Pain Management in the Neonate
1. An obstetrician is about to do a circumcision without using analgesia. When you question this practice, he replies that neonates cannot perceive pain. Is this correct?
This is not correct. The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.” The definition further states that although pain is subjective, the inability to communicate verbally does not negate the possibility that an individual is experiencing pain and requires adequate pain-relieving treatment. The issues of pain perception in newborns, its management, and its prevention were neglected for decades. The inability of newborns to “self-report” contributed significantly to the denial of the importance of neonatal pain and the consequences of inadequate treatment. In response to a painful stimulus, all newborns mount acute changes in endocrine, vegetative, immune, and behavioral functions. Multiple lines of evidence show that the pain system is intact and functional in preterm and term neonates, even among the tiniest preterm newborns. Acute pain is processed in the somatosensory cortex, and these responses are altered by the characteristics of neonates, their behavioral state at the time of painful stimulation, the intensity of stimulation, and contextual factors. Such a nuanced response suggests that term and preterm neonates may be capable of conscious sensory perception of acute pain ( Fig. 17-1). 1234567891011
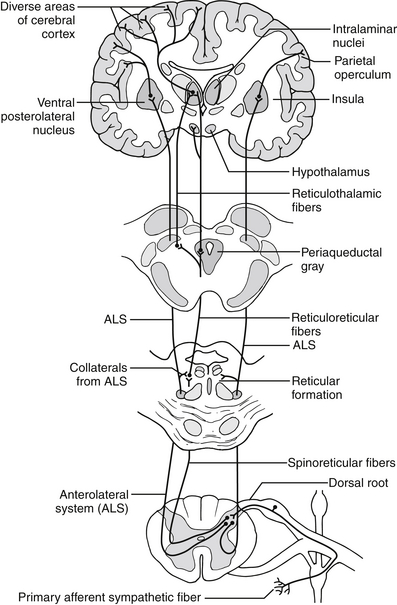
Figure 17-1 Schematic diagram showing the lateral and medial pain systems. Primary afferents from cutaneous, mucosal, visceral, joint and connective tissue, vascular, and other deep-tissue nociceptors enter the spinal cord via A-delta (thinly myelinated)-fibers, C (unmyelinated)-fibers, and sympathetic fibers. These afferents make rich connections in superficial layers of the dorsal horn of the spinal cord (called the substantia gelatinosa). They have direct and indirect links with projection neurons in deeper layers of the dorsal horn that project to the supraspinal pain-processing areas in the brainstem, medial and posterior thalamus, and various areas of the cortex. The lateral pain system mostly transmits somatic and mucosal pain, whereas the medial pain system transmits visceral pain. Cortical areas most closely associated with pain processing include the primary and secondary somatosensory areas, the anterior cingulate cortex, the insula, and parietal association areas. (From Bonaz B. Visceral sensitivity perturbation integration in the brain-gut axis in functional digestive disorders. J Physiol Pharmacol 2003;54[Suppl 4]:27–42.)
If pain is prolonged or repetitive, these physiologic and behavioral responses may be muted, transient, or absent. Neonates, especially preterm neonates, have limited energy reserves and cannot mount a prolonged psychophysiologic activation response to pain. 1213141516171819
The developing nervous system may be permanently modified after prolonged or repetitive pain, resulting in altered pain processing at the spinal and supraspinal levels. In addition, pain is associated with a number of adverse physiologic responses that include alterations in circulatory (tachycardia, hypertension, vasoconstriction), metabolic (increased catabolism, metabolic acidosis), immunologic (impaired immune response), and hemostatic (platelet activation) systems. 2021222324252627
Cutaneous sensory receptors first appear in the perioral area during the eighth week of gestation. They are present in all cutaneous and mucous surfaces by the 18th week of gestation. Synapses between peripheral sensory afferents and dorsal horn neurons in the spinal cord appear early in the first trimester and are mature by 20 weeks of gestation. Pain activates physiologic stress responses, which are associated with the release of catecholamines, cortisol, and other stress hormones. 2829303132333435363738
Stress responses to a painful stimulation are complex, but they can be detected from the 16th week of gestation. Physiologic stress is different from the pain felt by the more mature fetus, as this stress is mitigated by a pain medication such as fentanyl by 20 to 24 weeks. There is activation of the hypothalamic–pituitary–adrenal axis, autonomic nervous system, and hemodynamic changes in response to fetal pain. 3 In premature infants exposed to pain, there are significant increases of epinephrine, norepinephrine, and cortisol; hemodynamic changes; motor reflexes; and facial reactions. 39
They certainly are not. Developmentally regulated processes and behavioral studies show that pain thresholds increase progressively during late gestation and in the postnatal period. Preterm neonates have much greater sensitivity to pain than term neonates, and they manifest prolonged periods of hyperalgesia after tissue injury. These phenomena were further substantiated in the newborn and infant rat. Central sensitization and immaturity of the pain inhibitory systems are the main neurobiologic explanations for the increased pain sensitivity in newborns. 4041424344454647
Neonates admitted to a modern-day NICU are often exposed to acute or prolonged pain from a variety of sources. These include acute pain caused by heel sticks, venipunctures, tracheal suctioning, lumbar punctures, or chest tubes; postoperative pain resulting from circumcision, surgery to repair a hernia or ligate a patent ductus arteriosus; and prolonged pain from necrotizing enterocolitis, meningitis, birth trauma, or ventilation. Even routine care such as diaper changes, daily weighing, removal of adhesive tape, burns from transcutaneous probes, and rectal stimulation will cause low-level noxious stimulation and background excitability in the “pain system.” Although there is no definition for chronic pain in newborns, conditions associated with constant prolonged pain may include epidermolysis bullosa, necrotizing enterocolitis, scalded skin syndrome (staphylococcal), septic arthritis, tissue ischemia, and rare congenital conditions such as harlequin-type icthyosis. Identifying chronic pain is clinically relevant because it interferes with the infant’s growth, prolongs hospitalization, alters subsequent pain perception, and impairs cognitive and behavioral development (van Ganzewinkel CJ, et al. Unpublished data, 2012). 484950
Neonatal painful experiences cannot be accessed by conscious recall but may lead to long-term or permanent alterations in brain development that are expressed in unique ways during different stages of development, depending on the type, duration, and severity of neonatal painful stimuli; the neurologic maturity at which pain occurs; and the use of analgesia. Term neonates exposed to acute, short-term pain develop significant degrees of hyperalgesia after tissue injury, which includes the areas where the injury occurred (i.e., primary hyperalgesia) as well as areas adjacent to or remote from the original injury (i.e., secondary hyperalgesia). If pain is prolonged or repetitive, the developing nervous system will be permanently modified, with altered processing at spinal and supraspinal levels. Tissue damage in the early neonatal period causes profound and long-lasting dendritic sprouting of sensory nerve terminals, resulting in hyperinnervation that may continue into childhood and adolescence. Thus repeated heel sticks could lead to gait disorders in childhood, repeated perioral and nasal suctioning may promote oral aversion syndrome, surgical sites may maintain an increased pain sensitivity, and gastric suctioning at birth may increase the likelihood of irritable bowel syndrome or visceral pain in adolescence. 51525354555657585960616263646566676869
Twin pairs who were discordant only for the experience of surgery in infancy showed greater signs of attention-deficit/hyperactivity disorder, impulsivity, and socialization problems during early school years in the twin who was exposed to surgery compared with the other twin. Although it was speculated that these individuals may be at increased risk for developing chronic pain syndromes during adulthood, recent epidemiologic data from former preterm young adults suggest that this is not the case.
Repetitive pain in newborn rats accentuates neuronal excitation and cell death in developmentally regulated cortical and subcortical areas, associated with impaired short-term and long-term memory and altered pain thresholds. Morphine analgesia in newborn rats attenuated the long-term effects of neonatal pain on pain thresholds in adult male rats (but not females), whereas ketamine analgesia mediated similar long-term effects in adult female rats (but not males). 707172737475767778798081828384858687
Neonates need to be comfortable and as free of pain as possible to grow and develop normally. Valid, reliable, and regular pain assessments are a major prerequisite for attaining this goal. Behavioral indicators of pain include facial actions, body movements and tone, cry, behavioral state changes, sleep patterns, and consolability. Physiologic indicators of pain include increased heart rate, respiratory rate, and blood pressure, as well as decreased heart rate variability and oxygen saturations. Pain assessment in neonates is difficult in neurologically compromised, chemically paralyzed, or nonresponsive infants.
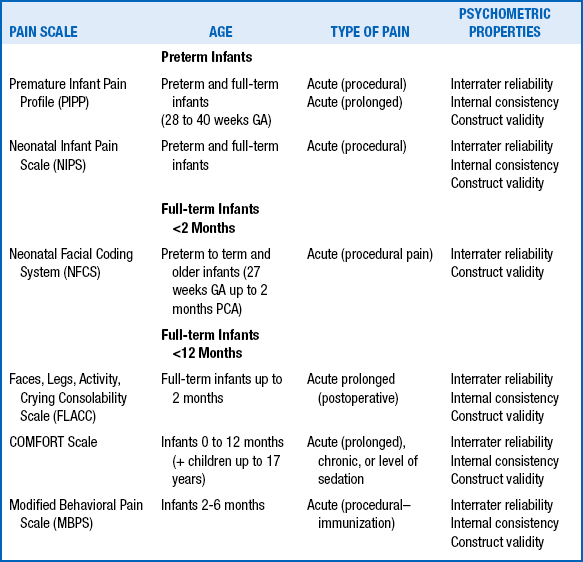
Many methods for measuring the intensity of acute pain in neonates have been validated, but other aspects of painful experiences (e.g., character, location, rhythmicity, duration of pain) have not been routinely assessed in neonates. Very few methods have been validated for the assessment of postoperative pain or chronic pain. The most commonly used methods include the Premature Infant Pain Profile (PIPP), the Neonatal Infant Pain Scale (NIPS), the CRIES score, the Neonatal Pain, Agitation, and Sedation Scale (NPASS), the Neonatal Facial Coding System (NFCS), and the Douleur Aiguë du Nouveau-né (DAN) scale. For acute procedural pain, results using the the NIPS, NFCS, and DAN scales were comparable. A challenge facing clinicians is to develop and validate objective measures of prolonged pain in preterm and term neonates ( Table 17-1). 888990919293949596979899100101102103104105106107108109110111112113114115116117118119120
TABLE 17-1
9. What are the clinical effects of continuous morphine or fentanyl infusions in ventilated preterm neonates?
In infants receiving morphine infusions, randomized controlled trials showed lower pain scores but no significant differences in intraventricular hemorrhage (IVH) (relative risk [RR] 1.13; 95% confidence interval [CI], 0.80-1.61), periventricular leukomalacia (RR, 0.81; 95% CI, 0.51-1.29), or mortality (RR, 1.14; 95% CI, 0.81-1.60) between the morphine and placebo groups. Intermittent bolus doses of open-label morphine, however, were associated with hypotension and increased rates of IVH and mortality. Morphine infusions did not improve short-term pulmonary outcomes among ventilated preterm neonates, whereas additional morphine doses were associated with worse respiratory outcomes among preterm neonates with respiratory distress syndrome. Infants receiving morphine spent more days on mechanical ventilation (weighted mean difference [WMD], 0.24 days; 95% CI, 0.11-0.36). 121122123124125126127128129130131132
Opiates have numerous side effects, including respiratory depression, nausea, vomiting, urinary retention, decreased gut motility, and histamine release causing hypotension or bronchospasm. Histamine release occurs more commonly with morphine than with fentanyl. In addition, morphine is associated with greater effects on gut motility, and very high doses may cause biliary spasm or even seizures. Chest wall rigidity or laryngospasm occur more commonly with fentanyl, with the rapid administration of intravenous doses. Fentanyl produces less sedation than morphine but has been associated with greater opioid tolerance because of its shorter duration of action. 133134135136137138139140141142143144145146147
Many of these signs were included in scoring systems designed to quantify opioid withdrawal in neonates born from heroin-addicted mothers. Their applicability to iatrogenic opioid tolerance and withdrawal resulting from prolonged use in the NICU has not been proved. Previous methods included the Neonatal Abstinence Score (NAS) by Finnegan et al. or the Neonatal Narcotic Withdrawal Index (NNWI) by Green and Suffet, but newer methods include the Withdrawal Assessment Tool (WAT-1) and the Sophia Observation Scale (SOS). Signs of opioid withdrawal include the following ( Box 17-1):
 Neurologic: high-pitched crying, irritability, increased wakefulness, hyperactive deep tendon reflexes, increased muscle tone, tremors, exaggerated Moro reflex, generalized seizures
Neurologic: high-pitched crying, irritability, increased wakefulness, hyperactive deep tendon reflexes, increased muscle tone, tremors, exaggerated Moro reflex, generalized seizures
 Gastrointestinal: poor feeding, uncoordinated and constant sucking, vomiting, diarrhea, dehydration
Gastrointestinal: poor feeding, uncoordinated and constant sucking, vomiting, diarrhea, dehydration
 Autonomic signs: increased sweating, nasal congestion, fever, mottling
Autonomic signs: increased sweating, nasal congestion, fever, mottling
 Other: poor weight gain, disorganized sleep states, skin excoriation 148149150151152153154
Other: poor weight gain, disorganized sleep states, skin excoriation 148149150151152153154
 Concomitant infusion of opioid agonists and N-methyl-D-aspartate (NMDA) antagonists such as low-dose ketamine (0.2-0.3 mg/kg/h) can delay the development of opioid tolerance. Opioid drugs such as ketobemidone and methadone also block NMDA receptors and produce less tolerance than morphine or fentanyl.
Concomitant infusion of opioid agonists and N-methyl-D-aspartate (NMDA) antagonists such as low-dose ketamine (0.2-0.3 mg/kg/h) can delay the development of opioid tolerance. Opioid drugs such as ketobemidone and methadone also block NMDA receptors and produce less tolerance than morphine or fentanyl.
 Continuous infusion of ultra-low-dose naloxone (0.1-0.5 μg/kg/h) selectively blocks the opioid receptors coupled with stimulatory Gs-proteins, thus blocking the mechanisms for superactivation of the cAMP pathway and inhibiting opioid tolerance.
Continuous infusion of ultra-low-dose naloxone (0.1-0.5 μg/kg/h) selectively blocks the opioid receptors coupled with stimulatory Gs-proteins, thus blocking the mechanisms for superactivation of the cAMP pathway and inhibiting opioid tolerance.
 Procedural changes in adult or pediatric ICU patients such as the daily interruption of sedatives, nurse-controlled sedation, sequential rotation of analgesics, or the use of neuraxial opioids may also decrease the incidence of opioid tolerance and withdrawal. 155156157158159160
Procedural changes in adult or pediatric ICU patients such as the daily interruption of sedatives, nurse-controlled sedation, sequential rotation of analgesics, or the use of neuraxial opioids may also decrease the incidence of opioid tolerance and withdrawal. 155156157158159160
 Methadone: This opioid agonist and NMDA antagonist has a long half-life (25 to 44 hours in neonates), can be given enterally (oral bioavailability, 80% to 90%), and reverses the tolerance produced by morphine or other opioid drugs. In one clinical study a methadone dose equivalent to 2.5 times the total daily fentanyl dose was effective in minimizing symptoms of opioid withdrawal.
Methadone: This opioid agonist and NMDA antagonist has a long half-life (25 to 44 hours in neonates), can be given enterally (oral bioavailability, 80% to 90%), and reverses the tolerance produced by morphine or other opioid drugs. In one clinical study a methadone dose equivalent to 2.5 times the total daily fentanyl dose was effective in minimizing symptoms of opioid withdrawal.
 Buprenorphine: This is a partial μ-opioid agonist, a nociception/orphanin receptor agonist, and delta-opioid antagonist with analgesic effects similar to those of morphine in preterm neonates. Buprenorphine was as potent as high-dose methadone for adult opioid addiction, and its clinical use in opioid-addicted mothers induced significantly less opioid withdrawal in their infants compared with methadone-treated mothers.
Buprenorphine: This is a partial μ-opioid agonist, a nociception/orphanin receptor agonist, and delta-opioid antagonist with analgesic effects similar to those of morphine in preterm neonates. Buprenorphine was as potent as high-dose methadone for adult opioid addiction, and its clinical use in opioid-addicted mothers induced significantly less opioid withdrawal in their infants compared with methadone-treated mothers.
 Clonidine: This alpha2-adrenergic receptor agonist has analgesic effects when administered intravenously, intramuscularly, intrathecally, orally, epidurally, or topically. Because the alpha2-adrenergic receptors activate the same inhibitory Gi-proteins, clonidine has been used to treat opioid withdrawal in neonates.
Clonidine: This alpha2-adrenergic receptor agonist has analgesic effects when administered intravenously, intramuscularly, intrathecally, orally, epidurally, or topically. Because the alpha2-adrenergic receptors activate the same inhibitory Gi-proteins, clonidine has been used to treat opioid withdrawal in neonates.
 Benzodiazepines: Benzodiazepines, such as diazepam or lorazepam, may be used for treating the seizures associated with opioid withdrawal, but they are not cross-tolerant with opioids and cannot be used as sole therapy for opioid withdrawal. 161162163164165166167168169170171172173174175176177
Benzodiazepines: Benzodiazepines, such as diazepam or lorazepam, may be used for treating the seizures associated with opioid withdrawal, but they are not cross-tolerant with opioids and cannot be used as sole therapy for opioid withdrawal. 161162163164165166167168169170171172173174175176177
Procedural pain should be prevented whenever possible. Procedural pain can be minimized with an appropriate awareness program involving nursing and respiratory therapy staff members; physicians; and, most important, parents. The most common sources of minor procedural pain are heel sticks and tracheal suctioning. Heel sticks can be treated with 25% sucrose, and tracheal suctioning can be treated with facilitated tucking. More pronounced pain should be treated with opiates. Remifentanyl, for example, is a good choice for short-term procedures such as intubation, whereas more prolonged pain should be treated with a longer-acting opiate such as morphine or fentanyl. Anxiolytics such as midazolam can be used as adjuncts, but they do not treat pain. Circumcision should be performed with sucrose and local anesthetic nerve block before the procedure and acetaminophen after the procedure. 178179180181182183184185
EMLA is an acronym for eutectic mixture of local anesthetics, containing lidocaine (2.5%) and prilocaine (2.5%). Prilocaine is metabolized to ortho-toluidine, which can oxidize hemoglobin to methemoglobin in neonates. Preterm newborns are particularly at risk because their stratum corneum is thinner, causing increased absorption and higher serum prilocaine levels. However,this appears mainly to be a theoretical concern because the incidence of clinically significant methemoglobinemia is strikingly low, even among the preterm neonates exposed to repeated daily doses of EMLA (up to four times a day). The use of EMLA and sucrose is effective in reducing venipuncture pain. 186187
Nonpharmacologic interventions are useful for minor pain and as adjunct therapy for severe pain. Sucrose solutions block the nociceptive transmission in the ascending pathways that transmit noxious stimuli to the brain, while activating the descending inhibitory pathways that modulate pain. Additionally, animal studies show that the gustatory receptors stimulated by sucrose lead to an activation of the endogenous opioid systems in the newborn brainstem, with reduced pain transmission to the thalamocortical circuits. These mechanisms are unlikely to lead to increased beta-endorphin levels in peripheral plasma, as noted in preterm newborns. Additional evidence for this mechanism is demonstrated by the fact that naloxone blocks the analgesic effects of sucrose. Until further evidence becomes available, the consensus opinion remains that sucrose induces effective analgesia for acute pain resulting from skin-breaking procedures in term and preterm newborns. Recently, however, safety of the long-term repeated use of sucrose solutions has been called into question, and protocols should be developed to limit sucrose dosing. 188189190191192
The goals of perioperative analgesic approaches are the relief of pain, the maintenance of physiologic stability, and the prevention of adverse events such as hypoventilation or shallow respiration owing to diaphragmatic splinting, paralytic ileus, protein catabolism, and pulmonary hypertension. The management of postoperative pain should ideally start before the operative procedure, with consideration given to the size and alignment of the surgical incision; the choice of anesthetic agents; infiltration of the surgical site with lidocaine or bupivacaine; and, if possible, the placement of an epidural catheter before or after surgery. Use of analgesics may improve postoperative outcomes with fewer adverse events, shorter duration of mechanical ventilation, rapid return of gastrointestinal function, and reduced incidence of postoperative apnea and other complications. Opiates are the mainstay of therapy; however, because of their known side effects, including respiratory depression, other drugs such as ketorolac and acetaminophen are being studied. 193194195
Postoperative analgesia is usually provided with opioids (e.g., morphine, fentanyl, methadone), antipyretic analgesics including acetaminophen or its intravenous analog propacetamol, or the nonspecific cyclooxygenase (COX) inhibitors (e.g., ibuprofen, ketorolac, diclofenac). The newer COX-2 inhibitors (e.g., parecoxib, valdecoxib, celecoxib, meloxicam) have not been studied in or approved for newborns and small children. Other options include epidural or caudal anesthesia with bupivacaine, or bupivacaine mixed with fentanyl infusions continued into the postoperative period. The use of nurse-controlled analgesia using a patient-controlled analgesia pump is also under investigation. 196
19. Are the doses of morphine and fentanyl for postoperative analgesia in neonates similar to the doses used for older children?
Neonates may receive lower morphine infusion rates than older children after surgery, starting as low as 0.005 mg/kg/h for preterm neonates and 0.01 mg/kg/h for term neonates. Neonates with cyanotic congenital heart defects also require lower morphine infusion rates than neonates undergoing noncardiac surgery. Depending on the dose and other patient characteristics, fentanyl and sufentanil provide variable degrees of suppression of autonomic and hormonal/metabolic responses to major surgery in neonates, although fentanyl may increase the risk of postoperative hypothermia. Critically ill neonates, whose vascular tone depends on sympathetic outflow, may become hypotensive after bolus doses of fentanyl or morphine. In preterm neonates undergoing ductal closure, higher fentanyl doses (>10.3 mg/kg) were associated with a decrease in an unstable postoperative respiratory course. Randomized controlled trials show no differences in the postoperative analgesia produced by bolus doses versus continuous infusions of morphine; however, apnea or other complications were greater in the bolus-dosing groups. Intravenous boluses of opioids should be given slowly (over 15 to 30 minutes) to postoperative neonates. 197198199
The majority of preterm neonates are capable of glucuronidating morphine, but birth weight and gestational and postnatal age influence the hepatic capacity for glucuronidation. Term and preterm neonates and older infants produce relatively greater proportions of morphine-3-glucuronide, which acts as an opioid antagonist and has a prolonged half-life. Older children and adults produce morphine-6-glucuronide, which is a potent analgesic, with 20 times the analgesic potency of morphine itself. Morphine-6-glucuronide was not detected in the plasma of any neonate, which may explain why neonates require relatively high plasma concentrations of unchanged morphine for effective analgesia. 200200
A meta-analysis performed from the reported pharmacokinetics parameters showed an increased volume of distribution for morphine, estimated to be 2.8 ± 2.6 L/kg, which seems to be unaffected by age. In contrast, the half-life and plasma clearance rates for morphine are clearly related to age, secondary to maturational changes in hepatic and renal function. Morphine half-life was estimated to be 9.0 ± 3.4 hours in preterm neonates, 6.5 ± 2.8 hours in term neonates ages 0 to 57 days and 2.0 ± 1.8 hours for older infants and children. Clearance was estimated to be 2.2 ± 0.7 mL/min/kg for preterm neonates, 8.1 ± 3.2 mL/min/kg for term neonates ages 0 to 57 days, and 23.6 ± 8.5 mL/min/kg for older infants and children. The prolonged half-life explains why effective analgesia can be maintained, following a loading dose, with very low infusion rates of morphine (5-15 μg/kg/h). Doses must be further decreased for neonates with impaired hepatic or renal functions, and a pharmacist should be consulted for these patients. 201
It is important to remember that preterm neonates are exposed to repeated invasive procedures that induce pain at a time of rapid brain growth and developmental programming of stress responses. In the few experiments that used opioids for surgical or inflammatory pain, no detrimental effects resulting from morphine exposure in infancy could be demonstrated. The long-term outcomes of former preterm children at 5 to 6 years of age who received morphine during their NICU course showed no differences in their cognitive, neuromotor, or behavioral outcomes, but there was a trend toward better performance in those receiving morphine. In children who were 5-years-old, de Graaf found that morphine-exposed neonates (compared with those in the placebo group) rated lower on a visual analysis subset. Other IQ subsets demonstrated no change when adjusted for other covariates using propensity scoring. Pilot data from the NEOPAIN cohort randomized to morphine or placebo groups in the NICU showed no differences in neuropsychologic outcomes at 5 to 7 years of age, but decreased body weight and head circumference, reduced socialization, and adaptive behaviors occurred in the morphine group. These children also had longer choice response latencies and 27% less task completion during a short-term memory task. However, in this same cohort at school age, the authors found a significant benefit of preemptive morphine analgesia compared with placebo controls among ventilated preterm neonates in math skills (8 to 10 years of age). Literacy placement was similar in the morphine and placebo groups. Limited data are available on the long-term developmental outcomes for neonates exposed to fentanyl therapy in the NICU. 202203
1Anand KJS, Johnston CC, Oberlander TF, et al. Analgesia and local anesthesia during invasive procedures in the neonate. Clin Ther 2005;27:844–76.
2Brady-Fryer B, Wiebe N, Lander JA. Pain relief for neonatal circumcision. Cochrane Database Syst Rev 2004;CD004217.
3IASP Pain Terminology, Committee on Taxonomy. International Association for the Study of Pain; 2001. [Accessed 21.05.01] at
4Anand KJS, Craig KD. New perspectives on the definition of pain. Pain 1996;67:3–6; discussion 209–11.
5Anand KJS, Rovnaghi C, Walden M, et al. Consciousness, behavior, and clinical impact of the definition of pain. Pain Forum 1999;8:64–73.
6Anand KJS, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987;317:1321–9.
7Weber F. Evidence for the need for anaesthesia in the neonate. Best Pract Res Clin Anaesthesiol 2010;24:475–84.
8Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med 2009;22:722–32.
9Anand KJS. Clinical importance of pain and stress in preterm neonates. Biol Neonate 1998;73:1–9.
10Bartocci M, Bergqvist LL, Lagercrantz H, et al. Pain activates cortical areas in the preterm newborn brain. Pain 2006;122:109–17.
11Slater R, Cantarella A, Gallella S, et al. Cortical pain responses in human infants. J Neurosci 2006;26:3662–6.
12Howard VA, Thurber FW. The interpretation of infant pain: physiological and behavioral indicators used by NICU nurses. J Pediatr Nurs 1998;13:164–74.
13Beacham PS. Behavioral and physiological indicators of procedural and postoperative pain in high-risk infants. J Obstet Gynecol Neonatal Nurs 2004;33(2):246–55.
14Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol 2007;31:283–8.
15Grunau RE, Holsti L, Whitfield MF, et al. Are twitches, startles, and body movements pain indicators in extremely low birth weight infants? Clin J Pain 2000;16:37–45.
16Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med 2009;22:722–32.
17Fuller BF, Conner DA. The effect of pain on infant behaviors. Clin Nurs Res 1995;4:253–73.
18Boyle EM, Freer Y, Wong CM, et al. Assessment of persistent pain or distress and adequacy of analgesia in preterm ventilated infants. Pain 2006;124:87–91.
19Hummel P, van Dijk M. Pain assessment: current status and challenges. Semin Fetal Neonatal Med 2006;11:237–45.
20Bouza H. The impact of pain in the immature brain. J Matern Fetal Neonatal Med 2009;22:722–32.
21Anand KJS, Grunau RE, Oberlander T. Developmental character and long-term consequences of pain in infants and children. Child Adolesc Psychiatr Clin N Am 1997;6:703–24.
22Grunau RE. Long-term consequences of pain in human neonates. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in neonates. 2nd ed. Amsterdam: Elsevier Science Publishers B.V.; 2000. p.55–76.
23Whitfield MF, Grunau RE. Behavior, pain perception, and the extremely low-birth weight survivor. Clin Perinatol 2000;27:363–79.
24Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol 2002;29:373–94, vii–viii.
25Peters JW, Schouw R, Anand KJS, et al. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain 2005;114:444–54.
26Anand KJS. Clinical importance of pain and stress in preterm neonates. Biol Neonate 1998;73:1–9.
27Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Semin Perinatol 2007;31:289–97.
28Vanhatalo S, van Nieuwenhuizen O. Fetal pain? Brain Dev 2000;22:145–50.
29Anand KJS, Maze M. Fetuses, fentanyl, and the stress response: signals from the beginnings of pain? Anesthesiology 2001;95:823–5.
30Fisk NM, Gitau R, Teixeira JM, et al. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology 2001 Oct;95(4):828–35.
31Lowery CL, Hardman MP, Manning N, et al. Neurodevelopmental changes of fetal pain. Semin Perinatol 2007;31:275–82.
32Anand KJS. Fetal Pain? Pain: Clinical Updates 2006;14:1–4.
33Lee SJ, Ralston HJ, Drey EA, et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA 2005;294:947–54.
34Anand KJS, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987;317:1321–9.
35Anand KJS, Rovnaghi C, Walden M, et al. Consciousness, behavior, and clinical impact of the definition of pain. Pain Forum 1999;8:64–73.
36Ward-Platt MP, Anand KJS, Aynsley-Green A. The ontogeny of the metabolic and endocrine stress response in the human fetus, neonate and child. Intensive Care Medicine 1989;15(Suppl 1):S44–5.
37Giannakoulopoulos X, Sepulveda W, Kourtis P, et al. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet 1994;344:77–81.
38Smith RP, Gitau R, Glover V, et al. Pain and stress in the human fetus. Eur J Obstet Gynecol Reprod Biol 2000;92:161–5.
39Partsch CJ, Sippell WG, MacKenzie IZ, et al. The steroid hormonal milieu of the undisturbed human fetus and mother at 16-20 weeks gestation. J Clini Endocrinol Metabol 1991;73:969–74.
40Anand KJS. Effects of perinatal pain and stress. Prog Brain Res 2000;122:117–29.
41Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Dev Med Child Neurol 1999;41:696–703.
42Thewissen L, Allegaert K. Analgosedation in neonates: do we still need additional tools after 30 years of clinical research? Arch Dis Child Educ Pract Ed 2011;96:112–8.
43Andrews K, Fitzgerald M. Wound sensitivity as a measure of analgesic effects following surgery in human neonates and infants. Pain 2002;99:185–95.
44Taddio A, Shah V, Gilbert-MacLeod C, et al. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 2002;288:857–61.
45Anand KJS, Coskun V, Thrivikraman KV, et al. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav 1999;66:627–37.
46Anand KJS, Garg S, Rovnaghi CR, et al. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res 2007;62:283–90.
47Howard RF, Hatch DJ, Cole TJ, et al. Inflammatory pain and hypersensitivity are selectively reversed by epidural bupivacaine and are developmentally regulated. Anesthesiology 2001;95:421–7.
48Carbajal R, Rousset A, Danan C, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 2008;300:60–70.
49Losacco V, Cuttini M, Greisen G, et al. Heel blood sampling in European neonatal intensive care units: compliance with pain management guidelines. Arch Dis Child Fetal Neonatal Ed 2011;96:F65–8.
50Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Res Manag 2008;13:25–32.
51Anand KJS, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med 1987;317:1321–9.
52Anand KJS, Grunau RE, Oberlander T. Developmental character and long-term consequences of pain in infants and children. Child Adolesc Psychiatr Clin N Am 1997;6:703–24.
53Whitfield MF, Grunau RE. Behavior, pain perception, and the extremely low-birth weight survivor. Clin Perinatol 2000;27:363–79.
54Grunau R. Early pain in preterm infants. A model of long-term effects. Clin Perinatol 2002;29:373–94, vii–viii.
55Anand KJS, Coskun V, Thrivikraman KV, et al. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav 1999;66:627–37.
56Anand KJS. Effects of perinatal pain and stress. Prog Brain Res 2000;122:117–29.
57Taddio A, Shah V, Gilbert-MacLeod C, et al. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 2002;288:857–61.
58Fitzgerald M, Millard C, MacIntosh N. Hyperalgesia in premature infants. Lancet 1988;1:292.
59Taddio A, Shah V, Atenafu E, et al. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain 2009;144:43–8.
60Liu JG, Rovnaghi CR, Garg S, Anand KJS. Opioid receptor desensitization contributes to thermal hyperalgesia in infant rats. Eur J Pharmacol. May 3 2004;491(2-3):127–136.
61Grunau RE. Long-term consequences of pain in human neonates. In: Anand KJS, Stevens BJ, McGrath PJ, editors. Pain in Neonates. 2nd ed. Amsterdam: Elsevier Science Publishers B.V.; 2000. p. 55–76.
62Anand KJS, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate 2000;77:69–82.
63Anand KJS. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med 2000;6:971–3.
64Narsinghani U, Anand KJS. Developmental neurobiology of pain in neonatal rats. Lab Animal 2000;29:27–39.
65Peters JW, Schouw R, Anand KJS, et al. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain 2005;114:444–54.
66De Lima J, Alvares D, Hatch DJ, et al. Sensory hyperinnervation after neonatal skin wounding: effect of bupivacaine sciatic nerve block. Br J Anaesth 1999;83:662–4.
67Beggs S, Alvares D, Moss A, et al. A role for NT-3 in the hyperinnervation of neonatally wounded skin. Pain 2012;153:2133–9.
68Kmita G, Urmanska W, Kiepura E, et al. Feeding behaviour problems in infants born preterm: a psychological perspective. Preliminary report. Med Wieku Rozwoj 2011;15:216–23.
69Anand KJS, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr 2004;144:449–54.
70Schultz AH, Jarvik GP, Wernovsky G, et al. Effect of congenital heart disease on neurodevelopmental outcomes within multiple-gestation births. J Thorac Cardiovasc Surg 2005;130:1511–6.
71Einaudi MA, Busuttil M, Monnier AS, et al. Neuropsychological screening of a group of preterm twins: comparison with singletons. Childs Nerv Syst 2008;24:225–30.
72Littlejohn C, Pang D, Power C, et al. Is there an association between preterm birth or low birthweight and chronic widespread pain? Results from the 1958 Birth Cohort Study. Eur J Pain 2012;16:134–9.
73Anand KJS. Pain, plasticity, and premature birth: a prescription for permanent suffering? Nat Med 2000;6:971–3.
74Vinall J, Miller SP, Chau V, et al. Neonatal pain in relation to postnatal growth in infants born very preterm. Pain 2012;153:1374–81.
75Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 2009;143:138–46.
76Berman JI, Mukherjee P, Partridge SC, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage 2005;27:862–71.
77Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 2000;284:1939–47.
78Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol 2012;71:385–96.
79Doesburg SM, Ribary U, Herdman AT, et al. Magnetoencephalography reveals slowing of resting peak oscillatory frequency in children born very preterm. Pediatr Res 2011;70:171–5.
80Brummelte S, Grunau RE, Zaidman-Zait A, et al. Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full-term children at 18 months corrected age. Dev Psychobiol 2011;53:184–95.
81Doesburg SM, Ribary U, Herdman AT, et al. Altered long-range alpha-band synchronization during visual short-term memory retention in children born very preterm. Neuroimage 2011;54:2330–9.
82Grunau RV, Whitfield MF, Petrie JH. Pain sensitivity and temperament in extremely low-birth-weight premature toddlers and preterm and full-term controls. Pain 1994;58:341–6.
83Grunau RV, Whitfield MF, Petrie JH, et al. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and fullterm children. Pain 1994;56:353–9.
84Grunau RVE, Whitfield MF, Petrie J. Children’s judgments about pain at age 8-10 years: do extremely low birthweight (<1000 g) children differ from full birthweight peers. J Child Psychol Psychiatr 1998;39:587–94.
85Anand KJS, Coskun V, Thrivikraman KV, et al. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav 1999;66:627–37.
86Anand KJS, Garg S, Rovnaghi CR, et al. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res 2007;62:283–90.
87Bhutta AT, Rovnaghi CR, Simpson PM, et al. Interactions of inflammatory pain and morphine treatment in infant rats: long-term behavioral effects. Physiol Behav 2001;73:51–8.
88Ranger M, Johnston CC, Anand KJ. Current controversies regarding pain assessment in neonates. Semin Perinatol 2007;31:283–8.
89Grunau RE, Holsti L, Whitfield MF, et al. Are twitches, startles, and body movements pain indicators in extremely low birth weight infants? Clin J Pain 2000;16:37–45.
90Boyle EM, Freer Y, Wong CM, et al. Assessment of persistent pain or distress and adequacy of analgesia in preterm ventilated infants. Pain 2006;124:87–91.
91Thewissen L, Allegaert K. Analgosedation in neonates: do we still need additional tools after 30 years of clinical research? Arch Dis Child Educ Pract Ed 2011;96:112–8.
92Stevens BJ, Johnston CC, Grunau RV. Issues of assessment of pain and discomfort in neonates. J Obstet Gynecol Neonatal Nurs 1995;24:849–55.
93Fuller BF. Infant behaviors as indicators of established acute pain. J Soc Pediatr Nurs 2001;6:109–15.
94Duhn LJ, Medves JM. A systematic integrative review of infant pain assessment tools. Adv Neonatal Care 2004;4:126–40.
95Pereira AL, Guinsburg R, de Almeida MF, et al. Validity of behavioral and physiologic parameters for acute pain assessment of term newborn infants. Sao Paulo Med J 1999;117:72–80.
96Franck LS, Ridout D, Howard R, et al. A comparison of pain measures in newborn infants after cardiac surgery. Pain 2011;152:1758–65.
97Suraseranivongse S, Kaosaard R, Intakong P, et al. A comparison of postoperative pain scales in neonates. Br J Anaesth 2006;97:540–4.
98Anand KJS. Pain assessment in preterm neonates. Pediatrics 2007;119:605–7.
99Stevens B, Johnston C, Taddio A, et al. The premature infant pain profile: evaluation 13 years after development. Clin J Pain 2010;26:813–30.
100Stevens B, Johnston C, Petryshen P, et al. Premature Infant Pain Profile: development and initial validation. Clin J Pain 1996;12:13–22.
101McNair C, Ballantyne M, Dionne K, et al. Postoperative pain assessment in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 2004;89:F537–41.
102Lawrence J, Alcock D, McGrath P, et al. The development of a tool to assess neonatal pain. Neonatal Netw 1993;12:59–66.
103Krechel SW, Bildner J. CRIES: a new neonatal postoperative pain measurement score. Initial testing of validity and reliability. Paediatr Anaesth 1995;5:53–61.
104Hummel P, Lawlor-Klean P, Weiss MG. Validity and reliability of the N-PASS assessment tool with acute pain. J Perinatol 2010;30:474–8.
105Hummel P, Puchalski M, Creech SD, et al. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol 2008;28:55–60.
106Uyan ZS, Bilgen H, Topuzoglu A, et al. Comparison of three neonatal pain scales during minor painful procedures. J Matern Fetal Neonatal Med 2008;21:305–8.
107Anand KJS, Aranda JV, Berde CB, et al. Summary proceedings from the neonatal pain-control group. Pediatrics 2006;117:S9–S22.
108Boyle EM, Freer Y, Wong CM, McIntosh N, Anand KJS. Assessment of persistent pain or distress and adequacy of analgesia in preterm ventilated infants. Pain 2006;124:87–91
109Saarenmaa E, Huttunen P, Leppaluoto J, et al. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. J Pediatr 1999;134:144–50.
110Vaughn PR, Townsend SF, Thilo EH, et al. Comparison of continuous infusion of fentanyl to bolus dosing in neonates after surgery. J Pediatr Surg 1996;31:1616–23.
111Guinsburg R, Kopelman BI, Anand KJS, et al. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr 1998;132:954–9.
112Lago P, Benini F, Agosto C, et al. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Arch Dis Child Fetal Neonatal Ed 1998;79:F194–7.
113Orsini AJ, Leef KH, Costarino A, et al. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. J Pediatr 1996;129:140–5.
114Anand KJS, Barton BA, McIntosh N, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med 1999;153:331–8.
115Anand KJS, Hall RW, Desai N, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363:1673–82.
116Bellu R, de Waal K, Zanini R. Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2010;95:F241–51.
117Dyke MP, Kohan R, Evans S. Morphine increases synchronous ventilation in preterm infants. J PaediatrChild Health 1995;31:176–9.
118Lemyre B, Doucette J, Kalyn A, et al. Morphine for elective endotracheal intubation in neonates: a randomized trial [ISRCTN43546373]. BMC Pediatr 2004;4:20.
119Simons SH, Anand KJS. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med 2006;11:260–7.
120Simons SH, van Dijk M, van Lingen RA, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290:2419–27.
121Saarenmaa E, Huttunen P, Leppaluoto J, et al. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. Journal of Pediatrics 1999;134:144–50.
122Vaughn PR, Townsend SF, Thilo EH, et al. Comparison of continuous infusion of fentanyl to bolus dosing in neonates after surgery. J Pediatr Surg 1996;31:1616–23.
123Guinsburg R, Kopelman BI, Anand KJS, et al. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr 1998;132:954–9.
124Lago P, Benini F, Agosto C, et al. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Arch Dis Child Fetal Neonatal Ed 1998;79:F194–7.
125Orsini AJ, Leef KH, Costarino A, et al. Routine use of fentanyl infusions for pain and stress reduction in infants with respiratory distress syndrome. J Pediatr 1996;129:140–5.
126Anand KJS, Barton BA, McIntosh N, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Arch Pediatr Adolesc Med 1999;153:331–8.
127Anand KJS, Hall RW, Desai N, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363:1673–82.
128Bellu R, de Waal K, Zanini R. Opioids for neonates receiving mechanical ventilation: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2010;95:F241–51.
129Dyke MP, Kohan R, Evans S. Morphine increases synchronous ventilation in preterm infants. J Paediatr Child Health 1995;31:176–9.
130Lemyre B, Doucette J, Kalyn A, et al. Morphine for elective endotracheal intubation in neonates: a randomized trial [ISRCTN43546373]. BMC Pediatr 2004;4:20.
131Simons SH, Anand KJS. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med 2006;11:260–7.
132Simons SH, van Dijk M, van Lingen RA, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290:2419–27.
133Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Semin Perinatol 2007;31:289–97.
134Simons SH, Anand KJS. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med 2006;11:260–7.
135Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 2—clinical use. Paediatr Anaesth 1997;7:93–101.
136Hall RW. Anesthesia and analgesia in the NICU. Clin Perinatol 2012;39:239–54.
137Hall RW, Shbarou RM. Drugs of choice for sedation and analgesia in the neonatal ICU. Clin Perinatol 2009;36:15–26.
138Semenikhin AA. Etiology of pruritus after epidural administration of narcotic analgesics. Anesteziol Reanimatol 1988:62–4.
139Saarenmaa E, Huttunen P, Leppaluoto J, et al. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: a randomized trial. J Pediatr 1999;134:144–50.
140Koren G, Butt W, Pape K, et al. Morphine-induced seizures in newborn infants. Vet Hum Toxicol 1985;27:519–20.
141Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Semin Perinatol 2007;31:289–97.
142Fahnenstich H, Steffan J, Kau N, et al. Fentanyl-induced chest wall rigidity and laryngospasm in preterm and term infants. Crit Care Med 2000;28:836–9.
143Lindemann R. Respiratory muscle rigidity in a preterm infant after use of fentanyl during Caesarean section. Eur J Pediatr 1998;157:1012–3.
144Wells S, Williamson M, Hooker D. Fentanyl-induced chest wall rigidity in a neonate: a case report. Heart Lung 1994;23:196–8.
145Anand KJS, Arnold JH. Opioid tolerance and dependence in infants and children. Crit Care Med 1994;22:334–42.
146Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010;125:e1208–25.
147Menon G, Anand KJS, McIntosh N. Practical approach to analgesia and sedation in the neonatal intensive care unit. Semin Perinatol 1998;22:417–24.
148Finnegan LP. Effects of maternal opiate abuse on the newborn. Fed Proc 1985;44.
149Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med 1994;22:763–7.
150Green M, Suffet F. The Neonatal Narcotic Withdrawal Index: a device for the improvement of care in the abstinence syndrome. Am J Drug Alcohol Abuse 1981;8:203–13.
151Franck LS, Harris SK, Soetenga DJ, et al. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med 2008;9:573–80.
152Franck LS, Scoppettuolo LA, Wypij D, et al. Validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) for monitoring iatrogenic withdrawal syndrome in pediatric patients. Pain 2012;153:142–8.
153Ista E, van Dijk M, Gamel C, et al. Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. Assessment remains troublesome. Intensive Care Med 2007;33:1396–406.
154Ista E, van Dijk M, Gamel C, et al. Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation. Crit Care Med 2008;36:2427–32.
155Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010;125:e1208–25.
156Bell RF. Low-dose subcutaneous ketamine infusion and morphine tolerance. Pain 1999;83:101–3.
157Eilers H, Philip LA, Bickler PE, et al. The reversal of fentanyl-induced tolerance by administration of “small-dose” ketamine. Anesth Analg 2001;93:213–4.
158Subramaniam K, Subramaniam B, Steinbrook RA. Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review. Anesth Analg 2004;99:482–95, table of contents.
159Anand KJS, Suresh S. Opioid tolerance in neonates: a state-of-the-art review. Paediatr Anaesth 2001;11:511–21.
160Chana SK, Anand KJS. Can we use methadone for analgesia in neonates? Arch Dis Child Fetal Neonatal Ed 2001;85:F79–81.
161Anand KJS, Arnold JH. Opioid tolerance and dependence in infants and children. Crit Care Med 1994;22:334–42.
162Anand KJS, Willson DF, Berger J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010;125:e1208–25.
163Anand KJS, Suresh S. Opioid tolerance in neonates: a state-of-the-art review. Paediatr Anaesth 2001;11:511–21.
164Chana SK, Anand KJS. Can we use methadone for analgesia in neonates? Arch Dis Child: Fetal Neonatal Ed 2001;85:F79–81.
165Franck L, Vilardi J. Assessment and management of opioid withdrawal in ill neonates. Neonatal Netw 1995;14:39–48.
166Siddappa R, Fletcher JE, Heard AM, et al. Methadone dosage for prevention of opioid withdrawal in children. Paediatr Anaesth 2003;13:805–10.
167Tobias JD, Schleien CL, Haun SE. Methadone as treatment for iatrogenic narcotic dependency in pediatric intensive care unit patients. Crit Care Med 1990;18:1292–3.
168Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev 2000:CD002025.
169Lacroix I, Berrebi A, Chaumerliac C, et al. Buprenorphine in pregnant opioid-dependent women: first results of a prospective study. Addiction 2004;99:209–14.
170O’Connor PG, Fiellin DA. Pharmacologic treatment of heroin-dependent patients. Ann Intern Med 2000;133:40–54.
171Gold MS, Pottash AC, Sweeney DR, et al. Opiate withdrawal using clonidine. A safe, effective, and rapid nonopiate treatment. JAMA 1980;243:343–6.
172Hoder EL, Leckman JF, Ehrenkranz R, et al. Clonidine in neonatal narcotic-abstinence syndrome. N Engl J Med 1981;305:1284.
173Kosten TR, Rounsaville BJ, Kleber HD. Comparison of clinician ratings to self reports of withdrawal during clonidine detoxification of opiate addicts. Am J Drug Alcohol Abuse 1985;11:1–10.
174Osborn DA, Jeffery HE, Cole MJ. Sedatives for opiate withdrawal in newborn infants. Cochrane Database Syst Rev 2005:CD002053.
175Pohl-Schickinger A, Lemmer J, Hubler M, et al. Intravenous clonidine infusion in infants after cardiovascular surgery. Paediatr Anaesth 2008;18:217–22.
176Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs 2004;20:344–51.
177Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Critical Care Medicine 2000;28:2122–32.
178Hall RW. Anesthesia and analgesia in the NICU. Clin Perinatol 2012;39:239–54.
179Walter-Nicolet E, Annequin D, Biran V, et al. Pain management in newborns: from prevention to treatment. Paediatr Drugs 2010;12:353–65.
180Cignacco EL, Sellam G, Stoffel L, et al. Oral sucrose and “facilitated tucking” for repeated pain relief in preterms: a randomized controlled trial. Pediatrics 2012;129:299–308.
181Ward-Larson C, Horn RA, Gosnell F. The efficacy of facilitated tucking for relieving procedural pain of endotracheal suctioning in very low birthweight infants. MCN Am J Matern Child Nurs 2004;29:151–6; quiz 7–8.
182Pereira e Silva Y, Gomez RS, Barbosa RF, et al. Remifentanil for sedation and analgesia in a preterm neonate with respiratory distress syndrome. [see comment] Paediatr Anaesth 2005;15:993–6.
183Anand KJ, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. [Republished in Lancet 1987 Jan 31;1(8527):243–8; PMID: 20928962]. Lancet 1987;1:62–6.
184Anand KJS, Johnston CC, Oberlander TF, et al. Analgesia and local anesthesia during invasive procedures in the neonate. Clin Ther 2005;27:844–76.
185Taddio A, Pollock N, Gilbert-MacLeod C, et al. Combined analgesia and local anesthesia to minimize pain during circumcision. [see comment] Arch Pediatr Adolesc Med 2000;154(6):620–3.
186Couper RT. Methaemoglobinaemia secondary to topical lignocaine/prilocaine in a circumcised neonate. J Paediatr Child Health 2000;36:406–7.
187Biran V, Gourrier E, Cimerman P, et al. Analgesic effects of EMLA cream and oral sucrose during venipuncture in preterm infants. Pediatrics 2011;128:e63–70.
188Bhattacharjee M, Mathur R. Antinociceptive effect of sucrose ingestion in the human. Indian J Physiol Pharmacol 2005;49:383–94.
189Mitchell A, Waltman PA. Oral sucrose and pain relief for preterm infants. Pain Manag Nurs 2003;4:62–9.
190Harrison D, Bueno M, Yamada J, et al. Analgesic effects of sweet-tasting solutions for infants: current state of equipoise. Pediatrics 2010;126:894–902.
191Holsti L, Grunau RE. Considerations for using sucrose to reduce procedural pain in preterm infants. Pediatrics 2010;125:1042–7.
192Mokhnach L, Anderson M, Glorioso R, et al. NICU procedures are getting sweeter: development of a sucrose protocol for neonatal procedural pain. Neonatal Netw 2010;29:271–9.
193Truog R, Anand KJS. Management of pain in the postoperative neonate. Clin Perinatol 1989;16:61–78.
194Anand KJS, Sippell WG, Aynsley-Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. [erratum appears in Lancet 1987 Jan 24;1(8526):234]. Lancet 1987;1(8524):62–6.
195Papacci P, De Francisci G, Iacobucci T, et al. Use of intravenous ketorolac in the neonate and premature babies. Paediatr Anaesth 2004;14:487–92.
196Papacci P, De Francisci G, Iacobucci T, et al. Use of intravenous ketorolac in the neonate and premature babies. Paediatric Anaesth 2004;14:487–92.
197Bouwmeester NJ, Anderson BJ, Tibboel D, et al. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 2004;92:208–17.
198Sammartino M, Garra R, Sbaraglia F, et al. Experience of remifentanil in extremely low-birth-weight babies undergoing laparotomy. Pediatr Neonatol 2011;52:176–9.
199Janvier A, Martinez JL, Barrington K, et al. Anesthetic technique and postoperative outcome in preterm infants undergoing PDA closure. J Perinatol 2010;30:677–82.
200Sammartino M, Garra R, Sbaraglia F, et al. Experience of remifentanil in extremely low-birth-weight babies undergoing laparotomy. Pediatr Neonatol 2011;52:176–9.
0205Anand KJS, Anderson BJ, Holford NHG, et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. Br J Anaesth 2008 Nov;101(5):680-9.
201Anand KJS, Anderson BJ, Holford NHG, et al. Morphine pharmacokinetics and pharmacodynamics in preterm and term neonates: secondary results from the NEOPAIN trial. British Journal of Anaesthesia 2008;101:680–9.
202MacGregor R, Evans D, Sugden D, et al. Outcome at 5-6 years of prematurely born children who received morphine as neonates. Arch Dis Childhood Fetal Neonatal Ed 1998;79(1):F40–3.
203de Graaf J, van Lingen RA, Simons SHP, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain 2011;152:1391–7.


