Chapter 9 Ocular Adnexa and Lacrimal System
Eyebrows
The eyebrows consist of thick skin covered by characteristic short, prominent hairs extending across the superior orbital margin, usually arching slightly but sometimes merely running horizontally. Generally, in men the brows run along the orbital margin, whereas in women the brows run above the margin.1 The first body hairs produced during embryologic development are those of the eyebrow.2
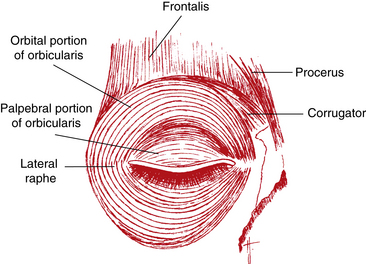
The muscles located in the forehead—the frontalis, procerus, corrugator superciliaris, and orbicularis oculi—produce eyebrow movements, an important element in facial expression (Figure 9-1). The frontalis muscle originates high on the scalp and inserts into connective tissue near the superior orbital rim. The fibers are oriented vertically and raise the eyebrow, causing a look of surprise or attention. The corrugator originates on the frontal bone and inserts into skin superior to the medial eyebrow. It is characterized as the muscle of trouble or concentration, and its fibers are oriented obliquely; it moves the brow medially, toward the nose, creating vertical furrows between the brows. The procerus, the muscle of menace or aggression, originates on the nasal bone and inserts into the medial side of the frontalis. It pulls the medial portion of the eyebrow inferiorly and produces horizontal furrows over the bridge of the nose.2,3 The orbicularis oculi (described later) lowers the entire brow. The fibers of these muscles blend with one another and are difficult to separate.2 All are innervated by the facial nerve—cranial nerve VII.
Eyelids
Clinical Comment: Lagophthalmos
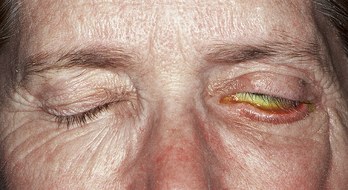
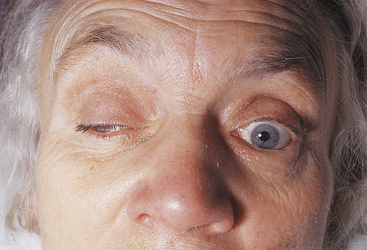
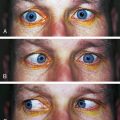
LAGOPHTHALMOS refers to incomplete closure of the eyelids (Figure 9-2). Its cause may be physiologic, mechanical (e.g., scarring), or paralytic. Lagophthalmos is most evident during sleep, when drying of the inferior cornea may result. Scratchy, irritated eyes are evident on awakening, and punctate keratitis can occur.4–6 Clinical assessment of the inferior cornea will show varying degrees of epithelial disruption, manifested as staining with fluorescein dye.
Palpebral Fissure
The palpebral fissure is the area between the open eyelids. Although numerous variations exist in the positional relationship of the lid margins to the limbus, generally the upper lid just covers the superior limbus when one’s eyes are open and looking straight ahead. The lower lid position is more variable, usually lying within 1 mm of the inferior limbus.7–9
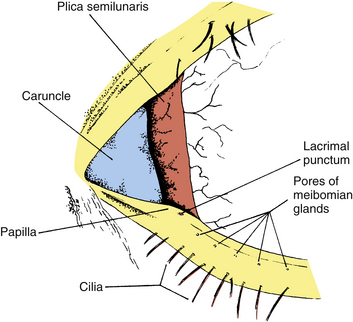
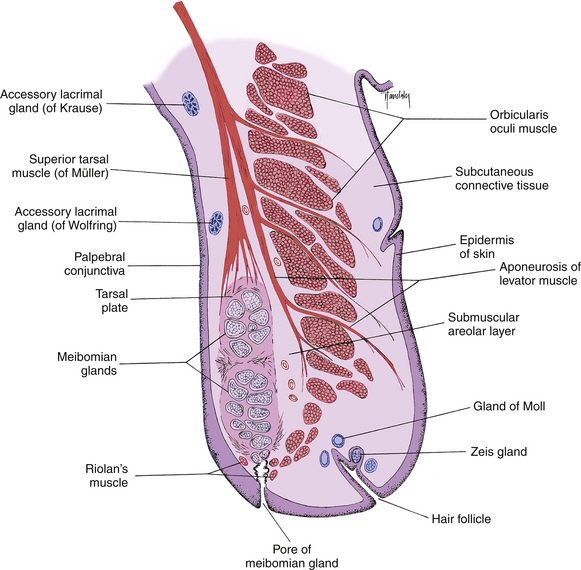
The upper and lower eyelids meet at the corners of the palpebral fissure in the lateral and medial canthi. The lateral canthus is located approximately 5 to 7 mm medial to the bony orbital margin and lies directly on the globe.9 The medial canthus is at the medial orbital margin but is separated from the globe by a reservoir for the pooling of tears, the lacrimal lake. The floor of the lacrimal lake is the plica semilunaris (Figure 9-3). This narrow, crescent-shaped fold of conjunctiva, located in the medial canthus, allows for lateral movement of the eye without stretching the bulbar conjunctiva. The caruncle is a small, pink mass of modified skin located just medial to the plica. It is covered with epithelium that contains goblet cells and fine hairs and their associated sweat and sebaceous glands.
Eyelid Topography
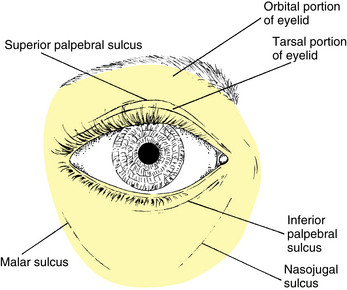
The upper eyelid extends to the eyebrow and is divided into the tarsal and the orbital (or preseptal) parts. The tarsal portion lies closest to the lid margin, rests on the globe, and contains the tarsal plate. The skin is thin, and the underlying loose connective tissue is devoid of adipose tissue. The orbital portion extends from the tarsus to the eyebrow, and a furrow—the superior palpebral sulcus—separates the tarsal portion from the orbital portion (Figure 9-4). This sulcus separates the pretarsal skin, which is tightly adherent to the underlying tissue, from the preseptal skin, which is only loosely adherent to its underlying tissue, which contains a cushion of fat. In the eyelids of those of Eastern Asian descent, the orbital septum fuses with the levator aponeurosis below the upper tarsal border, allowing the fat to descend further into the lid10 and eliminating the superior palpebral sulcus.2,11–13
In the lower eyelid the inferior palpebral sulcus, which separates the lower lid into tarsal and orbital parts, is often not very distinct. The tarsal portion rests against the globe, and the orbital portion extends from the lower border of the tarsus onto the cheek, extending just past the inferior orbital margin to the nasojugal and malar sulci (see Figure 9-4). These furrows occur at the attachment of the skin to the underlying connective tissue and become more prominent with age.
Eyelid Margin
The eyelid margin rests against the globe and contains the eyelashes and the pores of the meibomian glands. The cilia (eyelashes) are arranged at the lid margin in a double or triple row, with approximately 150 in the upper eyelid and 75 in the lower lid.14 The lashes curl upward on the upper and downward on the lower lid. Replacement lashes grow to full size in approximately 10 weeks, and each lash is replaced approximately every 5 months.9 The eyelashes are richly supplied with nerves, causing them to be sensitive to even the slightest unexpected touch, which will elicit a protective response—a blink.
Clinical Comment: Abnormalities Affecting the Cilia
Various epithelial diseases can cause madarosis (loss of eyelashes) or trichiasis (misdirected growth of eyelashes), in which the eyelashes grow toward rather than away from the palpebral fissure. Contact with the cornea can cause irritation and painful abrasions and can lead to ulceration.4 The problem lashes can be removed by epilation.
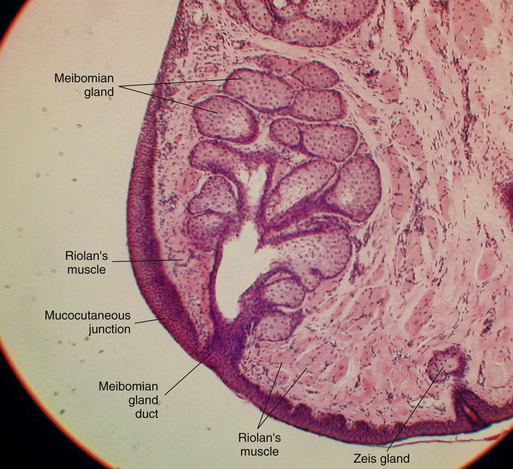
The pores of the meibomian glands are located posterior to the cilia (see Figure 9-3), and the transition from skin to conjunctiva, the mucocutaneous junction, occurs just posterior to these openings.15 A groove called the gray line runs along the eyelid margin between the cilia insertions and the pores of the meibomian glands. This groove is the location of a surgical plane that divides the lid into anterior and posterior portions.2
The eyelid margin can be divided into two parts: the medial one sixth is the lacrimal portion, and the lateral five sixths is the ciliary portion. The division occurs at the lacrimal papilla, a small elevation containing the lacrimal punctum, the opening that carries the tears into the nasolacrimal drainage system (see Figure 9-3). Usually, no cilia or meibomian pores are found medial to the punctum, in the lacrimal portion of the lid margin.
Clinical Comment: Epicanthus
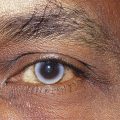
EPICANTHUS is a vertical fold of skin at the nasal canthus, arising in the medial area of the upper eyelid and terminating in the nasal canthal area. It is common in the newborn and may cause the appearance of esotropia (Figure 9-5). A parent of an infant with epicanthus might worry that the child’s eyes are crossed; however, a cover test will identify a true esotropia. As the bridge of the nose develops, epicanthus gradually disappears. A form of epicanthus arising from the tarsal fold and extending into the medial canthal area is common in those of Eastern Asian descent.2
Eyelid Structures
Orbicularis Oculi Muscle
Palpebral Portion
The palpebral portion of the orbicularis oculi muscle occupies the area of the eyelid that rests on the globe and is closest to the eyelid margin. It sometimes is divided further into pretarsal and preseptal parts. The palpebral portion is composed of semicircles of muscle fibers that run from the medial orbital margin and the medial palpebral ligament16 to the lateral palpebral raphe, where the superior and inferior fibers interdigitate with one another (see Figure 9-1). The lateral palpebral raphe overlies the lateral palpebral ligament.
Some fibers arise from deeper attachments on the posterior lacrimal crest. This section of the palpebral part of the orbicularis, the muscle of Horner or lacrimal part (pars lacrimalis), encircles the lacrimal canaliculi.9,11,16,17 Contraction of the orbicularis assists in moving tears through the canaliculi into the nasolacrimal drainage system.18 Another section of the palpebral orbicularis, the muscle of Riolan or ciliary part (pars ciliaris), lies near the lid margin on both sides of the meibomian gland openings; it maintains the lid margins close to the globe.19
Clinical Comment: Ectropion and Entropion
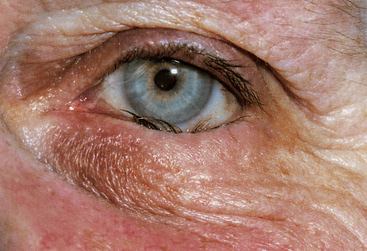
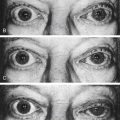
Eversion of the eyelid margin is called ectropion (Figure 9-6), the common cause of which is loss of muscle tone, a normal occurrence in the aging process. As the lid margin falls away from its position against the globe, the lacrimal punctum is no longer in position to drain the tears from the lacrimal lake. Epiphora, an overflow of tears onto the cheek, may occur, causing maceration of the delicate skin in this area.
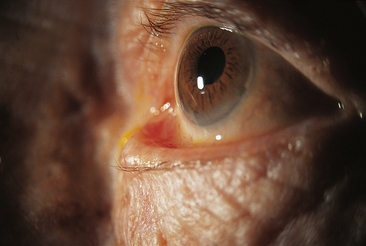
FIGURE 9-6 Severe involutional (senile) ectropion.
(From Kanski JJ: Clinical ophthalmology: a systematic approach, ed 5, Oxford, UK, 2003, Butterworth-Heinemann.)
Inversion of the lid margin is called entropion and may result from spasm of the orbicularis oculi muscle causing the lid margin to turn inward (Figure 9-7). This inward turning puts the eyelashes in contact with the globe and, unless relieved, can cause corneal abrasion. Scarring of the lid after trauma or disease may also cause entropion. Both ectropion and entropion are more common in the lower lid and can be corrected surgically, if necessary. The anatomic relationship of the muscular and connective tissue components is an important consideration when repair is done.20–22
Orbital Portion
The orbital portion of the orbicularis oculi muscle is attached superiorly to the orbital margin, medial to the supraorbital notch. The fibers encircle the area outer to the palpebral portion and attach inferiorly to the orbital margin, medial to the infraorbital foramen.2 These concentric circular fibers extend throughout the rest of the lid and over the orbital rim.
Orbicularis Action
The orbicularis oculi muscle is innervated by cranial nerve VII (the facial nerve). Contraction of the palpebral portion closes the eyelid gently, and the palpebral orbicularis is the muscle of action in an involuntary blink and a voluntary wink; relaxation of the levator muscle follows.23 Spontaneous involuntary blinking renews the precorneal tear film. A reflex blink is protective and may be elicited by a number of stimuli—a loud noise; corneal, conjunctival, or cilial touch; or the sudden approach of an object. When the orbital portion of the orbicularis contracts, the eye is closed tightly, and the areas surrounding the lids—the forehead, temple, and cheek—are involved in the contraction. Such eyelid closure is often a protective mechanism against ocular pain or after injury, and is called reflex blepharospasm. If the lids are closed tightly in a strong contraction, forces compressing the orbital contents can significantly increase the intraocular pressure.24
Superior Palpebral Levator Muscle
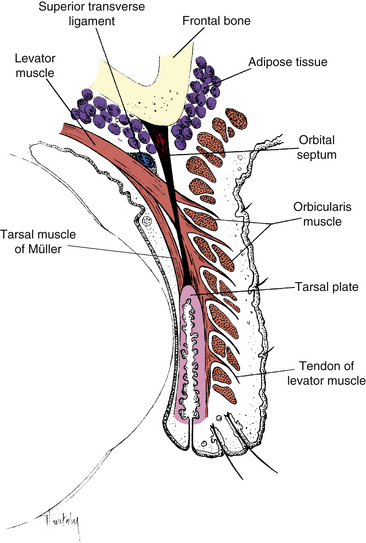
The superior palpebral levator muscle, the retractor of the upper eyelid, is located within the orbit above the globe and extends into the upper lid. It originates on the lesser wing of the sphenoid bone above and in front of the optic foramen, and its sheath blends with the sheath of the superior rectus muscle. As the levator approaches the eyelid from its posterior origin at the orbital apex, a ligament, the superior transverse ligament (Whitnall’s ligament) may act as a fulcrum, changing the anteroposterior direction of the levator to superoinferior10,12,25–28 (Figure 9-8). The superior transverse ligament is a fibrous band that spans the anterior superior orbit from the trochlea to the lacrimal gland fascia. It provides support for the upper lid and orbital structures as well as acting as a fulcrum. The ligament is located at the point where the levator muscle fibers end and the aponeurosis begins.29
Levator Aponeurosis
As it enters the eyelid, the levator becomes a fan-shaped tendinous expansion, the levator aponeurosis. Unlike a typical tendon, the aponeurosis spreads out into an extensive sheet posterior to the orbital septum. The fibers of the aponeurosis penetrate the orbital septum and extend into the upper lid, fanning out across its entire width. These tendinous fibers pass through the submuscular connective tissue; the posterior fibers insert into the lower anterior surface of the tarsal plate, and the anterior fibers run between the muscle bundles of the orbicularis to insert primarily into the skin of the eyelid, although some insert into the intermuscular septa of the orbicularis9,11,25–28 (see Figure 9-8). This attachment of the fibers from the levator aponeurosis anchors the skin to the underlying tissues in the pretarsal area of the eyelid and creates the palpebral sulcus. In those of Eastern Asian descent, the orbital septum attaches to the tarsal plate more inferiorly, and the aponeurotic fibers do not attach as extensively to the cutaneous tissue.2,11
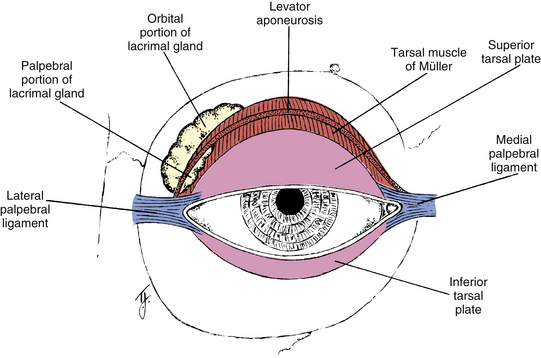
The two side extensions of the aponeurosis are referred to as horns. The lateral horn helps to support the lacrimal gland by holding it against the orbital roof, dividing the gland into orbital and palpebral lobes (Figure 9-9). The lateral horn then attaches to the lateral palpebral ligament and lateral orbital tubercle. The medial horn is attached to the medial palpebral ligament and medial orbital rim.
Levator Action
Contraction of the levator muscle causes elevation of the eyelid. The connection between the sheath of the levator and sheath of the superior rectus coordinates eyelid position with eye position so that as the eye is elevated, the lid is raised.27,28 The levator is innervated by the superior division of the oculomotor nerve, cranial nerve III.
The eyelids are closed by relaxation of the levator and contraction of the orbicularis oculi muscles. The tonic activity of the levator and the relaxation of the orbicularis holds the eyelid open. In a blink, tonic activity of the levator is suspended, and with a burst of activity the orbicularis rapidly lowers the lid, followed by a cessation of orbicularis activity and resumption of levator tonicity.30
Retractor of Lower Eyelid
The retractor of the lower lid is the capsulopalpebral fascia (lower eyelid aponeurosis).2,10 The capsulopalpebral fascia, an anterior extension from the sheath of the inferior rectus muscle and the suspensory ligament, inserts into the inferior edge of the tarsal plate.11,27,28 This insertion coordinates lid position with globe movement. The lower eyelid is depressed on globe depression, and the lower eyelid elevates slightly on upward movement of the globe.8 The capsulopalpebral fascia also fuses with the orbital septum and sends some fibers to insert into the inferior fornix.2,21,28
Tarsal Muscle (of Müller)
The superior tarsal muscle (muscle of Müller) is composed of smooth muscle and originates on the posteroinferior aspect of the levator muscle. These smooth muscle fibers begin to appear within the striated muscle at the point at which the muscle becomes aponeurotic.2,9,27,28 The superior tarsal muscle inserts on the superior edge of the tarsal plate (see Figures 9-8 and 9-9). Contraction of Müller’s muscle can provide 2 mm of additional lid elevation.10
A similar smooth muscle, the inferior tarsal muscle, is found in the lower eyelid. It arises from the inferior rectus muscle sheath and inserts into the lower conjunctiva and lower border of the tarsal plate.2,9,24 Investigators disagree about whether the inferior tarsal muscle actually inserts into the tarsal plate2,9,27 or inserts into the tissue below the tarsal plate.31 Both tarsal muscles are innervated by sympathetic fibers that widen the palpebral fissure when activated (as in situations associated with fear or surprise).9,28
Clinical Comment: Ptosis
PTOSIS is a condition in which the upper eyelid droops or sags. It can be caused by weakness or paralysis of the levator or Müller’s muscle. If Müller’s muscle alone is affected, a less noticeable form of ptosis occurs than when the levator is involved.32 An individual with ptosis might attempt to raise the lid by using the frontalis muscle, which results in elevation of the eyebrow and wrinkling of the forehead (Figure 9-10).
Tarsal Plate
Each eyelid contains a tarsal plate (tarsus) that gives the lid rigidity and structure and shapes it to the curvature of the globe. The tarsal plate in the upper lid is approximately 11 mm high, and the inferior tarsal plate is approximately 5 mm high.9 The anterior surface is adjacent to the submuscular connective tissue. The posterior surface is adherent to the palpebral conjunctiva. The orbital border of the tarsus is attached to the orbital septum, whereas the marginal border lies at the lid margin. The sides of the tarsal plates are attached to the bony orbital margin by the palpebral or tarsal ligaments (see Figure 9-9).
Palpebral Ligaments
The palpebral or tarsal ligaments are bands of dense connective tissue connecting the tarsal plates to the orbital rim and holding the tarsal plates in position against the globe during eye and lid movements. The medial palpebral ligament runs from the medial edge of each tarsal plate to the medial orbital rim, where it divides into two limbs. One limb attaches to the posterior lacrimal crest and the other to the anterior lacrimal crest. Both limbs lie anterior to the orbital septum33 (see Figure 8-18).
The lateral palpebral ligament is located posterior to the orbital septum and attaches the lateral edges of the tarsal plates to the lateral orbital margin at the lateral orbital tubercle34 (see Figure 8-18). Fibrous connections between the lateral palpebral ligament and the check ligament for the lateral rectus muscle allow a slight lateral displacement of the lateral canthus with extreme abduction.35
The upper borders of both the medial and the lateral ligaments are joined to the expansion of the levator tendon, and their lower borders are joined to an expansion of the ligament of Lockwood.9 The connective tissue structures at the canthi have been described as a retinaculum that is made up of the palpebral ligaments, the horns of the levator aponeurosis, the suspensory ligament, the check ligaments, and the superior transverse ligament.2
Glands of the Lids
The meibomian glands (tarsal glands) are sebaceous glands embedded in the tarsal plate. These long, multilobed glands resemble a large bunch of grapes and are arranged vertically such that their openings are located in a row along the lid margin posterior to the cilia (Figure 9-11). Approximately 30 to 40 meibomian glands are found in the upper lid and 20 to 30 in the lower lid.14 On eyelid eversion the vertical rows of the meibomian glands can sometimes be seen as yellow streaks through the palpebral conjunctiva. These glands secrete the outer lipid layer of the tear film.
Clinical Comment: Contact Lens Wear
Some studies have identified a loss in both the number and the length of meibomian glands in the contact lens wearer (Figure 9-12). Loss does not appear to be dependent on the type of lens but rather on the duration of wear and is speculated to be due to chronic irritation.36
The sebaceous glands of Zeis secrete sebum into the hair follicle of the cilia, coating the eyelash shaft to keep it from becoming brittle.9
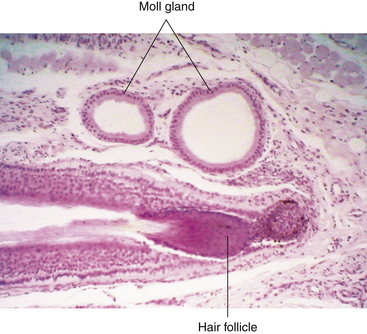
The glands of Moll have been called modified sweat glands but are more accurately described as specialized apocrine glands.37 They are located near the lid margin and their ducts empty into the hair follicle, into the Zeis gland duct, or directly onto the lid margin. Similar glands found in the axillae are scent organs, but that is likely not the function of the Moll glands.9,14
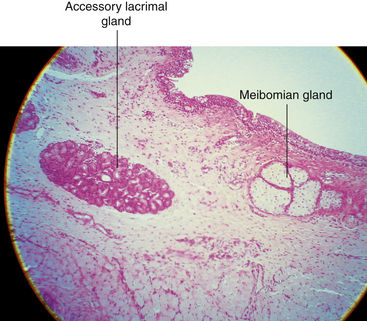
The accessory lacrimal glands of Krause are located in the stroma of the conjunctival fornix, and the accessory lacrimal glands of Wolfring are located along the orbital border of the tarsal plate2,9 (see Figure 9-11). These glands are oval and display numerous acini. In the upper fornix, 20 to 40 glands of Krause are found, although only six to eight such glands appear in the lower fornix.2 The glands of Wolfring are less numerous. The secretion of the accessory lacrimal glands appears similar to that of the main lacrimal gland and contributes to the aqueous layer of the tear film.
Histologic Features
Skin
The skin of the eyelid contains many fine hairs, sebaceous glands, and sweat glands. It is the thinnest skin in the body, easily forms folds and wrinkles, and is almost transparent in the very young.2 The epidermal layer consists of a basal germinal layer, a granular layer, and a superficial layer that is cornified. The underlying dermis is abundant in elastic fibers. A very sparse areolar connective tissue layer, the subcutaneous tissue, lies below the dermis. This thin layer is devoid of adipose tissue in the tarsal portion. A pad of fat often is located in this region in the orbital portion that separates the orbicularis from the skin.9
Muscles
The orbicularis oculi lies deep to the subcutaneous layer. These striated muscle bundles run throughout the eyelid. In a sagittal section of the lid prepared for microscopic examination, the orbicularis bundles are cut in cross section (Figure 9-13). Along the lid margin, small muscle bundles located on both sides of the meibomian gland represent a specific part of the orbicularis, the ciliary part (Riolan’s muscle), which holds the lid margin against the globe (see Figure 9-11).
Posterior to the orbicularis lies another layer of loose connective tissue, the submuscular areolar layer, which separates the muscle from the tarsal plate. Between this layer and the tarsal plate is a potential space, the pretarsal space, that contains the vessels of the palpebral arcades. The preseptal space is located between the orbicularis and the orbital septum; directly above is the preseptal cushion of fat.9
Tendinous fibers of the levator aponeurosis run through the submuscular tissue layer between the orbicularis and the superior tarsal muscle to insert into the tarsal plate and the skin of the lid (see Figure 9-11). It is this insertion of fibers that anchors the skin so firmly in the tarsal portion of the lid. There is no such attachment of the aponeurosis in the preseptal area. The smooth muscle fibers of the superior tarsal muscle are located above the superior tarsal plate and insert into its upper edge. Both the aponeurosis and the superior tarsal muscle are cut longitudinally in a microscope slide of a sagittal section of the lid.
Palpebral Conjunctiva
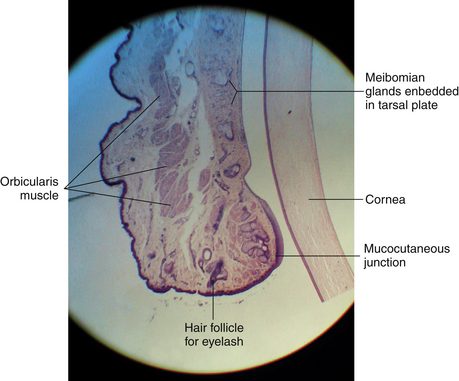
The palpebral conjunctiva is composed of two layers, a stratified epithelial layer and a connective tissue stromal layer, the submucosa. The epithelial layer of the conjunctiva is continuous with the skin epithelium at the mucocutaneous junction of the lid margin (see Figure 9-12). As the conjunctiva lines the lid, squamous cells are replaced by cuboidal and columnar cells, forming a stratified columnar mucoepithelial layer, the granular and keratinized layers having been discontinued.14,38 At the mucocutaneous junction, the epithelial layer is approximately five cells thick and may be a location for stem cells that repopulate the palpebral conjunctival epithelium.39 Over much of the upper lid the conjunctival epithelium is two or three cells thick, whereas over much of the lower lid, the epithelium is three or four cells thick.9 This stratified columnar epithelium continues throughout the fornices into the bulbar conjunctiva, where it changes to a stratified squamous layer near the limbus, becoming continuous with the corneal epithelium.
The surface of the superficial conjunctival cells and their microvilli and microplicae are covered with a glycocalyx similar to that of the corneal surface.40,41 Melanin granules often are found in the cytoplasm of conjunctival epithelial cells, especially near the limbus; these are particularly prevalent in individuals with heavily pigmented skin. Goblet cells, which produce the mucous component of the tear film, also are located in the epithelium. Subsurface vesicles, found below the outer membrane of the superficial conjunctival cell, may be an additional source of mucous material. As these vesicles fuse with the epithelial cell membrane, chains extend outward to form a chemical bond with the mucous layer secreted by the goblet cells. These chains increase the adherence of the tear film to the globe. These vesicle membranes may also contribute to the microvilli present on the surface epithelial cells.42
The goblet cells, which produce, store, and secrete the innermost mucous layer of the tear film, are scattered throughout the stratified columnar conjunctival epithelium (Figure 9-14). These cells are most numerous in the inferior nasal aspect of the tarsal conjunctiva.43 Their number decreases with advancing age and increases in inflammatory conditions. A goblet cell produces mucin droplets that accumulate, causing the cell to swell and become goblet shaped. The surface of the cell finally ruptures, releasing mucus. Parasympathetic and sympathetic nerves have been associated with goblet cells and may play a role in their secretion.44 Invaginations of conjunctival epithelium, often located near the fornix, are called crypts of Henle. Goblet cells release their mucus into the cavity formed by these invaginations, and the mucus may become trapped if the opening to the crypt is narrow. This accumulation of mucoid material may account for the application of the misnomer “glands” of Henle to describe these structures.
Clinical Comment: Vitamin A Deficiency
VITAMIN A DEFICIENCY has been associated with a loss of goblet cells. In dry-eye disorders showing a decrease in the number of goblet cells, treatment with vitamin A therapy can induce the reappearance of goblet cells.45 In acute disease cellular proteins may be activated causing keratinization of the surface epithelia.46
The submucosa (stroma, substantia propria) of the palpebral conjunctiva is very thin in the tarsal portion of the eyelid but becomes increasingly thick in the orbital portion. It is composed of loose, vascularized connective tissue that can be subdivided into an outer lymphoid layer and a deep fibrous layer. In addition to the normal connective tissue components (collagen fibrils, fibroblasts, ground substance, and a few fine elastic fibers), the lymphoid layer contains macrophages, mast cells, polymorphonuclear leukocytes, eosinophils, accumulations of lymphocytes, and occasional Langerhans cells.47 Immunoglobulin A (IgA) is found in the lymphoid layer, making the conjunctiva an immunologically active tissue.48–50 More lymphoid tissue is found in palpebral conjunctiva than in bulbar conjunctiva.51
Clinical Comment: Conjunctival Cysts and Concretions
CLEAR CONJUNCTIVAL CYSTS, either intraepithelial or subepithelial, are filled with mucoid material and are found most often in the palpebral conjunctiva.52 Conjunctival concretions are small, yellow-white nodules about the size of a pinhead and most often are located in the tarsal conjunctiva. They are composed of fine granular material and membranous debris, products of cellular degeneration. These nodules are hardened but contain no calcium deposits.53 Concretions are found more often in elderly patients and can be removed if they produce foreign body irritation.50
Glands
The meibomian glands are large sebaceous glands occupying the length of the tarsal plate. Each consists of 10 to 15 lobes or acini attached to a large central duct.54,55 The duct is arranged vertically such that the opening is located at the edge of the tarsal plate corresponding to the eyelid margin (Figure 9-15).
Meibomian glands are holocrine glands; their secretion is produced by the decomposition of the entire cell. Each acinus is surrounded by a layer of myoepithelial cells and is filled with actively dividing cells. The daughter cells become large and polyhedral, they begin to synthesize lipids and fill with lipid droplets.56 As each cell degenerates, the nucleus begins to diminish in size, and the cell membrane disintegrates.
Cells in varying stages of decomposition pack each saccule. Decomposed cells move down the duct toward the opening. The pressure exerted by a blink releases the secretion into the tear film,57 at which point the secretion (lipid droplets and cell debris) forms the outermost lipid layer of the tear film.15,54,55,58 The predominant innervation of meibomian glands is parasympathetic and may act to alter the lipid production or cause cell rupture.59,60
The secretion of the meibomian glands has been called meibum to distinguish it from sebum secreted by the sebaceous glands of the skin and hair follicles. Meibum is much more viscous than sebum; sebum is more polar and if mixed with the tear film will contaminate and disrupt it.61
Histologically, the sebaceous Zeis glands are similar to the meibomian glands. The Zeis glands, however, are composed of just one or two acini and are associated with the eyelash follicle (Figure 9-16). Generally, two Zeis glands are present per follicle. They release sebum into the follicle, thereby preventing the cilia from becoming dry and brittle.54
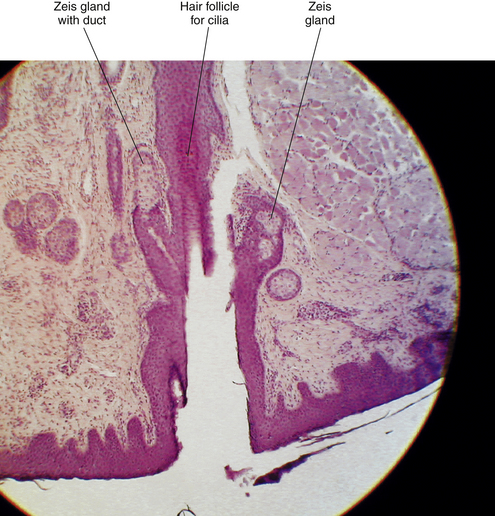
FIGURE 9-16 Light micrograph of lid margin. Zeis gland is located next to hair follicle; duct is evident.
Glands of Moll, modified apocrine glands, are also located near the eyelash follicle. They consist of a spiral that begins as a large cavity, the neck of which becomes narrow as it forms a duct. The large lumen often appears empty and is surrounded by a layer of cuboidal to columnar secretory cells (Figure 9-17).54 Myoepithelial cells surround these cells. As the Moll gland is an apocrine gland, its secretion is composed not of the whole cell but of parts of cellular cytoplasm. The duct might empty into the duct of a Zeis gland, or it might open directly onto the lid margin between cilia.37 Recent histochemical studies have identified antimicrobial peptides and proteins in Moll gland secretions that suggest a role in immune defense protecting the lash shaft and ocular surface.37,62
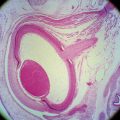
Accessory lacrimal glands are groups of secretory cells with a truncated-pyramid shape arranged in an oval pattern around a central lumen38 (Figure 9-18). The acini are surrounded, sometimes incompletely, by a row of myoepithelial cells.2 Animal studies suggest that the ducts of Wolfring glands have a tortuous course and open onto the palpebral conjunctiva.63 These are merocrine glands—that is, the cell remains intact and secretes a product—and they have the same histologic makeup as the main lacrimal gland.63 The secretion contains antibacterial agents, lysozyme, lactoferrin, and immunoglubulins.58 The accessory lacrimal glands are densely innervated, as is the main lacrimal gland.64
Clinical Comment: Common Eyelid Conditions
A hordeolum is an acute inflammation of an eyelid gland, usually caused by staphylococci.4 An infected Zeis or Moll gland is called an external hordeolum, or common stye, and usually comes to a head on the skin of the eyelid (Figure 9-19). A localized infection of a meibomian gland usually drains from the inside surface of the lid and thus is called an internal hordeolum. Mild cases usually resolve with hot compress treatment, but more severe cases might require antibiotic treatment.
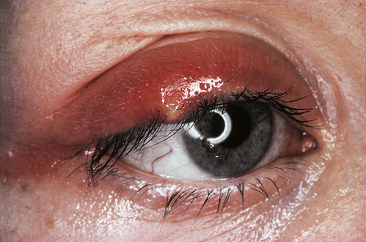
FIGURE 9-19 External hordeolum.
(From Kanski JJ: Clinical ophthalmology: a systematic approach, ed 5, Oxford, UK, 2003, Butterworth-Heinemann.)
A chalazion is a localized, noninfectious, and sometimes painless swelling of a meibomian gland, often caused by an obstructed duct (Figure 9-20). The gland may extrude its secretion into surrounding tissue, setting up a granulomatous inflammation. Medical or surgical therapy sometimes is necessary.65
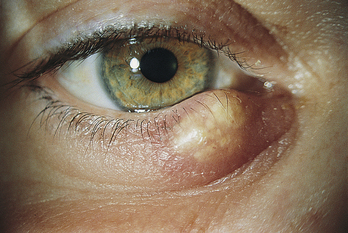
FIGURE 9-20 Painless chalazion.
(From Kanski JJ, Nischal KK: Ophthalmology: clinical signs and differential diagnosis, St Louis, 1999, Mosby.)
Blepharitis is an inflammatory disease of the lid; meibomian gland dysfunction is often the cause. Blockage of the gland pores can result in inflammation, sometimes complicated by bacterial infection.66 Clinical presentation might include crusting at the lash base and erythematosus of the lid margin. It can become a chronic condition that requires periodic treatments with hot packs, lid scrubs, and antibiotic ointment. Long-term inflammation can lead to hyperkeratinization and fibrosis of the glands and hyperemia and telangiectasia of the lid margin.67
Innervation of Eyelids
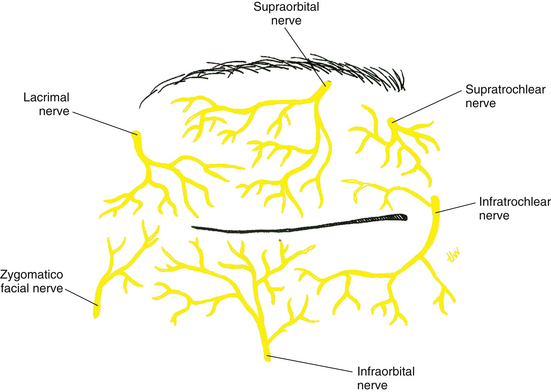
The ophthalmic and maxillary divisions of the trigeminal nerve provide sensory innervation of the eyelids. The upper lid is supplied by the supraorbital, supratrochlear, infratrochlear, and lacrimal nerves, branches of the ophthalmic division. The supply to the lower lid is by the infratrochlear branch of the ophthalmic nerve and the infraorbital nerve, a branch of the maxillary division (Figure 9-21). Motor control of the orbicularis muscle is through the temporal and zygomatic branches of the facial nerve, and that of the levator muscle is through the superior division of the oculomotor nerve. The tarsal smooth muscles are innervated by sympathetic fibers from the superior cervical ganglion.
Blood Supply of Eyelids
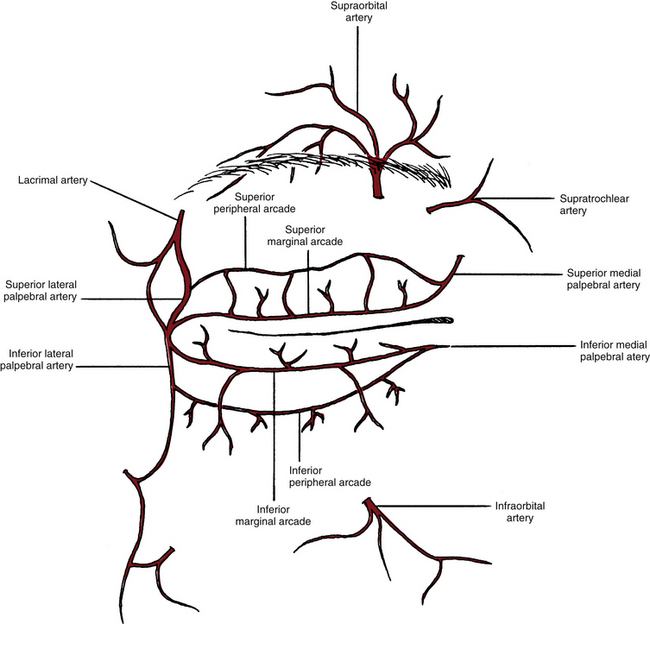
The blood vessels are located in a series of arcades or arches in each eyelid. The marginal palpebral arcade lies near the lid margin, and the peripheral palpebral arcade lies near the orbital edge of the tarsal plate (Figure 9-22). The vessels forming these arcades are branches from the medial and lateral palpebral arteries. The medial and lateral palpebral arteries are branches of the ophthalmic and lacrimal arteries, respectively. Normal variations occur in the blood supply, and the most common variation is a lack of the peripheral arcade in the lower lid.
Conjunctiva
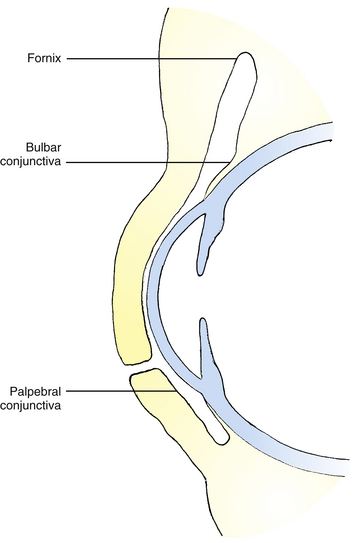
The conjunctiva is a thin, translucent mucous membrane that runs from the limbus over the anterior sclera, forms a cul-de-sac at the superior and inferior fornices, and turns anteriorly to line the eyelids. It ensures smooth movement of the eyelids over the globe. The conjunctiva can be divided into three sections that are continuous with one another: (1) the tissue lining the eyelids is the palpebral conjunctiva, or tarsal conjunctiva; (2) the bulbar conjunctiva covers the sclera; and (3) the conjunctival fornix is the cul-de-sac connecting palpebral and bulbar sections (Figure 9-23). Conjunctival stem cells are scattered in the basal layer throughout the conjunctiva, but are more numerous in the fornix region.68,69
At the mucocutaneous junction of the lid margin (see Figure 9-15), the nonkeratinized squamous palpebral conjunctival epithelium is continuous with the keratinized squamous epithelium of the epidermis of the eyelid. The conjunctiva forming the fornices is attached loosely to the fascial extensions of the levator, tarsal, and extraocular muscles, providing coordination of conjunctival movement with movement of the globe and lids. The fornices are present superiorly, inferiorly, and laterally, easing movement of the globe without creating undue stretching of the conjunctiva. The lateral fornix is the deepest and extends posterior to the equator of the globe.
The bulbar conjunctiva is translucent, allowing the sclera to show through, and is colorless except when its blood vessels are engorged. Bulbar conjunctiva is loosely adherent to the underlying tissue up to within 3 mm of the cornea, where it becomes tightly adherent and merges with the underlying Tenon’s capsule and sclera.
Plica Semilunaris
The plica semilunaris is a crescent-shaped fold of conjunctiva located at the medial canthus (see Figure 9-3). (It might be a remnant of the nictitating membrane seen in lower vertebrates.) The epithelium is 8 to 10 cells thick and contains numerous goblet cells, and the stroma is highly vascularized, containing smooth muscle fibers and adipose tissue.70 Because there is no deep fornix at the medial side as there is at the lateral side, the evident function of the plica is to allow full lateral movement of the eye without tissue stretching.
Caruncle
The function of the caruncle, a mound of tissue that overlies the medial edge of the plica semilunaris (see Figure 9-3), is poorly understood. The caruncle is similar to conjunctiva in that it contains nonkeratinized epithelium and accessory lacrimal glands, but it also has skin elements: hair follicles and sebaceous and sweat glands.70,71 The sebaceous glands are a likely source for the occasional accumulation of matter in the medial canthus of the healthy eye.
Conjunctival Lymphatics
The conjunctival lymphatic vessels are arranged in superficial and deep networks within the submucosa. These vessels drain into the lymphatics of the eyelids; those from the lateral aspect empty into the parotid lymph node, and those from the medial aspect empty into the submandibular lymph node (see Figure 11-14).
Conjunctival Innervation
Clinical Comment: Pingueculae and Pterygia
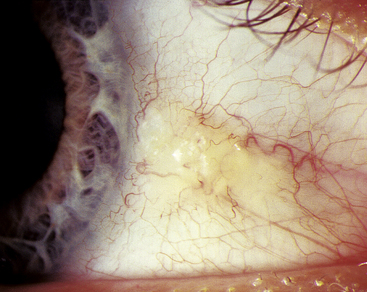
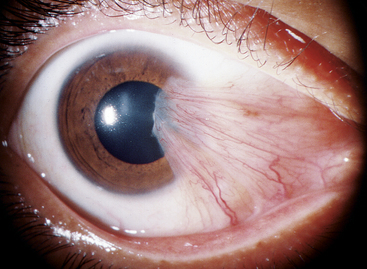
A PINGUECULA consists of an opaque, slightly elevated mass of modified conjunctival tissue in the interpalpebral area, usually at the 3-o’clock or 9-o’clock position. Pingueculae may vary considerably in size and appearance but usually are round or oval and yellowish (Figure 9-24). Two histologic changes occur in the submucosal layers, whereas the epithelial layers remain unchanged. The first submucosal change is hyalinization, which occurs in a zone just below the epithelium. This zone contains degenerating collagen and a granular material that probably results from the breakdown of connective tissue components.72,73 The second submucosal change in development of pinguecula is the formation of abnormal elastic fibers. Precursors of elastic fibers and abnormally immature forms of newly synthesized elastic fibers are found beneath the zone of hyalinization. These fibers degenerate, and elastic myofibrils are greatly reduced, which prevents normal assembly of elastic fibers.72,73 Fibroblasts in these regions show extensive alteration.
A pterygium is a fibrovascular overgrowth of bulbar conjunctiva onto cornea and is usually progressive. As with pinguecula, pterygium occurs in the 3-o’clock or 9-o’clock position of the interpalpebral area (Figure 9-25). The triangular pterygium may be gray in appearance, and its apex (often called the head) invades the cornea. This leading edge is composed of a zone of limbal epithelial tissue arising from altered basal stem cells. A zone of cells follows the head, migrates along the corneal basement membrane, and dissolves Bowman’s layer.72,74 The head is the only site of firm attachment to the corneal surface. Fibrovascular tissue with the same abnormal characteristics seen in pingueculae underlie the epithelium of a pterygium.74–76 An extensive network of blood vessels is evident.72
Pingueculae and pterygia show many of the same connective tissue changes but are different diseases. If mutational changes occur in the limbal epithelium at the corneal edge of a pinguecula, it may become a pterygium.76 Exposure to irritants such as wind and dust might initiate hyperplasia and be a precursor of both these degenerative changes. Molecular damage produced by chronic solar radiation, particularly high-energy ultraviolet rays, is the primary causal factor in pterygium, with irritants being predisposing factors.74,77–79 Biochemical studies have shown that oxidative stress can result in biochemical cellular changes that cause cellular proliferation, vascularization, and the adhesion to the corneal surface that occurs in pterygium.80–82
Pingueculae rarely are treated unless inflamed. Pterygia are surgically removed (1) when the apex approaches the visual axis, (2) if significant corneal astigmatism is induced, or (3) for cosmetic concerns. Complete removal is difficult because the altered cells appear as normal cornea, and the abnormal cell can only be discerned histologically.75 Thus pterygia often recur. Patients with either condition should be advised of the relationship of these conditions to irritants and sun exposure, and ultraviolet-filtering protective lenses should be prescribed, as well as artificial tears and ocular lubricants as needed.
Tenon’s Capsule
Below the conjunctival stroma is a thin, fibrous sheet called Tenon’s capsule (fascia bulbi). Tenon’s capsule serves as a fascial cavity within which the globe can move. It protects and supports the globe and attaches it to the orbital connective tissue.
The collagen fibrils that form Tenon’s capsule are arranged in a three-dimensional network of longitudinal, horizontal, and oblique groups.83 In young people, Tenon’s capsule contains collagen fibrils of uniform shape and diameters of 70 to 110 nm; in older individuals there is greater variation in fibril shape, and diameters vary from 30 to 160 nm.83,84 Few fibroblasts and some elastic fibers are present, but in a very small ratio compared with the number of collagen fibrils.84
Tear Film
The tear film, which covers the anterior surface of the globe, has several functions: (1) keeps the surface moist and serves as a lubricant between the globe and eyelids; (2) traps debris and helps remove sloughed epithelial cells and debris; (3) is the primary source of atmospheric oxygen for the cornea; (4) provides a smooth refractive surface necessary for optimum optical function85; (5) contains antibacterial substances (lysozyme, beta-lysin, lactoferrin, immunoglobulins) to help protect against infection86; (6) helps to maintain corneal hydration by changes in tonicity that occur with evaporation87; and (7) contains various growth factors and peptides that can regulate ocular surface wound repair.58
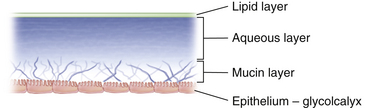
The tear film is composed of three layers (Figure 9-26). The outermost is a lipid layer containing waxy esters, cholesterol, and free fatty acids, primarily produced by the meibomian glands. The lipid layer retards evaporation and provides lubrication for smooth eyelid movement. The middle or aqueous layer contains inorganic salts, glucose, urea, enzymes, proteins, glycoproteins, and most of the antibacterial substances.2 It is secreted by the main and accessory lacrimal glands. The innermost or mucous layer acts as an interface that facilitates adhesion of the aqueous layer of the tears to the ocular surface.88,89 It is composed of the glycocalyx secretion from the surface epithelia and mucin produced and secreted by the conjunctival goblet cells. Mucins can also bind and entrap bacteria and viruses blocking binding sites on microbes and preventing them from penetrating the ocular surface.58
According to some sources, the tear film is 7 to 10 μm thick, with the aqueous layer accounting for 90% of the thickness.9,90 However, measurements using laser interferometry suggest that the full thickness of the mucous layer was not recognized using conventional measuring methods. Some have estimated tear layer thickness in the range of 34 to 45 μm, with the mucous layer the thickest.91–93 The mucous and aqueous portions are not static and may not remain as separate and distinct layers but may form a sort of gradient hampering accurate measurements.58
Lacrimal Secretory System
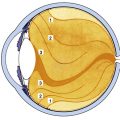
The lacrimal gland is divided into two portions, palpebral and orbital, by the aponeurosis of the levator muscle (see Figure 9-9). The superior orbital portion is larger and almond shaped. The superior surface lies against the periorbita of the lacrimal fossa, the inferior surface rests against the aponeurosis, the medial edge lies against the levator, and the lateral edge lies on the lateral rectus muscle. The palpebral lobe is one third to one half the size of the orbital lobe and is subdivided into two or three sections.38 If the upper lid is everted, the lacrimal gland can be seen above the edge of the upper tarsal plate. Ducts from both portions of the gland exit through the palpebral lobe.
The lacrimal gland consists of lobules made up of numerous acini. Each acinus is an irregular arrangement of secretory cells around a central lumen surrounded by an incomplete layer of myoepithelial cells.94 (Histologically, the main lacrimal gland is identical to the accessory lacrimal glands.) A network of ducts connects the acini and drains into one of the main excretory ducts. There are approximately 12 of these ducts, which empty into the conjunctival sac in the superior fornix.49 The secretion is composed of water, electrolytes, and antibacterial agents including lysozyme, lactoferrin, and immunoglobulins. The accessory glands are located in the subconjunctival tissue from the fornix area to near the tarsal plate. Basic secretion maintains the normal volume of the aqueous portion of the tears, and reflex secretion increases the volume in response to a stimulus. Both main and accessory glands play a role in basic and reflex secretion.
The lacrimal gland is supplied by the lacrimal artery, a branch of the ophthalmic artery. Sensory innervation is through the lacrimal nerve, a branch of the ophthalmic division of the trigeminal nerve. The gland receives vasomotor sympathetic innervation and secretomotor parasympathetic innervation.95 Reflex tearing occurs with stimulation of branches of the ophthalmic nerve or in response to external stimuli, such as intense light; the afferent pathway is through the trigeminal nerve, and the parasympathetic pathway is through the facial nerve.
Disagreement still exists regarding the relative contributions of the main and accessory lacrimal glands. In the traditional view, accessory glands provide basic secretion, and the main lacrimal gland is primarily active during reflex or psychogenic stimulation.95 A more recent view holds that all lacrimal glands produce the aqueous layer and that production is stimulus driven, with the rate of production ranging from low levels in sleep to high levels under conditions of stimulation.58,96
Clinical Comment: Tear Film Assessment
Various clinical tests are used to assess the extent of tear abnormalities, although no single test is thought to be diagnostically effective.97 The Schirmer test is a clinical measure of the adequacy of the aqueous portion of the tears. A special piece of filter paper is inserted over the inferior eyelid margin. Normally the strip should be moistened by at least 5 mm after 5 minutes.86,98 This test can be done with or without topical anesthesia; the test without topical anesthetic measures both reflex and basic secretion, and the test with anesthetic measures only basic secretion. The phenol red thread test, which uses a thread treated with a pH indicator, may be used in place of the Schirmer strips.
In another clinical assessment method, fluorescein dye is instilled into the lower cul-de-sac and it spreads throughout the tear film. After a blink the thin lipid upper layer begins to break down, and dry spots appear. The time between the completion of the blink and the first appearance of a dry spot is termed the tear film breakup time (TBUT) and gives an indirect measure of the evaporative rate. Normally the TBUT is greater than 10 seconds and longer than the time between blinks.2,98–100 A short TBUT can occur if irregularities or disturbances in the corneal surface prevent complete tear film adherence or if abnormalities exist in the lipid layer causing increased evaporation.101
Clinical Comment: Dry Eye
Alteration in any of the layers of the tear film or in normal lid anatomy and closure can result in depletion of the tear film and cause dry eye, one of the most common disorders seen in clinical eye care practice. Dry eye syndrome has a complex etiology and may be caused by a deficiency or alteration of any of the layers of the tear film or by an abnormal interaction between the layers.102 Aqueous deficiencies are common, and normal aging can cause a decrease of aqueous tear production. Patients with rheumatoid arthritis often develop Sjögren syndrome, an autoimmune disease that affects the lacrimal gland, causing a deficiency in the aqueous layer. This can cause an uncomfortable gritty, sandy feeling.103,104 Inflammation of the meibomian glands, meibomianitis, can modify the composition of the secretion producing a more viscous meibum that does not flow as easily through the pores.105,106 Loss of lipid secretion can lead to alterations in the lipid layer, allowing increased evaporation of the tear film and leading to dry eye symptoms of irritation and corneal epithelial compromise.36,67 Conditions with deficient secretion of mucus are associated with reduced goblet cell populations, such as chemical burns, Stevens-Johnson syndrome, and ocular pemphigoid.99,107 Complaints associated with dry eye include scratchy and foreign body sensations.
The diagnosis of dry eye is not usually based on a single definitive finding. The instillation of dye (fluorescein, lissamine green, or rose Bengal) can indicate tissue damage that occurs because of a defective tear film but does not indicate the reason.97 The tear film can be augmented by the application of ocular lubricants, consisting of artificial tears during the day and ointments at night. More serious dry eye problems can be treated with procedures that decrease tear drainage; punctual plugs are a temporary solution, and electrocautery can produce permanent closure of the punctum. Recent clinical studies suggest that a subclinical inflammatory condition contributing to dry eye may be successfully treated with topical antiinflammatory agents such as cyclosporin A.108–111
Tear Film Distribution
At the posterior edge of both upper and lower eyelid margins, there is a meniscus of tear fluid. The meniscus at the lower lid is more easily seen. The upper tear meniscus is continuous with the lower meniscus at the lateral canthus, whereas at the medial canthus the tear menisci lead directly to the puncta and drain into them.112 The lacrimal lake, a tear reservoir, is located in the medial canthus. The plica semilunaris makes up the floor of the lake, and the caruncle is located at its medial side.
Nasolacrimal Drainage System
Some tear fluid is lost by evaporation and some by reabsorption through conjunctival tissue, but approximately 75% passes through the nasolacrimal drainage system.86 The nasolacrimal drainage system consists of the puncta, canaliculi, lacrimal sac, and nasolacrimal duct, which empties into the nasal cavity (Figure 9-27).
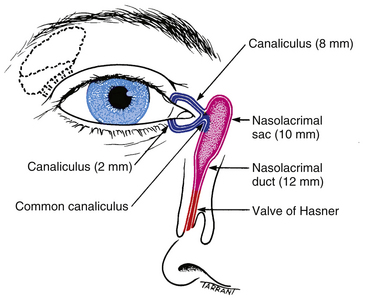
FIGURE 9-27 Anatomy of lacrimal drainage system.
(From Kanski JJ: Clinical ophthalmology, ed 3, Oxford, UK, 1995, Butterworth-Heinemann.)
Puncta and Canaliculi
The canaliculi are tubes in the upper and lower lids that join the puncta to the lacrimal sac. The walls of the canaliculi contain elastic tissue and are surrounded by fibers from the lacrimal portion of the orbicularis muscle (Horner’s muscle). The first portion of the canaliculus is vertical and extends approximately 2 mm; a slight dilation, the ampulla, is at the base of the vertical portion of the canaliculus.9,14,113 The canaliculus then turns horizontally to run along the lid margin for approximately 8 mm (see Figure 9-27). The canaliculi join to form a single common canaliculus that pierces the periorbita covering the lacrimal sac and enters the lateral aspect of the sac.114 The angle at which the canaliculus enters the sac produces a physiologic valve that prevents reflux.2,113–116
Lacrimal Sac and Nasolacrimal Duct
The lacrimal sac lies within a fossa in the anterior portion of the medial orbital wall. This fossa is formed by the frontal process of the maxillary bone and the lacrimal bone. The sac is surrounded by fascia, continuous with the periorbita, which runs from the anterior to the posterior lacrimal crests. The two limbs of the medial palpebral ligament straddle the sac to attach to the posterior and anterior crests.117 The orbital septum and the check ligament of the medial rectus muscle lie behind the lacrimal sac (see Figure 8-18).
The lacrimal sac empties into the nasolacrimal duct just as it enters the nasolacrimal canal in the maxillary bone. The duct is approximately 15 mm long and terminates in the inferior meatus of the nose. At this point the valve of Hasner is found. This fold of mucosal tissue prevents retrograde movement of fluid up the duct from the nasal cavity.9,17,113
Tear Drainage
During closure the eyelids meet first at the temporal canthus; closure then moves toward the medial canthus, where the tears pool in the lacrimal lake. The tear menisci are pushed toward the lacrimal puncta into which they drain. Theories explaining tear drainage report that the state of the lacrimal sac is either distended or compressed by contraction of the orbicularis muscle. According to some, contraction of the lacrimal part of the orbicularis compresses the canaliculi, forcing the tears into the lacrimal sac.118 Coincidentally, contraction of the muscle pulls on the fascial sheath attached to the lacrimal sac,16,118 which causes lateral displacement of the lateral wall, expanding the sac and creating negative pressure within it—in effect, pulling tears in from the canaliculus. On relaxation of the orbicularis, the lacrimal sac collapses, and the tears are driven into the nasolacrimal duct. In addition, the canaliculi open and act as siphons to pull tears in through the puncta.118 The tears drain into the nasolacrimal duct mainly by gravity, where most are absorbed by the mucosal lining before the remaining tears enter the inferior meatus.2
A number of studies, however, have measured an increase in pressure within the lacrimal sac during lid closure.119 Using high-speed photography, the medial edges of the eyelids have been observed to meet, halfway into a blink, occluding the puncta.112 According to this theory, the canaliculi and lacrimal sac are compressed, forcing all fluids into the nasolacrimal duct. As the eyelids open, compression of the canaliculi decreases, but the puncta remain occluded, creating a negative pressure in the canaliculi. When the puncta finally are opened, the negative pressure pulls the tears in immediately after the blink.2,120
The primary difference in these two theories is the state of the lacrimal sac. In the first scenario the sac is dilated, whereas in the second case the sac is compressed with orbicularis contraction. Capillary attraction plays a role in moving tears into the puncta and down into the canaliculi between blinks.86
Aging Changes in Orbital Adnexa and Lacrimal System
The eyebrow position heightens in both genders with increasing age.120
The aging process is apparent in the eyelids as tissue atrophies, the skin loses elasticity, and wrinkles appear. With age the distance between the center of the pupil and the lower eyelid margin increases due to sagging of the lower lid; this change is greater in males than females.120 More pronounced changes in lid margin position, including ectropion and entropion (previously described), increase in incidence with age-related changes in the orbicularis muscle tone.
Tearing may be caused by eversion of the lower punctum due to eyelid position or by stenosis of the passages in the lacrimal drainage system; both occur more frequently in elderly persons. Some studies find that the basal rate of tear secretion diminishes after age 40, contributing to dry eye, the incidence of which increases with age.121,122 Others have determined that tear reflex secretion decreases.123 The goblet cell population may decrease over age 80 and a decrease in lysozyme and lactoferrin are noted.123 Causative factors include loss of glandular tissue and a change in composition of the meibomian secretion forming a more viscous material that does not flow as easily.36,66 The incidence of vascular engorgement at the lid margin and plugged meibomian gland pores also increase with age.66
1. McCord C.D., Doxanas M.T. Browplasty and browpexy: an adjunct to blepharoplasty. Plast Reconstr Surg. 1990;86(2):248.
2. Doxanas M.T., Anderson R.L. Clinical orbital anatomy. Baltimore: Williams & Wilkins; 1984. p 89
3. Tarbet K.J., Lemke B.N. Clinical anatomy of the upper face. Int Ophthalmol Clin. 1997;37(3):11.
4. Bartlett J.D., Jaanus S.D. Clinical ocular pharmacology, ed 3. Boston: Butterworth-Heinemann; 1995. p 583
5. Katz J., Kaufman H.E. Corneal exposure during sleep (nocturnal lagophthalmos). Arch Ophthalmol. 1977;95:449.
6. Sturrock G.D. Nocturnal lagophthalmos and recurrent erosion. Br J Ophthalmol. 1976;60:97.
7. Jelks G.W., Jelks E.B. The influence of orbital and eyelid anatomy on the palpebral aperture. Clin Plast Surg. 1991;18(1):183.
8. Fox S.A. The palpebral fissure. Am J Ophthalmol. 1966;62:73.
9. Warwick R. Eugene Wolff’s anatomy of the eye and orbit, ed 7. Philadelphia: Saunders; 1976. p 181
10. Stewart J.M., Carter S.R. Anatomy and examination of the eyelids. Int Ophthalmol Clin. 2002;42(2):1.
11. Dailey R.A., Wobig J.L. Eyelid anatomy. J Dermatol Surg Oncol. 1993;18:1023.
12. Goldberg R.A., Wu J.C., Jesmanwicz A., et al. Eyelid anatomy revisited. Dynamic high-resolution magnetic resonance images at Whitnall’s ligament and upper eyelid structures with the use of a surface coil. Arch Ophthalmol. 1992;110(11):1598.
13. Jeong S., Lemke B.N., Dortzbach R.K., et al. The Asian upper eyelid: an anatomical study with comparison to the Caucasian eyelid. Arch Ophthalmol. 1999;117:907.
14. Jakobiec F.A., Iwamoto T. The ocular adnexa: lids, conjunctiva, and orbit. In Fine B.S., Yanoff M., editors: Ocular histology, ed 2, New York: Harper & Row, 1979. p 290
15. Jester J.V., Nicolaides N., Smith R.E. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981;20(4):537.
16. Ahl N.C., Hill J.C. Horner’s muscle and the lacrimal system. Arch Ophthalmol. 1982;100:488.
17. Fernandez-Valencia R., Pellico L.G. Functional anatomy of the human saccus lacrimalis. Acta Anat (Basel). 1990;139:54.
18. Shinohara H., Kominami R., Yasutaka S., et al. The anatomy of the lacrimal portion of the orbicularis oculi muscle (tensor tarsi or Horner’s muscle). Okajimas Folia Anat Jpn. 2001;77(6):225. (abstract)
19. Lipham W.J., Tawfik H.A., Dutton J.J. A histologic analysis and three-dimensional reconstruction of the muscle of Riolan, Ophthal Plastic Reconst Surg. 2002;18(2):93.
20. Morax S., Herdan M.L. The aging eyelid. Schweiz Rundsch Med Prax. 1990;9(48):1506. (abstract)
21. Dryden R.M., Leibsohn J., Wobig J. Senile entropion. Pathogenesis and treatment. Arch Ophthalmol. 1883;96:1978.
22. Fox S.A. Primary congenital entropion. Arch Ophthalmol. 1956;56:839.
23. Kikkawa D.O., Lucarelli M.J., Shovlin J.P., et al. Ophthalmic facial anatomy and physiology. In Kaufman P.L., Alm A., editors: Adler’s physiology of the eye, ed 10, St Louis: Mosby, 2003.
24. Hart W.M.Jr. The eyelids. In Hart W.M.Jr., editor: Adler’s physiology of the eye, ed 9, St Louis: Mosby, 1992.
25. Wobig J.L. Surgical technique for ptosis repair. Aust N Z J Ophthalmol. 1989;17(2):125.
26. Anderson R.L., Beard C. The levator aponeurosis. Attachments and their clinical significance. Arch Ophthalmol. 1977;95:1437.
27. Kuwabara T., Cogan D.G., Johnson C.C. Structure of the muscles of the upper eyelid. Arch Ophthalmol. 1975;93:1189.
28. Wobig J.L. The eyelids. In: Reeh M.J., Wobig J.L., Wirtschafter J.D., editors. Ophthalmic anatomy. San Francisco: American Academy of Ophthalmology; 1981:38.
29. Lim H.W., Paik D.J., Lee Y.J. A cadaveric anatomical study of the levator aponeurosis and Whitnall’s ligament. Korean J Ophthalmol. 2009;23:183-187.
30. Evinger C., Manning K.A., Sibony P.A. Eyelid movements: mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387.
31. Hawes M.J., Dortzbach R.K. The microscopic anatomy of the lower eyelid retractors. Arch Ophthalmol. 1982;100:1313.
32. Small R.G., Sabates N.R., Burrows D. The measurement and definition of ptosis. Ophthalmol Plast Reconstr Surg. 1989;5(3):171.
33. Anderson R.L. Medial canthal tendon branches out. Arch Ophthalmol. 1977;95:2051.
34. Rosenstein T., Talebzadeh N., Pogrel M.A. Anatomy of the lateral canthal tendon. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):24.
35. Gioia V.M., Linberg J.V., McCormick S.A. The anatomy of the lateral canthal tendon. Arch Ophthalmol. 1987;105:529.
36. Arita R., Itoh K., Inoue K., et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116:379-384.
37. Stoeckelhuber M., Stoeckelhuber B.M., Welsch U. Human glands of Moll: histochemical and ultrastructural characterization of the glands of Moll in the human eyelid. J Invest Dermatol. 2003;121(1):28.
38. Iwamoto T., Jakobiec F.A. Lacrimal glands. Tasman W., Jaeger E.A., editors. Duane’s foundations of clinical ophthalmology, vol 1. Philadelphia: Lippincott, 1994.
39. Wirtschafter J.D., Ketcham J.M., Weinstock R.J., et al. Mucocutaneous junction as the major source of replacement palpebral conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1999;40(13):3138.
40. Gipson I.K., Yankauckas M., Spurr-Michaud S.J., et al. Characteristics of a glycoprotein in the ocular surface glycocalyx. Invest Ophthalmol Vis Sci. 1992;33:218.
41. Nichols B., Dawson C.R., Togni B. Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 1983;24:570.
42. Dilly P.N. On the nature and the role of the subsurface vesicles in the outer epithelial cells of the conjunctiva. Br J Ophthalmol. 1985;69:477.
43. Kessing S.V. Investigations of the conjunctival mucin. (Quantitative studies of the goblet cells of conjunctiva). (Preliminary report). Acta Ophthalmol (Copenh). 1966;44:439.
44. Diebold Y., Rios J.D., Hodges R.R., et al. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2001;42(10):2270.
45. Sullivan W.R., McCulley J.P., Dohlman C.H. Return of goblet cells after vitamin A therapy in xerosis of the conjunctiva. Am J Ophthalmol. 1973;75:720.
46. Kruse F.E., Tseng S.C. Retinoic acid regulates clonal growth and differentiation of cultured limbal and peripheral corneal epithelium. Invest Ophthalmol Vis Sci. 1994;35:2405-2420.
47. Steuhl K.P., Sitz U., Knorr M., et al. [Age-dependent distribution of Langerhans cells within human conjunctival epithelium]. [German] Ophthalmologe. 1995;92(1):21-25.
48. Hogan M.J., Alvarado J.A. Histology of the human eye. Philadelphia: Saunders; 1971. p 112
49. Allensmith M.R., Greiner J.V., Baird R.S. Number of inflammatory cells in the normal conjunctiva. Am J Ophthalmol. 1978;86:250.
50. Jakobiec F.A., Iwamoto T. Ocular adnexa: introduction to lids, conjunctiva, and orbit. Tasman W., Jaeger E.A., editors. Duane’s foundations of clinical ophthalmology, vol 1. Philadelphia: Lippincott, 1994.
51. Knop N., Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest Ophthalmol Vis Sci. 2000;41:1270-1279.
52. Srinivasan B.D., Jakobiec F.A., Iwamoto T., et al. Epibulbar mucogenic subconjunctival cysts. Arch Ophthalmol. 1978;96:857.
53. Chin G.N., Chi E.Y., Bunt A. Ultrastructure and histochemical studies of conjunctival concretions. Arch Ophthalmol. 1980;98:720.
54. Weingeist T.A. The glands of the ocular adnexa. In: Zinn K.M., editor. Ocular structure for the clinician. Boston: Little, Brown; 1973:243.
55. Sirigu P., Shen R.L., Pinto-da-Silva P. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopes. Invest Ophthalmol Vis Sci. 1992;33(7):2284.
56. Efron N., Al-Dossarit M., Pritchard N. in vivo confocal microscopy of the palpebral conjunctiva and tarsal plate. Optom Vis Sci. 2009;86:1303-1308.
57. Butovich I.A., Millar T.J., Ham B.M. Understanding and analyzing meibomian lipids—A review. Curr Eye Res. 2008;33:405-420.
58. Dartt D.A., Hodges R.R., Zoukhri D. Tears and their secretion. The biology of the eye Fischbarg J, vol 10, Amsterdam: Elsevier, 2006, 18–82.
59. Butovich I.A. The meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009;28:483-498.
60. LeDoux M.S., Zhou Q., Murphy R.B., et al. Parasympathetic innervation of the meibomian glands in rats. Invest Ophthalmol Vis Sci. 2001;42(11):2434.
61. Krachmer J.H., Mannis M.J., Holland E.J. Seborrhea and meibomian gland dysfunction. Krachmer J.H., Mannis M.J., Holland E.J., editors. Cornea, vol 1. Mosby, 2005. St Louis
62. Stoeckelhuber M., Messmer E.M., Schubert C., et al. Immunolocalization of defensins and cathelicidin in human glands of Moll, Ann Anat. 2008;190:230-237.
63. Bergmanson J.P., Doughty M.J., Blocker Y. The acinar and ductal organization of the tarsal accessory lacrimal gland of Wolfring in rabbit eyelid. Exp Eye Res. 1999;68(4):411.
64. Seifert P., Stuppi S., Spitznas M. Distribution pattern of nervous tissue and peptidergic nerve fibers in accessory lacrimal glands. Curr Eye Res. 1997;16:298.
65. Kanski J.J. Clinical ophthalmology, ed 3. London: Butterworth-Heinemann; 1994.
66. Den S., Shimizu K., Ikeda T., et al. Association between meibomian gland changes and aging, sex, or tear function, Cornea. 2006;25:651-655.
67. McCann L.C., Tomlinson A., Pearce E.I., et al. Tear and meibomian gland function in blepharitis and normals. Eye Contact Lens. 2009;35:203-208.
68. Pe’er J., Zajicek G., Greifner H., et al. Streaming conjunctiva. Anat Rec. 1996;245(1):36.
69. Revoltella R.P., Papini S., Poselinni A., et al. Epithelial stem cells of the eye surface. Cell Prolif. 2007;40:445-461.
70. Fine B.S., Yanoff M. Ocular histology, ed 2. Hagerstown, Md: Harper & Row; 1979. p 310
71. Shields C.L., Shields J.A. Tumors of the caruncle. Int Ophthalmol Clin. 1993;33(3):31.
72. Austin P., Jakobiec F.A., Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983;90:96.
73. Li Z.Y., Wallace R.N., Streeten B.W., et al. Elastic fiber components and protease inhibitors in pinguecula. Invest Ophthalmol Vis Sci. 1991;32(5):1573.
74. Dushku N., John M.K., Schultz G.S., et al. Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithehal basal cells. Arch Ophthalmol. 2001;119:695.
75. Dushku N., Reid T.W. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997;16:1179.
76. Dushku N., Reid T.W. Immunohistochemical evidence that human pterygia originate from an invasion of vimentin-expressing altered limbal epithelial basal cells. Curr Eye Res. 1994;13:473.
77. Taylor H.R., West S.K., Rosenthal F.S., et al. Corneal changes associated with chronic UV radiation. Arch Ophthalmol. 1989;107:1481.
78. Mackenzie F.D., Hirst L.W., Battistutta D., et al. Risk analysis in the development of pterygia. Ophthalmology. 1992;99(7):1056.
79. Young R.W. The family of sunlight-related eye diseases. Optom Vis Sci. 1994;71(2):125.
80. John-Aryankalayil M., Dushku N., Jaworski C.J., et al. Microarray and protein analysis of human pterygium, Mol Vis. 2006;12:55-64.
81. Kase S., Osaki M., Sato I., et al. Immunolocalisation of E-cadherin and beta-catenin in human pterygium, Br J Ophthalmol. 2007;91:1209-1212.
82. Kau H.C., Tsai C.C., Lee C.F., et al. Increased oxidative DNA damage, 8-hydroxydeoxy- guanosine, in human pterygium, Eye. 2006;20:826-831.
83. Shauly Y., Miller B., Lichtig C. Tenon’s capsule: ultrastructure of collagen fibrils in normals and infantile esotropia. Invest Ophthalmol Vis Sci. 1992;33:651.
84. Meyer E., Ludatscher R.N., Miller B., et al. Connective tissue of the orbital cavity in retinal detachment: an ultrastructural study. Ophthal Res. 1992;24:365.
85. Reiger G. The importance of the precorneal tear film for the quality of optical imaging. Br J Ophthalmol. 1992;76:157.
86. Lemp M.A., Wolfley D.E. The lacrimal apparatus. In Hart W.M.Jr., editor: Adler’s physiology of the eye, ed 9, St Louis: Mosby, 1992.
87. Mishima S., Maurice D.M. The effect of normal evaporation on the eye. Exp Eye Res. 1961;1:46.
88. Lemp M.A., Holly F.J., Iwata S. The precorneal tear film. Arch Ophthalmol. 1970;83:89.
89. Watanabe H., Fabricant M., Tisdale A.S., et al. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36(2):337.
90. Ehlers N. The thickness of the precorneal tear film. Factors in spreading and maintaining a continuous tear film over the corneal surface. Acta Ophthalmol (Copenh). 1965;8(suppl 81):92.
91. Prydal J.I., Artal P., Woon H., et al. Study of human precorneal tear film thickness and structure using laser interferometry. Invest Ophthalmol Vis Sci. 2006;33(6):1992.
92. Prydal J.I., Campbell F.W. Study of precorneal tear film thickness and structure by interferometry and confocal microscopy. Invest Ophthalmol Vis Sci. 1992;33(6):2006.
93. Peral A., Pintor J. Ocular mucin visualization by confocal laser scanning microscopy, Cornea. 2008;27:395-401.
94. Egeberg J., Jenson O.A. The ultrastructure of the acini of the human lacrimal gland. Acta Ophthalmol (Copenh). 1969;47:400.
95. Jones L.T. Anatomy of the tear system. Int Ophthalmol Clin. 1973;13(1):3.
96. Jordan A., Baum J.L. Basic tear flow. Does it exist? Ophthalmology. 1980;95:1.
97. Khanal S., Tomlinson A., McFayden A., et al. Dry eye diagnosis Invest Ophthalmol Vis Sci. 2008;49:1407-1414.
98. Cho P., Yap M. Schirmer test. A review. Optom Vis Sci. 1993;70(2):152.
99. Lemp M.A., Dohlman C.H., Kuwabara T., et al. Dry eye secondary to mucous deficiency. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:1223.
100. Lemp M.A., Hamill J.R.J.r. Factors affecting tear film breakup in normal eyes. Arch Ophthalmol. 1973;89:103.
101. Mai G., Yang S. Relationship between corneal dellen and tear film breakup time. Yen Ko Hsueh Pao (Eye Sci). 1991;7(1):43. (abstract)
102. Khurana A.K., Chaudhary R., Ahluwalia B.K., et al. Tear film profile in dry eye. Acta Ophthalmol (Copenh). 1991;69(1):79.
103. Friedlaender M.H. Ocular manifestations of Sjögren’s syndrome: keratoconjunctivitis sicca. Rheum Dis Clin North Am. 1992;18(3):591.
104. Roberts D.K. Keratoconjunctivitis sicca. J Am Optom Assoc. 1991;62(3):187.
105. Borchman D., Yappert M.C., Foulks G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis, Exp Eye Res. 2010;91:246-256.
106. Ibrahim O.M.A., Matsumoto Y., Dogru M., et al. The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology. 2010;117:665-672.
107. Ralph R.A. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14(4):299.
108. Cross W.D., Lay L.F.Jr., Walt J.G., et al. Clinical and economic implications of topical cylosporin A for the treatment of dry eye. Manag Care Interface. 2002;15(9):44.
109. Calonge M. The treatment of dry eye. Surv Ophthalmol. 2001;Suppl 2:S227.
110. Stevenson D., Tauber J., Reis B.L. Efficacy and safety of cyclosporin: A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group, Ophthalmology. 2000;107(5):967.
111. Sall K., Stevenson O.D., Mundorf T.K., et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporin ophthalmic emulsion in moderate-to-severe dry eye disease. The Cyclosporin A Phase 3 Study Group. Ophthalmology. 2000;107(4):631.
112. Doane M.G. Blinking and the mechanics of the lacrimal drainage system. Ophthalmology. 1981;88(8):844.
113. Wobig J.L. The lacrimal apparatus. In: Reeh M.J., Wobig J.L., Wirtschafter J.D., editors. Ophthalmic anatomy. San Francisco: American Academy of Ophthalmology; 1981:55.
114. Yazici B., Yazici Z. Frequency of the common canaliculus: a radiological study. Arch Ophthalmol. 2000;118:1381.
115. Doane M.G. Interactions of eyelids and tears in corneal wetting and the dynamics of the normal human eye blink. Am J Ophthalmol. 1980;89:507.
116. Tucker N.A., Tucker S.M., Linberg J.V. The anatomy of the common canaliculus. Arch Ophthalmol. 1996;114:1231.
117. Milder B., Demorest B.H. Dacryocystography. The normal lacrimal apparatus. Arch Ophthalmol. 1954;51:181.
118. Jones L.T., Marquis M.M. Lacrimal function. Am J Ophthalmol. 1972;73:658.
119. Lucarelli M.J., Dartt D.A., Cook B.E., et al. The lacrimal system. In Kaufman P.L., Alm A., editors: Adler’s physiology of the eye, ed 10, St Louis: Mosby, 2003.
120. van den Bosch W.A., Leenders I., Mulder P. Topographic anatomy of the eyelids, and the effects of sex and age. Br J Ophthalmol. 1999;83:347.
121. Lin P.Y., Tsai S.Y., Cheng C.Y., et al. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye study. Ophthalmology. 2003;110(6):1096.
122. Schaumberg D.A., Sullivan D.A., Buring J.E., et al. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318.
123. Van Haeringen N.J. Aging and the lacrimal system, Br J Ophthalmol. 1997;81:824-826.