Ovarian Sex Cord–Stromal and Steroid Cell Tumors
General Features
Sex cord–stromal tumors account for approximately 8% of all ovarian tumors and are the most common functioning tumors associated with endocrine manifestations.1 These tumors contain granulosa cells, theca cells (and their luteinized derivatives), Sertoli cells, Leydig cells, and fibroblasts of gonadal stromal origin, either separately or in combination and exhibiting varying degrees of differentiation.1
Sex cord–stromal tumors arise from ovarian cells specialized in steroid hormone production. The designation sex cord–stromal tumors (which is favored by the World Health Organization (WHO) over gonadal stromal tumors) simply reflects the uncertainty about the origin of the gonadal sex cords;2 it is still unclear whether they derive from the celomic epithelium (sex cords) or the gonadal mesenchyme (stroma). Sex cords are identified in the developing testis by the fifth week of embryonic life but are not found in the developing ovary where nests of pregranulosa cells (of apparent stromal origin) enveloping the germ cells are evident later in embryonic life.1 Some authors do not accept the term ‘sex cords’ to describe progenitors of granulosa cells. However, the hyphenated term (sex cord–stroma) acknowledges that this category of ovarian tumors may be composed of sex cord derivatives (granulosa and Sertoli cells) that appear as epithelial elements, stromal derivatives (theca, lutein, and Leydig cell) that have the appearance of mesenchymal (gonadal stromal) cells, or derivatives of both precursors.1,2
Tumors composed of ovarian cell types are usually estrogenic, whereas those composed of cells of testicular type are androgenic, but tumors in either group may be nonfunctioning; rarely, tumors made up of female elements are androgenic, and tumors composed of Sertoli and Leydig cells are associated with estrogenic manifestations. Therefore, there is not always a strict correlation between the morphology of the tumor cells and the type of hormone they secrete.2
Because of the great diversity of histologic patterns, evaluation of sex cord–stromal tumors requires awareness of the patient’s age and clinical history, thorough sampling of the tumor, and immunohistochemical validation. The most helpful markers currently available are α-inhibin, calretinin, and epithelial membrane antigen (EMA). The first two are typically positive in sex cord–stromal tumors and the third is typically negative.3–5 Recently, a likely ‘driver’ point mutation of the FOXL2 gene has been identified in the vast majority of adult granulosa cell tumors (97%) but only rarely in juvenile granulosa cell tumors (10%).6 FOXL2, together with α-inhibin and calretinin, forms an immunomarker panel that will result in positive immunoreaction in essentially all cases of sex cord–stromal tumors.7
The distribution of the various sex cord–stromal tumors at the Massachusetts General Hospital over a 25 year period was as follows: tumors in the thecoma–fibroma group (87%), GCTs (12%), Sertoli–Leydig cell tumors (SLCTs) (0.05%), and unclassified (0.05%).1 Most clinically malignant sex cord–stromal tumors are GCTs.
Granulosa Cell Tumors
Granulosa cell tumors (GCTs) comprise two different clinicopathologic subtypes: adult and juvenile.1,2 The adult form occurs more often in middle-aged and postmenopausal women and characteristically contains microfollicles and uniform cells with scanty cytoplasm and pale, grooved nuclei; in contrast, the juvenile subtype occurs mainly in children and younger women, and typically shows large rudimentary follicles and cells with moderate to abundant cytoplasm and darker nuclei usually without grooves.1,2
Adult Granulosa Cell Tumor
Clinical Features
Adult granulosa cell tumors (AGCTs) account for 1–2% of all ovarian tumors and 95% of all GCTs. They occur mainly in menopausal and postmenopausal women of about 50–55 years of age and are the most common ovarian tumors associated with estrogenic symptoms (75% of cases).1, 8–12
Functioning AGCTs (which are typically estrogenic tumors) may be associated with various clinical syndromes depending upon the age of the patient: women in the reproductive age group usually present with irregular and excessive uterine bleeding (metropathia hemorrhagica), but amenorrhea, lasting from months to years, may precede the abnormal bleeding, or may be the only endocrine symptom.1 In postmenopausal women, the most common symptom is postmenopausal bleeding secondary to breakdown of endometrial hyperplasia or carcinoma. Occasionally, swelling, tenderness, and discharge from the breasts are prominent. Vaginal cytology typically shows increased maturation of squamous epithelial cells. Most of the clinical symptoms are related to the continuous estrogen stimulation of the endometrium: luteinizing hormone (LH) secretion from the anterior pituitary stops; ovulation does not occur, and progesterone is not secreted; the estrogenic stimulation of the endometrium results in endometrial hyperplasia (usually simple but occasionally complex atypical hyperplasia). Endometrial carcinoma occurs in approximately 5% of cases1 and is almost always a grade 1 endometrioid adenocarcinoma, which never metastasizes and almost never causes the death of the patient. Rare cases of AGCT appear before puberty and are associated with isosexual pseudoprecocity, due to estrogen production by the ovarian tumor. Although AGCTs are mostly estrogenic, very rare cases, usually large thin-walled cystic tumors, are associated with androgenic manifestations.13
In addition to endocrine symptoms, patients with AGCTs have signs and symptoms related to a pelvic mass such as abdominal pain and swelling; the tumor is palpable on pelvic examination in almost 90% of cases. Approximately 10% of patients present with acute abdominal symptoms due to rupture of the tumor with hemoperitoneum.1 AGCTs are confined to the ovary (International Federation of Gynecology and Obstetrics stage I) in 60–90% of cases.9–11,14 Extraovarian spread occurs to the peritoneum and omentum and occasionally to the liver or lungs.15 Lymph node metastases are uncommon.
Macroscopic Features
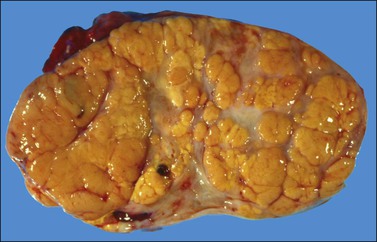
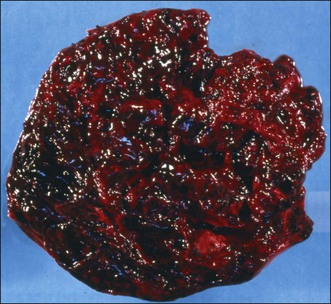
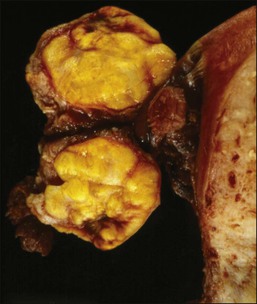
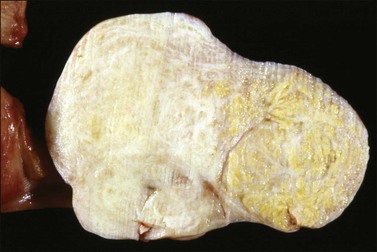
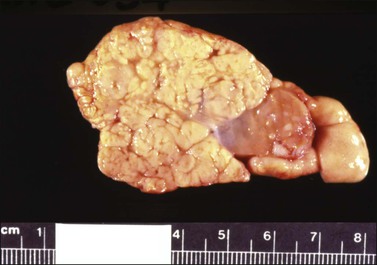
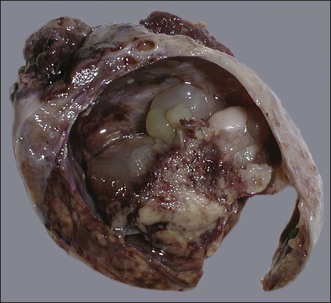
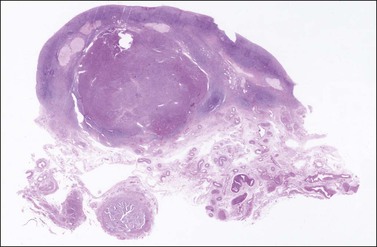
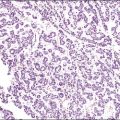
The tumors are unilateral in over 95% of cases. About 10–15% are ruptured. The average diameter is 12 cm but their size ranges from microscopic to very large masses. They are cystic and solid and the cysts characteristically contain blood clots. The solid component varies from yellow (Figure 28.1) to white depending upon the amount of lipid-containing cells, and from soft to firm depending on the quantity of granulosa cells and stromal cells.
Microscopic Features
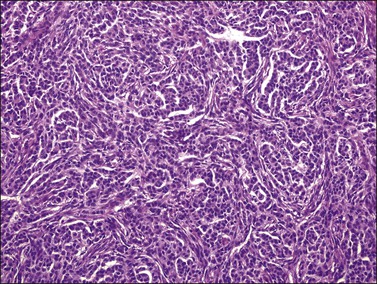
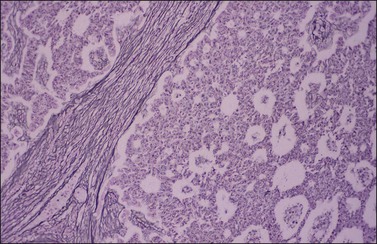
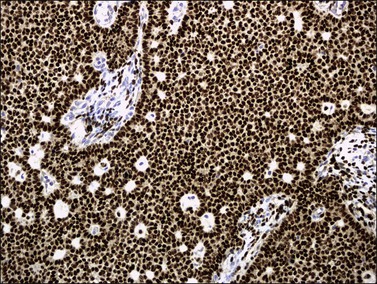
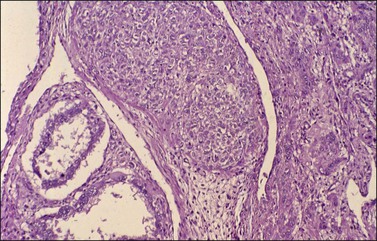
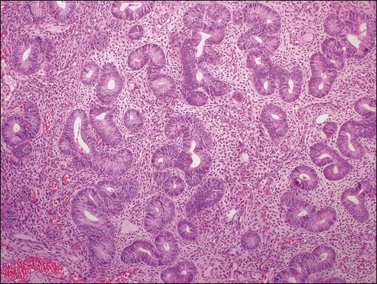
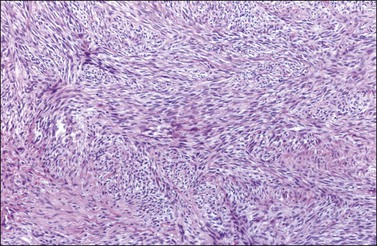
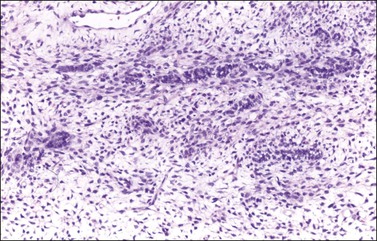
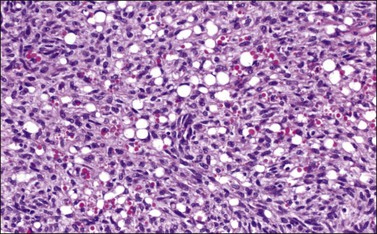
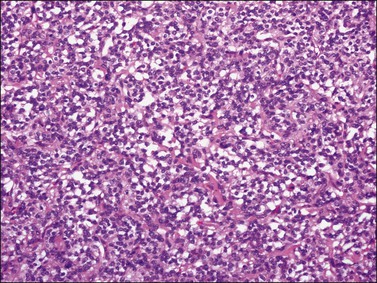
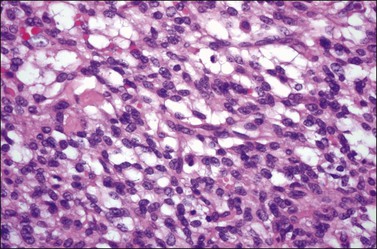
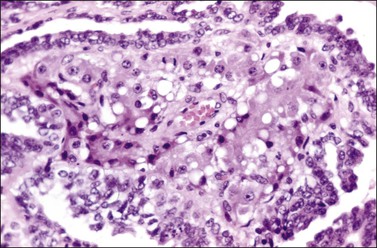
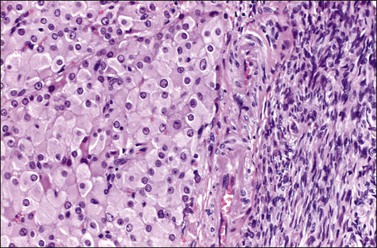
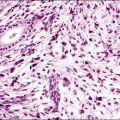
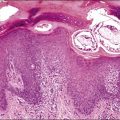
Histologically, there is a proliferation of granulosa cells often with a stromal component of theca cells, fibroblasts, or both. The granulosa cells grow in a wide variety of patterns, which are commonly admixed and lack prognostic significance. The most common pattern is the diffuse pattern in which the tumor cells grow in sheets with no organized structure (Figure 28.2). More characteristic but less common (30–50%) is the microfollicular pattern, which shows numerous rosette-like structures that simulate the Call–Exner bodies of the Graafian follicle (Figures 28.3 and 28.4). These small spaces contain eosinophilic debris or hyalinized basement membrane material and are surrounded by a circular row of typical granulosa cells. The cells contain scanty cytoplasm and pale, angular, or oval, often grooved, nuclei arranged haphazardly in relation to one another and to the follicles (Figure 28.4). The macrofollicular pattern is uncommon and shows cysts lined by well-differentiated granulosa cells and underlying theca cells (Figures 28.5). In totally cystic tumors, the cells lining the cysts may so closely mimic those of non-neoplastic ovarian follicles that distinction on a purely histologic basis is difficult. Tumor cells grow in anastomosing bands in the trabecular pattern, in undulating ribbons and cords in the gyriform, or in a watered-silk pattern (Figure 28.6), and in circumscribed nests and islands in the insular pattern (Figure 28.7). Some tumors may exhibit a diffuse sarcomatoid pattern characterized by somewhat spindle-shaped cells. Whatever the histologic pattern may be, the best morphologic markers of the AGCT are (1) the presence of Call–Exner bodies (microfollicular) (Figure 28.4) and (2) the cell nuclei, which appear uniform and haphazardly oriented to one another and are oval, round, and angular and pale, often grooved, and lack significant pleomorphism (Figure 28.8).
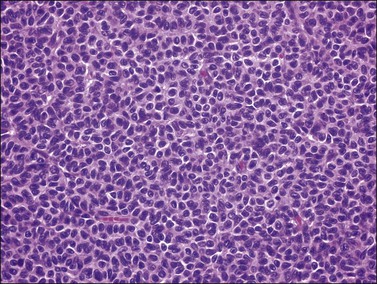
Figure 28.2 Adult granulosa cell tumor. Diffuse pattern. The regular-sized nuclei appear in a disorderly arrangement.
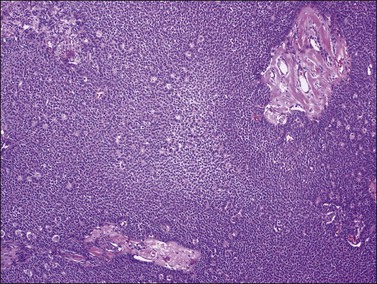
Figure 28.3 Adult granulosa cell tumor. Microfollicular pattern. Numerous Call–Exner bodies are seen.
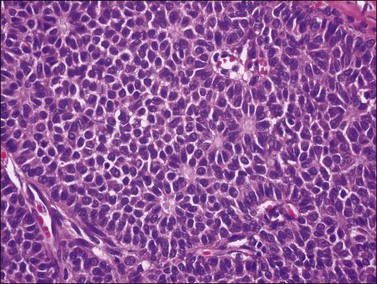
Figure 28.4 Adult granulosa cell tumor. Microfollicular pattern. Numerous Call-Exner bodies containing degenerated material are surrounded by granulosa cells with grooved nuclei.
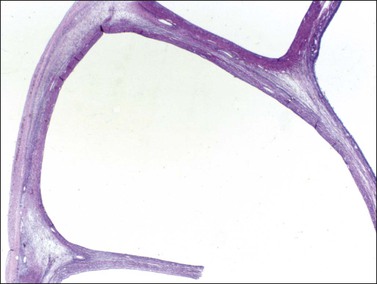
Figure 28.5 Adult granulosa cell tumor. Macrofollicular pattern. The large cystic cavities resemble follicle cysts.
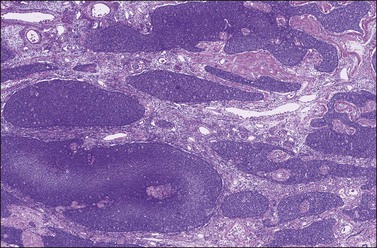
Figure 28.7 Adult granulosa cell tumor. Insular and microfollicular patterns. The tumor cells grow in well-defined islands containing Call–Exner bodies.
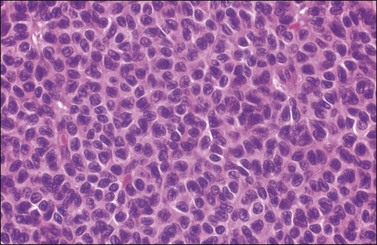
Figure 28.8 Adult granulosa cell tumor. The tumor cells have pale angular nuclei, many of which are grooved.
Approximately 2% of AGCTs contain scattered cells with bizarre, large, hyperchromatic nuclei (Figure 28.9).1 The mitotic activity in AGCTs varies, but is usually low; most tumors have 1–2 mitoses per 10 HPF. The presence of numerous mitoses, particularly abnormal forms, should raise the suspicion of poorly differentiated carcinoma.
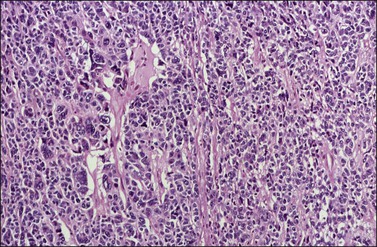
Figure 28.9 Adult granulosa cell tumor with bizarre nuclei. Large, hyperchromatic nuclei, including multinucleated forms, are present.
The stromal component of AGCTs varies from fibromatous to thecomatous (granulosa–theca cell tumor) and it is best enhanced by the reticulin stains (Figure 28.10). In contrast to theca cells and fibroblasts, which are individually invested by fibrils, the granulosa cell component of the tumor contains few fibrils.1 Occasional GCTs with hepatocellular differentiation have been reported.16,17 In contrast to lutein or Leydig cells, the hepatic-type cells are negative for inhibin.17
Immunohistochemistry
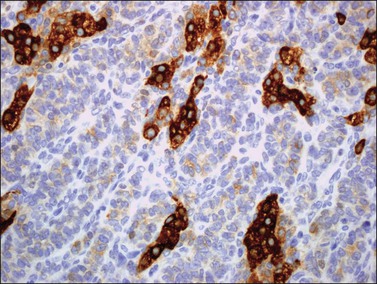
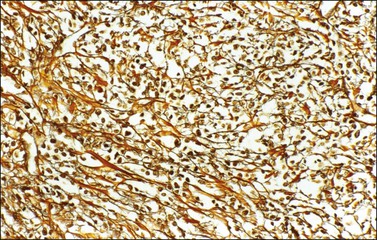
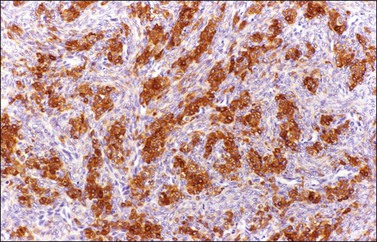
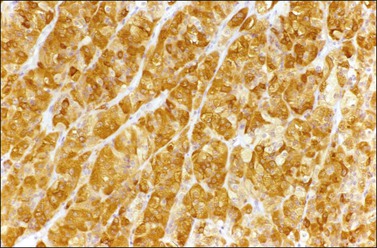
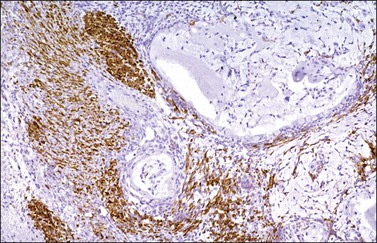
α-Inhibin (Figure 28.11), calretinin, and FOXL2 (Figure 28.12) are the most important positive immunoreactions.7 Calretinin is a more sensitive but less specific marker than α-inhibin.18,19 Steroidogenic factor-1 (SF-1), WT1, and CD56 are also positive.19,20 Almost all GCTs are vimentin positive. Monoclonal antibodies against low molecular weight cytokeratins (CK) 8 and 18 (CAM5.2, AE1/3) stain 30–60% of the tumors.3,21 Most GCTs are immunoreactive for smooth muscle actin but negative for desmin.21 Positive nuclear or cytoplasmic stain for S-100 protein has been reported in 50% of the tumors.3,21 There is membrane staining for CD99 (MIC2 gene product) in 70% of GCTs.22 Positive reaction for müllerian-inhibiting substance (MIS) has also been reported.23 The most significant negative immunoreactions are for EMA and CK7, which are almost always absent in GCTs.3,21
Genetic Profile
Over 80% of the GCTs are diploid. Cytogenetic abnormalities have been detected by comparative genomic hybridization and fluorescence in situ hybridization (FISH) and include trisomy 12 and 14 and monosomy 22.24,25 Over 90% of GCTs contain a missense point mutation 402 C→G (C134W) in the FOXL2 gene at 3q22.3, presumably an early event in tumorigenesis.6
Differential Diagnosis
1. Undifferentiated carcinoma versus diffuse AGCT. The typical nuclear features of AGCT (Figure 28.8) differ from those of undifferentiated carcinoma, namely nuclear hyperchromatism, pleomorphism, and atypical mitotic activity; carcinomas are often associated with higher stage and adverse clinical course. In difficult cases, positive immunostains for α-inhibin, calretinin, and vimentin and lack of staining for EMA favor the diagnosis of AGCT. FOXL2 immunoreaction is helpful (see Chapter 25, and Table 28.1).3,7,26,27
Table 28.1
Differentiation between AGCT and Undifferentiated Carcinoma
| AGCT | Undifferentiated Carcinoma | |
| Bilaterality | 5% | 15% |
| Stage | I | III |
| Nuclei | Paled, grooved, uniform | Hyperchromatic, pleomorphic |
| Prognosis | Favorable | Poor |
2. Small cell carcinoma of hypercalcemic type versus diffuse AGCT. The small cell carcinoma is often found in advanced stage and has an aggressive clinical course; it is associated with hypercalcemia in two-thirds of patients, lacks estrogenic symptoms, and contains hyperchromatic nuclei and numerous mitoses. Immunohistochemical staining may facilitate the diagnosis (see Chapter 27).
3. Endometrioid stromal sarcoma (primary or metastatic from the uterus) versus diffuse AGCT. The stromal sarcoma lacks endocrine symptoms and is frequently bilateral; microscopically, it shows numerous small arterioles, individual investment of tumor cells by reticulin fibers, and tongue-like pattern of infiltration mainly outside the ovary. Positive immunoreactions for α-inhibin, calretinin, and FOXL2 and negative CD10 staining support the diagnosis of AGCT (see Chapter 27).
4. Thecoma and cellular fibroma versus diffuse AGCT. The distinction may be aided by reticulin stains.
5. Large solitary luteinized follicle cyst of pregnancy and puerperium versus unilocular cystic AGCT. Patients with the former lesion have a history of recent pregnancy. The follicle cyst shows large luteinized cells with large bizarre nuclei. In contrast, the unilocular cystic AGCT is lined by non-luteinized cells with typical granulosa cell nuclei (see Chapter 24).
6. Endometrioid carcinoma (well differentiated and microglandular, resembling a sex cord–stromal tumor; sertoliform carcinoma) versus microfollicular, trabecular, or insular AGCT. Foci of typical endometrioid carcinoma with mucin-secreting glands are usually found in the former; also, carcinomatous (atypical) nuclei with numerous and often abnormal mitoses are seen. Squamous differentiation and adenofibromatous (müllerian) features are common. In carcinomas, α-inhibin and calretinin immunostains are negative (but positive in luteinized stromal cells), whereas cytokeratins and EMA are strongly positive (see Chapter 27).23,26
7. Insular carcinoid tumor versus microfollicular AGCT. The different nuclear features, calcification of acini of carcinoid tumors, and neuroendocrine markers should facilitate the differential diagnosis (see Chapter 29).
8. Sex cord tumor with annular tubules (SCTAT) versus microfollicular AGCT. The former tumor shows larger hyaline deposits than Call–Exner bodies as well as ring-shaped tubules; in cases associated with the Peutz–Jeghers syndrome (PJS) the SCTATs are small, multiple, and bilateral.
9. Gonadoblastoma versus insular or microfollicular AGCT. Background of intersexual condition, presence of germ cells, and larger hyaline deposits than Call–Exner bodies all favor gonadoblastoma (see Chapter 29).
10. Metastatic melanoma versus GCT. Histologic features of melanoma, melanin pigment, positive S-100, and HMB-45 and negative α-inhibin and calretinin all favor the former diagnosis (see Chapter 30).
11. Metastatic breast carcinoma (particularly of lobular type) versus diffuse AGCT. Bilaterality, history of breast cancer, presence of intracellular mucin, negative α-inhibin, positive EMA, and GCDFP-15 are all features of metastatic breast cancer (see Chapter 30).
Spread and Metastasis
All AGCTs are potentially malignant and capable of extending beyond the ovary, or recurring after apparently complete removal. Spread occurs mainly within the pelvis and lower abdomen; although rare, distant metastases have been reported in many locations.15 Most recurrences appear within 5 years but they can be detected many years postoperatively (occasionally after 20 or 30 years).1
Treatment and Prognosis
In menopausal or postmenopausal women, the optimal treatment is bilateral salpingo-oophorectomy with total hysterectomy. It is reasonable to conserve the opposite ovary and uterus of a young woman with an AGCT confined to one ovary. The recurrence rate is 10–15% for stage IA tumors and 20–30% overall. Recurrence is usually fatal, but reoperation, radiotherapy, and chemotherapy (similar to that used in malignant germ cell tumors) can be helpful.28
The 10 year survival varies from 60% to 90%.8–12 Patients with stage I tumors have much better prognosis than those with higher stage tumors (86% vs 49% 10 year survival).9 The most consistent indicator of aggressive behavior has been the presence of metastases or invasion of structures outside the ovary at the time of diagnosis.9,12 Also unfavorable but less significant factors are large tumor size (>6 cm in diameter)11 and tumor rupture.9 There is no correlation between the microscopic pattern and the clinical outcome. Flow cytometric results regarding DNA ploidy are contradictory.29
Juvenile Granulosa Cell Tumor
Clinical Features
Juvenile granulosa cell tumors (JGCTs) account for only 5% of GCTs and have been so designated because they usually occur in women less than 30 years of age, half of them in the first decade of life.1,30 These tumors differ histologically from the more common AGCTs and contain cells that appear less mature; they are often mistaken for other neoplasms, usually malignant germ cell tumors.
JGCTs occurring before puberty are associated in 80% of cases with isosexual pseudoprecocity, due to estrogen production by the ovarian tumor.30 They account for 10% of cases of sexual precocity. The large majority of cases (90%) of sexual precocity are central (idiopathic or constitutional), and result from premature release of gonadotropins by the hypothalamus (anterior pituitary gland) with no detectable organic lesion. In these cases (true precocity) ovulation occurs and there is a possibility of pregnancy. This does not occur in the cases of pseudoprecocity secondary to estrogen production by a functioning ovarian tumor. The GCTs that occur before puberty are almost always palpable on pelvic or rectal examination.1,30 Noteworthy, other ovarian tumors (of non-endocrine type) secreting human chorionic gonadotropin (hCG) can also be associated with isosexual pseudoprecocity in young females.1 In adult women, however, the most common symptom of the JGCT is uterine bleeding as a result of endometrial hyperplasia.1,30
In the largest series reported,30 which included 125 JGCTs, 44% occurred in the first decade, 34% in the second decade, 18% in the third decade, and only 3% after the age of 30 years. Eighty-two percent of the tumors encountered before the age of normal puberty were associated with isosexual pseudoprecocity. Eight cases of JGCTs that developed in patients with Ollier disease (multiple enchondromatosis),31 and three with Maffuci syndrome (Ollier disease with hemangiomas) have been reported, indicating that the JGCT is a rare component of these disorders.30 At operation, JGCTs are almost always unilateral (98%) and more than 95% of them are confined to the ovary (stage I). Extraovarian spread is found in only 2% of patients, mostly confined to the pelvis.30
Macroscopic Features
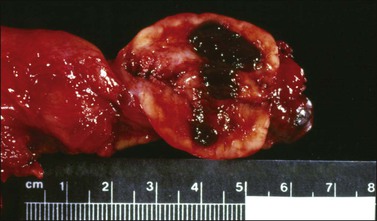
The tumors range from 3 to 32 cm (average 12.5) in diameter and appear similar to the AGCT. They are usually either uniformly solid or partly solid containing cysts that are often filled with blood (Figure 28.13); less commonly the tumors are composed predominantly of one or more thin-walled cysts. The neoplastic tissue is gray or yellow and occasionally large areas of necrosis or hemorrhage suggest a highly malignant tumor.1,30
Microscopic Features
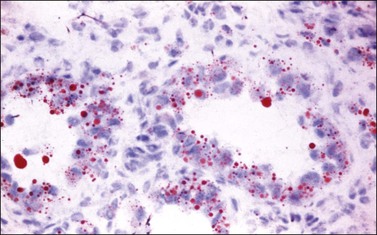
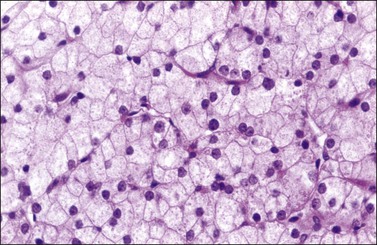
Microscopic examination shows diffuse sheets or nodules of neoplastic granulosa cells, which may be solid but more often are punctured by follicular spaces (Figure 28.14). Generally the follicles do not reach the large size of those encountered in the macrofollicular form of the AGCT; although they vary in size and shape, they are usually round to oval (Figures 28.15 and 28.16). The lumens typically contain eosinophilic or basophilic material that stains with mucicarmine in about two-thirds of cases. Papillae lined by granulosa cells grow into the cystic spaces in some tumors.32 Theca cells may be present in varying amounts and occasionally the granulosa and theca cells are arranged in a disorderly fashion; in such cases reticulin stains may help to differentiate them, with fibrils investing the theca cells individually, but the granulosa cells only in groups. Fat stains (ORO) are positive (Figure 28.17).
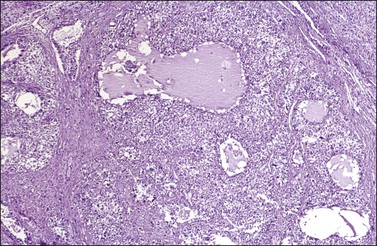
Figure 28.14 Juvenile granulosa cell tumor. Numerous oval to round follicles containing eosinophilic fluid.
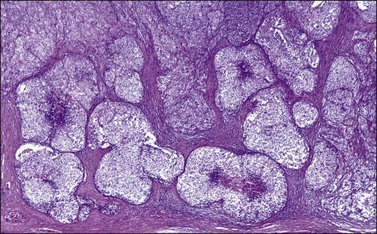
Figure 28.15 Juvenile granulosa cell tumor. The tumor cells form solid nodules, some of which exhibit central necrosis.
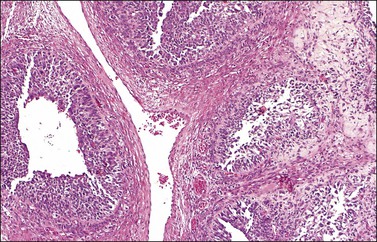
Figure 28.16 Juvenile granulosa cell tumor. Well-defined follicles of varying sizes separated by fibrovascular tissue.
The cytologic features of the JGCT also differ from those of the AGCT. The nuclei of both the granulosa and theca cells appear less mature in the former than the latter, showing hyperchromatism (Figure 28.18) and often considerable mitoses, which may be atypical; nuclear atypia varies from minimal to severe and nuclear grooves are absent. Rare cases of anaplastic JGCT exhibit a sheet-like growth with striking nuclear atypia resembling undifferentiated carcinoma. Another cytologic feature of these tumors is luteinization of both granulosa and theca cells (Figure 28.19) with fat stains typically showing abundant intracytoplasmic lipid in the two cell types.
Immunohistochemistry and Genetic Profile
Like the adult variant, JGCTs show immunoreaction for inhibin (Figure 28.20), calretinin, MIS, FOXL2, and SF-1.7,20 There is strong membrane reaction for CD99 (Figure 28.21) and CD56.22 The tumor cells are vimentin positive and react for low molecular weight cytokeratin in 25–50% of cases.21 EMA is usually negative, as is typical of sex cord–stromal tumors, but sometimes may be focally positive.33 Trisomy 12 has been demonstrated in many cases by FISH.34
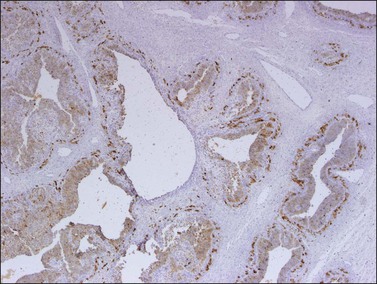
Figure 28.20 Juvenile granulosa cell tumor. The tumor cells are immunoreactive for α-inhibin, particularly the theca cells that surround rudimentary follicles.
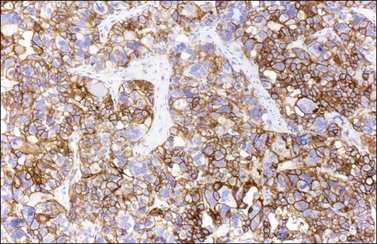
Figure 28.21 Juvenile granulosa cell tumor. The immunostaining for CD99 shows a characteristic membranous pattern.
The FOXL2 (C402G) mutation that is present in AGCTs is absent in JGCTs.6,35
Differential Diagnosis
1. Adult granulosa cell tumor. There are regular follicles, Call–Exner bodies, nuclear features (nuclear grooves), and lack of luteinization (except in pregnancy).
2. Malignant germ cell tumor (yolk sac tumor, embryonal carcinoma). Although the nuclei of JGCTs may occasionally appear severely atypical, they do not look as uniformly malignant (embryonal) as those of a primitive germ cell tumor. The latter lacks the typical follicular pattern and shows positive immunoreactions for hCG, α-fetoprotein, and cytokeratins, as well as nuclear transcription factors SALL4, LIN28, and OCT-4 or glypican-3 (see Chapter 29).
3. Thecoma. The patients are older and there is absence of follicles, predominance of theca cells, mild nuclear atypia, and characteristic reticulin stain.
4. Clear cell or undifferentiated carcinoma. Symptoms include older age of the patient, carcinomatous cells throughout the specimen, and negative α-inhibin in almost all cases.
5. Small cell carcinoma of hypercalcemic type. There is a lack of estrogenic symptoms, hypercalcemia, predominance of small cells (although it may contain large, rhabdoid-appearing cells focally), absence of the luteinized theca cell component, and higher mitotic rate. There are also negative α-inhibin and calretinin, and positive cytokeratin stains (see Chapter 27 and Table 28.2).
Table 28.2
Differentiation between JGCT and Small Cell Carcinoma
| JGCT | Small Cell Carcinoma |
| Rarely malignant | Highly malignant |
| No ↑ Ca2+ | ↑ Ca2+ frequent |
| Estrogenic | Never estrogenic |
| Follicles + + + | Follicles + |
| Thecomatous stroma | Nonspecific stroma |
| Abundant cytoplasm | Scanty cytoplasm |
6. Metastatic malignant melanoma. It is rare in young patients; there is a clinical history of melanoma, bilaterality, positive S-100 protein, and HMB-45 stains (Melan-A immunostaining can be positive in sex cord–stromal tumors; see Chapter 30).
Prognosis
Although the JGCT usually appears histologically more malignant than the AGCT, the follow-up data in four series of cases indicate a high cure rate.30,36–38 The stage of the tumor correlates best with prognosis. In the largest series reported, only 2 of 80 stage I tumors with follow-up information were clinically malignant. Both patients had stage IC tumors. All three stage II tumors were fatal.30 Contrary to the AGCT, almost all recurrences of the JGCT appear within 3 years postoperatively. The clinically malignant tumors were on average larger than those with a favorable outcome (20 vs 12 cm).30 Even if the FOXL2 (C402G) mutation, which is consistently present in AGCTs, is absent in JGCTs, patients with the latter tumors and higher FOXL2 protein expression had worse overall survival and disease-free survival than those with negative or weakly immunoreactive tumors.35 Neither DNA ploidy nor the S-phase fraction are predictive of the outcome. Some patients with advanced or recurrent disease respond to platinum-based combination chemotherapy.39
Thecoma
Thecomas are stromal tumors composed of lipid-containing cells resembling theca cells, lutein cells, and fibroblasts. These tumors can be divided into typical and luteinized variants.1
Typical Form
General Features
Typical thecomas are one-third as common as GCTs.1 They occur in older patients than those with GCTs (mean age, 59 years) and are estrogenic in almost all cases.1,40 About 60% of the patients present with uterine bleeding and 21% have an endometrial carcinoma.40
Macroscopic Features
Like GCTs, thecomas are unilateral in 97% of cases.1 Grossly, they are typically solid yellow masses (Figure 28.22) of about 7 cm in diameter. Cystic change and calcification may occur.
Microscopic Features
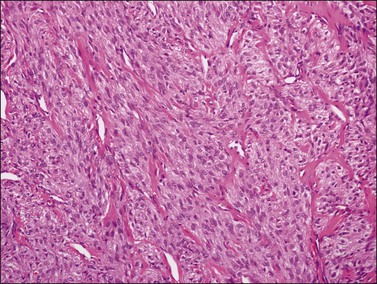
Typical thecomas are composed of sheets of round to spindle cells with ill-defined pale cytoplasm, often vacuolated and lipid-rich, alternating with collagen-producing fibroblasts (Figure 28.23). The nuclei vary from round to spindle shape and typically lacks atypia and mitoses. A fibromatous component often intersects sheets of theca-like cells. Hyaline plaques or even large zones of hyalinization may be present. Foci of calcification may also be found. Extensively calcified thecomas occasionally occur, especially in young women.41 Typical thecomas may also contain scattered ‘sex cord elements.’1 Contrary to GCTs, reticulin fibrils typically invest individual thecoma cells (Figure 28.24).
Rarely, mild nuclear atypia or even bizarre nuclei are seen in otherwise typical tumors, but severe atypia, high mitotic activity, and malignant behavior are extremely rare. Most of the tumors reported as ‘malignant thecomas’ probably represent examples of fibrosarcomas or diffuse GCTs.1
Immunohistochemistry and Cytogenetics
Thecomas are immunoreactive for vimentin, inhibin, calretinin, and other sex cord markers. Immunoreactions for cytokeratin are negative.18,26 Trisomy 12 can often be identified in thecomas.34
Differential Diagnosis
Thecomas and fibromas have overlapping features. They are essentially similar neoplasms, and typical thecomas are most frequently functioning tumors and contain lipid-rich cells that immunoreact for α-inhibin. Fat stains, however, are not helpful in distinguishing between thecomas and fibromas because otherwise typical-appearing fibromas can contain abundant lipid and some thecomas contain very little. The term ‘thecoma’ should be reserved for stromal tumors either containing moderate to large amounts of cytoplasmic lipid or associated with clinical or laboratory evidence of steroid hormone secretion. In contrast, fibromas are nonfunctioning tumors composed almost exclusively of spindle cells producing collagen and containing only small amounts of cytoplasmic lipid. Tumors with intermediate features are classified as ‘tumors in the thecoma–fibroma group.’
Luteinized Thecoma
General Features
Luteinized thecomas occur in a younger age group than typical thecomas. Although they are most common in postmenopausal women, 30% develop in women under 30 years of age.42 Approximately half of them are estrogenic, 40% nonfunctioning, and 10% androgenic.42 A rare variant of the luteinized thecoma is that associated with sclerosing peritonitis, which may be complicated by intestinal obstruction and can be fatal.43,44 Like luteinized thecomas of the usual type, these tumors occur mainly in young patients who present with abdominal swelling and ascites. The cause of the sclerosing peritonitis is unknown.
Macroscopic Features
Except for the lesions associated with sclerosing peritonitis, the gross features of luteinized thecomas are similar to those of typical thecomas. The former lesions are often bilateral ranging from normal-sized or slightly enlarged ovaries with a striking cerebriform appearance (Figure 28.26) to large tumors up to 31 cm in diameter.43 On section, there is prominent cortical edema and cystic change. The lack of a discrete mass in some cases suggests that they may represent a hyperplastic rather than neoplastic process. The sclerosing peritonitis involves the omentum, peritoneum, and intestinal serosa. There is fibroblastic proliferation, collagen, and inflammation with fibrin deposition.
Microscopic Features
Luteinized thecomas have the appearance of a fibroma or a typical thecoma but contain sharply defined lutein (steroid-type) cells, singly or forming nests (Figures 28.27 and 28.28) or masses. These cells are polyhedral or rounded cells with abundant eosinophilic cytoplasm and central, round nuclei with single prominent nucleoli.1,42,45,46 Occasionally, the steroid-type cells contain crystals of Reinke and these rare tumors, which may cause virilization, have been designated ‘stromal–Leydig cell tumors.’42 The variant associated with sclerosing peritonitis (Figure 28.29) is characterized by a dense proliferation of spindle cells admixed with lutein cells. There is minimal nuclear atypia but mitotic figures may be numerous (up to 40 per 10 HPF). In some cases there is diffuse edema with microcystic change.43
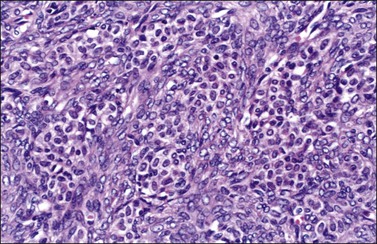
Figure 28.27 Luteinized thecoma in a patient with sclerosing peritonitis. Clusters of luteinized cells are admixed with spindle cells.
Genetic Profile
Loss of heterozygosity (LOH) at 9q22.3 (PTCH gene) but not 19p13.3 (STK11 gene) has been reported in luteinized thecomas.47
Differential Diagnosis
Extensively luteinized thecomas may be confused with steroid cell tumors,45 but the different clinical and pathologic features of both tumors help to distinguish them. The fibromatous component of steroid tumors usually represents <10% of the tumor.1 Luteinized thecomas may also be confused with stromal hyperthecosis, but the latter is always bilateral and characterized microscopically by nests of lutein cells on a background of normal stromal cells. In contrast, luteinized thecomas are rarely bilateral (except for those associated with sclerosing peritonitis) and contain large collagen-producing spindle cells and theca-like cells. The striking luteinization of thecomas in pregnant patients can create confusion with pregnancy luteomas. The latter, however, are multiple in half of the cases, contain no lipid, and lack fibromatous features.42
Luteinized thecomas associated with sclerosing peritonitis can be confused with various non-neoplastic and neoplastic lesions. The prominent nodular surface in some cases, or the marked ovarian enlargement in others, serves to distinguish these thecomas from stromal hyperthecosis. Furthermore, the stromal cells in hyperthecosis lack the mitotic activity often found in luteinized thecomas. Massive edema and fibromatosis are hypocellular and associated with either diffuse edema or extensive collagen deposition. Immunohistochemical stains (positive calretinin and α-inhibin (Figure 28.28) and negative EMA and cytokeratin reactions) may help to distinguish these rare lesions from metastatic lobular carcinoma of the breast, particularly in bilateral cases.
Treatment and Prognosis
The treatment of luteinized thecomas of the usual type is similar to that of typical thecomas. Exceptionally, mitotically active luteinized thecomas with nuclear atypia metastasize.42 The criteria for separating benign and malignant luteinized thecomas are similar to those used for distinguishing between cellular fibromas and fibrosarcomas48,49 (see later). Luteinized thecomas associated with sclerosing peritonitis are treated adequately by excision of the ovarian tumors and abdominal surgery, including lysis of adhesions, omentectomy, and bowel resection. None of the reported tumors have spread beyond the ovary, but three patients died of complications from their peritoneal disease.43
Fibroma
General Features
Fibromas account for about 4% of all ovarian tumors.1,50 They occur in women of all ages, but are most common between 50 and 60 years. Although most of them are asymptomatic, some patients may develop abdominal pain, abdominal enlargement, or urinary disturbances.50 In about 5% of cases there is an acute onset of abdominal pain because of torsion of the neoplasm. Although steroid hormone production is exceptional, the fibroma is occasionally accompanied by two unusual clinical syndromes: Meigs syndrome51 and the basal cell nevus (Gorlin) syndrome.52 The former, which is found in only 1–2% of cases, is defined as ascites and pleural effusion accompanying a fibrous ovarian tumor and disappearing after the resection of the tumor.51 When a fibroma is associated with Meigs syndrome and elevated CA125 levels, the clinical picture is very similar to that of an ovarian carcinoma and it may be only after laparotomy and surgical staging that the final diagnosis is established.53
Ascites alone is associated with 15–30% of ovarian fibromas over 10 cm in diameter.
Ovarian fibromas occur in a high proportion of young women with the basal cell nevus syndrome and in these patients the fibromas are almost always bilateral, multiple, and calcified.52
Macroscopic Features
Fibromas are bilateral in about 8% of cases and have an average diameter of 6 cm.1,50 They have a smooth, lobulated outer surface and on section appear as uniformly white or grayish white solid tissue (Figure 28.30); sometimes, however, the cut surface has a whorled appearance. Fibromas are cystic in about 20% of cases and calcification is not uncommon and is sometimes massive. Rarely, a fibroma may show ossification.1,50
Microscopic Features
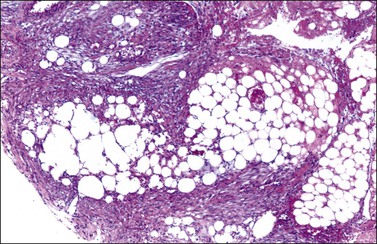
Fibromas are formed of interlacing bundles of spindle cells resembling fibroblasts (Figure 28.31), which often show a ‘feathertail’ pattern. Nuclear palisading is sometimes seen and occasionally a storiform pattern, similar to that of fibrous histiocytomas, is present. Although the cellularity varies, it is usually not marked; when striking the tumor is classified as a cellular fibroma (see later). The tumor cells are small and have narrow ovoid nuclei. Cytologic atypia and mitotic activity are usually absent. The cell cytoplasm may contain small amounts of lipid. Occasionally, it contains eosinophilic hyaline globules. Sharply delineated hyaline plaques are sometimes present and a more diffuse hyalinization is not uncommon; many fibromas show a variable degree of intercellular edema or myxoid change. Occasional fibromas contain scattered foci of sex cord elements (Figure 28.32). Ovarian fibromas immunoreact for calretinin (95–100%) and α-inhibin (15–70%).18
Cellular Fibroma
Approximately 10% of ovarian fibromas are densely cellular (almost as much as a diffuse GCT) and have been descriptively named ‘cellular fibromas.’48 The clinical features of these tumors are similar to those of typical fibromas. Grossly, however, cellular fibromas are larger (average size, 12 cm) and softer than the usual ovarian fibromas and their sectioned surfaces, which are solid (Figure 28.33), may exhibit hemorrhage and necrosis.
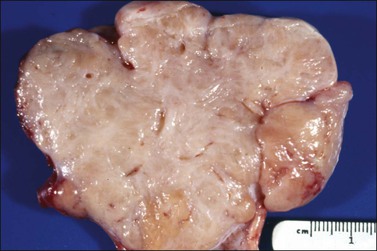
Figure 28.33 Cellular fibroma. The sectioned surface has a fleshy appearance which differs from that of a typical fibroma.48 (Reproduced with permission of John Wiley and Sons; Prat J, Scully RE. Cellular fibromas and fibrosarcomas of the ovary. A comparative clinicopathologic analysis of seventeen cases. Cancer 1981;47:2663–2670)
Microscopically, cellular fibromas are composed largely of densely cellular tissue with a few areas of hypocellular fibrous tissue. The cells are spindle shaped and arranged in intersecting bundles or in a storiform pattern. They have ill-defined cytoplasmic borders and round to oval hyperchromatic nuclei. The nuclei are slightly or moderately atypical, and between 1 and 3 mitotic figures per 10 HPF are present (Figure 28.34). Higher mitotic rates are still compatible with cellular fibroma (‘mitotically active cellular fibroma’) provided there is no appreciable cytologic atypia.48,49 A gain of trisomy 12 cells has been demonstrated by FISH in eight of these tumors.54
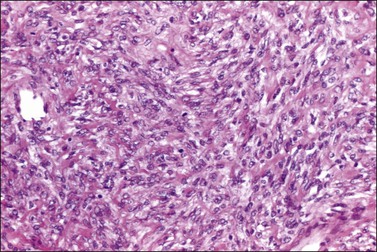
Figure 28.34 Cellular fibroma. The tumor is cellular but lacks significant atypia. A mitotic figure is seen. The patient died of recurrent tumor.
Because of the typically benign course and infrequent bilaterality of cellular fibromas, they can justifiably be treated by unilateral salpingo-oophorectomy in a young woman who wants to retain her fertility. If the tumor is adherent it should be removed as completely as is technically feasible. Patients with ruptured tumors should be followed carefully because of the possibility of intra-abdominal recurrence.48
Fibrosarcoma
Pure fibrosarcomas are among the most common form of ovarian sarcoma.1 They are often found in older women (average, 58 years) and are almost always unilateral and large masses (average size, 17 cm).48 On gross examination, they are soft and lobulated and completely replace the ovary. Sectioning typically shows solid, grayish white to tan tissue that often has areas of hemorrhage and necrosis.
Microscopically, the tumors are densely cellular, and the spindle-shaped cells are arranged in a herringbone or storiform pattern. The tumor cells have indistinct borders, eosinophilic cytoplasm, and hyperchromatic nuclei with prominent nucleoli. There is a moderate to marked degree of pleomorphism and the number of mitotic figures ranges from 4 to 25 per 10 HPF (Figure 28.35).48 Mitotic activity (particularly normal mitotic figures) in excess of 4 per 10 HPF in the absence of moderate to severe atypia does not signify a fibrosarcoma. In such cases, a diagnosis of mitotically active cellular fibroma is made.48,49
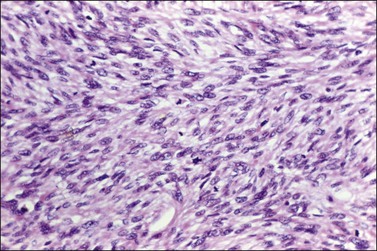
Figure 28.35 Fibrosarcoma. The tumor shows moderate nuclear atypia and several mitoses including abnormal forms.
The MIB1 labeling index and the proliferative index (percentage of cells in S + G2 + M phases) are higher in fibrosarcomas than in cellular fibromas of the ovary. Trisomy 12 and trisomy 8 have been reported in an ovarian fibrosarcoma.54 Fibrosarcomas are highly aggressive neoplasms associated with poor prognosis.
Sclerosing Stromal Tumor
This rare form of stromal tumor differs clinically from fibromas and thecomas in its age distribution. Approximately 80% of these tumors occur in patients who are less than 30 years old (average age 27 years).55,56 In contrast, only about 10% of fibromas and thecomas develop during that age period. Most patients present with nonspecific symptoms related to a unilateral adnexal mass. Unlike typical thecomas, sclerosing stromal tumors (SSTs) are usually nonfunctioning, and if hormonally active, are usually androgenic, most frequently during pregnancy.57
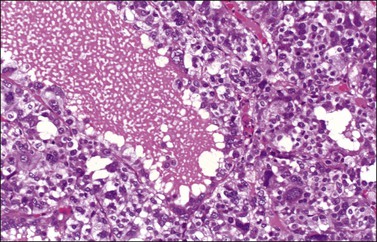
Grossly, the tumors are predominantly solid masses (average diameter, 10 cm) with yellow areas, edema, and cyst formation. The microscopic features of the SST are distinctive. Low-power microscopic examination typically shows ill-defined cellular pseudolobules separated by edematous fibrous tissue (Figure 28.36). The nodules contain two types of cells: spindle cells producing collagen and round to oval degenerated lutein cells (Figure 28.37). The latter have a vacuolated cytoplasm and contain lipid. Mitotic activity is low. A network of thin-walled blood vessels, ‘hemangiopericytoma-like,’ is usually present within the nodules.
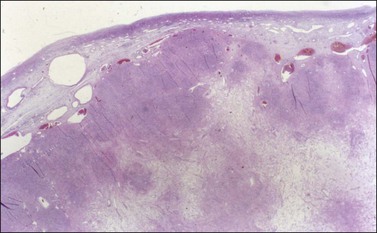
Figure 28.36 Slerosing stromal tumor. Cellular pseudolobules appear separated by edematous fibrous tissue.
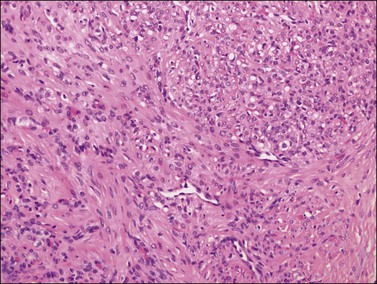
Figure 28.37 Slerosing stromal tumor. Spindle-shaped fibroblasts are admixed with rounded vacuolated cells. Note the presence of thin-walled blood vessels.
Immunohistochemical expression of vimentin, inhibin, calretinin, α-glutathione S-transferase, and CD34 has been demonstrated in SSTs.58 Smooth muscle actin and muscle-specific actin and desmin are usually positive.56 Immunoreactions for vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptor, fms-like tyrosine kinase 1 (flt-1), have also been detected in the lutein cells and small- to medium-sized blood vessels, respectively.59 These findings explain the prominent vasculature and edema characteristic of SSTs. Trisomy 12 has also been identified.59,60
Signet-Ring Stromal Tumor
This rare neoplasm occurs in adults and is nonfunctioning. Only eight cases have been reported.61–64 The clinical and macroscopic features are similar to those of ovarian fibromas. Microscopically, spindle cells are diffusely distributed and merge almost imperceptibly with rounded cells containing eccentric nuclei and single large vacuoles resembling signet-ring cells (Figure 28.38). Hyaline bodies are frequently present. Stains for lipid and mucin are negative. Ultrastructural studies have revealed that in some cases the vacuoles result from diffuse edema of the cytoplasmic matrix, in other cases from swelling of mitochondria, and in still others from cytoplasmic pseudoinclusions of edematous extracellular matrix.62,63 The main differential diagnosis is Krukenberg tumor, but negative mucin, EMA, and cytokeratin stains exclude that diagnosis.64 The signet-ring stromal tumor lacks the pseudolobulation, lipid-rich cells, and prominent vascularity of the SST. Inhibin has been negative in all cases tested.
Microcystic Stromal Tumor
This benign stromal tumor shows a characteristic microcystic pattern.65 In the only reported series of 16 cases, all patients were adults (mean 45 years) and the tumors were nonfunctioning, confined to the ovary (stage I), and had a mean size of 10 cm. They were cystic or solid and cystic. On microscopic examination, the appearance varies according to the relative prominence of the three fundamental components: microcysts, solid cellular regions, and fibrous stroma. These regions are punctuated by small cysts that anastomose with each other, giving a distinctive appearance (Figure 28.39). The neoplastic cells contain granular eosinophilic cytoplasm and round to oval nuclei with small nucleoli (Figure 28.40). Mitotic figures are rare. The tumors are typically negative for inhibin but positive for CD10. About one-third of the tumors examined to date have been cytokeratin positive but they have been EMA negative. The tumors show WT1 nuclear immunoreactivity and nuclear staining of β-catenin.66 Mutation analysis in two cases revealed the presence of an identical point mutation, c.98C>G, in exon 3 of CTNNB1 (β-catenin).66 This finding suggests that dysregulation of the Wnt/β-catenin pathway plays an important role in the pathogenesis of this ovarian stromal tumor.
Sertoli–Stromal Cell Tumors
Sertoli Cell Tumors
These uncommon tumors are composed of hollow or solid tubules intersected by stroma with only rare or no Leydig cells.1,67–69 They account for 4% of Sertoli–stromal cell tumors. They may occur at any age but are most common in women with an average age of 30 years. Sertoli cell tumors are usually nonfunctioning but may be estrogenic or rarely androgenic. Lipid-rich Sertoli cell tumors are associated with isosexual pseudoprecocity,1 and oxyphil Sertoli cell tumors with the Peutz–Jeghers syndrome (PJS).70 The endometrium may be hyperplastic. A decidual reaction caused by progestins produced by the tumor is occasionally seen.71 One tumor was malignant with distant metastases.68
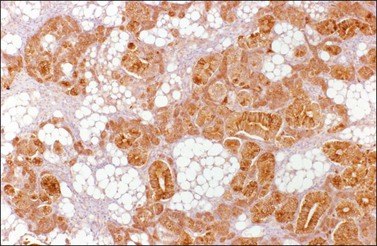
Sertoli cell tumors are unilateral and stage I.67–69 They average 9 cm in diameter and appear solid, lobulated, and yellow-brown. Microscopically, the tumors exhibit a prominent tubular architecture. The tubules are surrounded by a basement membrane that may coalesce to form hyalinized areas. Hollow tubules are lined by columnar to cuboidal cells with pale or eosinophilic cytoplasm (Figure 28.41). Other patterns include trabecular, diffuse, alveolar, pseudopapillary, and retiform. Spindle cells may be prominent. The Sertoli cells show mild nuclear atypia and very few mitoses. Bizarre nuclei may be rarely found. Vimentin, WT1,72 SF-1,73 cytokeratins, inhibin (Figure 28.42), calretinin, and CD99 are usually positive. EMA is negative.
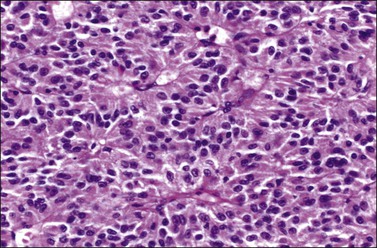
Figure 28.41 Sertoli cell tumor. The almost solid tubules are lined by cuboidal to columnar cells with eosinophilic cytoplasm. No mitotic activity is seen.
Endometrioid carcinomas may resemble Sertoli cell tumors (‘sertoliform’ variant).74,75 Struma ovarii and primary or metastatic carcinoid tumours may also mimic Sertoli cell tumors. The possibility that a sertoliform tumor could be a Sertoli cell adenoma in a phenotypic female with androgen insensitivity syndrome should also be considered.
Sertoli-Leydig Cell Tumors
Clinical Features
Sertoli-Leydig cell tumors (SLCTs) are rare and account for less than 0.5% of all ovarian tumors;1 they occur in all age groups but they peak during reproductive years (average age, 25 years).76–79 Tumors developing in patients with a germline DICER-1 mutation occur at a younger median age of 13 years.80 SLCTs are unilateral in over 98% of cases. They produce androgens and masculinize the patients in 40–60% of cases, but many tumors are nonfunctioning and some even have estrogenic effects.77,79 Testosterone and a variety of androgenic precursors may be secreted in variable proportions. Androgenic manifestations include amenorrhea, hirsutism, breast atrophy, enlargement of the clitoris, and hoarseness. In contrast to virilizing adrenal tumors, urinary 17-ketosteroid values are usually normal or only slightly raised. About 20% of cases are associated with elevated serum levels of α-fetoprotein.81 Patients may present with abdominal pain, ascites, or tumor rupture. SLCTs are stage IA in about 80% of cases.76–79 Only 2–3% of tumors have spread beyond the ovary, usually within the pelvis and rarely into the upper abdomen. Poorly differentiated tumors more often are ruptured and present at a more advanced stage than tumors of intermediate differentiation. Well-differentiated tumors are almost always stage IA.
Macroscopic Features
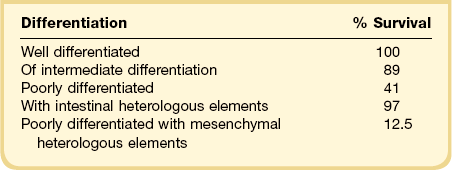
The tumors vary in their gross appearance from solid, lobulated, yellow masses (Figure 28.43) to unilocular or multilocular cysts containing blood. Their average diameter is 13 cm.76–79 Poorly differentiated tumors including those containing mesenchymal heterologous elements are often large, hemorrhagic, and necrotic.82 Retiform tumors and those with mucinous heterologous elements tend to be cystic or predominantly cystic and may contain numerous papillae (Figure 28.44).83–86
Microscopic Features
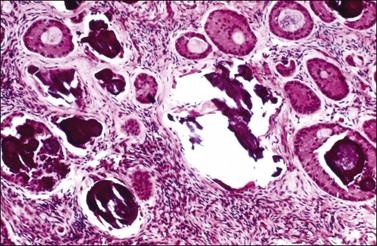
1. Well-differentiated tumors form hollow or solid tubules composed of cuboidal to columnar Sertoli cells, separated by a fibrous stroma, and admixed with nests of large round Leydig cells. The histologic appearance resembles that of the fetal testis (Figure 28.45) or even the tubular glands of a well-differentiated endometrioid adenocarcinoma. The nuclei are round and lack atypia and mitotic activity. Crystals of Reinke can be seen in some of the Leydig cells in 20% of cases.87
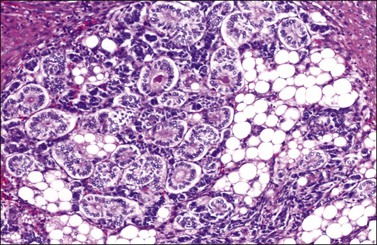
Figure 28.45 Well-differentiated Sertoli-Leydig cell tumor from a 29-year-old woman with virilization. The hollow tubules are lined by mature Sertoli cells. The intervening stroma contains numerous Leydig cells exhibiting large cytoplasmic vacuoles that were filled with lipid.
2. Tumors of intermediate differentiation contain cellular lobules of immature gonadal stromal cells separated by edematous stroma (Figures 28.46 and 28.47). The hyperchromatic spindle-shaped cells merge with cords and tubules of atypical Sertoli cells (Figure 28.48). The immature Sertoli cells contain small, round, oval, or angular nuclei; the abortive tubules resemble early sex cords of the embryonic testis (Figure 28.47).76–78 In tumors of intermediate differentiation, mitotic figures average 5 per 10 HPF. Rarely, bizarre nuclei may be seen. Leydig cells are found in clusters at the periphery of the cellular lobules or admixed with other elements. They may be vacuolated, contain lipofuscin, or rarely have Reinke crystals. The Leydig cells lack nuclear atypia and mitotic activity.
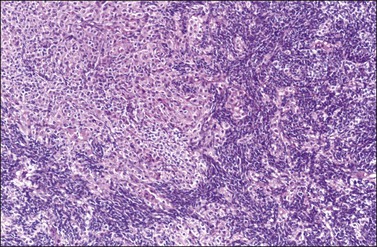
Figure 28.46 Sertoli-Leydig cell tumor of intermediate differentiation. Masses and clusters of Leydig cells with abundant eosinophilic cytoplasm separate aggregates of smaller Sertoli cells with scanty cytoplasm.
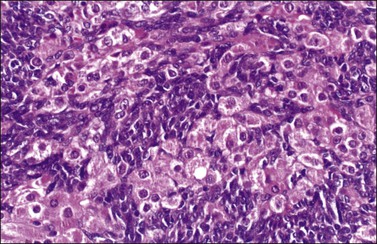
Figure 28.47 Sertoli-Leydig cell tumor of intermediate differentiation. The Sertoli cells have oval and angular nuclei whereas those of the Leydig cells are round and contain prominent nucleoli.
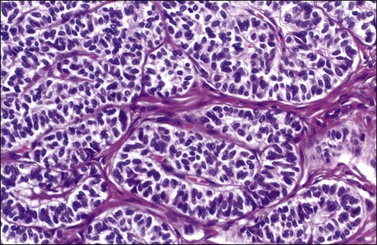
Figure 28.48 Sertoli-Leydig cell tumor of intermediate differentiation. Solid tubules of well-differentiated Sertoli cells resembling those of the fetal testis.
3. Poorly differentiated or sarcomatoid SLCTs are composed of spindle cells with vague trabecular arrangement resembling a fibrosarcoma (Figure 28.49). Leydig cells may or may not be present. Mitotic figures are usually numerous with a mean of over 20 per 10 HPF.77,78
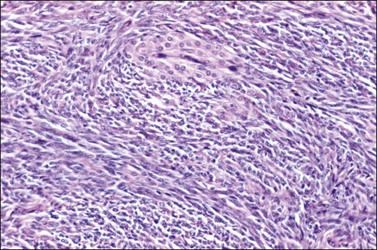
Figure 28.49 Sertoli-Leydig cell tumor, poorly differentiated (‘sarcomatoid’). The tumor cells are spindle shaped and resemble those of a sarcoma. A nest of Leydig cells is seen.
4. Fifteen percent of SLCTs show areas of slit-like spaces that mimic the rete of the ovary or testis (Figure 28.50); they have been designated retiform SLCTs.83 They occur on average about a decade earlier than other SLCTs, are less often virilizing, and are frequently papillary simulating epithelial–stromal tumors, particularly serous borderline tumors, and yolk sac tumors.83–85 The papillae often contain hyalinized cores. Some tumors show a multicystic pattern with sieve-like spaces lined by flattened cells. Approximately 30% of poorly differentiated LSCTs have retiform elements.
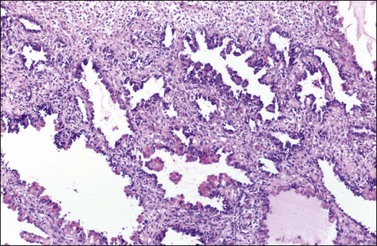
Figure 28.50 Sertoli-Leydig cell tumor, retiform. The tumor shows a papillary architecture. The papillae are partly covered by hyaline bodies.
5. Twenty percent of the tumors contain heterologous elements such as neoplastic mucinous glands (Figure 28.51), cartilage, and skeletal muscle (Figure 28.52).82,86 Glands and cysts are lined by well-differentiated mucinous epithelium of gastrointestinal type containing goblet cells, argentaffin cells, and Paneth cells; the mucinous epithelium may appear benign, borderline, or of low-grade malignancy. Focal insular carcinoid may be present.86 Mesenchymal heterologous elements are found in only 5% of all SLCTs and include areas of fetal cartilage, embryonal rhabdomyosarcoma (Figures 28.52 and 28.53), or both.82 Hepatic tissue has been found in 56% of the tumors with retiform differentiation (Figure 28.54).88,89 One recurrent heterologous tumor contained neuroblastoma.82
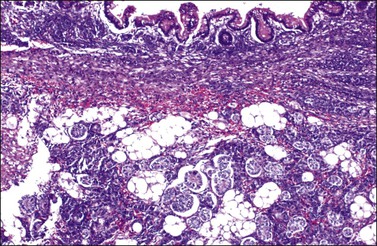
Figure 28.51 Well-differentiated Sertoli-Leydig cell tumor with heterologous elements. Well-differentiated gastrointestinal mucinous epithelium containing goblet cells (top).
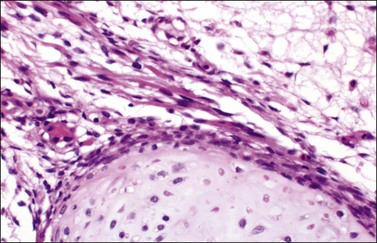
Figure 28.52 Sertoli-Leydig cell tumor with heterologous elements. Island of fetal-type cartilage (bottom) and bundles of rhabdomyoblasts.
SLCTs are divided into well-differentiated, intermediate, and poorly differentiated forms based on the degree of tubular differentiation of the Sertoli cell component and the quantity of spindle cell gonadal stroma. Both Sertoli cell elements and Leydig cells are seen less frequently in the poorly differentiated tumors. Heterologous elements and/or a retiform pattern may be seen in all but the well-differentiated variant. Unlike the theca cells in GCTs, the Leydig cell component is an integral part of the neoplastic process at least in some cases.
Immunohistochemistry
SLCTs are immunoreactive for vimentin, cytokeratins, inhibin (Figure 28.55), calretinin, progesterone receptor, androgen receptor, CD56, SF-1, and WT1. The intensity of expression differs between sex cord and stromal components. SLCTs react for FOXL2 in approximately half of cases.7 DICER1 immunoreaction is strong in Sertoli cells and weak in Leydig cells.80 Leydig cells in SLCTs do not react for FOX L2, WT1, and CD99, but express Melan-A.20
Genetic Susceptibility
Mutations in DICER1, a gene encoding an RNase III endoribonuclease, are found in 60% of SLCTs. Germline mutations have been identified in families affected by pleuropulmonary blastoma in childhood, multinodular goiter, and SLCTs.80,90,91
Differential Diagnosis
1. Sertoli cell tumors and hamartomas occur in the abdominal testes of patients with the androgen-insensitivity syndrome. Such patients have normal female habitus, absence of pubic or axillary hair, primary amenorrhea, no uterus, and 46,XY karyotype.
2. Endometrioid carcinomas resembling sex cord–stromal tumor. Endometrioid carcinomas show mucin secretion, areas of squamous differentiation (nests of cells, morules, and keratinizing foci), and an adenofibromatous component (see Chapter 27).
3. Endometrioid stromal sarcomas with sex cord-like differentiation (see Chapter 27).
4. Krukenberg tumors with tubular pattern and stromal luteinization. Krukenberg tumors are bilateral in most cases and contain markedly atypical cells, including signet-ring cells that contain mucin. Other general features of metastatic tumors will be present (see Chapter 30).
5. Carcinoid tumors of the trabecular type may be confused with SLCTs of intermediate differentiation. The ribbons of the carcinoid tumor are much longer and more evenly distributed than the sex cord structures of the latter tumors. The stroma of the carcinoid tumors is less cellular and more fibromatous than that of SLCTs and does not contain Leydig cells. Primary carcinoid tumors are associated with teratomatous elements in about 70% of cases (see Chapter 29), and metastatic carcinoids are usually bilateral and are frequently accompanied by peritoneal metastases (see Chapter 30).
6. Struma ovarii with solid tubular pattern may be difficult to distinguish from an SLCT. The finding of true thyroid follicles, calcium oxalate crystals, and positive immunostaining for thyroglobulin facilitate the correct diagnosis (see Chapter 29).
7. Ependymoma, primary or metastatic, may be confused with an SLCT. The finding of perivascular pseudorosettes composed of cells with fibrillary cytoplasmic processes and the positive glial fibrillary acidic protein immunoreactivity are helpful (see Chapter 29).
8. Female adnexal tumors of wolffian origin are rarely associated with endocrine symptoms (see Chapter 27)
9. Teratoma. SLCTs with heterologous elements may be misdiagnosed as teratomas. However, gonadal teratomas do not contain gonadal cell types and SLCTs do not contain ectodermal elements (except in a single reported case of neuroblastoma; see Chapter 29).82
10. Malignant mesodermal mixed tumors (MMMTs). Heterologous SLCTs containing cartilage or skeletal muscle may be erroneously interpreted as MMMTs. Nevertheless, the latter usually occur in postmenopausal patients and the cartilage component appears histologically malignant (chondrosarcoma; see Chapter 27).
11. Retiform SLCTs may be confused with yolk sac tumors because of the young age of the patient and the presence of a papillary architecture. The former, however, are accompanied by androgenic symptoms in 25% of cases and do not contain the primitive germ cells of yolk sac tumors. α-fetoprotein and glypican-3 immunoreactions are also helpful in this differential diagnosis (see Chapter 29).
Treatment and Prognosis
In young women, the treatment of choice is unilateral salpingo-oophorectomy. In those patients with virilizing SLCTs, normal menses return in about 4 weeks following removal of the tumor.79 Radical surgery and chemotherapy are indicated for tumors at an advanced stage. In contrast to GCTs, which often recur many years postoperatively, SLCTs tend to recur earlier, within 1–2 years (Table 28.3).77,78,82
Almost all SLCTs are benign, with the rare malignant cases falling into the category of poorly differentiated tumors (60%) and those with mesenchymal heterologous elements.82 Not enough cases of retiform SLCT have been reported to establish its prognosis, but it appears to be worse than that of SLCTs in general.84,85 Malignant behavior takes the form of intra-abdominal spread, ordinarily without distant metastases.77,78,82
Sex Cord Tumor with Annular Tubules
This tumor has distinctive morphologic features, which are intermediate between granulosa cell and Sertoli cell tumor.92,93 One-third of cases are associated with the Peutz–Jeghers syndrome (PJS) (mucocutaneous pigmentation, gastrointestinal hamartomatous polyps, rarely well-differentiated adenocarcinoma of the uterine cervix, and carcinomas of colon, pancreas, or breast) and have been found in patients of an average age of 27 years (Figure 28.56).92,93 The ovarian lesions are often incidental operative findings.93 In patients without the syndrome (average age, 34 years), the tumors often form unilateral palpable masses and are estrogenic in about half of the cases.93 In both cases, the tumors are composed of simple and complex ring-shaped tubules containing central hyaline nodules of basement membrane material (Figure 28.57).92,93 The cells lining the tubules have an antipodal arrangement with the nuclei located at opposite ends of the cells. Nuclear atypia and mitotic figures are rare. In patients with the PJS, multiple bilateral tumorlets are scattered within the ovarian stroma and calcification of the tubules is common (60%; Figure 28.58).92
Figure 28.56 Sex cord tumor with annular tubules. Section of ovary from a woman with Peutz–Jeghers syndrome. Several small yellow-pale nodules are seen.
Figure 28.57 Sex cord tumor with annular tubules. Complex annular tubules encircling numerous hyaline masses. Nuclei are arranged in antipodal manner at the periphery of the nests and around the hyaline mass.
Sex cord tumor with annular tubules (SCTAT) show positive immunoreaction for vimentin, inhibin, calretinin, FOXL2, SF-1, WT1, and CD56.18 There is positive reaction for cytokeratin but not for EMA. Germline-inactivating mutations of the PJS gene (STK11/LKB1) at chromosome 19p13.3 have been found in most PJS patients; however, somatic mutations have not been encountered in sporadic tumors.94 In sporadic sex cord–stromal tumors, LOH at 19p13.3 apparently targets a gene different from STK11 and may play a role in their pathogenesis, especially in tumors containing sex cord derivatives.95 LOH at the 19p13.3 region has also been found in three of eight well-differentiated adenocarcinomas of the cervix.
SCTATs associated with PJS have a favorable clinical course. About 20% of the SCTATs unassociated with PJS have been clinically malignant.93 Features associated with aggressive behavior include large size and invasive appearance, marked nuclear atypia, and high mitotic activity. The tumors spread via lymphatics. Recurrences are often late. Treatment is primarily surgical. MIS, α-inhibin, and progesterone are useful serum tumor markers.1
Steroid Cell Tumors
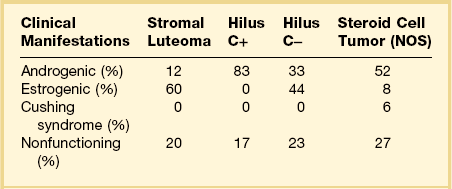
The term steroid cell tumors refers to a group of ovarian neoplasms composed of cells resembling steroid hormone-secreting cells (lutein cells, Leydig cells, or adrenal cortical cells). Although these tumors have been previously designated as lipid (lipoid) cell tumors, up to one-fourth of them contain little or no lipid.1,2 Consequently, the more appropriate and accurate term steroid cell tumors has been proposed for these neoplasms because it reflects both the cytologic features of the tumor cells and their capacity to secrete steroid hormones (Figure 28.59).1,2 Steroid tumors have been subdivided into four different subtypes: stromal luteomas, Leydig cell tumors, adrenal cortical-type tumors, and steroid cell tumors NOS (not otherwise specified) (Table 28.4).1,2 These neoplasms are uncommon and represent only approximately 0.1% of all ovarian tumors;1,2 nevertheless, they are clinically remarkable lesions, which are often associated with hyperestrogenism or masculinization (Table 28.5).96 All steroid cell tumors are immunoreactive for α-inhibin.
Stromal Luteoma
Stromal luteoma is a benign and small steroid cell tumor usually found within the ovarian stroma and presumably arising from it.1,97,98 The stromal origin of this tumor is supported by the finding of lutein cells in cases of stromal hyperthecosis, a non-neoplastic lesion associated with the neoplasm in over 90% of the cases (in the ipsilateral or contralateral ovary).97,98 In fact, in some cases of stromal hyperthecosis, nests of lutein cells may appear as nodules (nodular hyperthecosis).1 The stromal luteoma accounts for 20% of all steroid cell tumors, and 80% of them occur in postmenopausal women (mean age, 58 years).97,98 The tumor appears as a unilateral, well-circumscribed, solid, reddish yellow nodule, less than 3 cm in diameter (Figure 28.60). Microscopically, it is composed of lutein cells with relatively little lipid (Figure 28.61) and abundant lipochrome pigment. The nuclei are round and contain prominent nucleoli. Mitotic figures are uncommon. The tumor cells are arranged in a vague organoid pattern. In some cases, the artifactual formation of irregular spaces with hemorrhage can lead to an erroneous diagnosis of vascular tumor. In contrast to the other types of steroid cell tumor, stromal luteoma is commonly associated with hyperestrogenism (60%), although some rare cases have been androgenic (12%).97–99
Leydig Cell Tumor
Leydig cell tumors derive from Leydig cells, which are normally located in the ovarian hilus and contain crystals of Reinke in their cytoplasm on either light microscopy (Figure 28.62) or electron microscopy.96 Accordingly, the vast majority of reported tumors have been located in the hilus and have been thought to arise from hyperplastic hilus (Leydig) cells; they have been designated hilus cell tumors. Less frequently, Leydig cell tumors develop within the ovarian stroma, far from the hilus, and then are described as Leydig cell tumors (non-hilar type).1,96
Figure 28.62 Hilus cells. Reinke crystals are seen in the center of the field. A strand of a nerve is present in the right side of the figure.
Leydig cell tumors, which represent 15% of steroid cell tumors, usually occur in postmenopausal women (mean age, 58 years), and are associated with hirsutism or virilization in 80% of cases.1,96 They secrete predominantly testosterone and, therefore, the urinary 17-ketosteroid levels are often normal or only slightly elevated.1,96 Grossly, the tumors appear as small dark or reddish brown to yellow nodules; most of them are less than 5 cm in diameter (Figure 28.63). Microscopically, they are composed of polygonal cells arranged in nests. In some cases, Leydig cell tumors may contain cells with nuclear pseudoinclusions or marked nuclear pleomorphism (so-called endocrine atypia) (Figure 28.64). These pleomorphic tumors often exhibit Reinke crystals and lipochrome pigment. Leydig cell tumors with crystals are more frequently associated with virilization than Leydig cell tumors without crystals. When crystals are not found, the diagnosis of Leydig cell tumor is favored by its hilar location, the finding of hilus cell hyperplasia around non-medullated nerves, fibrinoid necrosis of vessel walls, and nuclear clustering with nucleus-free zones.1,96 Immunostains for α-inhibin are strongly positive. Practically all reported cases of hilus cell tumor have been benign.1,96
Figure 28.64 Leydig cell tumor. The polygonal cells have abundant eosinophilic cytoplasm and are arranged in nests. Some of them contain bizarre nuclei.
Mutations of LH receptor gene have been associated with the development of testicular Leydig cell tumors.100
Steroid Cell Tumor, Adrenal Cortical Type
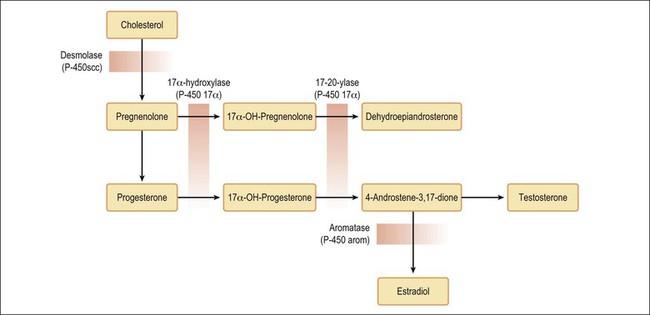
The morphologic similarity between steroid cell tumors and adrenal cortical tumors suggests that some of the former neoplasms may arise from adrenal cortical rests.1 These rests are extremely rare within the ovary, but they can be encountered in the mesovarium (broad ligament) in approximately 25% of women. In rare patients with steroid cell tumors, the responses of elevated levels of urinary 17-ketosteroids and 17-hydroxycorticosteroids to ACTH stimulation and dexamethasone suppression have been more concordant with an adrenal cortical tumor than one of ovarian type. Furthermore, the occasional association of such tumors with the adrenogenital syndrome or Cushing’s syndrome also supports their adrenal cortical nature.1 α-Inhibin immunostaining helps in the differential diagnosis.
Steroid Cell Tumor (NOS)
Steroid cell tumors NOS comprise a heterogeneous group of neoplasms that probably includes large stromal luteomas or Leydig cell tumors; however, since they lack Reinke crystals and their topographic origin is no longer evident, they cannot be classified accordingly.1,101 They represent 60% of steroid cell tumors.1 In one series, the clinically malignant tumors accounted for 28% of cases.101 The tumors may occur at any age, but characteristically at a younger age (mean, 43 years) than the other types of steroid cell tumors.1,101 They are associated with hirsutism or virilization in approximately 50% of cases, but may be estrogenic in 10% of cases. Four tumors have produced cortisol and caused Cushing’s syndrome and one has secreted aldosterone.1,102
Steroid cell tumors NOS are almost always unilateral. Bilaterality has been reported in only 6% of cases.96 Their size ranges from 1.2 to 45 cm with the malignant tumors having the largest diameters.101 The neoplasms may show different gross and microscopic features. Some of them are brown while others are yellow. Some tumors are composed of polygonal-shaped eosinophilic cells (Figure 28.65) whereas others show cells with clear cytoplasm containing abundant lipid (Figure 28.66). These tumors are positive for inhibin, calretinin, and Melan-A, a marker that can help in the distinction from other sex cord–stromal tumors;103 they are negative for FOXL2.
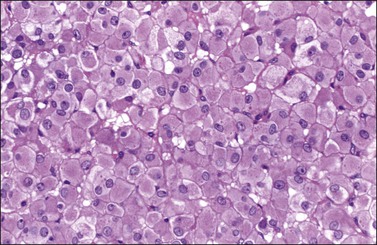
Figure 28.65 Steroid cell tumor NOS. The tumor cells are polygonal and have abundant eosinophilic cytoplasm and small round nuclei.
Differential Diagnosis
Steroid cell tumors, particularly those in the NOS category, may be confused with the tumors and tumor-like conditions contained in the following list. Positive immunohistochemical reactions for inhibin, calretinin, and SF-1 should exclude tumors in the differential diagnosis except for those in the sex cord–stromal category. However, steroid cell tumors are positive for Melan-A103 and negative for FOXL2.
1. Granulosa cell tumors and thecomas with marked luteinization. The presence of non-luteinized areas with some composed of spindle cells, the characteristic nuclear features, and the reticulin stains facilitate the correct diagnosis (See Figure 28.11).
2. Clear cell carcinoma (oxyphilic type). These tumors have glycogen-rich cytoplasm and excentric nuclei. Also, other patterns of clear cell carcinoma such as tubulocystic, papillary, and glandular are not found in steroid cell tumors.
3. Hepatoid yolk sac tumors and hepatoid carcinomas. Both tumors have epithelial cells, which may form glands. Immunoreaction for α-fetoprotein is characteristic.
4. Struma ovarii (oxyphilic type). The presence of true thyroid follicles, an association with other teratomatous elements, and immunostaining for thyroglobulin should help in establishing the correct diagnosis.
5. Paraganglioma and pheochromocytoma (primary or metastatic). Along with the clinical features, staining for chromogranin and the finding of dense core granules by ultrastructural examination permit the distinction of these tumors from steroid cell tumors.
6. Melanomas (primary or metastatic) have higher nuclear atypicality than steroid cell tumors. Nevertheless, the melanin granules of the former may be confused with the lipochrome pigment of the latter. Immunoreaction for S-100 protein and HMB-45 may be helpful in some cases.
7. Metastatic carcinomas from kidney, adrenal cortex, and liver. Clinical, radiologic, and immunohistochemical findings may facilitate the correct diagnosis.
8. Pregnancy luteomas resemble lipid-poor steroid cell tumor and are frequently virilizing (see Chapter 24). However, about half of the pregnancy luteomas are multiple and approximately one-third are bilateral. Nuclear atypia is absent but mitoses may be numerous. Steroid cell tumors with mitotic activity usually exhibit marked nuclear atypia.
Treatment and Prognosis
As with other types of endocrine tumor, it may be impossible to predict whether these tumors will have a malignant behavior on the basis of the clinicopathologic features. Nevertheless, some clinical and pathologic findings should be taken into account when considering the possibility of malignancy. Age of presentation is a good indicator of malignant behavior; the vast majority of malignant tumors occur in women older than 51 years.101 Other features to be considered are (1) 2 or more mitoses per 10 HPF, (2) grade 2 or 3 nuclear atypia, (3) diameter of 7 cm or greater, (4) hemorrhage, and (5) necrosis.101 Approximately 40% of the tumors show clinical signs of malignancy. In a young patient with a stage IA tumor, unilateral salpingo-oophorectomy is the standard treatment. Follow-up by measurement of hormone levels is important. In older patients with low-stage tumors, hysterectomy with bilateral salpingo-oophorectomy is advised. For advanced-stage tumors, bulk removal is recommended.101
Endocrine Syndromes Associated with Ovarian Tumors
Ovarian tumors with obvious endocrine manifestations represent <5% of all ovarian neoplasms, and malignant ‘functioning’ ovarian tumors <10% of all ovarian cancers.1,2 Nevertheless, the frequency of functioning ovarian tumors at a subclinical level is probably much higher according to various laboratory data. For example, it has been reported that over one-third of the cases of ovarian cancer in postmenopausal women are associated with abnormal cornification in vaginal smears, suggesting an increased estrogen level.104 Furthermore, approximately half of the postmenopausal women with common epithelial tumors (which are usually considered to be nonfunctioning) have elevated urinary estrogen and/or pregnandiol levels.105
Ovarian Tumors with Functioning Stroma
In 1958, Morris and Scully106 defined ovarian tumors with functioning stroma (OTFS) as tumors, other than sex cord–stromal or steroid cell neoplasms, whose stroma is consistent with steroid hormone secretion and are associated with estrogenic, androgenic, or progestogenic manifestations. In these tumors the neoplastic cells are not primarily responsible for steroid hormone production, but by growing within the ovarian stroma stimulate its cells or the adjacent hilus cells, which subsequently produce estrogens, androgens, progesterone, or combinations of these hormones. The mechanism by which this stimulation occurs is not well known. Possibly, secretion of hCG or hCG-like substance by the tumor cells is the triggering factor.107 This phenomenon appears to be unique in the ovary among all endocrine glands. Almost all types of ovarian tumors (benign or malignant and primary or metastatic) can exhibit a functioning stroma, but the frequency of such association varies considerably depending upon the histologic type. It is found most frequently in mucinous (Figure 28.67) and endometrioid tumors and metastatic carcinomas from the gastrointestinal tract.108
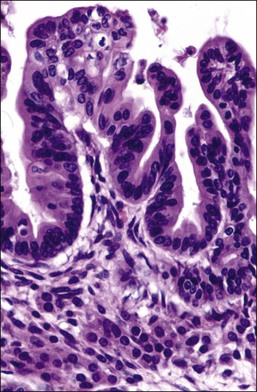
Figure 28.67 Mucinous borderline tumor with luteinization of stroma. Nests of lutein cells are seen below the neoplastic mucinous epithelium.
From a microscopical viewpoint, OTFS can be separated into two categories: the more common forms in which the stroma of the tumor is condensed or luteinized; and the less frequent tumors in which the peripheral stromal (Figure 28.68) or hilus cells become activated.109,110 Urinary estrogen levels have been correlated with the degree of stromal activation.108 In the same study, 40 of 80 (50%) postmenopausal women with benign and malignant surface epithelium stromal tumors or metastatic carcinomas to the ovaries had elevated urinary estrogen levels; 73% of these patients had activation of the ovarian stroma and 68% had a proliferative or hyperplastic endometrium. In these patients, the finding of mitoses in the fallopian tube epithelium is also indicative of estrogenic stimulation. Rare OTFS have been associated with progestational changes resulting in a decidual reaction or Arias-Stella change in the endometrium.111
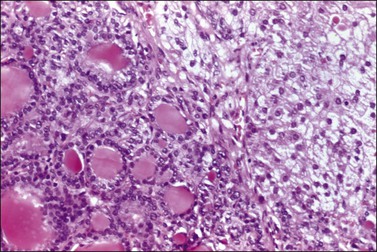
Figure 28.68 Struma ovarii with peripheral development of lutein cells. The lutein cells (right) are vacuolated. This tumor was associated with elevated estrogen levels and endometrial hyperplasia.
In a study of 100 non-selected primary and metastatic ovarian tumors for investigating the frequency of stromal activation and its possible relation to hCG or hCG-like secretion by tumor cells,107 stromal luteinization was present in 13 cases and stromal condensation in 16. The tumors most commonly associated with activation of the ovarian stroma were mucinous tumors (11 cases) and metastatic gastrointestinal adenocarcinomas (six cases). Two polyclonal and four monoclonal antibodies were used. A correlation between hCG production by the tumor cells, determined by immunohistochemistry, and activation (condensation or luteinization) of the ovarian stroma was encountered. Immunoreaction for hCG was more frequently found in tumors with morphologically active stroma than in those with an inactive stroma.107 These results indicate that hCG or hCG-like substances may play a role in activating the stroma of both primary and metastatic ovarian tumors.
Hypercalcemia
The small cell carcinoma, which is associated with hypercalcemia in two-thirds of cases, was first described in 1982112 (see Chapter 27). Several substances including osteoblast activating factor, prostaglandins, vitamin D-like substance, parathormone (PTH), and PTH-related peptide have been considered as potential responsible agents for the hypercalcemia. Attempts to demonstrate PTH immunostaining in these ovarian tumors have been unsuccessful in most cases.113 Immunostaining for PTH-related peptide has been described in five of seven cases of small cell carcinoma of the ovary, suggesting that PTH-related peptide is the responsible substance for the hypercalcemia associated with these ovarian tumors.114
Hyperthyroidism
Rare strumal carcinoids have been accompanied by evidence of hypersecretion of thyroid hormone in the form of postoperative thyroid storm or hypothyroidism.115 In cases of struma ovarii, biochemically documented thyroid hyperfunction has rarely been documented.
Carcinoid Syndrome
Of the three main types of carcinoid tumors of the ovary—insular, trabecular, and strumal—33% of the insular tumors and a single case of strumal carcinoid have been accompanied by the carcinoid syndrome.115,116 In these cases, the syndrome occurs in the absence of hepatic or other metastases since the secreted hormones enter the systemic circulation directly, bypassing the portal venous system and avoiding hepatic inactivation. The syndrome has been cured by removal of the ovarian tumor.
Zollinger–Ellison Syndrome
Mucinous ovarian tumors contain endocrine cells in their glandular epithelium in over 30% of the cases.117 A wide variety of peptide hormones have been demonstrated in these cells by immunohistochemistry. Eleven mucinous tumors of the ovary have produced the Zollinger–Ellison syndrome.118 Gastrin-containing cells were identified by immunohistochemistry in 10 of the cases. The patients had elevated serum gastrin levels that disappeared after removal of the tumors.
Hyperprolactinemia
Two cases of ovarian dermoid cyst containing prolactinomas of 2.5 cm and 1 mm each have been reported.119,120 The patients, aged 41 and 25 years, respectively, presented with amenorrhea and hyperprolactinemia.
Cushing Syndrome
Five cases of typical Cushing syndrome have been caused by cortisol production by steroid cell tumors.102 Four tumors occurred in adults, had spread beyond the ovary at the time of presentation, and were eventually fatal. The fifth tumor developed in a 2-year-old girl who presented with the syndrome and isosexual precocity, both of which regressed postoperatively. This tumor probably originated from adrenal cortical rests.
Hypoglycemia
Six ovarian tumors associated with hypoglycemia have been reported. They have included a dysgerminoma, a fibroma, a malignant schwannoma, a strumal carcinoid tumor, and a carcinoid tumor with a mixed insular and trabecular pattern.121
Hyperaldosteronism
Twelve cases of hypertension due to hormone secretion by an ovarian tumor have been reported.122,123 In eight cases, the hypertension was associated with a renin-secreting tumor and secondary hyperaldosteronism. In three cases, an aldosterone-secreting ovarian tumor resulted in primary hyperaldosteronism and low or normal plasma renin levels. In four cases the tumor also produced steroid hormones. Eight tumors were sex cord–stromal tumors, two were steroid cell tumors, one was a mucinous adenocarcinoma, and one was a leiomyosarcoma. One of the sex cord–stromal tumors occurred in a patient with Gorlin syndrome (see earlier) and another developed in a woman with Peutz–Jeghers syndrome (see earlier). Three tumors were clinically malignant and two were fatal. Immunoreaction for renin or prorenin was detected in five of the sex cord–stromal tumors and the leiomyosarcoma.
References
1. Scully, RE, Young, RH, Clement, PB. Sex cord-stromal tumors. In: Tumors of the ovary, maldeveloped gonads, fallopian tube, and broad ligament. Atlas of tumor pathology, 3rd series. Fascicle 23. Washington DC: Armed Forces Institute of Pathology; 1998:169–238.
2. Prat, J. Pathology of the ovary. Philadelphia: Saunders; 2004.
3. Aguirre, P, Thor, AD, Scully, RE. Ovarian endometrioid carcinomas resembling sex cord-stromal tumors: an immunohistochemical study. Int J Gynecol Pathol. 1989; 8:364–373.
4. Baker, PM, Oliva, E. Immunohistochemistry as a tool in the differential diagnosis of ovarian tumors: an update. Int J Gynecol Pathol. 2004; 24:39–55.
5. McCluggage, WG. Immunohistochemical markers as a diagnostic aid in ovarian pathology. Diagn Histopathol. 2008; 14:335–351.
6. Shah, SP, Kobel, M, Senz, J, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009; 360:2719–2729.
7. Al-Agha, OM, Huwait, HF, Chow, C, et al. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 2011; 35:484–494.
8. Bjorkholm, E, Pettersson, F. Granulosa-cell and theca-cell tumors. The clinical picture and long term outcome for the Radiumhemmet series. Acta Obstet Gynecol Scand. 1980; 59:361–365.
9. Bjorkholm, E, Silversward, C. Prognostic factors in granulosa cell tumors. Gynecol Oncol. 1981; 11:261–274.
10. Evans, AT, Gaffey, TA, Malkasian, GD, Jr., Annegers, JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980; 55:231–237.
11. Fox, H, Agrawal, K, Langley, FA. A clinicopathological study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975; 35:231–241.
12. Stenwig, JT, Hazekamp, JT, Beecham, JB. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 1979; 7:136–152.
13. Nakashima, N, Young, RH, Scully, RE. Androgenic granulosa cell tumors of the ovary. A clinicopathological analysis of seventeen cases and review of the literature. Arch Pathol Lab Med. 1984; 108:786–791.
14. Lauszus, FF, Petersen, AC, Greisen, J, Jakobsen, A. Granulosa cell tumor of the ovary: a population-based study of 37 women with stage I disease. Gynecol Oncol. 2001; 81:456–460.
15. Thrall, MM, Paley, P, Pizer, E, et al. Patterns of spread and recurrence of sex cord-stromal tumors of the ovary. Gynecol Oncol. 2011; 122:242–245.
16. Nogales, FF, Concha, A, Plata, C, Ruiz-Avila, I. Granulosa cell tumor of the ovary with diffuse true hepatic cell differentiation simulating stromal luteinization. Am J Surg Pathol. 1993; 17:85–90.
17. Ahmed, E, Young, RH, Scully, RE. Adult granulosa cell tumor of the ovary with foci of hepatic cell differentiation. A report of four cases and comparison with two cases of granulosa cell tumor with Leydig cells. Am J Surg Pathol. 1999; 23:1089–1093.
18. Deavers, MT, Malpica, A, Liu, J, et al. Ovarian sex cord-stromal tumors: an immunohistochemical study including a comparison of calretinin and inhibin. Mod Pathol. 2003; 16:584–590.
19. Cathro, HP, Stoler, MH. The utility of calretinin, inhibin, and WT1 immunohistochemical staining in the differential diagnosis of ovarian tumors. Hum Pathol. 2005; 36:195–201.
20. Zhao, C, Vinh, TN, McManus, K, et al. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. Am J Surg Pathol. 2009; 33:354–366.
21. Costa, MJ, DeRose, PB, Roth, LM, et al. Immunohistochemical phenotype of ovarian granulosa cell tumors: absence of epithelial membrane antigen has diagnostic value. Hum Pathol. 1994; 25:60–66.
22. Loo, KT, Leung, AKF, Chan, JKC. Immunohistochemical staining of ovarian granulosa cell tumours with MIC2 antibodies. Histopathology. 1995; 27:388–390.
23. Matias Guiu, X, Pons, C, Prat, J. Müllerian inhibiting substance, alpha-inhibin, and CD99 expression in sex cord-stromal tumors and endometrioid ovarian carcinomas resembling sex cord-stromal tumors. Hum Pathol. 1998; 29:840–845.
24. Lin, YS, Eng, HL, Jan, YJ, et al. Molecular cytogenetics of ovarian granulosa cell tumors by comparative genomic hybridization. Gynecol Oncol. 2005; 97:68–73.
25. Geiersbach, KB, Jarboe, EA, Jahromi, MS, et al. FOXL2 mutation and large-scale genomic imbalances in adult granulosa cell tumors of the ovary. Cancer Genet. 2011; 204:596–602.
26. Kommoss, F, Oliva, E, Bhan, AK, et al. Inhibin expression in ovarian tumors and tumor-like lesions: an immunohistochemical study. Mod Pathol. 1998; 11:656–664.
27. Riopel, MA, Perlman, EJ, Seidman, JD, et al. Inhibin and epithelial membrane antigen immunohistochemistry assist in the diagnosis of sex cord-stromal tumors and provide clues to the histogenesis of hypercalcemic small cell carcinomas. Int J Gynecol Pathol. 1998; 17:46–53.
28. Schwartz, PE, Smith, JP. Treatment of ovarian stromal tumors. Am J Obstet Gynecol. 1976; 125:402–411.
29. Evans, MP, Webb, MJ, Gaffey, TA, et al. DNA ploidy of ovarian granulosa cell tumors. Lack of correlation between DNA index or proliferative index and outcome in 10 patients. Cancer. 1995; 75:2295–2298.
30. Young, RH, Dickersin, GR, Scully, RE. Juvenile granulosa cell tumor of the ovary. A clinicopathologic analysis of 125 cases. Am J Surg Pathol. 1984; 8:575–596.
31. Velasco-Oses, A, Alonso-Alvaro, A, Blanco-Pozo, A, Nogales, FF, Jr. Ollier’s disease associated with ovarian juvenile granulosa cell tumor. Cancer. 1988; 62:222–225.
32. Irving, JA, Young, RH. Granulosa cell tumors of the ovary with a pseudopapillary pattern: a study of 14 cases of an unusual morphologic variant emphasizing their distinction from transitional cell neoplasms and other papillary ovarian tumors. Am J Surg Pathol. 2008; 32:581–586.
33. McCluggage, WG. Immunoreactivity of ovarian juvenile granulosa cell tumours with epithelial membrane antigen. Histopathology. 2005; 46:235–236.
34. Shashi, V, Golden, WL, von Kap Herr, C, et al. Interphase fluorescence in situ hybridization for trisomy 12 on archival ovarian sex cord-stromal tumors. Gynecol Oncol. 1994; 55:349–354.
35. D’Angelo, E, Mozos, A, Nakayama, D, et al. Prognostic significance of FOXL2 mutation and mRNA expression in adult and juvenile granulosa cell tumors of the ovary. Mod Pathol. 2011; 24:1360–1367.
36. Lack, EE, Perez-Atayde, AR, Murthy, AS, et al. Granulosa theca cell tumors in premenarchal girls. A clinical and pathologic study of ten cases. Cancer. 1981; 48:1846–1854.
37. Zaloudek, C, Norris, HJ. Granulosa tumors of the ovary in children. A clinical and pathologic study of 32 cases. Am J Surg Pathol. 1982; 6:503–512.
38. Calaminus, C, Wessalowski, R, Harms, D, Gobel, U. Juvenile granulosa cell tumors of the ovary in children and adolescents: results from 33 patients registered in a prospective cooperative study. Gynecol Oncol. 1997; 65:447–452.
39. Schneider, DT, Calaminus, G, Wessalowski, R, et al. Therapy of advanced ovarian juvenile granulosa cell tumors. Klin Padiatr. 2002; 214:173–178.
40. Bjorkholm, E, Silversward, C. Theca cell tumors. Clinical features and prognosis. Acta Radiol Oncol. 1980; 19:241–244.
41. Young, RH, Clement, PB, Scully, RE. Calcified thecomas in young women. A report of four cases. Int J Gynecol Pathol. 1988; 7:343–350.
42. Zhang, J, Young, RH, Arseneau, J, Scully, RE. Ovarian stromal tumors containing lutein or Leydig cells (luteinized thecomas and stromal Leyding cell tumors). A clinicopathological analysis of fifty cases. Int J Gynecol Pathol. 1982; 1:270–285.
43. Clement, PB, Young, RH, Hanna, W, Scully, RE. Sclerosing peritonitis associated with luteinized thecomas of the ovary. A clinicopathological analysis of six cases. Am J Surg Pathol. 1994; 18:1–13.
44. Staats, PN, McCluggage, WG, Clement, PB, Young, RH. Luteinized thecomas (thecomatosis) of the type typically associated with sclerosing peritonitis: a clinical, histopathologic, and immunohistochemical analysis of 27 cases. Am J Surg Pathol. 2008; 32:1273–1290.
45. Hughesdon, PE. Lipid cell thecomas of the ovary. Histopathology. 1983; 7:681–692.
46. Roth, LM, Sternberg, WH. Partly luteinized theca cell tumor of the ovary. Cancer. 1983; 51:1697–1704.
47. Tsuji, T, Catasus, L, Prat, J. Is loss of heterozygosity at 9q22.3 (PTCH gene) and 19p13.3 (STK11 gene) involved in the pathogenesis of ovarian stromal tumors? Hum Pathol. 2005; 36:792–796.
48. Prat, J, Scully, RE. Cellular fibromas and fibrosarcoma of the ovary: a comparative clinicopathologic analysis of seventeen cases. Cancer. 1981; 47:2663–2670.
49. Irving, JA, Alkushi, A, Young, RH, Clement, PB. Cellular Fibromas of the ovary: a study of 75 cases including 40 mitotically active tumors emphasizing their distinction from fibrosarcoma. Am J Surg Pathol. 2006; 30:928–938.
50. Dockerty, MB, Masson, JV. Ovarian fibromas: a clinical and pathologic study of two hundred and eighty three cases. Am J Obstet Gynecol. 1944; 47:741–752.
51. Meigs, JV. Fibroma of the ovary with ascites and hydrothorax. Meigs’ syndrome. Am J Obstet Gynecol. 1954; 67:962–987.
52. Gorlin, RJ. Nevoid basal-cell carcinoma syndrome. Medicine (Baltim). 1987; 66:98–113.
53. Domingo, P, Montiel, JA, Monill, JM, Prat, J. Pseudo-Meigs syndrome with elevated CA125 levels. Arch Intern Med. 1998; 158:1378–1379.
54. Tsuji, T, Kawauchi, S, Utsunomiya, T, et al. Fibrosarcoma versus cellular fibroma of the ovary: A comparative study of their proliferative activity and chromosome aberrations using MIB-1 immunostaining, DNA flow cytometry, and fluorescence in situ hybridization. Am J Surg Pathol. 1997; 21:52–59.
55. Chalvardjian, A, Scully, RE. Sclerosing stromal tumors of the ovary. Cancer. 1973; 31:664–670.
56. Zekioglu, O, Ozdemir, N, Terek, C, et al. Clinicopathological and immunohistochemical analysis of sclerosing stromal tumours of the ovary. Arch Gynecol Obstet. 2010; 282:671–676.
57. Cashell, AW, Cohen, ML. Masculinizing sclerosing stromal tumor of the ovary during pregnancy. Gynecol Oncol. 1991; 43:281–285.
58. Tiltman, AJ, Haffajee, Z. Sclerosing stromal tumors, thecomas, and fibromas of the ovary: an immunohistochemical profile. Int J Gynecol Pathol. 1999; 18:254–258.
59. Kawauchi, S, Tsuji, T, Kaku, T, et al. Sclerosing stromal tumor of the ovary: a clinicopathologic, immunohistochemical, ultrastructural, and cytogenetic analysis with special reference to its vasculature. Am J Surg Pathol. 1998; 22:83–92.
60. Moulla, A, Giakoustidis, D, Leontsini, M. Sclerosing stromal tumors of the ovary: a clinicopathologic, immunohistochemical and cytogenetic analysis of three cases. Eur J Gynaecol Oncol. 2004; 25:257–260.
61. Ramzy, I. Signet-ring stromal tumor of ovary. Histochemical, light, and electron microscopic study. Cancer. 1976; 38:166–172.
62. Suárez, A, Palacios, J, Burgos, E, Gamallo, C. Signet-ring stromal tumor of the ovary: a histochemical, immunohistochemical and ultrastructural study. Virchows Arch A. 1993; 422:333–336.
63. Dickersin, GR, Young, RH, Scully, RE. Signet-ring stromal and related tumors of the ovary. Ultrastructural Pathol. 1995; 19:401–419.
64. Vang, R, Bagué, S, Tavassoli, FA, Prat, J. Signet ring stromal tumor of the ovary: clinicopathologic analysis with comparison to Krukenberg tumor. Int J Gynecol Pathol. 2003; 23:45–51.
65. Irving, JA, Young, RH. Microcystic stromal tumor of the ovary: report of 16 cases of a hitherto uncharacterized distinctive ovarian neoplasm. Am J Surg Pathol. 2009; 33:367–375.
66. Maeda, D, Shibahara, J, Sakuma, T, et al. β-catenin (CTNNB1) S33C mutation in ovarian microcystic stromal tumors. Am J Surg Pathol. 2011; 35:1429–1440.
67. Tavassoli, FA, Norris, HJ. Sertoli tumors of the ovary. A clinicopathologic study of 28 cases with ultrastructural observations. Cancer. 1980; 46:2281–2297.
68. Young, RH, Scully, RE. Ovarian Sertoli cell tumors. A report of ten cases. Int J Gynecol Pathol. 1984; 2:349–363.
69. Oliva, EA, Alvarez, T, Young, RH. Sertoli cell tumors of the ovary. A clinicopathological and immunohistochemical study of 54 cases. Am J Surg Pathol. 2005; 29:143–156.
70. Ferry, J, Young, RE, Engel, G, Scully, RE. Oxyphil Sertoli cell tumor of the ovary: a report of three cases, two in patients with the Peutz-Jeghers syndrome. Int J Gynecol Pathol. 1994; 13:259–266.
71. Tracy, SL, Askin, FB, Reddick, RL, et al. Progesterone secreting Sertoli cell tumor of the ovary. Gynecol Oncol. 1985; 22:85–96.
72. Zhao, C, Bratthauer, GL, Barner, R, Vang, R. Diagnostic Utility of WT1 immunostaining in ovarian Sertoli cell tumor. Am J Surg Pathol. 2007; 31:1378–1386.
73. Zhao, C, Barner, R, Vinh, TN, et al. SF-1 is a diagnostically useful immunohistochemical marker and comparable to other sex cord-stromal tumor markers for the differential diagnosis of ovarian Sertoli cell tumor. Int J Gynecol Pathol. 2008; 27:507–514.
74. Young, RH, Prat, J, Scully, RE. Ovarian endometrioid carcinomas resembling sex cord-stromal tumors. A clinicopathologic analysis of 13 cases. Am J Surg Pathol. 1982; 6:513–522.
75. Ordi, J, Schammel, DP, Rasekh, L, Tavassoli, FA. Sertoliform endometrioid carcinomas of the ovary: a clinicopathologic and immunohistochemical study of 13 cases. Mod Pathol. 1999; 12:933–940.
76. Roth, LM, Anderson, MC, Govan, AD, et al. Sertoli-Leydig cell tumors. A clinicopathologic study of 34 cases. Cancer. 1981; 48:187–197.
77. Zaloudek, C, Norris, HJ. Sertoli-Leydig tumors of the ovary. A clinico-pathologic study of 64 intermediate and poorly differentiated neoplasms. Am J Surg Pathol. 1984; 8:405–418.
78. Young, RH, Scully, RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985; 9:543–569.
79. Gui, T, Cao, D, Shen, K, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol. 2012; 127:384–389.
80. Rio Frio, T, Bahubeshi, A, Kanellopoulou, C, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011; 305:68–77.
81. Gagnon, S, Tetu, B, Silva, EG, McCaughey, WTE. Frequency of alpha-fetoprotein production by Sertoli-Leydig cell tumors of the ovary: an immunohistochemical study of eight cases. Mod Pathol. 1989; 2:63–67.
82. Prat, J, Young, RH, Scully, RE. Ovarian Sertoli-Leydig cell tumors with heterologous elements. II. Cartilage and skeletal muscle: A clinicopathologic analysis of twelve cases. Cancer. 1982; 50:2465–2475.
83. Young, RH, Scully, RE. Ovarian Sertoli-Leydig cell tumors with retiform pattern: a problem in histopathologic diagnosis. Am J Surg Pathol. 1983; 77:755–771.
84. Roth, LM, Slayton, RE, Brady, LW, et al. Retiform differentiation in ovarian Sertoli–Leydig cell tumors. A clinicopathologic study of six cases from a gynecologic oncology group study. Cancer. 1985; 55:1093–1098.
85. Mooney, EE, Nogales, FF, Bergeron, C, Tavassoli, FA. Retiform Sertoli-Leydig cell tumors: clinical, morphological and immunohistochemical findings. Histopathology. 2002; 41:110–117.
86. Young, RH, Prat, J, Scully, RE. Ovarian Sertoli–Leydig cell tumors with heterologous elements. I. Gastrointestinal epithelium and carcinoid: a clinicopathologic analysis of thirty-six cases. Cancer. 1982; 50:2448–2456.
87. Young, RH, Scully, RE. Well-differentiated ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 23 cases. Int J Gynecol Pathol. 1984; 3:277–290.
88. Young, RH, Pérez-Atayde, AR, Scully, RE. Ovarian Sertoli-Leydig cell tumor with retiform and heterologous components. Report of a case with hepatocytic differentiation and elevated serum alpha-fetoprotein. Am J Surg Pathol. 1984; 8:709–718.
89. Mooney, EE, Nogales, FF, Tavassoli, FA. Hepatocytic differentiation in retiform Sertoli-Leydig cell tumors: distinguishing a heterologous element from Leydig cells. Hum Pathol. 1999; 30:611–617.
90. Heravi-Moussavi, A, Anglesio, MS, Cheng, SW, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012; 366:234–242.
91. Schultz, KA, Pacheco, MC, Yang, J, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1mutations: a report from the International Pleuropulmonary Blastoma Registry. Gynecol Oncol. 2011; 122:246–250.
92. Scully, RE. Sex cord tumor with annular tubules. A distinctive ovarian tumor of the Peutz-Jeghers syndrome. Cancer. 1970; 25:1107–1121.
93. Young, RH, Welch, WR, Dickersin, GR, Scully, RE. Ovarian sex cord tumor with annular tubules: review of 74 cases including 27 with Peutz–Jeghers syndrome and 4 with adenoma malignum of the cervix. Cancer. 1982; 50:1384–1402.
94. Connolly, DC, Katabuchi, H, Cliby, WA, Cho, KR. Somatic mutations in the STK11/LKB1 gene are uncommon in rare gynecological tumor types associated with Peutz-Jegher’s syndrome. Am J Pathol. 2000; 156:339–345.
95. Kato, N, Romero, M, Catasus, L, Prat, J. STK11/LKB1 Peutz-Jegher’s gene is not involved in the pathogenesis of sporadic sex cord-stromal tumors although LOH at 19p13.3 indicates another gene alterations in these tumors. Hum Pathol. 2004; 35:1101–1104.
96. Paraskevas, M, Scully, RE. Hilus cell tumor of the ovary. A clinico-pathological analysis of 12 Reinke crystal-positive and 9 crystal-negative cases. Int J Gynecol Pathol. 1989; 8:299–310.
97. Scully, RE. Stromal luteoma of the ovary. A distinctive type of lipoid-cell tumor. Cancer. 1964; 17:769–778.
98. Hayes, MC, Scully, RE. Stromal luteoma of the ovary: a clinicopathologic analysis of 25 cases. Am J Surg Pathol. 1987; 6:313–321.
99. Chico, A, Garcia, JL, Matias-Guiu, X, et al. A gonadotropin dependent stromal luteoma: a rare cause of post-menopausal virilization. Clin Endocrinol. 1995; 43:645–649.
100. Liu, G, Duranteau, L, Carel, JC, et al. Leydig-cell tumor caused by an activating mutation of the gene encoding the luteinizing hormone receptor. N Engl J Med. 1999; 341:1731–1736.
101. Hayes, MC, Scully, RE. Ovarian steroid cell tumor (not otherwise specified): a clinicopathological analysis of 63 cases. Am J Surg Pathol. 1987; 11:835–845.
102. Young, RH, Scully, RE. Ovarian steroid cell tumors associated with Cushing’s syndrome. A report of three cases. Int J Gynecol Pathol. 1987; 6:40–48.
103. Jones, MW, Harri, R, Dabbs, DJ, Carter, GJ. Immunohistochemical profile of steroid cell tumor of the ovary: a study of 14 cases and a review of the literature. Int J Gynecol Pathol.. 2010; 29:315–320.
104. Rubin, DK, Frost, JK. The cytologic detection of ovarian cancer. Acta Cytol. 1963; 7:191–195.
105. Rome, RM, Laverty, CR, Brown, JB. Ovarian tumours in postmenopausal women. Clinicopathological features and hormonal studies. J Obstet Gynecol Br Commonw. 1973; 80:984–991.
106. Morris, JM, Scully, RE. Tumors with ‘functioning’ stroma. In: Endocrine pathology of the ovary. St. Louis, MO: Mosby; 1958:131.
107. Matias-Guiu, X, Prat, J. Ovarian tumors with functioning stroma. An immunohistochemical study of 100 cases with human chorionic gonadotropin monoclonal and polyclonal antibodies. Cancer. 1990; 65:2001–2005.
108. Rome, RM, Fortune, DW, Quinn, MA, Brown, JB. Functioning ovarian tumors in postmenopausal women. Obstet Gynecol. 1981; 57:705–710.
109. Scully, RE, Ovarian tumors with functioning stroma. Haines and Taylor obstetrical and gynaecological pathology. 4th ed Fox, H, eds. Haines and Taylor obstetrical and gynaecological pathology; vol. 2. Churchill Livingstone, Edinburgh, 1995.
110. Rutgers, JL, Scully, RE. Functioning ovarian tumors with peripheral steroid cell proliferation: a report of twenty-four cases. Int J Gynecol Pathol. 1986; 5:319–337.
111. Zaloudek, CJ, Tavassoli, FA, Norris, HJ. Dysgerminoma with syncytiotrophoblastic giant cells. A histologically and clinically distinctive subtype of dysgerminoma. Am J Surg Pathol. 1981; 5:361–367.
112. Dickersin, GR, Kline, IW, Scully, RE. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer. 1982; 49:188–197.
113. Aguirre, P, Thor, AD, Scully, RE. Ovarian small cell carcinoma. Histogenetic considerations based on immunohistochemical and other findings. Am J Clin Pathol. 1989; 92:140–149.
114. Matias-Guiu, X, Prat, J, Young, RH, et al. Human parathyroid hormone-related protein in ovarian small cell carcinoma. Cancer. 1994; 73:1878–1881.
115. Robboy, SJ, Scully, RE. Strumal carcinoid of the ovary: an analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer. 1980; 46:2019–2034.
116. Robboy, SJ, Norris, HJ, Scully, RE. Insular carcinoid primary in the ovary: a clinicopathologic analysis of 48 cases. Cancer. 1975; 36:404–418.
117. Aguirre, P, Scully, RE, Dayal, Y, DeLellis, RA. Mucinous tumors of the ovary with argyrophil cells. An immunohistochemical analysis. Am J Surg Pathol. 1984; 8:345–356.
118. Boixeda, D, Roman, AL, Pascasio, JM, et al. Zollinger–Ellison syndrome due to gastrin-secreting ovarian cystadenocarcinoma. Case report. Acta Chir Scand. 1990; 156:409–410.
119. Kallenberg, GA, Pesce, CM, Norman, B, et al. Ectopic hyperprolactinemia resulting from an ovarian teratoma. JAMA. 1990; 263:2472–2474.
120. Palmer, PE, Bogojavlensky, S, Bhan, AK, Scully, RE. Prolactinoma in wall of ovarian dermoid cyst with hyperprolactinemia. Obstet Gynecol. 1990; 75:540–543.
121. Ashton, MA. Strumal carcinoid of the ovary associated with hyperinsulinemic hypoglycemia and cutaneous melanosis. Histopathology. 1995; 27:463–467.
122. Anderson, PW, d’Ablaing, G, Penny, R, et al. Secretion of prorenin by a virilizing ovarian tumor. Gynecol Oncol. 1992; 45:58–61.
123. Fox, R, Eckford, S, Hirschowitz, L, et al. Refractory gestational hypertension due to a renin-seceting ovarian fibrothecoma associated with Gorlin’s syndrome. Br J Obstet Gynaecol. 1994; 101:1015–1017.