CHAPTER 17 Ovulation induction
Introduction
The aim of ovulation induction is to achieve development of a single follicle and subsequent ovulation in anovulatory infertile women. The ability to induce ovulation is possible in most women, with excellent conception rates. Anovulation accounts for 20–25% of the causes of infertility, and clinicians who manage infertile couples ought to have a sound understanding of the control of follicular development in a normal cycle (see Chapter 15), causes of anovulation (see Chapter 16), appropriate investigations and different treatment options, including their indications and risks. This chapter will briefly review the physiology of ovulation, including the basic principles of ovulation induction, and describe treatment strategies based on the underlying cause of the anovulation.
Principles of Ovulation
Follicular development: recruitment, selection and dominance
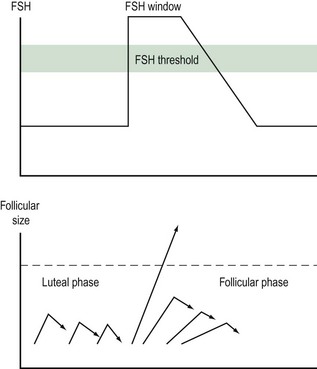
Follicular growth starts with the regression of the corpus luteum at the end of the previous cycle. As the levels of steroid hormones and inhibin drop, their inhibitory effect on FSH is abolished and FSH levels rise. This increase starts approximately 2 days prior to menstruation. As the follicles grow, the production of oestradiol and inhibin increases and FSH levels subsequently fall. The dominant follicle will be selected from day 5–7 of the menstrual cycle. The selection of a single dominant follicle appears to be the result of two oestrogen actions: a positive influence of oestrogen on FSH action within the maturing follicle, and a negative feedback mechanism on FSH at the hypothalamic–pituitary level. This latter action leads to a decline in gonadotrophin levels and ultimately to atresia of the less-developed follicles. Local paracrine–autocrine factors are also involved in this process, such as tumour necrosis factor and anti-Müllerian hormone. The dominant follicle is not affected by this decline in FSH levels, as it is more sensitive to FSH. Provided that a critical exposure of FSH was initially sustained, the dominant follicle continues to grow. In turn, FSH induces the development of LH receptors on the granulosa cells. Thus, LH plays a crucial role in the late stages of follicular development (Figure 17.1).
Ovulation Disorders
Classification
Investigations
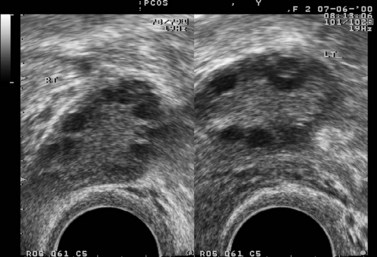
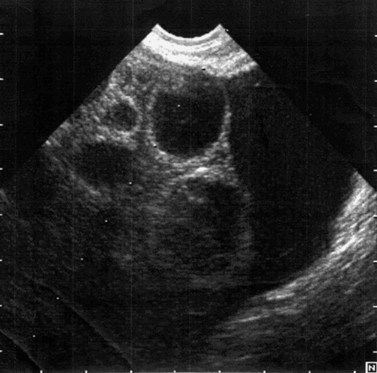
In the case of PCOS, the hormonal investigations should include serum androgens (testosterone, free testosterone, androstenedione and dehydroepiandrosterone sulphate), as they are often elevated, and baseline 17OH-progesterone. Depending on the results, adrenocorticotrophic hormone concentrations may be measured to exclude adrenal pathology. If PCOS is suspected, a transvaginal ultrasound should be performed to assess the ovaries and look for the characteristic peripheral follicles (‘necklace sign’). The criteria used for the ultrasonographic diagnosis of PCOS include the presence of more than 10 follicles with a diameter of 2–10 mm and increased density of the ovarian stroma (Adams et al 1986).
Diagnosis and Treatment
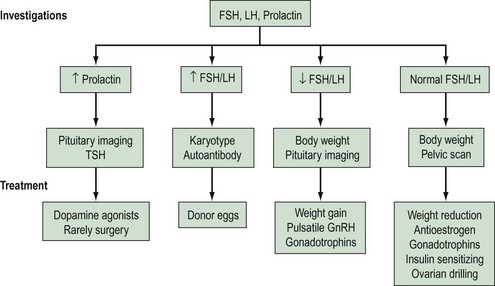
Based on the results of the above investigations, the causes of anovulation can be divided into four main categories (see Box 17.1).
Box 17.1 Classification of ovulation disorders
Hyperprolactinaemia
Causes of hyperprolactinaemia include:
Non-prolactin-secreting tumours are treated surgically in combination with radiotherapy.
Primary hypothyroidism causing hyperprolactinaemia is treated with thyroid replacement therapy.
Hypergonadotrophic hypogonadism
Hypogonadotrophic hypogonadism
Its causes can be either congenital or acquired. Kallman’s syndrome is one of the congenital causes. It consists of isolated gonadotrophin deficiency and anosmia. The syndrome is usually sporadic but it can also be inherited (see Chapter 16).
Acquired causes of hypogonadotrophic hypogonadism include:
If a central nervous system tumour is present, surgery is indicated.
Normogonadotrophic hypogonadism
This category includes women with anovulation with normal FSH, LH and prolactin levels. They may present with regular menses, oligomenorrhoea or even amenorrhoea. Predominantly, this group comprises women with PCOS. This is discussed in more detail in Chapter 18.
Other causes of normogonadotrophic hypogonadism and hyperandrogenism include:
A summary of investigations, classifications and treatments is shown in Figure 17.3, and an overview of assessments and treatments is given in the guidelines of the National Institute for Health and Clinical Excellence (2004).
Antioestrogens
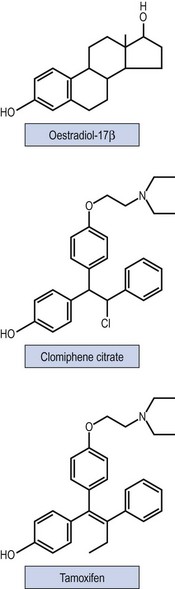
Non-steroidal selective oestrogen receptor modulators, including CC and tamoxifen, are used for ovulation induction. It is thought that they bind with oestrogen receptors in the hypothalamus, leading to a perceived drop in the endogenous oestrogen levels. As a consequence, GnRH and endogenous gonadotrophin secretion is increased, leading to induction of ovulation. Figure 17.4 shows the structure of the drugs.
Clomiphene citrate
Indications for treatment
CC has also been tried for the treatment of unexplained infertility. Nonetheless, when CC treatment was compared with placebo or no treatment, there was no evidence that CC was more effective in this subgroup of infertile women (Hughes et al 2000).
Treatment regimens and monitoring
If ovulation is not induced with the initial dose of 50 mg, the dose of CC can be increased in 50-mg increments in subsequent cycles up to a maximum dose of 250 mg/day. Approximately 70% of anovulatory women treated with CC respond to a dose of 100–150 mg/day (Gysler et al 1982). In practice, doses of 250 mg are rarely used. Women who do not ovulate while taking a dose of 150 mg are considered to be ‘clomiphene-resistant’ (Vandermolen et al 2001). The required CC dose is correlated to body weight, as there is a significant association between CC treatment failure and BMI greater than 27 kg/m2. Women who are overweight should be advised that a 5% reduction in weight may improve endocrine and ovarian function, increase spontaneous conception rates and improve the response to CC treatment. Lower doses of 25–50 mg/day should be considered for those women who are exceptionally sensitive to treatment with CC.
Monitoring of CC treatment is recommended. Ovulation is confirmed either with serum progesterone measurements or with the use of transvaginal ultrasonography. Serum progesterone concentrations are measured during the luteal phase. Ultrasound monitoring should be offered at least during the first treatment cycle to ensure that the woman is on the lowest necessary dose to achieve ovulation (National Institure for Health and Clinical Excellence 2004). Monitoring will minimize the risk of multiple pregnancy and ovarian hyperstimulation syndrome (OHSS), and is essential in patients in whom ovulation does not seem to have been induced.
Results
Studies with CC have shown an ovulation rate of 60–85% and a pregnancy rate of 30–40%. The discrepancy between ovulation and fecundity rates has not been explained, but may be due to other coexisting fertility factors, such as endometriosis or tubal damage. Some argue that it may be associated with possible antioestrogenic effects that CC exhibits on the endometrium and cervical mucus (Milson et al 2002).
A systematic review of 12 randomized controlled trials supported the effectiveness of CC therapy for anovulatory infertile women (Beck et al 2005). Clomiphene was found to be effective in increasing pregnancy rate compared with placebo [fixed odds ratio (OR) 5.8, 95% confidence interval (CI) 1.6–21.5; number needed to treat (NNT) 5.9, 95% CI 3.6–16.7].
Cumulative conception rates continue to rise after six cycles of treatment, reaching a plateau after 12 cycles. However, prolonged treatment with CC may be associated with an increased risk of ovarian malignancy. Therefore, after completion of 12 cycles of treatment, alternative therapies ought to be considered (National Institute for Health and Clinical Excellence 2004). Factors associated with increased risk of CC treatment failure include increased maternal age, raised BMI and history of severe oligomenorrhoea (Milson et al 2002).
Risks of treatment
With CC therapy, the risk of multiple gestation is approximately 2–13% (Venn and Lumley 1994). OHSS may occur in approximately 13% of cases, whereas severe OHSS is rare. Counselling of women undergoing ovulation induction with CC regarding the above risks is, therefore, essential.
The incidence of spontaneous miscarriage is 13–25% (Milson et al 2002). This figure is no different from the incidence of miscarriage in spontaneous conceptions. In addition to this, there is no evidence to indicate that CC treatment is associated with a higher incidence of congenital abnormalities.
Adjuvant treatment
A systematic review of 12 randomized controlled trials (Beck et al 2005) examined the concomitant administration of CC with other agents to infertile anovulatory women. The use of clomiphene in combination with tamoxifen did not provide any evidence of increased effect on pregnancy rate compared with clomiphene alone. The results were similar when CC plus ketoconazole was compared with CC alone, and CC plus bromocriptine was compared with CC alone.
Tamoxifen
Preliminary research has shown that tamoxifen has similar ovulation (44% with tamoxifen vs 45% with CC) and pregnancy rates (22% with tamoxifen vs 15% with CC) (Boostanfar 2001, Gerhard and Runnebaum 1979) compared with CC.
A meta-analysis, involving four trials, compared tamoxifen with CC in anovulatory women and showed that the two drugs were equally effective. Both drugs resulted in similar ovulation rates (OR 0.755, 95% CI 0.513–1.111). No benefit of tamoxifen over CC in achievement of pregnancy per cycle (OR 1.056, 95% CI 0.583–1.912) or per ovulatory cycle (OR 1.162, 95% CI 0.632–2.134) was demonstrated (Steiner et al 2005).
One randomized controlled trial showed that combination therapy of tamoxifen with CC did not improve pregnancy rates [8.6% with tamoxifen/CC vs 4.8% with CC alone; relative risk (RR) 1.80, 95% CI 0.20–16.21] (Suginami 1993).
Letrozole
Pharmacology and mechanism of action
Aromatase inhibitors were initially introduced for the treatment of breast cancer. Aromatase is a cytochrome P-450 haemoprotein that catalyses the rate-limiting step in the production of oestrogens using androgens as a substrate. Letrozole is a highly potent, selective and reversible aromatase inhibitor (Bayar et al 2006). Letrozole inhibits peripheral oestrogen production and, therefore, stimulates endogenous FSH secretion. Moreover, the accumulated androgens may increase follicular sensitivity to FSH (Mitwally and Casper 2004). Its high oral bioavailability and short half-life make it a suitable agent for ovulation induction. One potential advantage of letrozole over CC is its lack of direct antioestrogenic effects on the cervical mucus, the endometrium, uterine blood flow and embryo development (Barroso et al 2006).
Results
In a Cochrane review, Cantineau et al (2007) identified five trials comparing aromatase inhibitors with antioestrogens used as ovulation induction agents in ovarian stimulation protocols for intrauterine insemination (IUI) in subfertile women. No benefits of letrozole use were identified. Other studies involving women with anovulation or unexplained infertility have shown comparable pregnancy rates between letrozole and CC (Barroso et al 2006, Bayar et al 2006, Davar et al 2006, Jee et al 2006). One study reported higher pregnancy rates in PCOS patients treated with letrozole (Atay et al 2006). Results from larger randomized controlled trials are needed to determine the role of letrozole in ovulation induction.
Dopamine Agonists
Pharmacology and mechanism of action
Prolactin secretion from the anterior pituitary is mainly regulated by the inhibitory control of dopamine. Therefore, drugs that exhibit a dopaminomimetic activity reduce prolactin serum concentrations and restore gonadal function in hyperprolactinaemic women, with or without a pituitary adenoma. If a prolactinoma is present, dopamine agonists may also reduce tumour size (see Chapter 16).
Regimen, monitoring and results
Women with a microprolactinoma who are planning to conceive should be prescribed bromocriptine. If pregnancy occurs, bromocriptine may be discontinued as the risk of tumour growth is very small (2%). Nevertheless, in the case of macroprolactinomas, the risk of growth is significant (25%) and treatment with bromocriptine should be continued throughout pregnancy (Balen 2004). Monitoring of these women is done clinically, based on symptoms such as headaches and visual disturbances, and will include visual field assessments and management jointly with an endocrinologist.
Two large randomized controlled trials compared cabergoline with bromocriptine in women with hyperlactinaemic amenorrhoea. Cabergoline was found to be more effective than bromocriptine in achieving euprolactinaemia (83% and 93% with cabergoline vs 59% and 48% with bromocriptine). Moreover, cabergoline was more effective in restoring ovulation and increasing pregnancy rates (72% and 72% with cabergoline and 52% and 48% with bromocriptine) (Webster et al 1994, Pascal-Vigneron et al 1995). Nevertheless, consideration must be given to safety for use in pregnancy (National Institute for Health and Clinical Excellence 2004).
Insulin-Sensitizing Agents
PCOS is characterized by chronic anovulatory infertility and hyperandrogenism with clinical manifestations of oligo- or amenorrhoea, hirsutism and acne. Women with PCOS exhibit an increased prevalence of cardiovascular risk factors, including a higher incidence of obesity, insulin resistance and hyperinsulinaemia (Royal College of Obstetricians and Gynaecologists 2007). Increased insulin concentrations lead to hyperandrogenism and subsequently anovulation. In obese (BMI >30 kg/m2) PCOS women, weight reduction alone may decrease hyperinsulinaemia and hyperandrogenism, and restore ovulation. Therefore, prior to commencing drug treatment, women should be advised to lose weight, as this would improve their chance of spontaneous ovulation and improve their response to ovulation induction.
Metformin
Insulin-sensitizing agents have been tried as ovulation induction drugs in PCOS patients. The most commonly used is metformin, a biguanide oral hypoglycaemic drug used for the treatment of type 2 diabetes mellitus. Metformin has been proven to reduce serum insulin and androgen concentrations, and improve ovulation rates and hirsutism (Lord et al 2003). Metformin has been used as an ovulation induction agent in many trials; nevertheless, these trials are characterized by heterogeneity in terms of dosage, timing and duration of treatment (Al-Inany and Johnson 2006).
A Cochrane review, including women with clomiphene-resistant PCOS and a mean BMI greater than 25 kg/m2, concluded that metformin as a single agent did not increase pregnancy rates compared with placebo. Treatment with both metformin and CC did increase clinical pregnancy rates compared with CC alone (OR 4.88, 95% CI 2.46–9.67). Metformin as a single agent was, however, shown to induce ovulation compared with placebo (OR 3.88, 95% CI 2.25–6.69). Moreover, metformin in combination with CC was more effective at inducing ovulation compared with CC alone (OR 4.41, 95% CI 2.37–8.22) (Lord et al 2003).
Two large randomized controlled trials (Moll et al 2006, Legro et al 2007) concluded that metformin should not be used as a primary method for ovulation induction in PCOS patients. Moll et al (2006) compared the effect of CC plus metformin and CC plus placebo on ovulation induction in PCOS women. They concluded that there were no significant differences in ovulation, ongoing pregnancy or miscarriage rates. Nevertheless, a large proportion of women in the metformin group discontinued treatment because of side-effects. Legro et al (2007) compared CC plus placebo, metformin plus placebo and CC plus metformin. The livebirth rate was 22.5% in the CC group, 7.2% in the metformin group and 26.8% in the combination group. Conception rates were lower in the metformin group (21.7%) than in the CC group (39.5%) or the combination group (46%).
Therefore, infertile women with PCOS should not be offered metformin as a first-line agent, and CC remains the drug of choice. Nonetheless, PCOS patients who have not responded to CC and who have a BMI greater than 25 kg/m2 should be offered combined metformin and CC treatment (National Institute for Health and Clinical Excellence 2004).
Other drugs
Other insulin-sensitizing agents tried in the management of anovulatory infertility include the thiazolidinedione hypoglycaemic drugs. Experience with these drugs in PCOS women is limited. Troglitazone was shown to be effective in inducing ovulation. Nevertheless, it was withdrawn from the market after reports of hepatotoxicity. Rosiglitazone and pioglitazone are newer drugs used for the treatment of type 2 diabetes mellitus. Regular monitoring of liver enzymes is recommended, as these drugs may also have hepatotoxic effects. These agents are ‘class C’ drugs with evidence of teratogenicity in animals (Lord et al 2003), and far more data are required in ovulation induction patients to determine their potential role and safety.
Pulsatile Gonadotrophin-Releasing Hormone
Pulsatile GnRH has been used for ovulation induction since 1980. It is the treatment of choice for women with hypogonadotrophic hypogonadism with an intact pituitary function (Braat et al 1991).
Mechanism of action
In patients with hypogonadotrophic hypogonadism, the administration of exogenous pulsatile GnRH restores normal pulsatile pituitary gonadotrophin secretion. Subsequently, follicular recruitment and growth proceed as in a normal menstrual cycle, leading to the development of a single dominant follicle (Martin et al 1993).
Results
Pulsatile GnRH is a highly effective method of ovulation induction. Ovulation rates and pregnancy rates per treatment cycle of 70–93% and 18–29% have been reported (Braat et al 1991, Martin et al 1993, Filicori et al 1994).
Nevertheless, ovulation induction with pulsatile GnRH has been less successful in women with PCOS, raised BMI and high serum LH, testosterone or insulin levels (Filicori et al 1994).
Twin pregnancy rates ranged between 3.8% and 13.5% (Braat et al 1991, Martin et al 1993, Filicori et al 1994). This risk is significantly lower than that associated with gonadotrophin treatment (approximately 15%) (Martin et al 1993). Higher order pregnancies are rarely seen with pulsatile GnRH therapy.
The risk of OHSS is very low when exogenous GnRH is used. In comparison with gonadotrophin ovulation induction, GnRH treatment is associated with a lower risk of multiple folliculogenesis and ovarian enlargement (Martin et al 1993).
Spontaneous miscarriage rates have been approximately 10–30%. These appear to be higher in PCOS patients (40%) and lower in women with hypogonadotrophic hypogonadism (20%) (Filicori et al 1994). It is believed that in PCOS women, pretreatment with GnRH analogues may improve outcomes and lower the incidence of multiple pregnancies. Nonetheless, a systematic review by Bayram et al (2004) found no evidence to support the use of pulsatile GnRH in clomiphene-resistant PCOS women.
Gonadotrophins
Preparations
Recombinant human chorionic gonadotrophin
Thus far, research indicates that different gonadotrophin preparations are equally effective. When recombinant hCG was compared with urinary hCG, no differences in clinical outcomes (including ongoing pregnancy and livebirth rates, miscarriage and OHSS rates) were demonstrated (Al-Inany et al 2005). When recombinant FSH was compared with urinary hMG, no significant differences in pregnancy rates were observed (Nugent et al 2000).
Nevertheless, recombinant gonadotrophins have certain advantages in comparison with urinary products. They have high batch-to-batch consistency, unlimited supply, high purity and are associated with less risk of allergic reaction as they do not contain any non-gonadotrophin proteins. However, their increased cost is a major disadvantage. Thus, when deciding which preparation to use, factors such as patient safety, cost and drug availability must be taken into consideration (Table 17.1)
Table 17.1 Preparations of gonadotrophins for ovulation induction
| Formulation | Proprietary Name | Administered |
|---|---|---|
| Urinary | ||
| Human menopausal gonadotrophin FSH 75 U and LH 75 U/ampoule |
Merional Menopur |
IM SC |
| Urofollitrophin FSH 75 U and LH <1 U/ampoule |
Fostimon | IM/SC |
| Human chorionic gonadotrophin 5000 U/ampoule |
Choragon Pregnyl |
IM/SC |
| Recombinant | ||
| Follitrophin α FSH 75 U/ampoule |
Gonal-F | SC |
| Follitrophin β FSH 75 U or multiples in cartridges |
Puregon | SC |
| Lutrophin α LH 75 U/ampoule |
Luveris | SC |
| Follitrophin α with lutrophin α FSH 150 U and LH 75 U |
Pergoveris | SC |
| Choriogonadotrophin α 6500 U ≡ 250 µg |
Ovitrelle | SC |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; IM, intramuscular; SC, subcutaneous.
Indications
Ovulation induction with gonadotrophins is indicated for:
Hypogonadotrophic hypogonadism
Women with hypogonadotrophic hypogonadism have extremely low levels of endogenous gonadotrophins. Therefore, both exogenous FSH and LH are required. FSH is essential for follicular growth and oocyte maturation, whereas LH is required for steroidogenesis, luteinization and ovulation. The treatment of choice for this group of patients is hMG, as it contains both FSH and LH (Shoham et al 1991). Nonetheless, the use of recombinant FSH and LH may be an alternative. The regimen used for these women is the ‘step-up protocol’. The luteal phase is usually supported with the administration of hCG, as these patients have very low levels of LH.
Polycystic ovary syndrome
A systematic review of 14 randomized controlled trials found that urinary hMG and urinary FSH had similar effectiveness in terms of pregnancy rates. However, the incidence of OHSS was reduced with FSH compared with hMG. No significant differences were reported between the use of subcutaneous pulsatile and intramuscular injection of gonadotrophins, daily or alternate-day administration, and ‘step-up’ or ‘standard’ regimens (Nugent et al 2000).
A Cochrane review including four randomized controlled trials concluded that when recombinant FSH was compared with urinary FSH, there were no significant differences in terms of ovulation, pregnancy, miscarriage, multiple pregnancy and OHSS rates. No significant differences were demonstrated between administering recombinant FSH as a chronic low dose or as a standard regimen (Bayram et al 2001). The reader is referred to the review by Amer (2007) for further information.
Regimens and monitoring
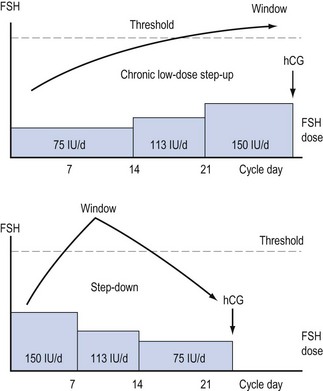
Varying regimens of administering exogenous gonadotrophins have been developed (see Figure 17.5).
Concomitant use of gonadotrophin-releasing hormone agonist
In a randomized clinical trial when GnRH agonists were used concomitantly with gonadotrophins, no significant differences were shown in ovulation or pregnancy rates compared with using gonadotrophins alone (Vegetti 1998). Moreover, Nugent et al (2000) found an increase in the incidence of OHSS when GnRH analogues were added to gonadotrophins. Therefore, the use of GnRH agonists is not recommended for PCOS women undergoing ovulation induction with gonadotrophins.
Results
In women with WHO Group I ovulation dysfunction, pregnancy rates of 25% per cycle and cumulative pregnancy rates of 90% after six cycles of treatment have been achieved (Amer 2007). Nevertheless, success rates were lower in PCOS women (5–25% and 30–60%, respectively).
Weight Reduction
The impact of obesity on assisted conception techniques has also been investigated. Regarding ovulation induction, it seems that obese women can be more resistant to CC than non-obese women. As far as IVF is concerned, the evidence from the literature is inconclusive. Although obese women appear to require higher doses of drugs for ovarian stimulation, have a lower chance of pregnancy following IVF and an increased miscarriage rate, it is not clear whether there is an effect of BMI on livebirth rates, cycle cancellation, oocyte recovery or ovarian hyperstimulation incidence (Maheshwari et al 2007).
In this group of patients, weight reduction improves biochemical indices and fertility rates. A 5–10% decrease in body weight will lead to a 30% reduction in body fat, which is sufficient to restore regular menstruation and ovulation. A prospective study was conducted by Clark et al (1995). This study looked at the effect of weight loss, with diet and exercise, on women with anovulation, clomiphene resistance and a BMI greater than 30. Weight reduction led to resumption of ovulation and subsequent pregnancy, as well as a reduction of testosterone levels and increased sex-hormone-binding globulin concentrations.
Surgical Management: Laparoscopic Ovarian Drilling
LOD was first described by Gjonnaess in 1984. Both laparoscopic ovarian cautery and laser vaporization (using carbon dioxide, argon or neodymium:yttrium aluminium garnet lasers) have been used since. The aim is to create approximately 10 holes per ovary in the ovarian surface and stroma. The mechanism of action is thought to be similar to that of ovarian wedge resection. With LOD, androgen-producing ovarian tissue is destroyed. This subsequently causes a decline in the serum levels of androgens, inhibin and LH and an increase in FSH levels. Therefore, disturbances in the ovarian–pituitary feedback mechanism are corrected, leading to follicular recruitment, maturation and ovulation. A long-term cohort study has also shown that up to 20 years after LOD, there was persistence of ovulation and normalization of serum androgens and sex-hormone-binding globulin levels in 60% of the participants (Gjonnaess 1998, Amer et al 2002).
With the employment of LOD, spontaneous ovulation rates of 30–90% and pregnancy rates of 13–88% have been described. In the literature, most data derive from randomized controlled trials comparing ovarian drilling with ovulation induction using exogenous gonadotrophins. There is no evidence of a difference in livebirth outcomes following either LOD (after 6–12 months of follow-up) or three to six cycles of ovulation induction with gonadotrophins in CC-resistant women. Moreover, there was no evidence of a difference in clinical pregnancy rate, miscarriage rate, ovulation rate and quality of life. Nonetheless, multiple pregnancy rates were reduced after LOD (Farquhar et al 2007). Moreover, there appears to be no difference between LOD of one ovary vs drilling of both ovaries in terms of ovulation induction.
In summary, as there is no evidence of a difference in efficacy between LOD and gonadotrophin ovulation induction, LOD may be the treatment of choice as it is associated with a lower risk of multiple pregnancy and OHSS. Nevertheless, LOD carries risks such as pelvic infection, postoperative adhesion formation, risks of general anaesthesia and the theoretical risk of premature ovarian failure. All these should be taken into consideration before embarking on this procedure. Careful counselling and selection of patients is therefore mandatory. The operation should only be performed by fully trained laparoscopic surgeons. At the moment, the use of LOD is restricted to anovulatory women with a normal BMI. Its use is not recommended as an attempt to decrease the risk of developing diabetes mellitus or coronary artery disease. Long-term risks for women with PCOS (Royal College of Obstetricians and Gynaecologists 2007).
Risks of Induction of Ovulation
Ovarian hyperstimulation syndrome
OHSS is a rare, serious and potentially life-threatening complication of ovulation induction. It is a systemic disease resulting from vasoactive products released from hyperstimulated ovaries. It occurs during the luteal phase of the cycle or during early pregnancy. The incidence of mild OHSS in ovulation induction with CC is 13.5%, whereas moderate and severe forms have only been described sporadically. In IVF/intracytoplasmic sperm injection (ICSI) cycles, the incidence of mild, moderate and severe OHSS may be 33%, 3–6% and 0.1–2%, respectively (Delvigne and Rozenberg 2002). Only a few cases of OHSS have occurred in spontaneous cycles. The true incidence of mortality from OHSS is unknown; nevertheless, deaths are rare.
Diagnosis/classification
Different classification systems of OHSS have been proposed. The latest system was suggested by Marthur in 2005 and is shown in Table 17.2 as adopted by the Royal College of Obstetricians and Gynaecologists. Four categories of OHSS are noted: mild, moderate, severe and critical. As the management of the condition will be dictated by its severity, it is mandatory to assess each case properly and classify it according to Table 17.2.
Table 17.2 Classification of severity of ovarian hyperstimulation syndrome (OHSS)
| Grade | Symptoms |
|---|---|
| Mild | |
| Moderate | |
| Severe | |
| Critical |
* Ovarian size may not correlate with severity of OHSS in cases of assisted reproduction because of the effect of follicular aspiration.
Risk factors
Prevention
Although several strategies have been developed, OHSS cannot be totally prevented. This mainly applies to late OHSS triggered by the rising serum hCG secreted in early pregnancy. Nevertheless, prevention and early recognition of OHSS are fundamental in ensuring patients’ safety. Identification of any risk factors may reduce the risk of developing OHSS, as adaptations can be made to the ovulation regimen. Careful monitoring of the stimulation with the use of ultrasonography should be implemented, as it has a good predictive value in the occurrence of OHSS (Blankstein et al 1987). For instance, starting with a lower dose of gonadotrophin, reducing the dose when OHSS is suspected or even cancelling the cycle and withholding hCG injection may prevent OHSS. In the latter case, the couple should be advised to refrain from intercourse or to use barrier methods of contraception.
Outpatient management
Women with mild OHSS and many with moderate OHSS may be managed as outpatients. Paracetamol and codeine are the analgesics of choice. Non-steroidal anti-inflammatory drugs are contraindicated, as they may compromise renal function. Women should be advised to drink to thirst and not to excess. A review is required every 2–3 days. Nevertheless, urgent reassessment is warranted if the woman develops severe abdominal pain, increasing abdominal distention, dyspnoea or has a subjective impression of decreasing urine output (Royal College of Obstetricians and Gynaecologists 2006).
Inpatient management
Women with severe OHSS and those with moderate OHSS whose symptoms cannot be controlled with oral medication should be admitted to hospital. Critical cases of OHSS may require admission to an intensive care unit for invasive monitoring (see Figure 17.6).
Multiple pregnancy
In many developed countries, there has been a 30–50% increase in twin births since 1980. Triplet deliveries have increased three- to five-fold during this period. The incidence of multiple births in the UK has risen from 10–15/100,000 maternities before 1981 to 12.1/1000 maternities in 1991 and 14.9/1000 maternities in 2004 (Office for National Statistics 2006). The number of multiple pregnancies conceived is even higher, as spontaneous and iatrogenic fetoreductions are not included in birth statistics.
Approximately 20% of the increase in multiple births can be attributed to advanced maternal age, as it is known that older women are more likely to have a multiple pregnancy. The remainder is associated with ovulation induction and assisted reproductive technologies, such as IVF and ICSI. The exact numbers of higher order pregnancies resulting from ovarian stimulation, with or without IUI, are unknown as there are no national registers that record the outcome of controlled ovarian simulation (ESHRE Task Force on Ethics and Law 2003).
Nonetheless, ovarian simulation, with or without IUI, contributes significantly to the occurrence of multiple births. A study by Lynch et al (2001) showed that 20% of all multiple pregnancies were attributable to ovulation induction agents, which is considerably higher than the 14% attributable to IVF. Similarly, 34% of higher order pregnancies in the UK in 1989 resulted from ovulation induction (Levene et al 1992). Multiple pregnancies occurred in 2–13% of women with all causes of infertility taking CC, whereas women with clomiphene-resistant PCOS treated with gonadotrophins have a multiple pregnancy rate of 36%.
Three different techniques of MFPR have been described. The most commonly used is the transabdominal needle insertion of potassium chloride to the fetal thorax above the diaphragm. This procedure is usually performed at 10–12 weeks of gestation. The selection of the embryo is based on which one is easiest to reach. The second method is the transcervical mini-suction, done between 8 and 11 weeks. Loss of the entire pregnancy has been reported in 50% of cases managed with this technique. The third method consists of transvaginal aspiration of the embryo, usually done at 10–12 weeks (Evans et al 1998).
It is widely accepted that for any higher order multiple pregnancy, reduction to twins has the highest survival rate. Reduction to a singleton pregnancy is not accepted because of the risk of losing that pregnancy if there is a problem later. Women with significant medical disease, such as cardiac disease, or uterine malformations are exceptions to this rule, and reduction to a singleton pregnancy may be considered in such cases (Evans et al 1998).
Prevention of multiple pregnancies should be preferred to MFPR, since it is associated with the ethical dilemmas of abortion. Moreover, it is acknowledged that the original higher order pregnancy may have detrimental effects on the development of the remaining fetuses, in terms of risk of preterm delivery, even after the event of fetocide (ESHRE Task Force on Ethics and Law 2003).
Ovarian cancer
Ovarian cancer is the fourth most common cancer among women in England and Wales, and is the most common cause of gynaecological cancer death. The possibility of a link between ovulation and ovarian oncogenesis led to concerns that fertility treatments may increase the risk of developing ovarian cancer. In particular, the possible association between drugs used for ovulation induction and the risk of ovarian cancer has been the subject of much debate. Concerns have been raised about the effects of multiple ovulations and trauma to the ovarian epithelium (Fathalla 1971), as well as the high levels of gonadotrophins reached during fertility treatment. It has been speculated that the latter may lead to the production of intraovarian carcinogens and malignant transformation (Daly 1992).
A collaborative analysis on data from 12 case–control studies of ovarian cancer conducted in the USA (Whittemore et al 1992) showed that the risk was increased among women who had used fertility drugs (OR 2.8, 95% CI 1.3–6.1) compared with women without a clinical history of infertility. On the other hand, infertile women who had not used fertility drugs experienced no increase in risk (OR 0.91, 95% CI 0.66–1.3). The above risk was higher among nulligravid women than among gravid women. Nevertheless, the information available on specific fertility medication was too incomplete to draw any conclusions.
A case–control study found that women taking clomiphene had a higher risk of developing the disease compared with women who were not taking clomiphene (RR 2.3, 95% CI 0.5–11.4). Prolonged use of clomiphene (12 months or more) was associated with a higher risk of ovarian cancer (RR 11.1, 95% CI 1.5–82.3). Nonetheless, treatment with the drug for less than 1 year was not associated with increased risk (Rossing et al 1994).
On the other hand, several reviews have reported insufficient evidence to support a direct causal relationship between fertility drug treatment and ovarian cancer (Nugent et al 1998, Klip et al 2000).
A UK epidemiological report of cancer incidence among women who had ovarian stimulation treatment found no evidence of a link between infertility treatment and ovarian cancer. This cohort study included 5556 women of whom 75% received ovulation induction drug treatment. The incidence rates of ovarian carcinoma were not significantly different from expectation based on national cancer rates (Doyle et al 2002).
Women who undergo fertility treatment ought to be counselled regarding the possible association between medical ovulation induction and ovarian cancer, and their informed consent should be sought (National Institute for Health and Clinical Excellence 2004). A survey of women attending a fertility clinic reported that the majority (67%) of women were aware of the potential link between fertility treatment and ovarian cancer. Moreover, 79% of participants were willing to accept this potential increased risk (Rosen et al 1997).
Other cancers
There is no current evidence to support a link between ovulation induction therapy and other cancers, such as breast, cervical, endometrial, colorectal and thyroid cancer and melanoma (Klip et al 2000).
Prion disease
It is common knowledge that products derived from or containing materials of human or bovine origin carry the theoretical risk of transmitting prion disease. This risk could apply to gonadotrophins of pituitary or urinary extraction origin. Nevertheless, to date, there has been no evidence of transmission of prion disease by any gonadotrophin (National Institute for Health and Clinical Excellence 2004). As a precaution, the Committee on Safety of Medicines recommended that no human urine used in the production of medicines should be sourced from a country with one or more indigenous cases of variant Creutzfeldt–Jakob disease.
Conclusions
KEY POINTS
Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. British Medical Journal. 1986;293:355-359.
Al-Inany HG, Aboulghar M, Mansour R, Proctor M 2005 Recombinant versus urinary human chorionic gonadotrophin for ovulation induction in assisted conception. Cochrane Database of Systematic Reviews 2: CD003719.
Al-Inany H, Johnson N. Drugs for anovulatory infertility in polycystic ovary syndrome. BMJ. 2006;332:1461-1462. (Clinical Research Ed.)
Amer SA. Gonadotrophin induction of ovulation. Obstetrics, Gynaecology and Reproductive Medicine. 2007;17:205-210.
Amer SA, Banu Z, Li TC, Cooke ID. Long-term follow-up of patients with polycystic ovary syndrome after laparoscopic ovarian drilling: endocrine and ultrasonographic outcomes. Human Reproduction. 2002;17:2851-2857.
Atay V, Cam C, Muhcu M, Cam M, Karateke A. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. Journal of International Medical Research. 2006;34:73-76.
Balen A. Ovulation induction. Current Obstetrics and Gynaecology. 2004;14:261-268.
Barroso G, Menocal G, Felix H, Rojas-Ruiz JC, Aswan M, Oehninger S. Comparison of the efficacy of the aromatase inhibitor letrozole and clomiphene citrate as adjuvants to recombinant follicle-stimulating hormone in controlled ovarian hyperstimulation: a prospective, randomized, blinded clinical trial. Fertility and Sterility. 2006;86:1428-1431.
Bayar U, Tanriverdi HA, Barut A, Ayoplu F, Ozcan O, Kaya E. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertility and Sterility. 2006;85:1045-1048.
Bayram N, van Wely M, van Der Veen F 2001 Recombinant FSH versus urinary gonadotrophins or recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2: CD002121.
Bayram N, van Wely M, Vanderkerckhove P, Lilford R, van der Veen F 2004 Pulsatile luteinizing hormone releasing hormone for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 1: CD000412.
Beck JI, Boothroyd C, Proctor M, Farquhar C, Hughes E 2005 Oral anti-oestrogens and medical adjuncts for subfertility associated with anovulation. Cochrane Database of Systematic Reviews 1: CD002249.
Blankstein J, Shalev J, Saadon T, et al. Ovarian hyperstimulation syndrome: prediction by number and size of preovulatory ovarian follicles. Fertility and Sterility. 1987;47:597-602.
Boostanfar R. A prospective randomized trial comparing clomiphene citrate with tamoxifen citrate for ovulation induction. Fertility and Sterility. 2001;75:1024-1026.
Braat DD, Schoemaker R, Schoemaker J. Life table analysis of fecundity in intravenously gonadotropin-releasing hormone-treated patients with normogonadotropic and hypogonadotropic amenorrhoea. Fertility and Sterility. 1991;55:266-271.
Cantineau AEP, Cohlen BJ, Heineman MJ 2007 Ovarian stimulation protocols (anti-oestrogens, gonadotrophins with and without GnRH agonists/antagonists) for intrauterine insemination (IUI) in women with subfertility. Cochrane Database of Systematic Reviews 2: CD005356.
Clark AM, Ledger W, Galletly C, et al. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Human Reproduction. 1995;10:2705-2712.
Daly MB. The epidemiology of ovarian cancer. Hematology/Oncology Clinics of North America. 1992;6:729-738.
Davar R, Asgharnia M, Tayebi N, Aflatoonian A. Comparison of the use of letrozole and clomiphene citrate in regularly ovulating women undergoing intrauterine insemination. Middle East Fertility Society Journal. 2006;11:113-118.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Human Reproduction Update. 2002;8:559-577.
Doyle P, Maconochie N, Beral V, Swerdlow AS, Tan SL. Cancer incidence following treatment for infertility at a clinic in the UK. Human Reproduction. 2002;17:2209-2213.
ESHRE Task Force on Ethics and Law. Ethical issues related to multiple pregnancies in medically assisted procreation. Human Reproduction. 2003;18:1976-1979.
Evans MI, Kramer RL, Yaron Y, Drugan A, Johnson MP. What are the ethical and technical problems associated with multifetal pregnancy reduction? Clinical Obstetrics and Gynecology. 1998;41:46-54.
Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P 2007 Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 3: CD001122.
Fathalla MF. Incessant ovulation — a factor in ovarian neoplasia. The Lancet. 1971;ii:163.
Filicori M, Flamigni C, Cognigni P, et al. Treatment of anovulation with pulsatile gonadotrophin-releasing hormone: prognostic factors and clinical results in 600 cycles. Journal of Clinical Endocrinology and Metabolism. 1994;79:1215-1220.
George K, Nair R, Tharyan P 2008 Ovulation triggers in anovulatory women undergoing ovulation induction. Cochrane Database of Systematic Reviews 3: CD006900.
Gerhard I, Runnebaum B. Comparison between tamoxifen and clomiphene therapy in women with anovulation. Archives of Gynecology. 1979;227:279-288.
Gjonnaess H. Late endocrine effects of ovarian electrocautery in women with polycystic ovary syndrome. Fertility and Sterility. 1998;69:697-701.
Gysler M, March CM, Mishell DRJr, Bailey EJ. A decade’s experience with an individualized clomiphene treatment regimen including its effect on the postcoital test. Fertility and Sterility. 1982;37:161-167.
Hughes E, Collins J, Vandekerckhove P 2000 Clomiphene citrate for unexplained subfertility in women. Cochrane Database of Systematic Reviews 2: CD000057. Update in Cochrane Database of Systematic Reviews 2000; 3: CD000057.
Jee BC, Ku SY, Suh CS, Kim KC, Lee WD, Kim SH. Use of letrozole versus clomiphene citrate combined with gonadotropins in intrauterine insemination cycles: a pilot study. Fertility and Sterility. 2006;85:1774-1777.
Klip H, Burger CW, Kenemans P, van Leeuwen FE. Cancer risk associated with subfertility and ovulation induction: a review. Cancer Causes and Control. 2000;11:319-344.
Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. New England Journal of Medicine. 2007;356:551-566.
Levene MI, Wild J, Steer P. Higher multiple births and the modern management of infertility in Britain. The British Association of Perinatal Medicine. BJOG: an International Journal of Obstetrics and Gynaecology. 1992;99:607-613.
Lord JM, Flight IH, Norman RJ 2003 Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database of Systematic Reviews 3: CD003053.
Lynch A, McDuffie R, Murphy J, et al. Assisted reproductive interventions and multiple birth. Obstetrics and Gynecology. 2001;97:195-200.
Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight on assisted reproductive technology — a systematic review. Human Reproduction Update. 2007;13:433-444.
Martin KA, Hall JE, Adams JM, Crowley JNWF. Comparison of exogenous gonadotropins and pulsatile gonadotropin-releasing hormone for induction of ovulation in hypogonadotropic amenorrhoea. Journal of Clinical Endocrinology and Metabolism. 1993;77:125-129.
Milson SR, Gibson G, Buckingham K, Gunn AJ. Factors associated with pregnancy or miscarriage after clomiphene therapy in WHO Group II anovulatory women. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2002;42:170-175.
Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotrophin required for controlled ovarian hyperstimulation. Journal of the Society for Gynecologic Investigation. 2004;11:406-415.
Moll E, Bossuyt PMM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomiphene citrate plus metformin and clomiphene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332:1485-1488. (Clinical Research Ed.)
National Institute for Health and Clinical Excellence. Clinical Guideline 11. Fertility: Assessment and Treatment for People with Fertility Problems. London: NICE; 2004.
Nugent D, Salha O, Balen AH, Rutherford AJ. Ovarian neoplasia and subfertility treatments. BJOG: an International Journal of Obstetrics and Gynaecology. 1998;105:584-591.
Nugent D, Vandekerckhove P, Hughes E, Arnot M, Lilford R 2000 Gonadotrophin therapy for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 4: CD000410.
Office for National Statistics. Population Censuses and Surveys. Birth Statistics. Births: 1938–2004, Maternities with Multiple Births. HMSO; 2006.
Pascal-Vigneron V, Weryha G, Bosc M, Leclere J. [Hyperprolactinemic amenorrhea: treatment with cabergoline versus bromocriptine. Results of a national multicenter randomized double-blind study]. Presse Medicale. 1995;24:753-757.
Royal College of Obstetricians and Gynaecologists. The Management of Ovarian Hyperstimulation Syndrome. Green-top Guideline No. 5. RCOG, London, 2006.
Royal College of Obstetricians and Gynaecologists. Long-term Consequences of Polycystic Ovary Syndrome. Green-top Guideline No. 33. RCOG, London, 2007.
Rosen B, Irvine J, Ritvo P, et al. The feasibility of assessing women’s perceptions of the risks and benefits of fertility drug therapy in relation to ovarian cancer risk. Fertility and Sterility. 1997;68:90-94.
Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. New England Journal of Medicine. 1994;331:771-776.
Shoham Z, Balen A, Patel A, Jacobs HS. Results of ovulation induction using human menopausal gonadotropin or purified follicle stimulating hormone in hypogonadotropic hypogonadism patients. Fertility and Sterility. 1991;56:1048-1053.
Steiner AZ, Terplan M, Paulson RJ. Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis. Human Reproduction. 2005;20:1511-1515.
Suginami H. A clomiphene citrate and tamoxifen citrate combination therapy: a novel therapy for ovulation induction. Fertility and Sterility. 1993;59:976-979.
Vandermolen DT, Ratts VS, Evans WS, Stovall DW, Kauma SW, Nestler JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertility and Sterility. 2001;75:310-315.
Vegetti W. Ovarian stimulation with low-dose pure follicle-stimulating hormone in polycystic ovarian syndrome anovulatory patients: effect of long-term pretreatment with gonadotrophin-releasing hormone analogue. Gynecologic and Obstetric Investigation. 1998;45:186-189.
Venn A, Lumley J. Clomiphene citrate and pregnancy outcome. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1994;34:56-66.
Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. New England Journal of Medicine. 1994;331:904-909.
Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case–control studies. II. Invasive epithelial ovarian cancers in white women. Collaborative Ovarian Cancer Group. American Journal of Epidemiology. 1992;136:1184-1203.