hospital, Massachusetts General Hospital, and the Mayo Clinic* (Table 46-7). This has not, however, translated into major improvements in either median or 2-year OS in view of high rates of abdominal relapse (liver, peritoneal). The delivery of EBRT plus concurrent chemotherapy before restaging and laparotomy plus IOERT or brachytherapy or resection plus IOERT translates into improved patient selection and some improvement in median and 2-year OS.166,167,168 Improvements in systemic agents will likely be necessary for the local control benefits of IORT to have survival implications.
An expanded discussion of results in brachytherapy and IOERT series is found
on the Expert Consult website. ![]()
Brachytherapy Series
Mohiuddin and colleagues152 reported on 81 patients with localized unresectable carcinoma of the pancreas managed at Thomas Jefferson University Hospital using intraoperative iodine-125 (125I) implants, EBRT, and perioperative systemic chemotherapy. The 125I implant was designed to deliver a minimum peripheral dose up to 120 Gy over 1 year. Patients were also treated with 50 to 55 Gy of EBRT with systemic chemotherapy consisting of 5-FU, mitomycin, and occasionally lomustine. Implants were performed at laparotomy. There was a 5% mortality rate, a 34% acute morbidity rate with cholangitis, upper gastrointestinal bleeding, and gastric outlet obstruction being the most common. In addition, there was a 32% late morbidity rate with gastrointestinal bleeding, cholangitis, and radiation enteritis being the most common late developments. Local control was obtained in 39 of 53 (71%) evaluable patients. Of 14 patients undergoing re-exploration more than 6 months after implantation, 86% showed extensive fibrosis and had negative biopsies from the region of the tumor. In 8 patients undergoing autopsy, 5 (63%) were without evidence of locoregional tumor. Nevertheless, 52 of these 81 patients (62%) failed treatment and had intra-abdominal disease (primarily hepatic and peritoneal). With a minimum follow-up of 2 years, the median survival for the total group was 12 months, with 2- and 5-year OS of 21% and 7%, respectively. For node-negative patients, the median survival was 13 months and the 2- and 5-year OS were 27% and 8%, respectively. For patients with histologically involved lymph nodes, the median survival was 9 months, with 2- and 5-year OS of 13% and 3%, respectively. Despite satisfactory local control in several patients, many centers would not be willing to accept this level of therapeutic intensity in a group of patients for whom management is ultimately primarily noncurative.
Nori and associates153 reported on a series of 15 patients undergoing similar management but using palladium-103 (103Pd) instead of 125I. The implant was designed to provide a matched peripheral dose of 110 Gy. Patients also received EBRT of 4500 cGy over  weeks and chemotherapy of 5-FU and mitomycin C. Median survival was 10 months, and the authors concluded that 103Pd is an alternative to 125I for interstitial brachytherapy for patients with unresectable disease and that symptom relief appeared to occur somewhat faster. The study did not show any improvement in the median survival compared with 125I.
weeks and chemotherapy of 5-FU and mitomycin C. Median survival was 10 months, and the authors concluded that 103Pd is an alternative to 125I for interstitial brachytherapy for patients with unresectable disease and that symptom relief appeared to occur somewhat faster. The study did not show any improvement in the median survival compared with 125I.
A note of caution was raised by Raben and colleagues154 on the use of palladium brachytherapy for locally unresectable carcinoma of the pancreas. In their series of 11 patients, they found an unacceptably high complication rate including gastric outlet obstruction, duodenal perforation, and sepsis. They did not find an improvement in median survival over other modalities and did not recommend this approach for further study.
IOERT plus EBRT
IORT with single-fraction electrons IOERT has been extensively studied.155–169 In experienced hands, IOERT can be given with acceptable morbidity. However, there are occasional reports of high complication rates.
Generally, IOERT has been given in combination with EBRT in the range of 45 to 50.4 Gy in 25 to 28 fractions with 5-FU alone or 5-FU–based combination chemotherapy. The dose of IOERT is generally in the range of 10 to 20 Gy (usually 20 Gy for patients with locally unresectable cancers; for those with resection before or after chemoradiation, the IOERT dose varies from 10 to 12.5 Gy).161,162,169
The RTOG reported on 51 patients with IOERT for locally unresectable pancreatic cancer. In this multi-institution phase II trial,159 there was a major postoperative complication rate of 12% and 2 patients had major morbidity that resulted in death. There was no obvious improvement in median survival.
The combination of EBRT (±5-FU) plus IOERT has resulted in an improvement in local control in series from both the Massachusetts General Hospital (MGH) and the Mayo Clinic, but this has not translated into an improvement in either median or 5-year survival* (see Table 46-7). In a Mayo Clinic analysis of 159 patients by Roldan and co-workers,158 the local control rate at 1 year was 82% for EBRT plus IOERT (n = 37 patients) versus 48% for EBRT alone (n = 122 patients) and 2-year local control was 66% versus 20% (p = .0005). Median and 2-year survival were similar, however, with median SR of 13.4 versus 12.4 months (IOERT vs. none) and 2-year OS of 12% versus 16.5%. The lack of survival improvement was related to a high incidence of abdominal relapse in both groups of patients (IOERT: 54% with liver or peritoneal metastases vs. 56% in non-IORT patients).
In the most recent update of results from study at the MGH,163 150 patients with locally unresectable pancreas cancer received IOERT as a component of treatment from 1978 to 2001 in conjunction with EBRT and 5-FU–based chemotherapy. Long-term survival was seen in 8 patients, and 5 were alive at or beyond the 5-year interval (see Table 46-7). Actuarial 1-, 2-, 3-, and 5-year OS for the 150 patients was 54%, 15%, 7%, and 4%, respectively, and median survival was 13 months. Survival was significantly related to the diameter of the IOERT treatment applicator (surrogate for tumor size). In the 26 patients treated with a 5- or 6-cm applicator, 2- and 3-year OS were 27% and 17%; none of 11 patients treated with a 9-cm diameter applicator survived beyond 18 months, and those treated with a 7- or 8-cm applicator had intermediate survival (p <.05).
A publication from Thomas Jefferson University Hospital (TJUH) in 2009 updated their significant IOERT experience.164 They compared 36 patients treated with pancreaticoduodenectomy and IORT with 46 patients who underwent surgery without IOERT using a propensity score analysis, which is a statistical method used to adjust for nonrandom treatment decisions in observational studies. Around 75% of the IOERT patients also received EBRT, and almost 90% received chemotherapy. Although they found that IOERT safely could be added to management approaches for resectable pancreatic cancer without increasing perioperative morbidity, it did not seem to increase OS. Locoregional control trended to being improved in the IOERT group (39% vs. 23%), but this did not reach statistical significance.
The M.D. Anderson Cancer Center experience also showed a numerical improvement that was not statistically significant in locoregional control in patients treated with IORT (9% vs. 4%).165 It is likely that these rates of recurrence are lower than with the TJUH experience because fewer of these patients had positive margins. The authors suggested that a local control advantage large enough to cause a survival benefit would only be possible in a group with very high risk of locoregional recurrence, such as those with R1 or R2 resections. Improvements in systemic agents may also be necessary for the possible local control benefits of IORT to have survival implications.
Preoperative Chemoradiation plus IOERT
In an attempt to improve patient selection and survival, a number of institutions have altered the sequencing of IORT and EBRT. Garton and colleagues166 from the Mayo Clinic Rochester used EBRT alone or plus concurrent 5-FU preoperatively in 27 patients with localized, unresectable disease and then delivered IORT. Median survival from diagnosis was 14.9 months with 2-year OS of 27% and 5-year OS of 7% (see Table 46-7). Local control was achieved in 78% of patients, but 52% developed liver or peritoneal relapse.
Investigators from Mayo Clinic in Arizona have used only the sequence of preoperative chemoradiation followed by restaging and surgical exploration with resection/IOERT, as indicated, for select patients with borderline resectable or unresectable pancreas cancer.167 A series of 26 patients with no prior treatment have received IOERT after preoperative chemoradiation; resection was performed in 12 of 26 patients before IOERT (R0 or R1, 9; R2, 3). Median survival for the total group was 19 months, with 2-year OS of 27% and 3-year OS of 20% (see Table 46-7). Survival outcomes appeared to be improved in patients with resection after preoperative chemoradiation versus those without resection (median 23 vs. 10 months; 2-year OS 40% vs. 17%; 3-year OS, 40% vs. 0%; p = .011, log-rank). Liver or peritoneal relapse has been documented in 13 of 26 patients (50%).
A pooled analysis of 270 patients from five European Institutions was presented at the 2008 meeting of the International Society for Intraoperative Radiation Therapy (ISIORT) by Valentini and colleagues.168 Radical surgery was performed in 247 cases (91.5%; R0 resection, 53.4%; R1, 27.4%; R2, 19.2%) and exploratory laparotomy in 8.5%. Surgery was preceded by EBRT in 63 patients (concurrent chemoradiation in 38%) and 106 received postoperative ERBT (concurrent chemoradiation in only 7.5%). Median survival was 19 months for the total group of patients, and 5-year OS was 17.7%. Survival and local control appeared better in patients treated with preoperative EBRT/CRT compared with postoperative EBRT (or chemoradiation) or IORT alone (median OS of 30 months vs. 22 months was 13 months; local control: median not reached with preoperative EBRT/CRT group vs. median 28 months with postoperative EBRT/CRT and median 8 months with IORT alone) (see Table 46-7). The authors believed that this may be because those patients destined to fail early systemically would do so during preoperative EBRT, sparing them from the attempt at surgery and IORT. On multivariate analysis, nodal status and timing of EBRT significantly affected survival. In the subset of patients who remained free from local relapse for more than 2 years, the 3- and 5-year OS were 32% and 28% versus 12% and 0% in patients with local relapse within 2 years.
Care delivered in the context of locoregionally unresectable, periampullary pancreatic cancer is expected to be palliative. As such, one of the treatment options available includes best supportive care without specific antineoplastic intervention. The supportive care needs of patients with locoregionally advanced, periampullary pancreatic cancer can be substantial (see web-only Table 46-1 available on the Expert Consult website![]() ). Attention to the management of these significant medical issues forms the foundation of excellent palliation and care, regardless of whether intervention with antineoplastic therapy (chemotherapy with or without RT) is selected. Moreover, adequate attention to the medical optimization of these needs before and during antineoplastic therapy is essential for optimal intervention and opportunity for benefit. Weight loss correlates closely with the inability to tolerate full-dose chemotherapy, and patients are not well served when they receive systemic chemotherapy with unrelieved obstructive jaundice.170 Similarly, patients with incipient or fully expressed gastric outlet obstruction have difficulty tolerating intervention with irradiation to the upper abdomen because this is often associated with severe nausea, vomiting, and anorexia. Especially during the early phase of patient presentation and management, “best supportive care” should not be a euphemism for a passive medical stance of minimal involvement and attention.
). Attention to the management of these significant medical issues forms the foundation of excellent palliation and care, regardless of whether intervention with antineoplastic therapy (chemotherapy with or without RT) is selected. Moreover, adequate attention to the medical optimization of these needs before and during antineoplastic therapy is essential for optimal intervention and opportunity for benefit. Weight loss correlates closely with the inability to tolerate full-dose chemotherapy, and patients are not well served when they receive systemic chemotherapy with unrelieved obstructive jaundice.170 Similarly, patients with incipient or fully expressed gastric outlet obstruction have difficulty tolerating intervention with irradiation to the upper abdomen because this is often associated with severe nausea, vomiting, and anorexia. Especially during the early phase of patient presentation and management, “best supportive care” should not be a euphemism for a passive medical stance of minimal involvement and attention.
WEB-ONLY TABLE 46-1 Common Complications Requiring Supportive Care in Patients with Locally Advanced Pancreatic Cancer
Irradiation Techniques
Guidelines are illustrated for a classic four-field approach in Figure 46-8. The information required to appropriately set these fields includes the preoperative CT scan to assess the location of the tumor before resection and a postoperative CT scan to confirm the absence of locally recurrent, persistent, or metastatic cancer and to accurately localize the kidneys and other normal tissues plus nodal target volumes.
Significantly more treatment planning certainty can be accomplished with CT-based treatment planning (Fig. 46-9). This allows contouring of the hepaticojejunostomy, the pancreaticojejunostomy, the pancreatic remnant, the celiac axis, the superior mesenteric artery, and critical normal structures, such as liver, spinal cord, and kidneys. Preoperative tumor volumes can be superimposed on these volumes. The ability to visualize all of these relevant normal tissues and treatment planning volumes allows for enhanced flexibility in field placement, gantry angulation, and treatment planning.
For critical structure tolerance, the RTOG 97-04 protocol offered some guidelines.171 Efforts should be made to exclude the small bowel and liver as much as possible. The liver must not have greater than 60% of its volume receive greater than 30 Gy. The spinal cord dose is limited to less than 45 Gy by use of posterior blocking in the lateral fields. The equivalent of one kidney should receive 20 Gy, or at least the equivalent of two thirds of one kidney must be spared from the RT fields. If only a single functioning kidney is present, at least two thirds of the functioning kidney must be excluded from any radiation port. For lesions of the head of the pancreas, 50% of the right kidney is often in the anterior-posterior/posterior-anterior fields and thus two thirds of the left kidney should be shielded. For body or tail pancreatic lesions, 50% of the left kidney is often in the anterior-posterior/posterior-anterior fields and thus two thirds of the right kidney should be shielded.
The importance of following standard guidelines has been demonstrated by a secondary analysis of RTOG 97-04 presented in abstract form by Abrams and associates.115 By using prospectively defined guidelines, patients’ radiation treatment plans were scored as per protocol, variation acceptable, and variation unacceptable. Of the 416 evaluable patients, 52% were per protocol, whereas 48% were less than per protocol. Survival was increased for all per protocol patients compared with less than per protocol (1.74 years vs. 1.47 years, p = .019). This result persisted on multivariate analysis. The Kaplan-Meier curves comparing per protocol and less than per protocol patients appeared to diverge at around 14 months, which the authors thought implied an effect on tumor control.
In RTOG 08-48 (available online at www.rtog.org) the principles utilized in two-dimensional RT planning from RTOG 97-04 have been extended to three-dimensional and IMRT planning. In addition, an atlas of images illustrating these principles has been posted on the RTOG website. This protocol targets the areas of greatest subclinical tumor concentration based on anatomic landmarks not readily apparent in two-dimensional imaging (celiac and superior mesenteric arteries, portal vein, pancreaticojejunostomy, gross tumor volume location of the resected tumor) and also specifies more precisely suggested tolerance parameters for critical normal organs. The clinical and planning target volumes resulting are significantly smaller than those standardly utilized in the past with two-dimensional imaging and planning.
The advent of IMRT has sparked interest in the treatment of both resected and unresectable pancreatic adenocarcinomas. Two studies analyzed the use of IMRT to treat patients with malignancies of the pancreas.172,173 The majority of patients received concurrent chemotherapy. The toxicity profile compared favorably with that of protocols based on continuous infusion 5-FU or gemcitabine.172 Compared with conventional RT, IMRT reduced the mean dose to the liver, kidneys, stomach, and small bowel.173 A recent dosimetric study from van der Geld and colleagues174 revealed that four-dimensional IMRT planning significantly reduced the mean volume of right kidney exposed to 20 Gy by more than 11%, while also lowering the mean dose to the stomach, liver, and small bowel. These dosimetric improvements may allow for larger, systemic doses of chemotherapy to be given concurrently with irradiation in a safe manner.
Treatment Algorithm, Controversies, and Future Possibilities
A treatment algorithm for pancreatic cancer is provided in Figure 46-10.
For resectable disease, surgical therapy is clearly required for any chance at curing pancreatic cancer. However, controversies regarding what is the most appropriate adjuvant therapy remain debatable. From both retrospective and prospective studies, the considerable rates of local and distant relapse postoperatively imply that chemoradiation should be most appropriate; however, the available phase III data (both in support and challenging this assertion) are flawed by study limitations. RTOG 97-04 did not test the question of chemoradiation versus chemotherapy. CONKO-001 did not test this question either, although this study did demonstrate that adjuvant chemotherapy with gemcitabine is superior to surgery alone for patients with postoperative CA19-9 levels less than or equal to 2.5 × the upper limit of normal. Specifically, the EORTC trial has been argued to be statistically underpowered and the ESPAC-1 trial has been criticized for nihilistically concluding that chemoradiation has no role in the adjuvant therapy of resected pancreatic cancer.175 Experts have challenged the ESPAC-1 trial for its lack of quality assurance monitoring, toxicity reporting, and nonadherence to the protocol, thus questioning the validity of the aforementioned conclusion.175,176 If patterns of failure continue to show substantial rates of local and distant relapse, it would seem impossible to improve survival by discarding chemoradiation.
Future possibilities for improvement in survival for patients with locally advanced pancreas cancer need to involve more effective systemic therapy and irradiation. The evaluation of high-dose preoperative EBRT plus simultaneous and maintenance chemotherapy is reasonable in both borderline and unresectable pancreas cancers in high performance status patients, if imaging and laparoscopy or laparotomy have ruled out peritoneal and liver metastases. In patients who have surgical exploration, dose escalation with IOERT should continue to be evaluated; and for nonsurgical patients, EBRT boosts with SBRT or IMRT should be evaluated. The results of RTOG 97-04 support the use of gemcitabine as the chemotherapeutic agent of choice before and after 5-FU–based EBRT. The trial also confirms the importance of separating results of tumors from the pancreatic head region from those of the body and tail, as well as the importance of extent of lymph node involvement. In secondary analyses, RTOG 97-04 also demonstrated that postoperative CA 19-9 levels and adherence to RT guidelines may significantly affect survival outcomes.91
The most optimal results for patients with potentially curable pancreatic cancer (gross total resection with negative margins) will likely be achieved with trimodality treatment (surgery, chemotherapy, and RT). The safest, most effective combination of all three components is yet to be determined. Future trials should address sequencing of the systemic components of treatment relative to the concurrent chemoradiation component with regard to giving several cycles of chemotherapy alone before starting concurrent chemoradiation.177,178 Moreover, future treatment possibilities include molecular-based regimens utilizing gene targeting. This approach is antibody based, designed to target and kill pancreatic cancer cells.
1 Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection. A key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009;115:3979-3990.
10 Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365-374.
19 Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma. Survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. Int J Radiat Oncol Biol Phys. 1999;44:1039-1046.
21 Berger AC, Garcia MJr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation. A prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918-5922.
27 Hruban RH, Offerhaus JA, Kern SE, et al. Tumor-suppressor genes in pancreatic cancer. J Hepatobiliary Pancreas Surg. 1998;5:383-391.
44 Cubilla AL, Fortner J, Fitzgerald PJ. Lymph node involvement in carcinoma of the head of the pancreas area. Cancer. 1978;41:880.
47 Tepper J, Nardi G, Suit H. Carcinoma of the pancreas. Review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519.
51 Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600-604.
65 Yeo CJ. Pylorus-preserving pancreaticoduodenectomy. Surg Oncol Clin North Am. 1988;7:143.
71 Assumpcao L, Cameron JL, Wolfgang CL, et al. 1423 pancreaticoduodenectomies for pancreatic cancer. A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210.
73 Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990’s. Pathology, complications, and outcomes. Ann Surg. 1997;226:248.
77 Brennan MF, Kattan M, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293.
83 Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899.
85 Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776.
87 Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation. Long-term results of EORTC trial 40891. Ann Surg. 2007;246:734-740.
89 Neoptolemos JP, Stocken DD, Friess H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer. A randomized controlled trial. Lancet. 2001;358:1576.
91 Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. A randomized controlled trial. JAMA. 2008;299:1019-1026.
93 Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma. Postoperative adjuvant chemoradiation improves survival. Ann Surg. 1997;225:621.
94 Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503-3510.
95 Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma. The Mayo Clinic experience (1975-2005). J Clin Oncol. 2008;26:3511-3516.
96 Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma. The Johns Hopkins Hospital–Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981-990.
97 Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer. A randomized trial. J Clin Oncol. 1997;15:2403.
98 Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer. A randomized controlled trial. JAMA. 2007;297:267-277.
101 Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
102 Neoptolemos J, Stocken DD, Bassi C, et alEuropean Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection. A randomized controlled trial. JAMA. 2010;304(10):1073-1081.
115 Abrams RA, Winter KA, Regine WF, et al. Correlation of RTOG 97-04 radiation therapy quality assurance scores with survival. 2007 ASCO annual meeting proceedings. J Clin Oncol. 2007;25(Suppl 18S):4523.
122 Talamonti MS, Small W, Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150-158.
123 Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502.
126 Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer. The importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846.
131 Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma. A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. Cancer. 1981;48:1705-1710.
136 Moertel C, Gunderson L, Mailliard J, et al. Early evaluation of combined fluorouracil and leucovorin as a radiation enhancer for locally unresectable, residual, or recurrent gastrointestinal carcinoma. J Clin Oncol. 1994;12:21-27.
147 Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 04-11. J Clin Oncol. 2009;27:4096-4102.
148 Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665-672.
160 Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764-2768.
163 Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-299.
166 Garton G, Gunderson L, Nagorney D, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
168 Valentini V, Calvo F, Reni M, et al. Intra-operative radiotherapy (IORT) in pancreatic cancer. Joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;91:54-59.
174 van der Geld YG, van Triest B, Verbakel WF, et al. Evaluation of four-dimensional computed tomography-based intensity-modulated and respiratory-gated radiotherapy techniques for pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1215-1220.
177 Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809-816.
178 Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47-55.
1 Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection. A key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009;115:3979-3990.
2 Jamal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
3 Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base Report on Pancreatic Cancer. Cancer. 1995;76:1671.
4 Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553-2562.
5 Warshaw AL, Fernandez-Del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455-465.
6 Arab L. Epidemiologic evidence, on coffee and cancer. Nutr Cancer. 2010;62(3):271-283.
7 Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients. Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
8 Hilgers W, Kern SE. The molecular genetic basis of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 1999;26:1-12.
9 Tersmette AC, Petersen GM, Offerhaus GJA, et al. Increased risk of incident pancreatic cancer among first-degree relatives of patients with familial pancreatic cancer. Clin Cancer Res. 2001;7:738-744.
10 Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med. 2009;133:365-374.
11 Johns Hopkins National Familial Pancreatic Tumor Registry. Available online at pathology.jhu.edu/pancreas/PartNFPTR.php
12 Moossa AR, Gamagami RA. Diagnosis and staging of pancreatic neoplasms. Surg Clin North Am. 1995;75:871.
13 Legmann P, Vignaux O, Dousset B, et al. Pancreatic tumors. Comparison of dual-phase helical CT and endoscopic sonography. AJR Am J Roentgenol. 1998;170:1315-1322.
14 Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410-1418.
15 Brand RE. Screening for familial pancreatic cancer. Is doing something better than doing nothing? Gut. 2009;58:1321-1322.
16 Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals. An EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606-621.
17 Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076.
18 Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin North Am. 1998;7:93-101.
19 Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma. Survival results and observations regarding patterns of failure, radiotherapy dose and CA 19-9 levels. Int J Radiat Oncol Biol Phys. 1999;44:1039-1046.
20 Montgomery RC, Hoffman JP, Riley LB, et al. Prediction of recurrence and survival by post-resection CA 19-9 values in patients with adenocarcinoma of the pancreas. Ann Surg Oncol. 1997;4:551-556.
21 Berger AC, Garcia MJr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation. A prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918-5922.
22 Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735-3742.
23 Iacobuzio-Donahue CA, Shen-Ong GL, Van Heek T, et al. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160:1239-1249.
24 Rosty C, Christa L, Kuzdzal S, et al. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002;62:1868-1875.
25 Bottger TC, Storkel S, Wellek S, et al. Factors influencing survival after resection of pancreatic cancer. Cancer. 1994;73:63-73.
26 Jorba R, Jaurrieta E, Bernat R, et al. DNA ploidy and S+G2M fraction (proliferative index) as prognostic determinants in human periampullary cancer. Hepatogastroenterology. 1995;42:740-747.
27 Hruban RH, Offerhaus JA, Kern SE, et al. Tumor-suppressor genes in pancreatic cancer. J Hepatobiliary Pancreas Surg. 1998;5:383-391.
28 Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115-4121.
29 Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K, ACVR1B, and BRCA2 in familial pancreatic cancer. Deleterious BRCA2 mutations in 17%. Cancer Res. 2002;62:3789-3793.
30 Goggins RT, Murray T, Bolden S, et al. Pancreatic adenocarcinomas with DNA replication errors (RER+) are associated with wild type K-ras and characteristic histopathology. Am J Pathol. 1998;152:1501-1507.
31 Griffin CA, Hruban RH, Morseberger L, et al. Consistent chromosome abnormalities and adenocarcinoma of the pancreas. Cancer Res. 1995;55:2394-2399.
32 Griffin CA, Hruban RH, Long PP, et al. Chromosome abnormalities in pancreatic adenocarcinoma. Gene chromosomes. Cancer. 1994;9:93-100.
33 Greenhalf W, Malats N, Nilsson M, et al. International registries of families at high risk of pancreatic cancer. Pancreatology. 2008;8:558-565.
34 Solcia E, Capella C, Kloppel G, editors. Tumors of the Pancreas, series ed 3, Washington, DC: Armed Forces Institute of Pathology, 1997.
35 Wilentz RE, Hruban RH. Pathology of cancer of the pancreas. Surg Oncol Clin North Am. 1998;7:43-65.
36 Adsay NV, Basturk O, Cheng JD, et al. Ductal neoplasia of the pancreas. Nosologic, clinicopathologic, and biologic aspects. Semin Radiat Oncol. 2005;15(4):254-264.
37 Hruban RH, Adsay NV. Molecular classification of neoplasms of the pancreas. Hum Pathol. 2009;40:612-623.
38 Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837.
39 Compton CC. Serous cystic tumors of the pancreas. Semin Diag Pathol. 1999;17:43-55.
40 Wilentz RE, Albores-Saavedra J, Zahurak M, et al. Pathologic examination accurately predicts prognosis in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 1991;23:1320-1327.
41 Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts. Intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diag Pathol. 2000;17:16-30.
42 Wilentz RE, Hruban RH. Pathology of pancreatic cancer. In: Cameron JL, editor. Pancreatic Cancer. Hamilton, Ontario: BC Decker; 2001:37-66.
43 Cubilla AL, Fortner J, Fitzgerald PJ. Pancreas cancer—duct cell adenocarcinoma. Survival in relation to site, size, stage, and type of therapy. J Surg Oncol. 1978;10:465.
44 Cubilla AL, Fortner J, Fitzgerald PJ. Lymph node involvement in carcinoma of the head of the pancreas area. Cancer. 1978;41:880.
45 Nagai H, Kuroda A, Morioka Y. Lymphatic and local spread of T1 and T2 pancreatic cancer. Ann Surg. 1986;204:65.
46 Nagakawa T, Kobayashi H, Ueno K, et al. Clinical study of lymphatic flow to the para-aortic lymph nodes in carcinoma of the head of the pancreas. Cancer. 1994;73:1115.
47 Tepper J, Nardi G, Suit H. Carcinoma of the pancreas. Review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519.
48 Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56.
49 Kayahara M, Nagakawa T, Ueno K, et al. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118.
50 Johnstone PA, Sindelar WF. Patterns of disease recurrence following definitive therapy of adenocarcinoma of the pancreas using surgery and adjuvant radiotherapy. Correlation of a clinical trial. Int J Radiat Oncol Biol Phys. 1993;27:831.
51 Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600-604.
52 Asiyanbola B, Gleisner A, Herman JM, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy. Effect of number of metastatic lymph nodes and lymph node ratio. J Gastrointest Surg. 2009;13:752-759.
53 Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806-1813.
54 Yeo TP, Hruban RH, Leach SD, et al. Pancreatic cancer. Curr Probl Cancer. 2002;26:176.
55 Wong D, Ko AH, Hwang J, et al. Serum CA19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas. 2008;37:269-274.
56 Olsen CC, Schefter TE, Chen H, et al. Results of a phase I trial of 12 patients with locally advanced pancreatic carcinoma combining gefitinib, paclitaxel, and 3-dimensional conformal radiation. Report of toxicity and evaluation of circulating K-ras as a potential biomarker of response to therapy. Am J Clin Oncol. 2009;32:115-121.
57 Rösch T, Dittler HJ, Strobel K, et al. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas. A blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469-477.
58 Farma JM, Santillan AA, Melis M, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465-2471.
59 Sauter PK, Coleman J. Pancreatic cancer, a continuum of care. Semin Oncol Nursing. 1999;15:36.
60 American Joint Committee on Cancer. Exocrine pancreas. In: Edge SB, editor. AJCC Staging Manual. ed 7. New York: Springer; 2010:285.
61 Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol. 2009;27:2855-2862.
62 Braash JW, Gagner M. Pylorus-preserving pancreaticoduodenectomy. Technical aspects. Langenbecks Arch Surg. 1991;376:50.
63 Kozuschek W, Reith HB, Waleczek H, et al. A comparison of long-term results of the standard Whipple procedure and the pylorus preserving pancreaticoduodenectomy. J Am Coll Surg. 1994;178:443.
64 Takao S, Aikou T, Shinchi H, et al. Comparison of relapse in long-term survival between pylorus-preserving and Whipple pancreaticoduodenectomy in periampullary cancer. Am J Surg. 1998;176:467.
65 Yeo CJ. Pylorus-preserving pancreaticoduodenectomy. Surg Oncol Clin North Am. 1988;7:143.
66 Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas, 201 patients. Ann Surg. 1995;221:721.
67 Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg. 1993;217:430.
68 Edis AJ, Kiernan PD, Taylor WF, et al. Attempted curative resection of ductal carcinoma of the pancreas. Review of the Mayo clinical experience. Mayo Clin Proc. 1980;55:531.
69 Yeo CJ. The Whipple procedure in the 1990’s. Adv Surg. 1999;32:271.
70 Yeo CJ. Management of complications following pancreaticoduodenectomy. Surg Clin North Am. 1995;75:913.
71 Assumpcao L, Cameron JL, Wolfgang CL, et al. 1423 pancreaticoduodenectomies for pancreatic cancer. A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210.
72 Michelassi FM, Erroi F, Dawson PJ, et al. Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Ann Surg. 1989;210:544.
73 Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990’s. Pathology, complications, and outcomes. Ann Surg. 1997;226:248.
74 Yeo CJ, Sohn A, Cameron JL, et al. Periampullary adenocarcinoma. Analysis of 5-year survivors. Ann Surg. 1998;227:821.
75 Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610-618.
76 Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68.
77 Brennan MF, Kattan M, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293.
78 Manabe T, Miyashita T, Ohshio G, et al. Small carcinomas of the pancreas. Cancer. 1988;62:135.
79 Chiang KC, Yeh CN, Lee WC, et al. Prognostic analysis of patients with pancreatic head adenocarcinoma less than 2 cm undergoing resection. World J Gastroenterol. 2009;14(15):4305-4310.
80 Pongprasobchai S, Pannala R, Smyrk TC, et al. Long-term survival and prognostic indicators in small (< or = 2 cm) pancreatic cancer. Pancreatology. 2008;8:587-592.
81 Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation ± 5 fluorouracil. Int J Radiat Oncol Biol Phys. 1993;26:483.
82 Willett CG, Warshaw AL, Convery K, et al. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993;176:33.
83 Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899.
84 Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006.
85 Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region. Phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230:776.
86 Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer: Letter to editor. Ann Surg. 2006;244:332-333.
87 Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation. Long-term results of EORTC trial 40891. Ann Surg. 2007;246:734-740.
88 Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200.
89 Neoptolemos JP, Stocken DD, Friess H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer. A randomized controlled trial. Lancet. 2001;358:1576.
90 Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001;358:1565.
91 Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. A randomized controlled trial. JAMA. 2008;299:1019-1026.
92 Whittington R, Bryer MP, Haller DG, et al. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1991;21:1137.
93 Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma. Postoperative adjuvant chemoradiation improves survival. Ann Surg. 1997;225:621.
94 Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. Results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503-3510.
95 Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma. The Mayo Clinic experience (1975-2005). J Clin Oncol. 2008;26:3511-3516.
96 Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma. The Johns Hopkins Hospital–Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981-990.
97 Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer. A randomized trial. J Clin Oncol. 1997;15:2403.
98 Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer. A randomized controlled trial. JAMA. 2007;297:267-277.
99 Neuhaus P, Riess H, Post S, et al. CONKO-001. Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer. J Clin Oncol. 26(May 20 Suppl; abstract LBA4504), 2008.
100 Royal RE, Wolff RA, Crane CH. Pancreatic cancer. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Principles and Practice of Oncology. ed 8. Philadelphia: Lippincott Williams & Wilkins; 2009:1086-1122.
101 Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer. A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
102 Neoptolemos J, Büchler M, Stocken DD, et al. ESPAC-3. A multicenter, international, open-label, randomized, controlled phase III trial of adjuvant 5-fluorouracil/folinic acid versus gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2009;27(Suppl [abstract LBA4505]):18s.
103 Nukui Y, Picozzi VJ, Traverso LW. Interferon based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy or pancreatic adenocarcinoma. Am J Surg. 2000;179:367-371.
104 Picozzi VJ, Kozarek RE, Jacobs AD, et al. Adjuvant therapy for resected pancreas cancer (PC) using alpha-interferon (IFN)–based chemoradiation. Completion of a phase II trial. Proc ASCO. 2003;22:265. [abstract 1061]
105 Picozzi VJ, Abrams RA, Decker PA, et al. ACOSOG Trial Z05031. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alpha-2b–based chemoradiation. Ann Oncol. 2011;22(2):348-354. Epub 2010 Jul 29
106 Linehan DC, Tan MC, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma. A single-institution phase II study. Ann Surg. 2008;248:145-151.
107 Lawrence T. Gemcitabine as a radiation sensitizer. Semin Oncol. 1995;22:68-71.
108 Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208-2212.
109 McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202-4208.
110 Epelbaum R, Rosenblatt E, Nasrallah S, et al. Phase II study of gemcitabine combined with radiation therapy in patients with localized, unresectable pancreatic cancer. J Surg Oncol. 2002;81:138-143.
111 Allen AM, Zalupski MM, Robertson JM, et al. Adjuvant therapy in pancreatic cancer. Phase I trial of radiation dose escalation with concurrent full-dose gemcitabine. Int J Radiat Oncol Biol Phys. 2004;59:1461-1467.
112 Blackstock AW, Mornex F, Partensky C, et al. Adjuvant gemcitabine and concurrent radiation for patients with resected pancreatic cancer. A phase II study. Br J Cancer. 2006;95:260-265.
113 Demols A, Peeters M, Polus M, et al. Adjuvant gemcitabine and concurrent continuous radiation (45 Gy) for resected pancreatic head carcinoma. A multicenter Belgian phase II study. Int J Radiat Oncol Biol Phys. 2005;62:1351-1356.
114 Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer. A randomized EORTC/FFCD/GERCOR phase II study. J Clin Oncol. 2010;28(29):4450-4456. Epub 2010 Sep 13
115 Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704-A phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 2011 Jan 28. [Epub ahead of print]
116 . RTOG protocol 08-48. Available online at www.rtog.org/members/protocols/0848/0848.pdf Accessed December 28, 2009
117 Ishikawa O, Ohigashi H, Imaoka S, et al. Is the long-term survival rate improved by preoperative irradiation prior to Whipple’s procedure for adenocarcinoma of the pancreas head? Arch Surg. 1994;129:1075-1080.
118 Yeung RS, Weese JL, Hoffman JP, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma—a phase II study. Cancer. 1993;72:2124-2133.
119 Pendurthi TK, Hoffman JP, Ross E, et al. Preoperative versus postoperative chemoradiation for patients with resected pancreatic adenocarcinoma. Am Surg. 1998;64:686-692.
120 Meszoely IM, Wang H, Hoffman JP. Preoperative chemoradiation therapy for adenocarcinoma of the pancreas. The Fox Chase Cancer Center experience, 1986-2003. Surg Oncol Clin North Am. 2004;13:685-696.
121 Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas. An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317-323.
122 Talamonti MS, Small W, Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150-158.
123 Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496-3502.
124 Small W, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer. A multicenter phase II trial. J Clin Oncol. 2008;26:942-947.
125 Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients. Results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567-579.
126 Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer. The importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833-846.
127 Ammori JB, Colletti LM, Zalupski MM, et al. Surgical resection following radiation therapy with concurrent gemcitabine in patients with previously unresectable adenocarcinoma of the pancreas. J Gastrointest Surg. 2003;7:766-772.
128 Brown KM, Siripurapu V, Davidson M, et al. Chemoradiation followed by chemotherapy before resection for borderline pancreatic adenocarcinoma. Am J Surg. 2008;195:318-321.
129 Lillemoe K, Cameron J, Yeo C, et al. Pancreaticoduodenectomy—does it have a role in palliation of pancreatic cancer? Ann Surg. 1996;223:718-728.
130 Ouchi K, Sugawara T, Ono H, et al. Palliative operation for cancer of the head of pancreas. Significance of pancreaticoduodenectomy and intraoperative radiation therapy for survival and quality of life. World J Surg. 1998;22:413-417.
131 Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma. A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. Cancer. 1981;48:1705-1710.
132 Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas. Comparison of combined modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751-755.
133 Flickinger J, Jawalekar K, Deutsch M, et al. Split course radiation therapy for adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;15:359-364.
134 Jeekel J, Treuniet-Donker AD. Treatment perspectives in locally advanced unresectable pancreatic cancer. Br J Surg. 1978;11:1332-1334.
135 Bruckner HW, Kalnicki S, Dalton J, et al. Combined modality therapy increasing local control of pancreatic cancer. Cancer Invest. 1993;11:241-246.
136 Moertel C, Gunderson L, Mailliard J, et al. Early evaluation of combined fluorouracil and leucovorin as a radiation enhancer for locally unresectable, residual, or recurrent gastrointestinal carcinoma. J Clin Oncol. 1994;12:21-27.
137 Blackstock AW, Bernard SA, Richards F, et al. Phase I trial of twice weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208-2212.
138 Blackstock AW, Tempero MA, Niedwiecki D, et al. Cancer and Leukemia Group B (CALGB) 89805. Phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer. 2003;34:107-116.
139 Wolff RA, Evans DB, Gravel DM, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Can Res. 2001;7:2246-2253.
140 McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202-4208.
141 Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 26S(abstract 4506), 2008.
142 Talamonti MS, Catalano PJ, Vaughn DJ, et al. Eastern Cooperative Oncology Group phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrent radiation therapy in patients with locally advanced pancreas cancer. A regimen with unexpected early toxicity. J Clin Oncol. 2000;18:3384-3389.
143 Martenson JA, Vigliotti APG, Pitot HC, et al. A phase I study of radiation therapy and twice-weekly gemcitabine and cisplatin in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55:1305-1310.
144 Brunner TB, Grabenbauer GG, Klein P, et al. Phase I trial of strictly time-scheduled gemcitabine and cisplatin with concurrent radiotherapy in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55:141-153.
145 Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine based chemoradiation than with 5-fluorouracil chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys. 2002;52:1293-1302.
146 Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374-3378.
147 Crane CH, Winter K, Regine WF, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer. Radiation Therapy Oncology Group RTOG 04-11. J Clin Oncol. 2009;27:4096-4102.
148 Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115(3):665-672.
149 Crane CH, Willett CG. Stereotactic radiotherapy for pancreatic cancer? Cancer. 2009;115:468-472.
150 Whittington R, Solin L, Mohiuddin M, et al. Multimodality therapy of unresectable pancreatic adenocarcinoma. Cancer. 1984;54:1991-1998.
151 Mohiuddin M, Cantor RJ, Bierman W, et al. Combined modality treatment of localized unresectable adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1988;14:79-84.
152 Mohiuddin M, Rosato F, Barbot D, et al. Long-term results of combined modality treatment with I-125 implantation for carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1992;23:305-311.
153 Nori D, Merimsky O, Osian AD, et al. Palladium-103. A new radioactive source in the treatment of unresectable carcinoma of the pancreas. A phase I-II study. J Surg Oncol. 1996;61:300-305.
154 Raben A, Mychalczak B, Brennan MF, et al. Feasibility study of the treatment of primary unresectable carcinoma of the pancreas with palladium-103 brachytherapy. Int J Radiat Oncol Biol Phys. 1996;25:351-356.
155 Shipley WU, Nardi GL, Cohen AM, et al. Iodine-125 implant and external beam irradiation in patients with localized pancreatic carcinoma. A comparative study to surgical resection. Cancer. 1980;45:709-714.
156 Shipley WU, Wood WC, Tepper JE, et al. Intraoperative electron beam irradiation for patients with unresectable pancreatic carcinoma. Ann Surg. 1984;200:25-32.
157 Tepper JE, Shipley WU, Warshaw AL, et al. The role of misonidazole combined with intraoperative radiation therapy in the treatment of pancreatic carcinoma. J Clin Oncol. 1987;5:579-584.
158 Roldan GE, Gunderson LL, Nagorney DM, et al. External beam versus intraoperative and external beam irradiation for locally advanced pancreatic cancer. Cancer. 1988;61:1110.
159 Tepper JE, Noyes D, Krall J, et al. Intraoperative radiation therapy of pancreatic carcinoma. A report of RTOG 85-05. Int J Radiat Oncol Biol Phys. 1991;21:1145-1149.
160 Mohiuddin M, Regine WF, Stevens J, et al. Combined intraoperative radiation and perioperative chemotherapy for unresectable cancers of the pancreas. J Clin Oncol. 1995;13:2764-2768.
161 Calvo FC, Viera JC, Gunderson LL, Willett CG. Pancreas and hepatobiliary tract cancer. In: Perez CA, Brady LW, Halperin EC, Schmidt-Ulrich RK, editors. Principles and Practice of Radiation Oncology. ed 4. Philadelphia: JB Lippincott; 2004:1574-1588.
162 Termuhlen P, Evans D, Willett CG. IORT in pancreatic carcinoma. In: Gunderson LL, Willett CG, Harrison LB, Calvo FC, editors. Intraoperative Irradiation: Techniques and Results. Totowa, NJ: Humana Press; 1999:201-222.
163 Willett CG, Del Castillo CF, Shih HA, et al. Long-term results of intraoperative electron beam irradiation (IOERT) for patients with unresectable pancreatic cancer. Ann Surg. 2005;241:295-299.
164 Showalter TN, Rao AS, Rani Anne P, et al. Does intraoperative radiation therapy improve local tumor control in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma? A propensity score analysis. Ann Surg Oncol. 2009;16:2116-2122.
165 Meyer JJ, Crane CH. Is there a role for intraoperative radiation therapy in patients with resected pancreatic adenocarcinoma? Ann Surg Oncol. 2009;16:2081-2083.
166 Garton G, Gunderson L, Nagorney D, et al. High-dose preoperative external beam and intraoperative irradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 1993;27:1153-1157.
167 Gunderson LL, Moss A, Callister MG, et al. Preoperative chemoradiation and IOERT for unresectable or borderline resectable pancreas cancer. ISIORT 2008 Proceedings. Rev Cancer. 2008;22:32-33.
168 Valentini V, Calvo F, Reni M, et al. Intraoperative radiotherapy (IORT) in pancreatic cancer. Joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;91:54-59.
169 Miller R, Valentini V, Moss A, et al. Pancreas cancer. In Gunderson LL, Willett CG, Calvo FC, Harrison LB, editors: Intraoperative Irradiation: Techniques and Results, ed 2, Totowa, NJ: Humana Press, 2011.
170 Andreyev HJN, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503-509.
171 . RTOG Protocol 97-04. Available online at www.rtog.org/members/protocols/97-04/97-04.pdf Accessed December 28, 2009
172 Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;50:454-459.
173 Milano MT, Chmura SJ, Garafalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies. Toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2004;59:445-453.
174 van der Geld YG, van Triest B, Verbakel WF, et al. Evaluation of four-dimensional computed tomography–based intensity-modulated and respiratory-gated radiotherapy techniques for pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1215-1220.
175 Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Rad Oncol Biol Phys. 2005;61:965-966.
176 Choti MA. Adjuvant therapy for pancreatic cancer—the debate continues. N Engl J Med. 2004;350:1249-1251.
177 Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:809-816.
178 Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47-55.