Ocular drug delivery
Hala Fadda, Ashkan Khalili, Peng Tee Khaw and Steve Brocchini
Chapter contents
Anatomy and physiology of the eye
Ocular drug delivery routes and elimination pathways
Some common ocular conditions and pharmacological interventions
Topical ophthalmic preparations
Formulating ophthalmic preparations
Hydrogen ion concentration (pH)
Topical, liquid ophthalmic preparations
Topical, semisolid ophthalmic preparations
Barriers to topical ocular drug absorption
Non-corneal routes of absorption
Improving drug solubility and absorption in topical ophthalmic preparations
Sterility of ophthalmic preparations
Drug half-life in the anterior chamber
Active transporters of the cornea
Targeting the posterior segment of the eye
Periocular drug delivery routes
Problems with traditional and new ocular drug delivery systems
Key points
Introduction
Drug delivery to the eye is one of the most important areas of modern ocular therapy and presents many opportunities and challenges. The current market for ophthalmic pharmaceuticals is now worth many billions of dollars a year. The front of the eye is accessible and conditions affecting it can be treated by simple topical eye drops. The back of the eye is, however, treated as an entirely separate ocular region, and more advanced delivery systems have been designed for its treatment, including intraocular injections and implants that can provide sustained drug release over two years. A range of new therapies have and are being developed for treating ocular conditions including cells, genes and proteins, not only the traditional small molecules.
This chapter will describe the anatomy and physiology of the eye as well as the most common conditions affecting the different ocular regions. The natural anatomical ocular barriers to drug bioavailability have a great impact on ocular pharmacokinetics. Understanding ocular physiology is essential for developing drug delivery systems that are effective, safe and acceptable to patients. The design of topical ophthalmic preparations ranging from solutions to ointments and in-situ forming gels will be discussed. Although eye drops have been used since the times of Cleopatra and comprise over 90% of the ophthalmic preparations in the clinic; they have poor bioavailability and short duration of action. The reason for these shortcomings and formulation efforts to overcome them will be described.
The remainder of the chapter will focus on intraocular systems including injections and implants that deliver drugs directly to the back of the eye. This direct delivery approach has greatly improved drug bioavailability and has been approved for the delivery of several drugs. Current treatment goals are to develop systems that provide therapeutic drug levels for prolonged periods and to minimize the invasiveness of drug delivery procedures. Intraocular implants, which have been developed through sophisticated pharmaceutical, material and biomedical engineering approaches, have helped achieve these goals. Several of them are available in the clinic or are in late stage clinical trials. However, there remain unmet clinical needs, and research in this area is flourishing.
Anatomy and physiology of the eye
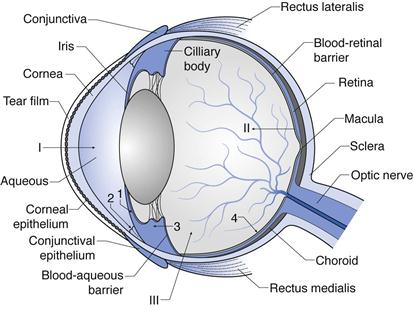
The structure of the eye is shown in Figure 41.1. This figure shows the layers and chambers of the eye and the routes and barriers to ocular drug delivery. Each of these is discussed below.
Layers of the eye
The outer layer of the eye can be considered as segments of two spheres: sclera and cornea. The sclera constitutes the back 5/6ths of the globe, and the transparent cornea provides the forward 1/6th of the globe. The sclera is a tough fibrous tissue that protects the eye from internal and external forces and maintains its shape. The front of the sclera is often referred to as the ‘white’ of the eye. The episclera is the outermost layer of the sclera and has a rich blood supply. The conjunctiva is a thin, transparent mucous membrane that covers the visible part of the sclera and extends to the inside of the eyelids. The optic nerve emerges from the sclera in the posterior part of the eye.
The cornea is the most anterior part of the eye, in front of the iris and pupil. It is densely innervated by nerves, particularly sensory nerves. While the central cornea is avascular, the region of the corneoscleral limbus is supplied by branches of the anterior ciliary arteries. The cornea refracts and transmits light to the lens and retina. It also protects the eye against infection and structural damage to the deeper parts. The cornea and sclera are connected at the limbus.
The surfaces of the cornea and conjunctiva are covered by a film of tears produced by the lacrimal gland. It lubricates the eye surface and protects it from chemicals, microbes and airborne solid particles. It comprises three layers: a mucous layer adhering to the epithelium, an aqueous layer and a superficial lipid layer. The aqueous layer constitutes electrolytes, proteins, glycoproteins, biopolymers, glucose and urea, and has a thickness of 8–12 µm. The lipid layer is composed of sterol esters, wax esters and fatty acids. The mucous layer interacts with the epithelial cells of the cornea, and so each eyelid blink allows spread of the tear film over the eye surface. A dynamic equilibrium exists in the pre-corneal tear film as it goes through a continuous cycle of production, evaporation and drainage.
The middle layer of the eyeball consists of the iris, ciliary body and choroid. The ciliary body is a ring of tissue that extends from the base of the iris to the choroid. The ciliary muscle is its most prominent structure and is in a contracted state to allow the lens to become convex. The ciliary body is also the site of production of aqueous humour. The iris is a fragile diaphragm with circular constrictor and radial dilator muscles positioned in front of the lens and ciliary body, which separates the anterior and posterior chambers. It controls the size of the pupil and thus the amount of light reaching the retina. The colour of the iris is determined by the amount of melanin expressed in it. The choroid is the vascular layer of the eye lying between the retina and sclera. It provides oxygen and nutrients to the outer layers of the retina.
The inner layer of the eye is the retina, which is a complex network of neurons that process light. The layer of the retina surrounding the vitreous cavity is the neural retina, the outer retinal wall surrounded by the choroid and sclera is the retinal pigment epithelium (RPE). The neural cells of the retina are arranged in several parallel layers and the major classes present are photoreceptors (the rods and cones that are responsible for the conversion of light into an electrical signal through the presence of pigments), bipolar cells, horizontal cells, amacrine cells, ganglion cells (which capture and process light signals) and the Müllerian glia (which form the organizational backbone of the neural retina). The RPE constitutes about 3.5 million epithelial cells arranged in a hexagonal pattern. Their important functions include the maintenance of photoreceptor function, storage and metabolism of vitamin A, production of growth factors required by nearby tissue, and wound healing after injury or surgery.
Chambers of the eye
The eye contains three main chambers: anterior chamber, posterior chamber and vitreous cavity. Aqueous humour fills the anterior and posterior chambers. It is a clear, colourless, watery fluid that comprises a vast array of electrolytes, organic solutes, growth factors and other proteins that nourish the non-vascularized tissue of the anterior chamber; particularly trabecular meshwork, lens and corneal endothelium. It is produced by the ciliary body epithelium and flows into the anterior chamber. Aqueous humour leaves the anterior chamber through the trabecular meshwork into Schlemm’s canal and aqueous veins (conventional pathway) or through the ciliary muscle and other downstream tissues (unconventional pathway). If the exit of aqueous humour from the eye is blocked, the amount of fluid within the eye increases, leading to an increase in pressure which may lead to glaucoma and cause damage to the optic nerve. The trabecular meshwork is made up of an extracellular matrix forming a porous like structure through which aqueous humour flows into the canal of Schlemm. Schlemm’s canal connects with the venous system through a network of 25 to 35 collector channels.
The vitreous cavity comprises 80% of the volume of the eye. It weighs approximately 3.9 g and contains vitreous humour. This is a hydrogel containing approximately 98% water. The other 2% of vitreous components are predominantly collagen fibrils and hyaluronic acid. Proteins, inorganic salts, glucose and ascorbic acid are also present. Vitreous humour has a pH of approximately 7.5 and a viscosity 2–4 times that of water. The presence of sodium hyaluronate is primarily responsible for the viscosity of the vitreous humour. Its viscous properties allow it to return to its normal shape when compressed. The vitreous body is surrounded by a thin membrane known as the hyaloid membrane and no blood vessels penetrate it; its nutrition is therefore carried by vessels of the retina and the ciliary body.
Ocular drug delivery routes and elimination pathways
Routes and barriers to ocular drug delivery can be summarized here with reference to Fig. 41.1 (see I, II and III).
Ocular drug elimination (Fig. 41.1) is via:
1. drug elimination from the aqueous humour into the systemic uveoscleral circulation
2. aqueous humour outflow through the trabecular meshwork and Schlemm’s canal.
3. Drug elimination from the vitreous humor via diffusion into the anterior chamber.
4. Drug elimination via posterior route across blood retinal barrier.
Some common ocular conditions and pharmacological interventions
Ocular drug delivery is undertaken for treatment of local disease at different sites in the eye, thus a brief introduction to common eye conditions is appropriate.
Dry eye syndrome.
Dry eye is a common disease which occurs when either the tear volume is inadequate or of poor quality (poor functional tear). This often results in unstable tears and consequently ocular surface disease. Dry eye is not curable and management is to control the symptoms and protect the ocular surface from being damaged. The initial treatments include use of tear substitutes and mucolytic eye drops. In advanced cases, the use of anti-inflammatory eye drops, surgical intervention to reduce punctual drainage and contact lenses have been shown to be beneficial.
Cataract.
Cataract is the opacity of lens, which often results from denaturation of the lens protein. Cataracts, which are usually age-related, are the most common cause of treatable blindness worldwide. Surgery is the only treatment and is very effective. It involves replacement of the clouded lens with a synthetically produced intraocular lens.
Glaucoma.
The glaucomas are a group of diseases in which there is a specific type of damage to the optic nerve (optic disc cupping), resulting in a characteristic pattern of visual field loss; first peripheral and then central vision loss. Glaucoma, a life-long condition, is the leading cause of irreversible blindness worldwide and is the second most common cause of blindness, after cataract. The most important and only modifiable risk factor in this group of diseases is raised intraocular pressure (IOP). It has been shown that reduction in intraocular pressure, medically (eye drops) or surgically, can halt or decrease the progression of the visual field loss.
Age-related macular degeneration (AMD).
AMD is a degenerative disorder that affects the macula, the most sensitive part of the retina, and consequently results in the loss of central vision. AMD is the leading cause of irreversible visual loss in industrialized countries and often occurs in the population over the age of 50 years. There are two forms of AMD: wet and dry. Wet AMD arises when abnormal new blood vessels grow underneath the macula and leak, thus raising the macula off the back of the eye and causing loss of central vision, often very quickly. With the more common dry AMD, the light-sensitive cells in the macula degenerate, with a gradual blurring of central vision. Recent anti-vascular endothelial growth factor (anti-VEGF) treatments including pegaptanib (Macugen) and ranibizumab (Lucentis) have shown beneficial effects in the majority of patients with wet AMD. This treatment, however, requires multiple intraocular injections with an average of 8–10 injections per year (this is discussed in detail in a later section).
Endophthalmitis.
Endophthalmitis is the inflammation of the internal layers of the eye. Infectious endophthalmitis most frequently occurs following ocular surgery and penetrating trauma, particularly with retained foreign body. Special care must be taken when injecting medicines into the back of the eye to ensure complete sterility is maintained. The most commonly cultured bacteria in post-operative endophthalmitis are gram positive (90%), including Staphylococcus epidermidis, Staphylococcus aureus and Streptococcus species. The most common bacteria found following trauma are staphylococcus and bacillus species. Non-infectious endophthalmitis may occur due to many causes including impurities in intraocular injections (e.g. endotoxin, silicone oil precipitates). The main treatments are antibiotics (peri-orbital, intra-ocular and parenteral).
Topical ophthalmic preparations
Topical ophthalmic preparations include: solutions, suspensions, ointments/gels and the newer dispersion systems. These have been traditionally used for treating pathological conditions of the anterior segment, such as infection, inflammation, allergy, dry eye, glaucoma, and corneal ulceration, as well as for instilling anaesthetic and diagnostic agents.
Designing ocular drug delivery formulations requires an understanding of what can be tolerated by the eye. Physiological and biochemical mechanisms exist to protect the eye from harmful stimuli. While these mechanisms are protective, they do sometimes present a barrier to drug absorption. The lacrimal system of the eye is extremely dynamic. The tear volume in the normal eye is 5–9 µL. Basal tears are continuously secreted by the lacrimal glands at an average rate of 1.2 µL/minute, thus giving a tear turnover rate of about 17%/minute. Reflex tears are triggered by irritants and can vary from 3 to 400 µL/minute, the intention being to rapidly eliminate the stimulus. Another protective mechanism is the eyelid movements associated with blinking. Blinking moves tear fluids and foreign matter to the nasal corner of the lid surface, where the liquid exits via the puncta and is then drained away by the nasolacrimal ducts into the inferior nasal passage (Fig. 41.2). Tears contain lysozymes and immunoglobulins which impart an anti-infectious activity. The combined mechanisms of lacrimal drainage and blinking means that administered eye drops are rapidly cleared from the conjunctival sac with residence time ranging from 4 to 23 minutes. Moreover, the rate of drainage from the eye has a positive, linear correlation with instilled volume. It has been found that the palpebral fissure in the open eye is capable of accommodating only 20 to 30 µL of added fluid temporarily without spilling.
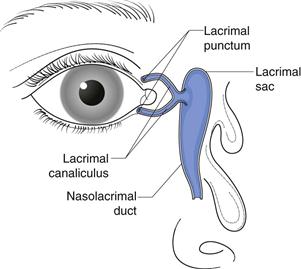
Fig. 41.2 Illustration of nasolacrimal drainage.
Ideally, administered eye drops should be spaced by several minutes to minimize washout. Punctal occlusion maximizes local absorption and minimizes unnecessary systemic exposure by up to 70%. To reduce the elimination rate of administered eye drops, it is important that the topical preparations do not cause irritation. This can be achieved by designing their properties to be as close as possible to the lacrimal fluids covering the surface of the eye.
Formulating ophthalmic preparations
Osmolality
The concentration of salts in lacrimal fluids determines its osmolality. Predominant inorganic ions in tears are sodium, potassium, calcium, chloride and bicarbonate. These have an important function in controlling the osmotic pressure of the intercellular and extracellular fluids of the epithelial spaces of the cornea and conjunctiva. Osmolality in healthy, non-dry eyes has an average value of 302 mmol/kg during the daytime. Patients with dry eye syndrome have been found to present with tear film hyperosmolality which contributes to the symptoms of the disease.
When the eye is exposed to a hypotonic ophthalmic solution, the corneal epithelium becomes more permeable and water flows into the cornea causing oedema. Hypertonic solutions have a dehydrating effect on the corneal epithelium. Hypotonic and hypertonic solutions are irritating to the eye and therefore induce an increased production rate of tears. The rate of tear production increases to several hundred microlitres per minute through reflex tear secretion and reflex blinks. This increased tear turnover rate reduces the retention half-life of a solution that has been applied to the eye.
Normal tear osmotic pressure is equivalent to 0.9% to 1.0% sodium chloride solution. Solutions of osmotic pressure equivalent to 0.6 to 1.3% sodium chloride appear to be well tolerated by the eye. Ophthalmic solutions can be made isotonic by the use of tonicity agents such as sodium chloride, potassium chloride, buffering salts, dextrose, mannitol and glycerol, as long as they are compatible with the other ingredients in the formulation.
Hydrogen ion concentration (pH)
The pH of tears is close to neutral and is controlled by various substances dissolved in the aqueous layer of tears: carbon dioxide, bicarbonate and proteins such as the basic lysozyme and an acidic tear prealbumin. The fatty acids produced by the Meibomian glands also mix with the aqueous phase of tears. The pH of tears is subject to diurnal variation and increases slowly from 6.9 to 7.5 during the waking hours of the day due to carbon dioxide evaporation. The buffer capacity of tear fluids is low but significant; it is predominantly controlled by the balance of bicarbonate and carbon dioxide, as well as proteins. Acidic or basic solutions instilled into the eye cannot be neutralized by the tears that are present and therefore reflex tears are generated to dilute the administered drop and eliminate it. The recovery to the original pH of the tear film can vary from a few minutes up to 20 minutes. The duration of recovery is influenced by the pH, volume, and buffer capacity of the administered solution, as well as the age of the patient. Strongly acidic or basic solutions should not be administered to the eye as they can cause damage to the ocular tissue. The eye can generally tolerate topical ophthalmic preparations at a pH within the range of 3.5 to 9. However, it is preferable to formulate as close to physiological tear pH as possible to reduce discomfort and the associated increased lacrimation.
pH is important in drug ionization and product stability. Pilocarpine is a natural alkaloid used in the treatment of glaucoma. It undergoes pH-dependent hydrolytic degradation and one of the ways to maintain stability of pilocarpine aqueous solution is to maintain the pH at 4–5 through the use of a weak acidic buffer. Since the pH deviates from the physiological pH of the lacrimal fluids, the constituting buffer must be weak to allow the lacrimal fluids to be restored to their normal pH within a short period of time following instillation. Drug ionization is also important in determining drug solubility and permeability across the corneal epithelium. The extent of ionization can be manipulated through control of the pH of ophthalmic preparations.
Commonly used buffers in ophthalmic solutions include borate and phosphate buffers. To prepare solutions of lower pH range acetic acid/sodium acetate and citric acid/sodium citrate buffers are used. It is important that strong buffers are not used and to use a low concentrations of weak buffers.
Surface tension
The surface tension of tear fluid at physiological temperature in a healthy eye is 43.6 to 46.6 mN m-1. Administration of solutions that have a surface tension much lower than that of the lacrimal fluid destabilizes the tear film and disperses the lipid layer into droplets that are solubilized by the drug or surfactants in the formulation. The oily film reduces the rate of evaporation of the underlying aqueous layer and therefore once it is lost, dry spots are formed which are painful and irritant. Surfactants are implicated in this.
Surfactants are typically included in ophthalmic preparations to solubilize or disperse drugs. Irritation power of surfactants decreases in the following order: cationic > anionic > zwitterionic > non-ionic. Non-ionic surfactants are therefore the most commonly used, examples include; polysorbate 20, polyoxyl 40 stearate, polyoxypropylene-polyoxyethylenediol. Despite being the least irritant, non-ionic surfactants have been shown to remove the mucus layer and disrupt the tight junctional complexes of the cornea; therefore increasing drug permeability. Surfactants may also interact with polymeric substances in the preparation and reduce the efficacy of preservatives. The concentration of surfactant is important not only in terms of drug solubility, safety and patient tolerance, but also because high concentrations can lead to foaming upon product manufacture or shaking.
Viscosity
Viscosity enhancing polymers are used in ophthalmic solutions to prolong drug retention in the precorneal tear film and thus enhance drug absorption. Mechanisms proposed are not just reduced drainage rate; the thickness of the precorneal tear film is also increased due to the ability of viscosity-enhancing polymers to drag water and stabilize the aqueous layer as they spread over the corneal surface on blinking. This increased volume acts as a reservoir for the drug so that it is re-spread in the tear film over the cornea with each blink. Water soluble polymers that have been used to increase solution viscosity include poly (vinyl alcohol), poly (vinylpyrrolidone), various cellulose derivatives, particularly; methylcellulose, hydroxypropyl methylcellulose and carboxymethyl cellulose (at concentrations of 0.2–2.5%) and poly(ethylene glycol)s (at concentrations of 0.2–1%).
Tears are non-Newtonian fluids whose coefficient of viscosity is shear dependent (shear-thinning). This is commonly seen with linear, multiple-charged polymers such as sodium hyaluronate and Carbopol. Zero shear viscosity values of 4.4 to 8.3 mPa s have been reported for normal tears. The force required by the eyelids to blink is 0.2 N and for a forceful blink it is 0.8 N. The pain threshold is 0.9 N and therefore if a higher force than this is required for blinking, then it would be painful for the patient. This limits the acceptable viscosity of administered ocular solutions since the force needed to move the instilled solution at the shear rates equivalent to those generated by blinking should be lower than 0.9 N. Furthermore, very viscous solutions can cause blurring of vision and may block the puncti and canaliculi. Nevertheless, solutions containing viscoelastic material can be used at higher viscosities. Since the viscosity of viscoelastic polymers is shear dependent; the viscosity of these polymer solutions can change in the eye due to blinking.
Topical, liquid ophthalmic preparations
Solutions
Ophthalmic solutions are the most common topical ophthalmic preparation. They are typically the easiest to manufacture (have the lowest cost of production) and are relatively easy for a patient or healthcare provider to administer. Ophthalmic solutions are also desirable where a rapid onset of action is required as they do not need to undergo dissolution. This would be the case for local anaesthetics (e.g. lignocaine, proxymetacaine hydrochloride); ocular diagnostics (fluorescein sodium) and ocular preoperative drugs. Moreover, solutions are homogeneous and therefore display a better dose uniformity. A limitation of solutions, however, is that they are rapidly drained out of the eye. Moreover, the rate of drainage is proportional to the size of the drop administered. The volume of eye drops administered from commercial eye dropper bottles has been reported to be in the range of 25 to 56 µL; this is dependent on the physical shape and orifice of the dropper opening, physicochemical properties of the liquid and the manner in which the dropper is used.
Suspensions
Several ocular preparations are available as suspensions. This approach has been used to administer drugs which are sparingly soluble in water, e.g. steroids, or to prolong drug release. Particles tend to be retained in the ocular cul-de-sac (the space between the eyeball and eyelid) and slowly go into solution thus increasing the contact time. Particle size and shape need to be carefully selected as some particles can cause irritation of the sensory nerves in the epithelium. The European Pharmacopoeia sets standards for particle size: in a sample corresponding to 10 µg of the solid phase, ‘not more than 20 particles should have a maximum dimension greater than 25 µm, and not more than two of these particles have a maximum dimension greater than 50 µm. None of the particles has a maximum dimension greater than 90 µm’.
The particles of a suspension need to be readily dispersible on shaking by the patient to ensure uniform dose administration. Homogeneity and dose uniformity need to be confirmed in multi-dose containers from first to last use. Problems that can arise with suspensions are conversions in crystal structure of the drug, i.e. polymorphic changes during storage, which can lead to changes in drug solubility and dissolution behaviour. If the drug is polydispersed, Ostwald ripening may arise on changes in storage temperature or prolonged storage. Cake formation can also be a problem, which may not be resolved by forming a flocculated suspension since large floccules can irritate the eye. Using a polymer solution as a viscosity enhancing agent can prevent caking and allow particle resuspension by shaking. Betaxolol and brinzolamide are available as suspensions. The formulation of the former contains Carbomer 934 P and ion exchange resins.
Submicron emulsions
Ciclosporin is an immunomodulator with anti-inflammatory effects. It is available at a concentration of 0.05% as a sub-micron emulsion (Restasis®, Allergan) for topical application to the eye. Ciclosporin is hydrophobic (log P=3.0) and has a very poor aqueous solubility of 6.6 µg/ml and cannot therefore be formulated in conventional aqueous ophthalmic vehicles. It has been successfully solubilized in an oil-in-water (o/w) submicron emulsion. The oil phase in Restasis is castor oil and the emulsion is stabilized with the non-ionic surfactant polysorbate 80 and glycerin, which behaves here as a co-surfactant. Ocular submicron emulsions with a droplet size of ~0.1 µm have also shown potential for prolonging drug release and achieving significantly higher drug concentrations in the cornea and aqueous humour compared to suspensions.
Topical, semisolid ophthalmic preparations
Ointments
Ophthalmic ointments have been used as an option to reduce drug drainage by tear flow and therefore increasing corneal residence time. Ointments can also be entrapped in the fornices whereby they serve as a reservoir for the drug. Sustained drug release effects of 2–4 hours are usually observed. Ointments also have the advantage of allowing the incorporation of drugs with poor aqueous solubility. Hydrophobic ointments sometimes improve the stability of hydrolysable compounds, particularly peptides. Soft paraffin and liquid paraffin are commonly used as bases for ophthalmic ointments. Anhydrous, water-soluble bases such as carbomer with poly(ethylene glycol) are also used. Antibiotics, antifungals and steroids are the classes of drugs most available as ointments. Drug bioavailability usually peaks later with ointment vehicles than with solutions or suspensions. Total bioavailability in the aqueous humour can also be significantly greater than with solutions or suspensions (Fig. 41.3).
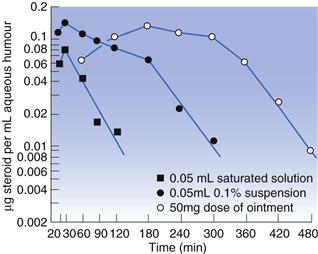
Fig. 41.3 Comparison of aqueous humour levels of fluorometholone following administration by different dosage forms. (Courtesy of Sieg and Robinson, 1975, with permission.)
Ointments are however more difficult to administer compared to solutions and may give rise to a more variable dose. Also blurring of vision arises which tends to reduce patient compliance making ointments more useful for night-time administration. Drug molecules may be entrapped within the ointment base due to favourable partitioning towards the base, therefore inhibiting drug release. The base is also sensitive to changes in temperature.
Gels
Gels, which are semisolid systems comprising water-soluble bases, are also available and are more favourable than ointments for water soluble drugs. These utilize polymers such as polyvinyl alcohol (PVA), poloxamer, hydroxypropyl methylcellulose (HPMC), Carbopol or Carbomer, dispersed in a liquid. Pilocarpine is a cholinomimetic agent which reduces intraocular pressure and is licensed for glaucoma. It is available as a gel (Pilogel®, Alcon) containing more than 90% water and employing Carbopol 940 (a synthetic high molecular weight polymer of acrylic acid). An equivalent duration of response has been shown with a single instillation of the gel compared to four instillations of the solution. The total daily dose of pilocarpine can be reduced from 8 mg/day to 2 mg/day with the gel formulation.
Gels that are activated by ions, pH and temperature have also been developed. These undergo a phase transition from liquid to solid in the ocular cul-de-sac to form a viscoelastic gel. These in situ forming gels have an advantage over the pre-formed gels in that the dose is more reproducible and administration is easier; thus improving patient compliance. Examples of polymers activated by temperature include poloxamers e.g. poloxamer 407. Smart Hydrogel™ has been developed which is a graft co-polymer of polyacrylic acid and a poloxamer which requires only 1–3% polymer concentration to undergo gelation at body temperature. Smart Hydrogel™ also has bioadhesive properties due to the presence of polyacrylic acid.
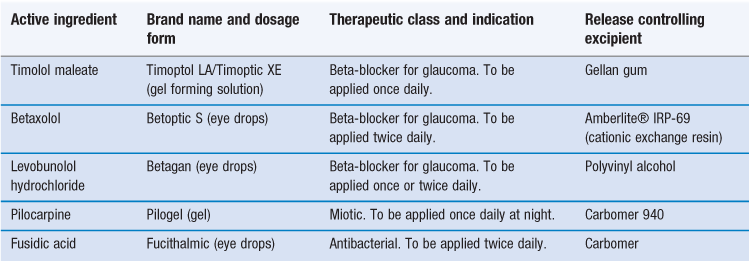
Timolol is a non-selective beta blocker licensed for glaucoma. Timolol maleate gel-forming solution (Timoptic-XE®, Merck) is available in the clinic and constitutes a purified anionic heteropolysaccharide derived from gellan gum. The gellan gum is in aqueous solution and forms a gel in the presence of cations which are present in the precorneal tear film. It is administered once a day, compared to the regular Timoptic® preparation which needs to be administered twice daily to achieve a similar hypotensive effect. This gel is subsequently removed by the flow and drainage of tears. Alginates also undergo sol to gel phase transition when exposed to the ionic strength of ocular fluids. See Table 41.1 for examples of gel and gel-forming ophthalmic preparations.
Other polymer systems that undergo physical changes when exposed to a changing environment include cellulose acetate phthalate latex which coagulates when its native pH of 4.5 is raised by the tear fluid to pH 7.4. Carbopols have pKas of 4 to 5 and ophthalmic solutions of these polymers are therefore prepared at this pH. When these poorly buffered systems are exposed to the near-neutral pH of ocular fluids, the polymer solubility is reduced and the system undergoes gelation.
Mucoadhesive systems
Other ways to increase the contact time of topical ophthalmic solutions with the ocular surface have been through the use of mucoadhesive polymers. These attach to the mucin coat that covers the conjunctiva and cornea. Mucin has a protein or polypeptide core with carbohydrate chains branching off. The mucin coat serves to protect, hydrate and lubricate the surface of the eye. Mucoadhesive polymers are commonly macromolecular hydrocolloids with numerous hydrophilic functional groups possessing the correct charge density. They should also exhibit good wetting of the ocular surface to facilitate maximum interaction with the mucin coat. Electrostatic, covalent and hydrogen interactions are the most common between mucoadhesive polymers and mucin.
Mucoadhesive polymers can be natural, synthetic or semi-synthetic in nature. The synthetic polymers include polyacrylic acid, polycarbophil as well as cellulose derivatives. The (semi)natural mucoadhesive polymers include chitosan and various gums such as guar, xanthan, carrageenan, pectin and alginate. Chitosan is a cationic polymer which has shown great promise for ophthalmic use. Besides being mucoadhesive it has good wetting properties and is biodegradable, biocompatible and has good ocular tolerance. It is also pseudoplastic and viscoelastic in solution. It is positively charged at neutral pH and therefore electrostatic forces arise between it and the negatively charged sialic acid residues of the mucus glycoproteins which contribute to its mechanism of mucoadhesion. Hyaluronic acid is another polymer which has also shown potential. It is a high molecular weight biological polymer consisting of linear polysaccharides and is present in the extracellular matrix as well as being the main component of the vitreous humour. It displays mucoadhesive properties as well as high water binding capacity and pseudoplastic behaviour. Hyaluronic acid is used in some ocular surgeries in the anterior chamber (e.g. cataract) in the sub-conjunctival space (e.g. glaucoma filtering surgery).
Polycarbophil (polyacrylic acid crosslinked with divinyl glycol) has been used in a topical azithromycin formulation which is available in the clinic under the name of AzaSite/Durasite® (Inspire Pharmaceuticals). It has been shown to display higher bioavailability compared to conventional aqueous eye drops. Therapeutic drug levels also persist for several days in the eyelids and conjunctiva following the administration of the last dose. Polycarbophil is insoluble in water and its swelling is pH dependent. Swelling is greatest at pH 6 to 7 which is the pH of lacrimal fluids. On exposure to tears, polycarbophil swells and entangles with mucin on the ocular surface. Hydrogen bonding also exists between the nonionized carboxylic acid of polycarbophil and the mucin.
Ion-exchange resins
The concept of ion-exchange resins has been available for more than 50 years and has been used and marketed in various dosage forms to control drug delivery. The drug (acidic or basic in nature) is ionically bound to an ion-exchange resin to form an insoluble complex. Drug can only be released from the complex through exchange of the bound drug ions with physiological ions in body fluids. The actual resin is an insoluble, ionic material composed of two parts, a structural portion comprising a polymer matrix, usually styrene cross-linked with divinylbenzene, and a functional portion, which is the ion-active group. The ion active group can either be negatively or positively charged thus functioning as either a cation or anion exchanger. These drug-resinates are usually spherical in shape, porous and hydrate on exposure to aqueous fluids. They are insoluble, non-absorbable and are considered safe for use in humans. They have had several applications in pharmaceuticals, including: taste masking, drug stabilization and sustained release solid dosage forms as well as liquid suspensions.
Betaxolol hydrochloride (a cardioselective beta-blocker) is available as an ion-resin suspension formulation (Betoptic-S®, Alcon, US). The positively charged drug is bound to a cation-exchange resin (Amberlite® IRP69). The matrix of Amberlite IRP69 is styrene-divinylbenzene polymer and the functional portion is sodium polystyrene sulphonate. Sulphonic acid acts as a strong cation exchanger. The mobile, or exchangeable, cation is sodium; this can be exchanged for many cationic species. Upon ocular instillation of the suspension, betaxolol is displaced from the resin by the sodium ions in the tear film. This exchange occurs over several minutes. The polar nature of betaxolol can cause ocular discomfort, therefore formulating it as an ion-exchange resin reduces the rate of drug release and minimizes this discomfort.
Resin particle size is one of the factors that controls the rate of drug release. In Betoptic-S, the resins have been finely milled to a diameter of 5 µm to achieve a fine suspension. The polymer, Carbomer 934P (a water-soluble acrylic polymer), is also included to improve the physical stability and ease of re-suspension of the product, as well as to enhance the ocular residence time.
Barriers to topical ocular drug absorption
The topical route of drug delivery is the most common way of treating the anterior segment and over 90% of the ophthalmic medicines on the market are in the form of eye drops. The topical route provides selectivity with an enhanced therapeutic index, it also circumvents first pass metabolism and drugs can be administered in a simple, non-invasive manner. Its main shortcoming, however, is its inefficiency, whereby only 1–5% of the instilled dose reaches the aqueous humour. The highly efficient lacrimal drainage system and the corneal barrier to drug permeation are the mechanisms mainly responsible for this low ocular drug bioavailability. Drug binding to proteins also reduces absorption, and protein levels of lacrimal fluids are higher in inflamed or infected eyes.
The corneal barrier
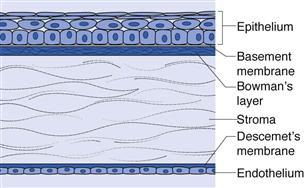
Drugs can permeate the cornea by passive diffusion, facilitated diffusion or active transport. Facilitated diffusion and active transport occur via transporter proteins expressed on the corneal epithelium. Passive diffusion does not require transporters, however it is determined by the physicochemical properties of the drug. The cornea is divided into five layers; epithelium, Bowman’s membrane, stroma, Descemet’s membrane and the endothelium. The layers which form substantial barriers to drug permeation are: epithelium, stroma and endothelium (from the outer to the inner surface) (Fig. 41.4). The epithelium and endothelium are rich in lipids while the stroma has high water content. The corneal epithelium is about 0.1 mm thick and is considered to be the rate limiting barrier to transcorneal drug permeation. It contributes ~90% of the barrier to hydrophilic drugs and ~10% of the barrier to lipophilic drugs. Drugs can penetrate this layer by partitioning through the cells (transcellular) or by passing between the cells (paracellular). The epithelium, however, has tightly adherent cells with tight junctions which excludes macmolecules having a radius > 1 nm. Only small drugs of MW < 350 Da and ions can permeate through the paracellular route. Most of the lipophilic compounds can pass through the corneal epithelium via the transcellular route. The cornea is considered a tight tissue, more than the intestine, lung and nasal mucosa; making drug absorption via the paracellular route more difficult compared to these other organs.
The stroma is a cellular, aqueous environment interdispersed with glycosaminoglycans and collagen fibrils that are organized in parallel lamellae. It is open knit allowing hydrophilic molecules to pass through relatively easily. It however limits the penetration of highly lipophilic or large molecular weight compounds. The corneal endothelium is a single cell layer with large intercellular junctions. It is in direct contact with the aqueous humour and partially resists the permeation of lipophilic compounds, though not hydrophilic ones.
Ophthalmic drugs with modest lipophilicity and low molecular weight are absorbed more efficiently via the corneal route compared to hydrophilic, ionized drugs. The optimal lipophilicity for the permeation of steroids and beta-blockers has been shown to correspond to a log P of 2–3. Compounds with a higher lipophilicity (log P > 3) have been shown to have lower permeability as their permeation is rate limited by the slow transfer through the hydrophilic stroma. Drugs need to have an appropriate balance of lipid and water solubility for good corneal permeation.
Good aqueous solubility is important as drugs must be in solution to permeate through the cornea. Since the instilled drops are diluted by tear fluids and in contact with the corneal epithelium for a very short time, making bioavailability low, high drug concentration is one important consideration, when possible, for ophthalmic solutions. For ionizable drugs, the pH of the formulation can be adjusted to obtain the optimum balance of solubility and trans-epithelial permeation. It is desirable to possess good aqueous solubility at the physiological pH of tears, without the loss of lipohilicity for corneal permeation.
Non-corneal routes of absorption
While the corneal route is the principal route of entry for drugs into the eye, studies have also shown that absorption can also occur via the conjunctiva-scleral layer; particularly for large hydrophilic molecules such as timolol maleate, and carbonic anhydrase inhibitors as well as proteins and peptides which can be used as carriers. The conjunctiva has 5–15 layers of squamous epithelial cells with tight junctions at the apical end. It is more permeable or leaky than the cornea and allows drugs to permeate through the paracellular as well as transcellular routes. The conjunctiva is highly vascularized so drug absorption often results in systemic distribution of the drug away from the eye. Efflux drug transporters on the epithelial cells have been identified. The conjunctival stroma comprises blood vessels, nerves and lymphatics that attach to the sclera. Drug permeation through the sclera occurs through the aqueous intercellular space between the collagen fibres. Drug permeability through the sclera is not dependent on lipophilicity or size. Drugs with a molecular weight of more than 1 kilodalton (kDa) are almost impermeable through the cornea; whereas dextran (40 kDa) and albumin (69 kDa) have good permeability through the sclera. Despite this, the conjunctival-scleral route is considered a non-productive route because the blood vessels in the conjunctiva rapidly absorb the instilled drug which dissipates into the systemic circulation rather than ending up in the aqueous humour.
Improving drug solubility and absorption in topical ophthalmic preparations
Drug ionization and salt form
The pKa of the drug (acid dissociation constant) and pH determine its degree of ionization in solution. While the pKa can only be altered through structural changes in the molecule, the pH of the drug vehicle can be controlled. A higher proportion of unionized species displays a greater degree of transcorneal permeability, as has been shown with pilocarpine. Pilocarpine is a weak base but to achieve good solubility and stability the eye drops are formulated with an acidic vehicle (pH 3.5–5.5). Interestingly, however, carbonic anhydrase inhibitors display a greater pharmacological effect (reduction in intraocular pressure) in the ionized form compared to the un-ionized form. This effect is observed, not because of greater drug permeability, but due to the ability of these inhibitors to sequester in the cornea and form a depot.
The physical form of the drug can also be an important determinant of its ocular bioavailability. The salt form can affect solubility and lipophilicity of the drug. Dexamethasone acetate ester displays the optimum balance of solubility and corneal permeability compared to the very water-soluble phosphate salt or lipophilic free base.
Cyclodextrins
Cyclodextrins (CDs) have shown great potential in improving the solubility of poorly water-soluble drugs (see Chapter 24). CDs are cyclical oligosaccharides with a lipophilic centre and hydrophilic outer surface. They can complex lipophilic drugs in their interior, thus forming water-soluble complexes. This maintains the structure, lipohilicity and hence the permeability of the compounds. The drug is associated with CD by hydrogen bonding, hydrophobic interactions or van der Waals forces. The hydrophilic CDs acts as carriers, delivering water-insoluble molecules to the corneal membrane where they can partition from the CD complex. A state of equilibrium exists between free and complexed drug which is dependent on the strength of the non-covalent interactions between the drug and CD. The relatively lipophilic membrane has low affinity to the large hydrophilic CD molecules and the biological membrane is not disrupted as is observed with penetration enhancers. Moreover, this provides an opportunity for delivering irritant drugs as they are not freely available and are entrapped in the complex. Research with this formulation approach has been shown to improve corneal penetration of dexamethasone, pilocarpine and carbonic anhydrase inhibitors.
Prodrugs
Improved corneal penetration can be gained through the use of prodrugs. A prodrug is a drug with added functionalities that converts into the active parent drug through enzymatic or chemical reactions. Approaches of enhancing corneal penetration through the use of prodrugs include; optimization of lipophilicity, enhancement of aqueous solubility, improved affinity for uptake transporters and evasion of efflux pumps.
Drugs with carboxylic acid groups, such as the prostaglandin analogues indicated for glaucoma, have low corneal permeation. This is due to the ionization of the carboxylic acid group at the near neutral pH of tears which reduces permeability through the lipophilic epithelium. One strategy to mitigate this has been esterification of the carboxylic acid group. Since the cornea has high esterase activity, these derivatives can easily revert to their parent form. One of the problems, however, with ester prodrugs is their increased susceptibility to hydrolysis. Bulky isopropyl esters have been used to achieve stability in aqueous solutions. The prostaglandin analogues, latanoprost and travoprost, are isopropyl esters. These prodrugs have been shown to improve corneal permeability and intraocular pressure lowering effects compared to their parent forms (i.e. free acids).
Dipivalyl epinephrine (dipivefrin) is also indicated for glaucoma and is the first marketed ophthalmic prodrug. It is metabolized to epinephrine (adrenaline), which was the drug originally used, though this was later found to give rise to severe side effects in patients. Epinephrine is a polar drug and was subject to rapid clearance from the ocular surface via nasal lacrimal drainage. Systemic absorption was therefore significant giving rise to cardiac arrhythmias and blood pressure elevation. The prodrug, dipavyl ester of epinephrine, was designed to be more lipophilic than the parent drug for improved corneal permeation.
Other examples of pro-drugs in the clinic include the beta-blocker, levobunolol (log P = 2.4). Levobunolol is converted in the cornea to the active dihydrolevobunolol by metabolic reduction of its keto group. Dihydrolevobunolol is more lipophilic and has a longer half-life compared to its parent form. Active research is being pursued in designing prodrugs of the antivirals aciclovir and ganciclovir. Amino acid and peptide derivatives of these prodrugs target the amino acid and peptide transporters of the cornea.
Sterility of ophthalmic preparations
It is a regulatory requirement that preparations intended for ophthalmic use, including those for cleansing the eyes, must be sterile at the time of filling and closing in a sealed container. Ocular infections are extremely dangerous and can rapidly lead to the loss of vision. Eye-cups, droppers and all other dispensers should also be sterile and regulated if packaged with the drug product. For ophthalmic preparations, terminal sterilization of products in their final containers should be adopted wherever possible. If the product cannot withstand terminal sterilization then filtration under aseptic conditions should be considered, usually performed using a filter pore size of 0.22 µm or less. Sterilization methods are discussed in greater detail in Chapters 16 and 17. The raw materials used for aseptic manufacture should be sterile, wherever possible, or should meet a low specified bioburden control limit. Ophthalmic preparations must furthermore be labelled with duration of use once opened.
Preservatives are included in multi-dose containers to destroy and inhibit the growth of microorganisms that may have been accidentally introduced on opening of the container (see Chapter 50). They are not to be used in products for intraocular administration as they can lead to irritation. Ideally, a preservative should have a broad-spectrum anti-microbial activity, exhibit compatibility and stability with all the ingredients in the preparation and the container, as well as being innocuous to the ocular tissue. Benzalkonium chloride is the most commonly used preservative, at concentrations ranging from 0.004 to 0.02%. It is a quaternary ammonium salt and causes epithelial toxicity on repeated administrations. Poor tolerance to treatment has been associated with preservatives and newer alternatives are being investigated.
Single dose units (SDUs) have been developed to circumvent the use of preservatives while maintaining stability. The manufacturing and packaging of these is however expensive and so they have not been embraced for all the marketed ophthalmic solutions. Several multi-dose bottles have been developed that maintain sterility without the use of a preservative; one of these is the ABAK patented filter system which uses a 0.2 µm nylon membrane to prevent bacteria from entering the bottle. It is known as Airless Antibacterial Dispensing System (AADS™, Pfizer) and works by preventing air, and therefore bacteria, from entering the container on dispensing. Furthermore, a silver coil is included in the bottle tip. Silver has antibacterial properties and therefore any bacteria contacting the tip do not contaminate the contents. This system guarantees three months of sterility.
Ocular drug pharmacokinetics
Drug half-life in the anterior chamber
Peak drug levels in the anterior chamber are reached 20–30 minutes after eye drop administration. These concentrations in the aqueous humour are typically, however, two-fold less than the administered concentration. From the aqueous humour the drug can diffuse to the iris and ciliary body where it may bind to melanin and form a reservoir allowing gradual drug release to the surrounding cells. Drug is eliminated from the aqueous humour by two main routes: aqueous turnover through the trabecular meshwork and Schlemm’s canal (route 2, Fig. 41.1) and by the venous blood flow of the anterior uvea across the blood aqueous barrier (route 1, Fig. 41.1). Aqueous humour turnover is at a rate of 2.2 to 3.1 µL/minute during wakefulness. For an individual with an average anterior chamber volume of 185 µL, the half-life of anterior chamber fluid is 43 minutes. The other mechanism of drug elimination by the uveal blood flow is dependent on the drug’s ability to permeate the endothelial cells of the blood vessels and is therefore more favourable for lipophilic drugs. The clearance of lipohilic drugs can be in the range of 10–30 µL/minute. Drug half-lives in the anterior chamber are typically short, about one hour. Drug distribution to the vitreous is extremely slow as the lens prohibits diffusion.
Active transporters of the cornea
Various uptake and efflux transporters have been shown to be present in the corneal epithelium. These transporters are also present in the epithelium of the intestine, blood-brain barrier and kidney tubuli. Efflux transporters protect cells from noxious stimuli and are also implicated in drug resistance. It is estimated that 25% of administered drugs are substrates for transporters. Since the cornea is in contact with the external environment it is not surprising that it expresses efflux transporters as part of a protective mechanism.
Efflux transporters that have been identified on the corneal epithelium include P-glycoprotein (P-gp, MDR1), breast cancer resistant protein (BCRP) and multi-drug resistant protein 5 (MRP 5). P-gp was found to be implicated in the transport of ciclosporin A (immunomodulator for treating dye eyes) in the cornea. The prostaglandin agonists used in the treatment of glaucoma; bimatoprost, latanoprost, travoprost, and their free acid forms, are substrates of the MRP 5 efflux pump on the cornea. Bimatoprost is also a substrate for P-gp. Co-administration of these prostaglandin agonists for the treatment of glaucoma has been proposed for overcoming efflux as well as achieving a synergistic pharmacological effect, since these molecules act at different receptors for reducing intraocular pressure.
One of the main uptake transporters in the cornea epithelium are the amino acid transporters. The corneal epithelium is a highly regenerative tissue with continuous protein synthesis, thus placing a demand on amino acid transport. The aqueous humour is the main source of nutrient provision for the corneal epithelium. Oligopeptide transporters have also been identified and shown to be involved in the transport of valaciclovir (L-valyl ester of aciclovir) through the cornea. They are also being utilized in prodrug delivery. Organic anion transporting polypeptide family (OATP) has substrates of mainly anionic, amphipathic nature. Their presence in the cornea may be implicated in the transport of the thyroid hormone, which has a role in the development and transparency of the cornea. Its involvement in drug transport has still not been determined.
The pharmacokinetic significance of the role of these ocular transporters still requires investigation. With respect to topical solutions, the contact time is short and most of the drug absorption takes place in 2 –3 minutes after instillation. Hence, these transporters may become saturated and passive diffusion becomes the predominant mechanism.
Blood retinal barrier
The blood retinal barrier (BRB) (route II, Fig. 41.1) restricts the entry of drugs from the systemic circulation into the posterior segment of the eye. It is composed of two parts; an outer part formed by the retinal pigment epithelium (RPE) and an inner part, comprising endothelial cells of the retinal vessels. These two parts are connected to each other by tight junctions which pose a barrier to the perfusion of hydrophilic drugs from the highly vascular choroid into the retina and vitreous; and vice versa. The blood retinal barrier has been shown to have some structural similarities to the blood brain barrier.
Transporters that have been identified in the RPE include amino acid transporters, oligopeptide transporters, monocarboxylate transporters, folate and vitamin C transporters as well as glucose transporters, organic anion transporter polypeptide (OATP), organic cation transporter (OCT) and organic anion transporter (OAT). The efflux transporters are P-gp, MRP1, MRP4, MRP5 and BCRP. Drugs that have been found to have interactions with transporters in the blood-retinal barrier are predominantly substrates of OAT, OCT or OATP. Substrates of OAT include various antibiotics (penicillin, erythromycin and tetracycline) and antivirals (aciclovir, zidovudine). The main substrates for OCT are the antiglaucoma drugs carbachol, dipivefrine, brimonidine and timolol. OATP substrates are penicillin, erythromycin, steroidal anti-inflammatory agents (dexamethasone, hydrocortisone, prednisolone) and ciclosporine. Transporters seem to play an important role in drug delivery to the posterior eye segment. Moreover, drug concentrations are low at the BRB which means that the transporters are unlikely to be saturated and therefore their role significant.
Ocular metabolism
Another ocular defence mechanism which protects the eye from the outside environment is the metabolism of xenobiotics. Both phase I and phase II metabolism reactions take place in ocular tissue. Phase I is whereby a polar functional group is introduced into the molecule which makes it more susceptible to phase II conjugation reactions. In some cases, however, the products of phase I reactions are eliminated from the body without further changes. Studies show that the most active metabolic sites in the eye are the ciliary body and pigmented epithelium of the retina. This may be attributable to the high perfusion of these sites by the blood circulation and consequently exposure to the xenobiotics circulating in the blood. Moreover, the main function of the ciliary body is to produce aqueous humour through ultrafiltration of plasma. It should therefore be able to handle the exogenous compounds to which it is exposed and convert them into harmless metabolites which could otherwise have toxic effects on the lens and other internal organs of the eye.
The enzymes involved in phase I reactions are the esterases, which have been identified in ocular tissue and include acetyl, butyrl and carboxy-cholinesterases. This hydrolysis of compounds containing ester linkages has been exploited in prodrug design including dipivalyl epinephrine and pilocarpine prodrugs. Various esterases have been identified in the cornea. Aldehyde and ketone reductases have also been reported in ocular tissue. Ketone reductase reduces levobunolol, a beta blocker indicated for glaucoma. Peptidase activity has also been determined. Cytochrome P-450 (CYP) enzyme expression is to date considered marginal in the human cornea, iris-ciliary body and retina/choroid.
Several ocular drugs are substrates for CYP enzymes and the level of expression of these enzymes in the liver have been implicated in the systemic response to these locally administered ophthalmic medicines. Timolol is a non-selective beta-blocker used as an anti-glaucoma medication. Although it is administered into the eye it is partially absorbed into the systemic circulation where it is metabolized by the enzyme CYP-2D6. Individuals that are poor metabolizers of timolol can be more prone to its adverse systemic effects, such as reductions in heart rate and blood pressure. Moreover, CYP enzymes are inducible by several pharmacological agents including phenobarbital, rifampicin and phenytoin. Such induction can increase drug metabolism. One of the phase II enzymes identified in the eye is Glutathione-S-transferase (GST). This binds to lipophilic compounds, such as bilirubin and haemetin, which is a critical step in the detoxification process. GST has been indentified in the lens and deficiency of it has been associated with cataract.
Targeting the posterior segment of the eye
Systemic drug delivery
Diseases affecting the posterior segment of the eye are the most responsible for vision impairment leading to blindness. These include age-related macular degeneration (AMD), diabetic retinopathy and optic nerve damage associated with glaucoma. The posterior segment can be reached by topical, systemic or direct drug delivery systems. Both paths are however tortuous for the drug and several barriers need to be overcome. Topical drug delivery presents a long diffusion pathway for the drug and the administered dose may end up in the systemic circulation through the conjunctival and nasal blood vessels. Adequate concentrations are therefore not reached in the posterior segment.
Systemic delivery of a drug means it needs to be able to cross the blood-retina barrier to reach the retina and vitreous. Moreover, only a small fraction of blood flow circulates through the posterior segment of the eye and therefore high systemic doses need to be administered which can lead to systemic side effects. Despite these absorption barriers, verteporfin (Visudyne®, Novartis) has been successfully developed and licensed for intravenous (IV) administration for the photodynamic treatment of wet AMD associated with predominantly classic subfoveal choroidal neovascularization (CNV). It is a liposomal drug delivery system available as a lyophilized cake which is reconstituted for intravenous injection. Liposomes are phospholipid vesicles with an aqueous core and phospholipid bilayers which allow the entrapment of hydrophilic and lipophilic drugs respectively. They are biocompatible and biodegradable and can vary in size from nanometres to tens of micrometres (Chapter 45). Verteporfin is a water-insoluble drug and is entrapped within lipid bilayers of liposomes. Following IV infusion, verteporfin is activated by local irradiation using non-thermal red laser (low energy laser) applied to the retina. Light is delivered to the retina as a single circular point, via a fibre optic. Cytotoxic derivatives are produced which cause local damage to the neovascular endothelium, thus occluding the targeted vessels. Treatment is administered to the patient every three months.
Intravitreal injections
Intravitreal injections provide the most efficient means of drug delivery to the back of the eye. The drug bypasses the blood ocular barriers thus achieving higher intraocular levels which improve treatment efficacy. Systemic side effects are also minimized. Intravitreal injections have been shown to be effective in patients for a variety of low molecular weight drugs and monoclonal antibodies. The intravitreal route is approved for two anti-vascular endothelial growth factors indicated for the treatment of neovascular AMD. Approximately two-thirds of people with AMD have the neovascular (or wet) form of the disease which can progress rapidly leading to irreversible sight loss within days or weeks. Vascular endothelial growth factor (VEGF) induces angiogenesis and augments vascular permeability and inflammation; which are thought to contribute to the progression of the wet form of AMD. VEGF has also been implicated in blood retinal barrier breakdown. The two approved treatments, pegaptanib sodium (Macugen®, Pfizer) and ranibizumab (Lucentis®, Genentech), bind to and inhibit the activity of different isoforms of VEGF. Pegaptanib is a PEGylated modified oligonucleotide and ranibizumab is monoclonal antibody. They need to be injected into the vitreous approximately every six and four weeks, respectively.
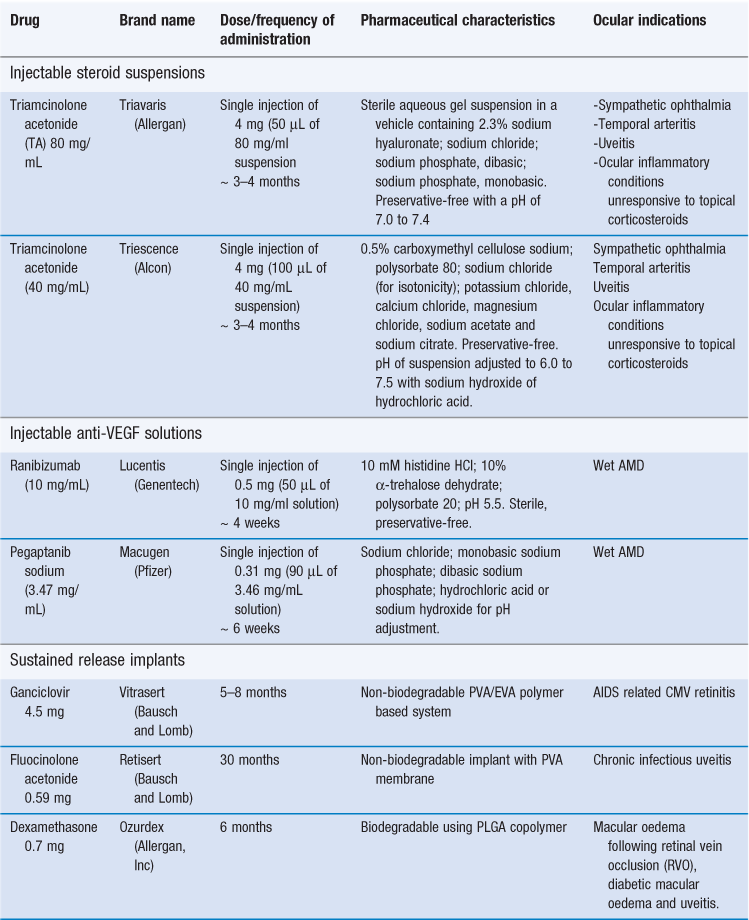
Drug retention in the vitreous space depends on the half-life of the drug which in turn determines the frequency of administration. The half-life of most drugs for the treatment of posterior segment disease ranges from a few hours to a few days. Dexamethasone has a vitreal half-life of 5.5 hours. The half-life of ranibizumab in the vitreous is 3 days and that of pegaptanib is 3–5 days. Triamcinolone acetonide is one of the few exceptions with a long half-life of 18.6 days; the injections are therefore only given every 3–4 months. Triamcinolone acetonide is injected as a suspension and its slow dissolution in the vitreous contributes to this long half-life.
Repeated intravitreal injections cause patient discomfort and associated complications include retinal detachment, endophthalmitis, vitreous haemorrhage and infection. The lens can also be affected and cataracts may form. Although these events have a low incidence they can be sight-threatening. Sustained-release implants are being developed to overcome these problems and to achieve steady concentrations of the drug while minimizing the peaks and troughs in drug levels. Patient compliance is also improved. Table 41.2 summarizes the different approaches for targeting the posterior segment of the eye.
Intraocular implants
Implantable drug delivery systems can be classified into bioerodible and non-bioerodible. In both, drug release kinetics are determined by the polymer system used, drug physicochemical properties and diffusion of the drug through the polymer. Biocompatibility is an essential property for all systems; the components should not interact with the surrounding tissue and should not elicit foreign body reactions through inflammatory or immune responses. Moreover, implants must not be affected by the host and they need to be relatively stable at the implant site. The inside of the eye is a viable location for implantation as evidenced by the use of intraocular lenses which are implanted to replace the clouded over natural lens during cataract surgery.
Non-biodegradable intraocular implants
Non-biodegradable systems are commonly ‘reservoir’ devices, whereby the drug core is coated by a semi-permeable polymer through which the drug can leave. Or the polymer coating may have an opening of a fixed area through which the drug can diffuse out. The other type of non-biodegradable system is the ‘monolithic’ type which is a homogeneous mix of drug and polymer. It is easier, however, to achieve zero-order kinetics from a reservoir system. Vitrasert® (ganciclovir 4.5 mg; Bausch and Lomb) is the first implantable intravitreal device to be available in the clinic and was approved by the FDA in 1996. It is indicated for the local treatment of cytomegalovirus (CMV) retinitis. Ganciclovir is embedded in a polyvinyl alcohol (PVA) and ethylene vinyl acetate (EVA) polymer based system. Drug is slowly released from this implant over 5–8 months. PVA is a hydrophilic polymer acting as the scaffold of the implant as well as controlling the rate of drug diffusion. EVA is hydrophobic polymer used to coat the implant to also control drug diffusion. Fluid imbibes into the implant and dissolves the drug; a saturated solution is formed within the core and drug molecules diffuse out of the system under a concentration gradient. The advantages of this system are that as long as a saturated drug solution remains in the core, the release rate will be constant. Moreover, no initial burst release of drug is observed. Intraocular insertion of the implant requires surgery; a 4–5 mm sclerotomy at the pars plana is necessary for implantation. Further surgery is required to remove the implant devoid of drug. The risks associated with this invasive procedure are vitreous haemorrhage, retinal detachment and endophthalmitis.
Retisert® (fluocinolone acetonide 0.59 mg; Bausch and Lomb) was approved by the FDA in 2005 and is indicated for the treatment of chronic infectious uveitis affecting the posterior segment of the eye. The pure drug is compressed into a 1.5 mm tablet die and coated with a PVA membrane and silicone laminate which has a release orifice. The initial drug release rate is 0.6 µg/day, decreasing over the first month to a steady state of 0.3 to 0.4 µg/day over approximately 30 months. With this course of treatment, uveitis recurrence rates are reduced. However, studies have shown patients to need cataract extraction and intraocular pressure lowering surgery.
Ongoing developments in this area include: i) a fluocinolone acetonide sustained-release implant which can be inserted intravitreally via injection instead of surgery, ii) a helical-shaped device, comprising a non-ferrous metal scaffold coated with a polymer-drug matrix for the delivery of triamcinolone acetonide administered by transconjunctival injection for diabetic macular oedema and iii) an implant containing genetically modified cells which produce growth factors, including ciliary neurotrophic factor. The pore size of the implant allows the growth factors to diffuse outwards into the eye and nutritional molecules to enter but prevents the entry of antibodies or cells that would attack the cells.
Biodegradable intraocular implants
Biodegradable systems are composed of polymers that are metabolized by enzymatic or non-enzymatic (e.g. hydrolysis) reactions in vivo into more soluble forms that can be safely eliminated by the body. Their main advantage over non-biodegradable systems is that they do not have to be removed from the body once the drug has been exhausted. Biodegradable polymers can be made into a variety of shapes and sizes including; pellets, sheets, discs and rods, through different processes. Hot melt extrusion has been used whereby the polymer and drug are subjected to elevated temperature and pressure causing polymer to undergo melting while being simultaneously propelled through a die to form uniform polymer strings or sheets. Solution casting has been used to produce polymer films. This involves forming a homogeneous solution or dispersion of polymer and drug in solvent which is spread onto a flat surface. The solvent is then allowed to evaporate and the dry film is peeled off.
Freeze-drying is another method employed, with the cake formed being subsequently shaped by heating and compression. Developing ocular biodegradable systems is however more complicated as a multitude of factors needs to be taken into consideration including device stability, as well as erosion of the polymer and surface area changes which will affect in vivo kinetics.
Ozurdex® (Allergan) is a dexamethasone (0.7 mg) bioerodible ocular implant with a six month duration of action. It is approved by the FDA for the treatment of macular oedema following retinal vein occlusion (RVO), diabetic macular oedema and uveitis. This implant is based on the copolymer poly(lactic-co-glycolic acid) (PLGA) which has been used for over 30 years in biodegradable sutures for ophthalmic surgery.
PLGA is a copolymer of poly(glycolic acid) (PGA) and polylactic acid (PLA) which are also biodegradable and biocompatible. Hydrolysis of the ester linkage of these polymers converts them to the original monomers, lactic acid and/or glycolic acid which are converted to carbon dioxide and water by the Krebs cycle. PLGA is a versatile copolymer; the lactide: glycolide ratio and the stereoisomeric composition (the amount of L- vs DL-lactide) are the critical factors for PLGA biodegradation as they regulate polymer chain hydrophilicity and crystallinity. A 1 : 1 ratio of lactic acid to glycolic acid provides the fastest biodegradation rate; increasing or decreasing the proportion of either prolongs degradation time.
Several other factors can modulate the degradation behaviour of PLGA and other polyester polymer implants including; morphology of the copolymer (extent of crystallinity), glass transition temperature (which determines if it exists in the glassy or rubbery state), molecular weight and molecular weight distribution (a large molecular weight distribution indicates a relatively large number of carboxylic end groups which expedite autocatalytic degradation of the polymer), porosity of the implant which influences water permeability, implant dimensions (size, shape, surface area), implant composition (acidic or basic drugs and excipients; basic compounds can catalyse ester linkage hydrolysis) as well as physicochemical factors of the environment (ionic composition, strength and pH).
Drug release from PLGA matrix implants can follow pseudo-first order with a triphasic pattern. The first phase is burst release whereby drug at the surface of the implant dissolves creating a high drug release rate over a short period. This burst release is likely to be exacerbated if the system is of large surface area, e.g. microparticles and for systems with a high drug loading and comprising hydrophilic drugs. The second phase is the diffusive phase involving drug dissolution and diffusion out of the matrix down a concentration gradient which is governed by drug solubility in the surrounding fluid. As water penetrates into the core, the implant swells and random hydrolytic cleavage of the polymer chains can also take place. This creates pores which increase the surface area available for drug diffusion. Eventually bulk erosion in the core of the matrix causes the polymer chains to lose their structural integrity and mass loss arises. This third phase results in rapid release of the remaining drug load when hydrolysis of the polymers reaches a threshold. The implant shape changes and it finally fragments. This burst drug release is the main disadvantage of these PLGA biodegradable polymer implants over the non-biodegradable ones. A recent study has combined two PLGA polymers of different molecular weight and in different ratios to achieve a pseudo-first order release with a minimum burst effect. The polymer with the higher molecular weight provides the scaffold for the implant while the lower molecular weight polymer undergoes gradual hydrolysis and regulated drug dissolution and release.
In addition to PLGA, other aliphatic polyesters have also been investigated for their use in ocular implants. Poly(ε-caprolactone)(PCL) is of particular interest as it has a slow rate of degradation and can therefore be used to achieve prolonged drug release over a year or more. It is a semi-crystalline polymer with a melting point between 59 and 64 °C. It is currently in use for sutures, artificial skin support as well as cellular regeneration. In a recent study, triamcinolone acetonide loaded PCL implants were prepared by homogenously mixing the drug and polymer in solvent followed by solvent evaporation. The powder formed was then hot melt extruded into thin filaments using a syringe. The filaments formed were of 150 µm diameter and were cut up into desired lengths of 2 mm. The rods were implanted into the subretinal space and drug release was observed for at least four weeks. An initial phase of fast drug release followed by a pseudo-first order release was observed.
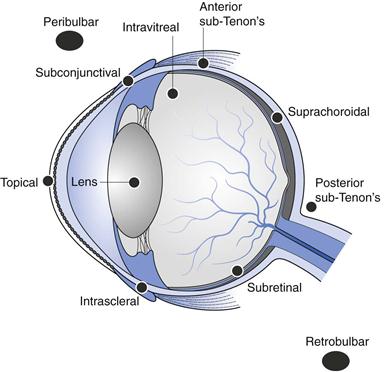
Periocular drug delivery routes
The periocular routes have become increasingly popular for drug delivery to the posterior segment of the eye. Periocular encompasses subconjunctival, subtenon, peribulbar and retrobulbar routes of administration which place the drug in close proximity to the sclera (Fig. 41.5). It is superior in safety compared to the systemic and intravitreal routes due to the lower systemic exposure and risks of injection, respectively. With respect to efficacy, it lies in the middle to low end compared to the other (intravitreal, topical and systemic) delivery routes. It does achieve, however, better bioavailability in the outer and middle regions of the eye and is therefore currently the preferred route for treating diseases of the uveal tract, sclera and cornea. It is also well suited for the treatment of mild to moderate acute posterior segment disease and for preventative drug therapy. The dynamic physiological clearance mechanisms encountered by drugs administered by the periocular compared to the intravitreal route are the subconjunctival-episcleral blood and lymph vessel flow, as well as the choroidal vasculature.
The subconjunctival route bypasses the permeability barrier of the conjunctiva and cornea and can therefore achieve both anterior and posterior drug levels. It is a popular route for the administration of antibiotics, for instance as prophylactic agents in cataract surgery. It is also used for the local delivery of cytotoxic injections of 5-fluorouracil and occasionally mitomycin-C following glaucoma filtration surgery (GFS). In GFS, a sclera flap is created forming a new channel for outflow of aqueous humour to reduce the intraocular pressure. As is the case with all surgeries, a wound healing response arises resulting in the formation of scar tissue. Here, scar tissue forms in the subconjunctival space which if excessive can gradually reduce and block the aqueous drainage resulting in a rise in intraocular pressure. This makes it necessary to administer cytotoxics repeatedly following surgery for the prevention of scarring.
Sub-tenon injection is between the sclera and Tenon’s capsule. The Tenon’s capsule is a sheet of connective tissue between the eyeball (globe) and socket (orbit) which provides a smooth socket allowing free movement of the globe. This route allows a prolonged contact time of the drug with the sclera. The sclera has a relatively large surface area of 16.3 cm2 and high permeability relative to the cornea. Molecules of size up to 70 000 Da have been shown to readily permeate through it. Drug permeability through the sclera is inversely proportional to molecular size. Although no clear correlation exists between drug lipophilicity and the steady-state permeability coefficient through the sclera, drugs with higher lipophilicities exhibit stronger binding to the sclera and longer transport lag times. Injection via the subtenon route is used to administer local anaesthetics, corticosteroids and anti-cancer agents.
Intravitreal pharmacokinetics
Drugs administered into the vitreous can be cleared by two routes: the anterior and posterior. The anterior route is where the drug diffuses into the anterior chamber and leaves with the aqueous humour, via the canal of Schlemn. The posterior route is across the retinal surface. Physicochemical properties that influence drug clearance are: molecular weight (MW), compound lipophilicity (measured by log P or log D) and dose number (DN=dose/solubility at pH 7.4).
Log MW has been found to positively correlate with the vitreal half-life of molecules. This can be explained by the slow diffusion of high MW compounds in the vitreous gel as well as the observation that high MW compounds are predominantly eliminated through the longer anterior pathway. Log D and log P correlate negatively with the vitreal half-life of the drug. Lipophilic compounds have a shorter half-life compared to hydrophilic ones. It has been proposed that hydrophilic molecules are eliminated by the anterior route, while lipophilic molecules are eliminated by the posterior route. The posterior route is the main elimination pathway for lipophilic drugs and offers a large surface area and active transporter mechanisms, thus providing a faster route of elimination compared to the anterior route. Dose number positively correlates with the vitreal half-life of molecules. If the dose administered exceeds the solubility of the molecule and is administered as a suspension, then the drug will need to undergo dissolution before it is absorbed and/or cleared, thus prolonging its half-life. This is the case for the administration of triamcinolone acetonide.
Problems with traditional and new ocular drug delivery systems
Deposits of triamcinolone acetonide particles and crystals have been identified in the vitreous and retina of patients who have been treated with intravitreal triamcinolone acetonide injections. These deposits have been observed in patients, months and even a couple of years, after the last administration of a triamcinolone injection. It is speculated that these insoluble deposits arise from aggregation or clumping of drug particles. It could even be that a polymorphic conversion of the drug occurs in the ocular fluids, resulting in an extremely stable, and therefore insoluble, form of triamcinolone which persists in the posterior segment of the eye.
Countless number of intraocular implants have been shown to perform successfully in vitro during preclinical development, however very few perform satisfactorily in vivo, and make it to the clinic. One of the main reasons for this failure is non-biocompatibility which triggers the formation of a thick fibrotic capsule around implants. This fibrotic tissue is an amalgamation of cells, fibrinogen, collagen fibres and other proteins. It is predominantly an inflammatory response, orchestrated by interleukins and TGFs synthesized by epithelial cells. This collagenous fibrotic tissue creates a diffusion barrier for drug molecules and retards biodegradation of the implant. Foreign body reactions are largely influenced by the surface properties of the implant, including contact angle, surface functional groups, water-polymer interactions, roughness, morphology, porosity and contact duration.
Repeated intravitreal injections are associated with increased risk of scleral damage and ocular infection, increase in intraocular pressure, incision-related subconjunctival and intravitreal haemorrhage as well as discomfort associated with foreign body sensation and pain in the patient.
A thorough characterization of the compatibility of excipients in the formulation with the active compound is absolutely necessary. Benzalkonium chloride is cationic and therefore is incompatible with anionic drugs. Its activity, and consequently preservative efficacy, is reduced in the presence of multivalent metal ions and anionic and non-ionic surfactants. Despite these interactions of benzalkonium chloride with drugs and other excipients, it may still be necessary to use in some cases. Examples of these include sodium cromoglicate and nedocromil sodium which form insoluble emulsion complexes with benzalkonium chloride through ion pairing. This insoluble complex is, however, removed by filtration during the manufacturing process.
Patient compliance and instillation of eye drops
The short half-life of drugs in the anterior chamber requires the frequent administration of eye drops. This presents problems with patient adherence to treatment regimens. Studies have demonstrated that almost 50% of glaucoma patients were found to be non-adherent to their medication for over 75% of the time. Eye drops are challenging to administer and require coordination, manual dexterity and vision, necessitating clear instructions and patient counselling. Administration is particularly difficult for elderly patients. Most glaucoma patients are older individuals and a study has shown that 17% of glaucoma sufferers rely on others to administer their eye drops. Dose timing and frequency have been strongly associated with non-adherence to eye drop therapy. Patients on a three times daily regimen were more likely to experience missed doses and also had irregular timing of doses compared to patients on twice daily regimens. This illustrates the importance of designing ophthalmic preparations that provide a sustained drug release and which require less frequent dosing.
References
1. Daniels J, Dart J, Tuft S, Khaw PT. Corneal stem cells in review. Wound Repair and Regeneration. 2001;9:483–493.
2. Sieg JW, Robinson JR. Vehicle effects on ocular drug bioavailability I Evaluation of fluoromethalone. Journal of Pharmaceutical Sciences. 1975;64:931–936.
3. Willoughby C, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function – a review. Clinical and Experimental Ophthalmology. 2010;38:2–11.
Bibliography
1. Bravo-Osuna I, Noiray, Briand E, et al. Interfacial interactions between transmembrane ocular mucins and adhesive polymers and dendrimers analyzed by surface plasmon resonance. Pharmaceutical Research. 2012;29:2329–2340.
2. del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discovery Today. 2008;13:135–143.
3. Durairaj C, Shah JC, Senapati S, Kompella UB. Prediction of vitreal half-life based on drug physicochemical properties: quantitative structure-pharmacokinetic relationships (QSPKR). Pharmaceutical Research. 2009;26:1236–1260.
4. Durrani AM, Farr SJ, Kellaway IW. Influence of molecular weight and formulation pH on the precorneal clearance rate of hyaluronic acid in the rabbit eye. International Journal of Pharmaceutics. 1995;118:243–250.
5. Edman P. Biopharmaceutics of Ocular Drug Delivery. Boca Raton, Florida: CRC Press; 1993.
6. Furrer P, Delie F, Plazonnet B. Ophthalmic drug delivery. In: Rathborne MJ, Hadgraft J, Roberts M, Lane M, eds. Modified-Release Drug Delivery Technology. New York: Informa Healthcare; 2008.
7. Gaudana R, Jwala J, Boddu SH, Mitra A. Recent perspectives in ocular drug delivery. Pharmaceutical Research. 2009;26:1197–1216.
8. Ghate D, Edelhauser H. Ocular drug delivery. Expert Opinion in Drug Delivery. 2006;3:275–287.
9. Guo X, Chang R-K, Hussain MA. Ion-exchange resins as drug delivery carriers. Journal of Pharmaceutical Sciences. 2009;98:3886–3902.
10. Lee S, Hughes P, Ross A, Robinson M. Biodegradable implants for sustained drug release in the eye. Pharmaceutical Research. 2010;27:2043–2053.
11. Mannermaa E, Vellonen K-S, Urtti A. Drug transport in corneal epithelium and blood-retinal barrier: Emerging role of transporters in ocular pharmacokinetics. Advanced Drug Delivery Reviews. 2006;58:1136–1163.
12. Robinson J, Mlynek G. Bioadhesive and phase-change polymers for ocular drug delivery. Advanced Drug Delivery Reviews. 1995;16:45–50.
13. Shirasaki Y. Molecular design for enhancement of ocular penetration. Journal of Pharmaceutical Sciences. 2008;97:2462–2496.
14. Weiner A. Drug delivery systems in ophthalmic applications. In: Wax M, Clark A, Yorio T, eds. Ocular Therapeutics: Eye on New Discoveries. New York: Academic Press; 2008.