Chapter 59 Ocular Ischemic Syndrome
In 1963, Kearns and Hollenhorst1 reported on the ocular symptoms and signs occurring secondary to severe carotid artery obstructive disease. They called the entity “venous stasis retinopathy” and noted that it occurred in approximately 5% of patients with severe carotid artery insufficiency or thrombosis. Some confusion has since arisen with this term because it has also been used to designate mild central retinal venous obstruction.2 A number of additional alternative names have been proposed, including ischemic ocular inflammation,3 ischemic oculopathy,4 and the ocular ischemic syndrome.5,6 Histopathologic examination of eyes with the entity generally does not reveal inflammation,7,8 and therefore the descriptive term the present authors and Dr. Larry Magargal have thought preferable is the ocular ischemic syndrome.5,6
Demographics and incidence
The mean age of patients with the ocular ischemic syndrome is about 65 years, with a range generally from the fifties to the eighties. No racial predilection has been identified, and males are affected more than females by a ratio of about 2 : 1. Either eye can be affected, and in approximately 20% of patients, ocular involvement is bilateral. The incidence of the disease has not been extensively studied, but from the work of Sturrock and Mueller9 an annual estimate of 7.5 cases/million persons can be made. This number may be falsely low, since it is possible that a number of cases are misdiagnosed.
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Etiology
In general, a 90% or greater stenosis of the ipsilateral carotid arterial system is present in eyes with the ocular ischemic syndrome.5 It has been shown that a 90% carotid stenosis reduces the ipsilateral central retinal artery perfusion pressure by about 50%.10,11 The obstruction can occur within the common carotid or internal carotid artery. In about 50% of cases, the affected vessel is 100% occluded, while in 10% there is bilateral 100% carotid artery obstruction.5
Occasionally, obstruction of the ipsilateral ophthalmic artery can also be responsible.5,12,13 Rarely, an isolated obstruction of the central retinal artery alone can mimic the dilated retinal veins and retinal hemorrhages seen in eyes with the ocular ischemic syndrome.14
Atherosclerosis within the carotid artery is the cause for the great majority of cases of the ocular ischemic syndrome.5 Dissecting aneurysm of the carotid artery has been reported as a cause,15 as has giant cell arteritis.16 Hypothetically, entities such as fibromuscular dysplasia,17 Behçet disease,18 trauma,19 and inflammatory entities that cause carotid artery obstruction could lead to the ocular ischemic syndrome.
Clinical presentation
Visual loss
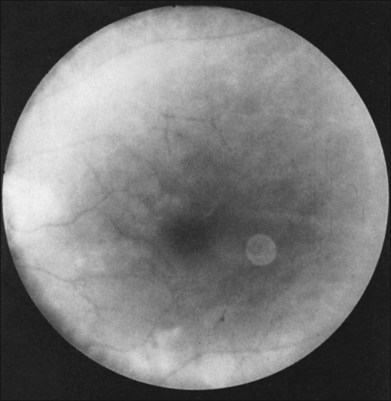
Greater than 90% of patients with the ocular ischemic syndrome relate a history of visual loss in the affected eye(s).5 In two-thirds of cases, it occurs over a period of weeks, but it is abrupt in approximately 12%. In this latter group, with sudden visual loss, there is often a cherry-red spot present on funduscopic examination (Fig. 59.1).
Prolonged light recovery
Prolonged recovery following exposure to a bright light has been described in patients with severe carotid artery obstruction.20 Concurrent attenuation of the visual evoked response has also been observed in these cases after light exposure. The phenomenon has been attributed to ischemia of the macular retina. In cases of bilateral, severe carotid artery obstruction, the visual loss after exposure to bright light occurs in both eyes, mimicking occipital lobe ischemia due to vertebrobasilar disease.21
Scintillating scotomas
Dissection of the internal carotid artery has been reported to cause scintillating scotomas that resemble a migraine aura.22 While these could theoretically be associated with the classic ocular ischemic syndrome, they have not been observed by the authors.
Amaurosis fugax
A history of amaurosis fugax is elicited in about 10% of ocular ischemic syndrome patients.5 Amaurosis fugax, or fleeting loss of vision for seconds to minutes, is thought to most commonly be caused by emboli to the central retinal arterial system, although vasospasm may also play a role.23 Although the majority of people with amaurosis fugax alone do not have the ocular ischemic syndrome, it can be an indicator of concomitant, ipsilateral carotid artery obstructive disease. About one-third of patients with amaurosis fugax have an ipsilateral carotid artery obstruction of 75% or greater.24 Rarely, it has been associated with a stenosis of the ophthalmic artery.24
Pain
Pain is present in the affected eye or orbital region in about 40% of cases,5 and has been referred to as “ocular angina.” Most often, it is described as a dull ache. It can occur secondary to neovascular glaucoma, but in those cases in which the intraocular pressure is normal, the cause may be ischemia to the globe and/or ipsilateral dura.
Visual acuity
The presenting visual acuities of patients with the ocular ischemic syndrome are bimodally distributed, with 43% of affected eyes having vision ranging from 20/20 to 20/50, and 37% having counting fingers or worse vision.25 Absence of light perception is generally not seen early, but can develop in the later stages of the disease, usually secondary to neovascular glaucoma. Among all eyes with the ocular ischemic syndrome after one year of follow-up, including those with and without treatment, approximately 24% remain in the 20/20–20/50 group and 58% have counting fingers vision or worse.
External collaterals
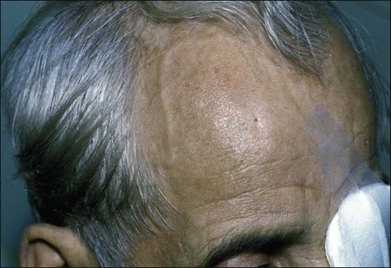
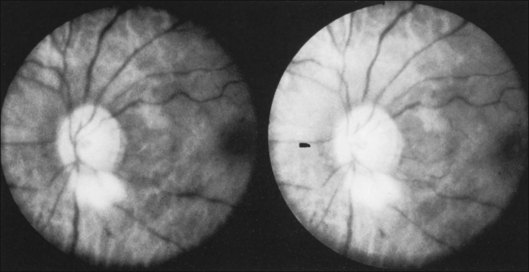
Prominent collateral vessels are occasionally seen on the forehead (Fig. 59.2). These vessels connect the external carotid system on one side of the head to that on the other. These vascular collaterals should not be mistaken for the enlarged tender vessels seen with giant cell arteritis, since temporal artery biopsy may shut down this important source of collateral blood flow to the brain.
Anterior segment changes
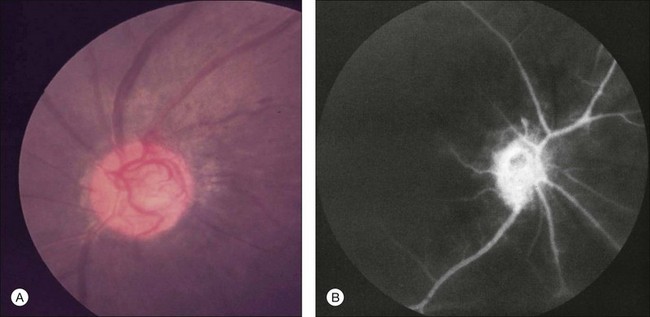
Neovascularization of the iris is encountered in approximately two-thirds of eyes with the ocular ischemic syndrome at the time of presentation5 (Fig. 59.3). Nevertheless, only slightly over half of these eyes have or develop an increase in intraocular pressure, even if the anterior chamber angle is closed by fibrovascular tissue. Impaired ciliary body perfusion, with a subsequent decrease in aqueous production, probably accounts for this phenomenon.
Flare in the anterior chamber is usually present in eyes with rubeosis iridis. An anterior chamber cellular response is seen in almost one-fifth of eyes with the ocular ischemic syndrome,5 but it rarely exceeds grade 2+, on a 0 to 4+ range, as per the Schlaegel classification.26 Keratic precipitates can be present, but are typically small.
Posterior segment findings
The retinal arteries are usually narrowed and the retinal veins are most often dilated, but not tortuous (Fig. 59.4). The venous dilation may be accompanied by beading, but usually not to the extent seen in eyes with marked preproliferative or proliferative diabetic retinopathy. Dilation of the veins is probably a nonspecific response to the ischemia from the inflow obstruction. Nevertheless, in some eyes both the retinal arteries and veins are narrowed. In contrast, eyes with central retinal vein obstruction usually also have dilated retinal veins, but they are often tortuous. The fact that the ocular ischemic syndrome occurs secondary to impaired inflow, while central retinal vein obstruction is usually associated with compromised outflow resulting from thrombus formation at or near the lamina cribrosa, may account for this difference.27
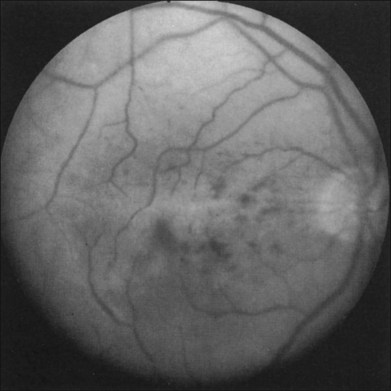
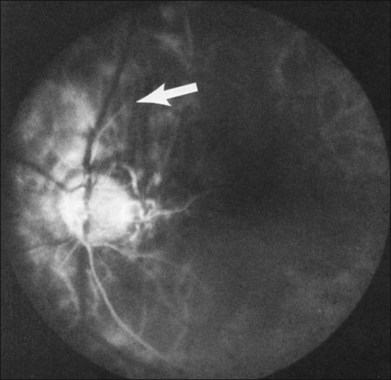
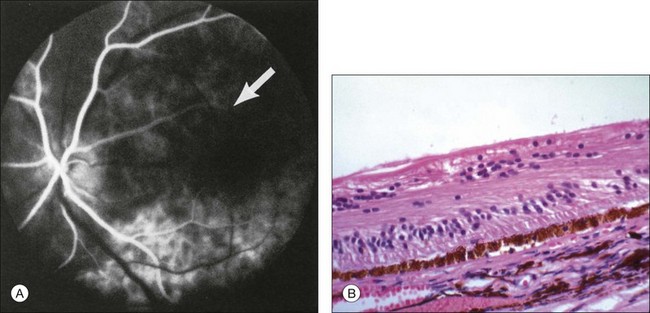
Retinal hemorrhages are seen in about 80% of affected eyes. They are most commonly present in the midperiphery, but can also extend into the posterior pole (Fig. 59.5, Fig. 59.6 online). While dot and blot hemorrhages are the most common variant, superficial retinal hemorrhages in the nerve fiber layer are occasionally seen. The hemorrhages probably arise secondary to leakage from the smaller retinal vessels, which have sustained endothelial damage as a result of the ischemia. Similar to the case with diabetic retinopathy, they may also result from the rupture of microaneurysms. In general, the hemorrhages seen with the ocular ischemic syndrome are less numerous than those accompanying central retinal vein obstruction. They are almost never confluent.
Microaneurysms are frequently observed outside the posterior pole, but can be seen in the macular region also. Hyperfluorescence with fluorescein angiography (Fig. 59.7) differentiates these abnormalities from hypofluorescent retinal hemorrhages. Retinal telangiectasia has also been described.28
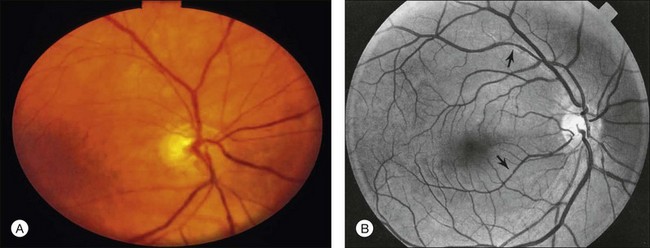
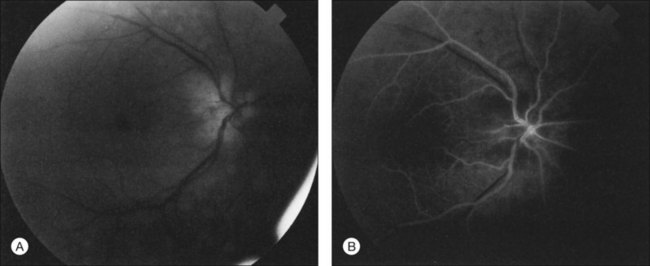
Posterior segment neovascularization can occur at the optic disc or on the retina. Neovascularization of the disc (Fig. 59.8) is encountered in about 35% of eyes, while neovascularization of the retina is seen in about 8%.5 Vitreous hemorrhage arising from traction upon the neovascularization by the vitreous gel has been reported to occur in 4% of eyes with the ocular ischemic syndrome in a retrospective study.5 Rarely, the neovascularization can progress to severe preretinal fibrovascular proliferation (Fig. 59.9 online). Neovascularization of the retina (Fig. 59.10 online) is encountered in 8% of eyes with ocular ischemia. It is usually present concomitant with neovascularization of the disc.
A cherry-red spot is seen in approximately 12% of eyes with the ocular ischemic syndrome (see Fig. 59.1).5 It can occur secondary to inner layer retinal ischemia from embolic obstruction of the central retinal artery, but probably more often develops when the intraocular pressure exceeds the perfusion pressure within the central retinal artery, particularly in eyes with neovascular glaucoma.
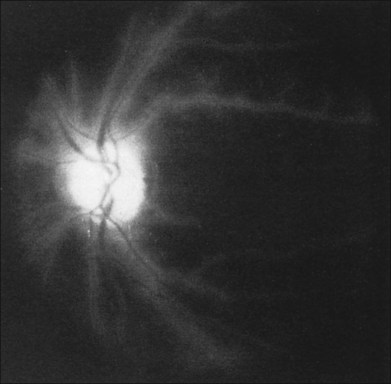
Additional posterior segment signs5 include cotton-wool spots (Fig. 59.11 online) in 6% of eyes, spontaneous retinal arterial pulsations in 4% (Fig. 59.12), and cholesterol emboli within the retinal arteries in 2%. In contrast to spontaneous retinal venous pulsations, which are a normal variant and located at the base of the large veins on the optic disc, the arterial pulsations are usually more pronounced, and may extend a disc diameter or more out from the optic disc into the surrounding retina. Anterior ischemic optic neuropathy (Fig. 59.13) has also been reported in ocular ischemic syndrome eyes.5,29,30 Acquired arteriovenous communications of the retina are rarely seen.31
A list of the anterior and posterior segment signs found with the ocular ischemic syndrome is shown in Table 59.1.1–5,9
Table 59.1 Anterior and posterior segment signs seen in eyes with the ocular ischemic syndrome
| Anterior segment | |
| Rubeosis iridis | 67% |
| Neovascular glaucoma | 35% |
| Uveitis (cells and flare) | 18% |
| Posterior segment | |
| Narrowed retinal arteries | Most |
| Dilated retinal veins | Most |
| Retinal hemorrhages | 80% |
| Neovascularization | 37% |
| Optic disc | 35% |
| Retina | 8% |
| Cherry red spot | 12% |
| Cotton-wool spot(s) | 6% |
| Spontaneous retinal arterial pulsations | 4% |
| Vitreous hemorrhage | 4% |
| Cholesterol emboli | 2% |
| Ischemic optic neuropathy | 2% |
(Adapted from Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol 1988;11:239–51.)
Ancillary studies
Fluorescein angiography
The intravenous fluorescein angiographic signs5 associated with the ocular ischemic syndrome are listed in Table 59.2.
Table 59.2 Fluorescein angiographic signs seen in eyes with the ocular ischemic syndrome
| Delayed and/or patchy choroidal filling | 60% |
| Prolonged retinal arteriovenous transit time | 95% |
| Retinal vascular staining | 85% |
| Macular edema | 17% |
| Other signs | |
| Retinal capillary nonperfusion | |
| Optic nerve head hyperfluorescence | |
| Microaneurysmal hyperfluorescence |
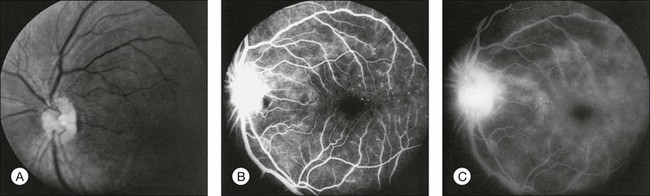
Delayed arm-to-choroid and arm-to-retina circulation times are frequently observed in the ocular ischemic syndrome. However, these measurements may be difficult to assess, since they depend upon whether the dye was injected in the antecubital fossa or hand, and also on the rate of injection. The observation of a well-demarcated, leading edge of fluorescein dye within a retinal artery after an intravenous injection is a distinctly unusual finding. It can be seen in eyes with the ocular ischemic syndrome, secondary to hypoperfusion (Fig. 59.14).
Normally, the choroidal filling is completed within 5 seconds after the first appearance of dye. Sixty percent of eyes with the ocular ischemic syndrome demonstrate patchy and/or delayed choroidal filling (Fig. 59.15). In some instances, the filling is delayed for a minute or longer. Although not the most sensitive sign, an abnormality in choroidal filling is the most specific fluorescein angiographic sign in ocular ischemic eyes.
Staining of the retinal vessels in the later phases of the study is seen in about 85% of eyes (Fig. 59.16 online, Fig. 59.17). Both larger and smaller vessels can be involved, the arteries generally more so than the veins. Chronic hypoxic damage to endothelial cells may account for the staining. In contrast, staining of the retinal vessels is uncommon, with central retinal artery obstruction alone. With central retinal vein obstruction, the veins can demonstrate late staining, but the retinal arteries are generally not affected.
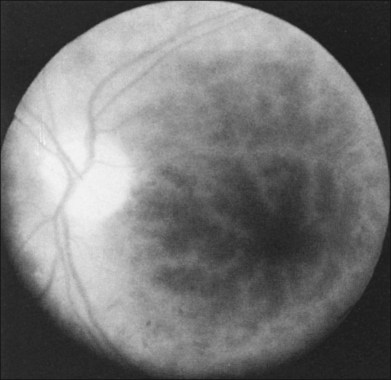
Fig. 59.16 Staining of the macular vessels in the ocular ischemic syndrome.
(Reproduced with permission from Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol 1988;11:239–51.)
Macular leakage and edema evident on fluorescein angiography is seen in about one-sixth of eyes with the ocular ischemic syndrome32 (Fig. 59.18). Hypoxia, and subsequent endothelial damage, within the smaller retinal vessels, as well as leakage from microaneurysms, may account for this phenomenon (Fig. 59.19). Dye accumulation may be mild or severe, and is usually associated with hyperfluorescence of the optic disc. The disc, however, is typically not swollen. Despite the prominent leakage with fluorescein angiography, the ophthalmoscopic cystic changes of macular edema are generally not as pronounced as those seen after ocular surgery or those associated with diabetic retinopathy.
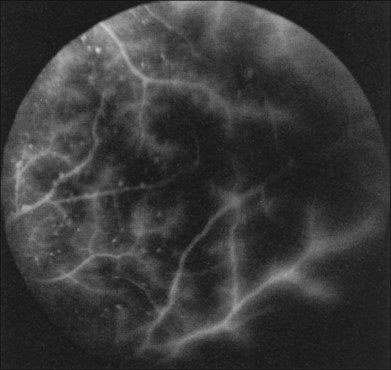
Fig. 59.19 Peripheral fluorescein angiogram of the same eye shown in Fig. 59.18. Many hyperfluorescent microaneurysms are seen, as is staining of the retinal vessels.
(Reproduced with permission from Brown GC. Macular edema in association with severe carotid artery obstruction. Am J Ophthalmol 1986;102:442; American Journal of Ophthalmology. Copyright © Ophthalmic Publishing Group.)
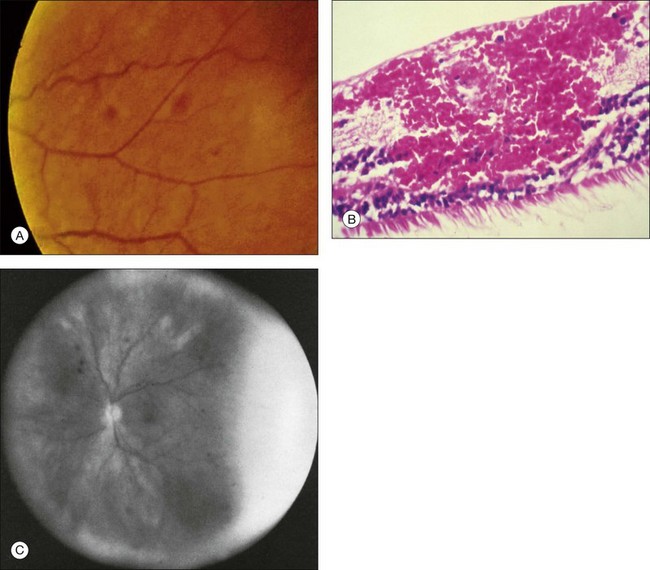
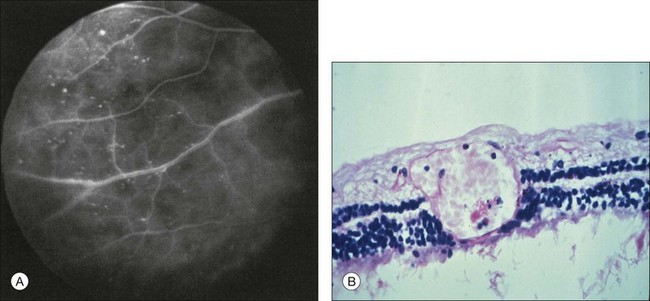
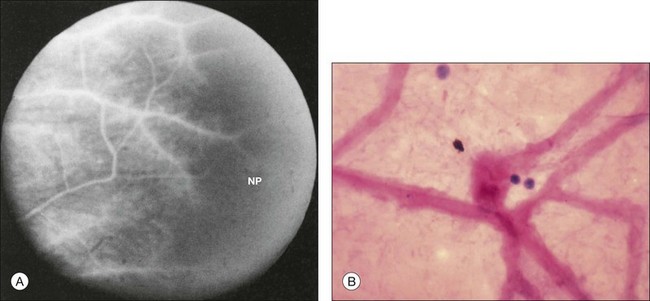
Retinal capillary nonperfusion can be seen in some eyes (Fig. 59.20 online). The histopathologically observed absence of endothelial cells and pericytes within the retinal capillaries most likely corresponds to the areas of nonperfusion seen with fluorescein angiography.7,8,33
Bilateral, simultaneous, intravenous fluorescein angiography is a technique that has been reported to be helpful diagnostically in patients with a unilateral ocular ischemic syndrome.34 However, the technique requires specialized equipment and is not generally available.
Electroretinography
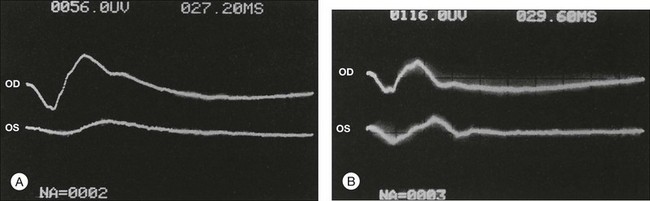
The electroretinogram often discloses a diminution of the amplitude, or absence, of both the a- and b-waves in eyes with the ocular ischemic syndrome5,6 (Fig. 59.21). The b-wave corresponds to activity of the Mueller and/or bipolar cells, and therefore to inner layer retinal function, while the a-wave correlates with activity of the photoreceptors in the outer retina.35,36 Therefore, with central retinal artery obstruction, in which there is essentially inner layer retinal ischemia, the b-wave amplitude is characteristically decreased. With the ocular ischemic syndrome there is both retinal vascular and choroidal compromise, leading to ischemia of the inner and outer retina, respectively. Thus, both the b-wave and a-waves are affected.
Reduction in the amplitude of the oscillatory potential of the b-wave has been noted in eyes with retinal ischemia secondary to carotid artery stenosis.37 This can be seen in patients with proven carotid artery disease, even in the presence of a normal fluorescein angiogram.
Carotid artery imaging
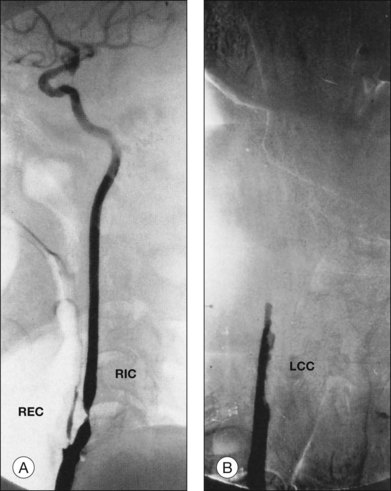
Carotid angiography typically discloses a 90% or greater obstruction of the ipsilateral internal or common carotid artery in persons with the ocular ischemic syndrome (Fig. 59.22). Given that noninvasive tests, such as duplex ultrasonography and oculoplethysmography, have an accuracy of approximately between 88 and 95% in detecting carotid stenosis of 75% or greater,38–40 carotid angiography is often indicated for potential surgical cases or cases in which there is doubt about the diagnosis.
Others
Visual evoked potentials have been used to study eyes with severe carotid artery stenosis. The recovery time of the amplitude of the major positive peak after photostress has been shown to improve in patients with severe stenosis after endarterectomy.41
Ophthalmodynamometry can be of benefit in detecting decreased ocular perfusion in cases of unilateral ocular ischemic syndrome.10,42
In the absence of an ophthalmodynamometer, Kearns42 has advocated light digital pressure on the upper lid of the affected eye during ophthalmoscopy. Retinal arterial pulsations can usually be readily induced in eyes with the ocular ischemic syndrome. This is generally not the case in eyes with central retinal vein obstruction, an entity that can be confused with the ocular ischemic syndrome.
Systemic associations
Diseases associated in one way or another with atherosclerosis are frequently seen in conjunction with the ocular ischemic syndrome. Systemic arterial hypertension has been reported in 73% of ocular ischemic syndrome patients and concomitant diabetes mellitus has been observed in 56%.43 In an age-matched historical control population from the Framingham Study,44 the corresponding prevalences for systemic arterial hypertension and diabetes mellitus were 26% and 6%.
At the time of presentation, almost one-fifth of patients relate a history of having peripheral vascular disease for which previous bypass surgery was required.43 The stroke rate for patients for people with the ocular ischemic syndrome is approximately 4% per year.45
A rare but serious cause of ocular ischemic syndrome is giant cell arteritis.46 This condition has been reported to cause bilateral loss of vision, which may occur despite treatment with steroids.47
Mortality data43 have shown that the 5-year death rate for patients with the ocular ischemic syndrome is 40%. The leading cause of death is cardiovascular disease, which accounts for about two-thirds of cases. Stroke is the second leading cause of death. Thus, most patients with the ocular ischemic syndrome should be considered for cardiac evaluation, in addition to a carotid workup. It is also important to note that Mizener et al. noted that in 69% of their patients, ocular ischemic syndrome was the first clinical manifestation of carotid occlusive disease, a fact that only further underscores the importance of timely systemic evaluation in these patients.48
Differential diagnosis
The entities that are most commonly confused with the ocular ischemic syndrome include mild central retinal vein obstruction and diabetic retinopathy. Features that differentiate these abnormalities are listed in Table 59.3. In contrast to the ocular ischemic syndrome, the veins in eyes with mild, or nonischemic, central retinal vein obstruction are often dilated and tortuous. Additionally, with light digital pressure on the lid it is difficult to induce retinal arterial pulsations in eyes with central retinal vein obstruction. While both entities usually have a prolonged retinal arteriovenous transit time, choroidal filling defects and prominent retinal arterial staining are usually absent on fluorescein angiography in eyes with central retinal vein obstruction.
Table 59.3 Features that differentiate the ocular ischemic syndrome (OIS), central retinal vein obstruction (CRVO), and diabetic retinopathy
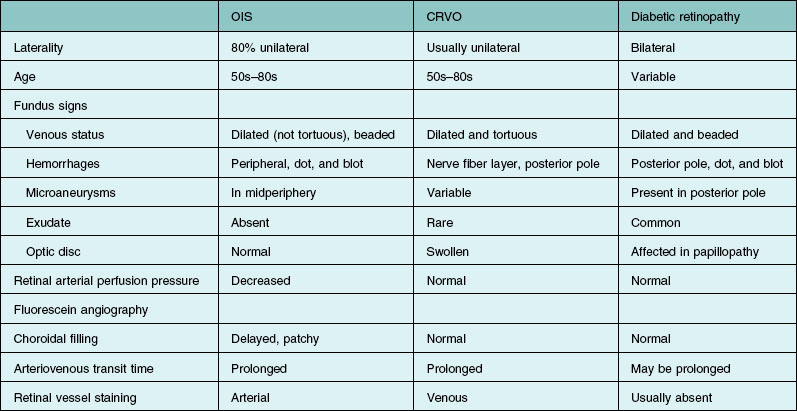
In some cases of diabetic retinopathy, the ocular ischemic syndrome can exacerbate the proliferative changes. It has not been proven that carotid stenosis is protective against the development of proliferative diabetic retinopathy.45
Treatment
With regard to vision, the natural course of the ocular ischemic syndrome is uncertain. Nonetheless, most eyes with the fully developed entity probably have a poor long-term outcome. When rubeosis iridis is present, well over 90% of eyes become legally blind within a year of discovery.25
Total carotid artery obstruction
When a carotid artery is 100% obstructed, endarterectomy is usually ineffective since a thrombus often propagates distally to the next major vessel. In these cases, extracranial to intracranial bypass surgery, usually from the superficial temporal artery to the middle cerebral artery, has been attempted to alleviate the obstruction. Although case reports suggest that this procedure can be of benefit initially in salvaging vision in eyes with the ocular ischemic syndrome,49–54 as well as causing regression of neovascular glaucoma,55 the visual prognosis at one year after the surgery is almost universally poor, despite the fact that 20% of patients have visual improvement within the first three months of surgery.25 Additionally, the procedure has not been shown in a large randomized study to be of benefit in preventing the risk of ischemic stroke.56
That said, some authors have offered objective support of improvement in perfusion following endarterectomy. Costa et al. were able to demonstrate increased mean peak systolic flow velocities and end diastolic velocities in the orbital vessels following surgery, with a significant reduction of the mean resistance indices in the central retinal and posterior ciliary arteries.57
Kawaguchi et al.58 recently evaluated the effects of superficial temporal to middle cerebral artery (STA-MCA) bypass in a series of patients with the OIS. These authors compared a number of clinical parameters including carotid Doppler flow imaging in 32 patients who received STA-MCA as compared to nine patients with OIS who did not have STA-MCA. Prior to surgery all 32 patients had reversal of flow in their ophthalmic arteries. The mean peak systolic flow improved to 0.15 m/s at 3 months as compared to –0.26 at baseline. In addition, whereas all patients had reversal of flow in the ophthalmic artery preoperatively, 56% developed antegrade flow at the 3-month period. In the final analysis, 47% developed visual improvement following surgery and the remainder visual stability.58
Less than total carotid artery obstruction
Although there are no randomized studies that compare the natural history of the disease to the course after carotid endarterectomy, this surgery may also stabilize or improve vision in the eyes of patients who undergo successful endarterectomy prior to the development of rubeosis iridis.25,59 Not withstanding, the visual results associated with this treatment are fair at best. In the series of Sivalingam et al.,25 at the end of one year 7% of eyes with the ocular ischemic syndrome that underwent endarterectomy had visual improvement, 33% were unchanged, and 60% had worse vision. Among the 60 total ocular ischemic syndrome eyes in the group, an endarterectomy was performed for only three without rubeosis. At the end of one year follow-up the vision was better in one, stable in one, and worse in the third. Endarterectomy appears to rarely cause regression of iris neovascularization in eyes with the ocular ischemic syndrome.60
More recent data demonstrated that carotid revascularization surgery results in improved retinal blood in 80% of cases, although the long-term visual implications are unclear.61 Stenting can also be undertaken in select cases to successfully restore blood flow within the carotid artery.62,63 Rarely, bilateral external carotid obstruction can cause the ocular ischemic syndrome.64 In this instance, external carotid endarterectomy can be considered.64 In cases of the ocular ischemic syndrome with ipsilateral internal carotid and external carotid obstructions, it would seem that reversal of both would yield the best ocular prognosis, but this is not known with certainty at the current time.
It should be noted that eyes with the ocular ischemic syndrome will occasionally develop a severe increase in intraocular pressure after ipsilateral carotid endarterectomy.65,66 This is most likely to occur in eyes with rubeosis iridis and anterior chamber angle compromise from fibrovascular tissue formation. Although aqueous outflow is impaired in such eyes, ciliary body perfusion and aqueous humor formation are also decreased secondary to the carotid stenosis. When the carotid obstruction is suddenly reversed, ciliary body perfusion and aqueous humor formation increase, but the outflow obstruction in the anterior chamber angle is still present. Thus, the intraocular pressure rises drastically. Ciliary body destructive procedures or glaucoma filtering surgery may be required in these cases.
Section on carotid endarterectomy in general available online.
Carotid endarterectomy in general
Several large randomized studies have recently been published concerning the indications for carotid endarterectomy in general.67–70 Carotid endarterectomy has been proven to be efficacious in both symptomatic patients with high-grade (70–99%) symptomatic carotid stenosis, and in asymptomatic patients with greater than (or equal to) 60% stenosis. Specifically, the investigators of the North American Symptomatic Carotid Endarterectomy Trial68 noted a 17% absolute risk reduction in the cumulative 2-year risk of ipsilateral stroke, and a 10% absolute risk reduction in fatal ipsilateral stroke when those randomized to endarterectomy were compared to those who were treated medically. The European Carotid Surgery Trialists’ Collaborative Group67 also were able to demonstrate a similar treatment effect of carotid endarterectomy for patients with 70–99% stenosis (6-fold reduction in 3-year risk of ipsilateral stroke), but also found that in the 0–29% stenosis group, the early risks of surgery (2.3% died or had a disabling stroke within 30 days of surgery) outweighed the 3-year benefit when compared to medical therapy. The investigators of the Asymptomatic Carotid Atherosclerosis Study65 were able to demonstrate an aggregate risk reduction of 53% in the incidence of death or stroke, when those randomized to surgery were compared to those who received medical treatment. Asymptomatic patients with carotid artery stenosis of 60% or greater reduction in diameter were eligible to benefit.
A Cochrane Database Systematic Review71 incorporating 35 000 years of patient follow-up recently demonstrated that carotid surgery: (1) increased the 5-year risk of ipsilateral ischemic stroke in patients with less than 30% stenosis (n = 1746, absolute risk reduction (ARR) −2.2%, P = 0.05); (2) had no significant effect in patients with 30–49% stenosis (n = 1429, ARR 3.2%, P = 0.6), was of marginal benefit in patients with 50–69% stenosis (n = 1549, ARR 4.6%, P = 0.04), and was highly beneficial in patients with 70% to 99% stenosis (n = 1095, ARR 16.0%, P < 0.001). Accordingly, any patient with the ocular ischemic syndrome and severe carotid artery stenosis should be considered for carotid endarterectomy.
Medical therapy
Since atherosclerosis is, by far, the most common cause of the ocular ischemic syndrome, medical therapy should be directed toward treating atherogenic disease by controlling risk factors such as systemic arterial hypertension, smoking, diabetes mellitus, and hyperlipidemia. Of considerable importance is the fact that high-dose (40 mg PO daily) rosuvastatin therapy has been shown to actually reverse coronary artery atherosclerosis, an event likely generalizable to atherosclerosis elsewhere in the body.72 Since cardiac disease is the leading cause of death associated with the ocular ischemic syndrome, evaluation by a cardiologist should be considered.43
Direct ocular therapeutic modalities
Full scatter panretinal laser photocoagulation has been advocated for ocular ischemic eyes with rubeosis iridis and/or posterior segment neovascularization.59,73,74 This generally consists of 1500–2000 500 µm burns with the argon green laser. Unlike the situation when rubeosis iridis occurs secondary to diabetic retinopathy, in which there is regression in a majority of cases with full scatter panretinal photocoagulation, approximately 36% of ocular ischemic syndrome eyes will demonstrate regression of the iris neovascularization after full scatter treatment.25 If the anterior chamber angle is completely closed by fibrovascular tissue and there is no posterior segment neovascularization, panretinal photocoagulation is probably not indicated unless a glaucoma filtering procedure is being considered, as higher success rates of filtration surgery have been reported when PRP has been performed.75
While there is little in the reported literature regarding the management of macular edema secondary to this condition, Klais and Spaide reported excellent clinical resolution of fluid and dramatic improvement in vision in a patient treated with intravitreal triamcinolone acetonide.76 Intravitreal bevacizumab has been used for the treatment of the iris neovascularization and macular edema associated with the ocular ischemic syndrome, but data are sparse.77
![]() Bonus images for this chapter can be found online at http://www.expertconsult.com
Bonus images for this chapter can be found online at http://www.expertconsult.com
Fig. 59.6 Right fundus of a 35-year-old man with a cherry-red spot, rubeosis iridis, and retinal hemorrhages in the macula. A 95% right internal carotid artery obstruction was present.
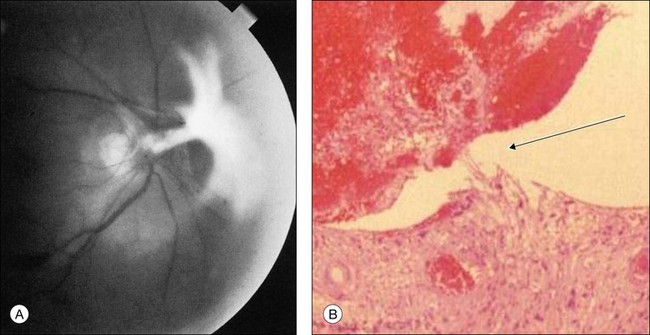
Fig. 59.9(A) Fibrovascular proliferation overlying the optic disc and causing retinal traction in an eye with the ocular ischemic syndrome. (Reproduced with permission from Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol 1988;11:239–51.) (B) Histopathology of an eye with ischemic retinopathy demonstrates a connection of neovascularization of the optic disc on stretch (arrow) between the inferior disc and the superior vitreous gel filled with blood. Presumably, the intravitreal blood occurred as a result of rupture of the thin-walled vessels on stretch (hematoxylin–eosin ×60).
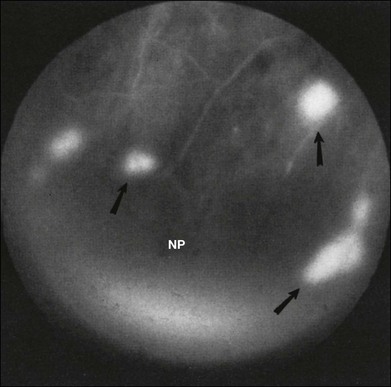
Fig. 59.10 Neovascularization of the retina (arrows) with fluorescein angiography in a nondiabetic person affected by the ocular ischemic syndrome. Retinal capillary nonperfusion (NP) can be seen. (Reproduced with permission from Brown GC, Magargal LE, Simeone FA, et al. Arterial obstruction and ocular neovascularization. Ophthalmology 1982;89:139–46. Copyright © 1982 American Academy of Ophthalmology.)
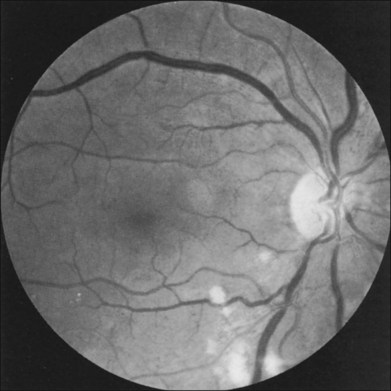
Fig. 59.11 Cotton-wool spots in the fundus of a man with the ocular ischemic syndrome. Irregularly dilated retinal veins are also evident.
Fig. 59.16 Staining of the macular vessels in the ocular ischemic syndrome. (Reproduced with permission from Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol 1988;11:239–51.)
Fig. 59.20(A) Fluorescein angiography reveals retinal capillary nonperfusion (NP) in an ocular ischemic syndrome eye. (Reproduced with permission from Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11:239–51.) (B) Trypsin digest of the retinal vessels of an ocular ischemic syndrome eye reveals loss of both the endothelial cells and pericytes (hematoxylin–eosin ×200). (Courtesy of Dr W. Richard Green.)
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
1 Kearns TP, Hollenhorst RW. Venous stasis retinopathy of occlusive disease of the carotid artery. Proc Mayo Clin. 1963;38:304–312.
2 Hayreh SS. So-called “central retinal vein occlusion.” Venous-stasis retinopathy. Ophthalmologica. 1976;172:14–37.
3 Knox DL. Ischemic ocular inflammation. Am J Ophthalmol. 1965;60:995–1002.
4 Young LHY, Appen RE. Ischemic oculopathy, a manifestation of carotid artery disease. Arch Neurol. 1981;38:358–361.
5 Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11:239–251.
6 Brown GC, Magargal LE, Simeone FA, et al. Arterial obstruction and ocular neovascularization. Ophthalmology. 1982;89:139–146.
7 Kahn M, Green WR, Knox DL, et al. Ocular features of carotid occlusive disease. Retina. 1986;6:239–252.
8 Michelson PE, Knox DL, Green WR. Ischemic ocular inflammation. A clinicopathologic case report. Arch Ophthalmol. 1971;86:274–280.
9 Sturrock GD, Mueller HR. Chronic ocular ischaemia. Br J Ophthalmol. 1984;68:716–723.
10 Kearns TP. Ophthalmology and the carotid artery. Am J Ophthalmol. 1979;88:714–722.
11 Kobayashi S, Hollenhorst RW, Sundt TM, Jr. Retinal arterial pressure before and after surgery for carotid artery stenosis. Stroke. 1971;2:569–575.
12 Bullock J, Falter RT, Downing JE, et al. Ischemic ophthalmia secondary to an ophthalmic artery occlusion. Am J Ophthalmol. 1972;74:486–493.
13 Madsen PH. Venous-stasis insufficiency of the ophthalmic artery. Acta Ophthalmol. 1965;40:940–947.
14 Magargal LE, Sanborn GE, Zimmerman A. Venous stasis retinopathy associated with embolic obstruction of the central retinal artery. J Clin Neuroophthalmol. 1982;2:113–118.
15 Duker JS, Belmont JB. Ocular ischemic syndrome secondary to carotid artery dissection. Am J Ophthalmol. 1988;106:750–752.
16 Hamed LM, Guy JR, Moster ML, et al. Giant cell arteritis in the ocular ischemic syndrome. Am J Ophthalmol. 1992;113:702–705.
17 Effeney DJ, Krupski WC, Stoney RJ, et al. Fibromuscular dysplasia of the carotid artery. Austral NZ J Surg. 1983;53:527–531.
18 Dhobb M, Ammar F, Bensaid Y, et al. Arterial manifestations in Behçet’s disease: four new cases. Ann Vasc Surg. 1986;1:249–252.
19 Sadun AA, Sebag J, Bienfang DC. Complete bilateral internal carotid artery occlusion in a young man. J Clin Neuroophthalmol. 1983;3:63–66.
20 Donnan GA, Sharbrough FW. Carotid occlusive disease. Effect of bright light on visual evoked response. Arch Neurol. 1982;39:687–689.
21 Wiebers DO, Swanson JW, Cascino TL, et al. Bilateral loss of vision in bright light. Stroke. 1989;20:554–558.
22 Ramadan NM, Tietjen GE, Levine SR, et al. Scintillating scotomata associated with internal carotid artery dissection: report of three cases. Neurology. 1991;41:1084–1087.
23 Winterkorn JM, Teman AJ. Recurrent attacks of amaurosis fugax treated with calcium channel blocker. Ann Neurol. 1991;30:423–425.
24 Aasen J, Kerty E, Russell D, et al. Amaurosis fugax: clinical, Doppler and angiographic findings. Acta Neurol Scand. 1988;77:450–455.
25 Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15–20.
26 Schlaegel T. Symptoms and signs of uveitis. In: Duane TD, ed. Clinical ophthalmology, vol. 4. Hagerstown: Harper and Row; 1983:1–7.
27 Green WR, Chan CC, Hutchins GM, et al. Central retinal vein occlusion. A prospective histopathologic study of 29 eyes in 28 cases. Retina. 1981;1:27–55.
28 Campo RV, Reeser FH. Retinal telangiectasia secondary to bilateral carotid artery occlusion. Arch Ophthalmol. 1983;101:1211–1213.
29 Brown GC. Anterior ischemic optic neuropathy occurring in association with carotid artery obstruction. J Clin Neuro-Ophthalmol. 1986;6:39–42.
30 Waybright EA, Selhorst JB, Combs J. Anterior ischemic optic neuropathy with internal carotid artery occlusion. Am J Ophthalmol. 1982;93:42–47.
31 Bolling JP, Buettner H. Acquired retinal arteriovenous communications in occlusive disease of the carotid artery. Ophthalmology. 1990;97:1148–1152.
32 Brown GC. Macular edema in association with severe carotid artery obstruction. Am J Ophthalmol. 1986;102:442–448.
33 Dugan JD, Green WR. Ophthalmic manifestations of carotid occlusive disease. Eye. 1991;5:226–238.
34 Choromokos EA, Raymond LA, Sacks JG. Recognition of carotid stenosis with bilateral simultaneous retinal fluorescein angiography. Ophthalmology. 1982;89:1146–1148.
35 Carr RE, Siegel JM. Electrophysiologic aspects of several retinal diseases. Am J Ophthalmol. 1964;58:95–107.
36 Henkes HE. Electroretinography in circulatory disturbances of the retina. II. The electroretinogram in cases of occlusion of the central retinal artery or one of its branches. Arch Ophthalmol. 1954;51:42–53.
37 Coleman K, Fitzgerald D, Eustace P, et al. Electroretinography, retinal ischaemia and carotid artery disease. Eur J Vasc Surg. 1990;4:569–573.
38 Bosley TM. The role of carotid noninvasive tests in stroke prevention. Semin Neurol. 1986;6:194–203.
39 Castaldo JE, Nicholas GG, Gee W, et al. Duplex ultrasound and ocular pneumoplethysmography concordance in detecting severe carotid stenosis. Arch Neurol. 1989;46:518–522.
40 Neale ML, Chambers JL, Kelly AT, et al. Reappraisal of duplex criteria to assess significant carotid artery stenosis with special reference to reports of the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. J Vasc Surg. 1994;20:642–649.
41 Banchini E, Franchi A, Magni R, et al. Carotid occlusive disease. An electrophysiological investigation. J Cardiovasc Surg. 1987;28:524–527.
42 Kearns TP. Differential diagnosis of central retinal vein obstruction. Ophthalmology. 1983;90:475–480.
43 Sivalingham A, Brown GC, Magargal LE, et al. The ocular ischemic syndrome II. Mortality and systemic morbidity. Int Ophthalmol. 1989;13:187–191.
44 Kannel WB, Gordon T, editors. The Framingham Study. Public Health Service Publication No. NIH 77–1247, Section 6, Tables 6–9, Section 29, Tables A-22 and A-23, Section 32, p. 84–5.
45 Duker J, Brown GC, Bosley TM, et al. Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology. 1990;97:869–874.
46 Casson RJ, Fleming FK, Shaikh A, et al. Bilateral ocular ischemic syndrome secondary to giant cell arteritis. Arch Ophthalmol. 2001;119:306–307.
47 Hwang JM, Girkin CA, Perry JD, et al. Bilateral ocular ischemic syndrome secondary to giant cell arteritis progressing despite corticosteroid treatment. Am J Ophthalmol. 1999;127:102–104.
48 Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–864.
49 Edwards MS, Chater NL, Stanley JA. Reversal of chronic ischaemia by extracranial–intracranial arterial by-pass. Neurosurgery. 1980;7:480–483.
50 Higgins RA. Neovascular glaucoma associated with ocular hypoperfusion secondary to carotid artery disease. Austral J Ophthalmol. 1984;12:155–162.
51 Katz B, Weinstein PR. Improvement of photostress recovery testing after extracranial–intracranial bypass surgery. Br J Ophthalmol. 1986;70:277–280.
52 Kearns TP, Younge BR, Peipgras PG. Resolution of venous stasis retinopathy after carotid artery bypass surgery. Proc Mayo Clin. 1980;55:342–346.
53 Kiser WD, Gonder J, Magargal LE, et al. Recovery of vision following treatment of the ocular ischemic syndrome. Ann Ophthalmol. 1983;15:305–310.
54 Shibuya M, Suzuki Y, Takayasu M, et al. Effects of STA–MCA anastomosis for ischaemic oculopathy due to occlusion of the internal carotid artery. Acta Neurochir. 1990;103:71–75.
55 Kearns TP, Siebert RG. The ocular aspects of carotid artery surgery. Tr Am Ophthalmol Soc. 1978;76:247–265.
56 The EC/IC Bypass Study Group. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Eng J Med. 1985;313:1191–1200.
57 Costa VP, Kuzniec S, Molnar LJ, et al. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. An investigation by color Doppler imaging. Ophthalmology. 1999;106:306–310.
58 Kawaguchi S, Sakaki T, Kamada K, et al. Effects of superficial temporal to middle cerebral artery bypass for ischaemic retinopathy due to internal carotid artery occlusion/stenosis. Acta Neurochir (Wien). 1994;129:166–170.
59 Johnston ME, Gonder JR, Canny CL. Successful treatment of the ocular ischemic syndrome with panretinal photocoagulation and cerebrovascular surgery. Can J Ophthalmol. 1988;23:114–119.
60 Hauch TL, Busuttil RW, Yoshizumi MO. A report of iris neovascularization. An indication for carotid endarterectomy. Surgery. 1984;95:358–362.
61 Cardia G, Porfido D, Guerriero S, et al. Retinal circulation after carotid artery revascularization. Angiology. 2011;62:372–375.
62 Fintelman R, Rosenwasser RH, Jabbour P, et al. An old problem, a new solution. Surv Ophthalmol. 2010;55:85–88.
63 Marx JL, Hreib K, Choi IS, et al. Percutaneous carotid artery angioplasty and stenting for ocular ischemic syndrome. Ophthalmology. 2004;111:2284–2291.
64 Alizai AM, Trobe JD, Thompson BG, et al. Ocular ischemic syndrome after occlusion of both external carotid arteries. J Neuroophthalmol. 2005;25:268–272.
65 Coppeto JR, Wand M, Bear L, et al. Neovascular glaucoma and carotid artery obstructive disease. Am J Ophthalmol. 1985;99:567–570.
66 Melamed S, Irvine J, Lee DA. Increased intraocular pressure following endarterectomy. Ann Ophthalmol. 1987;19:304–306.
67 European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild carotid stenosis. Lancet. 1991;337:1235–1243.
68 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453.
69 Asymptomatic Carotid Atherosclerosis Study Group. Carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. JAMA. 1995;273:1421–1428.
70 Mayberg MR, Wilson SE, Yatsu F, et al. for the Veterans Affairs Cooperative Studies Program 309 Trialist Group. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. JAMA. 1991;266:3289–3294.
71 Rerkasem K, Rothwell PM. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 13(4), 2011. Apr CD001081
72 Nissen SE, Nicholls SJ, Sipahi I, et al. for the ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565.
73 Carter JE. Panretinal photocoagulation for progressive ocular neovascularization secondary to occlusion of the common carotid artery. Ann Ophthalmol. 1984;16:572–576.
74 Eggleston TF, Bohling CA, Eggleston HC, et al. Photocoagulation for ocular ischemia associated with carotid artery occlusion. Ann Ophthalmol. 1980;12:84–87.
75 Allen RC, Bellows AR, Hutchinson BT, et al. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982;89:1181–1187.
76 Klais CM, Spaide RF. Intravitreal triamcinolone acetonide injection in ocular ischemic syndrome. Retina. 2004;24:459–461.
77 Amselem L, Montero J, Diaz-Llopis M, et al. Intravitreal bevacizumab (Avastin) injection in ocular ischemic syndrome. Am J Ophthalmol. 2007;144:122–124.
1 Kearns TP, Hollenhorst RW. Venous stasis retinopathy of occlusive disease of the carotid artery. Proc Mayo Clin. 1963;38:304–312.
2 Hayreh SS. So-called “central retinal vein occlusion.” Venous-stasis retinopathy. Ophthalmologica. 1976;172:14–37.
3 Knox DL. Ischemic ocular inflammation. Am J Ophthalmol. 1965;60:995–1002.
4 Young LHY, Appen RE. Ischemic oculopathy, a manifestation of carotid artery disease. Arch Neurol. 1981;38:358–361.
5 Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988;11:239–251.
6 Brown GC, Magargal LE, Simeone FA, et al. Arterial obstruction and ocular neovascularization. Ophthalmology. 1982;89:139–146.
7 Kahn M, Green WR, Knox DL, et al. Ocular features of carotid occlusive disease. Retina. 1986;6:239–252.
8 Michelson PE, Knox DL, Green WR. Ischemic ocular inflammation. A clinicopathologic case report. Arch Ophthalmol. 1971;86:274–280.
9 Sturrock GD, Mueller HR. Chronic ocular ischaemia. Br J Ophthalmol. 1984;68:716–723.
10 Kearns TP. Ophthalmology and the carotid artery. Am J Ophthalmol. 1979;88:714–722.
11 Kobayashi S, Hollenhorst RW, Sundt TM, Jr. Retinal arterial pressure before and after surgery for carotid artery stenosis. Stroke. 1971;2:569–575.
12 Bullock J, Falter RT, Downing JE, et al. Ischemic ophthalmia secondary to an ophthalmic artery occlusion. Am J Ophthalmol. 1972;74:486–493.
13 Madsen PH. Venous-stasis insufficiency of the ophthalmic artery. Acta Ophthalmol. 1965;40:940–947.
14 Magargal LE, Sanborn GE, Zimmerman A. Venous stasis retinopathy associated with embolic obstruction of the central retinal artery. J Clin Neuroophthalmol. 1982;2:113–118.
15 Duker JS, Belmont JB. Ocular ischemic syndrome secondary to carotid artery dissection. Am J Ophthalmol. 1988;106:750–752.
16 Hamed LM, Guy JR, Moster ML, et al. Giant cell arteritis in the ocular ischemic syndrome. Am J Ophthalmol. 1992;113:702–705.
17 Effeney DJ, Krupski WC, Stoney RJ, et al. Fibromuscular dysplasia of the carotid artery. Austral NZ J Surg. 1983;53:527–531.
18 Dhobb M, Ammar F, Bensaid Y, et al. Arterial manifestations in Behçet’s disease: four new cases. Ann Vasc Surg. 1986;1:249–252.
19 Sadun AA, Sebag J, Bienfang DC. Complete bilateral internal carotid artery occlusion in a young man. J Clin Neuroophthalmol. 1983;3:63–66.
20 Donnan GA, Sharbrough FW. Carotid occlusive disease. Effect of bright light on visual evoked response. Arch Neurol. 1982;39:687–689.
21 Wiebers DO, Swanson JW, Cascino TL, et al. Bilateral loss of vision in bright light. Stroke. 1989;20:554–558.
22 Ramadan NM, Tietjen GE, Levine SR, et al. Scintillating scotomata associated with internal carotid artery dissection: report of three cases. Neurology. 1991;41:1084–1087.
23 Winterkorn JM, Teman AJ. Recurrent attacks of amaurosis fugax treated with calcium channel blocker. Ann Neurol. 1991;30:423–425.
24 Aasen J, Kerty E, Russell D, et al. Amaurosis fugax: clinical, Doppler and angiographic findings. Acta Neurol Scand. 1988;77:450–455.
25 Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol. 1991;15:15–20.
26 Schlaegel T. Symptoms and signs of uveitis. In: Duane TD, ed. Clinical ophthalmology, vol. 4. Hagerstown: Harper and Row; 1983:1–7.
27 Green WR, Chan CC, Hutchins GM, et al. Central retinal vein occlusion. A prospective histopathologic study of 29 eyes in 28 cases. Retina. 1981;1:27–55.
28 Campo RV, Reeser FH. Retinal telangiectasia secondary to bilateral carotid artery occlusion. Arch Ophthalmol. 1983;101:1211–1213.
29 Brown GC. Anterior ischemic optic neuropathy occurring in association with carotid artery obstruction. J Clin Neuro-Ophthalmol. 1986;6:39–42.
30 Waybright EA, Selhorst JB, Combs J. Anterior ischemic optic neuropathy with internal carotid artery occlusion. Am J Ophthalmol. 1982;93:42–47.
31 Bolling JP, Buettner H. Acquired retinal arteriovenous communications in occlusive disease of the carotid artery. Ophthalmology. 1990;97:1148–1152.
32 Brown GC. Macular edema in association with severe carotid artery obstruction. Am J Ophthalmol. 1986;102:442–448.
33 Dugan JD, Green WR. Ophthalmic manifestations of carotid occlusive disease. Eye. 1991;5:226–238.
34 Choromokos EA, Raymond LA, Sacks JG. Recognition of carotid stenosis with bilateral simultaneous retinal fluorescein angiography. Ophthalmology. 1982;89:1146–1148.
35 Carr RE, Siegel JM. Electrophysiologic aspects of several retinal diseases. Am J Ophthalmol. 1964;58:95–107.
36 Henkes HE. Electroretinography in circulatory disturbances of the retina. II. The electroretinogram in cases of occlusion of the central retinal artery or one of its branches. Arch Ophthalmol. 1954;51:42–53.
37 Coleman K, Fitzgerald D, Eustace P, et al. Electroretinography, retinal ischaemia and carotid artery disease. Eur J Vasc Surg. 1990;4:569–573.
38 Bosley TM. The role of carotid noninvasive tests in stroke prevention. Semin Neurol. 1986;6:194–203.
39 Castaldo JE, Nicholas GG, Gee W, et al. Duplex ultrasound and ocular pneumoplethysmography concordance in detecting severe carotid stenosis. Arch Neurol. 1989;46:518–522.
40 Neale ML, Chambers JL, Kelly AT, et al. Reappraisal of duplex criteria to assess significant carotid artery stenosis with special reference to reports of the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. J Vasc Surg. 1994;20:642–649.
41 Banchini E, Franchi A, Magni R, et al. Carotid occlusive disease. An electrophysiological investigation. J Cardiovasc Surg. 1987;28:524–527.
42 Kearns TP. Differential diagnosis of central retinal vein obstruction. Ophthalmology. 1983;90:475–480.
43 Sivalingham A, Brown GC, Magargal LE, et al. The ocular ischemic syndrome II. Mortality and systemic morbidity. Int Ophthalmol. 1989;13:187–191.
44 Kannel WB, Gordon T, editors. The Framingham Study. Public Health Service Publication No. NIH 77–1247, Section 6, Tables 6–9, Section 29, Tables A-22 and A-23, Section 32, p. 84–5.
45 Duker J, Brown GC, Bosley TM, et al. Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology. 1990;97:869–874.
46 Casson RJ, Fleming FK, Shaikh A, et al. Bilateral ocular ischemic syndrome secondary to giant cell arteritis. Arch Ophthalmol. 2001;119:306–307.
47 Hwang JM, Girkin CA, Perry JD, et al. Bilateral ocular ischemic syndrome secondary to giant cell arteritis progressing despite corticosteroid treatment. Am J Ophthalmol. 1999;127:102–104.
48 Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–864.
49 Edwards MS, Chater NL, Stanley JA. Reversal of chronic ischaemia by extracranial–intracranial arterial by-pass. Neurosurgery. 1980;7:480–483.
50 Higgins RA. Neovascular glaucoma associated with ocular hypoperfusion secondary to carotid artery disease. Austral J Ophthalmol. 1984;12:155–162.
51 Katz B, Weinstein PR. Improvement of photostress recovery testing after extracranial–intracranial bypass surgery. Br J Ophthalmol. 1986;70:277–280.
52 Kearns TP, Younge BR, Peipgras PG. Resolution of venous stasis retinopathy after carotid artery bypass surgery. Proc Mayo Clin. 1980;55:342–346.
53 Kiser WD, Gonder J, Magargal LE, et al. Recovery of vision following treatment of the ocular ischemic syndrome. Ann Ophthalmol. 1983;15:305–310.
54 Shibuya M, Suzuki Y, Takayasu M, et al. Effects of STA–MCA anastomosis for ischaemic oculopathy due to occlusion of the internal carotid artery. Acta Neurochir. 1990;103:71–75.
55 Kearns TP, Siebert RG. The ocular aspects of carotid artery surgery. Tr Am Ophthalmol Soc. 1978;76:247–265.
56 The EC/IC Bypass Study Group. Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Eng J Med. 1985;313:1191–1200.
57 Costa VP, Kuzniec S, Molnar LJ, et al. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. An investigation by color Doppler imaging. Ophthalmology. 1999;106:306–310.
58 Kawaguchi S, Sakaki T, Kamada K, et al. Effects of superficial temporal to middle cerebral artery bypass for ischaemic retinopathy due to internal carotid artery occlusion/stenosis. Acta Neurochir (Wien). 1994;129:166–170.
59 Johnston ME, Gonder JR, Canny CL. Successful treatment of the ocular ischemic syndrome with panretinal photocoagulation and cerebrovascular surgery. Can J Ophthalmol. 1988;23:114–119.
60 Hauch TL, Busuttil RW, Yoshizumi MO. A report of iris neovascularization. An indication for carotid endarterectomy. Surgery. 1984;95:358–362.
61 Cardia G, Porfido D, Guerriero S, et al. Retinal circulation after carotid artery revascularization. Angiology. 2011;62:372–375.
62 Fintelman R, Rosenwasser RH, Jabbour P, et al. An old problem, a new solution. Surv Ophthalmol. 2010;55:85–88.
63 Marx JL, Hreib K, Choi IS, et al. Percutaneous carotid artery angioplasty and stenting for ocular ischemic syndrome. Ophthalmology. 2004;111:2284–2291.
64 Alizai AM, Trobe JD, Thompson BG, et al. Ocular ischemic syndrome after occlusion of both external carotid arteries. J Neuroophthalmol. 2005;25:268–272.
65 Coppeto JR, Wand M, Bear L, et al. Neovascular glaucoma and carotid artery obstructive disease. Am J Ophthalmol. 1985;99:567–570.
66 Melamed S, Irvine J, Lee DA. Increased intraocular pressure following endarterectomy. Ann Ophthalmol. 1987;19:304–306.
71 Rerkasem K, Rothwell PM. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 13(4), 2011. Apr CD001081
72 Nissen SE, Nicholls SJ, Sipahi I, et al. for the ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565.
73 Carter JE. Panretinal photocoagulation for progressive ocular neovascularization secondary to occlusion of the common carotid artery. Ann Ophthalmol. 1984;16:572–576.
74 Eggleston TF, Bohling CA, Eggleston HC, et al. Photocoagulation for ocular ischemia associated with carotid artery occlusion. Ann Ophthalmol. 1980;12:84–87.
75 Allen RC, Bellows AR, Hutchinson BT, et al. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982;89:1181–1187.
76 Klais CM, Spaide RF. Intravitreal triamcinolone acetonide injection in ocular ischemic syndrome. Retina. 2004;24:459–461.
77 Amselem L, Montero J, Diaz-Llopis M, et al. Intravitreal bevacizumab (Avastin) injection in ocular ischemic syndrome. Am J Ophthalmol. 2007;144:122–124.