Chapter 88 Ossification of the Posterior Longitudinal Ligament
Ossification of the posterior longitudinal ligament (OPLL), most typically found in males (2:1) in their mid 50s, contributes to approximately 25% of cervical myelopathy in the North American population and higher percentages in the Asian population.1,2 Originating as early hypertrophy of the PPL with accompanying punctate ossification centers (early OPLL), these foci coalesce and become loci of frank ossification in the PPL.1,2 Early OPLL appears in patients in their mid 40s, whereas classic, more ossified OPLL is found in people in their mid 50s and later. OPLL may be managed with either anterior surgery (single/multilevel corpectomies with fusion, with or without combined posterior instrumentation), or posterior surgery (laminectomy with or without fusion or laminoplasty) (Figs. 88-1 to 88-6).
Prevalence of Ossification of the Posterior Longitudinal Ligament
In asymptomatic North Americans, the frequency of OPLL is reportedly 0.12% on plain radiographs, and in the Japanese, it is 2.2%. In Koreans, 0.6% of 11,774 adults demonstrated OPLL involving the C3, C4, and C5 on plain radiographs; 32% had continuous OPLL, 31% segmental OPLL, 31% mixed OPLL, and 5.6% focal OPLL.3 In the presence of myelopathy, OPLL is evident, using CT and MRI studies in up to 25% of North Americans and in at least 27% of Japanese.4–6 Seventy percent of cervical OPLLs involve 2.7 to 4 levels, progressing in a caudal-rostral fashion, and the remaining 30% are evenly divided between the proximal thoracic (T1-4) and the proximal lumbar (L1-3) regions.
Genetics of Ossification of the Posterior Longitudinal Ligament
Human Leukocyte Antigen: Probable Site on Genome
Multiple studies increasingly document a genetic correlation among OPLL, diffuse idiopathic skeletal hyperostosis (DISH), and ossification of other ligaments—ossification of the yellow ligament (OYL) and ossification of the anterior longitudinal ligament (OALL).7,8 In evaluation of 91 sibling pairs of patients with OPLL in 53 Japanese families, the genetic locus for OPLL was found near the human leukocyte antigen (HLA) site on chromosome 6-p. In patients with DISH, 50% have concurrent OPLL and test positive for HLA.9 In the Chinese population, another susceptibility gene, COL6A1, appears to represent a “common susceptibility gene” for both OYL and OPLL.10
Autosomal Dominant Inheritance
An autosomal dominant mode of inheritance is supported by genetic and epidemiologic data from patients with OPLL. OPLL is found in 26.15% of parents and 28.89% of siblings of patients with OPLL, a finding that supports a likely autosomal dominant transmission.11 A 53% expression rate of OPLL is observed in patients with two concurrent HLA strands compared with 24% with one strand, which further supports this hypothesis.8 Autosomal recessive transmission has been suggested when 56% of a patient’s siblings with both HLA haplotypes were symptomatic with OPLL, whereas those with only one HLA haplotype were not.12 Two additional unique genetic factors were identified in 18 patients with OPLL compared with 51 age-matched controls: BamHI 10.0/10.0 kb and HindIII 19.0/19.0 kb genotypes.7,13
Other Contributing Factors (Hormones and Proteins)
Genetically modulated hormones and proteins also appear to contribute to the expression of OPLL. Increased concentrations of growth hormone receptors and activins have been shown to enhance OPLL expression/progression.14,15 Elevated concentrations of bone morphogenetic proteins have resulted in increased osteogenesis in originally nonossified ligaments of OPLL patients (347 families, 1030 relatives).15 In a more recent study, bone morphogenetic protein 2 (BMP-2) additionally positively correlated with the extent of OPLL progression.16 Insulin was also correlated with OPLL onset/progression in people with non–type 1 diabetes via direct and indirect stimulation of BMP-2 within the ligament.17 Fibronectin, a glycoprotein that plays a role in the development of bony tissues, was also found to be significantly elevated in patients with OPLL or OYL.18
Anatomy of Ossification of the Posterior Longitudinal Ligament
In Vitro Characteristics of Cultured Posterior Longitudinal Ligament in Patients with Ossification of the Posterior Longitudinal Ligament
In patients with OPLL, the PPL is osteogenic.7,8,13–15 Immunohistochemical evaluation of PPL cells for patients undergoing anterior cervical decompression for cervical disease revealed “up-regulation of proliferating cell nuclear antigen” in patients with OPLL.19 This finding of increased osteogenicity of the PPL was also confirmed in myelopathic patients with OPLL in North America.20 Collecting supernatants of PPL obtained intraoperatively from patients with OPLL compared with non-OPLL control patients (with spondylosis) revealed increased osteocalcin synthesis in the OPLL patients. The quantity of osteocalcin induced was determined by incubating these PPL cells with 1.25(OH)2 and vitamin D3 for 72 hours in serum-free medium.21 Those with OPLL grew to confluence, whereas those with spondylosis alone did not respond to vitamin D3 priming.
Classification of Ossification of the Posterior Longitudinal Ligament
Early Disease
OPLL represents a continuum of maturation that starts with hypertrophy of the PPL and ends with frank ossification.1,4,6,22 Early OPLL usually originates opposite multiple interspaces in patients in their mid 40s and is often misdiagnosed as multiple disc herniations. However, unlike disc herniations, OPLL begins with retrovertebral extension that can be seen on enhanced MRI studies performed with gadolinium (Gd)-DTPA and as punctate CT-documented ossification. Too often, patients undergo multilevel anterior discectomy and fusion procedures where the PPL is ignored, leading to many retained disc fragments, and in early OPLL, leaving patients with continued symptomatology.
Classic Disease
There are four classic types of mature/classic OPLL23 (Figs. 88-7 and 88-8). The segmental variant (39%), located behind the vertebral bodies, does not cross the intervening disc spaces. The continuous type (27%) extends from vertebra to vertebra, traversing the disc spaces. The mixed form (29%) simultaneously includes both continuous and segmental elements with “skip” areas. The “other” form (5%) is localized to the disc spaces, with limited degrees of rostral and caudal retrovertebral extension.
Myelopathy Scales
Japanese Orthopaedic Association Scale
The JOA scale categorizes the severity of myelopathy using a 17-point scale.24–27 In 2007, the JOA released a new evaluation tool for cervical myelopathy called the Cervical Myelopathy Evaluation Questionnaire (JOA CMEQ).24–27 The new tool consists of five categories, which include 24 questions devoted to upper extremity motor function, lower extremity motor function, bladder function, cervical spine function, and quality of life. The English version of JOA CMEQ, its calculation software, and users’ manual are available at the JOA home page (http://www.joa.or.jp/english/english_frame.html).
Clinical Presentation of Ossification of the Posterior Longitudinal Ligament
Patients with early OPLL become symptomatic in their mid 40s with mild radiculopathy/myelopathy, whereas those with classic (mature) OPLL are more typically affected in their mid 50s, presenting with more advanced myelopathic syndromes. Males are affected twice as frequently as females. Although most patients become subacutely symptomatic over a progressive 12-month period, 10% present with acute deterioration associated with minor trauma.28 In a series of 118 OPLL patients, minor trauma resulted in new myelopathy (13 patients), worsening of preexisting myelopathy (7 patients), or no new changes (7 patients). Eighteen of 19 patients with the narrowest cervical canals were most adversely affected.28 In another series of 91 patients operated on for OPLL, 26 sustaining minor trauma preoperatively experienced major myelopathic deficits postoperatively.29 Patients with more mobile spines associated with segmental, mixed, and other forms of OPLL exhibited poorer outcomes when compared with those with more rigid spines attributed to continuous OPLL. Typical symptoms included neck/arm pain or dysesthesias (>50% of the patients), and neurologic signs included arm/leg weakness (25%), spasticity, and ataxia.
Neurodiagnostic Studies in Ossification of the Posterior Longitudinal Ligament
Radiography
Based on lateral 6-foot plain radiographs, the normal anteroposterior dimension of the cervical spinal canal should measure 17 mm between the C3-7 levels. In absolute stenosis, the canal is narrowed to 10 mm or less, whereas with relative stenosis, the canal measures between 10 and 13 mm. The extent of OPLL is readily described by the occupancy ratio, which is determined by dividing the thickness of the measured ossified lesion by the anterior/posterior developmental canal diameter. If the ratio is greater than 40%, the risk of myelopathy increases.2
Computer-assisted measurements based on plain radiographs may be used to follow the postoperative progression of OPLL.30 In a multicenter study, lateral radiographs taken immediately as well as 1 and 2 years following posterior decompressions were assessed (131 patients). All radiographs were transformed into digital images and compared. Over 2 years, there was a 56.5% rate of progression, occurring more often in younger patients with the mixed and continuous forms of OPLL.
Magnetic Resonance Imaging
MRI examinations, particularly T1- and T2-weighted MRI studies, performed with and without contrast (Gd-DTPA) demonstrate the spinal column, spinal cord, nerve roots, intrinsic cord disease, and extrinsic cord compression from the occiput through the cervicothoracic junction in the transaxial, coronal, and sagittal planes. On noncontrast MRI studies, hypertrophied PPL often appears opposite multiple disc spaces, demonstrated by accompanying retrovertebral extension appearing slightly hyperintense and enhancing with Gd-DTPA. Classic OPLL is identified on 50% of T1-weighted MRI studies by a hyperintense signal, reflecting the presence of fat within mature Haversian canals actively engaged in bone marrow production. MRI examinations may also help identify disc herniations (hypointense masses) that occur in 81% of cases in conjunction with segmental OPLL.31
Ossified OPLL, which appears hypointense on MRI studies, may lead to an underestimation of the true extent of the OPLL and should therefore be combined with plain radiographic and CT-based studies to document the true degree of cord compression more accurately. In a multicenter study, 156 OPLL patients from 16 institutions were followed an average of 10.3 years with plain radiographs, MRI, and CT studies.32 Of interest, all 39 patients with OPLL occupying greater than 60% of the anteroposterior diameter of the spinal canal were myelopathic, whereas only 49% with OPLL occupying less than 60% of the spinal canal were myelopathic.
Intrinsic cord swelling/edema, myelomalacia, and gliosis produce hyperintense signals on T2-weighted MRI scans and are considered poorer prognostic signs for cervical spondylotic myelopathy (CSM) compared with OPLL.31,33 Despite the 43% incidence of increased preoperative cord signals on MRI studies that failed to resolve postoperatively, patients with OPLL exhibited better outcomes compared with those with CSM. Dynamic MRI studies may also prove useful in demonstrating “dynamic” compression preoperatively and residual cord compromise postoperatively.
Computed Tomography
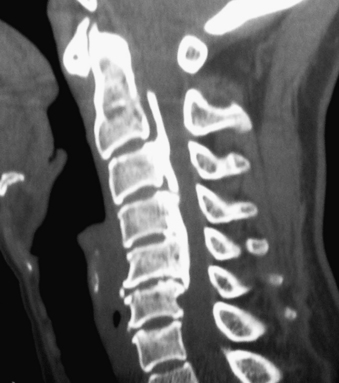
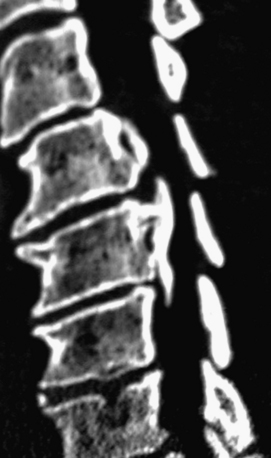
Noncontrast CT, intravenous-enhanced contrast CT, two-dimensional and three-dimensional reconstructed CT, and myelo-CT studies directly demonstrate punctate ossification characteristic of early OPLL or frank ossification typical of classic OPLL (Fig 88-9; see also Figs. 88-7 and 88-8). CT studies also demonstrate degenerative changes adjacent to levels of prior surgery.33 (Double-dose intravenous contrast-enhanced CT images may increase resolution of lateral or foraminal root pathology, helping to differentiate postoperative scar [enhancing] from new disc pathology [nonenhancing]). Two-dimensional and three-dimensional noncontrast CT reconstructed images provide a sagittal overview of the extent of cord compression without incurring the risk associated with myelo-CT studies. In particular, younger patients with OPLL and normal-sized spinal cords are at greater risk for acute deterioration following myelography compared with older individuals with OPLL and significant underlying cord atrophy.
Signs of Dural Penetrance on Computed Tomography
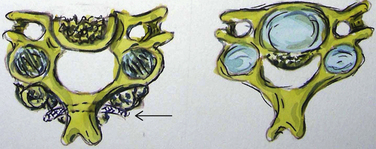
Bone-window CT examinations document two major signs of dural penetrance; the double-layer and single-layer CT signs (see Figs. 88-7 to 88-9). The double-layer sign is characterized by a hyperdense line of OPLL directly behind the vertebra, followed by a hypodense mass representing penetrated dura, and finally, an intradural hyperdense mass of OPLL.34,35 The single-layer sign is represented by a large central mass of OPLL. However, when the single mass is lateralized, the resulting positive C sign reflects an imbrication of the lateral dura and a greater likelihood for cerebrospinal fluid (CSF) fistula formation.
The double-layer sign is most highly correlated with absent dura, with CSF fistulas occurring between 52.6% and 84% of the time, whereas the single-layer sign (C sign) produces CSF fistulas 13.6% to 25% of the time. In one series, 10 of 12 OPLL patients with CT-documented double-layer signs developed dural defects at surgery, whereas only 1 of 9 patients showing the single-layer sign developed a CSF leak intraoperatively.35,36 As anticipated, the greatest focus of OPLL compression in these patients corresponded to the site of dural penetrance. From a series of 197 Korean patients undergoing anterior resection of OPLL, signs of dural penetration were observed in 30.5% of patients: 52.6% with double-layer signs (nonsegmental OPLL) and 13.6% with single-layer signs.36 For those with double-layer signs, the thicker the central mass of OPLL, the greater the incidence of intraoperative CSF fistula.
The majority of patients with OPLL who exhibit neither the single- nor the double-layer signs should not develop intraoperative CSF fistulas if careful dissection is carried out under an operating microscope. When Epstein performed multilevel simultaneous anterior corpectomies with posterior fusions in 54 OPLL patients, only one demonstrated the double-layer CT sign and one demonstrated the single-layer sign.34,37 In 85 similar patients, three CSF fistulas resulted, with one double-layer CT sign and two single-layer CT signs.34 In a more recent series of 110 patients undergoing similar procedures, five CSF fistulas resulted, with three with double-layer CT signs and two with single-layer CT signs.
Myelo-Computed Tomography Studies
Myelo-CT studies are now rarely performed, because combined MRI/CT studies are extremely accurate and avoid myelography’s inherent risks of precipitating neurologic deterioration. Dorsally and dorsolaterally, myelo-CT studies (dynamic, static) demonstrate shingling of the lamina, OYL, OPLL, spondylosis, and disc pathology contributing to significant cord compression/stenosis. Postoperatively, myelo-CT studies also confirm whether the posterior decompression has been adequate or if further possible anterior surgery is warranted. Following open-door laminoplasty performed in OPLL patients, there was less dorsal shift of the cord but greater cord expansion, the latter factor positively correlating with outcome.38
Studies Documenting Fusion Following Multilevel Anterior Cervical Surgery
Fusion criteria, documented on static and two-dimensional CT studies performed immediately postoperatively and repeated at 3, 6, and 12 months postoperatively included (1) the presence of bony trabeculation, (2) lack of bony lucency at the fibula strut allograft/vertebral body interface, and (3) ingrowth of bone centrally into the fibula. Dynamic radiographs additionally revealed (1) less than 3.5 mm of translation, (2) less than 5 degrees of angulation, and (3) less than 1 mm of motion demonstrated between the tips of adjacent spinous processes. An additional criterion for fusion following multilevel ACF performed with fibula strut allograft consists of cephalad or caudad bony ingrowth from the vertebral end plate into the central canal.39 Eighteen patients with OPLL had, on average, a 2.9-level ACF performed with fresh-frozen fibula strut allografts, accompanied by C2-T1 posterior wiring and fusion (PWF). CT documentation of bony ingrowth and other signs of fusion were observed in 17 (94%) of 18 patients (ingrowth documented utilizing 500–900 Hounsfield units). Bony ingrowth doubled from 3 to 6 months postoperatively, increasing both rostrally (1.5–3.5 mm) and caudally (2.1–4.6 mm). Although early bony ingrowth signaled progression toward fusion, its absence was not pathognomonic for a failure to fuse as observed in the one patient.
Diseases Involving Ossification of Ligaments
Diffuse Idiopathic Skeletal Hyperostosis
DISH, an ossifying diathesis involving extensive ossification of the anterolateral aspect of contiguous vertebral bodies, is readily demonstrated on preoperative CT examinations. It typically occurs (15–30% of cases) in adults older than 65 years of age, is often asymptomatic, and is far more prevalent than OALL in North Americans.9,40 OPLL contributes to the diffuse ligamentous ossification seen in up to 50% of patients with DISH but should only be resected either anteriorly or posteriorly in symptomatic patients.41,42 DISH often becomes massive before producing dysphagia; therefore, other etiologies of dysphagia must be sought before the surgical resection of DISH, which may result in several months of dysphagia.42,43
Ossification of the Anterior Longitudinal Ligament
OALL is defined by initial hypertrophy of the anterior longitudinal ligament, followed by progressive cartilaginous infiltration, and, ultimately, frank ossification. On T1-weighted MRI studies, although the ossified OALL mass appears hypointense, the fat reflecting active bone marrow production occurring within mature Haversian canals is hyperintense.44 On CT examinations, ventral OALL may become massive but rarely produces dysphagia; therefore, it should be resected only once other etiologies for the symptoms have been eliminated. At surgery, the extent of OALL resection may be assessed on intraoperative lateral radiographs or fluoroscopic images.
Conservative Treatment
Patients with OPLL, whether younger than or older than 65 years of age, with progressive myelopathy, with or without evidence of cord edema, and minimal to no significant medical risk factors are considered good surgical candidates. For those with marked radiographic evidence of cord compromise, operative intervention should be performed prior to the anticipated 10% incidence of even minor cervical trauma, which can precipitate severe and/or permanent myelopathic progression.28
In patients with OPLL who are older than 70 years of age with severe myelopathy and significant medical comorbidities, surgery is considered high risk at best. For patients undergoing either multilevel ACF or laminectomies for myelopathy/OPLL, studies have reported that poor prognostic factors include age older than 70; severe myelopathy; cardiovascular or peripheral vascular disease; and a recent trauma history.45,46 In two of Epstein’s initial 44 patients undergoing multilevel circumferential surgery for OPLL, two patients expired from acute or 3-week delayed myocardial infarction.47,48 Such older patients with significant major comorbidities or fixed deficits should alternatively be managed at comprehensive pain management centers.
Surgical Treatment
Role of Prophylactic Surgery
Asymptomatic patients, younger than 65 years of age, may be considered candidates for prophylactic decompression if severe cervical OPLL is radiographically or physiologically documented. This population, with a longer life expectancy, has a greater risk of inadvertent trauma and resultant irretrievable myelopathy.28,29 T2-weighted MRI studies demonstrating high cord signals, reflecting cord edema or myelomalacia, may signal the need for surgery. Similarly, abnormal somatosensory-evoked potential (SSEP) responses may indicate subclinical dorsal cord compromise and the need to consider operative intervention. Surgery, performed prior to the onset and/or progression of a neurologic deficit, correlated with better outcomes in 87% of patients in the Saunders series.45
Anesthetic Protocol for Patients with Ossification of the Posterior Longitudinal Ligament Undergoing Circumferential Cervical Surgery
Patients undergoing multilevel ACF, posterior stabilization, and halo application for complex OPLL, are managed with a strict anesthetic protocol to avoid emergent hypoxia, reintubation, tracheostomy, and death.49,50 Awake fiberoptic intubation and positioning are performed first under continuous intraoperative SSEP monitoring.49 Eliminating cervical motion limits the potential for inadvertent hyperextension or hyperflexion injury to the cord. Following surgery, patients are kept prophylactically intubated the first postoperative night. This eliminates acute respiratory distress, particularly attributable to postoperative tracheal swelling, and the need for emergent reintubation. The following day, patients are evaluated fiberoptically by a skilled anesthesiologist and either electively extubated or kept intubated. Parameters that have to be met prior to extubation include (1) direct fiberoptic evaluation of the trachea and vocal cords for swelling, (2) indirect assessment of swelling performed by letting down the endotracheal cuff checking for an air leak and the ability to verbalize, (3) review of the patient’s immediate postoperative CT scan for soft tissue swelling, and (4) assessment of other attendant medical risk factors. If the patient cannot be extubated between postoperative days 1 to 7, elective tracheostomies are scheduled. For 58 patients who underwent, on average, three-level ACF with, on average, 6.5-level PWF, spanning 10 hours, and requiring usually 2.6 units of blood transfusion, fiberoptic extubation was successfully performed the first postoperative day in 40 patients.49
For another 15 patients with major risk factors, extubation was delayed until postoperative days 2 to 7, and three required elective tracheostomy (day 7). Seven major risk factors identified in this study were found to positively correlate with delayed extubation or tracheostomy. In descending order, these included (1) surgical time of more 10 hours (12 patients); (2) obesity greater than 220 pounds (12 patients); (3) transfusions of more than four units of blood (10 patients); (4) secondary anterior cervical surgery (9 patients); (5) anterior surgery, including the C2 level (7 patients); (6) four-level ACF (5 patients); and (7) asthma (5 patients). In addition, minor risk factors included advanced age (>65 years), severe preoperative neurologic deficits (Nurick grade IV–V; moderate/severe myelopathy), and an intraoperative CSF fistula. Using this protocol, only one patient required emergent reintubation 20 minutes after being extubated on the third postoperative day. In this case, three major risk factors (prior C4-7 ACF 3 years earlier, asthma, and surgery lasting more than 10 hours [14 hours]) and one minor risk factor (CSF fistula with wound-peritoneal and lumboperitoneal shunts) were identified. Notably, no patients required an emergency tracheostomy. Other factors observed in the literature known to contribute to airway complications included angioedema, recurrent laryngeal nerve palsy, dysphagia with or without esophageal perforation, and new cord injuries.25,51
Somatosensory-Evoked Potential and Motor-Evoked Potential Monitoring
Continuous intraoperative SSEP monitoring limits morbidity associated with cervical surgery for OPLL.42,52,53 SSEP monitoring includes the evaluation of median, ulnar, and posterior tibial responses. Awake fiberoptic nasotracheal intubation and positioning are performed with the patient awake under continuous SSEP monitoring, avoiding any cervical motion (flexion-extension). For patients operated on in the supine position, the chin is taped/distracted slightly superiorly. For patients undergoing surgery in the prone position, the three-pin head holder is applied using local anesthesia (1% lidocaine injection), and the patient is positioned awake. Keeping the patient awake during positioning allows potentials to be more readily compared with prepositioning baseline data. To avoid SSEP changes or loss, inhalation anesthetics (i.e., isoflurane, nitrous oxide) are usually kept at concentrations below 0.4%; an alternative balanced narcotic technique is typically used.
Significant Somatosensory-Evoked Potential Changes
SSEP changes are defined by (1) a 50% decline in the amplitude and (2) a 10% decrease in latency.52 Such changes are initially observed over 50 seconds for the first recording and are reproduced within 100 seconds. Once reproduced, and not considered false positives, immediate medical and/or surgical resuscitative measures may be initiated. Medical measures include the induction of hypertension, warming of irrigating fluids, decreasing the concentration of inhalation anesthetic, and increasing the oxygen concentration. Surgical resuscitative measures include releasing distraction, removal of an oversized graft, and cessation of manipulation. Epstein demonstrated that no instances of quadriplegia or death were encountered in 100 prospectively SSEP-monitored cervical surgical cases. Eight of 218 previously unmonitored cervical operations resulted in quadriplegia (prior series included eight surgeons).52 Other series similarly monitored SSEPs and observed 10 new postoperative neurologic deficits in 182 cervical procedures.54
Intraoperative Motor-Evoked Potentials and Electromyographic Monitoring
Transcranial motor evoked potentials (MEP) or transcutaneously placed epidural electrodes are typically used to monitor anterior cervical cord function.55 Complications associated with MEP electrode placement are often minimal (seizures, headache, transient motor deficit), and successful monitoring typically correlates with positive outcomes. In a recent cervical surgical series (1055 patients) combining all three intraoperative monitoring modalities (SSEP [all 1055 patients], MEP [26 patients], and electromyography [EMG; 427 patients]), 34 patients (3.2%) had new postoperative deficits. SSEP sensitivity was 52% (specificity 100%), MEP sensitivity was 96% (specificity 100%), and EMG sensitivity was 46% (specificity 73%).56
Risk Factors
Major risk factors that correlated with new neurologic deficits following cervical surgery, including OPLL, are (1) multisegmental surgery, (2) severe preoperative neurologic deficits, (3) age older than 70, and (4) use of instrumentation. In many series, 50% of patients with complete intraoperative loss of potentials may show partial deficits, whereas intraoperative recovery of SSEP potentials is often correlated with eventual neurologic recovery or no deficit. Of the 34 (3.2%) of 1055 patients in Kelleher’s series who experienced new postoperative deficits following cervical surgery, 6 had sensory/motor deficits, 7 had new sensory deficits, 9 had motor weakness, and 12 had new root injuries. Of these, 21 fully resolved (average 3.3 months), 9 partially resolved, and 4 were permanent.56
Surgical Approaches
Much controversy surrounds whether anterior or posterior surgical approaches are superior for managing cervical OPLL. Anterior surgery offers direct OPLL removal typically through multilevel ACF, whereas posterior procedures allow for indirect dorsal decompression of multilevel pathology employing laminectomy with posterior fusion procedures or laminoplasty. Some authors advocate direct anterior resection of one- to two-level OPLL (111 patients), but in expansive laminoplasty for multilevel OPLL (10 patients), good to excellent outcomes resulting from this approach were being observed in 88% and fair outcomes in 12% of patients.57
Posterior Surgical Approach
Older high-risk patients (>65 years) with significant multilevel OPLL but an adequately preserved cervical lordotic curvature or an exaggerated lordosis may be managed with varied posterior surgical decompressive approaches: laminectomy alone, laminectomy with posterior fusion, or laminoplasty.26,37,58–62 Posterior removal of shingled laminae and a hypertrophied or ossified yellow ligament allows the cord to migrate away from ventrally situated OPLL and spondylotic/osteophytic changes. Nevertheless, posterior surgery is not appropriate in the presence of kyphosis, because removal of the posterior elements will leave the cord tethered over ventral disease.
Laminectomy
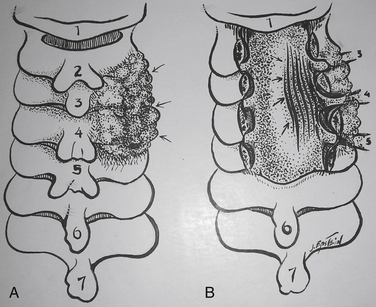
Laminectomy may sufficiently decompress the cervical spinal canal in patients with OPLL if the cervical spine is stable and the lordotic curvature is adequately preserved (see Figs. 88-1 to 88-3). Laminectomy and medial facetectomies/foraminotomies should include the resection of 25% or less of the medial aspect of the facet joint to preserve stability; greater than 50% to 75% facet removal correlates with greater pathologic motion/instability.63 Long-term results of laminectomy for patients with OPLL (44 patients) revealed a neurologic recovery rate of 44.2% 1 year postoperatively, and the rate of 42.9% was nearly unchanged 5 years later.59 However, outcomes worsened between 5 and 10 years postoperatively. Negative prognostic factors included (1) older age at the time of the original surgery, (2) more severe preoperative neurologic deficits, (3) history of new trauma, and (4) presence of OPLL. Despite OPLL progression in 70% of patients, only one patient experienced significant neurologic deterioration. Additionally, although kyphosis progressed in 47% of patients, it was not significantly correlated with deterioration.
Laminectomy with Posterior Fusion
Cervical laminectomy in conjunction with a posterior fusion is another alternative for the management of OPLL where the lordotic curvature is preserved. In some patients, prophylactic stabilization may be performed to avoid the evolution of instability, whereas in others, instability may already be present. Iatrogenic instability secondary to a failed laminectomy may also contribute to the need for simultaneous fusion.63 Preoperative documentation of chronic olisthy, partial swan-neck deformity, or hyperlordosis with excessive mobility constitute other reasons for considering posterior fusion.
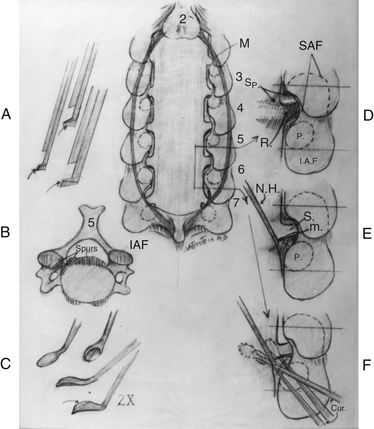
Limited Laminectomy with Spinous Process-Based Fusions
Utilizing more focal or limited laminectomies (one to three levels), with undercutting and/or removal of the OYL from respective cephalad and caudad laminae, leaves multiple spinous processes intact, which may be used for rod-eyelet-cable–based fusion constructs.64–67 Advantages of this technique include the avoidance of lateral mass and/or pedicle screws with the accompanying risks of critical breaches (1.4–10.6%) and neurologic/neurovascular injuries.66,67 In an initial study of 14 patients undergoing one- to two-level focal laminectomy with rod-eyelet-cable–instrumented fusions, maximal 36-Item Short Form Health Survey (SF-36) improvement occurred on five health scales within the initial 6 months postoperatively, and fusions preserved stability and avoided disease progression.66 In a series of 35 patients undergoing focal laminectomy (one to three levels) with posterior fusion (rod-eyelet-cable construct), patients averaged 65 years of age and exhibited severe myelopathy (Nurick grade IV–V) and cord compression (stenosis, OPLL, OYL, olisthy). Following average two-level laminectomy and seven-level fusions, patients neurologically improved (Nurick grade 0–I; mild radiculopathy, mild myelopathy), exhibiting two transient root injuries, with 100% fusion at 5.2 months postoperatively (dynamic radiographs, CT-documented). Iliac autograft supplemented with beta tricalcium phosphate plus autogenous bone marrow aspirate effectively promoted posterolateral cervical fusion in this series, a finding well documented in posterior iliac crest/posterior cervical and lumbar posterolateral fusion studies.68–70 When performing these procedures, the use of microfibrillar collagen (Duragen; Integra, Plainsboro, NJ) is also recommended. However, the use of porcine skin hemostatic gelatin (Gelfoam; Pfizer, Morris Plains, NJ), based on the literature and its own insert, is contraindicated, with risks including root compression/neurologic deficits due to severe swelling of the implant, infection, and allergic reactions to the porcine component of the product.71 In one case, Gelfoam resulted in the delayed (3 weeks) postoperative exacerbation of myelopathy in an elderly female; following the removal of hypertrophied/compressive and adherent Gelfoam, her symptoms resolved. The addition of silver-based dressings (Silverlon; Argentum Medical, Plainsboro, NJ) used with sterile water over a 10-day postoperative period also markedly limited the frequency of wound infections.72 SF-36 outcomes questionnaires revealed improvement on all 8 health scales, and Odom criteria demonstrated 29 good/excellent outcomes and 6 fair/poor outcomes.67
Laminectomy with Facet Fusion, Lateral Mass, and Pedicle Fixation Techniques
Fusion alternatives include facet wiring techniques, insertion of lateral mass screws, and dorsal pedicle screw fixation.60–62,73,74 Laminectomy with posterior wiring and fusion resulted in high fusion rates without significant complications in Epstein’s series of 85 OPLL patients.37,58 Alternatively, Hamanishi and Tanaka noted no significant difference in outcomes when comparing laminectomy performed in 35 patients without instability to laminectomy with fusion performed in 34 unstable patients.60 Using the SF-36, Kumar et al. evaluated patient-based outcomes in 25 patients undergoing laminectomy with lateral mass plating for unstable spondylotic myelopathy.61 No patients exhibited new postoperative instability or increased kyphosis, 80% showed good outcomes, 76% improved on myelopathy scores, and none developed delayed deterioration. Applying lateral mass plates in 43 patients, including 14 with postlaminectomy instability, Wellman et al. encountered no significant complications in 281 screws placed (average, seven screws per patient).62 However, dorsal decompression with or without fusion did not suffice in a subset of Abumi and Kaneda’s patients with significant OPLL.73 Following the application of pedicle screws for dorsal fixation after 26 laminectomies or laminoplasties, 15 patients required additional anterior procedures. In Abumi et al.’s update 2 years later, postoperative radiographic studies demonstrated that 10 of 190 (5.3%) screws perforated the cortex of the pedicles but did not result in neurovascular complications.74 Alternatively, of 58 patients with CSM, OPLL, or degenerative disease undergoing dorsal cervical decompressions with posterior pedicle screw–instrumented fusions, 8 developed screw-related complications, including 2 vertebral artery injuries.75
Laminoplasty
Some surgeons consider the laminoplasty to be an optimal approach to multilevel OPLL; the more levels of OPLL involved, the more likely a laminoplasty may be performed. Laminoplasty simultaneously offers dorsal decompression while augmenting stability without the need for traditional fusion.23,26,76 Results of cervical decompression in a goat model using the laminoplasty versus the laminectomy, radiographic and biomechanical results confirmed that laminoplasty was superior in maintaining cervical alignment and avoiding postoperative spinal deformity.77
Where OPLL extended up to the C2 level or down to the T1 level, these levels should be included in the original decompression, avoiding further OPLL expansion requiring secondary surgery. In one series, long-term recovery rates of 44.2% were observed after 1 year and 42.9% after 5 years (44 patients undergoing laminectomy); long-term deterioration occurred 5 to 10 years later and correlated with a 32.8% decline in JOA scores.59 Major negative prognostic factors included advanced age at the time of the original surgery, more severe preoperative myelopathy, and a history of trauma.
For 64 patients who underwent expansive laminoplasty, late recovery rates of 64% were maintained at 10 years with only 14% of patients demonstrating delayed deterioration at 5 to 15 years postoperatively.78 Of interest, OPLL progressed in only two cases at previously operated sites. When OPLL progression was evaluated over a 5-year period using plain radiographs for 55 postlaminectomy patients, 12 patients (21.8%) demonstrated OPLL progression/thickness; progression was greater in younger patients with continuous or mixed OPLL and was most marked at the C2-4 levels.79 Long-term follow-up (average, 10.2 years; range, 5–20 years) in 66 patients undergoing laminoplasty for myelopathy/OPLL revealed significantly poorer results for patients with a canal occupancy ratio of greater than 60% and a hill-shaped ossification. Other but lesser negative factors included poorer preoperative clinical status (poorer JOA), postoperative changes in alignment, and older age at the original surgery.80 In another study with an average 14-year follow-up following open-door laminoplasty for patients with either CSM or OPLL, average JOA scores and recovery rates improved markedly within 3 years postoperatively but showed slight deterioration at 5 years.81 Notably, although 66% demonstrated OPLL progression, this did not contribute to clinical symptomatic worsening.
Improvement following laminoplasty largely relies on whether there is a sufficient lordotic cervical curvature to allow for dorsal migration of the cord away from ventrally situated OPLL. In one study, comparing preoperative with postoperative myelo-CT studies in 65 patients undergoing laminoplasty for OPLL, a mean dorsal cord shift of greater than 3 mm correlated with good clinical outcomes (range, 0.0–6.6 mm).76 However, for OPLL lesions located at the rostral or caudal extremes of the canal, decompression had to be extended one level above or below this pathology to maximize dorsal cord migration. In another study involving patients undergoing laminoplasty, a 42% increase in the average postoperative anteroposterior canal diameter (3 years postoperatively), a 96% bone fusion rate, an 83% incidence of preserved range of motion, and significant neurologic improvement (preoperative JOA score, 9; postoperative JOA score, 14.1) were observed.23 For laminoplasty to be successful in OPLL patients, congenital stenosis had to be largely avoided, because a minimum canal diameter of 17 mm (31 patients) was typically required to achieve adequate cord decompression; in smaller canals, anterior approaches had to be seriously considered.82
Limitations of Outcomes with Posterior Decompressions
Lesser-quality clinical outcomes may be observed for patients with severe OPLL undergoing dorsal decompressions (laminectomy with or without fusion, laminoplasty). In one series of OPLL patients, better results were observed following anterior (48 patients) rather than posterior laminoplasty (27 patients) surgical procedures. This included both the mean overall improvement scores (neurosurgical cervical spine scale score of 78% [anterior] versus 46.1% [posterior] decompressions) and long-term follow-up scores (anterior scores rose from 9 and 13, and laminoplasty scores declined from 10.4 to 9.7).83 Other studies also observed better outcomes following anterior rather than posterior surgery for OPLL.5,6,47,48
Increased Ossification of the Posterior Longitudinal Ligament Progression Following Dorsal Decompression
A major concern remains whether dorsal decompression of OPLL increases the rate of OPLL progression. One study radiologically compared OPLL progression rates following 25 laminoplasties, 16 laminectomies, and 56 nonsurgical cases. Although no significant difference was observed in OPLL progression rates following either laminoplasty or laminectomy, both operations increased OPLL’s progression compared with those treated conservatively.84
Anterior Surgical Approaches
Single-Level Anterior Corpectomy with Fusion
Some surgeons have determined that direct anterior resection of OPLL results in improved postoperative neurologic outcomes when found at one level with retrovertebral extension or two-level discectomy/fusion.85,86 When patients demonstrated OPLL at one to two interspaces (focal), it was typically accompanied by significant retrovertebral extension and, therefore, one-level corpectomies were the preferred operation. Reoperation rates for 55 OPLL patients undergoing one-level ACF varied according to the type of plates applied: 3 Orion plates, using a fixed-plate/fixed-screw design (Sofamor Danek, Memphis, TN); 12 Atlantis plates, using a fixed-plate variable-screw design (Sofamor Danek); and 40 ABC dynamic plates (Aesculap, Tuttlingen, Germany).86 The overall failure rates were 7 of 15 (47%) for fixed compared with 4 of 40 (10%) for dynamic plates. The average cephalad migration of the dynamic plates was 6.6 mm (range, 3–10 mm), and the average caudad migration was 5.7 (range, 3–8 mm). The lower failure rate for dynamic plates that allowed several millimeters of rostral and caudal migration indicated that the dynamic design contributed to reduced stress shielding and increased compression, both of which contributed to fusion and stability.
Multilevel Anterior Corpectomy and Fusion
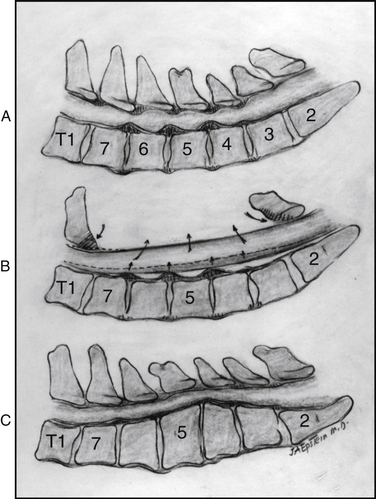
Better outcomes have been reported in some series for OPLL patients undergoing multilevel anterior corpectomy and fusion procedures, rather than posterior operations (see Figs. 88-4 to 88-6).87–98 In one study, Nurick scores improved in 86% of 93 patients undergoing anterior cervical corpectomy (average score, 1.24), whereas poorer outcomes followed posterior surgery (improvement of only 0.07).99 In another study comparing the results of ADF in patients with OPLL (27 patients) versus laminoplasty (66 patients), over a mean interval of 6 years postoperatively, ADF resulted in superior neurologic outcomes with patients exhibiting an occupying ratio of 60% or greater. However, this was accompanied by a 15% incidence of graft complications, and additional surgery was required in 26% of patients.100 Other series include the incidence of major complications that may be associated with multilevel ACF/posterior fusion.37,47,48,87,88,91,101–103 In 76 patients with OPLL undergoing either nonplated ADF (average, 3.5 levels) or ACF (average, 3 levels), Epstein found a 13% incidence of pseudarthrosis/instability within the first 6 months postoperatively.88 In another series, 10% of 31 nonplated four-level ACFs also failed acutely.45 In a third series of 36 patients undergoing two- to four-level ACF (15 performed with plates), the combined perioperative mortality/major morbidity rate was 22%, even though 97% ultimately fused.104 In a fourth series, involving two-level ACF with fixed anterior plates, a 9% incidence of graft extrusion occurred; the failure rate rose to 50% for three-level plated ACF.102 In a fifth series, involving one-level ACF (87 patients) and two- to three-level ACF (98 patients), a 98.8% fusion rate, a 3.2% neurologic complication rate, and an 86.5% improvement rate were observed.105
Complication Rates
Complication rates were lower when dynamic rather than fixed plates were used to perform multilevel ACF/PWF. When Epstein performed 22 multilevel (two- to four-level) ACFs without anterior plates but added PWF, three graft extrusions resulted.47 Adding a fixed plate to another 22 of these multilevel constructs resulted in two immediate inferior graft/plate extrusions.48 When Atlantis plates (Sofamor Danek) were applied in 16 similar patients, three extruded postoperatively (2 patients at 1 month, 1 patient at 4 months). All of these included inferior graft, plate, and screw extrusions.37 After having performed 25 multilevel ACF/PWFs with dynamic plates, only one patient developed a “partial” pseudarthrosis of the anterior graft demonstrated on sequential CT studies, warranting a second PWF.86 The average dynamic plate migration for these multilevel ACF/PWF constructs was 6.1 mm (range, 4–10 mm) cephalad and 5.8 mm (range, 4–9 mm) caudad. Dynamic plating similarly appeared to limit stress shielding, promoted graft settling, and fostered fusion in multilevel constructs.86,92
Posterior fusions may be completed using posterior wiring and fusion techniques, lateral mass screw/plating systems, or pedicle screw/rod instrumentation. The biomechanical advantage of a posterior construct, or posterior “tension band,” has been well documented. In a sagittal plane biomechanical study, Kirkpatrick et al. demonstrated that posterior fusion reduced the range of motion by 62% compared with 24% with strut grafting alone. This percentage was 43% following anterior strut graft and the application of an anterior plate.101 Posterior spinous process fusions may also readily be performed using a rod-eyelet–braided titanium cable construct, with iliac autograft supplemented with beta tricalcium phosphate applied laterally over the laminae and facet joints.
Cord and Root Injuries
Complications of anterior cervical surgery include a 2% to 10% incidence of quadriplegia and up to a 17% incidence of root injury (typically the C5 root).12 Root injuries may result from rapid dorsal cord migration, or the so-called untethering effect, more often following a posterior cervical procedure rather than anterior decompression.45 In one study, 9 of 49 patients undergoing laminectomy and lateral mass screw fixation for OPLL developed postoperative C5 root palsies within 6 hours to 6 days postoperatively. Although there was no increased cord signal observed on T2-weighted MRI studies in these patients, an exaggerated cervical lordosis combined with OPLL indicated that these injuries were due to a tethering effect on the C5 roots themselves.106 Although less frequent, anterior migration can also occur but may be mitigated by limiting the anterior trough diameter to 14 to 15 mm.45 Many patients with OPLL in North America are larger individuals and typically require resection of vertebral bodies/discs into the 18- to 20-mm range to achieve adequate decompression. In these cases, therefore, more limited 14- to 15-mm troughs would leave significant amounts of OPLL in place and would likely fail to result in resolution of radicular symptoms.
Outcomes of Circumferential Surgery
Higher rates of successful fusion without plate/graft-related complications were increasingly observed as dynamic plates (ABC; Aesculap, Tuttlingen, Germany) progressively replaced fixed-plated systems. Of 66 patients undergoing simultaneous multilevel ACF (2.6–3.0 levels) with posterior fusions (seven-level; circumferential procedures) for cervical OPLL, 13% of fixed plates (extrusion, fracture, pseudarthrosis) versus only 3.6% of dynamic ABC plates (1 plate; delayed pseudarthrosis) failed.96 Nurick grades, Odom criteria, and SF-36 outcomes were evaluated in 47 patients undergoing circumferential cervical surgery for OPLL.97 Patients averaged 54 years of age, exhibited severe myelopathy (average Nurick grade 3.6), and underwent average 2.6-level ACF with seven-level posterior fusions (C2-T1) accompanied by halo placement. Fixed plates (28 plates) and dynamic plates (19 plates) were applied. Determination of fusion was based on both dynamic radiographic and two dimensional–CT studies an average of 5 months postoperatively. At 1 year postoperatively, Nurick grades improved 2.8 to 3.2 points, Odom criteria showed 40 excellent/good and 7 fair/poor outcomes, and SF-36 outcomes revealed moderate improvement on five health scales: social function, bodily pain, role physical, physical function, and role emotional. Minimal additional improvement occurred over the succeeding second year.
Outcomes further improved as increasingly only dynamic plates were used. In a study involving multilevel ACF/posterior fusion using ABC plates in 40 patients, only one patient exhibited a delayed pseudarthrosis, patients fused an average of 6.3 months postoperatively, and there were no longer any plate/graft extrusions.98 At one postoperative year, Nurick grades improved from a preoperative severe myelopathy (average score, 3.9) to postoperative mild radiculopathy/myelopathy (average score, 0.4), and SF-36 improvement was maximal on role physical, bodily pain, and role emotional health scales.
Anterior Floating Method: An Option for Anterior Resection
The anterior floating method is an alternative technique proposed for ventral OPLL resection where it occupies more than 60% of the spinal canal.107 This technique includes marked lateral and cephalad/caudad resection and thinning of the vertebral bodies with air drills to avoid CSF fistulas associated with classic ventral OPLL resection. In theory, because this technique frees the ossified dura from its constraints, ventral migration of the remaining dura/OPLL mass allows for adequate spinal cord and nerve root decompression. Although this procedure offers long-term JOA recovery rates of 71%, it does not offer direct resection of the OPLL mass. Therefore, further progression of OPLL accompanied by retethering of the “floating OPLL” mass remains a major concern, as do the technical risks that include vertebral artery and root injury associated with the extreme lateral resection technique.
Abumi K., Kaneda K., Shono Y., et al. One-stage posterior decompression and reconstruction of the cervical spine by using pedicle screw fixation systems. J Neurosurg. 1999;90(Suppl 1):19-26.
Epstein N.E. An argument for traditional posterior cervical fusion techniques: evidence from 35 cases. Surg Neurol. 2008;70(1):45-55.
Epstein N.E. Circumferential cervical surgery for ossification of the posterior longitudinal ligament: a multianalytic outcome study. Spine. 2004;29(12):1340-1345.
Epstein N.E. Evaluation of intraoperative somatosensory evoked potential monitoring during 100 cervical operations. Spine. 1993;18(6):737-747.
Fukui M., Chiba K., Kawakami M., et al. Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). Part 3. Determination of reliability. J Orthop Sci. 2007;12:21-26.
Fukui M., Chiba K., Kawakami M., et al. Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ). Part 4. Establishment of equations for severity scores: subcommittee on low back pain and cervical myelopathy evaluation of the clinical outcome committee of the Japanese Orthopaedic Association. J Orthop Sci. 2008;13:25-31.
Hida K., Iwasaki Y., Kohanagi I., et al. Bone window computed tomography for detection of dural defect associated with cervical ossified posterior longitudinal ligament. Neurol Med Chir (Tokyo). 1997;37(2):173-175.
Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: part 2: advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32(6):654-660.
1. Epstein N.E. Ossification of the posterior longitudinal ligament in evolution in 12 patients. Spine. 1994;19(6):673-681.
2. Miyasaka H. Consideration on pathophysiology of OPLL. Clin Orthop Relat Res. 1975;10:1091-1096.
3. Kim T.J., Bae K.W., Uhm W.S., et al. Prevalence of ossification of the posterior longitudinal ligament of the cervical spine. Joint Bone Spine. 2008;75(4):471-474.
4. Epstein N.E. Ossification of the posterior longitudinal ligament: diagnosis and surgical management. Neurosurg Q. 1992;2(3):23-41.
5. Yonenobu K., Hosono N., Iwasaki M., et al. Neurologic complications of surgery for cervical compression myelopathy. Spine. 1991;16(11):1277-1282.
6. Epstein N.E. The surgical management of ossification of the posterior longitudinal ligament in 43 North Americans. Spine. 1994;19(6):664-672.
7. Koga H., Hayashi K., Taketomi E., et al. Restriction fragment length polymorphism of genes of the alpha 2(XI) collagen, bone morphogenetic protein-2, alkaline phosphatase, and tumor necrosis factor-alpha among patients with ossification of the posterior longitudinal ligament and controls from the Japanese population. Spine. 1996;21(4):469-473.
8. Matsunaga S., Yamaguchi M., Hayashi K., et al. Genetic analysis of ossification of the posterior longitudinal ligament. Spine. 1999;24(10):937-938.
9. Resnick D., Guerra J., Robinson C.A. Association of diffuse idiopathic skeletal hyperostosis (DISH) and calcification and ossification of the posterior longitudinal ligament. AJR Am J Roentgenol. 1978;131:1049-1053.
10. Kong Q., Ma Z., Lif F., et al. COL6A1 polymorphisms associated with ossification of the ligament flavum, and ossification of the posterior longitudinal ligament. Spine. 2007;32(25):2834-2838.
11. Terayama K. Genetic studies on ossification of the posterior longitudinal ligament of the spine. Spine. 1989;14(11):1184-1191.
12. Sakou T., Taketomie E., Matsunaga S., et al. Genetic study of ossification of the posterior longitudinal ligament in the cervical spine with human leukocyte antigen haplotype. Spine. 1991;16(11):1249-1252.
13. Koga H., Sakou T., Taketomi E., et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am J Hum Genet. 1998;62(6):1460-1467.
14. Ikegawa S., Kurokawa T., Hizuka N., et al. Increase of serum growth hormone-binding protein in patients with ossification of the posterior longitudinal ligament of the spine. Spine. 1993;18(13):757-760.
15. Yonemori I., Imamura T., Ishidou Y., et al. Bone morphogenetic protein receptors and activin receptors are highly expressed in ossified ligament tissues of patients with ossification of the posterior longitudinal ligament. Am J Pathol. 1997;150(4):1335-1347.
16. Wang H., Liu D., Yang Z., et al. Association of bone morphogenetic protein-2 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the spine and its severity in Chinese patients. Eur Spine J. 2008;17(7):956-964.
17. Li H., Liu D., Zhao C.O., et al. Insulin potentiates the proliferation and bone morphogenetic protein-2-induced osteogenic differentiation of rat spinal ligament cells via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Spine. 2008;33(22):2394-2402.
18. Miyamoto S., Yonenobu K., Ono K. Elevated plasma fibronectin concentrations in patients with ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spine. 1993;18(15):2267-2270.
19. Motegi H., Yamazaki M., Goto S., et al. Proliferating cell nuclear antigen in hypertrophied spinal ligaments. Immunohistochemical localization of proliferating cell nuclear antigen in hypertrophied posterior longitudinal ligament of the cervical spine. Spine. 1998;23(3):305-310.
20. Epstein N.E., Grande D.A., Breitbart A.S. In-vitro characteristics of cultured posterior longitudinal ligament tissue. Spine. 2002;27(1):56-58.
21. Breitbart A.S., Grande D.A., Kessler R., et al. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg. 1998;101:67-74.
22. Epstein N.E. Diagnosis and surgical management of ossification of the posterior longitudinal ligament. Contemp Neurosurg. 1992;22:1-6.
23. Morimoto T., Matsuyama T., Hirabayashi H., et al. Expansive laminoplasty for multilevel cervical OPLL. J Spinal Disord. 1997;10(4):296-298.
24. Fukui M., Chiba K., Kawakami, et al. An outcome measure for patients with cervical myelopathy: Japanese Orthopaedic Association Cervical Myelopthy Evaluation Questionnaire (JOACMEQ): Part I. J Orthop Sci. 2007;12:227-240.
25. Fukui M., Chiba K., Kawakami M., et al. Japanese Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 2: Endorsement of the alternative item. J Orthop Sci. 2007;12:241-248.
26. Fukui M., Chiba K., Kawakami M., et al. Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 3. Determination of reliability. J Orthop Sci. 2007;12:21-26.
27. Fukui M., Chiba K., Kawakami M., et al. Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 4. Establishment of equations for severity scores: subcommittee on low back pain and cervical myelopathy evaluation of the clinical outcome committee of the Japanese Orthopaedic Association. J Orthop Sci. 2008;13:25-31.
28. Katoh S., Ikata T., Hirai N., et al. Influence of minor trauma to the neck on the neurological outcome in patients with ossification of the posterior longitudinal ligament of the cervical spine. Paraplegia (Eng). 1995;33(6):330-333.
29. Fujimura Y., Nakamura M., Toyama Y. Influence of minor trauma on surgical results in patients with cervical OPLL. J Spinal Disord. 1998;11(1):16-20.
30. Chiba K., Yamamoto I., Hirabayashi H., et al. Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine. 2005;3(1):17-32.
31. Koyanagi I., Iwasaki Y., Hida K., et al. Magnetic resonance imaging findings in ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg. 1998;88(2):247-254.
32. Matsunaga S., Nakamura K., Seichi A., et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine. 2008;33(24):2648-2650.
33. Baba H., Furusawa N., Imura S., et al. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18(15):2167-2173.
34. Epstein N.E. Identification of ossification of the posterior longitudinal ligament extending through the dura on preoperative CT examination of the cervical spine. Spine. 2001;26:182-186.
35. Hida K., Iwasaki Y., Kohanagi I., et al. Bone window computed tomography for detection of dural defect associated with cervical ossified posterior longitudinal ligament. Neurol Med Chir (Tokyo). 1997;37(2):173-175.
36. Min J.H., Jang J.S., Lee S.H. Significance of the double-layer and single-layer signs in the ossification of the posterior longitudinal ligament of the cervical spine. J Neurosurg Spine. 2007;6(4):309-312.
37. Epstein N.E. Anterior approaches to cervical spondylosis and OPLL: review of operative techniques and assessment of 65 multilevel circumferential procedures. Surg Neurol. 2001;55:313-324.
38. Fujimura Y., Nishi Y., Nakamura M. Dorsal shift and expansion of the spinal cord after expansive open-door laminoplasty. J Spinal Disord. 1997;10(4):282-287.
39. Epstein N.E. Computed tomography (CT) validating bony ingrowth into fibula strut allograft: a criterion for fusion. Spine J. 2002;2:129-133.
40. Kissel P., Youmans J.R. Posttraumatic anterior cervical osteophyte and dysphagia: surgical report and literature review. J Spinal Disord. 1992;5(1):104-107.
41. Epstein N.E. Simultaneous cervical diffuse idiopathic skeletal hyperostosis and ossification of the posterior longitudinal ligament resulting in dysphagia or myelopathy in two geriatric North Americans. Surg Neurol. 2000;53(5):427-431.
42. McCafferty R.R., Harrison M.J., Tamas L.B., et al. Ossification of the anterior longitudinal ligament and Forrestier’s disease: an analysis of seven cases. J Neurosurg. 1996;85(3):524-525.
43. Oga M., Mashima T., Iwakuma T., et al. Dyaphagia complications in ankylosing spinal hyperostosis and ossification of the posterior longitudinal ligament. Roentgenographic findings of the developmental press of cervical osteophytes causing dysphagia. Spine. 1993;18(3):391-394.
44. Epstein N.E., Hollingsworth R. Ossification of the anterior longitudinal ligament (OALL) contributing to dysphagia: a case report. J Neurosurg. 1999;90(Suppl 2):261-263.
45. Saunders R.L., Pikus H.J., Ball P. Four-level cervical corpectomy. Spine. 1998;23(33):2455-2461.
46. Epstein N.E. Laminectomy for cervical myelopathy. Spinal Cord. 2003;41(6):317-327.
47. Epstein N.E. Circumferential surgery for the management of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1998;11(3):200-207.
48. Epstein N.E. The value of anterior cervical plating in preventing vertebral fracture and graft extrusion following multilevel anterior cervical corpectomy with posterior wiring/fusion: indications, results, and complications. J Spinal Disord. 2000;13:9-15.
49. Epstein N.E., Hollingsworth R., Nardi D., et al. Can airway complications following multilevel anterior cervical surgery be avoided? J Neurosurg. 2001;94(Suppl 2):185-188.
50. Emery S.E., Smith M.D., Bohlman H.H. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg [Am]. 1991;73(4):544-551.
51. Emery S.E., Bohlman H.H., Bolesta M.J., et al. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg [Am]. 1998;80(7):941-951.
52. Epstein N.E. Evaluation of intraoperative somatosensory evoked potential monitoring during 100 cervical operations. Spine. 1993;18(6):737-747.
53. Epstein N.E. Somatosensory evoked potential monitoring (SSEPs) in 173 cervical operations. Neuro-Orthopedics. 1996;20:2-21.
54. May D.M., Jones S.J., Crockard H.A. Somatosensory evoked potential monitoring in cervical surgery: identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85(4):566-573.
55. Gokaslan Z.L., Samudrala S., Deletis V., et al. Intraoperative monitoring of spinal cord function using motor evoked potentials via transcutaneous epidural electrode during anterior cervical spinal surgery. J Spinal Disord. 1997;10(4):299-303.
56. Kelleher M.O., Tan G., Sarjeant R., et al. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: a prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8(3):215-221.
57. Mizuno J., Nakagawa H. Ossified posterior longitudinal ligament: management strategies and outcomes. Spine J. 2006;6(Suppl 6):282S-288S.
58. Epstein N.E. Reoperation rates for acute graft extrusion and pseudarthrosis following one level anterior corpectomy and fusion with and without plate instrumentation: etiology and corrective management. Surg Neurol. 2001;56:73-81.
59. Kato Y., Iwasaki M., Fuji T., et al. Long-term follow-up results of laminectomy for cervical myelopathy caused by ossification of the posterior longitudinal ligament. J Neurosurg. 1998;89(2):217-223.
60. Hamanishi C., Tanaka S. Bilateral multilevel laminectomy with or without posterolateral fusion for cervical spondylotic myelopathy: relationship to type of onset and time until operation. J Neurosurg. 1996;85(3):447-451.
61. Kumar V.G., Rea G.L., Mervis L.J., et al. Cervical spondylotic myelopathy: functional and radiographic long-term outcome after laminectomy and posterior fusion. Neurosurgery. 1999;44(4):771-777.
62. Wellman B.J., Follett K.A., Traynelis V.C. Complications of posterior articular mass plate fixation of the subaxial cervical spine in 43 consecutive patients. Spine. 1998;23(2):193-200.
63. Nowinski G.P., Visarius H., Nolte L.P., et al. A biomechanical comparison of cervical laminaplasty and cervical laminectomy with progressive facetectomy. Spine. 1993;18(14):1995-2004.
64. Epstein N.E. Cervical posterior cervical approaches in the management of spondylosis and ossification of the posterior longitudinal ligament. Surg Neurol. 2002;58:194-207.
65. Epstein N.E. A review of laminoforaminotomy for the management of lateral and foraminal cervical disc herniations or spurs. Surg Neurol. 57(4), 2002. 266–233
66. Epstein N.E., Epstein J.A. Short form-36 outcomes following focal 1- and 2-level cervical laminectomy with multilevel-instrumented fusion. Surg Neurol. 2006;66(3):264-268.
67. Epstein N.E. An argument for traditional posterior cervical fusion techniques: evidence from 35 cases. Surg Neurol. 2008;70(1):45-55.
68. Epstein N.E. Bone void filler in posterior iliac crest reconstruction and to supplement intertransverse process fusion. Spine J. 2006;6(5):600-602.
69. Epstein N.E. Efficacy of different bone volume expanders for augmenting lumbar fusions [review article]. Surg Neurol. 2008;69(1):16-29.
70. Epstein N.E. An analysis of noninstrunmented posterolateral lumbar fusions performed in predominantly geriatric patients using lamina autograft and beta tricalcium phosphate. Spine J. 2008;8(6):882-887.
71. Epstein N.E., Silvergleid R.S., Hollingsworth R. Increased postoperative cervical myelopathy and cord compression due to Gelfoam. Spine J. 2009;9(2):19-21.
72. Epstein N.E. Do silver-impregnated dressings limit infections following lumbar laminectomy with instrumented fusion? [technical note]. Surg Neurol. 2007;68(5):483-485.
73. Abumi K., Kaneda K. Pedicle screw fixation for nontraumatic lesions of the cervical spine. Spine. 1997;22(16):1853-1863.
74. Abumi K., Kaneda K., Shono Y., et al. One-stage posterior decompression and reconstruction of the cervical spine by using pedicle screw fixation systems. J Neurosurg. 1999;90(Suppl 1):19-26.
75. Hasegawa K., Hirano T., Shimoda H., et al. Indications for cervical pedicle screw instrumentation in nontraumatic lesions. Spine. 2008;33(21):2284-2289.
76. Sodeyama T., Goto S., Mochizuki M., et al. Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine. 1999;24(15):1527-1531.
77. Baisden J., Voo L.M., Cusick J.F., et al. Evaluation of cervical laminectomy and laminoplasty. A longitudinal study in the goat model. Spine. 24(13), 1999. 1283–1238
78. Iwasaki M., Kawaguchi Y., Kimura T., et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96(Suppl 2):80-189.
79. Hori T., Kawaguch Y., Kimura T. How does the ossification of the posterior longitudinal ligament thicken following cervical laminoplasty? Spine. 2007;32(19):E551-E556.
80. Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: part 1: clinical results and limitations of laminoplasty. Spine. 2007;32(6):47-53.
81. Chiba K., Ogawa Y., Ishii K., et al. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31(26):2998-3005.
82. Ishida Y., Ohmori K., Suzuki K., et al. Analysis of dural configuration for evaluation of posterior decompression in cervical myelopathy. Neurosurgery. 1999;44(1):91-95.
83. Kawano H., Handa Y., Ishii H., et al. Surgical treatment for ossification of the posterior longitudinal ligament of the cervical spine. J Spinal Disord. 1995;8(2):145-150.
84. Takatau T., Ishida Y., Suzuki K., et al. Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord. 1999;12(3):271-273.
85. Epstein N.E. The management of 1 level anterior cervical corpectomy with fusion employing Atlantis hybrid plates: preliminary experience. J Spinal Disord. 2000;13(4):324-328.
86. Epstein N.E. Anterior dynamic plates in complex cervical reconstructive surgeries. J Spinal Disord. 2002;15(3):221-227.
87. Epstein N.E. Vertebral body fractures following extensive anterior cervical surgical procedures for ossification of the posterior longitudinal ligament. Neuro-Orthopedics. 1997;21:1-11.
88. Epstein N.E. Evaluation and treatment of clinical instability associated with pseudarthrosis after anterior cervical surgery for ossification of the posterior longitudinal ligament. Surg Neurol. 1998;49(3):246-252.
89. Epstein N.E. Complications following surgical procedures of ossification of the posterior longitudinal ligament in the cervical spine warranting additional surgical correction. Neuro-Orthopedics. 1998;22:85-97.
90. Epstein N.E. Anterior cervical micro-dural repair of cerebrospinal fluid fistula after surgery for ossification of the posterior longitudinal ligament [technical note]. Surg Neurol. 1999;52:511-514.
91. Epstein N.E. Circumferential surgery for spondylostenosis with kyphosis in two patients with athetoid cerebral palsy. Surg Neurol. 1999;52:339-344.
92. Epstein N.E. Postsurgical imaging: marked superior and inferior migration of a dynamic plate after multilevel anterior corpectomy and fusion with posterior wiring. Spine J. 2001;1:226.
93. Epstein N.E. A comparative analysis of plate/graft failure with correction following circumferential cervical spinal surgery. Spinal Surg (Japan). 2002;16(1):1-8.
94. Epstein N.E. Review article: diagnosis and surgical management of cervical ossification of the posterior longitudinal ligament. Spine J. 2002;2:436-449.
95. Epstein N.E. Ossification of the cervical posterior longitudinal ligament: a review. Neurosurg Focus. 2002;13(2):1-10.
96. Epstein N.E. Fixed versus dynamic plate complications following multilevel anterior cervical corpectomy and fusion with posterior stabilization. Spinal Cord. 2003;41:379-384.
97. Epstein N.E. Circumferential cervical surgery for ossification of the posterior longitudinal ligament: a multianalytic outcome study. Spine. 2004;29(12):1340-1345.
98. Epstein N.E. Dynamic anterior cervical plates for multilevel anterior corpectomy and fusion with simultaneous posterior wiring and fusion: efficacy and outcomes. Spinal Cord. 2006;44(7):432-439.
99. Fessler R.G., Steck J.C., Giovanini M.A. Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery. 1998;43(2):257-265.
100. Iwasaki M., Okuda S., Miyauchi A., et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: part 2: advantages of anterior decompression and fusion over laminoplasty. Spine. 2007;32(6):654-660.
101. Kirkpatrick J.S., Levy J.A., Carillo J., et al. Reconstruction after multilevel corpectomy in the cervical spine. A sagittal plane biomechanical study. Spine. 1999;24(12):1186-1190.
102. Vaccaro A.R., Falatyn S.P., Scuderi G.J., et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11(5):410-415.
103. Meding J.B., Stambough J.L. Critical analysis of strut grafts in anterior spinal fusions. J Spinal Disord. 1993;6(2):166-174.
104. Macdonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86(6):990-997.
105. Eleraky M.A., Llanos C., Sonntag V.K. Cervical corpectomy: report of 185 cases and a review of the literature. J Neurosurg. 1999;90(Suppl 1):35-41.
106. Chen Y., Chen D., Wang X., et al. C5 palsy after laminectomy and posterior cervical fixation for ossification of the posterior longitudinal ligament. J Spinal Disord Tech. 2007;20(7):533-535.
107. Yamamura I., Kurosa Y., Matuoka T., et al. Anterior floating method for cervical myelopathy caused by ossification of the posterior longitudinal ligament. Clin Orthop Relat Res. 1999;359:27-34.