Opioids
Perspective
Opioid is a term that applies to all natural, synthetic, and semisynthetic agents with morphine-like actions. It is more inclusive than the term opiate, which refers only to natural agents. Both terms are derived from opium, the Greek word for juice in reference to poppy juice. Poppy juice contains more than 20 distinct natural alkaloids with morphine-like activity. The nonspecific term narcotic refers to any agent that induces sleep and should not be used. Opioid is more precise and is the correct medical term for the agents that act on opiate receptors in the body. Finally, the term endorphin applies to any of the endogenous opioid peptides, including endomorphins, dynorphins, enkephalins, and nociceptin.1
The World Drug Report 2010 of the United Nations Office on Drugs and Crime estimates that between 155 and 250 million people (3.5 to 5.7% of the population aged 15 to 64 years) used illicit substances at least once in 2008. Globally, cannabis, amphetamine-type stimulant, and cocaine users outnumber opiate (heroin/opium) users, but opiates are associated with more harm with use. Annual prevalence of opiate (heroin/opium) use in the United States is estimated at 0.58% of the population aged 15 to 64 years. Mortality rate for dependent opiate users is between 6 and 20 times that expected for those in the general population of the same age and gender.2
In addition, nonmedicinal use of prescription opioids, in adults and teens, is an extensive and growing problem in the United States. According to the Centers for Disease Control and Prevention (CDC), in 2010, enough opioid analgesics were sold to medicate every American adult with a typical dose of 5 mg of hydrocodone every 4 hours for 1 month.3 Fatal poisonings involving opioid analgesics more than tripled from 4000 to 13,800 from 1999 to 2006, including a multistate epidemic of nonpharmaceutical fentanyl-related overdoses that resulted in more than 1000 deaths from April 2006 to March 2007.4 This trend continues to rise, and in 2010 there were more than 15,000 opioid analgesic–related fatalities reported to the CDC.
Principles of Disease
Although opioids have been used for more than 5000 years, opiate binding to brain membrane receptors was not shown until the 1970s, and the first opioid receptor gene was not isolated until 1992. Subsequent molecular characterization of the endogenous opioid system, including peptides and receptors, has improved the understanding of opioid physiologic roles.5
The three established opioid receptors, mu/MOP (OPRM1 gene), kappa/KOP (OPRK1 gene), and delta/DOP (OPRD1 gene), are transmembrane, G protein–coupled receptors distributed throughout the CNS, concentrated in areas associated with nociception as well as in areas of euphoria and respiratory control. Systemically, opioid receptors are localized in sensory nerve endings, the gastrointestinal tract, cardiovascular endothelial cells, and immune cells.5,6 Whereas opioid receptor ligand binding has demonstrated variable pharmacologic effects, whether it is through specific opioid receptor subtypes or receptor gene splice variants, receptor dimerization, or biased ligand agonism is unclear.7 Genetic variations in opioid receptors and in metabolic pathways account for some of the interindividual differences in response to both endogenous and exogenous opioids. Several polymorphisms of OPRM1 have been identified, including A118G polymorphism, present in up to 30% of individuals of European descent and 50% of Asian descent,8 which is associated with increased dosage requirements for pain control. Whereas it might also result in lower relative clinical toxicity after overdose, this has not been studied. Polymorphisms of several cytochrome P450 enzymes, including CYP2D6 (tramadol, codeine), CYP3A5 (fentanyl), and CYP2B6 (methadone), have been linked to variability in response to several opioids.9
Pathophysiology and Pharmacology
Opioids are well absorbed after gastrointestinal (oral and rectal) or parenteral administration but also through nasal, buccal, pulmonary, and transdermal routes, depending on the lipid solubility of the specific opioid.1 Heroin is usually abused through intravenous and subcutaneous routes, but it is also absorbed after nasal administration because it is lipid soluble. In general, opioid toxicity is less pronounced but more prolonged with ingestion than with parenteral administration.1 Absorption of opioids after ingestion occurs in the small intestine and with therapeutic doses is complete within 1 or 2 hours. However, because of delayed gastric emptying, absorption and clinical effects of toxicity may be prolonged after overdose.
Most opioids have a large volume of distribution. Clinical effects depend on lipid solubility, which affects the rate at which opioids and their metabolites cross the blood-brain barrier. All opioids undergo hepatic metabolism and renal elimination, and variations in hepatic and renal function are important because metabolite activity may contribute to clinical effects and toxicity.1
The pharmacokinetics of the specific agent determine the clinical course of opioid toxicity. Heroin peaks in the serum within 1 minute of intravenous injection, 3 to 5 minutes of intranasal administration, and 10 minutes of subcutaneous injection. Heroin’s lipophilic nature allows rapid transport across the blood-brain barrier into the CNS. In the CNS and blood, heroin is rapidly hydrolyzed to 6-monoacetylmorphine and then morphine, which is less lipid soluble. In the liver, morphine undergoes conjugation with glucuronic acid to form more water-soluble compounds that are excreted by the kidneys.1 Morphine and heroin are not metabolized by cytochrome P450 enzymes and are not directly affected by P450 polymorphisms.
Withdrawal
Because the half-life of heroin is 30 minutes and the half-life of methadone is 15 to 40 hours, withdrawal symptoms occur 4 to 6 hours after discontinuation of heroin and 24 to 48 hours after discontinuation of methadone.10,11 Duration of symptoms is 7 to 10 days and 2 weeks, respectively. The degree of physical dependence that has developed is also important. With chronic opioid exposure, cellular adaptation results in upregulation of cyclic adenosine monophosphate (cAMP). When the exposure is discontinued or an antagonist is administered, the result is a temporary elevation of cAMP levels and increased sympathetic activity above a normal baseline.
Clinical Features
Neurologic
Parkinsonian symptoms in injection drug abusers have been attributed to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), a side product during synthesis of a meperidine analogue in street laboratories. MPTP metabolite accumulates in CNS cell mitochondria and is associated with focal lesions in the substantia nigra and a syndrome clinically indistinguishable from idiopathic parkinsonism. The syndrome is irreversible in some patients.12
Spongiform leukoencephalopathy is associated with “chasing the dragon,” a practice of inhaling heroin heated typically on aluminum foil. Patients have psychomotor retardation, dysarthria, ataxia, tremor, and other neurologic abnormalities.13 This syndrome is thought to be related to a combination of mitochondrial injury and hypoxia from the heroin or, more likely, the method of preparation. One report also associates heroin-induced movement disturbance with basal ganglia lesions.14
Serotonin syndrome is a clinical triad of mental status changes, autonomic instability, and neuromuscular changes (see Chapter 151) and may be fatal. Most cases involve an interaction between a serotonergic agent and a second agent, usually a selective serotonin reuptake inhibitor or a monoamine oxidase inhibitor. Meperidine, dextromethorphan, methadone, and tramadol inhibit serotonin reuptake and are associated with serotonin syndrome, as are some opiate analogues without known serotonin reuptake effects, such as oxycodone and buprenorphine.15
Respiratory
By suppressing the sensitivity of the medullary respiratory center to hypercapnia, opioids decrease respiratory rate and tidal volume in a dose-dependent manner.1 Although it initially remains intact, the hypoxic drive is overridden in severe poisoning or when antagonistic stimuli (e.g., pain) are blocked. Overdose of an agonist-antagonist agent produces less significant respiratory depression, presumably because of mu receptor antagonism (Table 162-1). Central sleep apnea occurs with long-term opioid use and also with acute increased opioid use from baseline. Continuous positive airway pressure is usually ineffective for treatment of sleep apnea in these patients.16
Table 162-1
Select Opioid Doses and Associated Respiratory Depression
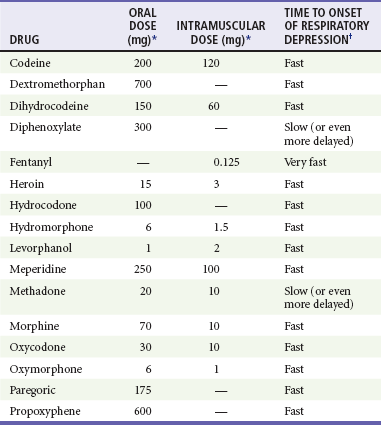
*Equivalent to 10 mg of intramuscular morphine.
†Varies with the drug and route of administration. In addition, the effects of a dose in any particular patient depend on multiple factors, including age, weight, and comorbid conditions. After intramuscular administration, very fast means 5 to 30 minutes, fast means 15 to 60 minutes, and slow means 1 to 4 hours. After oral administration, these time definitions are approximately doubled.
Bronchospasm is rare with heroin use in both asthmatic and nonasthmatic patients after inhalational exposure and rarely after other routes. When it occurs, the bronchospasm is often severe, prolonged, and refractory to beta-agonist therapy, and it may require mechanical ventilation for several days. It is unclear whether the heroin, an adulterant, or a combination triggers the bronchospasm and whether the response is histamine mediated or from direct irritation.17
Acute lung injury occurs with therapeutic opioid use but is much more common after overdose.18 The capillary leak is likely to be from hypoxia rather than a direct drug effect.
Otologic
Sensorineural hearing loss has been reported with acute and chronic use of some opioids, including heroin, methadone, and hydrocodone, especially in combination with acetaminophen. Genetic polymorphism producing altered metabolism, direct cochlear effects, and comorbidities have been suggested as causative factors.19,20
Cardiovascular
Opioids cause mild hypotension and relative bradycardia. Hypotension is from histamine release and can be blocked by antihistamines (H1 antagonists). The hypotension is typically orthostatic and resolves with supine positioning. In recent years, there are increasing reports of cardiovascular toxicity associated with certain opioids. Propoxyphene and especially norpropoxyphene, its cardioactive metabolite, are associated with sodium channel blockade, similar to type IA antidysrhythmic agents, and produce widening of the QRS complex.21 In November 2010, because of dose-dependent PR interval prolongation, QRS widening, and QT prolongation, manufacturers voluntarily withdrew all proprietary and generic products containing propoxyphene from the United States market in response to a request from the Food and Drug Administration.22 Levomethadyl, a three-dose per week opioid replacement for management of dependence, was discontinued in 2003 because of blockade of hERG potassium channels, resulting in QTc prolongation. Methadone also blocks this potassium channel and is associated with QTc prolongation, especially with other predisposing factors,23 at higher doses, or with CYP2B6 polymorphism (resulting in delayed inactivation of methadone).
Gastrointestinal
Decreased gastrointestinal motility is common with therapeutic use and overdose of opioids. Severe cases may develop ileus.1 Increased biliary tract pressures and choledochoduodenal sphincter spasm occur with therapeutic dosing of many opioids, including morphine, meperidine, and codeine. Spasm is not always reproducible within the same patient but seems related to individual susceptibility more than to a specific agent. Presenting clinical symptoms mimic biliary colic and may respond to naloxone or glucagon.24
Urologic
Opioids can cause urinary retention from urethral sphincter spasm and decreased detrusor tone. Alpha-adrenergic antagonists may reverse this effect. Glomerulosclerosis and renal amyloidosis are seen in end-stage “heroin nephropathy” of chronic opioid addicts.25
Metabolic
Hypoglycemia occurs after opioid overdose, but the mechanism is unclear. Coingestants, especially ethanol, may contribute to this finding. Hypothermia is reported, but the mechanism is unclear. Hyperthermia should prompt a search for infectious complications, particularly in injection drug users, and for coingestants (e.g., cocaine and other stimulants) or adulterants (e.g., tripelennamine and scopolamine).26
Withdrawal
Opioid withdrawal occurs in tolerant individuals when opioid use is discontinued or an antagonist is administered. Increased sympathetic discharge and adrenergic hyperactivity are responsible for the clinical symptoms and signs. In contrast to the typical toxidrome of opioid toxicity (CNS and respiratory depression and miosis), withdrawal is associated with the opposite symptoms of CNS excitation, tachypnea, and mydriasis. Pulse and blood pressure are also increased. Although opioid withdrawal can be uncomfortable, it is not life-threatening.10,11
Diagnostic Strategies
The diagnosis of opioid intoxication is usually based on history and physical examination. Other than hypoglycemia, specific laboratory abnormalities are not seen. Carbon dioxide and oxygen saturation monitoring is helpful for identification of respiratory depression and hypoxia. When the patient has hypoxemia and pulmonary rales, a chest radiograph can evaluate for acute lung injury. A 12-lead electrocardiogram can confirm a suspected dysrhythmia or evaluate for QRS widening or QT prolongation if propoxyphene or methadone overdose is suspected. An abdominal radiograph may identify packets of opioids or other illicit substances in a body packer (see Chapter 147 and Chapter 186). With ingestion of an unknown opioid preparation, acetaminophen and salicylate concentrations should be checked because many prescription opioids are available in combination products. Likewise, many illicit opioid users are exposed to additional drugs and contaminants.
Opiates are detected on most qualitative antibody-based enzymatic immunoassay urine toxicology screens. Semisynthetic and synthetic opioids, such as fentanyl and its derivatives, are often missed on typical urine screens unless they are specifically included or the assay cross-reacts. A urine test result is positive for days after last use, depending on the half-life. Poppy seed ingestion leads to a positive opiate screen for morphine and codeine; however, detection of 6-monoacetylmorphine, a specific metabolite of heroin, can confirm heroin use.27
As with opioid toxicity, no diagnostic test exists for opioid withdrawal.
Management
Gastrointestinal Decontamination
Gastrointestinal decontamination is not routinely recommended, and there is no convincing evidence that it provides any benefit. Despite this, whole-bowel irrigation has been used to hasten passage of drug packets from body packers, body stuffers, or patients with ingestions involving opioid combination products or multidrug ingestions. Although it is unnecessary because of an effective opioid antidote and of no demonstrated benefit, a single early dose of activated charcoal (1 g/kg in children and 50-100 g in adults) may be considered in rare circumstances after consultation with a poison center or toxicologist.28
Antidote with Opioid Antagonist
Naloxone can precipitate acute withdrawal in chronic opioid users. In this population, naloxone should be started at 0.04 to 0.2 mg and then slowly titrated. The duration of action of naloxone is 1 or 2 hours. Consequently, either repeated doses or a continuous infusion may be required, starting at two thirds of the effective initial dose, given per hour.1
Naloxone has an excellent safety profile. Acute lung injury, hypertension, and dysrhythmia have been rarely associated with use of naloxone after general anesthesia and in patients with underlying cardiac or pulmonary disease.29 Whether naloxone is the cause of these complications is unproven. Idiosyncratic reactions and sympathetic discharge with precipitation of acute withdrawal have been proposed as explanations. The risk may be greater in those with ongoing hypoventilation before naloxone administration. Complete clinical recovery in response to naloxone is strongly suggestive of opioid overdose. Naloxone has been given to overdoses with valproic acid, clonidine, tramadol, and captopril and ethanol intoxication because of a presentation similar to opioid intoxication. These have been reported to improve to lesser degrees and inconsistently with naloxone. The mechanism of these responses to naloxone is not established. Some of these drugs may have activity at opioid receptors.29
Nalmefene is an opioid antagonist with a long half-life (8-11 hours) and duration of clinical effect. Intravenous nalmefene has a rapid onset of action in reversal of opioid-induced CNS and respiratory depression. Alternative administration routes are oral, subcutaneous, and intramuscular. The initial intravenous dose is 0.5 to 1.5 mg (the pediatric dose is not established). Higher doses have been used but are associated with increased risk of adverse effects.30,31 When a clinical response has been achieved with nalmefene, repeated doses or continuous infusions are generally not required; however, the duration of induced withdrawal symptoms may be longer with nalmefene. Naloxone remains the preferred antidote in patients at risk for withdrawal or other adverse effects and in patients with anticipated short duration of opioid toxicity.
Withdrawal
Opioid withdrawal is not life-threatening, but supportive and symptomatic care can decrease the distress and avoid the associated complications. When withdrawal is produced by administration of naloxone, the symptoms are of short duration.10,11 Other patients with withdrawal can receive clonidine or symptomatic treatment (antiemetics, antidiarrheals, benzodiazepines) to alleviate symptoms in the emergency department (ED). In addition, fluid and electrolyte replacement is important for patients with dehydration from gastrointestinal symptoms.
Methadone, a long-acting opioid, provides opioid replacement to treat or to prevent withdrawal in chronic heroin users admitted for other medical conditions but should not be used for patients in the ED with uncomplicated acute withdrawal. The onset of action of an initial dose of methadone of either 20 mg orally or 10 mg intramuscularly is 30 to 60 minutes. This initial dose typically controls significant symptoms.10 Some symptoms, particularly drug craving, may be ongoing and require a subsequent dose after several hours. Maintenance methadone therapy requires doses every 24 hours or may be tapered daily. In October 2002, the Food and Drug Administration approved a buprenorphine monotherapy product (Subutex) as well as a buprenorphine-naloxone combination product (Suboxone) for treatment of opioid addiction. In contrast to methadone, these products may be prescribed by physicians outside of traditional opioid treatment programs, but prescribing physicians must have a specific Drug Enforcement Administration waiver per the Drug Addiction Treatment Act of 2000.32
Clonidine, a central alpha2-agonist, is used for treatment of opioid withdrawal without opioid replacement.10 Clonidine controls symptoms by suppressing sympathetic hyperactivity. It may also shorten the duration of withdrawal. The initial dose is 0.1 mg orally. Repeated doses may be given every 30 to 60 minutes; relatively large total doses may be required. As with opioid replacement, clonidine therapy is titrated to individual clinical response. Hypotension may limit the treatment but is not common in the setting of opioid withdrawal treatment. Patches for transdermal administration of clonidine are available, but onset of action is delayed 24 hours. Oral doses still must be given initially.
Buspirone is an azapirone compound used to treat ethanol and nicotine addiction. Studies suggest that it may also be an option to treat opioid addicts by decreasing serotonergic neurotransmission in withdrawal, but further investigation is needed before ED use.33
Disposition
Patients with opioid toxicity are often treated successfully in the ED, sometimes in conjunction with the ED observation unit. Patients who receive naloxone should be observed for 2 hours to assess the extent of resedation, hypoxia, and pulmonary complications.34 Full reversal of opioid toxicity may produce an awake patient who is uncooperative with the observation duration. If these patients are discharged against medical advice, they should demonstrate decision-making capacity as well as a full understanding of the risks of return of severe toxicity and development of complications, including apnea. Asymptomatic patients can be observed in the ED until 4 hours after an ingestion, at which time they can be discharged with appropriate psychiatric evaluation or drug abuse counseling. However, patients who have ingested an opioid with a longer half-life, active metabolite, or modified-release preparation, as well as patients with multiple drug ingestions involving an opioid, may require longer observation periods.
Asymptomatic patients who have a known or possible ingestion of diphenoxylate-atropine (Lomotil) should be observed in a monitored unit; this includes small children who may have ingested only a single tablet (2.5 mg of diphenoxylate and 0.025 mg of atropine). The metabolite of diphenoxylate, difenoxin, has a longer half-life and is five times more active than diphenoxylate, and it may cause delayed and prolonged toxicity. Delayed absorption, caused by each component of Lomotil and enterohepatic recirculation, may contribute as well.35
Patients with a known or potential ingestion of packets of illicit opioid drugs, body packers and body stuffers, remain asymptomatic until one or more of the packets leak. Minimum necessary observation duration for these patients if they are asymptomatic is not clear. Body packers are typically admitted until the packets are passed; body stuffers may be discharged after 6 hours.36
References
1. Yakesh, T, Wallace, MS. Opioids, analgesia, and pain management. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill; 2011:481.
2. United Nations Office on Drugs and Crime. World Drug Report 2010 (United Nations Publication, Sales No. E.10.XI.13). www.unodc.org.
3. Centers for Disease Control and Prevention. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999-2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492.
4. Warner, M, Chen, LH, Makuc, DM. Increase in Fatal Poisonings Involving Opioid Analgesics in the United States, 1999-2006. NCHS Data Brief, No 22. Hyattsville, Md: National Center for Health Statistics; 2009.
5. Kieffer, BL, Evans, CJ. Opioid receptors: From binding sites to visible molecules in vivo. Neuropharmacology. 2009;56(S1):205.
6. Dhawan, BN, et al. International Union of Pharmacology XII classification of opioid receptors. Pharmacol Rev. 1996;48:567.
7. Dietis, N, Rowbotham, DJ, Lambert, DG. Opioid receptor subtypes: Fact or artifact? Br J Anaesth. 2011;107:8.
8. Mague, SD, Blendy, JA. OPRM1 SNP (A118G): Involvement in disease development, treatment response and animal models. Drug Alcohol Depend. 2010;108:172.
9. Argoff, CE. Clinical implications of opioid pharmacogenetics. Clin J Pain. 2010;26:S16.
10. Olmedo, R, Hoffman, RS. Withdrawal syndromes. Emerg Med Clin North Am. 2000;18:273.
11. Jenkins, DH. Substance abuse and withdrawal in the intensive care unit. Surg Clin North Am. 2000;80:1033.
12. Ballard, PA, Tetrud, JW, Langston, JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): Seven cases. Neurology. 1985;35:949.
13. Chang, WC. MRI features of spongiform leukoencephalopathy following heroin inhalation. Neurology. 2006;67:504.
14. Mätzler, W, Nägele, T, Gasser, T, Krüger, R. Acute parkinsonism with corresponding lesions in the basal ganglia after heroin abuse. Neurology. 2007;68:414.
15. Isenberg, D, Wong, SC, Curtis, JA. Serotonin syndrome triggered by a single dose of suboxone. Am J Emerg Med. 2008;26:840.e.3.
16. Mogri, M, Khan, MI, Grant, BJ, Mador, MJ. Central sleep apnea induced by acute ingestion of opioids. Chest. 2008;133:1484.
17. Cygan, J, Trunsky, M, Corbridge, T. Inhaled heroin–induced status asthmaticus: Five cases and a review of the literature. Chest. 2000;117:272.
18. Soto, J, Sacristan, JA, Alsar, MJ. Pulmonary oedema due to fentanyl. Anaesthesia. 1992;47:913.
19. Yorgason, JG, Kalinec, GM, Luxford, WM, Warren, FM, Kalinec, F. Acetaminophen ototoxicity after acetaminophen/hydrocodone abuse: Evidence from two parallel in vitro mouse models. Otolaryngol Head Neck Surg. 2010;142:814.
20. Shaw, KA, Babu, KM, Hack, JB. Methadone, another cause of opioid-associated hearing loss: A case report. J Emerg Med. 2011;41:635.
21. Stork, CM, et al. Propoxyphene-induced wide QRS complex dysrhythmia responsive to sodium bicarbonate: A case report. J Toxicol Clin Toxicol. 1995;33:179.
22. FDA Drug Safety Communication. FDA Recommends Against the Continued Use of Propoxyphene. www.fda.gov/drugs/drugsafety/ucm234338.htm.
23. Hanon, S, Seewald, RM, Yang, F, Schweitzer, P, Rosman, J. Ventricular arrhythmias in patients treated with methadone for opioid dependence. J Interv Card Electrophysiol. 2010;28:19.
24. Bird, KJ. Narcotic-induced choledochoduodenal sphincter spasm reversed by naloxone. Anaesthesia. 1986;41:1120.
25. Dubrow, A, et al. The changing spectrum of heroin-associated nephropathy. Am J Kidney Dis. 1985;5:36.
26. Perrone, J, Shaw, L, DeRoos, F. Laboratory confirmation of scopolamine co-intoxication in patients using tainted heroin. J Toxicol Clin Toxicol. 1999;37:491.
27. Tenore, PL. Advanced urine toxicology testing. J Addict Dis. 2010;29:436.
28. Von Westphal, V, Jacobsen, P. Time limits for gastrointestinal decontamination in poisoning. Ugeskr Laeger. 2009;171:718.
29. Chamberlain, JM, Klein, BL. A comprehensive review of naloxone for the emergency physician. Ann Emerg Med. 1994;12:650.
30. Kaplan, JL, et al. Double-blind randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Ann Emerg Med. 1999;34:42.
31. Chumpa, A. Nalmefene hydrochloride. Pediatr Emerg Care. 1999;15:141.
32. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. Buprenorphine. www.buprenorphine.samhsa.gov.
33. Van den Brink, W, Haasen, C. Evidence-based treatment of opioid-dependent patients. Can J Psychiatry. 2006;51:635.
34. Etherington, J, et al. Is early discharge after naloxone reversal of presumed opioid overdose? CJEM. 2000;2:157.
35. McCarron, MM, Challoner, KR, Thompson, GA. Diphenoxylate-atropine (Lomotil) overdose in children: An update. Pediatrics. 1991;87:694.
36. Moreira, M, Buchanan, J, Heard, K. Validation of a 6-hour observation period for cocaine body stuffers. Am J Emerg Med. 2011;29:299.








