74 Other Neuromuscular Transmission Disorders
Lambert–Eaton Myasthenic Syndrome
Etiology and Pathophysiology
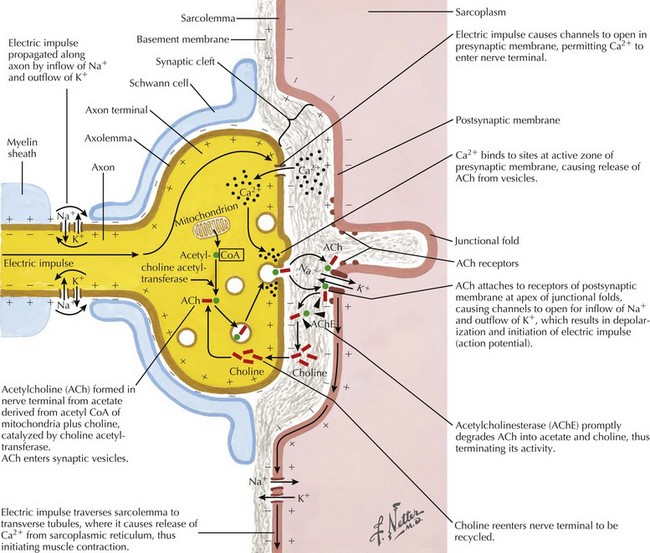
Under normal conditions, neuronal depolarization opens VGCCs on the presynaptic axon terminal membrane, resulting in calcium influx within the nerve terminal. Intracellular calcium binds to calmodulin and mobilizes acetylcholine (ACh) vesicles that are released into the synaptic cleft (Fig. 74-1). The VGCCs are the primary site of immunopathology in LEMS. Divalent IgG autoantibodies cross-link the calcium channels, disrupting their function, resulting in inadequate release of presynaptic ACh vesicles from motor and autonomic cholinergic nerve terminals. The release of fewer ACh quanta at the neuromuscular junction decreases the probability of reaching the “all or none” depolarization threshold of a muscle fiber and thus the likelihood of the muscle fiber action potential (MFAP) generation. It is this drop out of a significant number of MFAP generation that results in low CMAPs on routine NCS.
Clinical Presentation
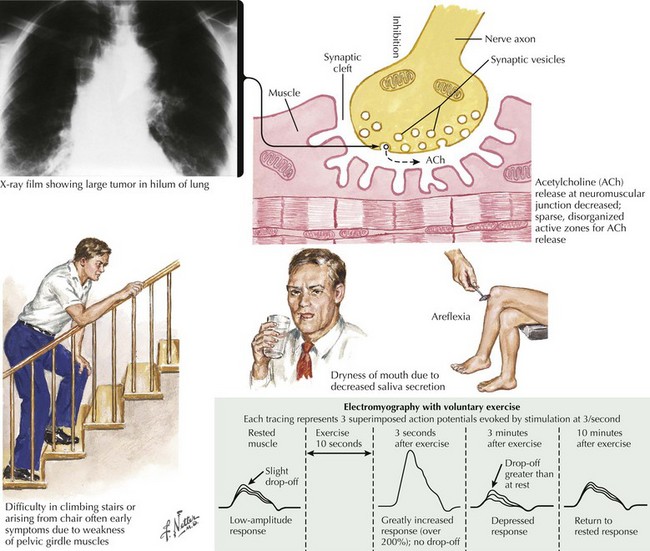
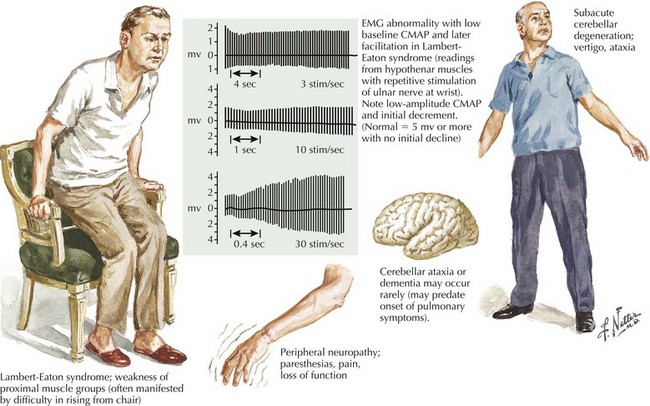
The clinical presentation is similar in primary autoimmune and secondary paraneoplastic LEMS. Fatigue is a prominent and early symptom in LEMS patients. Patients with LEMS also complain of proximal weakness, especially lower extremity weakness, sometimes mimicking a myopathy. The complaints of fatigue and proximal weakness often may seem disproportionate to the objective examination findings. When patients with suspected LEMS do not have objective detectable weakness, it may be better appreciated by watching individuals rise from a chair or climb stairs (Fig. 74-2). Muscle stretch reflexes are typically reduced or absent and this finding can be an important clue to the diagnosis. Postexercise potentiation of depressed or absent muscle stretch reflexes and of muscle strength is often demonstrable and signifies a presynaptic neuromuscular junction transmission disorder.
Diagnostic Approach
Appropriate NCS testing is crucial in the diagnosis of LEMS (Fig. 74-3). Most patients with LEMS have low-amplitude CMAPs on routine nerve conduction studies. The identification of low baseline CMAP amplitudes should prompt postexercise facilitation testing. This is performed by having the patient isometrically contract the muscle from which the CMAP is being recorded (e.g., abductor digiti quinti minimi) for 10 seconds followed by nerve stimulation and measurement of the CMAP. In patients without LEMS (or another presynaptic neuromuscular junction transmission disorder), there will be no change or only modest increase (<100%) in the size of the CMAP amplitude. The differentiation between a presynaptic and a postsynaptic neuromuscular junction transmission disorder is that brief (10–15 seconds) voluntary exercise, or rarely, if necessary, high-frequency (20–50 Hz) repetitive motor nerve stimulation, leads to marked (>100%) facilitation of the baseline CMAP in patients with LEMS (presynaptic) but not those with myasthenia gravis (MG) (postsynaptic). In addition to testing for postexercise facilitation, slow (e.g., 2- or 3-Hz) repetitive motor nerve stimulation demonstrating decrement of CMAP amplitude of more than 10% can further support the diagnosis of a neuromuscular transmission disorder. Many electromyography (EMG) laboratories routinely combine the slow repetitive motor nerve testing with the 10-second exercise testing in order to search simultaneously for both the postexercise facilitation (of the first CMAP following brief exercise) and the decrement (of the subsequent CMAPs during slow repetitive nerve studies) seen in postsynaptic disorders.
Botulism
Burns TM, Russell JA, Lachance DH, Jones HR. Oculobulbar involvement is typical with Lambert-Eaton Myasthenic Syndrome. Ann Neurol. 2003;53:270-273.
Gutmann L, Phillips LH, Gutmann L. Trends in the association of Lambert-Eaton myasthenic syndrome with carcinoma. Neurology. 1992;42:848-850.
Lambert EH, Eaton LM, Rooke ED. Defect of neuromuscular conduction associated with malignant neoplasms. Am J Physiol. 1956;187:612-613.
Lambert EH, Rooke ED. Myasthenic state and lung cancer. In: Brain WR, Norris FH, editors. The Remote Effects of Cancer on the Nervous System. New York, NY: Grune and Stratton; 1965:67-80.
Lennon VA, Kryzer TJ, Griesmann GE, et al. Calcium-channel antibodies in the Lambert-Eaton syndrome and other paraneoplastic syndromes. N Engl J Med. 1995;332:1467-1474.
McEvoy KM, Windebank AJ, Daube JR, Low PA. 3,4-Diaminopyridine in the treatment of Lambert-Eaton myasthenic syndrome. N Engl J Med. 1989;321:1567-1571.
O’Neil JH, Murray NMF, Newsom-Davis J. The Lambert Eaton myasthenic syndrome: a review of 50 cases. Brain. 1988;111:577-596.
Sabater L, Titulaer M, Saiz A, Verschuuren J, Güre AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert-Eaton myasthenic syndrome. Neurology. 2008;70:924-928.
Sanders DB. Lambert-Eaton myasthenic syndrome: clinical diagnosis, immune-mediated mechanisms, and update on therapies. Ann Neurol. 1995;37(suppl 1):S63-S73.
Sobel J. Botulism. Clinical Infectious Diseases. 2005;41:1167-1173.
Tim RW, Sanders DB. Repetitive nerve stimulation studies in the Lambert-Eaton myasthenic syndrome. Muscle Nerve. 1994;17:995-1001.
Tseng-Ong L, Mitchell WG. Infant botulism: 20 years’ experience at a single institution. J Child Neurol. 2007;22:1333-1337.