46
Obstetric emergencies
Introduction
Worldwide, one woman dies every minute of every day from a complication of pregnancy. In developed countries, maternal death is uncommon, but evidence from the UK Confidential Enquiry into Maternal Deaths found substandard care in around two-thirds of cases. This is partly due to the fact that most obstetric emergencies are rare and often unfold with such rapidity that junior medical staff can find themselves facing potentially catastrophic conditions that they may never have seen before, let alone have ever managed.
This chapter will examine the obstetric emergencies listed below:
![]() unexpected collapse
unexpected collapse
![]() amniotic fluid embolism
amniotic fluid embolism
![]() prolapsed umbilical cord
prolapsed umbilical cord
![]() retained placenta
retained placenta
![]() shoulder dystocia
shoulder dystocia
![]() uterine inversion
uterine inversion
![]() uterine rupture.
uterine rupture.
See also haemorrhage (Chapter 34), eclampsia (Chapter 36) and pulmonary embolism (p. 254):
Principles of management
Anticipation and preparation are essential – and they may lead to prevention. For example, if the mother has a history of postpartum haemorrhage, anticipation with i.v. access and blood sent for group and save in early labour may make an important difference to the outcome should the problem recur. If there are risk factors for shoulder dystocia, such as a presumed large fetus in a mother with diabetes and a long first stage of labour, it is important to ensure that an experienced midwife is allocated to care for her and that senior medical staff are present on the labour ward at the time of delivery.
In addition, as life-threatening emergencies are relatively rare, it is important that there should be regular ‘fire drills’ of obstetric emergencies to ensure that all staff are fully prepared, that equipment is fully functional and that supporting systems (portering, laboratory, etc.) are prepared. It should also be remembered that emergencies can arise anywhere in the unit, not just in the labour ward. There is now clear evidence of the benefits of formal team-training and use of simulation in rare obstetric emergencies. This applies to both technical aspects of delivery and non-technical (team working) aspects of care.
The principles outlined in Box 46.1 can be adapted for initial resuscitation in all obstetric emergencies which involve maternal compromise. Remember that there are often two lives at stake and in most emergencies, minutes or even seconds count. Remember too, however, that panicking is never helpful. A good principle to remember is that the fetus rarely needs to be resuscitated directly – ‘resuscitate the mother and you will resuscitate the fetus’. It should be noted that an obstetric emergency can cause profound lifelong psychological problems for both the mother and her partner. This can manifest itself as postnatal depression, post-traumatic stress syndrome and a real fear of becoming pregnant again. Counselling and debriefing after such experiences should be encouraged both while the woman is in hospital and some weeks later.
Resuscitation
Resuscitation should have a strong focus on the ABC of basic life support as noted in Box 46.1. The aim is to resuscitate the mother and then (and only then) to consider the welfare of the baby. Resuscitation in pregnancy has some differences from that in a non-pregnant person, as outlined below, but it is still essential to approach the problem by using ABC, and then consider possible causes. The 4 Hs and 4 Ts listed in Box 46.2 are helpful.
The key resuscitation differences are that:
![]() the aorta and vena cava are compressed by the gravid uterus, impeding venous return and reducing cardiac output
the aorta and vena cava are compressed by the gravid uterus, impeding venous return and reducing cardiac output
![]() there is an increased risk of aspiration of stomach contents due to relaxation of the oesophageal–gastric junction (progesterone effect) and the pressure of the uterus
there is an increased risk of aspiration of stomach contents due to relaxation of the oesophageal–gastric junction (progesterone effect) and the pressure of the uterus
![]() difficult intubation is more common in the pregnant than in the non-pregnant patient (1:300 vs 1:3000) – short neck and laryngeal oedema
difficult intubation is more common in the pregnant than in the non-pregnant patient (1:300 vs 1:3000) – short neck and laryngeal oedema
![]() chemical pneumonitis is more likely than in the non-pregnant state, owing to the decreased pH of the stomach contents and the increased chance of inhaling the contents because of the changes outlined above.
chemical pneumonitis is more likely than in the non-pregnant state, owing to the decreased pH of the stomach contents and the increased chance of inhaling the contents because of the changes outlined above.
It is therefore important, in the early stages of resuscitation, to:
![]() tilt the patient to the left by 15–30° (reduces aortocaval compression and increases potential cardiac output by 25%)
tilt the patient to the left by 15–30° (reduces aortocaval compression and increases potential cardiac output by 25%)
![]() apply cricoid pressure and intubate early, to avoid aspiration of gastric contents and to facilitate oxygenation
apply cricoid pressure and intubate early, to avoid aspiration of gastric contents and to facilitate oxygenation
![]() involve a senior obstetrician and anaesthetist immediately or as early as possible (to facilitate intubation and early caesarean section where and when appropriate).
involve a senior obstetrician and anaesthetist immediately or as early as possible (to facilitate intubation and early caesarean section where and when appropriate).
In cases of cardiorespiratory arrest, the revised Resuscitation UK guidelines (2010) should be followed. These have moved the emphasis for basic life support towards a single compression-ventilation (CV) ratio of 30:2. If the mother is not delivered, left lateral tilt can be achieved using firm support under the right hip (an assistant can be asked to kneel and use their knees to support the patient in left lateral tilt).
After about 20–22 weeks, it is essential to perform a caesarean section early if resuscitation is unsuccessful (at this gestation, the fundus of the uterus will be at or above the level of the umbilicus). The decision for perimortem caesarean section should be made by 4 min if there is no response to active resuscitation, and the delivery by 5 min (the ‘4-minute rule’). An anaesthetic is not required in order to proceed. This is primarily to save the life of the mother and forms part of the resuscitation technique. It makes CPR more efficient by:
![]() increasing venous return
increasing venous return
![]() improving ease of ventilation
improving ease of ventilation
![]() allowing CPR to be carried out in the supine position
allowing CPR to be carried out in the supine position
![]() reducing oxygen requirement after delivery.
reducing oxygen requirement after delivery.
Amniotic fluid embolism
Epidemiology
This is one of the most catastrophic conditions that can occur in pregnancy. It is rare, with an incidence somewhere between 1:10 000 and 1:30 000. As a precise diagnosis can be difficult, it is also difficult to establish an accurate mortality rate, but it is probably around 20–40%.
Aetiology
The exact pathophysiology remains unclear. It was believed that some breakdown occurred in the physiological barrier separating the mother and fetus, allowing a bolus of amniotic fluid to enter the maternal circulation. This bolus moved to the pulmonary circulation and produced massive perfusion failure, bronchospasm and shock. More recently, it has been suggested that the underlying mechanism may be an anaphylactoid reaction to fetal antigens entering the maternal circulation and individual variations in sensitivity to these antigens are reflected by the severity of the resulting clinical picture.
Risk factors
Amniotic fluid embolism can occur at any time in pregnancy but it most commonly occurs in labour (70%), after vaginal delivery (11%) and following caesarean section (19%). The following risk factors have been identified:
![]() multiparity
multiparity
![]() placental abruption
placental abruption
![]() intrauterine death
intrauterine death
![]() precipitate labour
precipitate labour
![]() suction termination of pregnancy
suction termination of pregnancy
![]() medical termination of pregnancy
medical termination of pregnancy
![]() abdominal trauma
abdominal trauma
![]() external cephalic version
external cephalic version
![]() amniocentesis.
amniocentesis.
Clinical features
The clinical picture usually develops almost instantaneously and the diagnosis must be considered in all collapsed obstetric patients. The mother may demonstrate some or all of the signs and symptoms listed in Box 46.3 but classically a woman in late stages of labour or immediately postpartum starts to gasp for air, starts fitting and may have a cardiac arrest. There is often a profound disseminated intravascular coagulopathy (DIC) with massive haemorrhage, coma and death. There are inevitably signs of fetal compromise.
Diagnosis
The definitive diagnosis is usually at autopsy and is made by confirming the presence of fetal squames in the pulmonary vasculature. It is also possible to confirm the diagnosis in a surviving patient, again by finding fetal squames in washings from the bronchus or in a sample of blood from the right ventricle. In the acute situation, as there is no single clinical or laboratory finding which can diagnose or exclude amniotic fluid embolism, the diagnosis is made clinically by exclusion.
Management
This is primarily supportive and should be aggressive. There is, however, no evidence that any specific type of intervention significantly improves maternal prognosis. Initial therapy is aimed at supporting cardiac output and management of DIC. If the woman is undelivered, an immediate caesarean section may be appropriate, providing the mother can be stabilized.
A chest X-ray will often show pulmonary oedema, and an increase in right atrial and right ventricular size. The ECG demonstrates right ventricular strain and there is a metabolic acidosis (reduction of pO2 and pCO2).
In addition to the initial management of an obstetric emergency (Box 46.1), therapy may include:
![]() aggressive fluid replacement
aggressive fluid replacement
![]() maintenance of cardiac output with a dopamine infusion
maintenance of cardiac output with a dopamine infusion
![]() treatment of anaphylaxis with adrenaline (epinephrine), salbutamol, aminophylline and hydrocortisone
treatment of anaphylaxis with adrenaline (epinephrine), salbutamol, aminophylline and hydrocortisone
![]() treatment of DIC with fresh frozen plasma and cryoprecipitate
treatment of DIC with fresh frozen plasma and cryoprecipitate
![]() treatment of haemorrhage after delivery with syntocinon, ergometrine, carboprost (haemabate) or misoprostol, and uterine massage (p. 282)
treatment of haemorrhage after delivery with syntocinon, ergometrine, carboprost (haemabate) or misoprostol, and uterine massage (p. 282)
![]() early transfer to an ITU for central monitoring, respiratory support and other therapy as appropriate.
early transfer to an ITU for central monitoring, respiratory support and other therapy as appropriate.
Prognosis
The outcome for the baby is very poor, with a perinatal mortality rate of approximately 60% and most survivors usually suffering neurological impairment. Maternal outcome in mothers who have suffered a cardiac arrest is complicated by the fact that many are left with serious neurological impairment.
Prolapsed umbilical cord
Definition
‘Cord presentation’ is defined as the presence of the cord between the presenting part and the membranes, prior to membrane rupture. ‘Prolapsed umbilical cord’ refers to the same situation after membrane rupture. The cord can remain in the vagina (occult prolapse) or can prolapse through the introitus with loops lying outside the vagina (Figs 46.1 and 46.2). It is a true obstetric emergency requiring immediate action.
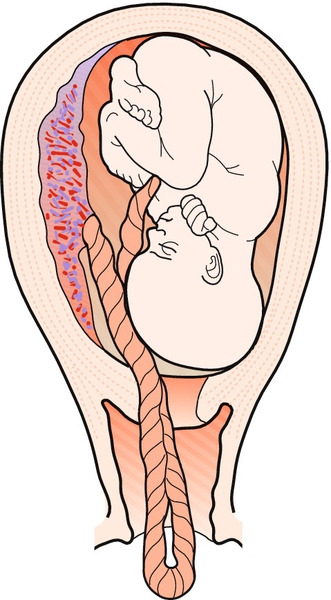
Fig. 46.1Umbilical cord prolapsing through incompletely dilated cervix.
This is due partially to a high presenting part.
This fetus requires immediate delivery.
Epidemiology
The incidence is related to presentation (Table 46.1). Any obstetric condition that precludes a close fit between the fetus and the pelvic inlet makes a cord prolapse more likely, particularly breech presentation, malposition, preterm gestation, polyhydramnios, fetal growth restriction and placenta praevia. Other predisposing factors include a long umbilical cord, artificial rupture of the membranes, and being a second twin.
Table 46.1
Incidence of cord prolapse in relation to presentation
| Presentation | Incidence |
| Vertex | 0.4% |
| Frank breech | 0.5% |
| Flexed breech | 4–6% |
| Footling breech | 15–18% |
Clinical features/investigation
There are two main insults to the cord, both of which may lead to cessation of fetal blood flow and fetal death. First, there is direct compression by the fetal body against the maternal pelvis and second, there is likely to be cord spasm from exposure to the cool external atmosphere or excessive handling of the cord.
Cardiotocography (CTG) usually indicates fetal compromise in the form of deep variable decelerations or a single prolonged deceleration (Fig. 46.3).
In some instances, the cord is clearly visible protruding through the vagina (‘overt’ cord prolapse), but it may be found at a vaginal examination carried out in response to some CTG abnormality (‘occult’ cord prolapse). It is important to routinely exclude cord prolapse following artificial rupture of the membranes or in the presence of variable decelerations of acute onset (this CTG pattern is associated with benign cord compression as well as cord prolapse).
Management
It is important to act swiftly, providing the maternal condition is stable. If there is any possibility that a fetal heartbeat is still present, the baby should be delivered immediately. If the cervix is fully dilated, preparation should be made for delivery by forceps or ventouse; if not, by immediate caesarean section. General anaesthesia is often required, but a spinal anaesthetic may be used by an experienced anaesthetist in some circumstances.
To protect the cord from occlusion during the transfer to theatre, the woman should be placed in the head-down position and a hand placed in the vagina to lift up the presenting part off the cord and prevent cord compression. An alternative is the knee–chest position (Fig. 46.4). Another reasonable approach is to insert a Foley catheter and fill the maternal bladder with 500 mL fluid. The catheter can be spigotted and this will relieve the pressure on the cord. This may be a useful approach when transporting the woman from a community setting. In this setting, if loops of cord are outside the vagina, a gentle attempt can be made to replace them within the vagina. There is a balance to be achieved between excessive cord handling versus leaving the loops exposed to a cold environment, both of which can lead to significant cord spasm.
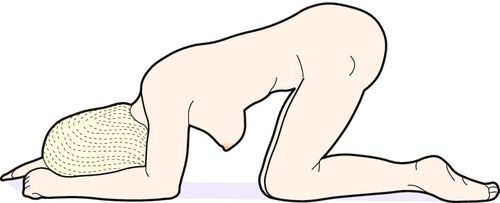
Fig. 46.4The knee–chest position should be adopted on the way to theatre.
Gravity ± an assistant’s hand displaces the presenting part away from the umbilical cord.
A tocolytic (e.g. terbutaline 0.25 mg s.c. or slow i.v.) should be given to minimize uterine contractions. The bladder should be emptied before starting caesarean section. If there is doubt as to fetal viability, for instance if the cord has prolapsed at home or silently on the antenatal ward, it is important to establish fetal viability before embarking on an unnecessary caesarean section. The absence of cord pulsation does not necessarily indicate fetal death, particularly if the prolapse is acute, and the fetal heart itself should be visualized directly by ultrasound. If fetal death has occurred, the mother should be allowed to labour and deliver spontaneously.
Prognosis
Fetal mortality has been reduced over the years with the increasing use of caesarean section and improvement in neonatal intensive care, but still remains around 10%.
Retained placenta
Definition
Retained placenta (see also p. 281) is defined as failure to deliver the placenta within 30 min of delivery of the fetus. A retained placenta increases the risk of postpartum haemorrhage by a factor of 10 owing to the inability of the uterus tocontract down completely. This risk appears to be maximal at 40 min after delivery. Such haemorrhage can be severe and life-threatening, particularly if there has been a partial separation.
Epidemiology
Retained placenta occurs in 2–3% of all vaginal deliveries and is more likely with pre-term gestations: if the baby is delivered before 37 weeks, the incidence increases by a factor of three, and if delivered at 26 weeks, the risk is increased by a factor of 20. It is also more common after a previous caesarean section, and rarely this can be associated with a morbidly adherent placenta.
Pathology
During normal childbirth, 90% of placentas are usually delivered within the first 15 min. Placental delivery is usually preceded by signs of placental separation, i.e. lengthening of the cord, a sudden small gush of dark blood and increased mobility of the uterus. Failure of the placenta to deliver may occur because of an unusually adherent unseparated placenta, or because the placenta has separated successfully but is retained within the uterus by a partially closed cervix. Failure of separation is much the more worrying of these two situations.
An adherent placenta is the result of abnormal placental implantation during the first trimester. Normally, the invading fetal trophoblast cells are arrested by the maternal decidual barrier, probably by the action of a specific form of leucocyte. If this maternal decidual layer is in some way ineffective, the trophoblast cells may invade further than usual and may extend through the myometrium or even as far as the outer serosal layer. The decidual barrier may be rendered ineffective by a number of factors, and is, for example, often thin and scarred following caesarean section. When over-invasion occurs, the placenta becomes abnormally adherent and is referred to as placenta accreta (Box 46.4). Those with a placenta praevia overlying a previous caesarean section scar are at very high risk of this serious complication.
Morbidly adherent placenta is subdivided into three subgroups: placenta accreta, placenta increta and placenta percreta, depending on the depth of invasion (see Table 46.2), though it is often difficult to differentiate these clinically. Both ultrasound assessment (looking for abnormal Doppler blood flow) and MRI scanning have been used to help diagnose the degree of invasion. However, there is no definitive test which can absolutely exclude this complication prior to delivery. There is loss of the physiological cleavage plane such that the placenta is unable to separate after delivery of the baby, and partial separation or iatrogenic effort at separation may lead to profound haemorrhage.
Table 46.2
Classification of abnormal placental attachment
| Type | Incidence | Pathology |
| Placenta accreta | 75–78% | Invades superficially into the myometrium |
| Placenta increta | 17% | Invades deeply into the myometrium |
| Placenta percreta | 5–7% | Invades through the myometrium and penetrates the outer serosal layer of the uterus. It may invade adjacent structures, including bladder and bowel |
A particular problem is the increasing prevalence of mothers who have had previous caesarean sections. If there is a low anterior placenta in a subsequent pregnancy, there will be a significant risk of placenta accreta and subsequent haemorrhage. If there is a suspicion of abnormal placentation in elective cases, the theatre team must be made aware well in advance. Consultant obstetrician and anaesthetist should be present and cell salvage equipment, cross-matched blood, etc. must be available.
Management
If the patient is bleeding heavily, a retained placenta is an obstetric emergency and treatment must be immediate. Aside from the initial resuscitation measures (above) the patient should be transferred to theatre for a manual removal of placenta.
If there is no bleeding, an initial conservative approach can be adopted. Intravenous access should be established and cross-match arranged in case bleeding begins, and it is reasonable to wait an hour or so for spontaneous expulsion of the placenta to occur. In the interim, the use of syntocinon, the ‘rubbing-up’ of a contraction, or breastfeeding, with its resultant physiological release of oxytocin, may help to aid expulsion. Early randomized trials suggest there may be some advantage from use of sublingual nitroglycerin tablets, but confirmation from larger trials is required.
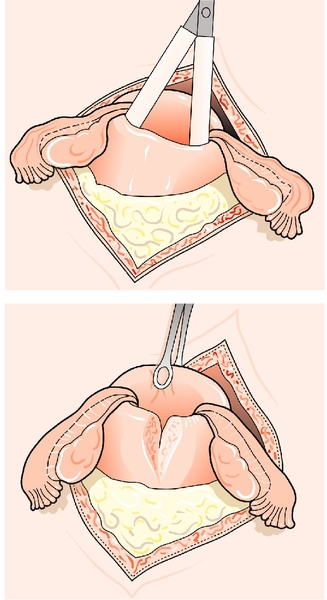
If the placenta is still retained after 1 h, the mother should be transferred to theatre for regional or general anaesthesia. Then, under aseptic conditions, a hand is passed into the uterus through the cervix in order to identify the cleavage plane between the placenta and the uterine wall. During the procedure, the uterine fundus is supported through the abdominal wall using the opposite hand. The placenta can then be gently stripped off the uterine wall and delivered (Fig. 46.5). Once it is out, a contraction should be ‘rubbed up’ and a bolus of syntocinon given i.v. to reduce the risk of postpartum haemorrhage due to an atonic uterus. The procedure must be covered with antibiotics, as there is a significant association between manual removal of the placenta and postpartum endometritis.
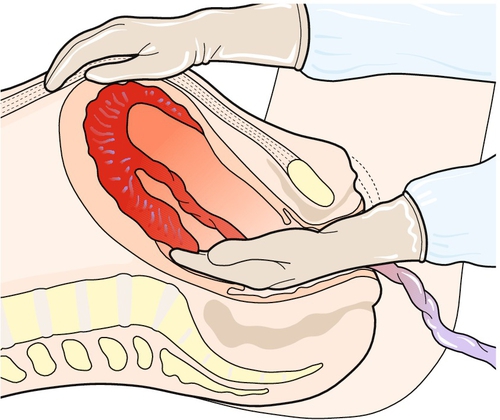
Fig. 46.5Manual removal of the placenta.
A cleavage plane is identified with the fingers, which then continue along the plane until the placenta is fully separated from the uterine wall.
If the cleavage plane cannot be found and the placenta is so firmly adherent to the uterine wall as to make removal impossible or dangerous (uterine rupture), the clinical diagnosis is ‘placenta accreta’. Subsequent management then depends on the degree of haemorrhage. If there is persistent uncontrollable haemorrhage, a hysterectomy is often required. It may be possible to arrest the haemorrhage using tamponade techniques (using either a balloon or packing within the uterine cavity). If there is no active haemorrhage, conservative management can be considered. With conservative management, when the placenta is left in situ to be absorbed over time, there is a significant incidence of major complications from infection and bleeding and the patient must be monitored closely for several weeks following discharge. Senior staff must always be involved in management of abnormal placentation when it is suspected or diagnosed.
Sepsis
Systemic maternal sepsis has recently become the leading cause of direct maternal death in the UK, with predominant organisms being E. coli, the Group A Streptococcus, and the Group B Streptococcus. Early presentation of sepsis (< 12 h post-birth) is more likely to be caused by streptococcal infection, particularly Group A, and severe continuous pain suggests necrotizing fasciitis. Early recognition and aggressive management of suspected sepsis is important to prevent progression to severe sepsis with multi-organ dysfunction and septic shock.
Puerperal pyrexia is discussed on chapter infection can lead to sepsis (Box 46.5) and may progress to severe sepsis (Box 46.6) If severe sepsis does not respond to an initial fluid resuscitation, then the patient is said to be in septic shock.
Full bacteriological and radiological investigation for the source of sepsis is essential, but should not significantly delay fluid resuscitation and antibiotic therapy. Arterial blood gases, lactate, U&Es, LFTs, glucose and clotting are very useful in monitoring the systemic response to sepsis, and this is often best carried out in a high dependency or intensive care setting. In rare cases of severe uterine sepsis, hysterectomy may be required.
Shoulder dystocia
Shoulder dystocia is one of the most frightening and threatening obstetric emergencies. There is a need to act quickly in order to prevent serious fetal morbidity and mortality.
Definition
The fetal anterior shoulder becomes impacted behind the symphysis pubis, preventing delivery. Clinically, it is defined as difficulty delivering the shoulders requiring obstetric manoeuvres beyond episiotomy and moderate downward traction. Although the incidence overall is around 0.2%, it rises to 0.5% with a fetal weight of over 3.5 kg and 10% with a weight of over 4.5 kg. Shoulder dystocia accounts for 8% of all intrapartum fetal deaths.
Risk factors
Although risk factors have been identified (Box 46.7), they have only very limited predictive value; 50% of shoulder dystocia occurs in normal-sized fetuses and 98% of large fetuses do not have dystocia. It is estimated that 3695 elective caesarean sections would have to be performed in non-diabetic mothers with babies estimated to weigh > 4.5 kg in order to avoid one permanent brachial plexus injury.
Clinical features
The baby’s head is often delivered as far as the chin and the fetal body is in the pelvis. The head often retracts tightly against the perineum and vulva – this is called the ‘turtle sign’ and should raise the possibility of an impending shoulder dystocia.
The umbilical cord is trapped and occluded between the fetal trunk and the maternal pelvis, leading to rapid fetal hypoxia and death. The pH drops by an estimated 0.04/min and it therefore takes around 7 min for the pH of a previously uncompromised fetus to fall below pH 7.00. It is estimated that 50% of deaths occur within 5 min.
Neonatal morbidity may result from brachial plexus damage due to excessive downward traction of the head during attempts at delivery. It is possible to damage nerve roots at the level of C5–T1, C5–6 (Erb palsy, Fig. 46.6) or C7–T1 (Klumpke palsy).
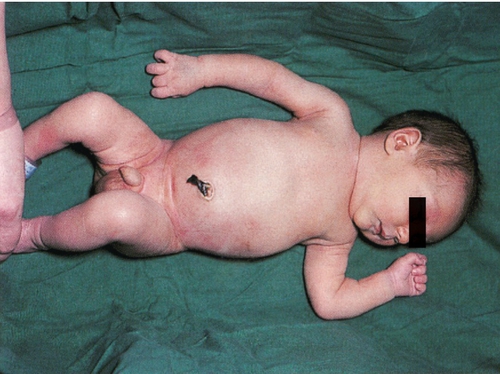
The baby was otherwise well.
While the main concerns for shoulder dystocia relate to the fetus, there may also be maternal complications in the form of genital tract trauma and atonic postpartum haemorrhage. Uterine rupture is rare.
Management
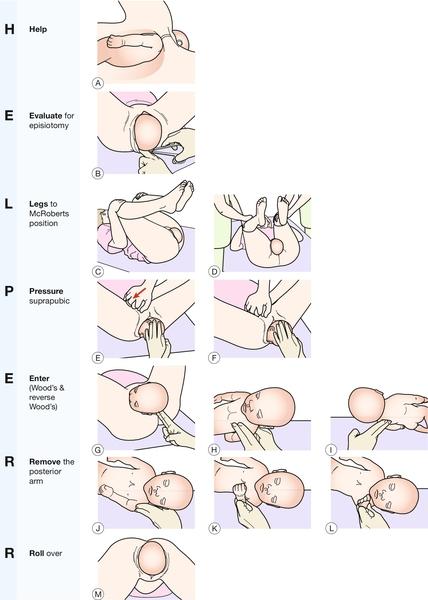
This is an obstetric emergency where seconds count. The aim is to disimpact the anterior shoulder and allow the fetus to be delivered. The mnemonic ‘HELPERR’ (ALSO programme, AAFP, Kansas, USA) is useful to help the clinician through a set of detailed manoeuvres in a calm, logical way. Each manoeuvre is attempted for a maximum of 30 s before moving to the next (Fig. 46.7). Recent simulation evidence suggests that compared with internal rotation, removal of the posterior arm as the initial internal manoeuvre may be more successful at reducing the degree of brachial plexus stretch and potentially be less traumatic to the fetus. However, in clinical practice, practitioners should initially use the manoeuvre they are most familiar with but be prepared to use other manoeuvres if that is unsuccessful.
If all else fails, there are three ‘last resort’ measures. These are described here in brief:
1. Posterior axillary traction: this manoeuvre involves placing the index fingers of each hand into the posterior fetal axilla which lies in the sacral curve. A similar technique involves passing a soft catheter between the body and humerus and this allows traction to be applied to the axilla (posterior axillary sling technique – PAST)
2. Symphysiotomy: the symphyseal joint is split with a scalpel, thereby increasing the pelvic diameters (Fig. 46.8). Both legs must be supported during the process to prevent excessive abduction of the hips
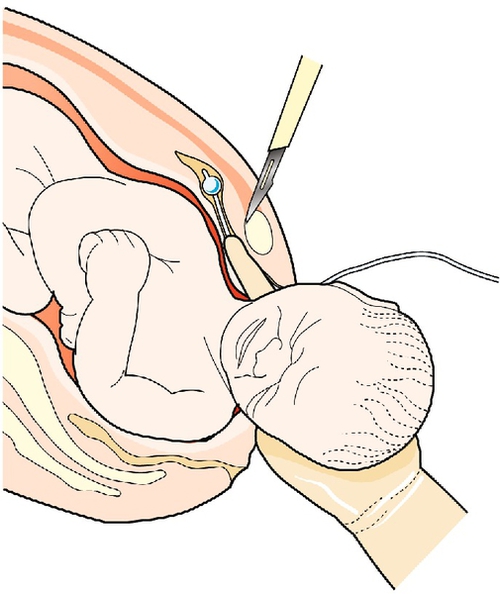
The left forefinger is shown displacing the urethra to the maternal left. A scalpel is positioned above the pubic symphysis and the joint divided anterior to posterior.
3. One or both clavicles of the fetus may be deliberately fractured to reduce the bisacromial distance
4. The Zavanelli manoeuvre: this involves replacing the head with flexion and rotation, and then delivering by caesarean section. In the largest series to date, out of 59 such procedures, 53 were successful. Generally, the fetal outcome is poor, often because this is a manoeuvre of last resort.
Shoulder dystocia remains an extremely serious, unpredictable and relatively rare event. Fetal survival and neurological normality are proportional to the speed of successful resolution.
Uterine inversion
Definition
Uterine inversion is rare, occurring in 1/2000–1/20 000 pregnancies, but as it may quickly lead to maternal death, it is an extremely significant third-stage complication. The uterus may undergo varying degrees of inversion and, in its extreme form, the fundus may pass through the cervix, such that the whole uterus is turned completely inside out. As there is a rich vagal supply to the cervix, the inversion leads to profound vasovagal shock, and this may be exacerbated by massive postpartum haemorrhage secondary to uterine atony.
Pathology
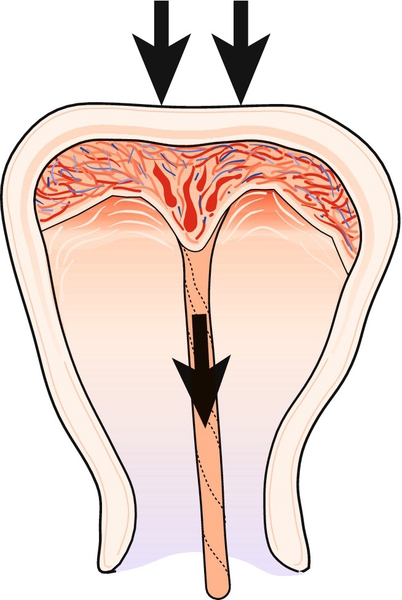
Inversion occurs with active management of the third stage, that is to say it is usually iatrogenic, associated with cord traction before the uterus contracts (Fig. 46.9). It is more likely with a fundal placenta and is found in association with the factors listed in Box 46.8.
Clinical presentation
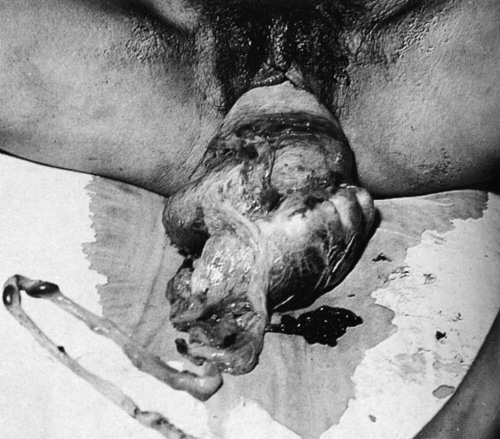
With complete inversion, the uterus will appear as a bluish-grey mass protruding from the vagina, and in extreme cases there may also be vaginal eversion. The placenta remains attached in about 50% of cases. If the inversion is partial, the only obvious sign may be that of profound shock out of proportion to any blood loss. The diagnosis will require a vaginal examination although an abnormally shaped uterine fundus on abdominal palpation often suggests the diagnosis. Rarely, the presentation is sudden death following neurogenic shock (Fig. 46.10).
Management
Some 90% of patients will have immediate, potentially major life-threatening haemorrhage. In order to minimize vasovagal-induced shock and also haemorrhage, it is imperative to replace the uterus as quickly as is practicable. Immediate resuscitation is required (Box 46.1) and should involve all available obstetric and anaesthetic help. Simultaneous attempts should be made to replace the uterus either within the vagina or possibly back through the cervix if possible. No attempt should be made to separate the placenta, as this may exacerbate the haemorrhage.
One method of reduction is to grasp the uterine fundus with the fingers directed towards the posterior fornix and replace the uterus back into the vagina, pushing the fundus towards the umbilicus and allowing the uterine ligaments to pull the uterus back into position (Fig. 46.11). Alternatively, the centre of the uterus may be indented with three or four fingers and only the centre of the fundus pushed up until it re-inverts. Once re-inversion has occurred, the hand inside the uterus should maintain pressure on the uterine fundus until oxytocics have been given, in order to maintain a contracted uterine state and prevent recurrence.
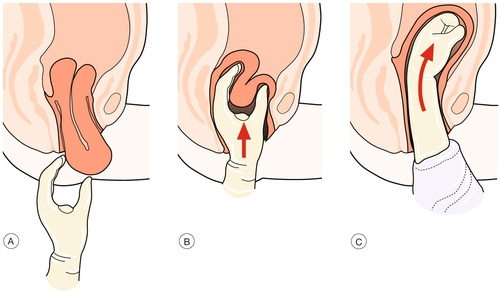
Fig. 46.11Replacing an inverted uterus.
(A) Recognition of uterine inversion. (B) Replacement of the uterus through the cervix. (C) Restitution of the uterus.
Should these methods fail, O’Sullivan’s hydrostatic technique should be employed. This involves passing 2 litres of warmed fluid into the vagina using either a ventouse cup or anaesthetic gas tubing. The resulting vaginal distension, especially at the vault, is extremely effective at allowing the uterus to return to the normal position. Up to 5 litres of fluid may be required to achieve uterine replacement. If successful, the fluid should be allowed to drain and oxytocics given as above.
Should all of these attempts fail, a laparotomy is required to aid re-inversion. An incision may be required at the rim of the inversion (Fig. 46.12). Hysterectomy is an option.
Uterine rupture
Loss of the integrity of the wall of the uterus may occur either suddenly, or more gradually during the progress of labour. The uterine cavity may communicate directly with the peritoneal cavity (a complete uterine rupture, see Fig. 29.4) or be separated from the peritoneal cavity by the visceral peritoneum of the uterus (incomplete uterine rupture or uterine dehiscence).
A complete uterine rupture is a life-threatening emergency often resulting in fetal death, and may lead to maternal death from massive intra-abdominal haemorrhage. Early recourse to caesarean section in ‘high-risk’ parous labours with signs of obstruction is likely to reduce the incidence.
Epidemiology
This obstetric emergency is rare in multiparous women who have had previous vaginal deliveries and virtually unheard of in primigravidae. It does, however, complicate 0.6% of deliveries in those who have had a previous caesarean section, with the rupture occurring at the site of the caesarean section incision. This risk increases further when oxytocin is used injudiciously and when the number of previous caesarean sections increases. Prostaglandin use is a particular risk and it is assumed that the consequent powerful contractions place a greater strain on the scar. Induction of labour with prostaglandin increases the risk of scar rupture by 14-fold. The risk of rupture is increased yet again if the previous caesarean section was ‘classical’ rather than ‘lower segment’ (i.e. midline rather than low transverse uterine incision) and up to a third of pregnancies with classical incisions may be complicated by rupture even several weeks before term. Most obstetricians would offer those with a midline uterine scar an early elective caesarean section.
Pathology
With complete rupture, the fetus may be extruded into the abdominal cavity. As the rupture can extend laterally into the uterine arteries or broad ligament plexus of veins, there is often severe haemorrhage. Rarely, rupture may occur following direct abdominal trauma, for example a road traffic accident.
In caesarean section scar dehiscence, the fetal membranes remain intact. There is usually minimal bleeding and the rupture does not usually involve the entire scar length. Occasionally, these are found incidentally at caesarean section carried out for other reasons.
Risk factors
There are many risk factors which increase the risk of uterine rupture (Box 46.9), and most of the intrapartum causes are the consequence of increased force being applied to the uterine muscle.
Clinical features
The most common sign of uterine rupture is that of fetal compromise identified by acute onset of significant CTG changes. This occurs in 70%. Other features include maternal tachycardia, vaginal bleeding (4%), abdominal pain (8%) and easily palpable fetal parts per abdomen. Occasionally, the fetal head is felt to have risen higher on vaginal examination. Dehiscence or rupture may occasionally be identified at a vaginal examination for postpartum haemorrhage. In severe instances, there may be cardiovascular collapse.
Management
If uterine rupture is suspected, the initial drill of summoning immediate help and resuscitation is followed by an immediate emergency laparotomy to deliver the baby. At the time of laparotomy, it may be possible to repair the defect, especially if this is simple dehiscence of a previous caesarean section scar. If there is massive haemorrhage (more likely if the rupture is complete) or if it does not involve a previous scar, or has led to extension of a scar, an emergency hysterectomy is likely to be required. Most cases of incomplete uterine rupture are not identified at the time of the acute rupture and only become apparent at caesarean section for fetal compromise.
Prognosis
With complete rupture and expulsion of the fetus into the abdominal cavity, the perinatal mortality rate approaches 75%. If untreated, most women would die from haemorrhage and infection.