Chapter 30 Oral Cavity Cancer
The estimated number of newly diagnosed oral cancers in 2010 was 23,880.1 The estimated number of deaths was 5470.
The oral cavity consists of the lips, floor of the mouth, oral tongue (the anterior two-thirds of the tongue), buccal mucosa, upper and lower gingiva, hard palate, and retromolar trigone. Table 30-1 shows the frequency of involvement of various locations.2 After a general discussion of etiology and epidemiology, issues relative to the various subsites will be presented separately. A discussion of preradiotherapy and postradiotherapy dental care is included.
TABLE 30-1 Distribution of Oral Cavity Cancer: 14,253 Cases
| Site | Percentage |
|---|---|
| Lower lip | 38 |
| Tongue | 22 |
| Floor of mouth | 17 |
| Gingiva | 6 |
| Palate | 6 |
| Retromolar trigone | 5 |
| Upper lip | 4 |
| Buccal mucosa | 2 |
Reprinted from Krolls SO, Hoffman S: Squamous cell carcinoma of the oral soft tissues. A statistical analysis of 14,253 cases by age, sex, and race of patients, J Am Dent Assoc 92:571, 1976.
Etiology and Epidemiology
Oral cavity cancer is predominately a disease of middle-aged men who use tobacco and alcohol. Approximately 95% of carcinomas appear after age 45 years, with an average age of 60 years.2 The use of tobacco in any form is associated with an increased risk of oral cancer.3–5 Some evidence suggests that patients with oral cavity cancer who continue to smoke during radiation therapy have poorer outcomes.6 The risk of tobacco-related cancers of the upper aerodigestive tracts declines among former smokers after 5 years, and after 10 years of abstention the risk may approach that of nonsmokers.7 Although the effects of alcohol and tobacco in inducing cancers of the upper aerodigestive tract seem to be additive, the risk of alcohol consumption without tobacco use is unclear. Some studies indicate a slightly increased risk with alcohol use in the absence of tobacco, whereas others show no apparent increased risk.8,9
Human papillomavirus (HPV) infection, marijuana smoking, betel quid use, and drinking the beverage “mate” have also been implicated as causative factors in the formation of squamous cell carcinomas of the upper aerodigestive tract.10–13 In recent years, oral cancers have increased among relatively young females who have never consumed alcohol or smoked. The reason for this is unclear.14
Smokeless tobacco (snuff) can promote carcinomas of the buccal gingival sulcus, which are diagnosed most often in older Caucasian women living in the southeastern United States. Carcinoma of the buccal mucosa is also associated with chewing tobacco. It is commonly seen in the southeastern United States, with a male to female ratio of 3 or 4 to 1.15 Leukoplakia is seen with oral carcinoma in approximately 15% of cases.16
Persons with a “Scotch-Irish” complexion (red hair and blue eyes) and/or prolonged exposure to sunlight are most susceptible to lip carcinoma.17 In one series, 82% were previous or present tobacco smokers.18 Pipe smoking is an alleged risk factor, but this has not been substantiated by most studies. Lip cancer is often associated with poor dental hygiene or edentulous patients.18,19 Lip trauma and a history of alcohol abuse are also related factors.18 Most cases appear after age 40 years, but approximately 10% occur before age 40 years and a few before age 30 years. This disease is uncommon in blacks.
Oral Care
A complete dental examination should be performed on all patients, whether dentate or edentulous, before irradiating any portion of the mandible or maxilla. The radiation oncologist should inform the patient’s dentist of the anticipated radiation treatment plan, including dose and location of the radiotherapy (RT) fields. To make appropriate pretherapy recommendations, the dentist should be familiar with possible postradiotherapy complications, such as caries and osteoradionecrosis. There is a lifelong risk of impaired healing that can lead to osteoradionecrosis, especially when teeth are extracted from hypovascularized and hypocellular bone.20 Therefore, one objective of the pretherapy oral evaluation is to determine whether teeth in the proposed irradiated area can be reasonably maintained in a healthy state for the remainder of the patient’s life.
Medical, dental, and psychosocial issues that affect a person’s future dental health should be assessed at the pretherapy evaluation. The patient’s compliance, motivation for daily oral hygiene procedures, dental awareness, and access to dental care are predictors of dental health. A panoramic radiograph, intraoral radiographs, and hard and soft tissue examinations should be performed to identify high-risk dental factors such as deep caries, nonrestorable teeth, root tips, bony pathology, endodontically treated teeth, periapical and pulpal pathology, and nonfunctional teeth. Teeth exhibiting periodontal disease should be evaluated to determine their long-term prognosis. Some prognostic factors for poor periodontal health include probing depths more than 6 mm, gingival recession, furcation involvement, and mobility.21,22 Because of the numerous reported cases of progression of gingival recession and periodontal disease after RT, it may be difficult to assess the longevity of each tooth.
To reduce the future risk of osteoradionecrosis, teeth with high-risk dental factors should be removed before the patient receives doses of more than 55 Gy.21 Whether extraction of teeth with moderate disease is indicated remains controversial. If the patient has poor resistance to dental disease or an unwillingness to perform routine dental care or fluoride applications, pretherapy extraction of moderately diseased teeth may be justified. A healing time of 14 to 21 days is recommended after extraction, before initiating radiation therapy.20 Extraction should be accomplished as atraumatically as possible, with alveoloplasty to remove sharp, bony projections. The dentist should coordinate dental appointments with the radiation oncologist to minimize the delay in cancer therapy. However, extraction of healthy teeth does not reduce the risk of osteoradionecrosis and should be avoided.23
Clinical practice guidelines for the treatment of cancer therapy-induced oral mucositis have been published.24 Radiation–induced mucositis cannot be prevented; however, excellent oral hygiene can reduce the risk of oral infections. Supersoft toothbrushes and mild toothpastes are available for patients to facilitate proper oral hygiene during and after RT.
Lip Cancers
Anatomy
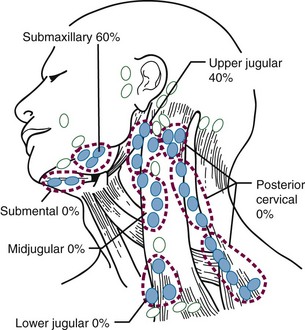
Lymph vessels from both lips drain into the submandibular lymph nodes. In addition, lymph from the central part of the lower lip drains into the submental lymph nodes. The submental nodes drain either to the submandibular lymph nodes or to the jugulo-omohyoid node. The submandibular lymph nodes drain to the deep cervical chain of lymph nodes.25
Pathology and Patterns of Spread
The most common neoplasms are moderately to well differentiated squamous cell carcinomas; approximately 5% are poorly differentiated.26 Basal cell carcinomas usually arise on the skin above or below the lip and invade the vermilion border, but rarely arise from the vermilion border. Squamous cell carcinomas start on the vermilion of the lower lip, and less commonly on the upper lip. The commissure is rarely the site of origin. Leukoplakia is a common problem on the lower lip and may precede carcinoma by many years.16
Early lesions can initially invade adjacent skin and the orbicularis oris muscle. Advanced lesions can invade the adjacent commissures of the lip and buccal mucosa, the skin and wet mucosa of the lip, the adjacent mandible, and eventually the mental nerve. The incidence of perineural invasion is approximately 2%.27 Lymph node involvement at presentation occurs in approximately 5% to 10% of patients. An additional 5% to 10% of patients with a clinically negative neck subsequently develop lymph node metastases. The risk of lymph node involvement increases with depth of invasion, poor differentiation, larger lesions, invasion of the commissure, and recurrence after prior treatment.19
Hendricks and colleagues28 from the Mayo Clinic reported the following incidence of positive cervical lymph nodes by T stage: T1, 2%; T2, 9%; and T3, 30%. The overall incidence of adenopathy was 19% when the commissure was involved.26 De Visscher and associates29 reported a nodal recurrence rate of 5.4% after primary surgical resection in 184 patients with squamous cell carcinoma of the lower lip. Ninety-three percent of lesions were stage I at presentation.
Clinical Manifestations and Staging
Carcinoma of the lip usually presents as a slowly enlarging exophytic lesion with an elevated border. Occasionally, there is minor bleeding. Erythema of the adjacent skin may suggest dermal lymphatic invasion. Anesthesia or paresthesia of the skin indicates perineural invasion.30
The American Joint Committee on Cancer (AJCC) staging for lip cancer applies to lesions arising from the vermilion surface31 (Table 30-2).
TABLE 30-2 American Joint Committee on Cancer: Oral Cavity Primary Tumor Staging
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor 2 cm or less in greatest dimension |
| T2 | Tumor more than 2 cm but not more than 4 cm in greatest dimension |
| T3 | Tumor more than 4 cm in greatest dimension |
| T4 (lip) | Tumor invades through cortical bone, inferior alveolar nerve, floor of mouth, or skin of face (i.e., chin or nose) |
| T4 (oral cavity) | Tumor invades through cortical bone into deep (extrinsic) muscle of tongue (genioglossus, hyoglossus, palatoglossus, and styloglossus muscles), maxillary sinus, or skin of face |
| T4b | Tumor involves masticator space, pterygoid plate, or skull base and/or encases internal carotid artery |
Reprinted from American Joint Committee on Cancer: Lip and oral cavity. In Edge SB, Byrd D, Compton CC, et al, editors: AJCC Cancer Staging Handbook, ed 7. Chicago, 2010, Springer, pp 49-61.
Treatment
Early Lesions (<2 cm)
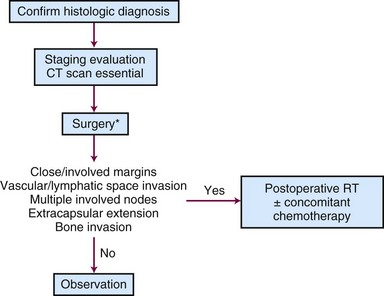
The majority of these lesions can be surgically excised with primary closure as an outpatient procedure. Surgery is satisfactory if the lip commissure does not need to be resected and if the resulting aperture of the oral cavity permits the insertion of dentures. Postoperative irradiation is recommended for positive margins or perineural invasion.27,32,33
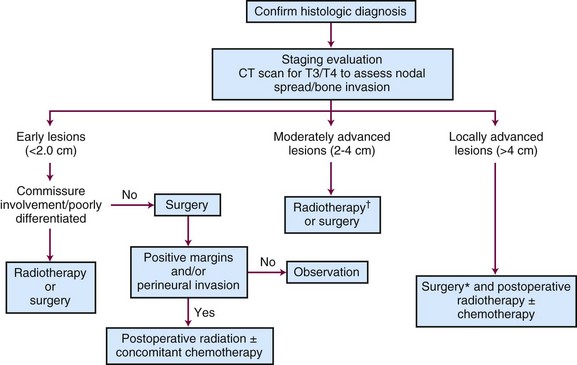
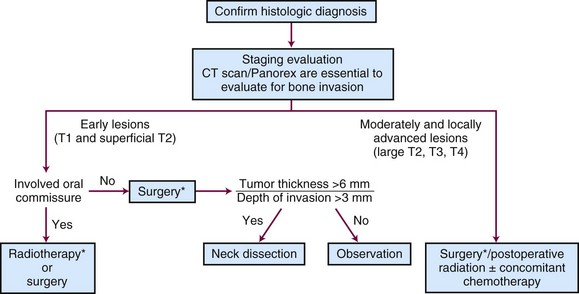
Tumors that should be treated with radiotherapy include those involving a commissure in order to obtain better cosmesis and improved local control.34 The uncommon, poorly differentiated lesions are also preferably treated by irradiation to cover a more generous treatment volume and the first-echelon lymph nodes. An algorithm for treatment planning is shown in Figure 30-1.
Moderately Advanced Lesions (2 to 4 cm)
The length of the lower lip is approximately 7 cm. Removal of more than half of the lower lip with simple closure produces a poor cosmetic and functional result so that a reconstructive procedure is usually necessary. In these cases, irradiation has the advantage of a better functional and cosmetic result. Traditionally, the reconstructed lip may look normal in a photograph but may lack sensory and motor innervation as well as elasticity. However, there have been recent improvements in the functional and cosmetic results of various reconstructive surgical procedures.35–37
Stranc and colleagues34 studied lip function in 37 patients after surgery (19 cases) or radiation therapy (18 cases) and compared results with normal controls. Compared with surgery, irradiation produced better preservation of lip sensation, intercommissural distance, and elasticity. Inadequate lip seal was observed in 2 of 18 patients (11%) after RT compared with 8 of 19 patients (42%) treated surgically.
Teichgraeber and Larson reviewed the M.D. Anderson Hospital experience for patients with lip cancer involving the commissure.38 Of the 22 patients with T2 lesions who were treated with surgery, 10 patients (45%) had recurrence. There are no data for commissure involvement treated with irradiation, but the relative ease of RT and the cosmetic and functional results lead us to recommend irradiation for lesions exhibiting this pattern of spread.
Locally Advanced Lesions (> 4 cm)
Large lesions are managed by resection and postoperative irradiation.33 Erythema of the skin adjacent to the lesion may indicate dermal lymphatic involvement; wide-field irradiation is recommended followed by consideration of surgical resection depending on the response to radiation therapy. Management by definitive radiotherapy and concomitant chemotherapy is generally preferred in patients who are not surgical candidates.
Management of the Neck
Regional lymphatics are not electively treated for T1 and T2 lesions unless commissure involvement is present. Patients with advanced (>4 cm), poorly differentiated, and/or recurrent tumors often require elective neck treatment. Other factors associated with an increased risk of nodal spread include perineural invasion, maximal thickness of more than 6 mm, or low p27Kip1 protein expression.39 The decision to employ elective neck irradiation or elective neck dissection depends on the modality selected for treatment of the primary tumor.
Results
T1 to T3 Lesions
Interstitial Irradiation with or without External Beam Irradiation (EBRT)
Jorgensen and co-workers40 reviewed 869 patients with squamous cell carcinoma who were treated with an interstitial radium implant alone; 90% of the lesions were less than 2 cm in size. The local recurrence and survival rates are shown in Table 30-3; 99% of the primary tumors were ultimately controlled with RT alone or combined with salvage surgery. Thirty-eight percent of patients with T3 tumors who developed a local recurrence developed lymph node metastases. Only 4% of patients died of lip cancer. Twenty-nine complications arose, of which two were bone necrosis.
TABLE 30-3 Lip Carcinoma Treated with Interstitial Radiotherapy (n = 869 Patients)
| Category | 5-yr Local Recurrence | 5-yr Survival |
|---|---|---|
| T1 | 7.4% | 99.5% |
| T2 | 12.7% | 97.4% |
| T3 | 26.4% | 81.4% |
Data from Jorgensen K, Elbrond O, Andersen AP: Carcinoma of the lip. A series of 869 patients, Acta Otolaryngol (Stockh) 75:312-313, 1973.
Pierquin and colleagues41 reported on 50 patients with carcinoma of the lower lip treated with brachytherapy. Only one local recurrence (2%) was observed.
MacKay and Sellers26 reviewed 2854 patients, 92% of whom were initially treated with brachytherapy and EBRT. The primary lesion was controlled in 84% of the cases and 8% were salvaged later for an overall local control rate of 92%. Regional control was achieved in 58% of patients who presented with positive nodes. However, the ultimate rate of neck control was only 35% when neck nodes appeared at a later date. The cause-specific and absolute 5-year survival rates were 89% and 65%, respectively.
Tombolini and associates42 reported on 57 patients with squamous cell carcinoma of the lower lip treated with low-dose-rate brachytherapy alone. Patients with clinically positive neck nodes received EBRT to the involved side of the neck. International Union Against Cancer (UICC) T stages were T1 in 27 cases (47%), T2 in 20 cases (35%), and T3 in 10 cases (18%). The 5-year local control rate was 90%.
Orecchia and colleagues43 treated 47 patients with T1 (n = 21) and T2 (n = 26) lip cancers with iridium-192 (192Ir) brachytherapy. The 5-year and 10-year disease-free survival (DFS) rates were 92% and 85%, respectively.
Guinot and associates44 treated 39 patients with lip carcinoma using high-dose-rate brachytherapy twice daily to total doses ranging from 40.5 to 45 Gy and observed a 3-year local control rate of 88%. Acute and chronic reactions were similar to those observed after low-dose-rate brachytherapy.
Petrovich and associates45 reported on 250 patients with lip cancer treated with RT; half were treated with brachytherapy and the remainder with EBRT. Two hundred forty-seven patients (99%) had squamous cell carcinomas and 240 patients (96%) had lower lip carcinomas. The incidence of lymph node metastasis was 9%. Eleven percent experienced recurrences after irradiation, and half were salvaged; 18 patients (7%) died of lip cancer. Moderately advanced tumors and tumors near the commissures were best treated with EBRT.
EBRT or Surgery
Babington and colleagues33 reported on 130 patients with lip carcinoma; 75% had T1 tumors. Initial treatment consisted of surgery (39%) or RT (48%), or both (13%). Close (≤ 2 mm) or positive margins were observed in 27% of those treated surgically. The 2-year relapse-free survival (RFS) rates were 82% and 54% after RT and surgery, respectively (p < .001). The recurrence rate after surgery was significantly higher for those with close or positive margins.
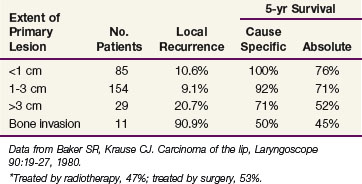
Baker and Krause18 reported on 279 patients treated with either radiotherapy (47%) or surgery (53%) at the University of Michigan (Table 30-4). There was no difference in the 5-year cause-specific survival (CSS) rates between the two groups. Patients with positive regional nodal metastases had a 5-year CSS of 29%. Regional lymph node metastases developed in 31% of those treated for locally recurrent lesions, indicating the need for elective neck treatment for this subset of patients.
De Visscher and associates29 treated 184 patients with squamous cell carcinoma of the lower lip surgically; 93% had stage I cancers. The local and regional recurrence rates were 4.9% and 5.4%, respectively.
Mohs’ Surgery
Mohs and Snow46 reported on 1148 patients with squamous cell carcinomas of the lower lip treated with microscopically controlled surgery. The 5-year local control rates were 94.2% for those with T1 lesions and 59.6% for patients operated on for T2 lesions. The 5-year local control rates were 96.3% for well to moderately differentiated tumors compared with 66.7% for those with poorly differentiated cancers.
Holmkvist and Roenigk47 reported on 50 consecutive patients with squamous cell carcinoma of the lip treated with Mohs’ micrographic surgery (MMS). Four patients (8%) experienced a recurrence; all were successfully salvaged with additional MMS. The average time to recurrence after the initial MMS was 2.5 years. Hruza48 also reported a relatively long interval between MMS and initial recurrence, with 20% of recurrences developing after 5 years.
T4 Lesions
Cancers that present with bone or nerve involvement are usually treated with a combination of surgery and EBRT. There are limited data pertaining to the local control rates after RT or surgery alone, ranging from 0% to 74%26,44; therefore, combined treatment is usually recommended. Byers and colleagues27 observed that 80% of patients with histologically proven perineural invasion developed cervical node metastases. Eight of 25 patients (32%) in their series who presented with either perineural invasion, tumors larger than 3 cm, or regional metastases died of disease.
The postoperative EBRT portals should include the primary site as well as the regional lymphatics (levels IA, IB, and II). The low neck is usually treated to doses sufficient for subclinical disease, and doses are frequently higher in patients with positive nodes. The total dose ranges from 60 to 70 Gy, at 2 Gy per once-daily fraction to the primary site, depending on the pathologic findings. Higher doses with altered fractionation schemes (e.g., 74.4 Gy at 1.2 Gy per fraction twice daily), as well as concomitant chemotherapy, should be considered in patients with positive margins or other high-risk factors such as extracapsular extension.49–51,52,53,54
Recurrent Lesions
Cross and colleagues19 reported on 563 patients treated with surgery for recurrent lip cancer. The prognosis was particularly poor for those with high-grade tumors of whom 16.7% were salvaged compared with 31.8% and 42.9% for those with well and moderately differentiated cancers, respectively. Holmkvist and Roenigk47 reported on four patients treated with MMS; all four were salvaged.
Irradiation Techniques
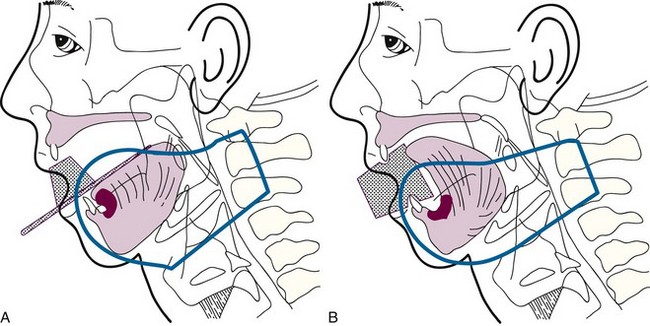
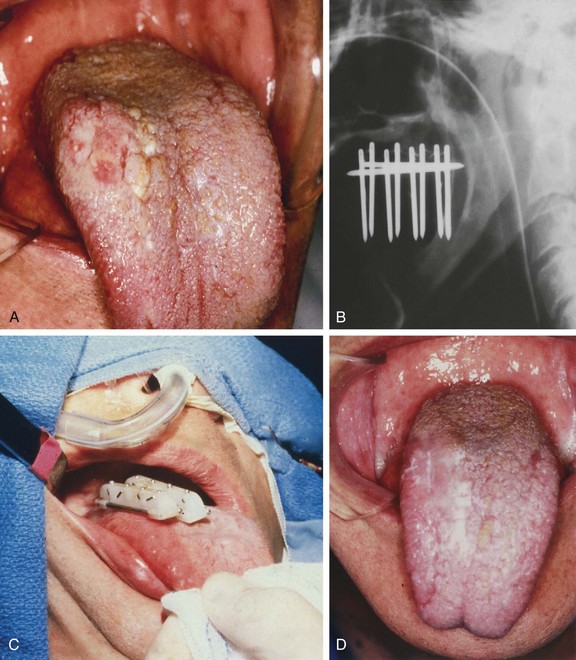
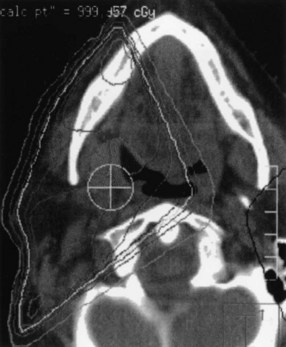
Brachytherapy may be used as the sole treatment or in conjunction with EBRT. Implantation is usually performed under local anesthesia using 192Ir sources and a single-plane plastic tube technique. The sources are arranged horizontally 10 to 12 mm apart with crossing sources on the lateral aspects of the implant. Three to five horizontal sources are used depending on the size of the lesion. The advantage of the plastic tube technique is that the volume of the implant is more easily adapted to the extent of the tumor and the commissure is readily included, if necessary. Alternatively, cesium needles mounted in a nylon bar may be employed (Fig. 30-2). The sources are spaced 1 cm apart and the dose is specified 0.5 cm from the plane of the implant. A gauze roll is placed between the lip and gum to increase the distance between the radioactive sources and the alveolar ridge. The recommended dose is 60 to 70 Gy at a dose rate of 0.4 to 0.5 Gy per hour for an implant alone. Large infiltrative lesions may be first treated with EBRT, 30 Gy at 2.5 Gy per fraction to shrink the tumor, followed by an interstitial brachytherapy boost to deliver an additional 35 to 40 Gy. Treatment of lip cancer with high-dose-rate interstitial needles is advocated by some.44
T3 and T4 Tumors
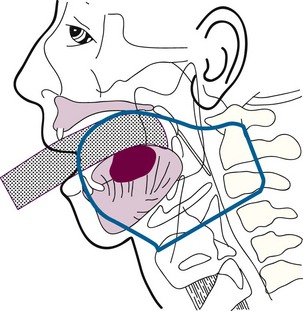
Low-volume T3 cancers may be treated with primary irradiation, preferably combining EBRT to the primary lesion and neck followed by a brachytherapy boost. EBRT is administered with parallel opposed fields, including the lip lesion and the level I and II lymph nodes55 (Fig. 30-3). A cork is placed in the mouth to displace the maxilla and upper lip and reduce the volume of normal tissue included in the fields. A separate anterior field is used to treat the level III and IV lymph nodes with a tapered midline block over the larynx. The supraclavicular lymph nodes are at low risk and are not included in the fields. Both sides of the neck are treated with irradiation because it is unlikely that T3 and T4 primary lesions would be well lateralized.
Complications
Fitzpatrick56 reported a 3.3% incidence of late complications that required surgical intervention. Orecchia and colleagues43 observed a 10.6% incidence of mucosal necrosis after RT for 47 T1 and T2 lip cancers treated with 192Ir brachytherapy. The risk of late complications increased with dose, dose per fraction, and volume.
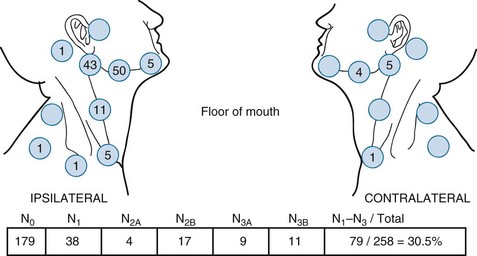
Floor of the Mouth
Anatomy
The first-echelon nodes for the floor of mouth are the submandibular lymph nodes (level IB), which may be stratified into preglandular, intraglandular, and postglandular groups. Lymph may also drain into the submental (level IA) nodes. These lymph nodes eventually drain to the jugulo-omohyoid (level II) nodes.25
Pathology and Patterns of Spread
Small to moderate-size (stage T1 and T2) lesions are associated with metastases to the ipsilateral regional lymph nodes in 15% to 38% of cases, depending on the size and depth of invasion of the primary tumor.57,58,59
Mohit-Tabatabai and colleagues60 reviewed 84 patients with squamous cell carcinoma of the floor of the mouth and concluded that lesion thickness was related to the probability of subclinical cervical metastases in clinically node negative (N0) patients (Table 30-5). Patients with a primary lesion more than 1.5 mm thick had a 20% or higher risk of lymph node metastases.60 Histologic grade and configuration of the primary lesion did not have a statistically significant correlation with subsequent development of neck node metastases. O’Brien and associates58 analyzed the impact of tumor thickness on the incidence of regional lymph node metastases and found that the risk increased significantly for lesions of 4 mm or more in thickness. The reported incidence of recurrence in the untreated clinically negative neck varies from 20% to 35%.59,61 Histologic grade and extent of vascular and perineural invasion have been implicated as predictors of lymph node spread.62
TABLE 30-5 Floor of Mouth Carcinomas: Correlation of Primary Tumor Thickness with Neck Failure*
| Thickness (mm) | T1N0 | T2N0 |
|---|---|---|
| 0.1 to 1.5 | 1/38 (3%) | 0/19 |
| 1.6 to 3.5 | 1/5 (20%) | 3/7 (43%) |
| ≥3.6 | 7/11 (64%) | 2/4 (50%) |
* Number of treatment failures/total number of patients.
Reprinted from Mohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A: Relation of thickness of floor of mouth stage I and II cancers to regional metastasis, Am J Surg 152:351-353, 1986.
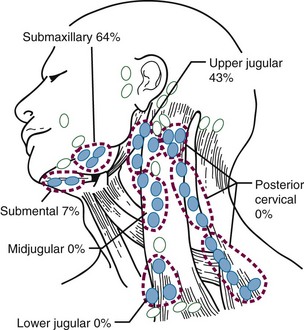
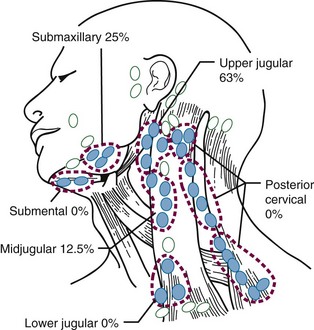
Figure 30-4 shows the distribution of clinically positive neck nodes at diagnosis,63 and Figure 30-5 depicts the distribution of pathologically positive nodes after elective neck dissection in 62 patients with carcinoma of the floor of the mouth.64 The incidence of positive lymph nodes was 19% for those with T1 or T2 lesions and 26% for patients with T3 or T4 cancers.
Clinical Manifestations, Patient Evaluation, and Staging
The AJCC staging system65 (see Table 30-2) is based on tumor size and invasion of adjacent structures such as bone or soft tissue of the neck. Radiographic studies may facilitate staging with reference to (1) the status of the mandible and teeth, (2) the deep extent of the tumor, and (3) the evaluation of the regional lymph nodes. CT scans should be obtained in essentially all patients. The mandible may be evaluated by panoramic x-ray films, dental films, and CT scanning. Magnetic resonance imaging (MRI) is useful to evaluate marrow space invasion and perineural involvement.66
The role of PET scanning as part of the initial staging workup for oral cavity cancers has not gained widespread acceptance. It may, however, be useful in early detection of recurrences and in prediction of which patients may benefit from elective neck dissection after chemoradiation.67–69
Treatment
Early Lesions (T1 and Superficial T2)
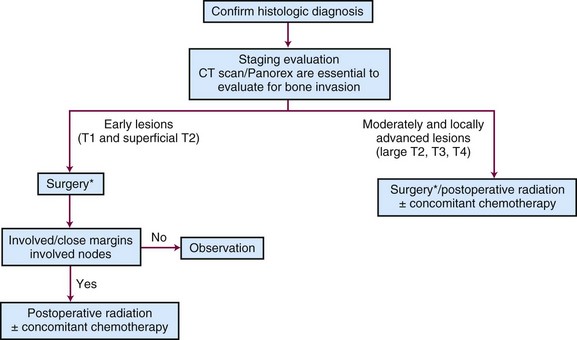
Surgery and radiation therapy produce equal cure rates for T1 and superficial T2 lesions. The risk of irradiation-induced bone and soft tissue necrosis is significant. Therefore, surgery is usually the treatment of choice. The neck is also treated with an elective neck dissection,70 although some advocate observation of the neck in select patients with clinically negative nodes (cN0).71 A treatment algorithm is shown in Figure 30-6.
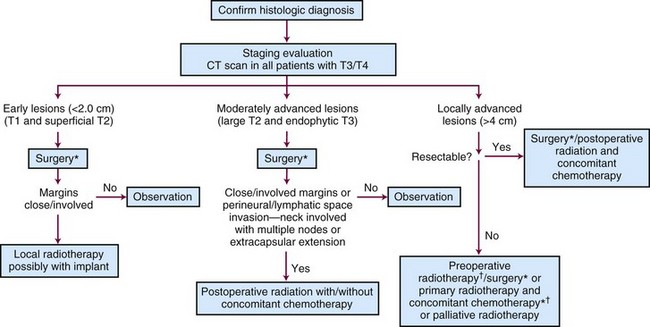
Figure 30-6 Treatment algorithm for de novo floor of mouth cancer. *Treat neck with neck dissection or radiotherapy in any patient with a primary lesion that is more than 1.5 mm thick.60 † Concomitant chemotherapy may be used.154
Sentinel lymph node biopsy is being investigated for possible use in oral cavity cancers.72,73 However, a recent study showed that this procedure was less sensitive for floor of mouth cancers (80%) compared with other oral sites (100%).74
Some patients present after excisional biopsy of the primary tumor. If the margins are either close or involved and there is no evidence of visible or palpable residual tumor, an interstitial implant alone to the primary site is a good alternative provided that the depth of invasion is less than 1.5 mm. Re-excision may not be feasible, because the surgeon does not know exactly what to remove. An additional advantage of RT is the ability to treat a larger area. Six patients were treated in this manner at M.D. Anderson Hospital75 and seven patients at the University of Florida.76 All had disease locally controlled, and none of the patients developed regional lymph node metastases. Conversely, Sessions and colleagues71 have recommended re-excision rather than irradiation if close or positive margins are found on permanent sections.
Moderately Advanced Lesions (Large T2 and Exophytic T3)
One common indication for adjuvant RT is the inability of the surgeon to obtain adequate margins of resection, because this often leads to local recurrence,77 even if immediate re-excision is performed.78 Jacobs and associates79 and Laramore and colleagues80 reported a large intergroup study where adjuvant postoperative EBRT (60 Gy) was administered for locally advanced cancers. They found that the relapse rate was 11% in patients with satisfactory margins and 26% in those with unsatisfactory margins. Unsatisfactory margins tend to reflect a higher residual tumor burden; therefore it may be prudent to deliver a higher dose of postoperative RT. At the University of Florida, patients with involved margins receive hyperfractionated irradiation to increase the dose given to the primary site while minimizing the potential late morbidity.52
Lapeyre and colleagues81 reported on 36 patients with oral tongue or floor of mouth carcinomas treated with brachytherapy after resection with close or positive margins. The local control rate was 88.5% at 2 years. Grade 2 to 3 chronic sequelae were seen in 3 of 19 patients (16%) with floor of mouth cancers.
The neck should be electively dissected if it is clinically negative. If there are multiple positive nodes or extracapsular extension, postoperative irradiation is indicated.32,52 Based on the latest results of the European Organization for Research and Treatment of Cancer (EORTC) and Radiation Therapy Oncology Group (RTOG) trials, concomitant chemotherapy is generally recommended as well in high-risk postoperative settings such as tumors with positive margins and/or extracapsular extension.49,51
Concomitant Postoperative Chemoradiation
The issue of whether concomitant chemotherapy is beneficial when administered with postoperative irradiation for head and neck cancer was recently addressed by two randomized trials (RTOG 9501 and EORTC 22931).49,51 Each of these showed an improvement in locoregional control and DFS when cisplatin (100 mg/m2) was given on days 1, 22, and 43 of the EBRT regimen.49,51 Severe acute effects are seen more frequently with chemoradiation compared with postoperative RT alone.
Results
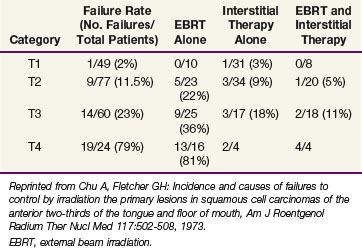
Outcomes after radiation therapy alone vary with the stage of disease and treatment technique. The irradiation schedules used at the University of Florida are shown in Table 30-6.82 EBRT alone results in lower local control rates compared with brachytherapy alone or combined with EBRT.83 Table 30-7 provides the M.D. Anderson Hospital experience with RT alone.84 The failure rates range from 2% for T1 lesions to 23% and 79% for T3 and T4 lesions, respectively. For T2 and T3 lesions, brachytherapy with or without EBRT appears to result in better local control rates than EBRT alone.
TABLE 30-6 Carcinoma of the Floor of Mouth: Radiation Therapy Schedules Currently Prescribed at the University of Florida
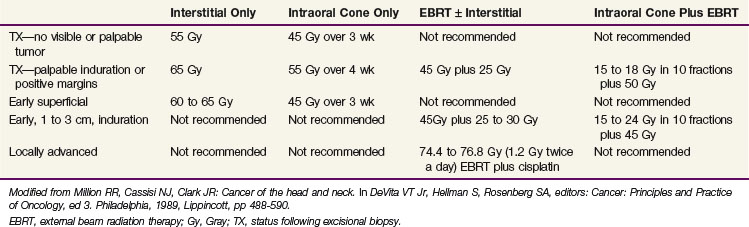
TABLE 30-7 Floor of Mouth Cancer: Failure to Control the Primary Lesion versus Radiation Therapy Technique (M.D. Anderson Hospital, January 1948 through December 1968)
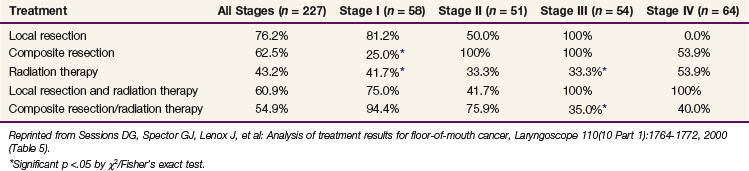
Sessions and colleagues71 reported on 280 patients who were treated with surgery alone, RT alone, or combined surgery and RT. Tumor extent was T1, 106 patients; T2, 107 patients; T3, 40 patients; and T4, 27 patients. The local recurrence rate was 41%. The 5-year CSS by treatment modality are shown in Table 30-8.
TABLE 30-8 Floor of Mouth Carcinoma: 5-yr Cause-Specific Survival Rates versus Treatment Modality and cTNM Stages (Washington University) (n = 227 Patients)
One hundred sixty patients with T1 (79 patients) and T2 (81 patients) floor of mouth cancers were treated at the Institut Gustave-Roussy with low-dose-rate 192Ir brachytherapy.85 One hundred twenty-seven patients had a clinically negative neck and 33 patients (21%) had N1 neck disease. Patients with T2 and/or N1 lesions underwent a neck dissection. With a minimum follow-up of 9 years, local control rates were 93% for T1 cancers and 88% for T2 tumors.
Pernot and associates86 reported on 207 patients with floor of mouth carcinomas treated with EBRT and 192Ir brachytherapy (105 patients) or brachytherapy alone (102 patients). Tumor stages were 41%, Tl; 48%, T2; 8%, T3; 2%, T4; and 1%, TX. Neck stages were 83%, N0; 12%, Nl; 3%, N2; and 2%, N3. The 5-year local control rates were 97%, 72%, and 51% for Tl, T2, and T3 tumors, respectively. The 5-year CSS were T1, 88%; T2, 47%; and T3, 36%.
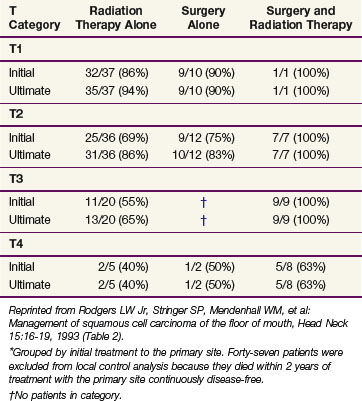
Rodgers and associates87 reviewed 194 patients treated at the University of Florida. Patients with advanced lesions (T3 or T4) had lower control rates when treated with RT or surgery alone compared with those treated with combined surgery and RT (Table 30-9). The 5-year CSS were stage I, 96%; stage II, 70%; stage III, 67%; and stage IVA, 44%.
TABLE 30-9 Floor of Mouth Carcinoma: Initial and Ultimate Local Control Rates*
(University of Florida) (n = 194 Patients)
Irradiation Techniques
T1 and T2 Cancers
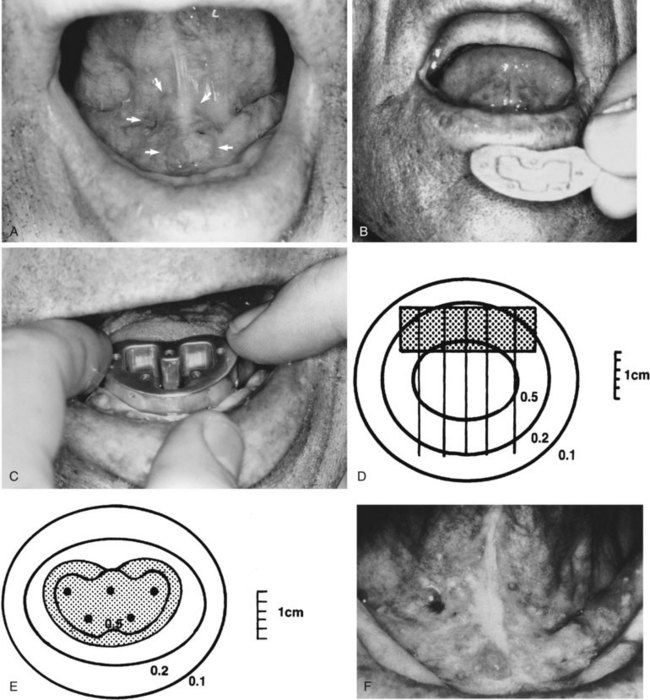
Patients with superficial (≤4 mm thick), well-differentiated squamous cell carcinomas of the floor of the mouth may be treated either with brachytherapy alone or, when accessible, with intraoral cone irradiation. Brachytherapy is not feasible if the tumor abuts or extends onto the mandibular alveolar ridge because of the risk of bone exposure. Brachytherapy may be performed with either rigid cesium needles mounted in a customized template or with iridium using the plastic tube technique. The rigid needles are preferable because although the needles are active, the implant can be accomplished rapidly because the needles are mounted in a rigid template88,89 (Figs. 30-7 and 30-8). An additional advantage of this technique is that the geometry of the implant is optimal and dosimetry can be obtained before the implant. The vertical needles are spaced approximately 1 cm apart with a crosser to ensure an adequate surface dose. The implant is anchored in place by a suture placed through the submentum into the floor of the mouth.
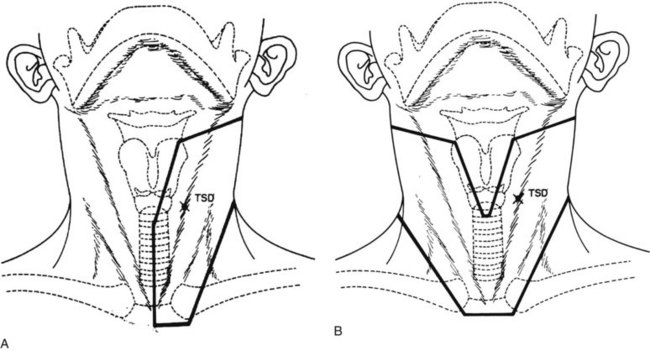
Cancers thicker than 4 mm and those that are poorly differentiated have an increased risk of subclinical disease in the regional nodes. The first-echelon nodes for the floor of the mouth are the level I and II nodes. EBRT is delivered with either 4-MV or 6-MV x-rays using parallel opposed fields that encompass the primary tumor as well as the first-echelon nodes. An intraoral stent is placed in the mouth to displace the maxilla and upper lip out of the fields55 (Fig. 30-9). The EBRT fields are treated to 46 Gy in 23 fractions once daily or 38.4 Gy at 1.6 Gy per fraction twice daily. Brachytherapy follows the EBRT if that is the technique selected to boost the tumor. If intraoral cone radiation therapy is selected to boost the tumor, it precedes the EBRT so the extent of the tumor can be optimally defined and because it may be difficult to place the cone after EBRT because of patient discomfort. The total dose ranges from 65 to 70 Gy.
The low neck is irradiated with an anterior field, using 4-MV or 6-MV x-rays to 50 Gy in 25 fractions or 40.5 Gy in 15 fractions. The latter schedule is preferred if treatment to the upper neck will be completed in less than 25 fractions. A tapered midline block is used to shield the larynx. The junction between the parallel opposed fields and the low neck field is at the thyroid notch90 (Fig. 30-10).
T3 and T4 Cancers
The likelihood of cure without a major complication after primary RT is low. Therefore, if patients are treated with curative intent, postoperative irradiation is combined with resection of the primary tumor and neck dissection. The fields are similar to those described for patients treated with RT alone. The superior border of the field is extended to the skull base if there are multiple positive nodes and/or extracapsular extension. The portals are reduced off of the spinal cord at 44 to 46 Gy and, if necessary, the neck posterior to the reduced fields may be irradiated with 8-MeV to 10-MeV electrons. Patients with ipsilateral positive nodes may be treated with intensity-modulated radiotherapy (IMRT) to reduce the dose to the contralateral parotid gland and, thus, the risk of long-term xerostomia.91,92 Patients with bilateral positive nodes are treated with parallel opposed fields because parotid-sparing IMRT may result in an increased risk of a marginal recurrence. Those with negative nodes may be treated with parallel opposed fields excluding most of the parotid glands. A petroleum jelly gauze bolus is placed on the incision to ensure an adequate surface dose. Patients with negative margins generally receive 60 Gy at 2 Gy per fraction. Altered fractionation should be considered for patients with positive margins and for those with an interval of more than 6 weeks between surgery and initiation of postoperative irradiation. The preferred schedule at the University of Florida is 74.4 Gy at 1.2 Gy per fraction administered twice daily in a continuous course over 6.5 weeks, usually with concomitant cisplatin.
Complications
A limited soft tissue necrosis may develop in the floor of the mouth, usually at the site of the original lesion. These ulcers are painful and usually respond to local anesthetic measures (lidocaine), antibiotics, and time. If the ulceration develops on the gingiva, the underlying mandible may be exposed. It is recommended to discontinue wearing of dentures, apply a local anesthetic, and administer antibiotics. Recent data suggest that pentoxifylline, 400 mg three or four times daily, may be beneficial in this setting, and may also reduce late irradiation-induced fibrosis.93 If the soft tissue or bone necrosis progresses, hyperbaric oxygen and surgical debridement may be necessary.94,95 Eighteen of 194 patients (9%) treated at the University of Florida developed severe complications87 (Table 30-10).
TABLE 30-10 Carcinoma of Floor of the Mouth: Treatment Complications (n = 194 Patients)
| Initial Treatment | Patients with Severe* Complications | Patients with Mild to Moderate† Complications |
|---|---|---|
| Radiotherapy alone | 6/117 (5%) | 49/117 (42%) |
| Surgery alone | 6/36 (17%) | 3/36 (8%) |
| Surgery and radiotherapy‡ | 6/41 (15%) | 8/41 (20%) |
* Postoperative complications (e.g., myocardial infarction, pulmonary embolism), wound infection or dehiscence, fistula formation, or osteoradionecrosis requiring hospitalization or surgical intervention.
† Small bone exposure, soft tissue necrosis, or minor infection. Osteoradionecrosis or surgical wound complication requiring outpatient therapy only.
‡ Preoperative or postoperative radiotherapy.
Reprinted from Rodgers LW Jr, Stringer SP, Mendenhall WM, et al: Management of squamous cell carcinoma of the floor of mouth, Head Neck 15:16-19, 1993 (Table 3).
Sessions and colleagues71 reported on 280 patients treated with surgery alone and/or RT. They observed late irradiation complications in 41% of patients treated by EBRT and brachytherapy, most of which appeared within 1 year of treatment. Surgical complications included infection (5%), wound slough (9.6%), orocutaneous fistula (6.1%), carotid artery exposure/blowout (1.1%), and delayed fatality (1.4%). Fifty-eight percent of these complications were related to the use of combined surgery and EBRT.
Bone necrosis was seen in 18% of patients treated with brachytherapy for T1 to T2 floor of mouth cancers at the Institut Gustave-Roussy; 2.5% were severe.85
Oral Tongue Cancers
Anatomy
The tongue has a submucosal plexus of lymph vessels. There are three routes of lymphatic drainage from the oral tongue: the tip of the tongue drains to the submental lymph nodes; lymph from the lateral aspects of the tongue drains to the submandibular lymph nodes and from there into the deep cervical lymph nodes; lymph from the medial tongue drains directly to the inferior deep cervical lymph nodes. Approximately 15% of patients have lymphatic metastases that bypass level II and present in levels III and IV.96 Lymphatic drainage is unilateral; drainage to the contralateral lymphatics is unlikely.
Pathology and Patterns of Spread
Ninety-five percent of oral tongue cancers are squamous cell carcinomas. Leukoplakia is also common.97 Verrucous carcinoma and minor salivary gland tumors occur infrequently.
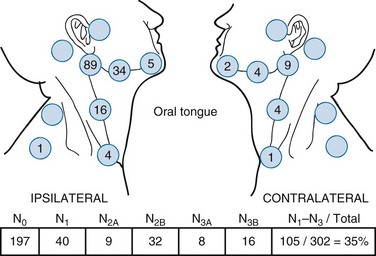
The distribution of clinically positive neck node metastases on presentation is shown in Figure 30-11.63 Forty-five percent of patients with cancers of the oral tongue have clinically positive nodes at diagnosis; 5% are bilateral. Byers and associates64 reported a 19% incidence of subclinical disease for T1N0 and T2N0 lesions, and 32% for T3N0 to T4N0 cancers after elective neck dissection. Ozeki and colleagues98 have reported three cases with metastases to the lingual lymph nodes.
Clinical Manifestations, Patient Evaluation, and Staging
Palpation of the tongue and floor of the mouth, visual examination, and assessment of tongue mobility will help define the extent of the primary tumor. CT and, to a lesser extent MRI, is useful for defining the deep extent of larger primary tumors and to assess the neck. The staging system is depicted in Table 30-2.31
Treatment
A treatment algorithm is shown in Figure 30-12.
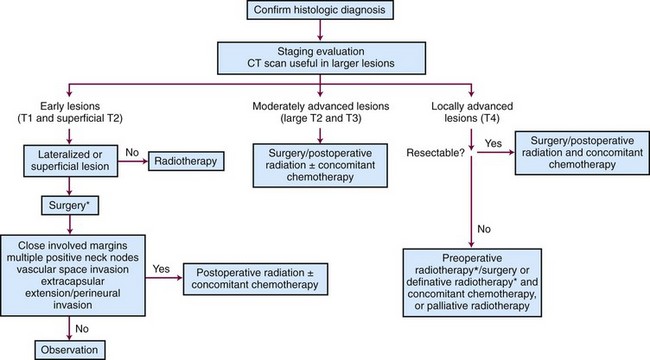
Figure 30-12 Treatment algorithm for de novo oral tongue cancer. *Concomitant chemotherapy may be used.154
Early Lesions (T1 and Superficial T2)
Surgery and radiotherapy are equally effective in controlling small oral tongue cancers. Superficial well-defined lesions can be cured using resection alone with good functional results.99,100 Postoperative irradiation is recommended for close or positive margins,77,101 multiple positive neck nodes, vascular space invasion, extracapsular extension, or perineural invasion.32,52 Although the risk of a significant RT complication is low, surgery is the preferred treatment in the authors’ institution because of a lower risk of bone exposure or soft tissue necrosis that may persist for months or years after RT.
Patients are treated with definitive irradiation if they decline surgery or are at high risk for operative complications.102 Radiation therapy for early-stage oral tongue carcinoma can be given with EBRT combined with an interstitial implant or an intraoral cone boost, or with an interstitial implant alone.84,103 The results of EBRT alone are suboptimal.
Results
Wang104 evaluated results of various boost techniques combined with EBRT for patients with T1 and T2 oral tongue lesions and observed a 5-year actuarial local control rate of 54% after an interstitial implant, 50% after intraoral cone orthovoltage irradiation, and 86% after intraoral cone electron beam therapy. The reason that local control was better after the electron beam intraoral cone boost compared with the orthovoltage intraoral cone treatment is unclear. Compared with interstitial implantation, intraoral cone irradiation is associated with a lower dose to the mandible and does not require anesthesia or hospitalization. It may also be associated with fewer complications. Currently, physicians at Massachusetts General Hospital give 3 Gy per fraction for eight or nine daily fractions with intraoral electron beam RT (five fractions per week) followed immediately by EBRT with 1.6 Gy twice daily for 12 days.104 The patient is given a 1.5- to 2-week split and resumes radiotherapy to an EBRT dose of 51.4 Gy; the total dose is approximately 75 Gy.
Intraoral cone irradiation with orthovoltage x-rays is preferred at the University of Florida.89 Patients usually receive 3 Gy per fraction with intraoral cone treatment for 7 to 9 days for a total of 21 to 27 Gy followed by EBRT to 30 Gy in 10 once-daily fractions or 32 Gy at 1.6 Gy per fraction twice daily over 2 weeks to the primary site and neck.
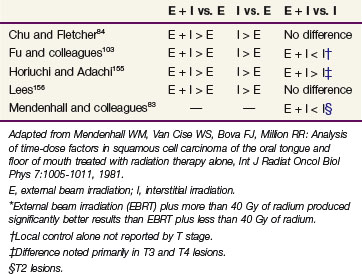
Brachytherapy may also be combined with EBRT to treat oral tongue cancer.100,103,105 Patients treated at the University of Florida with EBRT followed by an interstitial implant had a local control rate of 83%.102 Wendt and colleagues105 reported on the M.D. Anderson Cancer Center experience with T1N0 and T2N0 oral tongue carcinomas in which patients underwent either brachytherapy alone or combined with EBRT. Of eight patients who were treated with EBRT alone, five (63%) developed a local recurrence. Six of 18 patients (33%) treated with interstitial therapy failed at the primary site. In those patients treated with a combination of EBRT and brachytherapy, the 2-year local control rate was 92% for those treated with EBRT doses less than 40 Gy combined with a moderately high brachytherapy dose, compared with 65% for patients who received EBRT doses of 40 Gy or more with a lower brachytherapy dose.105 A review of the pertinent literature supports the efficacy of brachytherapy83 (Table 30-11).
Subclinical disease in the neck nodes is common in patients with oral tongue cancer. Matsuura and colleagues stressed the importance of elective treatment to the neck, especially as tumor thickness increases.106 In their experience, patients with tumor thickness of 8 mm or more were at an increased risk of nodal failure in the clinically negative neck. Wendt and associates105 also showed the importance of elective therapy to the clinically negative neck. Failure in the neck occurred in 44% of patients receiving no elective neck irradiation; 27% of the failures were in patients receiving less than 40 Gy compared with an 11% failure rate for those who received 40 Gy or more. Byers and associates107 recommended elective treatment to the clinically negative neck in patients with T2 to T4 primary tumors. Haddadin and colleagues108 observed that patients with T2 tongue carcinomas had a significantly better outcome after an elective neck dissection compared with those who were followed with salvage reserved until development of a regional failure (75% vs. 39% 5-year OS, respectively). Based on a review of the Memorial Sloan-Kettering Cancer Center experience, Spiro and colleagues99 recommended that elective neck therapy be given for primary tumors exceeding 2 mm in thickness because the risk of cervical metastasis approached 40% in this cohort of patients. O-Charoenrat and associates109 found that increasing tumor thickness predicted for occult cervical node metastasis and poor outcomes in patients with stage I and II oral tongue squamous cell carcinoma treated at the Royal Marsden Hospital. Univariate analysis of DFS showed poorer outcomes for patients older than 60 years (p = .0423) and with tumors thicker than 5 mm (p = .0067). The impact of tumor thickness on outcome (p = .005) was also appreciated in a multivariate analysis. They recommended elective neck treatment for tumors more than 5 mm thick.
The use of sentinel lymph node biopsy and PET scanning may alter the treatment paradigm as more experience is gained in their use for oral cavity cancers.110
Bourgier and colleagues reported on 279 patients treated with exclusive low-dose-rate brachytherapy with or without an elective neck dissection for T2N0 squamous cell carcinoma of the oral tongue.111 The incidence of subclinical cervical node metastases was 45%. The 2-year local and regional control rates were 79% and 76%, respectively. The incidence of grade 3 complications was 3%.
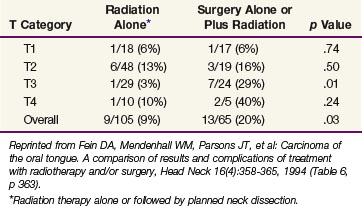
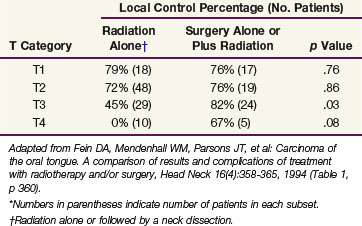
Fein and colleagues102 compared the results and complications of treatment with irradiation and/or surgery for squamous cell carcinoma of the oral tongue. The control rates for RT alone or surgery alone or in combination with RT were the same for stage T1 and T2 lesions; combined-modality treatment resulted in higher cure rates for T3 and T4 tumors (Table 30-12). In an analysis of patients with T1 and T2 lesions, those receiving less than 30 Gy of EBRT plus an interstitial implant had a better outcome than those receiving surgery plus or minus postoperative RT.102 Currently, for patients receiving radiation therapy alone, a dose of 1.6 Gy per fraction is given twice daily to 32 Gy followed immediately by an interstitial implant for an additional 35 to 40 Gy. This technique decreases the overall treatment time and avoids the large dose per fraction associated with 30 Gy in 10 fractions over 2 weeks.102
TABLE 30-12 Oral Tongue Carcinoma: Probability of Local Control at 2 yr According to T Category and Treatment Method (University of Florida)*
Chao and associates101 reported on 55 patients with stage T1 and T2 lesions treated with surgery and postoperative RT. Thirty-nine patients received EBRT alone, and 16 patients had an interstitial implant as part of the treatment. By adding an interstitial implant for patients with positive margins, the local control rate was equivalent to that observed for patients with negative margins treated with EBRT alone.
Sessions and colleagues100 reported on 332 patients with oral tongue cancer treated with surgery and/or RT at Washington University. Tumor stages at presentation were T1, 116 patients; T2, 128 patients; T3, 71 patients; and T4, 17 patients. Local recurrences occurred in 34%, and 31% suffered a neck recurrence. The 5-year CSS by treatment group for 279 of the 332 patients are shown in Table 30-13.
Irradiation Techniques
T1 and T2 Cancers
Reduction of overall treatment time is key to the successful treatment of oral tongue carcinomas with RT alone. Patients with well-differentiated carcinomas that are 4 mm or less are optimally treated with brachytherapy alone (Fig. 30-13). Interstitial implantation may be accomplished using rigid cesium needles mounted in a bar or with iridium using the plastic tube technique. Rigid cesium needles mounted in a bar are difficult to position because of the length of the needles, unless the lesion is relatively superficial. Therefore the plastic tube technique is preferred. The total dose varies from 65 to 70 Gy over 5 to 7 days.
Patients who have poorly differentiated carcinomas, as well as those with 5 mm or more depth of invasion, should be treated with a combination of EBRT and brachytherapy. Parallel opposed fields include the primary tumor as well as the level I and II lymph nodes. A cork and tongue block displaces the tongue inferiorly and the maxilla superiorly to minimize the amount of normal tissue included in the portals (Fig. 30-14). The fields are weighted 3 : 2 to the side of the tumor and either 30 Gy in 10 fractions once daily or 32 to 38.4 Gy at 1.6 Gy per fraction twice daily is delivered over 2 to 2.5 weeks with 4-MV or 6-MV x-rays. Although 60Co is an ideal beam for treatment of patients with oral cavity cancers, it is not available in most clinics. The level III and IV nodes are included in an anterior field as previously described. After EBRT, 35 to 40 Gy is added with an interstitial implant.
T3 and T4 Cancers
Patients with T3 and T4 oral tongue carcinomas are difficult to cure with RT alone. The ability to adequately encompass the primary tumor with an interstitial implant is difficult for all but the occasional patient with a favorable low-volume T3 cancer. The risk of a major complication, such as a soft tissue necrosis and/or bone necrosis, is high after successful RT. Therefore most patients are treated with surgery and postoperative irradiation. As for other sites, the dose depends on the margin status: Patients with negative margins generally receive 60 Gy at 2 Gy per fraction. Altered fractionation should be considered for patients with positive margins and/or for those with multiple risk factors or an interval of more than 6 weeks between surgery and initiation of postoperative irradiation. At our institution, the preferred schedule is 74.4 Gy at 1.2 Gy per fraction administered twice daily in a continuous course over 6.5 weeks. Concomitant cisplatin is also recommended for high-risk situations.52
Complications
Small, self-limited soft tissue necroses are fairly common. If these occur, recurrent cancer must be ruled out. If the lesion is thought to be necrotic, conservative treatment is instituted. The patient is examined frequently and broad-spectrum antibiotics such as tetracycline are initiated as well as pentoxifylline.93 Viscous lidocaine can be applied to the ulcer for local analgesia. Hyperbaric oxygen treatment is indicated for larger progressive necroses that do not respond to conservative management. Surgery is employed as a last resort for large persistent necroses that are often associated with bone necrosis.
Osteoradionecrosis occurs infrequently; the onset varies from 1 month to many years after radiation therapy.112 It is more common in patients receiving higher doses per fraction and/or with tumor invading the bone.113 If a patient has dentures, the dentures must be removed or altered by the dentist to avoid trauma to areas of exposed bone; healing may require many months. Management is similar to that for soft tissue necroses.
Oral pilocarpine has been advocated by some for xerostomia. Pilocarpine exercises a broad spectrum of pharmacologic effects, including increasing the secretions of exocrine glands, most notably the salivary glands. In a multicenter, randomized, double-blind, placebo-controlled trial published in 1993, pilocarpine produced a clinically significant benefit.114 However, its efficacy has been questioned by the outcomes of recent randomized trials.115,116 The recommended dosage is 5 mg, three or four times a day. The time interval necessary to achieve optimal results is 8 to 12 weeks.
Amifostine has been used more recently in an effort to lessen the untoward effects of RT. It can be administered either intravenously or subcutaneously.117,118 The latter is generally felt to be more convenient and associated with less toxicity.119 A full course of treatment with amifostine is quite expensive. This fact, coupled with its potential to cause nausea and skin reactions, has limited its acceptance in some centers.
At the University of Florida, the severe complication rate for 170 patients treated with surgery alone or in combination with RT for oral tongue cancer has been compared with the severe complication rate for patients treated with RT alone; the findings are presented in Table 30-14.102
Sessions and colleagues100 reported major treatment complications in 21 of 270 patients (12.8%), most of which occurred in those receiving composite resection and RT. Complications associated with RT included orocutaneous fistula (6/224 [2.7%]), flap necrosis (1/224 [0.4%]), carotid hemorrhage (1/224 [0.4%]), xerostomia (7/224 [3.1%]), trismus (5/224 [2.2%]), radiation caries (3.1%), soft tissue ulcer (22/224 [9.8%]), bone necrosis (14/224 [6.2%]), and dysphagia (3/224 [1.3%]).100
Buccal Mucosa
Pathology and Patterns of Spread
The majority of tumors originating from the buccal mucosa are low-grade squamous cell carcinomas that are frequently associated with leukoplakia. Verrucous carcinomas are observed more frequently in the buccal mucosa than in other parts of the oral cavity.120
The first-echelon lymphatics are the submandibular and subdigastric lymph nodes. The incidence of positive nodes at diagnosis ranges from 9% to 31%; the risk of subclinical disease is 16%.121 Bilateral cervical node metastases are very unusual. Advanced cancers have a higher propensity (60%) for lymph node metastases.120
Clinical Manifestations and Staging
CT imaging can be used to evaluate the deep extension of the lesion, to detect bone invasion, and to assess the parotid and facial nodes.66 The AJCC staging system is depicted in Table 30-2.31
Treatment
Early Lesions (T1 and Superficial T2)
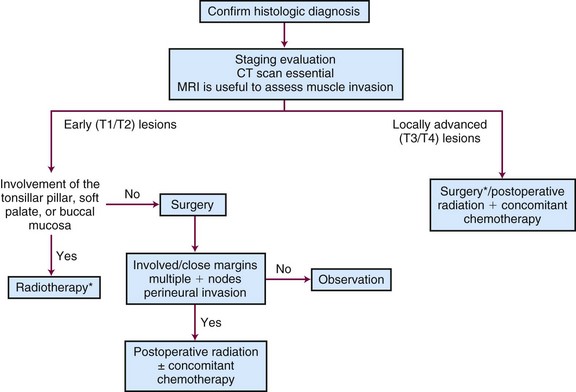
The preferred treatment for patients with carcinoma of the buccal mucosa is surgery. Radiotherapy alone is used to treat patients by default in the uncommon situation where surgery is not feasible. Patients with T1 and T2 cancers may be treated with a combination of EBRT and an interstitial implant.120 A treatment algorithm is shown in Figure 30-15.
Moderate to Locally Advanced Cancers (Large T2, T3, and T4)
Superficial T2 and T3 cancers may be treated with radiation therapy; however, if there is deep muscle invasion, the cure rates after RT are poor.122,123 The preferred treatment for patients with large T3 and T4 cancers is resection of the primary tumor in conjunction with a neck dissection followed by postoperative irradiation. Patients who are not surgical candidates are treated with EBRT and concomitant chemotherapy. Although it would be desirable to include brachytherapy as part of the treatment, the likelihood of adequately encompassing an advanced tumor with an interstitial implant is remote, and so these patients are treated with EBRT to 76.8 Gy in 64 twice-daily fractions over 6.5 weeks. The concurrent chemotherapy regimen that is used most often at our institution is once-weekly cisplatin, 30 mg/m2.
The management of verrucous carcinomas is controversial because of the perceived risk that the tumor may become more aggressive if it recurs after RT. However, there is little evidence in the literature to support this theory.124 Many tumors that recur after treatment (surgery, RT, or chemotherapy) are biologically more aggressive. Therefore, it is reasonable to treat these lesions with irradiation if surgery is not feasible.125 The dose is essentially the same as that prescribed for other squamous cell carcinomas. Wang126 reported a series of patients with verrucous carcinoma treated with RT; the results were comparable to those for patients treated for squamous cell carcinoma.
Results
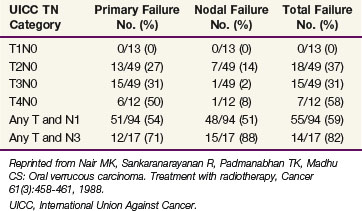
Five-year DFS after RT range from 50% to 60%, depending on the stage of the primary lesion and the presence or absence of lymph node metastases.61,126 Ash61 reviewed the outcomes of 374 patients; 97% were initially treated with RT. The primary lesion was controlled in 52% of the patients; however, only 25% of advanced lesions were controlled. Nair and colleagues127 evaluated the role of RT in the management of carcinomas of the buccal mucosa; irradiation was either administered with an interstitial implant consisting of 65 Gy over 6 days (45 patients), continuous course EBRT consisting of 52.5 Gy in 15 fractions over 19 days or 60 Gy in 25 fractions over 33 days (139 patients), or as planned split-course EBRT consisting of 35 Gy in 15 fractions over 19 days—3-week split—25 Gy in 10 fractions over 12 days (46 patients). The split-course technique tended to be used in elderly patients or for those with massive tumors. The results are shown in Table 30-15. Relapse at the primary site increased with T stage.
TABLE 30-15 Results of Radiotherapy in Cancer of the Buccal Mucosa: Site of Failure versus TN Category
Urist and associates128 reviewed results of 105 patients with squamous cell carcinomas of the buccal mucosa treated by resection of the primary tumor and neck dissection (Table 30-16). In a multivariate analysis, tumor thickness greater than 6 mm was the only significant prognostic factor. Depth of invasion greater than 3 mm was also highly significant but only in the univariate analysis.
TABLE 30-16 Carcinoma of the Buccal Mucosa Treated Surgically: Primary Tumor Parameters versus Outcome
| Clinical Factors | No. Patients | Recurrence |
|---|---|---|
| T Category | ||
| T1 | 23 | 5 (22%) |
| T2 | 32 | 11 (34%) |
| T3 | 20 | 6 (30%) |
| T4 | 14 | 6 (43%) |
| Thickness | ||
| <3 mm | 18 | 4 (22%) |
| 3 to 5.9 mm | 26 | 6 (23%) |
| ≥6 mm | 26 | 14 (54%) |
| Depth of Invasion | ||
| <1 mm | 13 | 2 (15%) |
| 1 to 2.9 mm | 18 | 6 (33%) |
| ≥3 mm | 22 | 12 (54%) |
| Total cases | 89 | 28 (31%) |
Reprinted from Urist MM, O’Brien CJ, Soong SJ, et al: Squamous cell carcinoma of the buccal mucosa. Analysis of prognostic factors, Am J Surg 154:411-414, 1987.
Diaz and colleagues123 reported on 119 patients with buccal mucosal carcinomas treated at the M.D. Anderson Cancer Center. Eighty-four were treated with surgery alone (71%), whereas 22 received postoperative RT and 13 received preoperative RT. None were treated with irradiation alone. Thirty-eight patients (32%) experienced local recurrences. Five-year OS by T category were T1, 79%; T2, 65%; T3, 56%; and T4, 69%. Muscle invasion, Stensen’s duct involvement, and extracapsular spread of involved nodes were significantly associated with decreased survival.123
Irradiation Techniques
T1 and T2 Lesions
The patient is immobilized in the supine position with an Aquaplast face mask. EBRT is administered with an ipsilateral field arrangement that includes the primary lesion and the level I and II lymph nodes. The anterior and superior borders of the field should be at least 2 cm from the borders of the primary tumor. The posterior border should be at the posterior aspect of the spinous processes if the nodes are to be irradiated; the inferior border is at the thyroid notch. The oral commissures and lips are shielded if possible to reduce the acute effects. Patients may be treated with either a “wedge pair” of 6-MV x-ray beams or an en face “mixed beam” of 6-MV x-rays and electrons (Fig. 30-16). The electron energy used in the mixed beam technique varies depending on the depth of the tumor. Because of the steep fall-off of the electron beam at depth, it is preferable to risk overshooting rather than underdosing the deep extent of the tumor, and to use higher electron beam energies such as 15-MeV or 20-MeV beams. The EBRT dose fractionation schedule is 38.4 Gy at 1.6 Gy per fraction, twice daily. The ipsilateral low neck is treated with an en face 6-MV x-ray field matched at the level of the thyroid notch.
T3 and T4 Lesions
For postoperative irradiation, the target volume includes the primary tumor bed and ipsilateral submandibular (level I) and subdigastric (level II) nodes. Patients with extensive positive ipsilateral nodes should be considered for RT to both sides of the neck. Patients with a tumor of T2 category or higher, tumor thickness of more than 6 mm, or depth of invasion of more than 3 mm have a greater than 30% risk of local recurrence and should be treated with postoperative RT.128
Gingiva
Pathology and Patterns of Spread
Squamous cell carcinomas of the mandibular gingiva may invade the underlying bone, retromolar trigone, adjacent buccal mucosa, and floor of the mouth. Byers and colleagues129 reported that 22% had associated leukoplakia, 36% had mandibular invasion, and 5% had perineural involvement. Bone invasion usually starts because there is no complete bony barrier in the edentulous portion of the mandible. The lingual and buccal plates are relatively resistant to tumor penetration. Tumor entry through the mental, mandibular, and other small foramina tends to occur in less than 10% of patients.130
Metastatic spread occurs first to the submandibular (level I) and upper internal jugular (level II) lymph nodes. Byers and associates129 reported 16% clinically positive nodes at diagnosis; contralateral lymph node involvement was found in only 3% of cases. Subclinical lymph node disease has been reported in 17% to 19% of cases.129 The incidence of involved lymph nodes was 12% for T1 and T2 lesions and 13% for T3 and T4 stage cancers.64 Figure 30-17 shows the distribution of positive lymph nodes after elective neck dissection.64
Clinical Manifestations and Staging
Because bone invasion compromises the results of RT, careful radiographic examination of the mandible with panoramic x-ray films, dental radiographs, and CT scanning is essential. The AJCC staging system is shown in Table 30-2.
Treatment
Early Lesions (T1 and Superficial T2)
The majority of these lesions are managed by surgery. Small lesions may be removed by transoral resection; however, rim resection is necessary in most cases. When direct bone invasion is present, removal of a segment of the mandible (segmental mandibulectomy) or maxilla is required. A treatment algorithm is shown in Figure 30-18.
Moderate to Locally Advanced Lesions (Large T2, T3, and T4)
Large lesions may require a hemimandibulectomy or partial maxillectomy.131 Because of the likelihood of local invasion through or along the subperiosteal lymphatics, radiation therapy is often indicated after resection to eradicate microscopic disease at the margins, to sterilize subclinical disease in the cervical lymph nodes, and thus improve the likelihood of cure.32 Postoperative RT is also indicated in the presence of perineural invasion, multiple positive nodes, or extranodal extension.52 Concomitant chemotherapy during EBRT is also recommended in the postoperative setting, as outlined elsewhere in this chapter.
Results
Cady and Catlin131 reviewed 606 patients with squamous cell carcinoma of the gingiva treated by surgery. The survival rate was 43% for patients with mandibular gingival lesions and 40% for those with maxillary alveolar ridge cancers. Soo and colleagues132 reviewed a 20-year experience with squamous carcinoma of the gums in 347 patients treated at the Memorial Sloan-Kettering Cancer Center. Sixty-four percent of patients presented with a clinically negative neck (N0). Ninety-seven percent of patients were treated surgically. The 5-year CSS was 54%. Advanced clinical stage (stages III and IV), prior dental extractions, bone invasion, and involvement of surgical margins were predictive of a lower survival rate on univariate analysis. Clinical stage was the only significant predictor of survival on multivariate analysis.
Byers and associates129 reported a 5-year OS of 43% for patients with squamous cell carcinoma of the mandibular gingiva. Fifty-one of these patients were treated with surgery that included resection of bone in 28 patients with early lesions. Only 1 of 28 patients developed a recurrence. Segmental mandibulectomy and neck dissection were used for 23 patients with advanced disease; there were no local failures. Postoperative irradiation was given for close margins, nerve invasion, or extensive nodal metastases. For those treated with surgery and postoperative RT, control above the clavicles was achieved in 95% of patients.
Overholt and colleagues133 retrospectively reviewed results of 155 patients treated surgically for carcinoma of the mandibular gingiva. Decreased local control was observed for primary lesions larger than 3 cm (p = .021) and persistently positive surgical margins (p = .027). Survival was adversely affected by advanced T category (p = .001), positive initial and final surgical margins (p = .004), mandibular invasion (p = .014), and cervical metastases (p < .001). Local control and survival were not affected by extent of mandibular resection, tumor extension beyond the mandibular gingiva, recent dental extractions in the region of the primary tumor, perineural invasion, or histologic grade.
Patients treated with RT alone for T3 and T4 lesions have 5-year OS ranging from 30% to 40%.121,129 Wang126 reported a 78% control rate for patients with T1 lesions and 27% for those with T2 cancers after treatment with RT alone. Fayos134 reported a 50% local control rate after irradiation alone for lesions with early bone invasion and a 25% local control rate after irradiation alone for those with extensive invasion.
Irradiation Techniques
T1 and T2 Lesions
EBRT may be administered with either an ipsilateral en face combination of 4-MV or 6-MV x-rays and electrons, or with two 4-MV or 6-MV x-ray beams arranged in a “wedge pair” (Fig. 30-19). The latter technique is preferred because it is possible to vary the depth of the target volume more precisely and underdosing of the medial extent of the tumor is less likely. Lesions that exhibit significant extension onto the soft palate or into the tongue (unusual for a T1 or T2 tumor) would be treated with parallel opposed fields weighted 3 : 2 to the side of the tumor. Alternatively, IMRT may be employed to spare the contralateral parotid gland. The doses used are from 60 to 65 Gy over 6 to 6.5 weeks for T1 tumors, and 70 Gy over 7 weeks or 74.4 Gy in 62 fractions twice daily for T2 tumors. The low neck is treated with an anterior 4-MV or 6-MV x-ray field matched at the thyroid notch and receives 50 Gy in 25 fractions.
T3 and T4 Lesions
Patients are treated with parallel opposed portals that include the primary tumor and upper neck nodes (Fig. 30-20). The fields are weighted 3 : 2 toward the side of the tumor. The anterior low neck is treated with an en face 6-MV x-ray field matched at the level of the thyroid notch. A petroleum jelly gauze bolus is placed on the incisions to ensure an adequate surface dose. Fields are reduced off of the spinal cord at approximately 45 Gy. An electron beam may be used to irradiate the posterior strips if additional RT to these sites is indicated after off-cord reduction.
For postoperative cases, radiation portals include the adjacent segment of the mandible or maxilla. The entire hemimandible or hemimaxilla from the distal neural foramen to the pterygopalatine ganglion must be treated when perineural invasion is present. The low neck must be irradiated if involved nodes are present or if the primary tumor is advanced. Postoperative EBRT doses range from 60 to 75 Gy at 1.8 to 2.0 Gy per day. If the resection margins are positive, 74.4 Gy at 1.2 Gy per fraction, twice daily with a minimum 6-hour interfraction interval, is currently used at our institution. Cisplatin is administered concomitantly, based on the results of recently published randomized data from the RTOG and EORTC.49,51
Retromolar Trigone
Pathology and Patterns of Spread
The vast majority of these tumors are squamous cell carcinomas. The primary tumor may spread to the adjacent buccal mucosa, anterior tonsillar pillar, mandibular gingiva, and mandible. Posterior spread to the pterygomandibular space and medial pterygoid muscle may occur. Invasion of the periosteum may occur early. Byers and colleagues135 reported a 25% incidence of bone invasion at diagnosis.
The incidence of clinically positive ipsilateral nodes on presentation is 39%, and the risk of subclinical disease in the cervical lymph nodes is approximately 25%.135 The distribution of positive nodes after an elective neck dissection64 is shown in Figure 30-21.
Clinical Manifestations and Staging
Retromolar trigone cancers tend to produce pain that may be referred to the external auditory canal and preauricular area. Invasion of the pterygoid muscles can produce trismus and is better demonstrated by MRI than by CT.66 CT scan of the head and neck (and MRI in selected cases) is useful for determining the deep extent of the primary tumor, bone invasion, and presence of positive neck nodes. The staging system is depicted in Table 30-2.
Treatment
Early Lesions (T1 and T2)
Local control rates for T1 and T2 lesions are similar following surgery or RT.135 If the tumor extends to the tonsillar pillar, soft palate, or buccal mucosa and an extensive resection is required, RT is selected. If possible, intraoral cone irradiation should be used for a portion of the treatment. Small lesions without detectable bone invasion can be resected with removal of the periosteum or a rim resection of the mandible. A treatment algorithm is shown in Figure 30-22.
Results
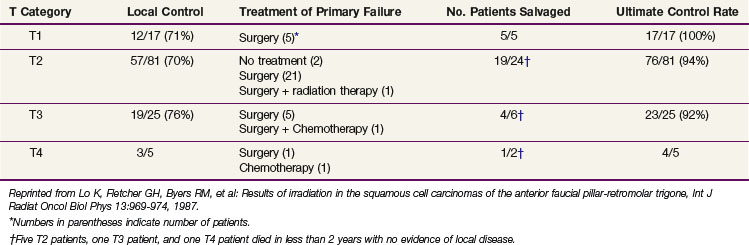
Lo and associates136 reported on a series of patients treated with radiation therapy at the M.D. Anderson Hospital (Table 30-17). Patients with retromolar trigone cancers were grouped with those having anterior tonsillar pillar lesions. Local control varied from 70% to 76% after RT for those with T1, T2, and T3 cancers. Salvage surgery resulted in ultimate local control rates varying from 92% to 100%.
TABLE 30-17 Local Control Rates after Radiotherapy: Anterior Faucial Pillar-Retromolar Trigone (M.D. Anderson Hospital)
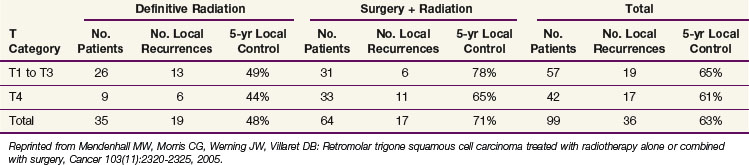
Mendenhall and colleagues137 reported on 99 patients treated with RT alone or combined with surgery at the University of Florida. The likelihood of cure was influenced by extent of disease and treatment; those treated with surgery and RT had a better chance of cure than those treated with RT alone (Table 30-18).
TABLE 30-18 Local Control of Squamous Cell Carcinoma of the Retromolar Trigone Treated at the University of Florida
Kowalski and associates138 reported on 114 patients who underwent an extended “commando” operation between 1960 and 1991 for carcinoma of the retromolar trigone. Sixty-six patients received postoperative RT (median, 50 Gy). Tumor categories were T1, 5 patients; T2, 44 patients; T3, 24 patients; T4, 28 patients; and TX, 13 patients. Forty-one patients (36%) experienced a recurrence at one or more sites: local, 31 (27%); dissected neck, 9 (22%); contralateral neck, 3 (7%); and distant, 7 (17%). The 5-year OS by T category was T1, 80%; T2, 57.8%; T3, 46.5%; T4, 65.2%; and overall, 55.3%.
Byers and colleagues135 reported on 110 patients with squamous carcinoma of the retromolar trigone who were treated at the M.D. Anderson Hospital from 1965 to 1977 and followed for 5 years or longer. Seventy patients had T1 and T2 tumors, and 77 patients had a clinically negative neck. Sixty patients received either surgery alone (n = 46) or combined with preoperative or postoperative radiation therapy (n = 14). Fifty patients were treated with irradiation alone. Failure at the primary site occurred in 7 of 60 patients (12%) treated surgically, and in 8 of 50 patients (16%) treated with RT alone.
Complications
Thirty percent of the patients reported by Lo and colleagues136 developed some degree of bone exposure, but only nine patients (5.6%) required a segmental mandibulectomy. The probability of bone exposure was not found to be dose related. Huang and associates139 reported on 65 patients treated for retromolar trigone cancer and observed complications that included bone necrosis, soft tissue necrosis, and severe trismus, occurring in 12% after surgery and postoperative RT, 11% after RT alone, and none after preoperative RT and surgery.
Kowalski and colleagues138 observed complications in 51.8% of 114 patients who underwent an extended “commando” operation between 1960 and 1991; 21 patients (18.4%) had a wound infection.
Hard Palate
Squamous cell carcinoma of the hard palate is relatively rare. In a series of about 5000 patients with oral cavity cancers, only 25 patients (0.5%) had squamous cell carcinoma of the hard palate.140 In a similar study, carcinoma of the hard palate represented 3% of all oral cavity carcinomas.141
The male to female rate is approximately 1 : 1. For both males and females, the peak incidence tends to occur in the seventh decade, with over 98% of patients older than 40 years of age.142
Anatomy
The greater palatine foramina are medial to the third maxillary molars; the greater palatine vessels and nerves emerge from this foramen. The greater palatine nerve innervates the gingiva, mucous membrane, and glands of the hard palate. The nasopalatine nerve innervates the mucous membrane of the anterior part of the hard palate. Because of the rich blood supply from the greater palatine artery, the incidence of osteoradionecrosis and soft tissue necrosis is lower than that reported for the mandible.143
Pathology and Patterns of Spread
Malignant tumors of the hard palate are most often adenoid cystic carcinomas and mucoepidermoid carcinomas arising from minor salivary glands. Squamous cell carcinoma is relatively uncommon. Kaposi’s sarcoma and melanomas may also originate on the hard palate.142
Most squamous cell carcinomas originate on the gingiva and spread secondarily to the hard palate. Perineural invasion occurs via the greater palatine foramen. The risk of positive lymph nodes is 13% to 24% at diagnosis.144,145 The incidence of subclinical disease in the cervical lymphatics is 22%.61
Clinical Manifestations, Patient Evaluation, and Staging
Imaging of the hard palate is primarily with CT scanning and is occasionally supplemented with MRI. Imaging of the neck nodes must include the facial and retrozygomatic nodes.66 Facial nodes are best assessed by bimanual examination, particularly in patients with dental fillings. If the lesion is an adenoid cystic carcinoma, it is essential to search for perineural spread. The AJCC staging system31 is shown in Table 30-2.
Treatment
Surgery is the usual initial treatment for most lesions; postoperative RT is indicated for patients with more extensive cancers. The role of primary RT in the management of hard palate carcinomas is ill defined. If the lesion has a large surface area and is superficial, irradiation can be used as initial treatment. However, most patients are treated with surgery because the underlying bone is often involved and, in that scenario, RT is unlikely to be effective.146 Postoperative radiotherapy is indicated for close or involved margins, perineural or vascular invasion, multiple involved nodes, extracapsular extension, or bone invasion.52 Dose and fractionation recommendations are similar to those given for other sites. A treatment algorithm is shown in Figure 30-23.
Malignant salivary gland tumors of the hard palate are often treated by a combination of surgery and postoperative RT, particularly if they are high-grade tumors. Low-grade minor salivary gland carcinomas can be treated by surgery alone if negative margins can be achieved. Some malignant minor salivary gland tumors have been successfully controlled by high-dose RT alone.147
Results
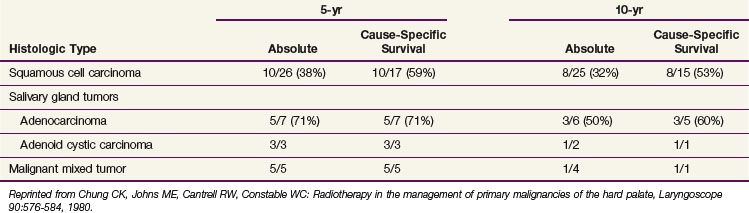
Shibuya and colleagues148 reported the radiation therapy results for malignant lesions of the hard palate; the 5-year OS for those with squamous cell carcinomas was approximately 45%. For patients with minimal bone invasion and a clinically negative neck, the 5-year OS was 75%. Data from the University of Virginia are presented in Table 30-19 for 41 patients treated with surgery, RT, or both.149
TABLE 30-19 Hard Palate Cancers Treated by Surgery, Radiotherapy, or Both: 5-yr and 10-yr Survival Rates
Yorozu and colleagues150 reported on 31 patients treated with EBRT at the Christie Hospital between 1990 and 1997. Twenty-six received RT alone and five were treated postoperatively for positive surgical margins. The 5-year local and ultimate local control rates after salvage surgery were 53% and 69%, respectively. Survival rates were 48% for patients with squamous carcinomas and 63% for those with minor salivary gland carcinomas. T category was the only significant predictor of 5-year local control rates, which were 80% for T1 and T2 lesions and 24% for T3 and T4 lesions, respectively. The only significant predictor for survival was N category.
Tran and associates151 reported on 38 patients treated for salivary gland tumors of the palate at the University of California at Los Angeles. Twenty-three of 38 tumors were on the hard palate; adenoid cystic carcinoma was the most common histologic type. Twenty-five patients received surgery alone; 13 were treated with surgery and postoperative irradiation. The local control rates were comparable for the two groups of patients (88% and 85%, respectively).
Kovalic and colleagues152 reported on 13 patients with carcinoma of the hard palate treated at Washington University. Histologic types included adenoid cystic carcinoma, nine patients; squamous cell carcinoma, three patients; and mucoepidermoid carcinoma, one patient. T stages were T1, one patient; T2, five patients; T3, three patients; and T4, four patients. All had a clinically negative neck. Ten patients received excision and postoperative RT; three patients were treated with RT alone. The 10-year actuarial DFS and local control rates were 77% and 92%, respectively.
A group of 50 patients was treated surgically at the University of Cincinnati; it included 25 patients with squamous cell carcinomas, 11 with adenoid cystic carcinomas, 6 with adenocarcinomas, and 8 with tumors of miscellaneous histologic types.153 The 5-year OS by histologic type were squamous carcinoma, 76%, and adenoid cystic carcinoma, 90%; the overall survival rate was 85%. The 10-year OS for adenoid cystic carcinomas fell to 75%, consistent with their tendency to develop late recurrences.
Most relapses are found at the primary site. Evans and Shah142 reported a 53% incidence of isolated primary site failure. The incidence of recurrence in the cervical nodes was 30%; failure involving both the primary site as well as the cervical lymph nodes was 10%. No patient treatment failed with results of distant metastases only. Seven percent of the patients experienced treatment failure in both distant and locoregional sites.142
The risk of developing metachronous carcinoma is high. In the University of Virginia’s experience,144 28% of the patients developed a metachronous primary carcinoma during their lifetime and 13% developed a third, fourth, or fifth carcinoma. The oral cavity was the most common site for metachronous cancers.
Irradiation Techniques: Hard Palate and Maxillary Alveolar Ridge
T1 and T2 Lesions
The preferred initial treatment for patients with T1 and T2 carcinomas of the hard palate is surgery. Radiation therapy is used by default to treat the occasional patient who is not a surgical candidate. Most of these lesions are not well lateralized; consequently, irradiation is administered with parallel opposed fields encompassing the primary tumor, with a margin of 2 cm or less. A cork and tongue block are placed in the mouth to displace the tongue, mandible, and lower lip inferiorly and to reduce the amount of normal tissue included in the fields (Fig. 30-24A). These lesions are not amenable to brachytherapy and so patients are treated with EBRT alone. The likelihood of cure with RT alone, even for early-stage lesions, is relatively low, so that an altered fractionation technique should be employed. We prefer hyperfractionated irradiation and use 74.4 to 76.8 Gy at 1.2 Gy per fraction, twice daily, over 6 to 6.5 weeks. The fields are extended to include the regional lymph nodes (levels I and II) for patients with aggressive, poorly differentiated cancers (Fig. 30-24B). The disadvantage of irradiating the regional lymph nodes is that the acute toxicity of the treatment is significantly increased. Fields are reduced at 45.6 Gy in 38 fractions to limited portals that adequately encompass the primary cancer. The low neck is irradiated with an anterior field that abuts the primary fields at the level of the thyroid notch and receives 50 Gy in 25 fractions over 5 weeks.
T3 and T4 Lesions
Patients with T3 and T4 cancers are optimally treated with surgery and postoperative RT using fields encompassing the primary tumor and regional lymph nodes as previously described. Postoperative irradiation is initiated within 6 weeks of surgery. Patients with negative margins generally receive 60 Gy at 2 Gy per fraction. Reduction off of the spinal cord is performed at 44 to 46 Gy, and the posterior strips are treated with 8-MeV to 10-MeV electrons if it is necessary to irradiate these areas to a higher dose. An altered fractionation technique should be considered for those who have positive margins, multiple risk factors, and/or a prolonged interval between surgery and RT.52 Systemic cisplatin chemotherapy is usually administered concomitantly with RT in the postoperative setting.49,51
Complications
No severe complications occurred in the patients who received radiation therapy (60 Gy in 5 to 6 weeks) at the University of Virginia.149 Most patients developed xerostomia and temporary loss of taste.144
Yorozu and colleagues150 reported results for 31 patients treated with EBRT at the Christie Hospital between 1990 and 1997. Twenty-six patients received irradiation alone, and five patients were treated postoperatively for positive surgical margins; necrosis occurred in one patient.
1 Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
23 Chang DT, Sandow PR, Morris CG, et al. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528-536.
27 Byers RM, O’Brien J, Waxler J. The therapeutic and prognostic implications of nerve invasion in cancer of the lower lip. Int J Radiat Oncol Biol Phys. 1978;4:215-217.
49 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
50 Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843-850.
51 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
54 Langendijk JA, Slotman BJ, van der Waal I, et al. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer. 2005;104:1408-1417.
58 O’Brien CJ, Lauer CS, Fredricks S, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer—but what thickness? Head Neck. 2003;25:937-945.
63 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
64 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160-167.
77 Scholl P, Byers RM, Batsakis JG, et al. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg. 1986;152:354-360.
78 Byers RM, Bland KI, Borlase B, Luna M. The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978;136:525-528.
83 Mendenhall WM, Van Cise WS, Bova FJ, Million RR. Analysis of time-dose factors in squamous cell carcinoma of the oral tongue and floor of mouth treated with radiation therapy alone. Int J Radiat Oncol Biol Phys. 1981;7:1005-1011.
86 Pernot M, Hoffstetter S, Peiffert D, et al. Epidermoid carcinomas of the floor of mouth treated by exclusive irradiation. Statistical study of a series of 207 cases. Radiother Oncol. 1995;35:177-185.
91 Mendenhall WM, Mancuso AA. Radiotherapy for head and neck cancer—is the “next level” down? Int J Radiat Oncol Biol Phys. 2009;73:645-646.
92 Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006;24:2618-2623.
96 Byers RM, Weber RS, Andrews T, et al. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14-19.
99 Spiro RH, Huvos AG, Wong GY, et al. Predictive value of tumor thickness in squamous cell carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345-350.
105 Wendt CD, Peters LJ, Delclos L, et al. Primary radiotherapy in the treatment of stage I and II oral tongue cancers: importance of the proportion of therapy delivered with interstitial therapy. Int J Radiat Oncol Biol Phys. 1990;18:1287-1292.
107 Byers RM, El-Naggar AK, Lee AK, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous cell carcinoma of the oral tongue? Head Neck. 1998;20:138-144.
115 Warde P, O’Sullivan B, Aslanidis J, et al. A phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;54:9-13.
123 Diaz EMJr, Holsinger FC, Zuniga ER, et al. Squamous cell carcinoma of the buccal mucosa: one institution’s experience with 119 previously untreated patients. Head Neck. 2003;25:267-273.
129 Byers RM, Newman R, Russell N, Yue A. Results of treatment for squamous carcinoma of the lower gum. Cancer. 1981;47:2236-2238.
133 Overholt SM, Eicher SA, Wolf P, Weber RS. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996;106:1335-1339.
135 Byers RM, Anderson B, Schwarz EA, et al. Treatment of squamous carcinoma of the retromolar trigone. Am J Clin Oncol. 1984;7:647-652.
137 Mendenhall WM, Morris CG, Amdur RJ, et al. Retromolar trigone squamous cell carcinoma treated with radiotherapy alone or combined with surgery. Cancer. 2005;103:2320-2325.
147 Cianchetti M, Sandow PS, Scarborough LD, et al. Radiation therapy for minor salivary gland carcinoma. Laryngoscope. 2009;119:1334-1338.
154 Mendenhall WM, Riggs CE, Amdur RJ, et al. Altered fractionation and/or adjuvant chemotherapy in definitive irradiation of squamous cell carcinoma of the head and neck. Laryngoscope. 2003;113:546-551.
1 Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
2 Krolls SO, Hoffman S. Squamous cell carcinoma of the oral soft tissues. A statistical analysis of 14,253 cases by age, sex, and race of patients. J Am Dent Assoc. 1976;92:571-574.
3 Taybos G. Oral changes associated with tobacco use. Am J Med Sci. 2003;326:179-182.
4 Kulkarni V, Saranath D. Concurrent hyperthermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol. 2004;40:145-153.
5 Znaor A, Brennan P, Gajalakshmi V, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681-686.
6 Browman GP, Wong G, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159-163.
7 Wynder EL. The epidemiology of cancers of the upper alimentary and upper respiratory tracts. Laryngoscope. 1978;88:50-51.
8 Castellsague X, Quintana MJ, Martinez MC, et al. The role of type of tobacco and type of alcoholic beverage in oral carcinogenesis. Int J Cancer. 2004;108:741-749.
9 Talamini R, La Vecchia C, Levi F, et al. Cancer of the oral cavity and pharynx in nonsmokers who drink alcohol and in nondrinkers who smoke tobacco. J Natl Cancer Inst. 1998;90:1901-1903.
10 Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-1131.
11 Goldenberg D, Golz A, Joachims HZ. The beverage mate: a risk factor for cancer of the head and neck. Head Neck. 2003;25:595-601.
12 Zhang ZF, Morgenstern H, Spitz MR, et al. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8:1071-1078.
13 Chen PC, Kuo C, Pan CC, Chou MY. Risk of oral cancer associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan—an integrated molecular and epidemiological study of 58 cases. J Oral Pathol Med. 2002;31:317-322.
14 Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J Clin. 1995;45:328-351. Review
15 McGuirt WF. Snuff dipper’s carcinoma. Arch Otolaryngol. 1983;109:757-760.
16 Shklar G. The oral cavity, jaws, and salivary glands. In: Robbins S, editor. Pathologic basis of disease. ed 3. Philadelphia, W.B.: Saunders; 1984:783-784.
17 Veness M. Lip cancer. Important management issues. Australas J Dermatol. 2001;42:30-32.
18 Baker SR, Krause CJ. Carcinoma of the lip. Laryngoscope. 1980;90:19-27.
19 Cross JE, Guralnick E, Daland EM. Carcinoma of the lip. A review of 563 case records of carcinoma of the lip at the Pondville Hospital. Surg Gynecol Obstet. 1948;81:153-162.
20 Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Path. 1987;64:379-390.
21 Schiodt M, Hermund NU. Management of oral disease prior to radiation therapy. Support Care Cancer. 2002;10:40-43.
22 Meraw SJ, Reeve CM. Dental considerations and treatment of the oncology patient receiving radiation therapy. J Am Dent Assoc. 1998;129:201-205.
23 Chang DT, Sandow PR, Morris CG, et al. Do pre-irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528-536.
24 Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2001;100:2026-2046.
25 Rouviére H. Anatomy of the Human Lymphatic System. Tobias MJ (translator). Edwards Brothers, Ann Arbor, Michigan, 1938. pp 1-28, 44-56, 77-78
26 MacKay EN, Sellers AH. A statistical review of carcinoma of the lip. Can Med Assoc J. 1964;90:670-672.
27 Byers RM, O’Brien J, Waxler J. The therapeutic and prognostic implications of nerve invasion in cancer of the lower lip. Int J Radiat Oncol Biol Phys. 1978;4:215-217.
28 Hendricks JL, Mendelson BC, Woods JE. Invasive carcinoma of the lower lip. Surg Clin North Am. 1977;57:837-844.
29 de Visscher JG, van den Elsaker K, Grond AJ, et al. Surgical treatment of squamous cell carcinoma of the lower lip. Evaluation of long-term results and prognostic factors—a retrospective analysis of 184 patients. J Oral Maxillofac Surg. 1998;56:814-820.
30 Petrovich Z, Kuisk H, Tobochnik N, et al. Carcinoma of the lip. Arch Otolaryngol. 1979;105:187-191.
31 American Joint Committee on Cancer. Lip and oral cavity. In: Greene FL, Page DL, Fleming ID, et al, editors. AJCC cancer staging manual. ed 6. New York: Springer; 2002:23-32.
32 Amdur RJ, Parsons JT, Mendenhall WM, et al. Postoperative irradiation for squamous cell carcinoma of the head and neck. An analysis of treatment results and complications. Int J Radiat Oncol Biol Phys. 1989;16:25-36.
33 Babington S, Veness MJ, Cakir B, et al. Squamous cell carcinoma of the lip: is there a role for adjuvant radiotherapy in improving local control following incomplete or inadequate excision? ANZ J Surg. 2003;73:621-625.
34 Stranc MF, Fogel M, Dische S. Comparison of lip function: surgery vs radiotherapy. Br J Plast Surg. 1987;40:598-604.
35 Zilinsky I, Winkler E, Weiss G, et al. Total lower lip reconstruction with innervated muscle-bearing flaps. Modification of the Webster flap. Dermatol Surg. 2001;27:687-691.
36 Liu J, Okutomi T, Cao Z, Tatematsu N. Modified labial tissue sliding flaps for repairing large lower lip defects. J Oral Maxillofac Surg. 2001;59:887-891.
37 Chang KP, Lai CS, Tsai CC, et al. Total upper lip reconstruction with a free temporal scalp flap: long-term follow-up. Head Neck. 2003;25:602-605.
38 Teichgraeber JF, Larson DL. Some oncologic considerations in the treatment of lip cancer. Otolaryngol Head Neck Surg. 1988;98:589-592.
39 Rodolico V, Barresi E, Di Lorenzo R, et al. Lymph node metastasis in lower lip squamous cell carcinoma in relation to tumour size, histologic variables and p27Kip1 protein expression. Oral Oncol. 2004;40:92-98.
40 Jorgensen K, Elbrond O, Andersen AP. Carcinoma of the lip. A series of 869 patients. Acta Otolaryngol (Stockh). 1973;75:312-313.
41 Pierquin B. Basic techniques of endocurietherapy. In: Pierquin B, Wilson JF, Chassagne D, editors. Modern brachytherapy. New York: Masson; 1987:69-79.
42 Tombolini V, Bonanni A, Valeriani M, et al. Brachytherapy for squamous cell carcinoma of the lip. The experience of the Institute of Radiology of the University of Rome “La Sapienza.”. Tumori. 1998;84:478-482.
43 Orecchia R, Rampino M, Gribaudo S, Negri GL. Interstitial brachytherapy for carcinomas of the lower lip. Results of treatment. Tumori. 1991;77:336-338.
44 Guinot JL, Arribas L, Chust ML, et al. Lip cancer treatment with high dose rate brachytherapy. Radiother Oncol. 2003;69:113-115.
45 Petrovich Z, Parker RG, Luxton G, et al. Carcinoma of the lip and selected sites of head and neck skin. A clinical study of 896 patients. Radiother Oncol. 1987;8:11-17.
46 Mohs FE, Snow SN. Microscopically controlled surgical treatment for squamous cell carcinoma of the lower lip. Surg Gynecol Obstet. 1985;160:37-41.
47 Holmkvist KA, Roenigk RK. Squamous cell carcinoma of the lip treated with Mohs micrographic surgery. Outcome at 5 years. J Am Acad Dermatol. 1998;38:960-966.
48 Hruza GJ. Mohs micrographic surgery local recurrences. J Dermatol Surg Oncol. 1994;20:573-577.
49 Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
50 Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers. A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27:843-850.
51 Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945-1952.
52 Hinerman RW, Mendenhall WM, Morris CG, et al. Postoperative irradiation for squamous cell carcinoma of the oral cavity. 35 year experience. Head Neck. 2004;26:984-994.
53 Winquist E, Oliver T, Gilbert R. Postoperative chemoradiotherapy for advanced squamous cell carcinoma of the head and neck. A systematic review with meta-analysis. Head Neck. 2007;29:38-46.
54 Langendijk JA, Slotman BJ, van der Waal I, et al. Risk-group definition by recursive partitioning analysis of patients with squamous cell head and neck carcinoma treated with surgery and postoperative radiotherapy. Cancer. 2005;104:1408-1417.
55 Parsons JT, Mendenhall WM, Moore GJ, Million RR. Radiotherapy of tumors of the oral cavity, 2nd ed. Thawley SE, Panje WR, Batsakis JG, Lindberg RD, editors. Comprehensive management of head and neck tumors, Vol. 1. Saunders, Philadelphia, W.B., 1999;695-719.
56 Fitzpatrick PJ. Cancer of the lip. J Otolaryngol. 1984;13:32-36.
57 Yamazaki H, Inoue T, Yoshida K, et al. Lymph node metastasis of early oral tongue cancer after interstitial radiotherapy. Int J Radiat Oncol Biol Phys. 2004;58:139-146.
58 O’Brien CJ, Lauer CS, Fredricks S, et al. Tumor thickness influences prognosis of T1 and T2 oral cavity cancer—but what thickness? Head Neck. 2003;25:937-945.
59 Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth. Treatment rationale. Head Neck Surg. 1984;7:15-21.
60 Mohit-Tabatabai MA, Sobel HJ, Rush BF, Mashberg A. Relation of thickness of floor of mouth stage I and II cancers to regional metastasis. Am J Surg. 1986;152:351-353.
61 Ash CL. Oral cancer. A twenty-five year study. Am J Roentgenol Radium Ther Nucl Med. 1962;87:417-430.
62 Woolgar JA, Scott J. Prediction of cervical lymph node metastasis in squamous cell carcinoma of the tongue/floor of mouth. Head Neck. 1995;17:463-472.
63 Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446-1449.
64 Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160-167.
65 American Joint Committee on Cancer. Lip and oral cavity. In: Edge SB, Byrd D, Compton CC, et al, editors. AJCC cancer staging handbook. ed 7. Chicago: Springer; 2010:49-61.
66 Mancuso AA, Harnsberger HR, Dillon WP. Oropharynx, oral cavity, and floor of mouth. In: Grayson TH, editor. Workbook for MRI and CT of the head and neck. 2nd ed. Baltimore: Williams & Wilkins; 1989:151-170.
67 Kubota K, Yokoyama J, Yamaguchi K, et al. FDG-PET delayed imaging for the detection of head and neck cancer recurrence after radio-chemotherapy. Comparison with MRI/CT. Eur J Nucl Med Mol Imaging. 2004;31:590-595.
68 Rogers JW, Greven KM, McGuirt WF, et al. Can post-RT neck dissection be omitted for patients with head-and-neck cancer who have a negative PET scan after definitive radiation therapy? Int J Radiat Oncol Biol Phys. 2004;58:694-697.
69 Yao M, Graham MM, Hoffman HT, et al. The role of post-radiation therapy FDG-PET in prediction of necessity for post-radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001-1010.
70 Dias FL, Kligerman J, Matos de Sa G, et al. Elective neck dissection versus observation in stage I squamous cell carcinomas of the tongue and floor of the mouth. Otolaryngol Head Neck Surg. 2001;125:23-29.
71 Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for floor-of-mouth cancer. Laryngoscope. 2000;110:1764-1772.
72 Ross GL, Soutar DS, MacDonald DG, et al. Improved staging of cervical metastases in clinically node-negative patients with head and neck squamous cell carcinoma. Ann Surg Oncol. 2004;11:213-218.
73 Hoft S, Maune S, Muhle C, et al. Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer. 2004;91:124-128.
74 Ross GL, Soutar DS, MacDonald DG, et al. Sentinel node biopsy in head and neck cancer. Preliminary results of a multicenter trial. Ann Surg Oncol. 2004;11:690-696.
75 Ange DW, Lindberg RD, Guillamondegui OM. Management of squamous cell carcinoma of the oral tongue and floor of mouth after excisional biopsy. Radiology. 1975;116:143-146.
76 Mendenhall WM, Parsons JT, Stringer SP, et al. Radiotherapy after excisional biopsy of carcinoma of the oral tongue/floor of the mouth. Head Neck. 1989;11:129-131.
77 Scholl P, Byers RM, Batsakis JG, et al. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue. Prognostic and therapeutic implications. Am J Surg. 1986;152:354-360.
78 Byers RM, Bland KI, Borlase B, Luna M. The prognostic and therapeutic value of frozen section determinations in the surgical treatment of squamous carcinoma of the head and neck. Am J Surg. 1978;136:525-528.
79 Jacobs JR, Ahmad K, Casiano R, et al. Implications of positive surgical margins. Laryngoscope. 1993;103:64-68.
80 Laramore GE, Scott CB, Al-Sarraf M, et al. Adjuvant chemotherapy for resectable squamous cell carcinomas of the head and neck. Report on Intergroup Study 0034. Int J Radiat Oncol Biol Phys. 1992;23:705-713.
81 Lapeyre M, Hoffstetter S, Peiffert D, et al. Postoperative brachytherapy alone for T1-2 N0 squamous cell carcinomas of the oral tongue and floor of mouth with close or positive margins. Int J Radiat Oncol Biol Phys. 2000;48:37-42.
82 Million RR, Cassisi NJ, Clark JR. Cancer of the head and neck. In: DeVita VTJr, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. ed 3. Philadelphia: J.B. Lippincott; 1989:488-580.
83 Mendenhall WM, Van Cise WS, Bova FJ, Million RR. Analysis of time-dose factors in squamous cell carcinoma of the oral tongue and floor of mouth treated with radiation therapy alone. Int J Radiat Oncol Biol Phys. 1981;7:1005-1011.
84 Chu A, Fletcher GH. Incidence and causes of failures to control by irradiation the primary lesions in squamous cell carcinomas of the anterior two-thirds of the tongue and floor of mouth. Am J Roentgenol Radium Ther Nucl Med. 1973;117:502-508.
85 Marsiglia H, Haie-Meder C, Sasso G, et al. Brachytherapy for T1-T2 floor-of-the-mouth cancers. The Gustave-Roussy Institute experience. Int J Radiat Oncol Biol Phys. 2002;52:1257-1263.
86 Pernot M, Hoffstetter S, Peiffert D, et al. Epidermoid carcinomas of the floor of mouth treated by exclusive irradiation. Statistical study of a series of 207 cases. Radiother Oncol. 1995;35:177-185.
87 Rodgers LWJr, Stringer SP, Mendenhall WM, et al. Management of squamous cell carcinoma of the floor of the mouth. Head Neck. 1993;15:16-19.
88 Marcus RBJr, Million RR, Mitchell TP. A preloaded, custom-designed implantation device for stage T1-T2 carcinoma of the floor of mouth. Int J Radiat Oncol Biol Phys. 1980;6:111-113.
89 Million RR, Cassisi NJ, Mancuso AA. Oral cavity. In: Million RR, Cassisi NJ, editors. Management of head and neck cancer: a multidisciplinary approach. ed 2. Philadelphia, J.B.: Lippincott; 1994:321-400.
90 Parsons JT, Mendenhall WM, Moore GJ, Million RR. Radiotherapy of tumors of the oropharynx, ed 2. Thawley SE, Panje WR, Batsakis JG, Lindberg RD, editors. Comprehensive management of head and neck tumors, Vol. 1. Saunders, Philadelphia, W.B., 1999;861-875.
91 Mendenhall WM, Mancuso AA. Radiotherapy for head and neck cancer—is the “next level” down? Int J Radiat Oncol Biol Phys. 2009;73:645-646.
92 Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer. Promises and pitfalls. J Clin Oncol. 2006;24:2618-2623.
93 Okunieff P, Augustine E, Hicks JE, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207-2213.
94 King GE, Scheeetz J, Jacob RF, Martin JW. Electrotherapy and hyperbaric oxygen. Promising treatments for postradiation complications. J Prosthet Dent. 1989;62:331-334.
95 Engelmeier RL, King GE. Complications of head and neck radiation therapy and their management. J Prosthet Dent. 1983;49:514-522.
96 Byers RM, Weber RS, Andrews T, et al. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14-19.
97 Braakhuis BJ, Leemans CR, Brakenhoff RH. A genetic progression model of oral cancer. Current evidence and clinical implications. J Oral Pathol Med. 2004;33:317-322.
98 Ozeki S, Tashiro H, Okamoto M, Matsushima T. Metastasis to the lingual lymph node in carcinoma of the tongue. J Maxillofac Surg. 1985;13:277-281.
99 Spiro RH, Huvos AG, Wong GY, et al. Predictive value of tumor thickness in squamous cell carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345-350.
100 Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112:616-625.
101 Chao KS, Emami B, Akhileswaran R, et al. The impact of surgical margin status and use of an interstitial implant on T1, T2 oral tongue cancers after surgery. Int J Radiat Oncol Biol Phys. 1996;36:1039-1043.
102 Fein DA, Mendenhall WM, Parsons JT, et al. Carcinoma of the oral tongue. A comparison of results and complications of treatment with radiotherapy and/or surgery. Head Neck. 1994;16:358-365.
103 Fu KK, Ray JW, Chan EK, Phillips TL. External and interstitial radiation therapy of carcinoma of the oral tongue. A review of 32 years’ experience. Am J Roentgenol. 1976;126:107-115.
104 Wang CC. Radiotherapeutic management and results of T1N0, T2N0 carcinoma of the oral tongue. Evaluation of boost techniques. Int J Radiat Oncol Biol Phys. 1989;17:287-291.
105 Wendt CD, Peters LJ, Delclos L, et al. Primary radiotherapy in the treatment of stage I and II oral tongue cancers. Importance of the proportion of therapy delivered with interstitial therapy. Int J Radiat Oncol Biol Phys. 1990;18:1287-1292.
106 Matsuura K, Hirokawa Y, Fujita M, et al. Treatment results of stage I and II oral tongue cancer with interstitial brachytherapy. Maximum tumor thickness is prognostic of nodal metastasis. Int J Radiat Oncol Biol Phys. 1998;40:535-539.
107 Byers RM, El-Naggar AK, Lee AK, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous cell carcinoma of the oral tongue? Head Neck. 1998;20:138-144.
108 Haddadin KJ, Soutar DS, Oliver RJ, et al. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999;21:517-525.
109 O-Charoenrat P, Pillai G, Patel S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral Oncol. 2003;39:386-390.
110 Civantos FJ, Gomez C, Duque C, et al. Sentinel node biopsy in oral cavity cancer: correlation with PET scan and immunohistochemistry. Head Neck. 2003;25:1-9.
111 Bourgier C, Coche-Dequeant B, Fournier C, et al. Exclusive low-dose-rate brachytherapy in 279 patients with T2N0 mobile tongue carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:434-440.
112 Fujita M, Hirokawa Y, Kashiwado K, et al. An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue. Int J Radiat Oncol Biol Phys. 1996;34:333-339.
113 Glanzmann C, Gratz KW. Radionecrosis of the mandibula. A retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36:94-100.
114 LeVeque FG, Montgomery M, Potter D, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol. 1993;11:1124-1131.
115 Warde P, O’Sullivan B, Aslanidis J, et al. A phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;54:9-13.
116 Gornitsky M, Shenouda G, Sultanem K, et al. Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:45-52.
117 Kouloulias VE, Kouvaris JR, Kokakis JD, et al. Impact on cytoprotective efficacy of intermediate interval between amifostine administration and radiotherapy. A retrospective analysis. Int J Radiat Oncol Biol Phys. 2004;59:1148-1156.
118 Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer. Report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369-1377.
119 Rades D, Fehlauer F, Bajrovic A, et al. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004;70:261-264.
120 Conley J, Sadoyama JA. Squamous cell cancer of the buccal mucosa. A review of 90 cases. Arch Otolaryngol. 1973;97:330-333.
121 MacComb WS, Fletcher GH, Healey JE. Intra-oral cavity. In: MacComb WS, Fletcher GH, editors. Cancer of the head and neck. Baltimore: Williams & Wilkins; 1967:89-151.
122 Vegers JW, Snow GB, van der Waal I. Squamous cell carcinoma of the buccal mucosa. A review of 85 cases. Arch Otolaryngol. 1979;105:192-195.
123 Diaz EMJr, Holsinger FC, Zuniga ER, et al. Squamous cell carcinoma of the buccal mucosa. One institution’s experience with 119 previously untreated patients. Head Neck. 2003;25:267-273.
124 Koch BB, Trask DK, Hoffman HT, et al. National survey of head and neck verrucous carcinoma. Patterns of presentation, care and outcome. Cancer. 2001;92:110-120.
125 Jyothirmayi R, Sankaranarayanan R, Varghese C, et al. Radiotherapy in the treatment of verrucous carcinoma of the oral cavity. Oral Oncol. 1997;33:124-128.
126 Wang CC. Radiation Therapy for Head and Neck Neoplasms: Indications, Techniques and Results. Littleton, Massachusetts: John Wright-PSG; 1983.
127 Nair MK, Sankaranarayanan R, Padmanabhan TK, Madhu CS. Oral verrucous carcinoma. Treatment with radiotherapy. Cancer. 1988;61:458-461.
128 Urist MM, O’Brien CJ, Soong SJ, et al. Squamous cell carcinoma of the buccal mucosa. Analysis of prognostic factors. Am J Surg. 1987;154:411-414.
129 Byers RM, Newman R, Russell N, Yue A. Results of treatment for squamous carcinoma of the lower gum. Cancer. 1981;47:2236-2238.
130 McGregor AD, MacDonald DG. Routes of entry of squamous cell carcinoma to the mandible. Head Neck Surg. 1988;10:294-301.
131 Cady B, Catlin D. Epidermoid carcinoma of the gum. A 20-year survey. Cancer. 1969;23:551-569.
132 Soo KC, Spiro RH, King W, et al. Squamous carcinoma of the gums. Am J Surg. 1988;156:281-285.
133 Overholt SM, Eicher SA, Wolf P, Weber RS. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996;106:1335-1339.
134 Fayos JV. Carcinoma of the mandible. Results of radiation therapy. Acta Radiol Ther Phys Biol. 1973;12:378-386.
135 Byers RM, Anderson B, Schwarz EA, et al. Treatment of squamous carcinoma of the retromolar trigone. Am J Clin Oncol. 1984;7:647-652.
136 Lo K, Fletcher GH, Byers RM, et al. Results of irradiation in the squamous cell carcinomas of the anterior faucial pillar-retromolar trigone. Int J Radiat Oncol Biol Phys. 1987;13:969-974.
137 Mendenhall WM, Morris CG, Amdur RJ, et al. Retromolar trigone squamous cell carcinoma treated with radiotherapy alone or combined with surgery. Cancer. 2005;103:2320-2325.
138 Kowalski LP, Hashimoto I, Magrin J. End results of 114 extended “commando” operations for retromolar trigone carcinoma. Am J Surg. 1993;166:374-379.
139 Huang CJ, Chao KS, Tsai J, et al. Cancer of retromolar trigone. Long-term radiation therapy outcome. Head Neck. 2001;23:758-763.
140 New GB, Hallberg OE. The end results of the treatment of malignant tumors of the palate. Surg Gynecol Obstr. 1941;73:520-524.
141 Chierici G, Silverman SJr, Forsythe B. A tumor registry study of oral squamous carcinoma. J Oral Med. 1968;23:91-98.
142 Evans JF, Shah JP. Epidermoid carcinoma of the palate. Am J Surg. 1981;142:451-455.
143 Barker GJ, Barker BF, Gier RE. Oral Management of the Cancer Patient: A Guide for the Health Care Professional. Kansas City, Missouri: The Curators of the University of Missouri; 1981.
144 Chung CK, Rahman SM, Lim ML, Constable WC. Squamous cell carcinoma of the hard palate. Int J Radiat Oncol Biol Phys. 1979;5:191-196.
145 Martin H. Tumors of the palate (benign and malignant). Arch Surg. 1942;44:599-635.
146 Ratzer ER, Schweitzer RJ, Frazell EL. Epidermoid carcinoma of the palate. Am J Surg. 1970;119:294-297.
147 Cianchetti M, Sandow PS, Scarborough LD, et al. Radiation therapy for minor salivary gland carcinoma. Laryngoscope. 2009;119:1334-1338.
148 Shibuya H, Horiuchi JI, Suzuki S, et al. Oral carcinoma of the upper jaw. Results of radiation treatment. Acta Radiol Oncol. 1984;23:331-335.
149 Chung CK, Johns ME, Cantrell RW, Constable WC. Radiotherapy in the management of primary malignancies of the hard palate. Laryngoscope. 1980;90:576-584.
150 Yorozu A, Sykes AJ, Slevin NJ. Carcinoma of the hard palate treated with radiotherapy: a retrospective review of 31 cases. Oral Oncol. 2001;37:493-497.
151 Tran L, Sadeghi A, Hanson D, et al. Salivary gland tumors of the palate. The UCLA experience. Laryngoscope. 1987;97:1343-1345.
152 Kovalic JJ, Simpson JR. Carcinoma of the hard palate. J Otolaryngol. 1993;22:118-120.
153 Truitt TO, Gleich LL, Huntress GP, Gluckman JL. Surgical management of hard palate malignancies. Otolaryngol Head Neck Surg. 1999;121:548-552.
154 Mendenhall WM, Riggs CE, Amdur RJ, et al. Altered fractionation and/or adjuvant chemotherapy in definitive irradiation of squamous cell carcinoma of the head and neck. Laryngoscope. 2003;113:546-551.
155 Horiuchi J, Adachi T. Some considerations on radiation therapy of tongue cancer. Cancer. 1971;28:335-339.
156 Lees AW. The treatment of carcinoma of the anterior two-thirds of the tongue by radiotherapy. Int J Radiat Oncol Biol Phys. 1976;1:849-858.