Opportunistic Atypical Fungus
Pneumocystis jiroveci
1. Describe the symptoms of Pneumocystis jiroveci infection and the cells affected by this organism.
2. List the appropriate specimen types collected for diagnosis of pneumocystis pneumonia.
3. Discuss the laboratory tests used in the diagnosis of P. jiroveci infection, including the methodology and biochemical principles.
4. List the four stains most commonly used in the diagnosis of P. jiroveci infection.
Laboratory Diagnosis
Specimen Collection and Transport
Direct Detection Methods
Stains
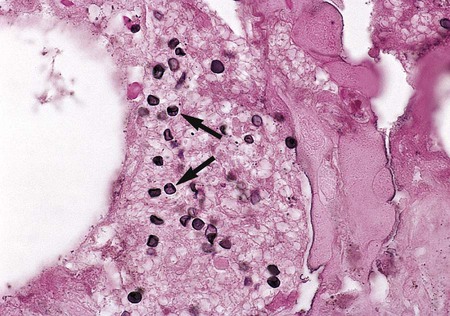
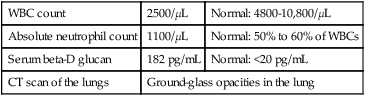
The diagnosis of P. jiroveci pneumonia currently is based on the clinical presentation, radiographic studies, and direct and/or pathologic examination of bronchoalveolar lavage fluid or biopsy material. The flexible-walled trophozoites are the predominant form of the organism, but these are difficult to visualize. They are somewhat discernible in Giemsa-stained material, but their pleomorphic appearance makes this form of the organism difficult to identify. A firm-walled cystic form also exists, although the cysts are outnumbered by the trophozoites 10 to 1. Cysts are more easily recognized than trophozoites and may be definitively identified using a variety of stains, such as calcofluor white, methenamine silver, and immunofluorescent staining (Figure 62-1). The cysts are spherical to concave, uniform in size (4 to 7 μm in diameter), do not bud, and contain distinctive intracystic bodies.