CHAPTER 31 ORGANIZATION: PYRAMIDAL AND EXTRAPYRAMIDAL SYSTEM
The large brains of primates have afforded remarkable behavioral adaptability. In humans, this adaptability is manifested in many ways, including the capacity for movements generated volitionally or somewhat automatically. Such movements include those required for speech and communication, for independent hand use, and for locomotion. Furthermore, these movements can be engaged in simultaneously, a feat that requires extraordinary adaptive control of the motoneuronal outputs to muscles. This occurs, for example, when a person talks and uses a mobile phone when walking or bicycling. This performance of skilled and complex movements can be generated by all components of the body’s musculature.
Motoneuronal output to generate movements under volitional control is achieved by descending neural drive from so-called motor cortical centers that generate corticospinal and corticobulbar outputs, as well as corticoreticulospinal outputs.1 Since work in the late 19th and early 20th centuries on stimulation of motor cortex to elicit movements and on sectioning of the pyramidal tract to impair them, the important role of motor cortical outputs with axons in the medullary pyramids has been recognized. This emphasis has fitted with observations of paralysis and weaknesses after development of lesions of this system and with evidence for evolution of the size and terminations of the corticospinal system in primates. There are corticospinal outputs not only to interneurons within the spinal cord but also directly to motoneurons (termed corticomotoneuronal connections). The pyramidal tract itself, so named from the pyramidal decussation in the medulla, contains the bulk of direct corticofugal outputs destined to recruit spinal motoneurons.1
A primary role for the motor cortex in movement control has been paralleled by recognition that subcortical nuclei are also critically involved. Two subcortical systems appear crucial for adequate volitional movement: cortical interactions with the motor thalamus and the integration of information through the basal ganglia.2 The basal ganglia comprise a large number of integrated regions (see later discussion) that affect the motor system at the level of the thalamus and brainstem. Just as lesion and stimulation studies show the importance of motor cortical outputs, comparable approaches to study of the basal ganglia and thalamus have revealed that they profoundly gate and modify movements. Depending on the location of lesions or stimulation, there can be extreme poverty of all movements, abnormal postures, and uncontrollable rhythmic motor outputs.3
THE EXTRAPYRAMIDAL MOTOR SYSTEMS
The basal ganglia and thalamus comprise a number of anatomically integrated regions involved in motor control.4,5 They also play important roles in emotional, motivational, associative, and cognitive functions. Basal ganglia regions include the caudate nucleus, putamen, internal and external segments of the globus pallidus, subthalamic nucleus, and substantia nigra. These regions have complex anatomical interconnections that are not fully characterized electrophysiologically. Thalamic regions participating in motor control include specific ventral, posterior, and intralaminar nuclei.6 The understanding of how these regions integrate motor information is based largely on knowledge of their relationship to the corticospinal pyramidal system. There are three types of subcortical extrapyramidal regions to consider: those that receive direct information from pyramidal or cortical regions with major influences on pyramidal regions; those with projections to pyramidal or directly related regions; and those that perform internal monitoring and regulation of extrapyramidal regions.
Subcortical Regions Receiving Pyramidal Input and Their Influence on the Pyramidal System
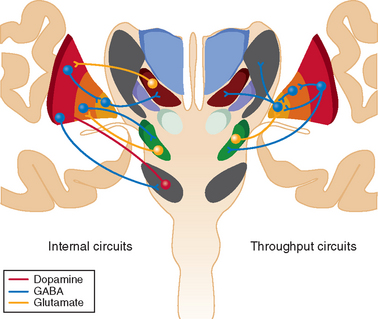
The largest subcortical extrapyramidal region receiving pyramidal input from both supragranular and infragranular regions of the motor cortices is the putamen (Figs. 31-1 and 31-2).4 Together with the caudate nucleus, this striatal area receives an information from all cortical regions. The putamen passes on processed information through both a direct activating and an indirect inactivating pathway to the basal ganglia output nuclei, the internal globus pallidus, and the substantia nigra pars reticulata.4 These basal ganglia regions are small in comparison with the striatum (100-fold fewer neurons) and contain large nonspiny γ-amino butyric acid–ergic (GABAergic) inhibitory projection neurons.
Striatal neurons also receive excitatory thalamic input from the ventral anterior, ventrolateral nuclei, and caudal intralaminar nuclei; receive inhibitory input from the external globus pallidus; and are modulated by dopamine from the substantia nigra pars compacta and mesencephalic tegmentum.4,7 Overall dopamine enhances activity in the direct pathway and decreases activity in the indirect pathway.8 The striatum con tains mainly GABAergic spiny projection neurons and a small population of GABAergic and cholinergic interneurons (≈3% of striatal neurons). Striatal spiny neurons project to the globus pallidus and substantia nigra, as well as giving rise to dense local arbors that contact other spiny neurons.4 They are usually silent and discharge only when cortical information is received. The GABAergic interneurons establish contacts with the dendritic shafts of neighboring spiny neurons and form the structural basis for feedforward striatal surround inhibition. Striatal cholinergic interneurons, in contrast, are tonically active and play a major role in the learning of reward behavior.
The smallest subcortical extrapyramidal region receiving pyramidal input from layer V neurons of the motor cortices is the subthalamic nucleus.9 This is the only cortical input to this nucleus, and this hyperdirect pathway conveys powerful excitatory effects from the motor cortices to the globus pallidus and substantia nigra pars reticulata, bypassing the striatum, with shorter conduction times than in the direct striatal pathway.9 It also receives significant inhibitory input from the globus pallidus and striatum.10 The subthalamic nucleus contains nonspiny excitatory glutamatergic neurons that conduct short-latency excitatory responses to the globus pallidus and substantia nigra after cortical excitation.
Specific regions of the thalamus also receive pyramidal input from motor cortices.5 There is a reciprocal excitatory component from small layer VI neurons to the ventrolateral thalamus and a fast-conducting nonreciprocal excitatory component from layer V neurons that allows the synchronization of thalamocortical oscillations and information flow across functionally related cortical fields. Cortical regions associated with executive function (such as dorsolateral prefrontal cortex) have nonreciprocal connections to thalamic regions that project to premotor cortices (ventral anterior thalamus), whereas premotor cortices have nonreciprocal connections with thalamic regions that project to the primary motor cortex (ventrolateral thalamus).11 The thalamus contains nonspiny excitatory glutamatergic neurons that conduct short-latency excitatory responses after appropriate cortical excitation. These interconnections between the cortex and thalamus largely determine cortical activity, facilitating information transfer from one cortical region to another through a feedforward mechanism. The output nuclei of the basal ganglia inhibit thalamocortical activity in the ventral anterior, anterior ventrolateral, and caudal intralaminar nuclei.11
Subcortical Regions Participating in the Internal Regulation of Extrapyramidal Systems
There is significant internal modulation of the hyperdirect, direct, and indirect basal ganglia pathways (see Fig. 31-1). One of the basal ganglia regions that most influence striatal processing is the dopaminergic substantia nigra pars compacta, as discussed previously, although pallidal and thalamic projections also significantly modify striatal output. The large, tonically active, nonspiny dopaminergic neurons of the substantia nigra pars compacta receive input directly from striatal spiny projection neurons, which modifies their firing rate and patterns.8 This reinforces wanted behaviors and suppresses unwanted behaviors. These dopaminergic neurons also receive significant innervation from cortical, limbic, and brainstem regions and play a role in shifting attentional sets.
The basal ganglia region with the most internal connections is the external segment of the globus pallidus.4 These pallidal neurons receive most input from the striatum and the subthalamic nucleus, as well as a small dopaminergic projection from the substantia nigra. Individual neurons in the external globus pallidus innervate the output nuclei, the subthalamic nucleus, and the substantia nigra pars compacta.4 About 25% of them also innervate the striatal GABAergic spiny neurons. These neurons provide the anatomical substrate for the synaptic integration of functionally diverse cortical information within the basal ganglia and appear to work in parallel with the subthalamic nucleus and striatum to set up appropriate oscillatory activity within the basal ganglia.10 They are therefore in a position to provide level-setting control of the activity through virtually the whole of the extrapyramidal basal ganglia system.
THE MOTOR CORTICES AND PYRAMIDAL SYSTEM
Pyramidal neurons in the somatomotor cortices send corticospinal and corticobulbar axons through the pyramidal tract. The primary motor cortex is usually known as area 4, defined according to Brodmann’s classic analysis of the cytoarchitecture of the human brain.12 In addition to its own local circuitry, it contains a topographically organized motor output to the bulbar muscles and to the trunk and extremities. The precise borders of this and other areas have been disputed partly because it is difficult to depict the areas precisely on maps of the cortical surface, which cannot reveal the borders in the depths of sulci (for review, see Zilles13). The caudal border of the primary motor cortex is clearly demarcated in the fundus of the central sulcus with its rostral border close to the anterior of the central sulcus. Hence, laterally over the hemisphere, the main part of area 4 lies in the central sulcus rather than on the external cortical surface. This delineation of the rostral edge of the primary motor cortex varies between individuals and relies on several architectural and cytoarchitectural features (see later discussion), and its existence is supported by high-resolution imaging and cytoarchitectonic mapping.14
Before some of the specific features of the primary motor cortex are described, other cortical areas that have a motor function must be recognized. Although several nomenclatures exist for these areas, there is broad agreement that the following cortical areas are involved. The ventral premotor cortex corresponds to the lower part of Brodmann’s area 6 and may be functionally subdivided into two or three regions. An alternative nomenclature derived by Matelli and colleagues15 refers to these areas as F4 and F5, F1 being the traditional primary motor cortex. The ventral premotor cortex areas are likely to be involved in the transformation of information about peripersonal space and visual space into motor commands for movements, particularly of the upper limb. The dorsal premotor cortex corresponds to the superior part of area 6, which also can be subdivided on functional grounds (F2 and F7). The precise role of F2 is debated, but it is involved in reaching and visual signaling. The F7 area is involved in eye movement control and perhaps also in stimulus-response associations for movements.16 The mesial part of area 6, once considered to be a single area, the supplementary motor area, is now subdivided into the supplementary motor area proper (F3) and the presupplementary motor area (F6). The supplementary motor area was originally defined in humans by Penfield and Welch.17 The main supplementary motor area is involved in preparation and selection of movements and perhaps in the initial learning of motor sequences.18 Finally, there are motor cortical areas within the cingulate sulcus (Brodmann areas 23 and 24), termed the rostral, dorsal, and ventral cingulate motor areas. The motor function of these nonprimary motor cortical areas has been discerned by a mixture of methods, including electrical stimulation and neurophysiological mapping, neuroanatomical tracing, and functional neuroimaging in primates. These areas not only contain some somatotopic organization (e.g., face, arm, and leg separations) but also have some direct projections of varying strength to the primary motor cortex and to the brainstem and spinal cord.1,19,20
Primary Motor Cortex
Certain anatomical features are unusual for this cortical region. First, it has the greatest cortical thickness (≈3.8 mm, the adjacent sensory cortex being narrowest at ≈1.8 mm), and it has a relatively low density of neuronal cell bodies.13 Presumably this allows substantial synaptic integration for flexible selection of motor outputs. Second, the area is agranular, lacking an obvious layer IV, and it contains giant Betz cells. These pyramidal neurons are characterized by large and variable size and the presence of many dendrites originating from their cell bodies, in addition to their major apical and basal dendrites.21 Pyramidal cells with output to subcortical and cortical regions are distributed throughout layers II to VI, with the majority in layers III and V. Layer V has a low density of neuronal packing, and approximately 15% of cells have projections through the pyramidal tract and are thus corticospinal cells. Such projections probably make up about 30% of the descending pyramidal tract (see later discussion). The projecting axons are largely myelinated with a range of conduction velocities.
The intrinsic connectivity of the primary motor cortex, as in most of the cortex, is arranged radially in columns. Intrinsic nonpyramidal neurons (including stellate and basket cells) have radially oriented dendrites and make largely local connections. Basket cells exert GABAergic inhibition of pyramidal cell output, part of a recurrent, laterally spreading inhibitory circuit from local corticofugal cells.22 About a third of local pairs of primary cortical cells show evidence of correlated drive during tasks, which indicates that they are receiving common inputs.23,24
Cortical and Thalamic Input to the Pyramidal System
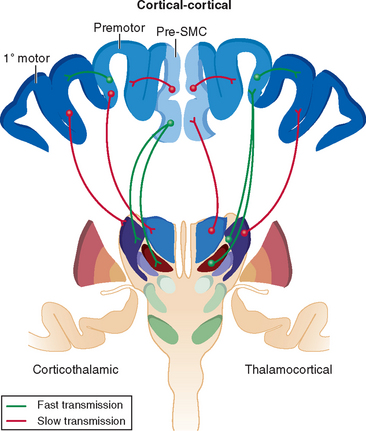
According to findings with various neuroanatomical techniques and electrophysiological mapping, many cortical areas project directly to the primary motor cortex (see Fig. 31-2).15 These include the ventral and dorsal premotor areas (both subfields of area 6), supplementary motor areas, and cingulate (rostral, dorsal, and ventral) motor areas. Some degree of somatotopic organization is maintained in these projections to the primary motor cortex. Sensory information from parietal areas also project either directly or indirectly through sensory cortex to the motor cortex.25 Corticocortical projection neurons are usually derived from the small supragranular pyramidal neurons and provide excitatory drive to supragranular neurons in nearby cortical regions. Within the cortical columns, these supragranular neurons provide strong excitatory drive to the large layer V pyramidal output neurons, thus reinforcing the thalamic input to these neurons.
As discussed previously, a number of thalamic nuclei assist in determining cortical activity by facilitating information transfer from one cortical region to another through a feedforward mechanism.11 In particular, the ventral anterior thalamus feeds forward executive information to premotor cortices (under basal ganglia influence), the ventrolateral anterior thalamus feeds forward premotor information to the primary motor cortex (under basal ganglia and cerebellar influence), and the ventrolateral posterior thalamus provides feedback from primary motor cortex (under cerebellar influence). These reciprocal and nonreciprocal corticothalamocortical connections form cohesive integrated circuits for the control of movement.11
Corticospinal Outputs and Their Origin
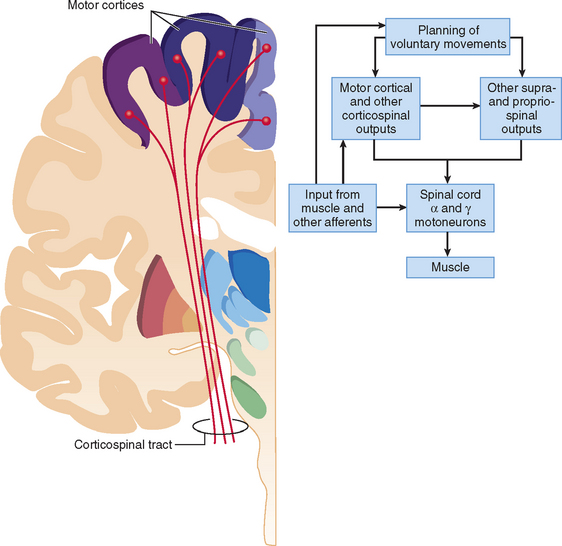
In addition to major corticofugal outputs to the medullary motor nuclei and the spinal cord from the lower part of layer V in the primary motor cortex, the upper part of its layer V has outputs to the striatum, red nucleus, pons, and reticular formation (Fig. 31-3). The focal outputs to the principal relay nuclei of the thalamus originate in layer VI, whereas corticocortical, corticostriate, and corticocallosal fibers arise from layer III.26,27 Evolution of mammals and ultimately primates appears to have shaped the outputs to the medulla and spinal cord similarly, with a progressive shift away from projections to sensory nuclei and toward the motor nuclei. The most direct projections—that is, monosynaptic corticomotoneuronal connections—arise largely from the caudal part of area 4. These outputs diverge at the motoneuronal level in such a way that one corticospinal axon can supply more than one motoneuron pool monosynaptically, and it probably synapses on many motoneurons within each pool.28 The primary motor cortex is concerned not with contraction of single muscles but with the actions of whole groups of functionally related muscles. Its function is to bring them to action both in specific movements and when a more static posture is maintained.29 The divergence of primary motor cortical output and the presence of multiple motor cortical areas allows for parallel control of muscle activity. The densest projections to the motoneuron pools arise in the primary motor cortex.19
COMMENTS ON FUNCTION AND CONCLUSIONS
1 Matelli M, Luppino G, Geyer S, et al. Motor cortex. In: Paxinos G, editor. The Human Nervous System. New York: Academic Press; 2004:973-996.
2 Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889-901.
3 Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford, UK: Clarendon, 1993.
4 Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461-466.
5 Bolam JP, Hanley JJ, Booth PA, et al. Synaptic organization of the basal ganglia. J Anat. 2000;196:527-542.
1 Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677-682.
2 Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators.”. Proc Natl Acad Sci U S A. 1998;95:7121-7126.
3 Vilensky JA, Gilman S. Integrating the work of D. Denny-Brown and some of his contemporaries into current studies of the primate motor cortex. J Neurol Sci. 2001;182:83-87.
4 Bolam JP, Hanley JJ, Booth PA, et al. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527-542.
5 Guillery RW, Sherman SM. The thalamus as a monitor of motor outputs. Philos Trans R Soc Lond B Biol Sci. 2002;357:1809-1821.
6 Darian-Smith C, Darian-Smith I. Thalamic projections to areas 3a, 3b, and 4 in the sensorimotor cortex of the mature and infant macaque monkey. J Comp Neurol. 1993;335:173-199.
7 Smith Y, Raju DV, Pare JF, et al. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520-527.
8 Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48-S56.
9 Hamani C, Saint-Cyr JA, Fraser J, et al. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4-20.
10 Bevan MD, Magill PJ, Terman D, et al. Move to the rhythm: oscillations in the subthalamic nucleus–external globus pallidus network. Trends Neurosci. 2002;25:525-531.
11 Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315-324.
12 Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth, 1909;324.
13 Zilles K. Architecture of the human cerebral cortex: regional and laminar organization. In: Paxinos G, editor. The Human Nervous System. New York: Academic Press; 2004:997-1055.
14 Geyer S, Ledberg A, Schleicher A, et al. Two different areas within the primary motor cortex of man. Nature. 1996;382:805-807.
15 Matelli M, Luppino G, Geyer S, et al. Motor cortex. In: Paxinos G, editor. The Human Nervous System. New York: Academic Press; 2004:973-996.
16 Passingham RE. The Frontal Lobe and Voluntary Action. Oxford, UK: Oxford University Press, 1993.
17 Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951;66:289-317.
18 Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol. 1996;6:782-787.
19 Lemon RN, Maier MA, Armand J, et al. Functional differences in corticospinal projections from macaque primary motor cortex and supplementary motor area. Adv Exp Med Biol. 2002;508:425-434.
20 Miyachi S, Lu X, Inoue S, et al. Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J Neurosci. 2005;25:2547-2556.
21 Scheibel ME, Scheibel AB. The dendritic structures of the human Betz cell. In: Braxier AAB, Pets H, editors. Architectonics of the Cerebral Cortex. New York: Raven Press; 1978:43-57.
22 Hendry SH, Houser CR, Jones EG, et al. Synaptic organization of immunocytochemically identified GABA neurons in the monkey sensory-motor cortex. J Neurocytol. 1983;12:639-660.
23 Fetz EE, Shupe LE. Neural network models of the primate motor system. In: Eckmiller R, editor. Advanced Neural Computers. Amsterdam: Elsevier; 1991:43-50.
24 Baker SN, Olivier E, Lemon RN. An investigation of the intrinsic circuitry of the motor cortex of the monkey using intracortical microstimulation. Exp Brain Res. 1998;123:397-411.
25 Jones EG. Ascending inputs to, and internal organization of, cortical motor areas. Ciba Found Symp. 1987;132:21-39.
26 Jones EG, Wise SP. Size, laminar and columnar distribution of efferent cells in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;175:391-438.
27 Jones EG. Laminar distribution of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex. New York: Plenum Press, 1984.
28 Lemon RN, Baker SN, Davis JA, et al. The importance of the corticomotoneuronal system for control of grasp. Novartis Found Symp. 1998;218:202-218.
29 Kurtzer I, Herter TM, Scott SH. Random change in cortical load representation suggests distinct control of posture and movement. Nat Neurosci. 2005;8:498-504.
30 Strens LH, Fogelson N, Shanahan P, et al. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13:1201-1205.
31 Pierrot-Deseilligny E. Propriospinal transmission of part of the corticospinal excitation in humans. Muscle Nerve. 2002;26:155-172.
32 Fetz EE, Perlmutter SI, Prut Y, et al. Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev. 2002;40:53-65.