Pain and Pain Management
Despite national and international efforts, guidelines, standards of practice, position statements, and many important discoveries in the field of pain management in the past three decades, critically ill patients suffer from moderate to severe pain that can be experienced at rest or during routine care.1,2 For instance, chest tube removal, turning, drain removal, and wound care were identified as the most painful procedures by critically ill adults in previous studies.2–4 Despite this situation, pain remains undertreated in most critically ill patients.5 Poor treatment of acute pain may lead to the development of serious complications6,7 and chronic pain syndromes,8–10 which may seriously impact the patient’s functioning, quality of life, and well-being. Such evidence reinforces the importance of providing attention to pain in this specific context of care.
Importance of Pain Assessment
Appropriate pain assessment is the foundation of effective pain treatment. Because pain is recognized as a subjective experience, the patient’s self-report is considered the most valid measure for pain and should be obtained as often as possible.11 Unfortunately in critical care, many factors such as the administration of sedative agents, the use of mechanical ventilation, and altered levels of consciousness may impact communication with patients.12,13 These obstacles make pain assessment more complex. Nevertheless, except for being unable to speak, many mechanically ventilated patients can communicate that they are in pain by using head nodding, hand motions or by seeking attention with other movements.4 In such a situation, use of appropriate communication methods may reduce patients’ distress associated with the presence of the endotracheal tube by enabling them to report the presence of pain or discomfort in a comprehensive way.14
Self-report pain intensity scales have been used with postoperative mechanically ventilated patients who were asked to point on the scale.2,15 However, in a study of mechanically ventilated adults with various diagnoses (trauma, surgical, or medical), only one third of mechanically ventilated patients were able to use a pain intensity scale.15 With a greater degree of critical illness, providing a pain intensity self-report becomes more difficult because it requires concentration and energy. When the patient is unable to communicate in any way, observable behavioral indicators become unique indices for pain assessment and are part of clinical guidelines and recommendations developed in North America.16,17 Pain is frequently encountered in critical care, and there is increased emphasis on the professional responsibility to manage the patient’s pain effectively. The critical care nurse must understand the mechanisms, assessment process, and appropriate therapeutic measures for managing pain.
Definition and Description of Pain
Pain is described as an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.18 This definition emphasizes the subjective and multidimensional nature of pain. More specifically, the subjective characteristic implies that pain is whatever the person experiencing it says it is and that it exists whenever he or she says it does.19 This definition also suggests that the patient is able to self-report. However, in the critical care context, many patients are unable to self-report their pain. Some authors20 have proposed an alternative definition for nonverbal patients, in this case infants, but the same principle applies to any non-verbal population, stating that changes in behaviors caused by pain are valuable forms of self-report and should be considered as alternative measures of pain. Based upon this, pain assessment must be designed to conform to the communication capabilities of the patient.
Components of Pain
The experience of pain includes sensory, affective, cognitive, behavioral, and physiologic components21:
The sensory component is the perception of many characteristics of pain, such as intensity, location, and quality.
The affective component includes negative emotions such as unpleasantness, anxiety, fear, and anticipation that may be associated with the experience of pain.
The cognitive component refers to the interpretation or the meaning of pain by the person who is experiencing it.
The behavioral component includes the strategies used by the person to express, avoid, or control pain.
The physiologic component refers to nociception and the stress response.
Types of Pain
Pain can be acute or chronic, with different sensations related to the origin of the pain.
Acute Pain
Acute pain has a short duration, and it usually corresponds to the healing process (30 days), but should not exceed 6 months. It implies tissue damage that is usually from an identifiable cause. If undertreated, acute pain may bring a prolonged stress response and lead to permanent damage to the patient’s nervous system. In such instance, acute pain can become chronic.8,9
Chronic Pain
Chronic pain persists for more than 6 months after the healing process from the original injury, and it may or may not be associated with an illness.22 It develops when the healing process is incomplete or, as described earlier, when acute pain is poorly managed.23
Both acute and chronic pain can have a nociceptive or neuropathic origin.11
Nociceptive Pain
Nociceptive pain arises from activation of nociceptors,11 and it can be somatic or visceral. Somatic pain involves superficial tissues, such as the skin, muscles, joints, and bones. Its location is well defined. Visceral pain involves organs such as the heart, stomach, and liver. Its location is diffuse, and it can be referred to a different location in the body.24 Interestingly, not all organs are sensitive to pain and some can be damaged quite extensively without the patient feeling a thing. For instance, many diseases of the liver, the lungs or the kidneys are completely painless and the only symptoms felt are those derived from the abnormal functioning of these organs. On the other hand, relatively minor lesions in viscera such as the stomach, the bladder, or the ureters can produce excruciating pain, as these organs are abundantly innervated by sensory neurons that signal harmful events.25
Neuropathic Pain
Neuropathic pain arises from a lesion or disease affecting the somatosensory system.11 The origin of neuropathic pain may be peripheral or central. Neuralgia and neuropathy are examples related to peripheral neuropathic pain, which implies a damage of the peripheral somatosensory system. Central neuropathic pain involves the central somatosensory cortex and can be experienced by patients after a cerebral stroke. Neuropathic pain can be difficult to manage and frequently requires a multimodal approach (i.e., the combinations of several pharmacologic and/or nonpharmacologic treatments).24
Physiology of Pain
Nociception
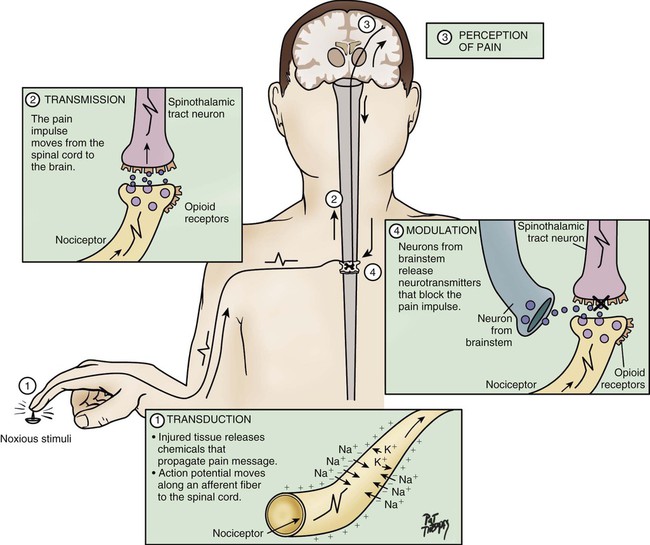
Nociception represents the neural processes of encoding and processing noxious stimuli necessary, but not sufficient, for pain.11 Pain results from the integration of the pain-related signal into specific cortical areas of the brain associated with higher mental processes and consciousness. In other words, pain is the conscious experience that emerges from nociception.26 Four processes are involved in nociception24:
The four processes are shown in Figure 9-1, which integrates pain assessment with nociception, and in Figure 9-2.
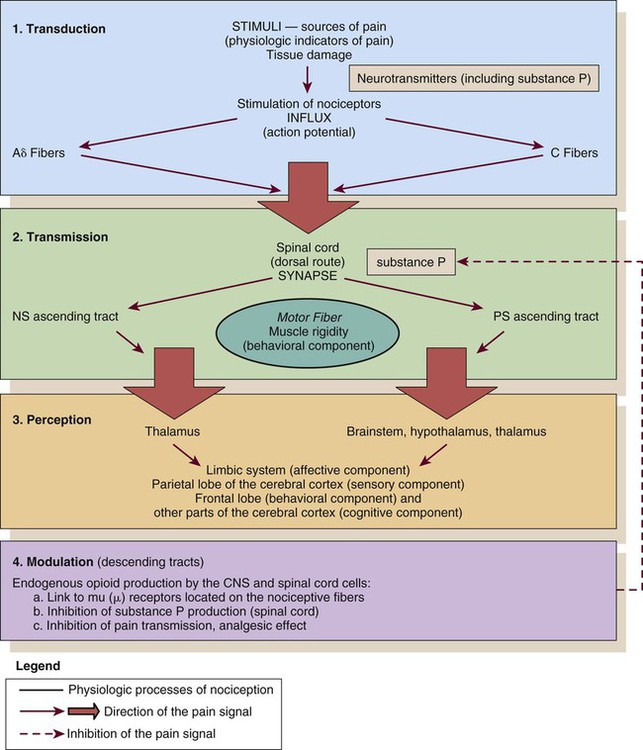
CNS, central nervous system; NS, neospinothalamic pathway; PS, paleospinothalamic pathway. (Courtesy Céline Gélinas, Ingram School of Nursing, McGill University, Canada.)
Transmission
As a result of transduction, an action potential is produced and is transmitted by nociceptive nerve fibers in the spinal cord that reach higher centers of the brain. This is called transmission, and it represents the second process of nociception. The principal nociceptive fibers are the A-delta (Aδ) and C fibers. Large-diameter, myelinated Aδ fibers that transmit well-localized, sharp pain are involved in “first pain” sensation, which leads to reflex withdrawal. Small-diameter, unmyelinated C fibers transmit diffuse, dull, aching pain, which is referred to as “second pain.”26 These fibers transmit the noxious sensation from the periphery through the dorsal root of the spinal cord. With the liberation of substance P, these fibers then synapse with ascending spinothalamic fibers to the central nervous system (CNS). These spinothalamic fibers are clustered into two specific pathways: neospinothalamic (NS) and paleospinothalamic (PS) pathways. Generally, the Aδ fibers transmit the pain sensation to the brain within the NS pathway, and the C fibers use the PS pathway.27
Through synapsing of nociceptive fibers with motor fibers in the spinal cord, muscle rigidity can appear because of a reflex activity.28 Muscle rigidity can be a behavioral indicator associated with pain. It can contribute to immobility and decrease diaphragmatic excursion. This can lead to hypoventilation and hypoxemia. Hypoxemia can be detected by a pulse oximeter (Spo2) and by oxygen arterial pressure (Pao2) monitoring. A ventilated patient’s interaction with the machine (e.g., activation of alarms, fighting the ventilator) also may indicate the presence of pain.29
Perception
The pain message is transmitted by the spinothalamic pathways to centers in the brain, where it is perceived. Pain sensation transmitted by the NS pathway reaches the thalamus, and the pain sensation transmitted by the PS pathway reaches brainstem, hypothalamus, and thalamus.27 These parts of the CNS contribute to the initial perception of pain. Projections to the limbic system and the frontal cortex allow expression of the affective component of pain.30 Projections to the sensory cortex located in the parietal lobe allow the patient to describe the sensory characteristics of his or her pain, such as location, intensity, and quality.30,31 The cognitive component of pain involves many parts of the cerebral cortex and is complex. These three components (affective, sensory, and cognitive) represent the subjective interpretation of pain. Parallel to this subjective process, certain facial expressions and body movements are behavioral indicators of pain occurring as a result of pain fiber projections to the motor cortex in the frontal lobe.
Modulation
Modulation is a process by which noxious stimuli that travel from the nociceptive receptors to the CNS may be enhanced or inhibited. Pain can be modulated by ascending and descending mechanisms. A typical example of ascending pain modulation is rubbing an injury site, thus activating large A-beta (Aβ) fibers in the periphery. Stimulation of these fibers activates inhibitory interneurons in the dorsal horn of the spinal cord, effectively preventing nociceptive signal transmission from the periphery to the higher brain regions. The physiologic basis of this mechanism of pain modulation was elucidated by Melzack and Wall in 196532 and refers to the Gate Control Theory (GCT). Analgesia may also be produced at the level of the spinal cord and the brainstem (spinothalamic pathway) via the release of endogenous opioids and neurotransmitters. Endogenous opioids are naturally occurring morphine-like pentapeptides found throughout the nervous system and exist in three general classes: beta-endorphins, enkephalins, and dynorphins. These substances block neuronal activity related to nociceptive impulses by binding to opioid mu (µ) receptor sites in the central and peripheral nervous systems.27 In the ascending pain modulation mechanism, endogenous opioids may be produced in the brainstem, and the dorsal horn or exogenous opioids may be introduced by administration of an opioid analgesic. The released or introduced opioids bind to the mu-opioid receptors on nociceptive nerve fibers, blocking the release of substance P. In the descending pain modulation mechanism, the efferent spinothalamic nerve fibers that descend from the brain can inhibit the propagation of the pain signal by triggering the release of endogenous opioids in the brain stem and in the spinal cord. Serotonin and norepinephrine are important inhibitory neurotransmitters that act in the CNS. These substances are also released by the descending fibers of the descending spinothalamic pathway.33 The use of distraction, relaxation and imagery techniques can facilitate the release of endogenous opioids, and has been shown to reduce the overall pain experience.34
Biologic Stress Response
A biologic stress response is activated by pain, an obvious stressor.29 This stress response involves the nervous, endocrine, and immune systems in the hypothalamic-pituitary-adrenal axis (HPA).35 The biologic stress response includes a short-term direct response, a midterm response, and a long-term indirect response. Stress mechanisms are depicted in Figure 9-3.
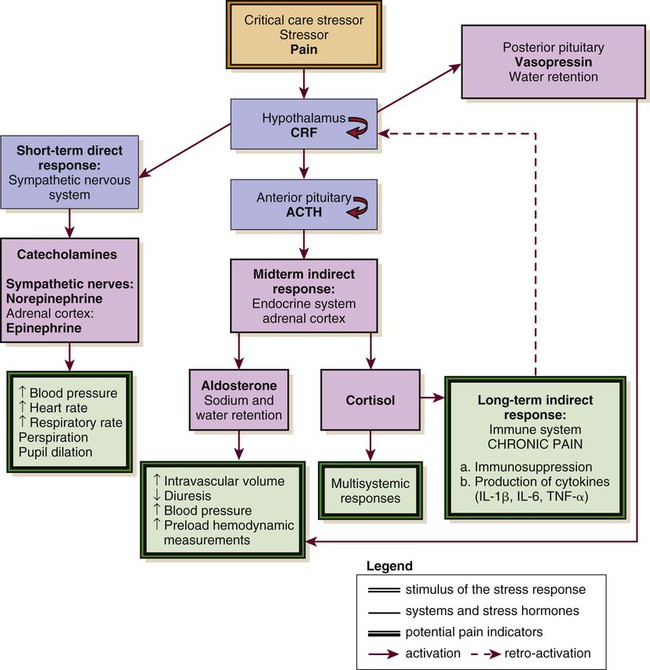
ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor; IL, interleukin; TNS, tumor necrosis factor. (Courtesy Céline Gélinas, Ingram School of Nursing, McGill University, Canada.)
Short-Term Direct Response
In the presence of a stressor such as pain, the hypothalamus releases corticotropin-releasing factor (CRF), which activates the sympathetic nervous system (SNS). Norepinephrine is then released from the terminals of sympathetic nerves, and epinephrine is released from the adrenal cortex. This mechanism constitutes the short-term direct stress response. The effects of these stress hormones allow observation of physiologic responses associated with activation of SNS. For instance, increased blood pressure, increased heart rate, and increased respiratory rate are common signs of acute pain.15,29,36 Moreover, pupil dilation can be observed.37
If pain persists over time or injuries are located in the bladder or the intestines, the parasympathetic nervous system (PNS) may be dominant. The blood pressure and heart rate may decrease rather than increase. The absence of pain-related indicators related to the activation of the SNS does not necessarily imply an absence of pain sensation.38
Long-Term Indirect Response
Long term, the stress hormones, specifically cortisol, influence the immune system in two ways: immunosuppression and release of cytokines.39 Cytokines may prolong by retroactivation the release of cortisol, which may exacerbate tissue damage, contributing to the chronic pain process.23
In summary, the biologic stress response allows observation of fluctuations in physiologic signs that represent a source of stress and may be associated with acute pain. The short-term signs are mainly related to SNS activation. Other signs, such as decreased diuresis and increased CVP and PAOP, relate to the midterm indirect stress response. The immune system is involved in the long-term indirect response of stress. No acute pain indicators have been associated with this process. All the indicators identified within the biologic stress response are not specific to pain because they can be attributed to other distress conditions, homeostatic changes, and medications.16
Framework for Pain Assessment and Definition
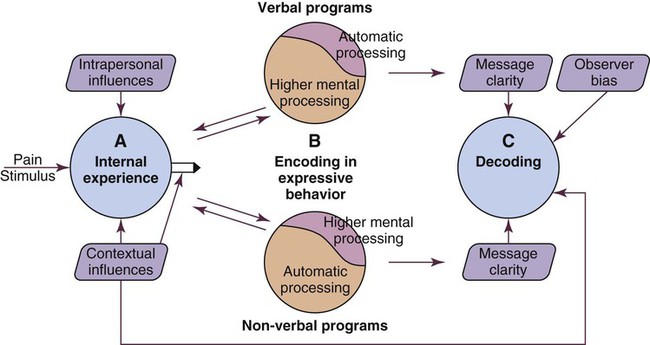
As previously stated, self-report of pain is not always possible to obtain in critically ill patients as many of them may be unable to communicate during their stay in the critical care unit. When the patient is unable to self-report, the expression of pain can be examined from the perspective of the Communications Model of Pain.40 The foundation of this model is that observational measures capture behaviors that are less subject to voluntary control and more automatic in comparison with self-report measures that depend on higher mental processes. Consequently, observational measures should be used to assess pain when the individual’s self-report is not available, as is often the case in critically ill patients. This A → B → C model conceptualizes pain as an internal state (A) that may be encoded in particular features of expressive behaviors (B), allowing observers (in this case nurses) to draw inferences (C) about the nature of the sender’s experience (Fig. 9-4).
Pain Assessment
Pain Assessment: The Subjective Component
Pain is known as a subjective experience. The subjective component of pain assessment refers to the patient’s self-report about his or her sensorial, affective, and cognitive experience of pain. Because it is considered the most valid measure of pain, the patient’s self-report must be obtained whenever possible.16 A simple yes or no (presence versus absence of pain) is a valid self-report. Mechanical ventilation should not be a barrier for nurses to document patients’ self-reports of pain. Many mechanically ventilated patients can communicate that they have pain or can use pain scales by pointing to numbers or symbols on the scale.2,4,15 Attempts should be made before concluding that a patient is unable to self-report. More importantly, sufficient time should be allowed for the patient to respond with each attempt.16
If sedation and cognition levels allow the patient to give more information about pain, a multidimensional assessment can be documented. Multidimensional pain assessment tools including the sensorial, emotional, and cognitive components are available, such as the Brief Pain Inventory,41 the Initial Pain Assessment Tool,24 and the McGill Pain Questionnaire–Short Form.42 However, because of the administration of sedative and analgesic agents in mechanically ventilated patients, the tool must be short enough to be completed. For instance, the McGill Pain Questionnaire–Short Form takes 2 to 3 minutes to complete and has been used to assess mechanically ventilated patients who were in a stable condition.2
The patient’s self-report of pain can also be obtained by questioning the patient using the mnemonic PQRSTU43:
Q: Quality
The Q in the mnemonic refers to the quality of the pain or the pain sensation that the patient is experiencing. For instance, the patient may describe the pain as dull, aching, sharp, burning, or stabbing. This information provides the nurse with data regarding the type of pain the patient is experiencing (e.g., somatic or visceral). The differentiation between types of pain may contribute to the determination of cause and management. A patient who has had open-heart surgery may complain of chest pain that is shooting or burning.4 This information can lead the nurse to investigate for cutaneous or bone injuries as a result of a sternotomy. Another patient may describe a sharp thoracic pain that may lead the nurse to consider visceral pain as a result of pulmonary embolism. A verbal description of pain is important because it provides a baseline account, allowing the critical care nurse to monitor changes in the type of pain, which may indicate a change in the underlying pathology.
R: Region or Location, Radiation
R usually is easy for the patient to identify, although visceral pain is more difficult for the patient to localize.24 If the patient has difficulty naming the location or is mechanically ventilated, ask the patient to point to the location on himself or herself or on a simple anatomic drawing.44
S: Severity and Other Symptoms
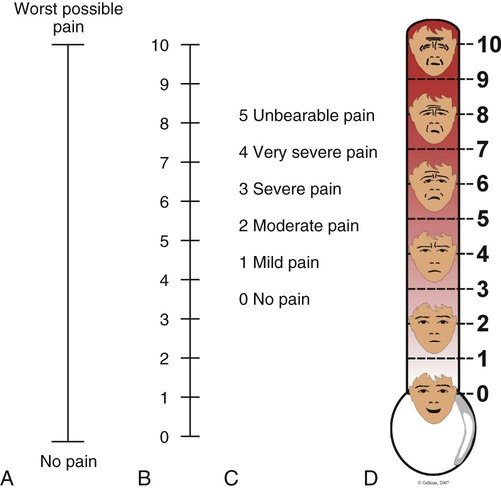
S, the severity or intensity of pain, is a measurement that has undergone much investigation. Many pain intensity scales are available, including the descriptive and numeric pain rating scales that are often used in the critical care environment (Fig. 9-5). Many critical care units use a specific pain intensity scale. The use of a single tool provides consistency of assessment and documentation. Employment of a pain intensity scale is useful in the critical care environment. Asking the patient to grade his or her pain on a scale of 0 to 10 is a consistent method and aids the nurse in objectifying the subjective nature of the patient’s pain. However, the patient’s tool preference should be considered.
Pain Assessment: The Observable or Objective Component
When the patient’s self-report is impossible to obtain, nurses can rely on the observation of behavioral indicators, which are strongly emphasized in clinical recommendations and guidelines for pain management in nonverbal patients.16,17 Fluctuations in vital signs should never be used alone but rather considered as a cue to begin further assessment for pain.
Pain-related behaviors have been described in critically ill patients and were also studied in the AACN Thunder Project II.45 Patients who experienced pain during nociceptive procedures were three times more likely to have increased behavioral responses such as facial expressions, muscle rigidity, and vocalization than patients without pain. Similar observations were found in a study of 257 mechanically ventilated critically ill adults.30 Patients who experienced pain during turning showed significantly more intense facial expressions (e.g., grimacing), muscle rigidity, and less compliance with the ventilator (e.g., fighting the ventilator) compared with patients without pain. Behavioral indicators are strongly recommended for pain assessment in nonverbal patients,16 and several tools have been developed and tested in critically ill adults including the Behavioral Pain Scale (BPS),46 the Critical-Care Pain Observation Tool (CPOT),47 the NonVerbal Pain Scale (NVPS),48 the Pain Behavioral Assessment Tool (PBAT),46 and the Pain Assessment and Intervention Notation (PAIN) algorithm.39 The BPS and the CPOT are supported by experts in critical care49,50 and are suggested for use in medical, postoperative, and non-brain trauma critically ill adults unable to self-report in the clinical guidelines of the Society of Critical Care Medicine (SCCM).17 Moreover, their implementation in critical care units has led to enhanced nursing practices of pain assessment and management,51 and improved patient outcomes including shorter durations of mechanical ventilation and stay in the critical care unit.52
Behavioral Pain Scale
The BPS shown in Table 9-1 was tested mostly in nonverbal mechanically ventilated patients with altered levels of consciousness.46,53–56 Its validity was supported with significantly higher BPS scores during nociceptive procedures (e.g., turning, endotracheal suctioning, peripheral venous cannulation) compared with rest or nonnociceptive procedures (e.g., arterial catheter dressing change, compression stocking applications, eye care). The authors of the BPS determined a cut-off score greater than 5 for the presence of pain. A positive association was found between nurses’ BPS ratings and conscious sedated patients’ self-report of pain intensity during turning.54 Good interrater reliability of BPS scores between several raters including nurses was reached. The BPS can be used quickly (2 to 5 minutes), and most clinicians were satisfied with its ease of use.46 However, some expressed concerns about the lack of conceptual clarity of certain items.49 For instance, scores of 3 (i.e., fighting ventilator) and 4 (i.e., unable to control ventilation) for compliance with the ventilator category may be ambiguous. Similarly, movements with upper limbs category may be confused with muscle tension.
TABLE 9-1
| ITEM | DESCRIPTION | SCORE |
| Facial expression | Relaxed | 1 |
| Partially tightened (e.g., brow lowering) | 2 | |
| Fully tightened (e.g., eyelid closing) | 3 | |
| Grimacing | 4 | |
| Upper limbs | No movement | 1 |
| Partially bent | 2 | |
| Fully bent with finger flexion | 3 | |
| Permanently retracted | 4 | |
| Compliance with ventilation | Tolerating movement | 1 |
| Coughing but tolerating ventilation for most of the time | 2 | |
| Fighting ventilator | 3 | |
| Unable to control ventilation | 4 | |
| Total | 3 to 12 |
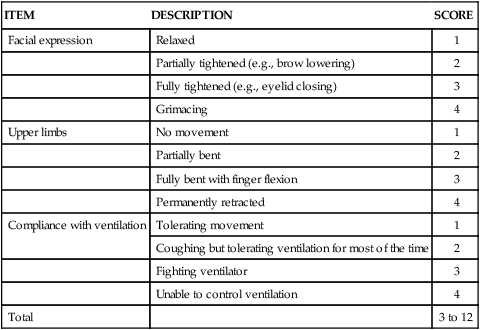
From Payen JF, et al. Assessing pain in the critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258.
Critical-Care Pain Observation Tool
The CPOT shown in Table 9-2 was tested in verbal and nonverbal, critically ill adult patients.15,29,47,57 Content validity was supported by critical care unit expert clinicians, including nurses and physicians.58 Validity of the CPOT was supported with significantly higher CPOT scores during a nociceptive procedure (e.g., turning with or without other care) compared with rest or a nonnociceptive procedure (e.g., taking blood pressure). Positive associations were found between the CPOT scores and the patient’s self-report of pain.15,29,47 A cut-off score greater than 2 was established with the CPOT in postoperative critical care unit adults.59 Similarly to the BPS, good interrater reliability of CPOT scores was achieved with critical care nurses.15,47 Feasibility and clinical utility of the CPOT were positively evaluated by critical care nurses.60 Nurses agreed that the CPOT was quick enough to be used in the critical care unit, simple to understand, easy to complete, and helpful for nursing practice. An online teaching video to learn how to use the CPOT at the bedside is available (see Table 9-2).
TABLE 9-2
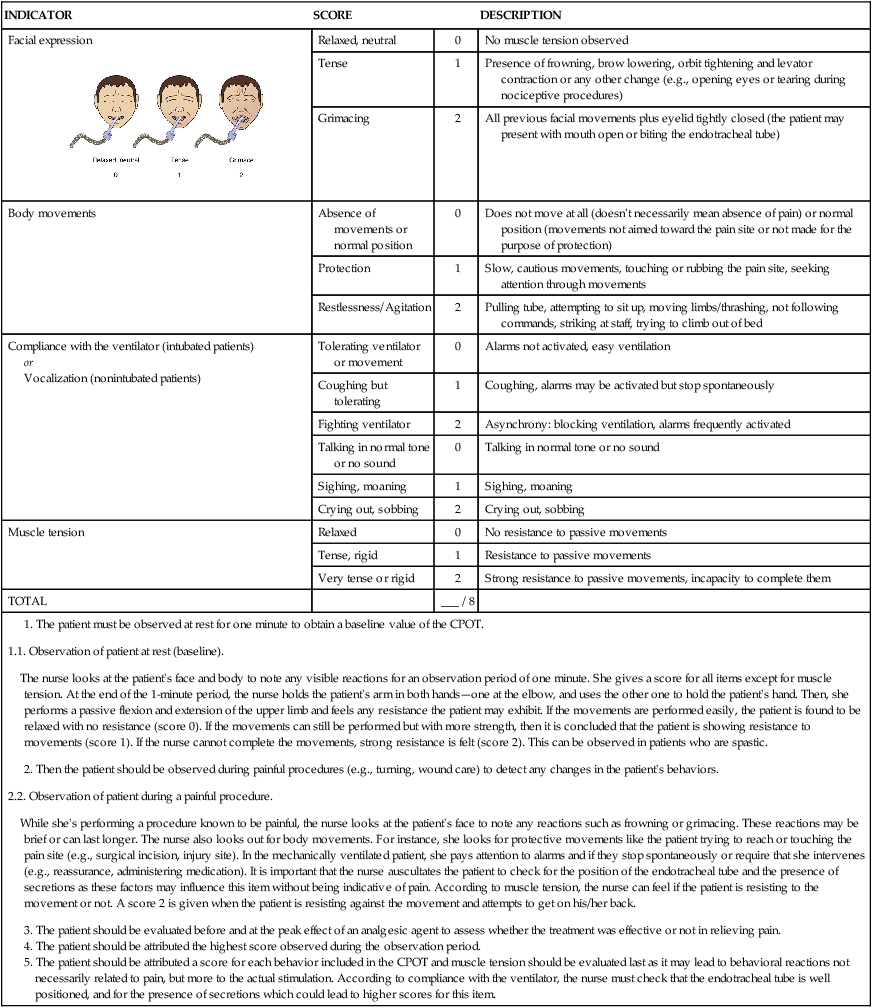
CRITICAL CARE PAIN OBSERVATION TOOL (CPOT)
| INDICATOR | SCORE | DESCRIPTION | |
Facial expression
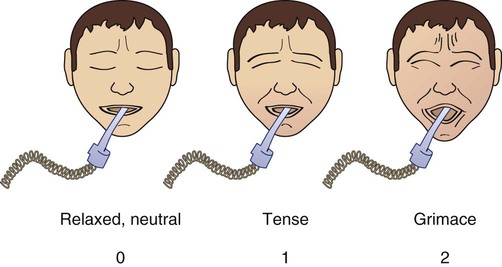 |
Relaxed, neutral | 0 | No muscle tension observed |
| Tense | 1 | Presence of frowning, brow lowering, orbit tightening and levator contraction or any other change (e.g., opening eyes or tearing during nociceptive procedures) | |
| Grimacing | 2 | All previous facial movements plus eyelid tightly closed (the patient may present with mouth open or biting the endotracheal tube) | |
| Body movements | Absence of movements or normal position | 0 | Does not move at all (doesn’t necessarily mean absence of pain) or normal position (movements not aimed toward the pain site or not made for the purpose of protection) |
| Protection | 1 | Slow, cautious movements, touching or rubbing the pain site, seeking attention through movements | |
| Restlessness/Agitation | 2 | Pulling tube, attempting to sit up, moving limbs/thrashing, not following commands, striking at staff, trying to climb out of bed | |
| Compliance with the ventilator (intubated patients) or Vocalization (nonintubated patients) |
Tolerating ventilator or movement | 0 | Alarms not activated, easy ventilation |
| Coughing but tolerating | 1 | Coughing, alarms may be activated but stop spontaneously | |
| Fighting ventilator | 2 | Asynchrony: blocking ventilation, alarms frequently activated | |
| Talking in normal tone or no sound | 0 | Talking in normal tone or no sound | |
| Sighing, moaning | 1 | Sighing, moaning | |
| Crying out, sobbing | 2 | Crying out, sobbing | |
| Muscle tension | Relaxed | 0 | No resistance to passive movements |
| Tense, rigid | 1 | Resistance to passive movements | |
| Very tense or rigid | 2 | Strong resistance to passive movements, incapacity to complete them | |
| TOTAL | ___ / 8 | ||
|
1. The patient must be observed at rest for one minute to obtain a baseline value of the CPOT. 2. Then the patient should be observed during painful procedures (e.g., turning, wound care) to detect any changes in the patient’s behaviors. 3. The patient should be evaluated before and at the peak effect of an analgesic agent to assess whether the treatment was effective or not in relieving pain. 4. The patient should be attributed the highest score observed during the observation period. 5. The patient should be attributed a score for each behavior included in the CPOT and muscle tension should be evaluated last as it may lead to behavioral reactions not necessarily related to pain, but more to the actual stimulation. According to compliance with the ventilator, the nurse must check that the endotracheal tube is well positioned, and for the presence of secretions which could lead to higher scores for this item. |
|||
An online teaching video funded and created by Kaiser Permanente Northern California Nursing Research (KPNCNR) to learn how to use the CPOT at the bedside is available at http://pointers.audiovideoweb.com/stcasx/il83win10115/CPOT2011-WMV.wmv/play.asx.
Modified from Gélinas C, et al. Validation of the Critical-Care Pain Observation Tool (CPOT) in adult patients. Am J Crit Care. 2006;15:420. Figure of facial expressions a courtesy of Caroline Arbour, RN, BSc, PhD candidate, McGill University, Canada, and redrawn by Elsevier.
Use of Cut-Off Scores
A cut-off score refers to the score on a specific scale associated with the best probability of correctly ruling in or ruling out a patient with a specific condition—in this case pain. The use of a cut-off score with behavioral pain scales can help to identify when pain is highly likely to be present, and guide nurses in determining whether an intervention to alleviate pain is required or not. Also, a cut-off score can help to evaluate the effectiveness of pain-management interventions. It is important to highlight that cut-off scores are established using a criterion (i.e., a gold standard in the field). As mentioned previously, in the case of pain, the patient’s self-report is known as the gold standard criterion.11 For a case example showing how a cut-off score can be used in practice, refer to Box 9-1.
Limitations Related to the Use of Behavioral Pain Scales
Behaviors have been validated for pain assessment in critically ill patients, but they present some limitations. In fact, they are impossible to monitor in patients unable to respond behaviorally to pain such as those suffering from paralysis or under the effects of neuromuscular blocking agents. Also, behavioral responses may be blurred with the administration of high doses of sedative agents.15 Indeed, minimal behavioral responses to painful procedures were found in unconscious, mechanically ventilated critically ill adults who were more heavily sedated compared with conscious patients.29 Similar results were found in previous studies in which patients who received a higher dose of midazolam obtained a lower score on the BPS.56
In addition, behavioral pain scales developed for nonverbal critically ill patients may not be applicable for those with a brain injury and an altered level of consciousness as they were found to exhibit atypical behavioral responses to pain.29,61,62 Instead of frowning and grimacing, brain-injured patients with altered levels of consciousness seemed to react mostly by opening their eyes, showing tears, opening their mouth, and exhibiting repetitive movements of the lower limbs when exposed to pain. Further studies are needed to better understand how brain-injured patients react to painful procedures. Therefore existing behavioral pain scales may be inappropriate for this specific vulnerable group. When selecting a scale, nurses should make sure that is has been tested in a patient population and context in which they plan to use it. Indeed, a scale can only be shown to be valid with a specific group of people and in a given context.63
Physiologic Indicators
When patients cannot react behaviorally to pain, the only possible clues left for the detection of pain are physiologic indicators (i.e., vital signs). Although vital sign values generally increase during painful procedures,15,29,36,46,56 they are not consistently related to the patient’s self-report of pain, nor are they predictive of pain.15,29 For example, none of the monitored vital signs (heart rate, mean arterial pressure [MAP], respiratory rate, transcutaneous oxygen saturation [Spo2], and end-tidal CO2) predicted the presence of pain in critically ill patients.29
In the ASPMN recommendations and the SCCM guidelines, it is stated that vital signs should not be considered as primary indicators of pain because they can be attributed to other distress conditions, homeostatic changes, and medications. Changes in vital signs should instead be considered a cue to begin further assessment of pain or other stressors.16,17 Physiologic measures other than vital signs can support the nurses in detecting the presence of pain in nonverbal critically ill patients especially when behavioral indicators are no longer available.
Cerebral Monitoring and Pain Assessment
Other than vital signs, human brain reactivity has been studied using brain imaging technology such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) in healthy individuals and in patients with clinical pain conditions.64 Many regions of the brain are involved in the perception of pain, including the somatosensory cortex, the frontal cortex, and the thalamus. The anatomic connections between these regions suggest that they function in an interactive way in encoding the different aspects of pain (sensory and affective components of pain). For instance, the somatosensory cortex plays a major role in processing the sensory component of pain, whereas the frontal cortex appears to reflect the affective component of pain.26 A closer look into brain activity may elucidate how pain inputs are first received and processed within the cerebral cortex, offering a direct and more precise indicator of pain.
Electroencephalogram
Continuous electroencephalographic (cEEG) activity is being used more frequently in critical care units, especially in the brain-injured population, to detect epileptic activity and ischemia.65 So far, few studies have examined the EEG reactivity of patients when exposed to pain. Frontal EEG activation was observed in 34 critically ill infants when exposed to a noxious stimulation (i.e. heelstroke).66 In a recent study with 32 cardiac surgery patients in a critical care unit, EEG activation was observed over the somatosensory cortex during chest tube removal only in patients who did not receive preprocedural analgesics (i.e., morphine). Interestingly, self-reported pain intensity of patients who did not receive morphine was higher than those who were administered morphine prior to chest tube removal.67
Bispectral Index
Another innovative technology, the Bispectral Index (BIS), is being explored for its relevance in the pain assessment process of critically ill, sedated patients.37,68 The primary utility of the BIS is as an objective measure of sedation levels during surgery in the operating room or during neuromuscular blocking in the critical care unit. This noninvasive monitor uses electrodes placed on the forehead, and displays a signal-processed electroencephalogram (EEG) with a digital number from 0 (flat EEG activity) to 100 (fully awake) that relates to the depth of sedation. An electromyographic (EMG) sensor that reflects muscle stimulation of the forehead is included to identify EMG artifact.
A study of 48 mechanically ventilated and sedated critically ill patients after cardiac surgery reported that the BIS value significantly increased when patients were exposed to a noxious stimulation (turning or endotracheal suctioning) instead of a nonnoxious procedure (gentle touch). However, the most commonly reported pain-related behaviors (e.g., facial expressions, body movements, tense posture, ventilator asynchrony) were not induced by the noxious stimulation in deeply sedated patients, highlighting the limitation of behaviors for pain assessment in heavily sedated critically ill patients. In a more recent study with nine sedated and mechanically ventilated critically ill patients,68 the median BIS value increased from 20% to 30% between rest and the painful procedures (turning and endotracheal suctioning). As opposed to the previous study, behavioral responses were exhibited by the patients with an increase in the median CPOT score from 0 to 3 during the procedures.
The new bilateral BIS is an alternative option to the conventional BIS monitoring system. The bilateral BIS allows the recording of EEG and EMG information of both hemispheres separately (i.e., BIS-Left and BIS-Right). As a result, the bilateral BIS could be particularly useful for pain assessment in patients with suppression of unilateral brain function, such as those with a cerebral stroke or a traumatic brain injury (TBI). For instance, in a recent pilot study with 12 critically ill TBI with altered level of consciousness,69 increases in BIS-Left (>6.6%) and BIS-Right (>7.2%) were observed in patients when exposed to turning compared with rest or a nonpainful procedure. Interestingly, the BIS increase was more pronounced on the noninjured side of the brain. Based on available study findings, the BIS may be an interesting technique to further study in critical care pain assessment because of its noninvasive nature and its suitability for use at the bedside.
Pain as a Vital Sign
Because pain is considered as another vital sign, including pain assessment with other routinely documented vital signs may help ensure that pain is assessed and controlled for in all patients on a regular basis. This approach can ensure that pain is detected and treatment implemented before the patient develops complications associated with unrelieved pain. The use of a pain flow sheet in critical care settings allows for a visible and ongoing pain assessment before and after an intervention for pain that is accessible to all clinicians involved in the assessment and management of pain.51,70
Patient Barriers to Pain Assessment and Management
Communication
The patient’s family can contribute significantly in the assessment of pain. The family is intimately familiar with the patient’s normal responses to pain and can assist the nurse in identifying clues. A family member’s impression of a patient’s pain should be considered in the pain assessment process of the critically ill patient.16
Altered Level of Consciousness and Unconsciousness
The patient either unconscious or with an altered level of consciousness presents a dilemma for all clinicians. Because pain relies on cortical response to provide recognition, the belief that the patient with a brain injury altering higher cortical function has no perception of pain may persist. Conversely, the inability to interpret the nociceptive transmission does not negate the transmission. Interviews by Lawrence71 with 100 patients, who recalled their experiences from a time when they were unconscious in critical care, revealed that they could hear, understand, and respond emotionally to what was being said. Experts recommend assuming that patients who are unconscious or with an altered level of consciousness have pain and that they be treated the same way as conscious patients are treated when they are exposed to sources of pain.16 It has been demonstrated that behavioral and physiologic indicators of pain can be observed in reaction to a painful procedure in critically ill patients, no matter what their level of consciousness.29 Moreover, it has been shown that some cortical activation related to pain perception is still present in unconscious patients in a neurovegetative state.72 Knowing this, the critical care nurse can initiate a discussion with the other members of the health care team to formulate a plan of care for the patient’s comfort.
Older Adult Patients
Many older adult patients do not complain much about pain. Some misconceptions, such as believing that pain is a normal consequence of aging or being afraid to disturb the health care team, are barriers to pain expression for older adults.24 Cognitive deficits or delirium present additional pain assessment barriers. Many older adult patients with mild to moderate cognitive impairments and even some with severe impairment are able to use pain intensity scales.73,74 Vertical pain intensity scales are more easily understood by this group of patients and are recommended16 (see Fig. 9-5). Older adult patients with cognitive deficits should receive repeated instructions and be given sufficient time to respond. When the self-report of pain is impossible to obtain, direct observation of pain-related behaviors is highly recommended in this population.16,74 More than 24 behavioral tools have been developed for older adult patients with cognitive deficits.75 The Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC),76 Doloplus-2,77 and the Pain Assessment in Advanced Dementia (PAINAD)78 are promising tools that are recommended by experts.75,79
Delirium is a form of transient cognitive impairment that is highly prevalent among older adult patients in the critical care unit.80 A major challenge with delirium is that there is overlap between delirium behaviors and pain-related behaviors. It remains unclear whether pain behavioral tools may assist the nurse in the detection of pain in older adult patients during episodes of delirium. Because pain is a modifiable factor of delirium, it can be controlled with adequate pain management.81
Neonates and Infants
Two-way verbal communication is impossible with critically ill infants, and this remains an important barrier to pain assessment. The misconception that preterm neonates were incapable of pain sensation has persisted for a long time. Evidence supports that term and preterm neonates have the anatomic and functional capacity for pain sensation at birth.82 One critical review examined more than 35 instruments used to assess pain in neonates and infants.83 The Premature Infant Pain Profile (PIPP)84 is the most recommended valid tool for pain assessment of infants, and it has been implemented in many clinical settings. The PIPP includes behavioral and physiologic indicators. The emphasis in pain assessment should be on behavioral indicators. Physiologic indicators should be interpreted with caution because they are also affected by disease, medications, and physiologic status.16
Cultural Influences.
Another barrier to accurate pain assessment is cultural influences on pain and pain reporting.85 Cultural influences are compounded when the patient speaks a language other than that of the health team members. To facilitate communication, the use of a pain intensity scale in the patient’s language is vital. The 0 to 10 numeric pain scales have been translated into many different languages.24
Some cultures believe that God’s test or punishment takes the form of pain. Persons with these cultural backgrounds do not necessarily believe that the pain should be relieved. Other cultures perceive pain as being associated with an imbalance in life. Persons from these cultures believe they need to manipulate the environment to restore balance to control pain.85
Lack of Knowledge
A relatively overlooked patient barrier to accurate pain assessment is the public knowledge deficit regarding pain and pain management. Many patients and their families are frightened by the risk of addiction to pain medication. They fear that addiction will occur if the patient is medicated frequently or with sufficient amounts of opioids necessary to relieve the pain. This concern is so powerful for some that they will deny or deliberately underreport the frequency or intensity of pain. Another misconception held by some patients is the expectation that unrelieved pain is simply part of a critical illness or procedure.86 Many patients have no memory of receiving an explanation of their pain-management plan.87 With that in mind, it is important that the critical care nurse teach the family and the patient about the importance of pain control and the use of opioids in treating pain in the critically ill.
Health Professional Barriers to Pain Assessment and Management
Addiction and Tolerance
Tolerance is defined as a diminution of opioid effects over time. Physical dependence and tolerance to opioids may develop if the medication is given over a long period. Physical dependence is manifested by withdrawal symptoms when the opioid is abruptly stopped. If this is an anticipated problem, withdrawal may be avoided by weaning the patient from the opioid slowly to allow the brain to reestablish neurochemical balance in the absence of the opioid.24
Respiratory Depression
Another concern of the health care professional is the fear that aggressive management of pain with opioids will cause critical respiratory depression. Opioids can cause respiratory depression, but in the critically ill, this is a rare phenomenon. The incidence of respiratory depression is less than 2%.88,89 Respiratory depression from the administration of opioids can be managed with diligent assessment practices, which are discussed later.
Organizational Barriers to Pain Assessment and Management
The organizational system influences pain and pain-management practices as well as associated outcomes. Failure to make pain management a priority is the initial barrier. Failure to adopt standard pain assessment tools or to provide staff with sufficient time to assess and document pain, and a lack of accountability for pain-management practices are observed in some organizations. Evidence has demonstrated the lack of documentation of pain assessment and the undertreatment of pain in critical care settings.5,90, 91 The lack of collaboration between physicians and nurses is identified as a barrier to effective pain management.92
Because unrelieved pain is harmful to patients and increases the cost of care, it must be a priority for the health organization. Every organization must analyze their pain-management issues and practices and provide education about pain and pain management to staff. Now that pain is considered as the fifth vital sign, pain assessment must be included in documentation systems as a standard. Pain has to be assessed in all critically ill patients, regardless of their clinical condition or their level of consciousness. The implementation of pain assessment tools is essential so that the health care team can establish a common language of communication. This can facilitate interprofessional collaboration. There is an increased commitment to clinical practice guidelines and standards for pain assessment and management by organizations such as The Joint Commission (http://www.jointcommission.org), which have considerable influence in health care institutions. In addition, strategies to enhance collaboration among health professionals may include interdisciplinary care rounds or case reviews.92 Patients have the right to be consulted about their pain care plan and should be involved in making decisions. The organization must continually evaluate outcomes and work to improve the quality of pain management.
Pain Management
Pharmacologic Control of Pain
Pharmacologic management of pain has infinite variety in the critical care unit. Although this chapter is not an in-depth discussion of pharmacology, some commonly administered agents are discussed. Pain pharmacology is divided into three categories of action: opioid agonists, nonopioids, and adjuvants. Elements of the pain and analgesia clinical guidelines17 of the Society of Critical Care Medicine (SCCM) for the treatment of pain in the critically ill adult are presented in Box 9-2. A care bundle for pain assessment and management in the critical care unit was created to facilitate the translation of the SCCM practice guidelines to the bedside and is shown in Figure 9-6. How pain is approached and managed is a progression or combination of the available agents, the type of pain, and the patient response to the therapy. Figure 9-7 illustrates the analgesic action sites in relation to nociception.
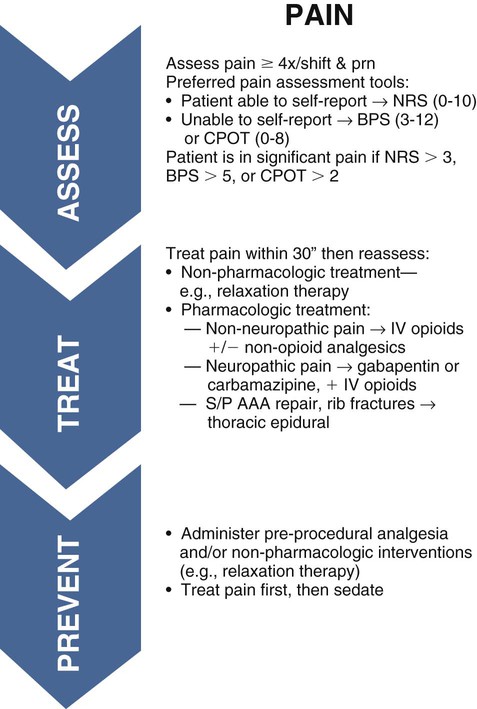
BPS, Behavioral Pain Scale; CPOT, Critical-Care Pain Observation Tool; NRS, Numeric Rating Scale; S/P AAA, status post abdominal aortic aneurysm.
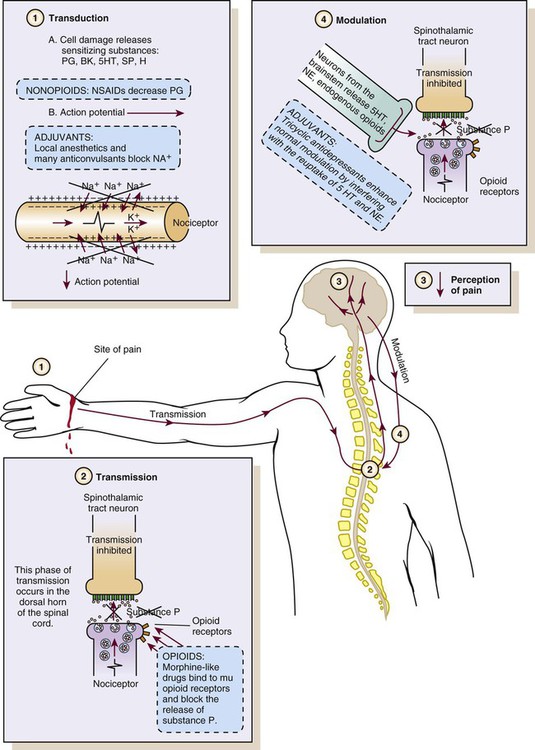
BK, Bradykinin; H, histamine; PG, prostaglandins; SP, substance P; 5HT, serotonin. (From McCaffery M, Pasero C. Pain: Clinical Manual for Nursing Practice. 2nd ed. St. Louis: Mosby; 1999.)
Opioid Analgesics
The opioids most commonly used and recommended as first-line analgesics are the agonists. These opioids bind to mu (µ) receptors (transmission process; see Fig. 9-7), which appear to be responsible for pain relief. Additional pharmacologic information is presented in Table 9-3. In the SCCM guidelines, opioids should be used as first line therapy for treatment of non-neuropathic pain.17
TABLE 9-3
| MEDICATION | DOSAGE | ONSET (min) | DURATION (hr) | AVAILABLE ROUTES | PROPERTIES | SIDE EFFECTS AND COMMENTS |
| Morphine | 1-4 mg IV bolus | 5-10 | 3-4 | PO, SL, R, IV, IM, SC, EA, IA | Analgesia, antianxiety | Standard for comparison |
| 1-10 mg IV infusion | Side effects: sedation, respiratory depression, euphoria or dysphoria, hypotension, nausea, vomiting, pruritus, constipation, urinary retention | |||||
| M6G can accumulate in renal failure or hepatic dysfunction patients. | ||||||
| Fentanyl | 25-100 mcg IV bolus | 1-5 | 0.5-4 | OTFC, IV, IM, TD, EA, IA | Analgesia, antianxiety | Same side effects as morphine |
| 25-200 mcg IV infusion | Rigidity with high doses | |||||
| Hydromorphone (Dilaudid) | 0.2-1 mg IV bolus 0.2-2 mg IV infusion |
5 | 3-4 | PO, R, IV, IM, SC, EA, IA | Analgesia, antianxiety | Same side effects as morphine |
| Codeine | 15-30 mg IM, SC | 10-20 | 3-4 | PO, IM, SC | Analgesia (mild to moderate pain) | Lacks potency (unpredictable absorption; not all patients convert it to an active form to achieve analgesia) |
| Most common side effects: light-headedness, dizziness, shortness of breath, sedation, nausea, and vomiting | ||||||
| Methadone (Dolophine) | 5-10 mg IV | 10 | 4-8 | PO, SL, R, IV, SC, IM, EA, IA | Analgesia | Usually less sedating than morphine, but repeated doses can result in accumulation and can cause serious sedation (2-5 days). |
| Acetaminophen | 650 mg maximum of 4 g/day | 20-30 | 4-6 | PO, R | Analgesia, antipyretic | Rare side effects Hepatotoxicity |
| Ketorolac (Toradol) | 15-30 mg IV | <10 | 6-8 | PO, IM, IV | Analgesia, minimum antiinflammatory effect | Short-term use (<5 days) |
| Side effects: gastric ulceration, bleeding, exacerbation of renal insufficiency | ||||||
| Use with care in older adult and renal failure patients. |
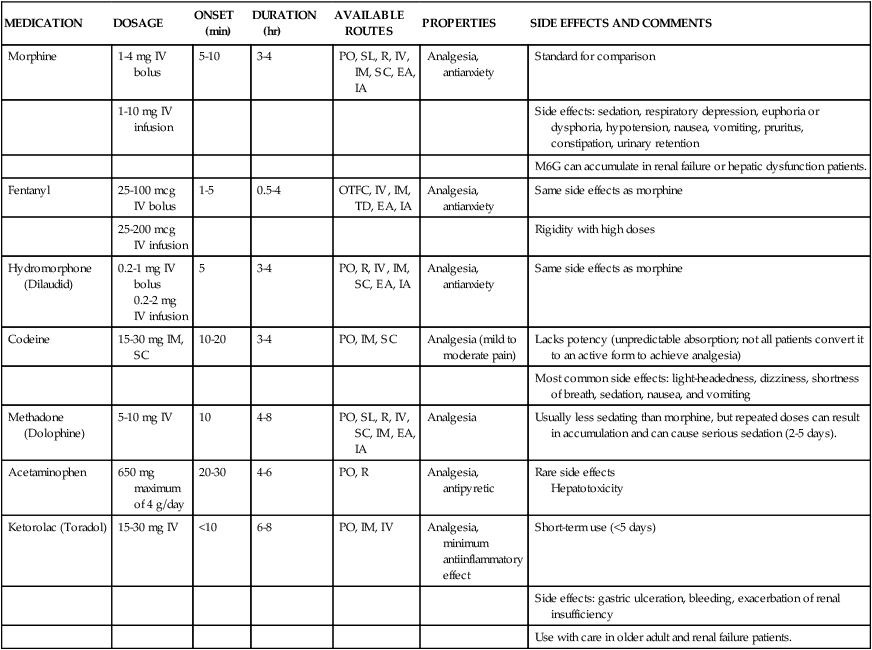
Morphine.
Morphine is the most commonly prescribed opioid in the critical care unit. Because of its water solubility, morphine has a slower onset of action and a longer duration compared with the lipid-soluble opioids (e.g., fentanyl). Morphine has two main metabolites: morphine-3-glucuronide (M3G, inactive) and morphine-6-glucuronide (M6G, active). M6G is responsible for the analgesic effect but may accumulate and cause excessive sedation in patients with renal failure or hepatic dysfunction.93 Morphine is available in a variety of delivery methods. It is the standard by which all other opioids are measured. It is also the agent that most closely mimics the endogenous opioids in the human pain modification system.
Many side effects have been reported with the use of morphine (see Table 9-3). The hypotensive effect can be particularly problematic in the hypovolemic patient. The vasodilation effect is potentiated in the volume-depleted patient, and the hemodynamic status must be carefully monitored. Volume resuscitation restores blood pressure in the event of a prolonged hypotensive response.
A more serious side effect requiring diligent monitoring is the respiratory depressant effect. Opioids may cause this complication because they reduce the responsiveness of carbon dioxide chemoreceptors in the respiratory center located in the medulla.94 Although infrequent, this effect can have significant sequelae for the critically ill patient. Many risk factors for opioid-induced respiratory depression have been identified.24 From these, advanced age, obesity, sleep apnea, impaired renal/pulmonary/hepatic/cardiac functioning, patients in whom pain is controlled after a period of poor control, patients who are opiate naïve (i.e., receiving opiates for less than a week), concurrent use of central nervous system depressants, and postoperative day 1 were described.89 The critical care nurse must monitor the patient intensively to prevent this complication. Monitoring of patients receiving opioid analgesics is discussed in more detail later in this chapter. In addition to side effects common to all opioids, morphine may stimulate histamine release from mast cells, resulting in cardiac instability and allergic reactions.
Fentanyl.
Fentanyl is a synthetic opioid preferred for critically ill patients with hemodynamic instability or morphine allergy. It is a lipid-soluble agent that has a more rapid onset than morphine and a shorter duration.95 The metabolites of fentanyl are largely inactive and nontoxic, which makes it an effective and safe opioid. Fentanyl or hydromorphone are preferred in hemodynamically unstable as well as in renal impaired patients.95 It is available in intravenous, intraspinal, and transdermal forms. The transdermal form is commonly referred to as the Duragesic patch or the 72-hour patch.
Because the side effects of fentanyl are similar to those of morphine, the nurse must monitor carefully the hemodynamic and respiratory response. When fentanyl is given by rapid administration and at higher doses, it has been associated with the additional hazard of bradycardia and rigidity in the chest wall muscles.93,96 The use of transdermal fentanyl is indicated rarely in the critically ill patient. The customary use of the “fentanyl patch” is for those experiencing chronic pain or cancer pain, and in critical care, it is used for the patient who requires extended pain control. Transdermal delivery requires 12 to 16 hours for onset of action, and it has a duration of 72 hours.24 If this delivery method is used, the patient will require other opioid management until the transdermal fentanyl takes effect.
Hydromorphone.
Hydromorphone is a semisynthetic opioid that has an onset of action and a duration similar to those of morphine.96 It is an effective opioid with multiple routes of delivery. It is more potent than morphine. Hydromorphone produces an inactive metabolite (i.e., hydromorphone-3-glucuronide), making it the opioid of choice for use in patients with end-stage renal disease.99 Studies have shown that some side effects (e.g., pruritus, sedation, nausea, vomiting) may occur less with hydromorphone than morphine.97
Meperidine.
Meperidine (Demerol) is a less potent opioid with agonist effects similar to those of morphine. It is considered the weakest of the opioids, and it must be administered in large doses to be equivalent in action to morphine. Because the duration of action is short, dosing is frequent. A major concern with this medication is the metabolite normeperidine, which is a CNS neurotoxic agent. At high doses in patients with kidney failure or liver dysfunction or in older adult patients, it may induce CNS toxicity, including irritability, muscle spasticity, tremors, agitation, and seizures.24 Although meperidine is useful in short-term specific conditions (e.g., treating postoperative shivering98), it should not be used routinely for analgesia in the critical care unit.95,99,100
Codeine.
Codeine has limited use in the management of severe pain. It is rarely used in the critical care unit. It provides analgesia for mild to moderate pain. It is usually compounded with a nonopioid (e.g., acetaminophen). To be active, codeine must be metabolized in the liver to morphine.24 Codeine is available only through oral, intramuscular, and subcutaneous routes, and its absorption can be reduced in the critical care patient by altered gastrointestinal motility and decreased tissue perfusion.
Methadone.
Methadone is a synthetic opioid with morphine-like properties but less sedation. It is longer acting than morphine and has a long half-life. This makes it difficult to titrate in the critical care patient. Methadone lacks active metabolites, and routes other than the kidney eliminate 60% of the medication. This means that methadone does not accumulate in patients with kidney failure. Methadone can be used to treat chronic pain syndromes when patients experience tolerance with other opioids and may help facilitate the down-titration of opioid infusions in the critical care unit.99 However, prolongation of the QT interval, which can lead to torsades de pointes, has been reported with its use.93
More Potent Opioids: Remifentanil and Sufentanil.
Remifentanil is 250 times more potent than morphine, and it has a rapid onset and predictable offset of action. For this reason, it allows a rapid emergence from sedation, facilitating the evaluation of the neurologic state of the patient after stopping the infusion.101,102 As opposed to fentanyl, the use of remifentanil was associated with a lower incidence of postoperative delirium.103
Sufentanil is 7 to 13 times more potent than fentanyl and 500 to 1000 times more potent than morphine. It has more pronounced sedation properties than fentanyl and other opioids. Patients under sufentanil require minimal sedative agent doses to achieve an adequate sedation level. It has a rapid distribution and a high clearance rate, preventing accumulation when given for a long period.104 Sufentanil has a longer emergence from sedation compared with remifentanil, but it allows a longer analgesic effect after stopping its administration.102
Preventing and Treating Respiratory Depression
Respiratory depression is the most life-threatening opioid side effect. The risk of respiratory depression increases when other medications with CNS depressant effects (e.g., benzodiazepines, antiemetics, neuroleptics, antihistamines) are concomitantly administered to the patient. While no universal definition of respiratory depression exists, it is usually described in terms of decreased respiratory rate (fewer than 8 or 10 breaths/min), decreased Spo2 levels, or elevated ETCO2 levels.94 A change in the patient’s level of consciousness or an increase in sedation normally precedes respiratory depression.
Monitoring.
Recent guidelines on monitoring for patients receiving opioid analgesia of ASPMN were established.94 In addition to assessing pain intensity as a targeted outcome of analgesia, regular sedation and respiratory assessments should be done. Valid and reliable sedation scales developed for use in critically ill patients should be used. Respirations should be evaluated over 1 minute and qualified according to rate, rhythm, and depth of chest excursion. The use of technology-supported monitoring (e.g., continuous pulse oximetry and capnography) can be useful in high-risk patients. More vigilant monitoring should be performed when patients may be at greater risk, such as during the first 24 hours after surgery, after an increase in the dose of an opioid, or a change in opioid agent or route of administration. For instance, Pasero et al have suggested monitoring these parameters at least every 2 hours for the first 24 hours and every 4 hours thereafter in stable patients.105
Snoring is a warning sign. It can be a sign of respiratory depression associated with airway obstruction by the tongue, leading to hypoxemia and possibly to cardiorespiratory arrest.24 A patient snoring after the administration of an opioid requires the critical care nurse to observe closely.
Opioid Reversal.
Critical respiratory depression can be readily reversed with the administration of the opiate antagonist naloxone.24 The usual dose is 0.4 mg, which is mixed with 10 mL of normal saline (for a concentration of 0.04 mg/mL). Naloxone is normally given intravenously very slowly (0.5 mL over 2 minutes) while the patient is carefully monitored for reversal of the respiratory signs. Naloxone administration can be discontinued as soon as the patient is responsive to physical stimulation and able to take deep breaths. However, the medication should be kept nearby. Because the duration of naloxone is shorter than most opioids, another dose of naloxone may be needed as early as 30 minutes after the first dose. The nurse must monitor sedation and respiratory status and remind the patient to breathe deeply every 1 to 2 minutes until he or she becomes more alert. The benefits of reversing respiratory depression with naloxone must be carefully weighed against the risk of a sudden onset of pain and the difficulty achieving pain relief. To prevent this from occurring, it is important to provide a nonopioid medication for pain relief. Moreover, the use of naloxone is not recommended after prolonged analgesia, because it can induce withdrawal and may cause nausea and cardiovascular complications (e.g., dysrhythmias).
New Sedative with Analgesic Properties: Dexmedetomidine
Dexmedetomidine (Precedex) is a short-acting alpha 2 agonist that is indicated for the short-term sedation (<24 hours) of mechanically ventilated patients in the critical care unit.106 Its mechanism of action is unique and differs from those of other commonly used sedatives in critical care. Indeed, compared to midazolam (Versed) or lorazepam (Ativan)—whose hypnotic effects act mainly on the limbic system and/or the cortex—the effect of dexmedetomidine is located in the locus ceruleus section of the brainstem. As a result, patients receiving dexmedetomidine IV infusions are calm and sleepy, yet they remain easily arousable.107 For this reason, dexmedetomidine is ideal for mild to moderate sedation, often referred to as conscious sedation. Refer to the Sedation and Delirium Management chapter for details on dosage and administration.
Dexmedetomidine also possesses an analgesic property. The analgesic effects of dexmedetomidine are principally due to spinal antinociception via binding to non-noradrenergic receptors (heteroreceptors) located on the dorsal horn neurons of the spinal cord.105 Recently, dexmedetomidine was found to decrease postoperative opioid requirements in both adults and children.109,110 These results suggest that dexmedetomidine could be of interest with respect to improving postoperative pain in critical care.
While dexmedetomidine is increasing in popularity for short-term sedation in the critical care unit, it is not without adverse effect. Indeed, inhibition of noradrenergic receptors in the brainstem and the spinal cord often causes hypotension and bradycardia.107 Less common adverse effects include decreased salivation, decreased secretion, and decreased bowel motility in the gastrointestinal tract; contraction of vascular and other smooth muscle; inhibition of renin release, increased glomerular filtration, and increased secretion of sodium and water in the kidney; decreased intraocular pressure; and decreased insulin release from the pancreas.108
Nonopioid Analgesics
In the SCCM guidelines, the use of nonopioids in combination with an opioid is recommended to reduce opioid requirements and opioid related side effects.17 This strategy provides greater analgesic effect through action at the peripheral and central levels. Pharmacologic information is presented in Table 9-3.
Acetaminophen.
Acetaminophen is an analgesic used to treat mild to moderate pain. It inhibits the synthesis of neurotransmitter prostaglandins in the CNS, and this is why it has no antiinflammatory properties.111 Acetaminophen is metabolized by two pathways: major (nontoxic metabolite) and minor (toxic metabolite that is rapidly converted into a nontoxic form by glutathione). In an acetaminophen overdose, a larger amount is processed by the minor pathway, and this results in a larger quantity of toxic metabolites and may cause damage to the liver. Side effects are rare at therapeutic doses (total daily dose should not exceed 4 g in 24 hours). Nonopioids are rarely used alone in critically ill patients. The nurse must consider the other products containing acetaminophen that the patient may receive when calculating the total daily dose of acetaminophen. Special care must be taken for patients with liver dysfunction, malnutrition, or a history of excess alcohol consumption, and their acetaminophen total dose should not exceed 2 g/day.17
Nonsteroidal Antiinflammatory Drugs.
The use of NSAIDs in combination with opioids is indicated in the patient with acute musculoskeletal and soft tissue inflammation.24 The mechanism of action of NSAIDs is to block the action of cyclooxygenase (COX, which has two forms: COX-1 and COX-2), the enzyme that converts arachidonic acid to prostaglandins. This inhibits the production of prostaglandins (transduction process; see Fig. 9-7). This action occurs in the PNS and the CNS components of pain. NSAIDs can be grouped as first-generation (COX-1 and COX-2 inhibitors, such as aspirin, ibuprofen, naproxen, and ketorolac) or second-generation (COX-2 inhibitors, such as celecoxib) agents. The inhibition of COX-1 is thought to be responsible for many of the side effects, such as gastric ulceration, bleeding as a result of platelet inhibition, and acute renal failure. In contrast, the inhibition of COX-2 is responsible for the suppression of pain and inflammation.111 Second-generation NSAIDs are associated with minimal risks of serious adverse effects, but their role in critically ill patients remains unknown.17
Ketorolac is the most appropriate NSAID for use in the critical care setting. Research has shown that it is a safe and effective agent for postoperative pain.112 Not all critically ill patients are candidates for ketorolac therapy because of its side effects. Caution is advised for using ketorolac in older adults or patients with kidney dysfunction because of their slower clearance rates. Because ketorolac is an NSAID, monitoring for clumping of platelets is of primary importance. Laboratory data should be evaluated for an increase in bleeding time, and the patient should be assessed for any signs of abnormal bleeding. Moreover, prolonged use of ketorolac for more than 5 days was associated with an increase in kidney failure and bleeding.113,114 It is important to consider the concurrent use of opioids and NSAIDs to affect pain modification at both areas of transmission. This combination of agents often significantly reduces the amount of opioids required for effective pain management.
Adjuvants.
Although not widely mentioned in the critical care literature, adjuvants can be helpful for pain relief in patients with complex pain syndromes such as neuropathic pain or for other specific purposes (e.g., procedural pain). Anticonvulsants (e.g., carbamazepine, phenytoin, gabapentin, pregabalin) are first-line analgesics for lancing neuropathic pain. The use of gabapentin or carbamazepine, in addition to intravenous opioids, is recommended for treatment of neuropathic pain in the SCCM guidelines.17 Even if the specific mechanism for pain relief is unknown, analgesia probably results from the suppression of sodium ion (Na+) discharges, reducing the neuronal hyperexcitability (action potential) in the transduction process24 (see Fig. 9-7). Antidepressants are also considered as analgesics in a variety of chronic pain syndromes, such as headache, fibromyalgia, low back pain, neuropathy, central pain, and cancer pain. The analgesic dose is often lower than that required to treat depression. Antidepressant adjuvant analgesics are usually divided into two main groups: tricyclic antidepressants (e.g., amitriptyline, imipramine, desipramine), and biogenic amine reuptake inhibitors (e.g., venlafaxine, paroxetine, sertraline). The mechanism of analgesia most widely accepted is the ability of antidepressants to block the reuptake of neurotransmitters serotonin and norepinephrine in the CNS.24 This increases the activity of the modulation process (see Fig. 9-7).
Ketamine.
Anesthetics may be used to treat pain in the critical care setting. Ketamine is a dissociative anesthetic agent that has analgesic properties. It was traditionally used intravenously for procedural pain in burn patients. It is also available in enteral routes. Compared with opioids, ketamine has the benefit of sparing the respiratory drive, but it has many side effects related to the release of catecholamines and the emergence of delirium. For this reason, ketamine is not recommended for routine therapy in critically ill patients.96,112 Before administering ketamine, the dissociative state should be explained to the patient. Dissociative state refers to the feelings of separateness from the environment, loss of control, hallucinations, and vivid dreams. The use of benzodiazepines (e.g., midazolam) can reduce the incidence of this unpleasant effect.24
Delivery Methods
The most common route for medication administration is the intravenous route by means of continuous infusion, bolus administration, or patient-controlled analgesia (PCA). Traditionally, the choice has been intravenous bolus administration. The benefits of this method are the rapid onset of action and the ease of titration. The major disadvantage is the rise and fall of the serum level of the opioid, leading to periods of pain control with periods of breakthrough pain.111
Patient-Controlled Analgesia
PCA is a method of medication delivery that uses the intravenous route and an infusion pump. It allows the patient to self-administer small doses of analgesics. Different opioids can be used, but the most extensively used is morphine. This method of medication delivery allows the patient to control the level of pain and sedation and to avoid the peaks and valleys of intermittent dosing by the health care professional. The patient can self-administer a bolus of medication the moment the pain begins, acting preemptively. Nursing management for the patient receiving analgesia medication via a PCA pump is described in the Nursing Interventions Classification (NIC) feature in Box 9-3.
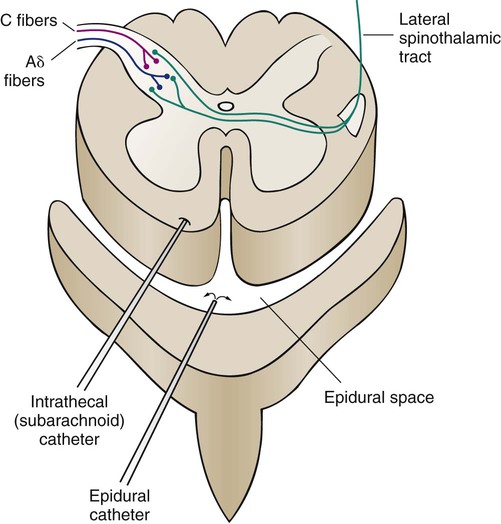
Intraspinal Pain Control
Intraspinal anesthesia is particularly appropriate for pain in the thorax, upper abdomen, and lower extremities. The two intraspinal routes are intrathecal and epidural (Fig. 9-8). Regardless of the route, the effects of the opioid agonist used is the same, and assessment parameters are the same as those used for other routes. Nursing management of the patient receiving intraspinal analgesia is described in the Nursing Interventions Classification (NIC) feature in Box 9-4.
Epidural Analgesia
Epidural analgesia is commonly used in the critical care unit after major abdominal surgery, nephrectomy, thoracotomy, and major orthopedic procedures. Per the SCCM guidelines, its use should be considered in postoperative abdominal aortic aneurysm patients, and for those with traumatic rib fractures.17 Certain conditions preclude the use of this pain control method: systemic infection, anticoagulation, and increased intracranial pressure. Epidural delivery of opiates provides longer-lasting pain relief with less dosing of opiates. When delivered into the epidural space, 5 mg of morphine may be effective for 6 to 24 hours, compared with 3 to 4 hours when delivered intravenously. Opioids infused in the epidural space are more unpredictable than those administered intrathecally. The epidural space is filled with fatty tissue and is external to the dura mater. The fatty tissue interferes with uptake, and the dura acts as a barrier to diffusion, making diffusion rate difficult to predict.
Equianalgesia
When a modification of opioid is considered, the nurse must be aware of equianalgesic dosages. In doing any conversion, the goal is to provide equal analgesic effects with the new agents. This concept is referred to as equianalgesia. Morphine is the standard for the conversion of opioids. Prescribed dosages must take into account the patient’s age and health status.24 The critical care nurse must have access to a chart for easy referral on the unit to administer the correct dosages of opioids to critically ill patients. Because of the variety of agents and routes, the professional pain organizations have developed equianalgesia charts for use by the health care professional. All critical care units need to have an equianalgesic chart posted for easy reference. Box 9-5 presents a guide to using these charts. Table 9-4 provides the equianalgesic dose for different medications used in clinical practice. Table 9-5 presents an equianalgesic chart with PO nonopioid and opioid doses for mild to moderate pain.
TABLE 9-4
EQUIANALGESIC CHART
Approximate Equivalent Doses of Opioids for Moderate-to-Severe Pain
| ANALGESIC | PARENTERAL (IM, SC, IV) ROUTE1,2 (mg) | PO ROUTE1 (mg) | COMMENTS |
| Mu Opioid Agonists | |||
| Morphine | 10 | 30 | Standard for comparison; multiple routes of administration; available in immediate-release and controlled-release formulations; active metabolite M6G can accumulate with repeated dosing in renal failure |
| Codeine | 130 | 200 NR | IM has unpredictable absorption and high side-effect profile; used PO for mild-to-moderate pain; usually compounded with nonopioid (e.g., Tylenol No. 3) |
| Fentanyl | 100 mcg/hr parenterally and transdermally ≅ 4 mg/hr morphine parenterally; 1 mcg/hr transdermally ≅ 2 mg/24 hr morphine PO | — | Short half-life, but at steady state, slow elimination from tissues can lead to a prolonged half-life (up to 12 hr); start opioid-naïve patients on no more than 25 mcg/hr transdermally; transdermal fentanyl NR for acute pain management; available by oral transmucosal route |
| Hydromorphone (Dilaudid) | 1.5 | 7.5 | Useful alternative to morphine; no evidence that metabolites are clinically relevant; shorter duration than morphine; available in high-potency parenteral formulation (10 mg/mL) useful for SC infusion; 3 mg rectal ≅ 650 mg aspirin PO; with repeated dosing (e.g., PCA), it is more likely than 2-3 mg parenteral hydromorphone =10 mg parenteral morphine |
| Levorphanol (Levo-Dromoran) | 2 | 4 | Longer-acting than morphine when given repeatedly; long half-life can lead to accumulation within 2-3 days of repeated dosing |
| Meperidine | 75 | 300 NR | No longer preferred as a first-line opioid for the management of acute or chronic pain due to potential toxicity from accumulation of metabolite, normeperidine; normeperidine has 15-20 hr half-life and is not reversed by naloxone; NR in elderly or patients with impaired renal functions; NR by continuous IV infusion |
| Methadone (Dolophine) | 10 | 20 | Longer-acting than morphine when given repeatedly; long half-life can lead to delayed toxicity from accumulation within 3-5 days; start PO dosing on PRN schedule; in opioid-tolerant patients converted to methadone, start with 10%-25% of equianalgesic dose |
| Oxycodone | — | 20 | Used for moderate pain when combined with a nonopioid (e.g., Percocet, Tylox); available as single entity in immediate-release and controlled-release formulations (e.g., OxyContin); can be used like PO morphine for severe pain |
| Oxymorphone (Numorphan) | 1 | 10 rectal | Used for moderate to severe pain; no PO formulation |
| Agonist-Antagonist Opioids: Not recommended for severe, escalating pain. If used in combination with mu agonists, may reverse analgesia and precipitate withdrawal in opioid-dependent patients. | |||
| Buprenorphine (Buprenex) | 0.4 | — | Not readily reversed by naloxone; NR for laboring patients |
| Butorphanol (Stadol) | 2 | — | Available in nasal spray |
| Dezocine (Dalgan) | 10 | — | |
| Nalbuphine (Nubain) | 10 | — | |
| Pentazocine (Talwin) | 60 | 180 | |
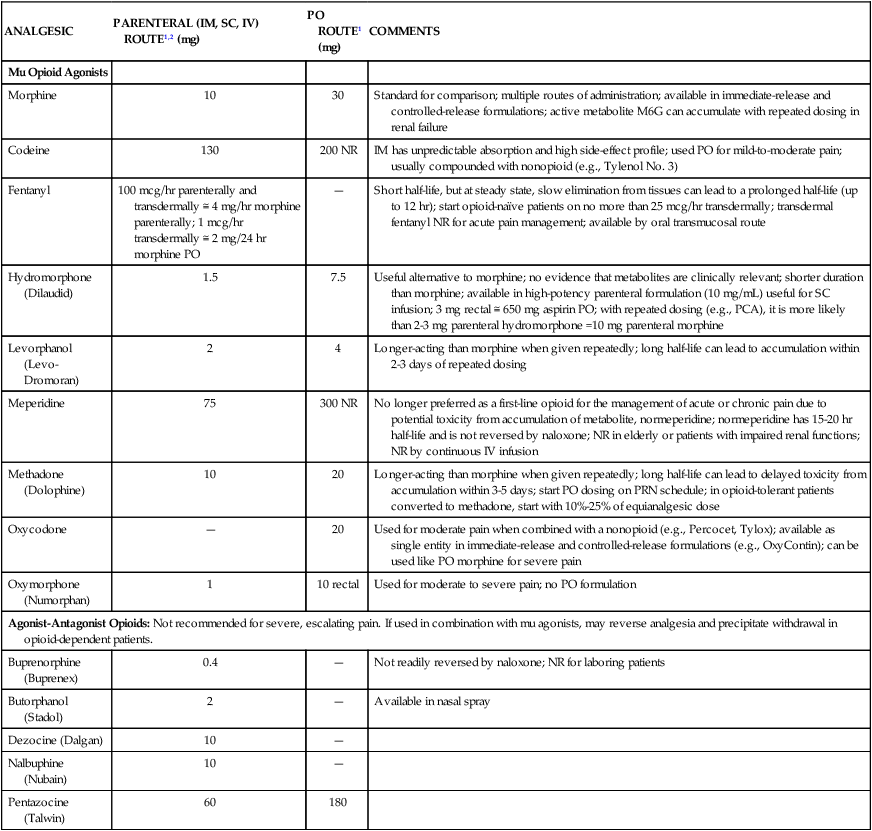
Duration of analgesia is dose dependent; the higher the dose, usually the longer the duration.
Reference: Pasero C, McCaffrey M. Pain Assessment and Pharmacologic Management, St. Louis: Elsevier; 2011. Table 16-1, p. 444-445.
TABLE 9-5
EQUIANALGESIC CHART
Approximate Equivalent Doses of PO Nonopioids and Opioids for Mild to Moderate Pain
| ANALGESIC | PO DOSAGE (mg) |
| Nonopioids | |
| Acetaminophen | 650 |
| Aspirin (ASA) | 650 |
| Opioids* | |
| Codeine | 32-60 |
| Hydrocodone† | 5 |
| Meperidine (Demerol) | 50 |
| Oxycodone‡ | 3-5 |
| Propoxyphene (Darvon) | 65-100 |
*Often combined with acetaminophen; avoid exceeding maximum total daily dose of acetaminophen (4000 mg/day).
†Combined with acetaminophen (e.g., Vicodin, Lortab).
‡Combined with acetaminophen (e.g., Percocet, Tylox); also available alone as controlled-release OxyContin and immediate-release formulations.
Modified from Pasero C, McCaffery M. Pain Assessment and Pharmacologic Management. St. Louis: Elsevier; 2011.
Nonpharmacologic Methods of Pain Management
Although numerous methods of pain management other than medications appear in the critical care literature,115 very few studies have been done to provide evidence of their effectiveness in the critical care settings. Nonpharmacologic methods can be used to supplement analgesic treatment, but they are not intended to replace analgesics. In most instances, these therapies may enhance the pharmacologic management of the patient’s pain. Per the SCCM guidelines, administration of preemptive analgesic therapy and/or non-pharmacological interventions, it is suggested to alleviate pain during invasive or potentially painful procedures in critically ill adults.17
Critical care nurses identify many barriers to the use of non-pharmacologic methods for pain management, including a lack of knowledge, training, and time.116 Despite these problems, more than 60% of them are willing to use the methods to relieve their patients’ pain. It is crucial that critical care nurses be provided with the appropriate training and equipment required to apply nonpharmacologic methods for pain management in the critically ill.
Physical Techniques
Cold Application
Ice therapy was found to be helpful to reduce procedural pain in critically ill patients. In a study with 50 cardiac surgery patients in a critical care unit, a significant decrease in pain intensity was obtained after chest tube removal when ice packets were placed around the site for 10 minutes prior to removal.117 The analgesic property of ice therapy was also studied by Demir and Khorshid118 using a 20-minute application of cold packs before chest tube removal in the critical care unit. Pain intensity was significantly lower after chest tube removal in patients who received the cold packs and a dose of analgesic compared with a placebo and a control group.
Massage
The effect of massage on pain relief was explored in two studies with postoperative cardiac surgery patients after they have been transferred from the critical care unit.119,120 In these two studies with a total of 171 patients, a significant decrease in pain intensity scores was obtained in patients who received a 20-minute massage intervention between postoperative day 2 and day 5 compared to a control group who received standard care and a 20-minute quiet time during the same period. Similar findings were found in the critical care unit. Indeed, in a recent pilot study121 with 40 postoperative cardiac surgery patients, a decrease in pain intensity scores was observed in patients who were administered two to three 15-minute hand massage sessions during the first 24 hours after surgery compared to a control group who did not receive hand massage but had the nurse holding their hands and reported no change in pain intensity.
Cognitive-Behavioral Techniques
Relaxation
Relaxation is a well-documented method for reducing the distress associated with pain. Although not a substitute for pharmacology, relaxation is an excellent adjunct for controlling pain. Relaxation decreases oxygen consumption and muscle tone, and it can decrease heart rate and blood pressure. Relaxation gives the patient a sense of control over the pain and reduces muscle tension and anxiety. Not all patients are interested in relaxation therapy. For those patients, deep-breathing exercises may be helpful. Indeed, in a recent study,3 lower pain scores were found both after chest tube removal in 40 critically ill patients executing deep breathing and receiving an analgesic compared to those who solely received an analgesic. Excellent references for thorough techniques in relaxation therapy are available.24
Guided Imagery
Guided imagery is a technique that uses the imagination to provide control over pain. It can be used to distract or relax. Guiding a patient to a place that is pain free and relaxing takes a considerable time commitment on the part of the nurse. Although this may be difficult in the critical care environment, guiding a patient to a place in his or her imagination that is free from pain may be beneficial.122
Music Therapy
Music therapy is a commonly used intervention for relaxation. Music that is pleasing to the patient may have soothing effects, but its effects on reducing pain are controversial.123 Ideally, the music should be supplied by a small set of headphones. It is important to educate the patient and family regarding the role of music in relaxation and pain control and to provide music of the patient’s choice.
Summary
• Pain in the critically ill patient is difficult to assess and manage. There are many sources of pain in the critical care setting, and the effects of unrelieved acute pain can have a significant impact on the patient’s recovery.
• When possible, the patient’s self-report of pain must be obtained. A simple yes or no communicated by head nodding from a mechanically ventilated patient is considered a valid self-report of pain.
• When the patient’s self-report is not available, behavioral indicators represent alternative measures of pain assessment, and assessment tools (e.g., BPS, CPOT) have been developed for assessment of pain in nonverbal critically ill patients.
• In some situations, behavioral indicators may be impossible to assess accurately. The use of physiologic indicators is then crucial. However, vital signs do not represent valid information for pain assessment. Innovative physiologic measures (e.g., BIS, EEG) are being explored and may support the nurses in the pain assessment process.
• The critical care nurse must collaborate with the multidisciplinary team to ensure a plan of care for management of the patient’s pain is developed. To fully participate, the nurse must have extensive knowledge of nonpharmacologic and pharmacologic therapies designed to achieve effective pain relief.