31 Outcome of Fetal Congenital Heart Defects
I. INTRODUCTION
A. Rationale
1. The goal of prenatal diagnosis is the near-term delivery of a nonhydropic infant whose postnatal management is anticipated.
2. Fetuses with structural cardiac defects are at increased risk for heart failure, premature delivery, and fetal demise.
3. One quarter of all infant deaths are due to congenital anomalies, and one third of these are cardiac defects.
4. In utero interventions for structural defects are not without risk, and data suggesting that intervention substantially alters the natural history are limited. In this chapter we review the outcome of fetuses presenting with structural cardiac defects.
B. Prenatal examination
1. It is estimated that 80% of all pregnant women have at least one ultrasound during pregnancy.
2. Both the International Society of Ultrasound in Obstetrics and Gynecology and the American Institute of Ultrasound in Medicine recommend that assessment of the fetal heart in the structural survey include both the four-chamber and outflow-tract views.
a. The four-chamber view alone can detect at best 60% of congenital cardiac defects.
b. Incorporating views of the left and right outflow tracts can further increase the detection of cardiac defects to 85% to 90%, including ductus-dependent lesions.
c. Secular trends show an increasing number of infants presenting for surgery with in utero diagnoses.
II. OVERALL OUTCOME OF FETAL CONGENITAL HEART DEFECTS
A. Historical outcomes
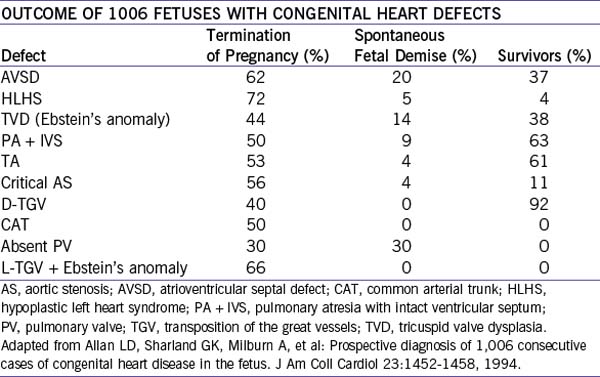
1. In Allen and colleagues’ (2005) series, the overall outcome of 1006 fetuses with diagnosed congenital heart defects (1980-1993) was fairly bleak, with a high percentage of interrupted pregnancies.
2. About 17% had associated chromosomal anomalies.
3. Survivors of continuing pregnancies ranged from 92% with D-transposition of the great vessels to 0% with common arterial trunk, tetralogy of Fallot with absent pulmonary valve, or L-transposition of the great vessels with Ebstein’s malformation of the tricuspid valve (Table 31-1).
B. Current outcomes
1. Outcomes of the fetus with prenatally diagnosed congenital heart disease have improved somewhat in more recent studies, with overall survival ranging from 45% to 60% in 93 to 408 cases.
2. Pregnancy interruptions decreased to 23% to 35% in these newer series.
3. Very few series have shown improved survival in infants with heart defects diagnosed before birth.
III. OUTCOME OF SPECIFIC HEART DEFECTS
A. Heterotaxy syndromes
1. The heterotaxy syndromes include right and left atrial isomerisms (RAI and LAI).
2. In general, mortality of RAI and LAI is high due to the complexity of the structural defects in both and to the high percentage of patients with complete atrioventricular block (AVB) in LAI.
a. Long-term survival with RAI is 13% to 40%.
b. Long-term survival for LAI is 14% to 52%.
4. Nonsurvival correlates with atrioventricular block and hydrops.
a. Many (33%-45%) of these pregnancies are interrupted or have intrauterine demise.
b. Prenatal diagnosis might confer no survival advantage for either LAI or RAI.
B. Common arterial trunk
1. There have been two small series, one of 17 and the other of 23 fetuses.
b. Overall survival was 35% or 42%. Integrity of the truncal valve influences long-term survival.
2. It is unknown if other factors frequent in common arterial trunk, such as the 22q11 deletion, branch pulmonary artery stenosis, and interrupted aortic arch, affect either prenatal or postnatal outcomes.
C. Tricuspid dysplasia (Ebstein’s anomaly)
1. Tricuspid valve abnormalities in the fetus range from mild displacement of the septal leaflet with mild insufficiency to severe displacement, absent valve coaptation with severe insufficiency, atrialization of the right ventricle, and functional pulmonary atresia.
2. Because of the truly massive proportions assumed by the RV, severe forms of tricuspid insufficiency that manifest early can restrict growth of functional lung tissue, further decreasing the likelihood of postnatal survival.
3. Even with anticipatory care and more novel approaches to postnatal management, some affected fetuses are at very high risk for intrauterine or neonatal demise.
4. However, there have been improvements in outcome in more recent years, with better understanding of the pathophysiology. In a study by Wald and colleagues (2005), 93% of infants with Ebstein’s anomaly, including 10 with a fetal diagnosis, survived the neonatal period largely as a result of limiting ductal patency.
D. Hypoplastic left heart syndrome
1. Infants with a prenatal diagnosis of hypoplastic left heart syndrome (HLHS) in general have less preoperative acidosis than those with postnatal diagnoses. Based on one study, they might have decreased mortality at the time of the bidirectional Glenn procedure.
2. Prenatal diagnosis can improve the outcome of fetuses with HLHS at highest risk.
a. It can identify those with restrictive foramen ovale who will need urgent postnatal balloon atrial septostomy.
b. It can identify fetuses who could benefit from in utero balloon interventions: either atrial septostomy for restrictive foramen ovale, or aortic valvuloplasty for critical aortic stenosis, which may progress to postnatal HLHS.
3. The influences of these factors on outcome of HLHS have not been evaluated.
E. Transposition of the great vessels
1. A patent ductus arteriosus and unrestricted patent foramen ovale are essential for immediate neonatal survival before surgical correction of D-transposition of the great vessels (TGV). Surgical correction at the best centers has a mortality rate of less than 5%.
2. If the foramen ovale is recognized to be restrictive, the cardiology team can be prepared for a lifesaving immediate postnatal balloon septostomy.
3. In one series, a restrictive foramen or ductus arteriosus was found in 20% of fetal D-TGV. Half of those required immediate balloon septostomy. Despite this resuscitation, 15% died.
4. Other series found significant differences between cases diagnosed before and after birth.
a. First-week mortality was 0% and 15.4%, respectively.
b. Preoperative and postoperative mortality was 6% and 8.5%, respectively, with a postnatal diagnosis, compared to 0% with a prenatal diagnosis.
F. Coarctation of the aorta
1. A retrospective review of infants with normal karyotype with coarctation of the aorta diagnosed between 1994 and 1998 showed a higher likelihood of cardiovascular collapse or death in infants with postnatal diagnoses.
2. In this group, 30% died before surgery could be performed.
3. Conversely, both preoperative and postoperative mortality was 0% in the infants with prenatal diagnoses by fetal echocardiography.
IV. DOES PRENATAL DIAGNOSIS IMPROVE THE OUTCOME?
A. Defining improved outcome
1. Intuitively, as prenatal recognition of cardiac defects has increased and postnatal care, including stabilizing infants with ductus-dependent lesions, is anticipated, the outcome of these infants is expected to improve. But as we have seen, this has not been shown definitively to be true.
2. One explanation is that there is no universal agreement on the definition of “improved outcome.”
a. Does this mean that there are fewer prenatal deaths of fetuses at risk for heart failure or with isolated cardiac defects?
b. Does it mean there are fewer births of infants with more complex cardiac defects or associated noncardiac or chromosomal abnormalities?
c. Should it simply mean a greater number of infants surviving surgery with normal development?
B. Results of therapy
1. In fetuses that have nonstructural cardiac defects and are at risk for heart failure, prenatal detection clearly improves in utero survival by appropriate pharmacologic therapy and an optimal delivery time. Examples include the fetus with sustained tachycardia, heart block, or ductal constriction, or monochorionic twins with twin-to-twin transfusion syndrome.
2. With the increasing technical success of fetal intervention, prenatal diagnosis recognizes an important subset of fetuses with milder defects that can progress into more significant problems by the time of birth.
a. Anticipatory management of the fetus with structural cardiac defects needing immediate intervention by delivery in a cardiac center of excellence can improve immediate postnatal outcome.
b. Such a plan also eliminates the need for neonatal transfer and separation of mother and baby.
a. In a fetus with critical aortic stenosis and a small LV, in utero valvuloplasty at 20 weeks can improve LV growth and a biventricular repair.
b. Although there is less experience with right-sided defects, early results suggest that prenatal valvuloplasty of the pulmonary valve can encourage growth of the RV, resulting in a biventricular or ventricle- and-a-half repair after birth.
a. Balloon septostomy of a restrictive foramen ovale recognized by fetal echocardiography has been performed successfully (at least technically) in the fetus with HLHS.
b. Antenatal recognition of HLHS allows the interventional team to perform immediate septostomy in the newborn after delivery in the catheterization suite.
6. Multidisciplinary management.
a. Which procedure is the most beneficial—balloon valvuloplasty, cardiac transplantation, or cardiac surgery, with all inherent risks and benefits—can be fully explored by the multidisciplinary team caring for the mother, fetus, and infant.
V. TAKE-HOME MESSAGE
A. Diagnosis
1. The four-chamber view alone can detect at best 60% of congenital cardiac defects.
2. Prenatal recognition of cardiac defects has increased. However, it is still not clear whether prenatal diagnosis significantly improves the clinical outcome of many heart defects, particularly structural pathology. One possible explanation is that there is no universal agreement on the definition of “improved outcome.”
3. Successful fetal intervention opens new frontiers in the value of prenatal diagnosis of fetuses with more mild defects that can progress into more significant problems by the time of birth.
Allan LD, Sharland GK, Milburn A, et al. Prospective diagnosis of 1,006 consecutive cases of congenital heart disease in the fetus. J Am Coll Cardiol. 1994;23:1452-1458.
Allen RH, Benson CB, Haug LW. Pregnancy outcome of fetuses with a diagnosis of hypoplastic left ventricle on prenatal sonography. J Ultrasound Med. 2005;24:1199-1203.
American Institute of Ultrasound in Medicine. Performance of the basic fetal cardiac ultrasound examination. J Ultrasound Med. 1998;117:601-607.
Achiron R, Glaser J, Gelernter I, et al. Extended fetal echocardiographic examination for detecting cardiac malformations in low risk pregnancies. BMJ. 1992;304:671-674.
Bartlett JM, Wypij D, Bellinger DC, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics. 2004;113:335-340.
Berg C, Geipel A, Kamil D, et al. The syndrome of left isomerism: Sonographic findings and outcome in prenatally diagnosed cases. J Ultrasound Med. 2005;24(7):921-931.
Berg C, Geipel A, Smrcek J, et al. Prenatal diagnosis of cardiosplenic syndromes: A 10-year experience. Ultrasound Obstet Gynecol. 2003;22(5):451-459.
Boldt T, Andersson S, Eronen M. Outcome of structural heart disease diagnosed in utero. Scand Cardiovasc J. 2002;36(2):73-79.
Brick DH, Allan LD. Outcome of prenatally diagnosed congenital heart disease: An update. Pediatr Cardiol. 2002;23(4):449-453.
Cedergren MI, Kallen BA. Obstetric outcome of 6346 pregnancies with infants affected by congenital heart defects. Eur J Obstet Gynecol Reprod Biol. 2006;125(2):211-216.
Donofrio MT, Bremer YA, Moskowitz WB. Diagnosis and management of restricted or closed foramen ovale in fetuses with congenital heart disease. Am J Cardiol. 2004;94(10):1348-1351.
Fountain-Dommer RR, Bradley SM, Atz AM, et al. Outcome following, and impact of, prenatal identification of the candidates for the Norwood procedure. Cardiol Young. 2004;14(1):32-38.
Franklin O, Burch M, Manning N, et al. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002;87(1):67-69.
Jouannic JM, Gavard L, Fermont L, et al. Sensitivity and specificity of prenatal features of physiological shunts to predict neonatal clinical status in transposition of the great arteries. Circulation. 2004;110(13):1743-1746.
Khoshnood B, De Vigan C, Vodovar V, et al. Trends in prenatal diagnosis, termination and perinatal mortality of newborns with congenital heart disease in France 1983-2000: A population-based evaluation. Pediatrics. 2005;115:95-101.
Lim JS, McCrindle BW, Smallhorn JE, et al. Clinical features, management, and outcome of children with fetal and postnatal diagnoses of isomerism syndromes. Circulation. 2005;112(16):2454-2461.
Mohan UR, Kleinman CS, Kern JH. Fetal echocardiography and its evolving impact 1992-2002. Am J Cardiol. 2005;96:134-136.
Perolo A, Prandstraller D, Ghi T, et al. Diagnosis and management of fetal cardiac anomalies: 10 years of experience at a single institution. Ultrasound Obstet Gynecol. 2001;18(6):615-618.
Sullivan ID. Prenatal diagnosis of structural heart disease: Does it make a difference to survival? Heart. 2002;87(5):405-406.
Tegnander E, Eik-Nes SH, Johansen OJ, et al. Prenatal detection of heart defects at the routine fetal examination at 18 weeks in a non-selected population. Ultrasound Obstet Gynecol. 1995;5:372-380.
Tulzer G, Arzt W, Franklin RC, et al. Fetal pulmonary valvuloplasty for critical pulmonary stenosis or atresia with intact septum. Lancet. 2002;360(9345):1567-1568.
Tworetzky W, McElhinney DB, Reddy VM, et al. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103(9):1269-1273.
Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: Potential for prevention of hypoplastic left heart syndrome: Candidate selection, technique, and results of successful intervention. Circulation. 2004;110(15):2125-2131.
Verheijen PM, Lisowski LA, Stoutenbeck P, et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg. 2001;121(4):798-803.
Volpe P, Paladini D, Marasini M, et al. Common arterial trunk in the fetus: Characteristics, associations, and outcome in a multicentre series of 23 cases. Heart. 2003;89(12):1437-1441.
Wald RM, Adatia I, Van Arsdell GS, Hornberger LK. Relation of limiting ductal patency to survival in neonatal Ebstein’s anomaly. Am J Cardiol. 2005;96(6):851-856.
Wigton TR, Sabbagha RE, Tamura RK, et al. Sonographic diagnosis of congenital heart disease: Comparison between the four-chamber view and multiple cardiac views. Obstet Gynecol. 1993;82:219-224.
Yates RS. The influence of prenatal diagnosis on postnatal outcome in patients with structural congenital heart disease. Prenat Diagn. 2004;24:1143-1149.