Chapter 105 Paediatric poisoning
EPIDEMIOLOGY
The peak incidence of poisoning in childhood is among 1–4 year olds. It usually occurs in the home when the child ingests a single prescribed or over-the-counter medication or a household product. Approximately 3500 young children are admitted to hospital each year in Australia.1 This mode of poisoning is called ‘accidental’ – erroneously because it is usually the result of inadequate supervision or improper storage of poisons. The mortality is very low and if hospitalisation is required, it is usually brief (1–3 days). In this circumstance, care must be taken to ensure that whatever treatment is applied, it does not impose additional risk.
Occasionally, poisoning in childhood is either truly accidental as in ingestion of a decanted chemical, or is part of a syndrome of child abuse (Munchausen syndrome by proxy), or is iatrogenic as when a parent mistakes medications at home or when medical or nursing staff make errors in drug administration in hospital. Medication errors occur in approximately 5% of paediatric inpatient medication.2 Self-poisoning in older children is usually with the intention to manipulate their psychosocial environment or to commit suicide, or is the result of substance abuse. All circumstances of poisoning require remedial action.
DIAGNOSIS
A knowledge of important poisons is indispensable. A number of poisons or classes of poison are potentially fatal to a small child if taken as a single tablet or a teaspoonful. These include opiates (methadone, buprenorphine, lomotil), camphor, quinine derivatives, cyclic antidepressants, clonidine, sulphonylureas, salicylates and calcium channel blockers.3
PRINCIPLES OF MANAGEMENT
| Poison | Antidotes | Comments |
|---|---|---|
| Amfetamines | Esmolol i.v. 500 μg/kg over 1 min, then 25–200 μg/kg per min | Treatment for tachyarrhythmia |
| Labetalol i.v. 0.15–0.3 mg/kg or phentolamine i.v. 0.05–0.1 mg/kg every 10 minutes | Treatment for hypertension | |
| Benzodiazepines | Flumazenil i.v. 3–10 μg/kg, repeat 1 minute, then 3–10 μ/kg per hour | Specific receptor antagonist. Beware convulsions |
| β-Blockers | Glucagon i.v. 7 μg/kg, then 2–7 μg/kg per min | Stimulates non-catecholamine cAMP (preferred antidote) |
| Isoprenaline i.v. 0.05–3 μg/kg per min | Beware β2 hypotension | |
| Noradrenaline i.v. 0.05–1 μg/kg per min | Antagonises at receptors | |
| Calcium channel blocker | Calcium chloride i.v. 10%, 0.2 ml/kg | Antagonises at receptors |
| Carbon monoxide | Oxygen 100% | Decreases carboxyhaemoglobin. May need hyperbaric oxygen |
| Cyanide | Dicobalt edetate i.v. 4–7.5 mg/kg | Give 50 ml 50% glucose after dose |
| Hydroxocobalamin (vitamin B12) i.v. 70 mg/kg | Beware anaphylaxis, hypertension | |
| Amyl nitrite 0.2 ml perles by inhalation until sodium nitrite 3% i.v. 0.13–0.33 ml/kg over 4 min, then sodium thiosulphate 25% i.v. 1.65 ml/kg (max 50 ml) at 3–5 minutes | Beware hypotension. Nitrites form methaemoglobin–cyanide complex. Beware excess methaemoglobin >20%. Thiosulphate forms non-toxic thiocyanate from methaemoglobin–cyanide | |
| Digoxin | Magnesium sulphate i.v. 25–50 mg/kg (0.1–0.2 mmol/kg) | Antagonises digoxin at sarcolemma |
| Digoxin Fab i.v: acute – 10 vials per 25 tablets (0.25 mg each), 10 vials per 5 mg elixir; steady state: vials, serum digoxin(ng/ml) × BW(kg)/100 | Binds digoxin | |
| Ergotamine | Sodium nitroprusside infusion 0.5–5.0 μg/kg per min | Treats vasoconstriction. Monitor BP continuously |
| Heparin i.v. 100 units/kg then 10–30 units/kg per hour | Monitor partial thromboplastin time | |
| Lead | Dimercaprol (BAL) i.m. 75 mg/m2 4-hourly, six doses, then i.v. CaNa2 edetate (EDTA) 1500 mg/m2 over 5 days if blood level >3.38 μmol/l If asymptomatic and blood level 2.65–3.3 μmol/l, infuse CaNa2EDTA 1000 mg/m2 per day 5 days or oral succimer 350 mg/m2 8-hourly 5 days, then 12-hourly 14 days | Chelating agents |
| Heparin | Protamine 1 mg/100 units heparin | Direct neutralisation |
| Iron | Desferrioxamine 15 mg/kg per hour 12–24 hours if serum iron >90 μmol/l (500 μg/dl) or >63 μmol/l (350 μg/dl) and symptomatic | Give slowly, beware anaphylaxis |
| Methanol, ethylene glycol, glycol ethers | Ethanol i.v. loading dose 10 ml/kg 10% diluted in glucose 5%, then 0.15 ml/kg per hour to maintain blood level 0.1% (100 mg/dl) | Competes with poison for alcohol dehydrogenase |
| Fomepizole (4-methylpyrazole) 15 mg/kg over 30 minutes, then 10 mg/kg 12-hourly, four doses. (Not available in Australia) | Inhibits alcohol dehydrogenase | |
| Methaemoglobinaemia | Methylene blue i.v. 1–2 mg/kg over several minutes | Reduces methaemoglobin to haemoglobin |
| Opiates | Naloxone i.v. 0.01–0.1 mg/kg, then 0.01 mg/kg per hour as needed | Direct receptor antagonist |
| Organophosphates and carbamates | Atropine i.v. 20–50 μg/kg every 15 minutes until secretions dry | Blocks muscarinic effects |
| Pralidoxime i.v. 25 mg/kg over 15–30 minutes then 10–20 mg/kg per hour for 18 hours or more. Not for carbamates | Reactivates cholinesterase | |
| Paracetamol | N-acetylcysteine i.v. 150 mg/kg in dextrose 5% over 60 minutes then 10 mg/kg per hour for 20–72 hours OR oral 140 mg/kg then 17 doses of 70 mg/kg 4-hourly (total 1330 mg/kg over 68 hours) | Restores glutathione inhibiting metabolites. Give if serum paracetamol exceeds 1500 μmol/l at 2 hours, 1000 at 4 hours, 500 at 8 hours, 200 at 12 hours, 80 at 16 hours, 40 at 20 hours. Beware anaphylaxis |
| Phenothiazine dystonia | Benzatropine i.v or i.m. 0.01–0.03 mg/kg | Blocks dopamine reuptake |
| Potassium | Calcium chloride 10% i.v. 0.2 ml/kg | Antagonises cardiac effects |
| Sodium bicarbonate i.v. 1 mmol/kg | Decreases serum potassium (slight effect). Beware hypocalcaemia | |
| Glucose i.v. 0.5 g/kg plus insulin i.v. 0.05 units/kg | Decreases serum potassium (rapid marked effect). Monitor serum glucose | |
| Salbutamol aerosol 0.25 mg/kg | Decreases serum potassium (rapid marked effect) | |
| Resonium oral or rectal 0.5–1 g/kg | Absorbs potassium (slow effect) | |
| Sulphonyl ureas | Glucose Octreotide 1–2 μg/kg 8-hourly | Inhibits insulin release |
| Tricyclic antidepressants | Sodium bicarbonate i.v. 1 mmol/kg to maintain blood pH >7.45 | Reduces cardiotoxicity |
Individual poisons may require specific measures. Consult toxicology texts4–6 for details.
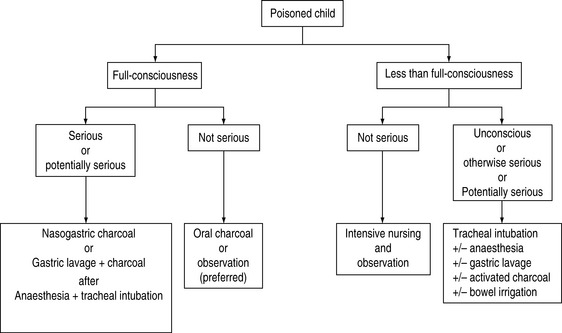
The vast majority of poisoning in childhood is by ingestion. The correct choice of a gastrointestinal decontamination technique is crucial to uncomplicated recovery. The choices are induced emesis, gastric lavage, activated charcoal, whole-bowel irrigation or a combination of these techniques. The efficacy, indications, contraindications and disadvantages and complications of these techniques are discussed below. A general plan of management is presented in Figure 105.1.
The decision to attempt removal of a poison should always be made with due reference to two facts
INDUCED EMESIS
Induced emesis is quickly disappearing from hospital practice and should not be performed routinely in this setting.7 It does not improve outcome and may reduce effectiveness of the alternative treatments of activated charcoal, oral antidotes and whole-bowel irrigation.
Specific contraindications include actual or impending loss of full consciousness or ingestion of corrosives or hydrocarbons.7
The efficacy of ipecacuanha (ipecac) is limited and decreases with time from ingestion. Although it causes vomiting in a high percentage (93–100%) of children within 25 minutes, the percentage of stomach contents ejected is small (28%) even when administered immediately after ingestion.8 Moreover, solids are retained in the stomach or may even be propelled into the duodenum.9
In experimental drug ingestions, approximately 50–83% of ingested experimental drug is removed if ipecac is given after 5 minutes,10 but falling to 2–44% if given at 30 or 60 minutes.11–15 In paediatric paracetamol poisoning, the 4-hour postingestion serum level was approximately 50% of controls if ipecac-induced vomiting occurred within 60 minutes of ingestion, but no benefit was derived if emesis occurred beyond 90 minutes after ingestion.16 Similarly, serum levels of paracetamol were reduced approximately 50% if ipecac was administered at home, inducing emesis at a mean of 26 minutes after ingestion, compared with ipecac administered at a medical facility at a mean of 83 minutes.17
In adults, ipecac is even less useful and has to be given immediately to have quantifiable effects.18
Induced emesis appears superior to gastric lavage but inferior to activated charcoal. In children poisoned with salicylate, emesis retrieved twice as much compared with gastric lavage.19 In adult volunteers ipecac-induced vomiting, occurring at an average of 19 minutes after ingestion, removed 54% of a tracer compared with 30% with gastric lavage performed at the equivalent times after ingestion.20–22
The use of ipecac has potential complications:
More serious, but rare complications include:
Critics claim that induced emesis merely creates work, delays discharge from the emergency department,24 increases complications24 and does not benefit the patient who presents more than 1 hour after ingestion.25 Importantly, ipecac did not alter the clinical outcome of patients who presented awake and alert to the emergency department.26
Induced emesis has been largely abandoned by emergency departments but its use in the home is safe and is associated with fewer paediatric emergency department attendances.27 Its use at home is still recommended by authoritative paediatric health organisations in the USA28 and by approximately half of poison centre staff.29 Although ipecac was recommended inappropriately in 20% of cases to poisons information centres, it caused little morbidity.30
GASTRIC LAVAGE
It involves passage of a large bore oro- or nasogastric tube into the stomach and the repeated instillation of fluid, usually water but some authorities advocate normal or half-normal saline. The oral route is preferred because of less potential for traumatic injury but an oropharyngeal airway may be needed to prevent tube occlusion by chomping. A smaller tube may be used if the poison is a liquid. Traditionally, the child should be placed in the left lateral position to limit stomach emptying but volume of intragastric contents rather than body position determines gastric emptying.31
Experimental studies with therapeutic substances in volunteers, or with liquid medicines or substances instilled into the stomachs of overdose victims, reported that gastric lavage retrieved 90% at 5 minutes,32 45% at 10 minutes21 and 30% at 19 minutes20 and reduced absorption or bioavailability by 20–32% at 1 hour after ingestion.11,33 When gastric lavage was performed 5 minutes after ingestion of tablet drugs, it failed to prevent absorption, presumedly because the tablets had not disintegrated.34 In true overdose situations, gastric lavage within 4 hours of admission reduced serum paracetamol levels by 39%.35
Efficacy of gastric lavage, even with large bore tubes, is poor because tablets are not removed and lavage encourages propulsion into the duodenum.8 In symptomatic patients, gastric lavage alone compared with gastric lavage and activated charcoal increased pneumonic aspiration and did not alter the duration of intubation or the stay in the emergency department or the intensive care unit (ICU),36 and was not beneficial unless performed within 1 hour of ingestion.26 A prospective randomised trial of gastric lavage in acute overdose,37 although criticised on methodological grounds,37,38 suggested that it made no difference to outcome of obtunded patients when preceding activated charcoal.
CONTRAINDICATIONS
ACTIVATED CHARCOAL
Treatment of charcoal with chemicals and heat increases its surface area to approximately 950 m2/g in so-called low surface area activated charcoal and to 2000 m2/g in superactivated charcoal. The latter adsorbs paracetamol better38 and is more palatable.39,40 Activated charcoal is not pleasant to drink and was associated with vomiting in 20% of poisoned children.41
It is superior to induced emesis and gastric lavage in treatment of symptomatic poisoned patients.22,26,36 Its efficacy diminishes with time after ingestion. Activated charcoal reduces absorption of ingested experimental drugs in volunteers by 85–100% when administered 5 minutes after ingestion,10,34,42 by 40–75% at 30 minutes9 and by 30–50% at 60 minutes.14,42 At a mean time of 98 minutes (SD 44) or more than 2 hours after poisoning, it was not effective at all.43,44
Studies have suggested that charcoal alone is as effective as when combined with either emesis or with lavage.37,38 Furthermore, combined methods have a higher incidence of aspiration, 8.5% versus none.34 Activated charcoal failed to show a benefit in asymptomatic poisoned patients.
Repeated doses of activated charcoal enhance elimination of some drugs by increasing adsorption and by achieving postabsorption elimination by interrupting enterohepatic circulation and by removing drug from the gastrointestinal mucosa (‘gastrointestinal dialysis’). Although there are multiple reports in experimental and clinical practice (Table 105.2), there is no hard evidence to show that this therapy reduces mortality or morbidity. Only in life-threatening poisoning by carbamazepine, dapsone, phenobarbital, quinine or theophylline should multiple-dose activated charcoal be considered.45
Table 105.2 Elimination and lack of elimination of drugs by multiple dose activated charcoal45
| Elimination increased in experimental and clinical studies | Elimination increased in volunteer studies | Elimination not increased in experimental or clinical studies |
|---|---|---|
| Carbamazepine | Amitriptyline | Astemizole |
| Dapsone | Dextropropoxyphene | Chlorpropamide |
| Phenobarbital | Digitoxin | Doxepin |
| Quinine | Digoxin | Imipramine |
| Theophylline | Disopyramide | Meprobamate |
| Nadolol | Methotrexate | |
| Phenylbutazone | Phenytoin | |
| Phenytoin | Sodium valproate | |
| Piroxicam | Tobramycin | |
| Sotalol | Vancomycin |
See text for details.
A suitable single dose is 1–2 g/kg. A multiple-dose regimen for children is 1–2 g/kg stat followed by 0.25–0.5 g/kg 4–6-hourly. An alternative is 0.25–0.5 g/kg hourly for 12–24 hours.46
COMPLICATIONS
Aspiration of charcoal causes severe and often fatal pneumonitis, bronchiolitis obliterans and adult respiratory distress syndrome. Intratracheal instillation of activated charcoal causes a significant increase in lung microvascular permeability and arterial blood gas derangements.47
Constipation is common after charcoal but bowel obstruction is fortunately rare. The addition of a laxative (e.g. sorbitol or magnesium sulphate) is not recommended because although transit time through the gut is decreased, efficacy of the charcoal is reduced and life-threatening fluid and electrolyte imbalance may occur.48
WHOLE-BOWEL IRRIGATION
Irrigation of the bowel with an iso-osmolar solution of polyethylene glycol and electrolytes is effective in reducing absorption of experimental drug by 24–67% at 1 hour after ingestion49,50 and up to 73% at 4 hours after ingestion.51 However, it has not been shown conclusively to improve the outcome of poisoned patients. The technique has limited applications to sustained-release or enteric-coated drugs and remains a theoretical option for ingestions of iron, lead, zinc (substances not adsorbed by activated charcoal), packets of illicit drugs52 and for lithium.53 Whole-bowel irrigation may be useful in delayed presentations when poisons have progressed beyond the stomach.
Irrigation solutions are adsorbed by activated charcoal and cause desorption of drug – necessitating prior administration of charcoal if a combined technique is used.54 However, whole-bowel irrigation did not add to the benefits of activated charcoal in an experimental model of sustained-release theophylline poisoning.55
A suitable regimen is approximately 30 ml/kg per hour for 4–8 hours until rectal effluent is clear. A regimen of 25 ml/kg per hour has been used safely for 5 days (total 44.3 litres),56 but a total of 3 litres performed as well as 8 litres in a simulated poisoning.57
PLAN OF MANAGEMENT
A general plan of management is suggested in Figure 105.1, but each case of poisoning mandates a specific plan of management according to circumstances. The primary determinant is the state of consciousness which relates to the risk of aspiration. Apart from that, the timing of presentation in relation to the severity of poisoning and the existence or otherwise of an effective antidote dictate if removal should be attempted, and by what means.
POISONING BY SPECIFIC SUBSTANCES
Like adults, children are poisoned regularly by therapeutic prescription or over-the-counter therapeutic drugs and substances. The poisons probably reflect their availability in the home. In a survey, the most common agents responsible for hospital admission were benzodiazepines, anticonvulsants, anti-parkinsonism drugs, paracetamol, major tranquillisers, antidepressants and cardiovascular drugs.58 The inquisitive nature of young children, however, may lead to poisoning by substances not normally regarded as dangerous – a few of these and others which have notably different management from similar poisoning in adults are considered here, briefly.
BUTTON OR DISC BATTERIES
Impaction in the oesophagus is the most significant situation; this can result in oesophageal perforation and tracheo-oesophageal fistula or aorto-oesophageal fistula. An impacted battery must be removed endoscopically as soon as possible. Lithium batteries are large and impact readily and they have higher voltages than other types. Electrolysis commences as soon as the battery surfaces are immersed in oesophageal fluid. The consequence is local and surrounding tissue destruction, including the trachea.59 Strong alkali is produced at the cathode and strong acid is produced at the anode. Mercury batteries are more likely to fragment60 but mercury poisoning is very uncommon.
PETROLEUM DISTILLATES
Pneumonitis is the most significant and it may occur during ingestion or subsequent vomiting. Although variable, these substances have low surface tensions which enable their rapid dispersement throughout contiguous mucosal surfaces which include the respiratory tree. Prime examples are petrol, kerosene, lighter fluid, lamp oil and mineral spirits. Any child who ingests a distillate must be assessed for pneumonitis and this should include clinical examination, a chest X-ray and at least a non-invasive measurement of oxygenation such as pulse oximetry. Although most children who ingest petroleum distillates do not develop pneumonitis, the onset of this complication may be within 30 minutes61 and progress rapidly to severe lung disease. An adequate period of observation (6 hours) is necessary to exclude this complication. There is a poor correlation between the amount ingested and the severity of pulmonary toxicity.
ESSENTIAL OILS
The oils from certain plants contain mixtures of terpenes, alcohols, aldehydes, ketones and esters, which are used domestically for various purposes. The well-known oils are eucalyptus, turpentine, citronella, cloves, melaleuca, peppermint, wintergreen and lavender. In general, small amounts cause depression of conscious state, irritation of gastrointestinal tract, liver dysfunction and pneumonitis if inhaled. Each has different toxicity. For example, as little as 5 ml of eucalyptus oil62 or 15 ml of turpentine may cause depression of conscious state.
Emesis should not be induced and gastric lavage performed only if airway protection is required.
LEAD
Treatment consists of gastric decontamination in the case of recent ingestion of lead salts. The use of chelating agents may be indicated. In the case of ingestion of a lead foreign body, serial X-rays should be taken to ensure elimination, otherwise surgical removal is indicated. In the case of multiple embedded gunshot pellets and in all cases of chronic poisoning, serum lead levels should be measured to guide chelation therapy (see Table 105.1).
PARACETAMOL
This is the most common drug ingested by children in an accidental, iatrogenic or deliberate overdose situation. Overdose has the potential for hepatic failure and, less commonly, renal failure. The onset of toxicity is delayed – up to several days. Consequently, the need for acute gastric decontamination is uncommon, but would be justifiable for acute large dose presentations. The toxicity in part is caused by the hepatic metabolite of the drug (N-acetyl-p-benzoquinoneimine) which accumulates when endogenous glutathione, which normally facilitates conversion of N-acetyl-p-benzoquinoneimine to non-toxic substances, becomes exhausted. Adequate supply of glutathione is ensured by administration of the antidote, N-acetylcysteine, a glutathione precursor which can be administered intravenously or orally (see Table 105.1).
The time of presentation after ingestion, as well as the dose, determines the management. If presentation is within 1 hour of ingestion, effective gastric removal or administration of activated charcoal may be all that is required pending a serum paracetamol level. In contrast, if presentation is several hours after ingestion, administration of the antidote, according to serum paracetamol level, takes precedence and although it may be administered orally or intravenously, the intravenous route is preferred.63 Near-simultaneous administration of activated charcoal may be of some benefit but it may also cause vomiting or desorption, thus decreasing the effectiveness of the antidote. If presentation is many hours after the poisoning, activated charcoal would not be indicated, so the antidote could be administered orally if necessary according to serum paracetamol levels. In all cases, evidence of liver dysfunction mandates administration of the antidote.
The threshold single toxic dose of paracetamol indicating N-acetylcysteine administration is generally regarded as 150 mg/kg, although it has been suggested that a single dose less than 200 mg/kg in children less than 6 years of age may be safely managed at home.64 However, in situations of repeated sub-150 mg/kg doses hepatotoxicity has a high mortality among children65 and in adults.66 Unfortunately, toxic overdosing by chronic administration is not uncommon even in paediatric institutions.67
No sufficient data exist on which to firmly base a decision to administer N-acetylcysteine to children. Time-related serum levels of paracetamol should be measured and reference made to a guideline to administer the antidote (see Table 105.1) as derived from single toxic dose adult data.68 The serum paracetamol level indicating antidote has been predicted as > 225 mg/l (1500 μmol/l) at 2 hours after ingestion of a single overdose of elixir in children 1–5 years of age.43 If serum levels of paracetamol cannot be obtained and > 150 mg/kg has been ingested as a single dose or liver dysfunction is present after chronic poisoning, the antidote should be given.
Adverse reactions to N-acetylcysteine (approximately 8%) respond to an antihistamine69 and its temporary cessation.
IRON
Small quantities of elemental iron (> 20 mg/kg) are toxic to children. This dose may be reached by ingestion of few iron tablets. The initial effects are gastrointestinal, which may include gastric erosion, followed sometimes by an interval before cardiovascular failure occurs at 6–24 hours and then followed by multiorgan failure (including encephalopathy) and hepatic and renal failure up to some 48 hours after ingestion. In addition to general supportive measures, specific management should include abdominal X-ray to determine if unabsorbed tablets are present, in which case gastric lavage or whole-bowel irrigation may be useful. Activated charcoal is useless. Chelation therapy with desferrioxamine (see Table 105.1) should be guided by serum iron level and clinical status.
CAUSTIC SUBSTANCES
In a study of 743 children,70 the incidence of oesophageal burns caused by ingestion of automatic machine dishwashing detergents was 59%, caustic soda 55% and drain cleaners 55%. All are strongly alkaline and are corrosive. Dishwasher detergents are presented as liquids, powders or tablet blocks and are commonly accessed in an open dishwasher.71 Pharyngeal and oesophageal irritation, burns or corrosion may occur. There may be simultaneous ocular and dermal toxicity. Any child presenting with a history of ingestion of a caustic substance, irrespective of clinical signs, should be considered for oesophagoscopy and follow-up since the correlation between symptoms and signs and oesophageal burns is poor70 and significant oesophageal damage may occur in the absence of more proximal injury.72
1 Cripps R, Steel D. Childhood poisoning in Australia. Australian Government, Australian Institute of Health and Welfare. http://www.nisu.flinders.edu.au/pubs/reports/2006/injcat90.php
2 Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in paediatric inpatients. JAMA. 2001;285:2114-2120.
3 Michael JB, Sztajnkrycer MD. Deadly pediatric poisons: nine common agents that kill at low doses. Emerg Med Clin North Am. 2004;22:1019-1050.
4 Haddad LM, Shannon MW, Winchester JF. Clinical Management of Poisoning and Drug Overdose, 3rd edn. Philadelphia: WB Saunders, 1998.
5 Ellenhorn MJ, Schonwald S, Ordog G, et al. Ellenhorn’s Medical Toxicology, 2nd edn. Baltimore: Williams and Wilkins, 1997.
6 Bates N, Edwards N, Roper J, et al. Paediatric Toxicology. London: Macmillan, 1997.
7 Krenzelok EP, McGuigan M, Lheur P. Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Associations of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:699-709.
8 Corby DG, Decker WJ, Moran MJ, et al. Clinical comparisons of pharmacologic emetics in children. Pediatrics. 1968;4:361-364.
9 Saetta JP, March S, Gaunt ME, et al. Gastric emptying procedures in the self-poisoned patient: are we forcing gastric content beyond the pylorus? J Roy Soc Med. 1991;84:274-276.
10 Neuvonen PJ, Vartiainen M, Tokola O. Comparison of activated charcoal and ipecac syrup in prevention of drug absorption. Eur J Clin Pharmacol. 1983;24:557-562.
11 Tenenbein M, Cohen S, Sitar DS. Efficacy of ipecac-induced emesis, orogastric lavage, and activated charcoal for acute drug overdose. Ann Emerg Med. 1987;16:838-841.
12 Curtis RA, Barone J, Giacona N. Efficacy of ipecac and activated charcoal/cathartic. Prevention of salicylate absorption in a simulated overdose. Arch Intern Med. 1984;144:48-52.
13 Danel V, Henry JA, Glucksman E. Activated charcoal, emesis, and gastric lavage in aspirin overdose. BMJ. 1988;296:1507.
14 McNamara RM, Aaron CK, Gemborys M, et al. Efficacy of charcoal cathartic versus ipecac in reducing serum acetaminophen in a simulated overdose. Ann Emerg Med. 1989;18:934-938.
15 Vasquez TE, Evans DG, Ashburn WL. Efficacy of syrup of ipecac-induced emesis for emptying gastric contents. Clin Nucl Med. 1988;13:638-639.
16 Bond GR, Requa RK, Krenzelok EP, et al. Influence of time until emesis on the efficacy of decontamination using acetaminophen as a marker in a pediatric population. Ann Emerg Med. 1993;22:1403-1407.
17 Amitai Y, Mitchell AA, McGuigan MA, et al. Ipecac-induced emesis and reduction of plasma concentrations of drugs following accidental overdose in children. Pediatrics. 1987;80:364-367.
18 Saincher A, Sitar DS, Tenenbein M. Efficacy of ipecac during the first hour after drug ingestion in human volunteers. J Toxicol Clin Toxicol. 1997;35:609-615.
19 Boxer L, Anderson FP, Rowe MD. Comparison of ipecac-induced emesis with gastric lavage in the treatment of acute salicylate ingestion. Ped Pharmacol Ther. 1969;74:800-803.
20 Young WFJr, Bivins HG. Evaluation of gastric emptying using radionuclides: gastric lavage versus ipecac-induced emesis. Ann Emerg Med. 1993;22:1423-1427.
21 Tandberg D, Diven BG, McLeod JW. Ipecac-induced emesis versus gastric lavage: a controlled study in normal adults. Am J Emerg Med. 1986;4:205-209.
22 Albertson TE, Derlet RW, Foulke GE, et al. Superiority of activated charcoal alone with ipecac and activated charcoal in the treatment of acute toxic ingestions. Ann Emerg Med. 1989;18:56-59.
23 Czajka PA, Russell SL. Nonemetic effects of ipecac syrup. Pediatrics. 1985;75:1101-1104.
24 Kornberg AE, Dolgin J. Pediatric ingestions: charcoal alone versus ipecac and charcoal. Ann Emerg Med. 1991;20:648-651.
25 Foulke GE, Albertson TE, Derlet RW. Use of ipecac increases emergency department stays and patient complication rates. Ann Emerg Med. 1990;17:402.
26 Kulig K, Bar-Or D, Cantrill SV, et al. Management of acutely poisoned patients without gastric emptying. Ann Emerg Med. 1985;14:562-567.
27 Bond GR. Home use of syrup of ipecac is associated with a reduction in pediatric emergency department visits. Ann Emerg Med. 1995;25:338-343.
28 Manoguerra AS, Cobaugh DJ. Guidelines for the Management of Poisoning Consensus Panel. Guidelines on the use of ipecac syrup in the out-of-hospital management of ingested poisons. Clin Toxicol. 2005;43:1-10.
29 Marchbanks B, Lockman P, Shum S, et al. Trends in ipecac use: a survey of poison center staff. Vet Hum Toxicol. 1999;41:47-49.
30 Wrenn K, Rodewald L, Dockstader L. Potential misuse of ipecac. Ann Emerg Med. 1993;22:1408-1412.
31 Doran S, Jones KL, Andrews JM, et al. Effects of meal volume and posture on gastric emptying of solids and appetite. Am J Physiol. 1998;275:R1712-R1718.
32 Auerbach PS, Osterich J, Braun O, et al. Efficacy of gastric emptying: gastric lavage versus emesis induced with ipecac. Ann Emerg Med. 1986;15:692-698.
33 Grierson R, Green R, Sitar DS, et al. Gastric lavage for liquid poisons. Ann Emerg Med. 2000;35:435-439.
34 Lapatto-Reiniluoto O, Kivisto KT, Neuvonen PJ. Gastric decontamination performed 5 min after ingestion of temazepam, verapamil and moclobemide: charcoal is superior to lavage. Br J Clin Pharmacol. 2000;49:274-278.
35 Underhill TJ, Greene MK, Dove AF. A comparison of the efficacy of gastric lavage, ipecacuanha and activated charcoal in the emergency management of paracetamol overdose. Arch Emerg Med. 1990;7:148-154.
36 Merigian KS, Woodard M, Hedges JR, et al. Prospective evaluation of gastric emptying in the self-poisoned patient. Am J Emerg Med. 1990;8:479-483.
37 Pond SM, Lewis-Driver DJ, Williams GM, et al. Gastric emptying in acute overdose: a prospective randomised controlled trial. Med J Aust. 1995;163:345-349.
38 Whyte IM, Buckley NA. Progress in clinical toxicology: from case reports to toxicoepidemiology. Med J Aust. 1995;163:340-341.
39 Roberts JR, Gracely EJ, Schoffstall JM. Advantage of high-surface-area charcoal for gastrointestinal decontamination in a human acetaminophen ingestion model. Acad Emerg Med. 1997;4:167-174.
40 Fischer TF, Singer AJ. Comparison of the palatabilities of standard and superactivated charcoal in toxic ingestions: a randomized trial. Acad Emerg Med. 1999;6:895-899.
41 Osterhoudt KC, Durbin K, Alpern ER, et al. Risk factors for emesis after therapeutic use of activated charcoal in acutely poisoned children. Pediatrics. 2004;113:806-810.
42 Neuvonen PJ, Elonen E. Effect of activated charcoal on absorption and elimination of phenobarbitone, carbamazepine and phenylbutazone in man. Eur J Clin Pharmacol. 1980;17:51-57.
43 Anderson BJ, Holford NHG, Armishaw JC, et al. Predicting concentrations in children presenting with acetaminophen overdose. J Pediatr. 1999;135:290-295.
44 Yeates PJ, Thomas SH. Effectiveness of delayed activated charcoal administration in simulated paracetamol (acetaminophen) overdose. Br J Clin Pharmacol. 2000;49:11-14.
45 Anonymous. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1999;37:731-751.
46 Ohning BL, Reed MD, Blumer JL. Continuous nasogastric administration of activated charcoal for the treatment of theophylline intoxication. Ped Pharmacol. 1986;5:241-245.
47 Arnold TC, Willis BH, Xiao F, et al. Aspiration of activated charcoal elicits an increase in lung microvascular permeability. J Toxicol Clin Toxicol. 1999;37:9-16.
48 Palatnick W, Tenenbein M. Activated charcoal in the treatment of drug overdose. Drug Safety. 1992;7:3-7.
49 Smith SW, Ling LJ, Halstenson CE. Whole bowel irrigation as a treatment for acute lithium overdose. Ann Emerg Med. 1991;29:536-539.
50 Tenenbein M, Cohen S, Sitar DS. Whole bowel irrigation as a decontamination procedure after acute drug overdose. Arch Intern Med. 1987;147:905-907.
51 Kirshenbaum LA, Mathews SC, Sitar DS, et al. Whole-bowel irrigation versus activated charcoal in sorbitol for the ingestion of modified-release pharmaceuticals. Clin Pharmacol Ther. 1989;46:264-271.
52 Tenenbein M. Position statement: whole bowel irrigation. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:753-762.
53 Scharman EJ. Methods used to decrease lithium absorption or enhance elimination. J Toxicol Clin Toxicol. 1997;35:601-608.
54 Makosiej FJ, Hoffman RS, Howland MA, et al. An in vitro evaluation of cocaine hydrochloride adsorption by activated charcoal and desorption upon addition of polyethylene glycol electrolyte lavage solution. J Toxicol Clin Toxicol. 1993;31:381-395.
55 Burkhart KK, Wuerz RC, Donovan JW. Whole bowel irrigation as adjunctive treatment for sustained-release theophylline overdose. Ann Emerg Med. 1992;21:1316-1320.
56 Kaczorowski JM, Wax PM. Five days of whole-bowel irrigation in a case of pediatric iron ingestion. Ann Emerg Med. 1996;27:258-263.
57 Olsen KM, Gurley BJ, Davis GA, et al. Comparison of fluid volumes with whole bowel irrigation in a simulated overdose of ibuprofen. Ann Pharmacother. 1995;29:246-250.
58 Hoy JL, Day LM, Tibballs J, et al. Unintentional poisoning hospitalizations among young children in Victoria. Inj Prev. 1999;5:31-35.
59 Tibballs J, Wall R, Velandy Koottayi S, et al. Tracheo-oesophageal fistula caused by electrolysis of a button battery impacted in the oesophagus. J Paediatr Child Health. 2002;38:201-203.
60 Litovitz T, Schmitz BF. Ingestion of cylindrical and button batteries: an analysis of 2382 cases. Pediatrics. 1992;89:747-757.
61 Anas N, Namasonthi V, Ginsburg CM. Criteria for hospitalizing children who have ingested products containing hydrocarbons. JAMA. 1981;246:840-843.
62 Tibballs J. Clinical effects and management of eucalyptus oil ingestion in infants and young children. Med J Aust. 1995;163:177-180.
63 Buckley NA, Whyte IM, O’Connell DL, et al. Oral or intravenous N-acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning? J Toxicol Clin Toxicol. 1999;37:759-767.
64 Bond GR, Krenzelok EP, Normann SA, et al. Acetaminophen ingestion in childhood – cost and relative risk of alternative referral strategies. J Toxicol Clin Toxicol. 1994;32:513-525.
65 Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: hepatotoxicity after multiple doses in children. J Pediatr. 1998;132:22-27.
66 Schiodt FV, Rochling FA, Casey DL, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112-1117.
67 Hynson JJ, South M. Childhood hepatotoxicity with paracetamol doses less than 150 mg/kg per day. Med J Aust. 1999;171:497.
68 Smilkstein MJ, Bronstein AC, Linden C, et al. Acetaminophen overdose: a 48 hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20:1058-1063.
69 Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Pharmacol. 2001;51:87-91.
70 Bautista Casasnovas A, Estevez Martinez E, Varela Cives R, et al. A retrospective analysis of ingestion of caustic substances by children. Ten-year statistics in Galicia. Eur J Pediatr. 1997;156:410-414.
71 Cornish LS, Parsons BJ, Dobbin MD. Automatic dishwasher detergent poisoning: opportunities for prevention. Aust N Z J Public Health. 1996;20:278-283.
72 Krenzelok EP, Clinton JE. Caustic esophageal and gastric erosion without evidence of oral burns following detergent ingestion. JACEP. 1979;8:194-196.