Chapter 99 Paediatric fluid and electrolyte therapy
Children need a much higher intake of water and electrolytes per kilogram of body weight than adults; this makes children more susceptible to dehydration if they have abnormal losses of water or a reduced intake. On the other hand, an inability to excrete a water load, due to immature kidneys (in neonates) or high levels of antidiuretic hormone (ADH), means that children can easily be given too much water intravenously. There are good reviews of fluid and electrolyte therapy in paediatric and neonatal intensive care.1–5
WATER
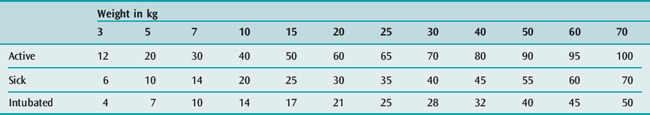
The full-term neonate is 80% water; this figure falls to about 60% by 12 months of age, and then remains almost constant throughout childhood. The intravenous (i.v.) fluid requirements for children in hospital are shown in Tables 99.1 and 99.2. The widely used formula for an active child shown in Table 99.1 was published in 1957,6 and some of the measurements of energy expenditure were made almost 100 years ago. The formula is a compromise between the requirements of a fully active child and the much lower requirements at basal metabolic rate – for example, the estimate of 100 ml/kg per day for an active 10-kg child in hospital is double the 50 ml/kg per day needed for a sick child at basal state (Table 99.1).6,7
Table 99.1 Approximate intravenous fluid requirements for children (ml/kg per day)
| Active child in hospital | |
| < 10 kg | 100 ml/kg per day |
| 10–20 kg | 1000 ml + (50 ml/kg per day for each kg over 10 kg) |
| > 20 kg | 1500 ml + (20 ml/kg per day for each kg over 20 kg) |
| Sick, but not intubated | |
| < 10 kg | 50 ml/kg per day |
| 10–20 kg | 500 ml + (30 ml/kg per day for each kg over 10 kg) |
| > 20 kg | 800 ml + (20 ml/kg per day for each kg over 20 kg) |
| Intubated, with humidified inspired gases | |
| < 10 kg | 35 ml/kg per day |
| 10–20 kg | 350 ml + (20 ml/kg per day for each kg over 10 kg) |
| > 20 kg | 550 ml + (12.5 ml/kg per day for each kg over 20 kg) |
Table 99.3 shows that very ill children in intensive care often need much less water than the ‘standard’ amounts that are often given. For example, if a 15-kg child (‘standard’ maintenance fluid of 50 ml/hour,6Table 99.2) sustains a head injury and has evidence of high levels of ADH (maintenance fluid × 0.7),8 is ventilated with humidified gas (maintenance × 0.75),9 is paralysed (basal state, maintenance × 0.7),6 and is maintained at a rectal temperature of 36°C (maintenance – 12%),1 then his actual full maintenance fluid requirement is (50 × 0.7 × 0.75 × 0.7) – 12% = 16 ml/hour. Even less water should be given initially if the child is overhydrated.
Table 99.3 Modifications to the fluid intakes for active children shown in Table 99.2
| Decrease | Adjustment |
|---|---|
| Humidified inspired air | × 0.75 |
| Basal state (e.g. paralysed) | × 0.7 |
| High ADH (IPPV, brain injury) | × 0.7 |
| Hypothermia | − 12% per °C |
| High room humidity | × 0.7 |
| Renal failure | × 0.3 (+ urine output) |
| Increase | |
|---|---|
| Full activity + oral feeds | × 1.5 |
| Fever | + 12% per °C |
| Room temperature > 31°C | + 30% per °C |
| Hyperventilation | × 1.2 |
| Neonate preterm (1–1.5 kg) | × 1.2 |
| radiant heater | × 1.5 |
| phototherapy | × 1.5 |
| Burns first day | + 4% per 1% area burnt |
| subsequently | + 2% per 1% area burnt |
ADH, antidiuretic hormone; IPPV, intermittent positive pressure ventilation.
The estimates of water requirements in Tables 99.1–99.3 are only approximate, and water balance must be monitored closely in any child in intensive care. Unfortunately, regular, accurate weighing of very sick children is often impractical, and hydration has to be assessed using:
In a child with oliguria following a severe ischaemic or hypoxic insult (such as birth asphyxia, near drowning or cardiac arrest), it may be helpful to measure the urine sodium concentration.10 In oliguria due to acute tubular necrosis, where restriction of fluid intake may be necessary, the urine sodium is usually more than 40 mmol/l. In oliguria due to hypovolaemia, the urine sodium is usually less than 20 mmol/l.
SODIUM11,12
Hyponatraemia may be due to:11
Acute hyponatraemia causes cerebral oedema, with a grave risk of cerebral herniation and death or severe brain damage.13 Hyponatraemia due to sodium deficit should be corrected by careful administration of sodium (Table 99.4). However, hyponatraemia is usually due to water excess rather than sodium deficiency, and this should be treated with restriction of water intake. If symptomatic, it can be corrected by careful administration of 3% sodium at 0.5 ml/kg per hour (Table 99.4), and furosemide 0.5 mg/kg i.v. if there are high ADH levels. Hyponatraemia must be corrected slowly: the serum sodium should increase by no more than 8 mmol/l each 24 hours, and even less in patients with long-standing hyponatraemia.11
Table 99.4 Doses and formulae in paediatric fluid and electrolyte therapy
| Albumin 20% | undiluted: 2–4 ml/kg 5% in 5% dextrose or saline: 10–20 ml/kg |
| Bicarbonate (number of mmol of deficit) | under 5 kg: base excess × wt(kg) × 0.5 (give half of this) over 5 kg: base excess × wt(kg) × 0.3 (give half of this) |
| Blood volume | 85 ml/kg in neonate 70 ml/kg in older children |
| Calcium | chloride 10% (0.7 mmol/ml Ca++): maximum 0.2 ml/kg i.v. stat, requirement 1.5 ml/kg per day gluconate 10% (0.22 mmol/ml Ca++): maximum 0.5 ml/kg i.v. stat, requirement 5 ml/kg per day |
| Dextrose | for hypoglycaemia: 1 ml/kg 50% dextrose i.v. in neonate: 4 mg/kg per min (2.4 ml/kg per hour 10% dextrose) day 1, increasing to 8 mg/kg per min (up to 12 mg/kg per min with hypoglycaemia) for hyperkalaemia: 0.1 U/kg insulin and 2 ml/kg 50% dextrose i.v. stat |
| Magnesium | chloride 0.48 g/5 ml (1 mmol/ml Mg++): 0.4 mmol (0.4 ml)/kg per dose slow i.v. 12-hourly sulphate 50% (2 mmol/ml Mg++): 0.4 mmol (0.2 ml)/kg per dose slow i.v. 12-hourly |
| Mannitol | 0.25–0.5 g/kg per dose i.v. (1–2 ml/kg of 25%) 2-hourly, provided serum osmolality < 330 mosm/kg |
| Packed cells | 10 ml/kg raises Hb 3 g%, 1 ml/kg raises PCV 1%. |
| Potassium | maximum 0.3 mmol/kg per hour, requirement 2–4 mmol/kg per day, 1 g KCl = 13.3 mmol K+. Hyperkalaemia: see dextrose |
| Sodium | depletion: 3% saline at 0.5 ml/kg per hour (maximum Na rise 8 mmol/l in 24 hours); requirement 2–6 mmol/kg per day, 1 g NaCl = 17.1 mmol/Na+ |
| Urine | minimum acceptable is 0.5–1.0 ml/kg per hour |
PCV, packed cell volume.
Hypernatraemia may be due to:12
With hypernatraemic dehydration, shock should be treated with boluses of 10 ml/kg of 0.9% saline. The water deficit should then be corrected very slowly using 0.9% saline so that the serum sodium falls no faster than 0.5 mmol/l per hour to prevent cerebral oedema.12 If salt ingestion causes severe acute hypernatraemia without dehydration, peritoneal dialysis or haemofiltration may be indicated.
CALCIUM, MAGNESIUM AND PHOSPHATE
Hypocalcaemia in children occurs in:
Hypocalcaemia and hypomagnesaemia cause jitters, tetany, cardiac arrhythmias and convulsions. The doses of calcium and magnesium are given in Table 99.4. The normal intravenous maintenance requirements in infants are 1 mmol/kg per day of calcium and 0.3 mmol/kg per day of magnesium.
STANDARD PAEDIATRIC MAINTENANCE FLUIDS
‘Maintenance solution’ is an unfortunate term, since these solutions do not provide maintenance calorie or protein requirements (see Parenteral nutrition, below). Until recently, it was common practice to give children a hypotonic maintenance solution of approximately 0.2% saline in 4% dextrose in generous amounts, based on the water requirements of an active child.6 This policy resulted in a high incidence of hyponatraemia – and far too many children developed cerebral oedema that resulted in death or severe brain damage.13,15–18
Several factors have contributed to the high incidence of hyponatraemia in children.
There is general agreement that an isotonic fluid such as 0.9% saline should be used to correct hypovolaemia, but there has been spirited debate about whether hypotonic 0.2% saline or isotonic 0.9% saline should be used for maintenance.5,23,24 The supporters of using 0.2% saline argue that it is logical and safe providing the volume of fluid given is never in excess of actual requirements,25–27 but there is limited empirical evidence to support this theory.27 The supporters of using 0.9% saline for initial maintenance therapy in sick children (with careful monitoring of the serum sodium) argue that, in practice, this has been preferable,13,15–18 because it is difficult to ensure that no excess fluid is administered, and there is a lower risk of hyponatraemia and cerebral oedema if any excess fluid that is given is isotonic. It is especially important to use isotonic fluid during and after surgery, and whenever there is a high risk of cerebral oedema (e.g. amongst children with diabetic ketoacidosis or brain injury).5,15–18,23,24
Ill children with clear signs of hypovolaemia should be treated with 5–10 ml/kg boluses of 0.9% saline until the intravascular volume has been restored, and then given 0.9% saline (with potassium and dextrose as required) in the volumes shown in Tables 99.1 and 99.2 (or calculated from Table 99.3). The serum sodium concentration should be monitored carefully. If the serum sodium is less than 138 mmol/l and falling, then less 0.9% saline should be infused. If the serum sodium is more than 142 mmol/l and rising, then 0.45% or 0.2% saline should be infused with a cautious increase in the rate.
Great care should be taken to avoid hypoglycaemia in children. Hypoglycaemia is particularly dangerous in young infants,28 while hyperglycaemia is probably less dangerous in children than it is in adults in intensive care.29
DEHYDRATION AND SHOCK
Weight loss is the best guide to the degree of dehydration, if a recent weight is known. Many of the commonly used clinical signs of dehydration in children are inaccurate, and this leads to dehydration being diagnosed when it is not present, and to overestimation of the degree of dehydration.19 The clinical signs of mild to moderate dehydration become apparent with only 3–4% dehydration in children.19 The most reliable signs are:30
In children with shock, intravenous access may be difficult. In these circumstances, parenteral fluid can be given rapidly into the bone marrow, which is an intravascular compartment.31 The usual sites chosen are the junction of the upper and middle third of the tibia (0–12 months of age), the medial malleolus (1–5 years) and the iliac crest (over 5 years). A 0.9 mm (20 gauge) lumbar puncture needle or an intraosseous needle can be used; the needle is held perpendicular to the bone and pushed in gently with a rotary motion about its long axis – a slight decrease in resistance will be felt as the needle enters the medulla. In infants with a patent anterior fontanelle, the superior sagittal sinus is an excellent way to gain intravenous access in an emergency.32
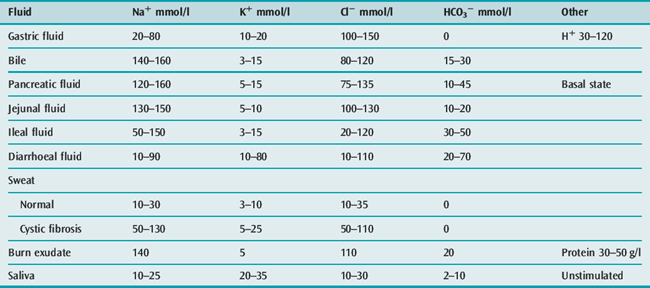
Shock should be treated with an initial bolus of 10 ml/kg of 0.9% saline, followed by further boluses of 10 ml/kg until the intravascular volume has been restored.33 After shock has been corrected with a rapid infusion of fluid, the remainder of the deficit is replaced over the next 48–72 hours, while giving maintenance requirements using 0.9% saline with dextrose and potassium chloride. Thus, a 5-kg child with 10% dehydration from diarrhoea (500 ml deficit) might receive 100 ml (20 ml/kg) of isotonic saline rapidly to restore the circulation, leaving a 400 ml deficit to be replaced over 48 hours. If the maintenance requirement is 250 ml/day (Table 99.1), then the child should be given a further 450 ml of 0.9% saline per day for the next 2 days (approximately 20 ml/hour) in addition to the initial 100 ml. Further abnormal fluid losses should be replaced with an appropriate fluid (see Table 99.5).
With hypernatraemic dehydration, shock should be treated as above, with rapid infusion of 10–20 ml/kg of isotonic saline. The remaining deficit should then be replaced slowly with 0.9% saline so that the serum sodium falls no faster than 0.5 mmol/l per hour to prevent cerebral oedema.12
OEDEMA
Oedema is common in children in intensive care units. It may be due to:
Several possible causes are often present in one child, and it can be difficult to decide which is the most important. Children with oedema and high levels of ADH will have a serum osmolality less than 270 mmol/kg (with hyponatraemia) and a urine osmolality greater than 270 mmol/kg; the appropriate treatment is fluid restriction. On the other hand, in children with oedema due to capillary leak, fluid restriction and attempts to remove water (diuretics, dialysis) are unlikely to cure the oedema, and often cause hypovolaemia; in fact, large amounts of fluid (e.g. blood and normal saline) may be needed to preserve the intravascular volume in these children – the oedema will only disappear when the capillary damage resolves.
CRYSTALLOID OR COLLOID
There have been many reviews of the contentious issue of the relative merits of crystalloid or colloid fluids in critically ill patients. A widely quoted review concluded that, compared to crystalloids, albumin increased mortality by 6% (95% CI, 3–9%).34 However, a subsequent large study in 6997 adults in Australia found no significant difference in overall mortality between normal saline and 4% albumin;35 the mortality with albumin tended to be lower in sepsis and higher in trauma, but the differences just failed to reach statistical significance in both subgroups. The current Cochrane analysis is heavily influenced by the large Australian study, and it concludes that there is no evidence that albumin reduces mortality when compared with cheaper alternatives such as saline.36
There have been very few studies comparing crystalloid to colloid fluids in children, so most of the evidence comes from studies in adults. The use of albumin in paediatric and neonatal intensive care has been reviewed.37,38 The meta-analyses do not suggest that colloid is better than crystalloid, and colloid is more expensive and carries a small risk of transmitting infection. Until more evidence is available, it seems sensible to use crystalloid as routine, but give concentrated albumin to children with severe hypoalbuminaemia (perhaps if their serum albumin is less than 25 g/l), unless the low albumin is caused by chronic malnutrition, when albumin should not be given.39
PARENTERAL NUTRITION40–42
‘Maintenance solution’ is an unfortunate medical term, particularly when small children are concerned – a solution of 5% dextrose with sodium and potassium chloride provides maintenance amounts of water, sodium, potassium and chloride, but little or no calories, protein, trace elements or vitamins. For example, 100 ml/kg per day of 5% dextrose provides 20 cal/kg per day (84 kJ/kg per day), which is only 20% of the requirement of a normal infant (let alone a child with increased calorie requirements). It has been estimated that, although an adult has the energy reserve to survive for about a year on 3 litres of 10% dextrose a day, a small preterm infant will survive only 11 days on 75 ml/kg per day of 10% dextrose.43
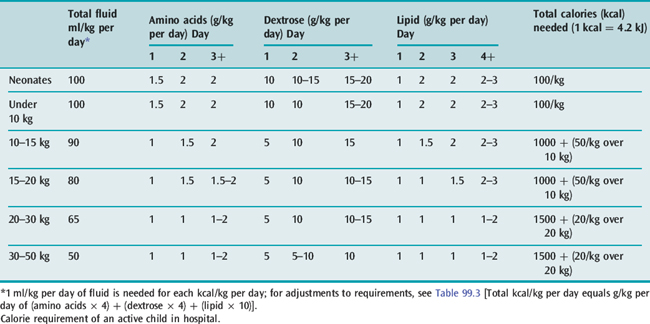
The usual requirements for amino acids, dextrose and fat in parenteral nutrition in children are shown in Table 99.6. The amino acid solution can be mixed with dextrose in the Pharmacy Department to make a ‘nutrient solution’. A standard nutrient solution might provide 4 mmol/kg per day of sodium, 3 mmol/kg per day of potassium, and 7.5 mmol/day of calcium and phosphate (with up to 12 mmol/l for neonates). The standard solution should also contain 4 mmol/l of magnesium, 0.2 μmol/kg per day of manganese, 3 μmol/kg per day of zinc, 0.5 μmol/kg per day of copper, 0.04 μmol/kg per day of iodide, 0.005 μmol/kg per day of chromium, 20 μg/l of hydroxycobalamin, 2 mg/l of phytomenadione, 1 mg/l of folic acid, and a multivitamin preparation. For short-term nutrition, the solution need not provide fluoride, iron or vitamins A, D and E, but a paediatric multivitamin preparation should be given to children on long-term parenteral nutrition. Fat can be given as a 20% emulsion, either through a separate i.v. line or alternating with the nutrient solution. Nutrient solutions for paediatric use contain high concentrations of calcium, magnesium and phosphorus, and they should not be mixed with the fat emulsion, even in a Y-connection placed just before the cannula.
In a child on parenteral nutrition, it is important that abnormal fluid losses (Table 99.5) be replaced with an appropriate solution (in addition to the nutrient solution), and that parenteral nutrition always be introduced and withdrawn slowly. A dislodged i.v. cannula should be replaced immediately in a child on parenteral nutrition to prevent rebound hypoglycaemia. If nutrient solution is not available at any time, it should be replaced with an infusion of a similar amount of dextrose (e.g. 20% dextrose with 40 mmol/l of sodium chloride and 20 mmol/l of potassium chloride).
Children receiving parenteral nutrition are liable to develop:
Frequent, careful monitoring is essential (Table 99.7). Initially, monitoring may need to be more frequent than suggested in Table 99.7, particularly in preterm babies. Monitoring can be less frequent once a child is stabilised on parenteral nutrition.
1 Winters RW, editor. The Body Fluids in Pediatrics. Boston: Little Brown, 1973.
2 Finberg L, Kravath RE, Hellerstein S. Water and Electrolytes in Pediatrics: Physiology, Pathophysiology and Treatment, 2nd edn. Philadelphia: Saunders, 1993.
3 Greenbaum LA. Pathophysiology of body fluids and fluid therapy. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson: Textbook of Pediatrics. 3rd edn. Philadelphia: Saunders; 2004:191-252.
4 Dell KM, Davis ID. Fluid and electrolyte management. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s Neonatal–Perinatal Medicine. 8th edn. Philadelphia: Mosby; 2005:695-712.
5 Paut O, Lacroix F. Recent developments in the perioperative fluid management for the paediatric patient. Curr Opin Anaesthesiol. 2006;19:268-277.
6 Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823-832.
7 Talbot FB. Basal metabolism standards for children. Am J Dis Child. 1938;55:455-459.
8 Bouzarth WF, Shenkin HA. Is ‘cerebral hyponatraemia’ iatrogenic? Lancet. 1982;1:1061-1062.
9 Sousulski R, Polin RA, Baumgart S. Respiratory water loss and heat balance in intubated infants receiving humidified air. J Pediatr. 1983;103:307-310.
10 Harrington JT, Cohen JJ. Measurement of urinary electrolytes – indications and limitations. N Engl J Med. 1975;293:1241-1243.
11 Androgué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581-1589.
12 Androgué HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342:1493-1499.
13 Choong K, Kho ME, Menon K, et al. Hypotonic versus isotonic saline in hospitalized children: a systematic review. Arch Dis Child. 2006;91:828-835.
14 Gennari FJ. Hypokalemia. N Engl J Med. 1998;339:451-458.
15 Taylor D, Durward A. Pouring salt on troubled waters. Arch Dis Child. 2004;89:411-414.
16 Hoorn EJ, Geary D, Robb M, et al. Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics. 2004;113:1279-1284.
17 Moritz ML, Ayus JC. Preventing neurological complications from dysnatremias in children. Pediatr Nephrol. 2005;20:1687-1700.
18 Neville KA, Verge CF, Rosenberg AR, et al. Isotonic is better than hypotonic saline for intravenous rehydration of children with gastroenteritis: a prospective randomized study. Arch Dis Child. 2006;91:226-232.
19 Mackenzie A, Barnes G, Shann F. Clinical signs of dehydration in children. Lancet. 1989;1:605-607.
20 Steele A, Gowrishankar M, Abrahamson S, et al. Postoperative hyponatremia despite near-isotonic saline infusion: a phenomenon of desalination. Ann Intern Med. 1997;126:20-25.
21 Gerigk M, Gnehm HE, Rascher W. Arginine vasopressin and renin in acutely ill children: implications for fluid therapy. Acta Paediatr. 1996;85:550-553.
22 Neville KA, Verge CF, O’Meara MW, et al. High antidiuretic hormone levels and hyponatremia in children with gastroenteritis. Pediatrics. 2005;116:1401-1407.
23 Cunliffe M, Potter F. Four and a fifth and all that. Br J Anaesth. 2006;97:274-277.
24 Sterns RH, Silver SM. Salt and water: read the package insert. Q J Med. 2003;96:549-552.
25 Holliday MA, Friedman AL, Segar WE, et al. Acute hospital-induced hyponatremia in children: a physiological approach. J Pediatr. 2004;145:584-587.
26 Hatherill M. Rubbing salt in the wound. Arch Dis Child. 2004;89:414-418.
27 Sanchez-Bayle M, Alonso-Ojembarrena A, Cano-Fernandez J. Intravenous rehydration of children with gastroenteritis: which solution is better? Arch Dis Child. 2006;91:716.
28 Kinnala A, Rikalainen H, Lapinleimu H, et al. Cerebral magnetic resonance imaging and ultrasonography findings after neonatal hypoglycemia. Pediatrics. 1999;103:724-729.
29 de Ferranti S, Gauvreau K, Hickey PR, et al. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology. 2004;100:1345-1352.
30 Steiner MJ, De Walt DA, Byerley JS. Is this child dehydrated? JAMA. 2004;291:2746-2754.
31 Spivey WH. Intraosseous infusions. J Pediatr. 1987;111:639-643.
32 Shann F. Cannulation of superior sagittal sinus. Lancet. 2002;359:1700.
33 Scheinkestel CD, Tuxen DV, Cade JF, et al. Fluid management of shock in critically ill patients. Med J Aust. 1989;150:508-517.
34 Roberts E. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235-240.
35 The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
36 Alderson P, Bunn F, Li Wan Po A, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. (Issue 4):2004. Art No CD0001208
37 Bohn D, Carcillo JA, editors. The use of albumin in pediatric and neonatal critical care. Pediatr Crit Care Med. 2001;2(Suppl 1):S1-S39.
38 Uhing MR. The albumin controversy. Clin Perinatol. 2004;31:475-488.
39 World Health Organization. Management of the Child with a Serious Infection or Severe Malnutrition. Geneva: WHO, 2000. WHO/FCH/CAH/00.1
40 Baker RD, Baker SS, Davis AM. Pediatric Parenteral Nutrition. New York: Aspen, 1997.
41 Shulman RJ, Phillips S. Parenteral nutrition in infants and children. J Pediatr Gastroenterol Nutr. 2003;36:587-607.
42 Poindexter BB, Leitch CA, Denne SC. Parenteral nutrition. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Martin’s Neonatal–Perinatal Medicine. 8th edn. Philadelphia: Mosby; 2005:679-693.
43 Heird WC, Driscoll JM, Schullinger JN, et al. Intravenous alimentation in pediatric patients. J Pediatr. 1972;80:351-372.