Chapter 43 Obesity, Sleep Apnea, the Airway, and Anesthesia
II. Definitions of Obesity and Obstructive Sleep Apnea
III. Pathophysiology of Obstructive Sleep Apnea
IV. Diagnosis of Obstructive Sleep Apnea
V. Obesity, Obstructive Sleep Apnea, and the Airway
VI. Effects of Anesthesia and Surgery on Postoperative Sleep
VII. Perioperative Risks of Obesity and Obstructive Sleep Apnea
VIII. Intraoperative Considerations
I Introduction
Up to 80% of people with obstructive sleep apnea (OSA) are obese, and with obesity at epidemic proportions worldwide,1 OSA remains a major contributing factor to airway management difficulties. Numerous studies have reported major respiratory complications, including brain damage and death, in surgical patients with OSA.2,3 These disastrous outcomes result from failure to secure the airway during the induction of anesthesia, airway obstruction immediately after tracheal extubation, and respiratory arrest after the administration of opioids or sedation in the postoperative period.
The prevalence of OSA is about 20% in the U.S. population. It occurs as a result of partial or complete airway obstruction during sleep,4 and it is associated with episodic hypoxemia and hypercarbia.5–7 However, with the U.S. population aging and becoming obese, the prevalence of OSA is expected to increase significantly. Among the surgical population, patients with morbid obesity and OSA tend to be overrepresented due to the higher rates of obesity and OSA-related complications requiring surgical therapy.3,8 More than 75% of patients with OSA are undiagnosed or untreated.
II Definitions of Obesity and Obstructive Sleep Apnea
Obesity is a degree of excess weight associated with adverse health consequences.9 It is defined as a body mass index (BMI, or weight in kg divided by height in m2) greater than 29, and overweight is defined as a BMI of 25 to 29.9.9 A BMI of 40 or greater is classified as morbid obesity, and a BMI of 50 or greater designates super-obesity. Morbid obesity is associated with an increased risk of comorbidities,10 which may influence perioperative morbidity and mortality.11
OSA is a disorder characterized by repetitive upper airway collapse during sleep. Airflow ceases for more than 10 seconds, five or more times per hour, despite continuing ventilatory effort. It usually is associated with a decrease in arterial oxygen saturation (SaO2) of more than 4%.12 Obstructive sleep hypopnea is a greater than 50% decrease in airflow for more than 10 seconds occurring 15 times or more per hour of sleep. It is usually associated with a decrease of 4% or more in SaO2.
III Pathophysiology of Obstructive Sleep Apnea
A Pharyngeal Muscle Activity and Airway Patency
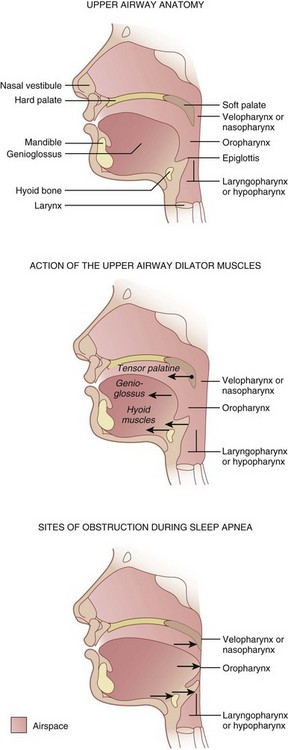
Three pharyngeal segments—nasopharynx (i.e., retropalatal pharynx), oropharynx (i.e., retroglossal pharynx), and laryngopharynx (i.e., retroepiglottic pharynx)—form the upper airway, which is a long, soft-walled tube that lacks bony support on the anterior and lateral walls, which makes it collapsible (Fig. 43-1).6 The transmural pressures across the pharyngeal walls (i.e., difference between extraluminal and intraluminal pressure) determine the patency of the upper airway. Activation of pharyngeal dilator muscles—the tensor veli palatini, the genioglossus, and the muscles of the hyoid bones (geniohyoid, sternohyoid, and thyrohyoid)—during inspiration counteracts the narrowing effects of reduced intraluminal pressure associated with inspiration. In addition to this inspiration-associated activation, tonic activity of these muscles during wakefulness helps to stabilize the pharyngeal walls.
B Sleep Pattern, Airway Obstruction, and Arousal
Normal sleep consists of four to six cycles of non–rapid-eye-movement (NREM) sleep followed by rapid-eye-movement (REM) sleep. The four stages of NREM sleep and one stage of REM sleep represent progressive slowing of the electroencephalographic waves. Rhythmic activity of the upper airway muscles decreases during deeper stages of sleep, which is accompanied by significant increase in upper airway resistance and consequent upper airway collapse.13
Contraction of the diaphragm during inspiration creates a subatmospheric pressure within the airway that may lead to narrowing of the collapsible segments of the pharynx.14 As pharyngeal pressure becomes more negative, pharyngeal collapse progressively increases. The lateral pharyngeal walls, a major site of pharyngeal adipose tissue deposition, are the most compliant and therefore the most common site of pharyngeal collapse.15 In obese patients, deposition of fat around the pharyngeal walls narrows the upper airway and increases the extraluminal pressure and collapse.16,17 For a given degree of loss of pharyngeal muscle tone and pharyngeal muscle collapse, a greater degree of pharyngeal obstruction is observed in patients with a posteriorly set tongue (caused by micrognathia and retrognathia or a receding mandible), large tongue, large tonsils, and nasal obstruction. Other factors that contribute to upper airway narrowing and subsequent collapse during sleep include: large neck circumference, anatomic or craniofacial abnormalities affecting the airway, and age.18,19
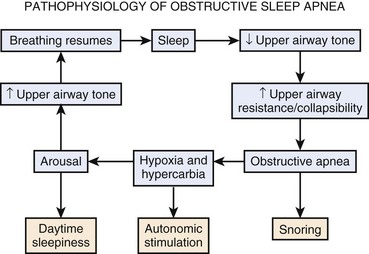
Airway collapse leads to obstructive apnea and consequently causes a decrease in arterial oxygen tension (PaO2) and increase in arterial carbon dioxide tension (PaCO2), which increases neural traffic in the reticular activating system, progressively increasing ventilatory efforts20,21 and causing arousal from sleep. Arousal, expressed as extremity twitching, gasping or snorting, vocalization, and increased electroencephalographic activity, reactivates the pharyngeal muscles and opens the upper airway. As the upper airway opens, ventilation resumes, which corrects hypoxia and hypercarbia.22 Hyperventilation after arousal reverses the blood gas disturbance to correspondingly decrease the central drive. The cycle repeats itself when the patient again falls asleep (Fig. 43-2).
Frequent arousals result in sleep disruption and excessive daytime somnolence, and oxygen desaturation, sympathetic hyperactivity, and a systemic inflammatory response may contribute to cardiovascular comorbidities such as systemic hypertension, cardiac arrhythmias, myocardial ischemia, pulmonary hypertension, and heart failure.23
IV Diagnosis of Obstructive Sleep Apnea
Because OSA is undiagnosed in an estimated 60% to 70% of patients and failure to recognize OSA preoperatively is one of the major causes of perioperative complications,5–7 all patients must be screened for OSA. Obtaining a thorough history and physical examination helps to determine a presumptive diagnosis of OSA, and polysomnography can confirm the diagnosis and severity of OSA and can determine the need for and level of continuous positive airway pressure (CPAP).
A Clinical Diagnosis
A systematic review and meta-analysis of clinical screening tests for OSA reported that the STOP-BANG screening tool was easy to use and a good predictor of severe OSA (i.e., apnea-hypopnea index [AHI] >30) (Box 43-1).24,25 The STOP-BANG questionnaire has a sensitivity of 93% and specificity of 43% at an AHI greater than 15 and a sensitivity of 100% and specificity of 37% at an AHI greater than 30.25 Other questionnaires, including the Berlin questionnaire and the American Society of Anesthesiologists (ASA) checklist, are also in clinical use and have a similar predictive accuracy for OSA.26–28
Box 43-1
STOP-BANG Scoring System
S = Snoring. Do you snore loudly (louder than talking or loud enough to be heard through closed doors)?
T = Tiredness. Do you often feel tired, fatigued, or sleepy during daytime?
O = Observed apnea. Has anyone observed you stop breathing during your sleep?
P = Pressure. Do you have or are you being treated for high blood pressure?
From Chung F, Yegneswaran B, Liao P, et al: STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 108:812–821, 2008.
When the cricomental space, defined as the perpendicular distance from a line between the cricoid cartilage and the inner mentum to the skin of the neck, is more than 1.5 cm, the diagnosis of OSA can be excluded with a negative predictive value of 100%.29 A decision rule developed to diagnose OSA using three predictors (i.e., cricomental space ≤1.5 cm, pharyngeal grade greater than II, and presence of an overbite) has a positive predictive value of 95% and may provide an alternative to polysomnography.29
B Polysomnography
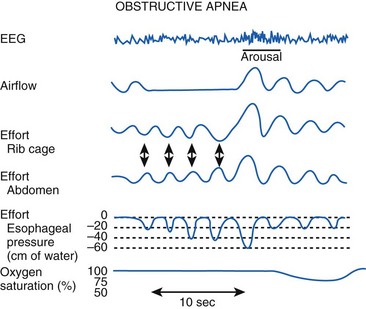
Polysomnography remains the gold standard in the diagnosis of OSA. Polysomnography consists of monitoring the electroencephalogram (EEG), electrooculogram (EOG), and submental electromyogram (EMG) for staging sleep. Oral and nasal airflow, respiratory efforts (i.e., inductance or impedance pneumography to monitor thoracoabdominal motion or the diaphragmatic EMG), oximetry, and capnography are also monitored. Body position, sound, arterial blood pressure, and the electrocardiogram are monitored (Fig. 43-3).30
The results of a sleep study are reported as events and indices. Events include apnea (no airflow ≥10 seconds), hypopnea (tidal volume [VT] ≤50% of the control awake value ≥10 seconds), desaturation (>4% decrease in SaO2), and arousal, which may be detected clinically (i.e., vocalization, turning, or extremity movement) or by an electroencephalographic burst.12 Indices are measured as events per hour, which include the AHI (i.e., number of times a patient was apneic or hypopneic per hour), oxygen desaturation index (i.e., number of times a patient had a more than 4% decrease in SaO2 per hour), and arousal index (i.e., number of times a patient was aroused per hour). If the patient has OSA, the entire sleep study is repeated with CPAP titration to determine the level of CPAP that causes a significant decrease in the AHI.
Because polysomnography may not always be available, other screening devices with single or multiple channels have been explored and may represent alternative methods to diagnose OSA. One study suggested that an O2 saturation value of more than 94% on room air in the absence of other causes should lend consideration to the diagnosis of long-standing OSA.31 The American Academy of Sleep Medicine recommended that the portable monitoring used as an alternative to a polysomnogram must record airflow, respiratory effort, and blood oxygenation. The device also must allow display of raw data with a capability for manual scoring or editing of automated scoring.32
It is unclear whether a routine preoperative sleep study (i.e., polysomnogram or home sleep study) could improve perioperative outcomes, because the optimal duration of preoperative CPAP therapy before proceeding with elective surgical procedures is unknown, and compliance with CPAP varies. For those suspected of having OSA based on clinical criteria, anesthesiologists may elect to proceed with a presumptive diagnosis of OSA, unless the patients have significant comorbidities.5,7
V Obesity, Obstructive Sleep Apnea, and the Airway
Obesity (determined by the BMI) is considered a predictor of difficult mask ventilation (DMV) and difficult intubation (DI).33 Morbidly obese patients have deposits of excess adipose tissue in the neck, breast, thoracic wall, and abdomen that may impede patency of and access to the upper airway. Magnetic resonance imaging studies of obese patients found greater amounts of fat in areas surrounding the collapsible segments of the pharynx in those with OSA,34,35 which may explain the difficulty in airway management in obese patients with OSA but not in all obese patients. The distribution pattern of body fat may be a more relevant factor contributing to difficult airway management than the BMI itself.34 Clinical studies have found that BMI alone is not a good predictor of a difficult airway.36–40
Patients with severe OSA (AHI ≥40) have been shown to be at a significantly higher risk for DMV and DI, leading to speculation that they may have different anatomic characteristics compared with patients who have less severe OSA.41 Obese patients with OSA have larger neck circumferences than equally obese patients (i.e., similar BMI) without OSA.16,42 This neck “mass loading” (up to 28% increase in neck soft tissue) may be responsible for a more collapsible airway, leading to DMV and DI.16 Men have a higher percentage of soft tissue and fat in the neck compared with women,43,44 which may explain greater airway difficulties in male OSA patients compared with female OSA patients. A logistic regression model identified neck circumference at the level of the thyroid cartilage as the single most predictor of problematic intubation.45 Probability of a DI increases significantly with a neck circumference of 40 cm or more.45,46 Neck circumference corrected for height (i.e., neck circumference/height) is sensitive and specific for detecting OSA compared with neck circumference alone.47 Racial differences in craniofacial anatomy may contribute to the severity of OSA and to a DI.48,49 Other factors that may contribute to difficult airway management include diabetes mellitus and abnormal facial morphology.50,51
Ultrasonography has been used to quantify neck soft tissue at the level of the vocal cords and suprasternal notch to determine potential predictors of difficult laryngoscopy in morbidly obese patients.52 The amount of pretracheal soft tissue was found to be a strong measure distinguishing an easy laryngoscopy from a difficult one.52 Lateral head and neck radiography can easily identify caudal soft tissue displacement, which shifts the hyoid bone caudally to increase the distance between the mandible and the hyoid bone. When this distance is more than 20 mm, the presence of OSA and a possible difficult airway should be suspected.53 Radiographic evaluation has affirmed strong relationships among DI and higher Mallampati scores, OSA, greater mandibular depth, and smaller mandibular and cervical angles.54
VI Effects of Anesthesia and Surgery on Postoperative Sleep
Other factors that influence sleep patterns and can exacerbate sleep disorders include the stress response to surgical insult and postoperative anxiety, pain, and opioids.55 These factors reduce REM sleep in the immediate postoperative period, which is followed by a rebound REM sleep that can last for several days after surgery.56 The rebound REM sleep makes patients with OSA even more vulnerable to airway obstruction. Postoperative sleep disturbances appear to be related to the location and invasiveness of the surgical procedure.57 For example, fewer sleep disturbances occur after mild or moderately invasive surgery than after major surgical procedures.
VII Perioperative Risks of Obesity and Obstructive Sleep Apnea
Factors that determine the perioperative risks in obese and OSA patients include the degree of obesity (i.e., BMI) and the severity of OSA, invasiveness of anesthesia and surgery, and postoperative opioid requirements.27 The ASA practice guidelines propose a scoring system that may be used to estimate whether an OSA patient is at increased risk for perioperative complications27 and to determine perioperative management (Box 43-2).
Box 43-2
American Society of Anesthesiologists Scoring System for Estimating Perioperative Complications
A. Severity of sleep apnea is based on sleep study (i.e., AHI) results or clinical indicators if a sleep study is not available:
B. Invasiveness of surgery and anesthesia:
Adapted from Gross JB, Bachenberg KL, Benumof JL, et al: Practice guidelines for the perioperative management of patients with obstructive sleep apnea: A report by the Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 104:1081–1093, 2006.
VIII Intraoperative Considerations
The major concerns during induction of anesthesia or sedation/analgesia technique in obese and in OSA patients include DMV, DI, and increased risk of regurgitation of gastric contents and potential pulmonary aspiration.58 General anesthesia with a secure airway is considered preferable to deep sedation without an airway,27 which is increasingly used in modern anesthesia practice.59
A Preinduction Considerations
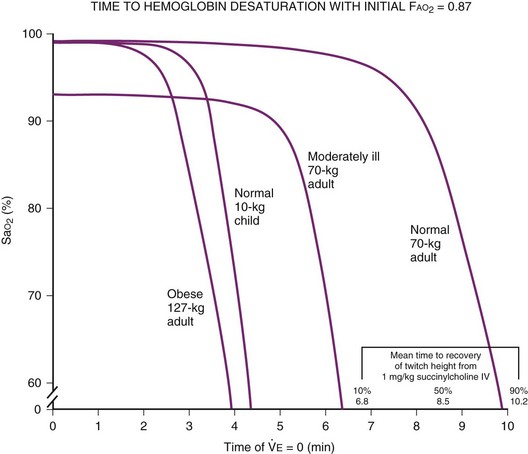
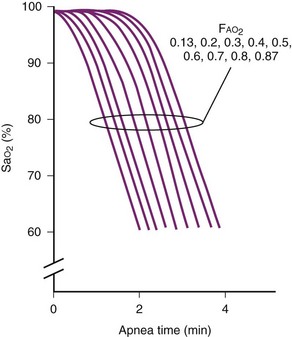
Alterations in pulmonary function (e.g., reduced functional residual capacity [FRC] and O2 reserves) in obese patients may result in severe hypoxemia even after short periods of apnea (Fig. 43-4).60,61 Positioning of the patient in the head-elevated laryngoscopy position (HELP), which can be achieved by stacking blankets or a specially designed foam pillow (Troop Elevation Pillow, CR Enterprises, Frisco, TX)62 or the inflatable Rapid Airway Management Positioner (RAMP, Airpal Inc., Center Valley, PA),63 can compensate for the exaggerated flexed position from posterior cervical fat. The objective of this maneuver is to elevate the head, upper body, and shoulders above the chest so that an imaginary horizontal line connects the sternal notch with the external auditory meatus to create a better alignment among the oral, pharyngeal, and laryngeal axes (see Fig. 9-1 in Chapter 9). This position structurally improves maintenance of the passive pharyngeal airway, facilitates bag-mask ventilation, and improves the success of endotracheal intubation.
Other techniques used to avoid postinduction hypoxemia include preoxygenation with 100% O2 until the end-tidal oxygen value is at least 90% and use of 10 cm H2O of CPAP or bi-level positive airway pressure (BiPAP) ventilation (i.e., intermittent positive-pressure ventilation [PPV] with positive end-expiratory pressure [PEEP]) with the patient in a 25-degree head-up position.60,64–66 Preinduction techniques followed by 10 cm H2O of PEEP during bag-mask ventilation and after intubation can reduce post-intubation atelectasis and improve arterial oxygenation.67 A poorly sealed preoxygenation system results in difficulty with achieving adequate preoxygenation within a reasonable period of time (Fig. 43-5).6
B Awake Endotracheal Intubation
One of the critical decisions regarding induction of general anesthesia in the obese patient with OSA is to determine whether awake intubation should be performed. Awake intubation should be considered when any component of the triple maneuver (i.e., mandible advancement, neck extension, and mouth opening) is unattainable.68 Obese OSA patients typically are more difficult to intubate than their non-OSA counterparts,51 but obesity by itself has not been largely associated with difficult laryngoscopy or DI.36,37,41,68 A retrospective analysis performed to identify patient characteristics that influence the choice of awake fiberoptic intubation or intubation after general anesthesia in obese patients revealed that awake intubation patients were more likely to be male, have a BMI of 60 kg/m2 or greater, and be assigned to a Mallampati class of III or IV.69 Because no single factor predicts DMV or DI, it may be prudent to combine multiple predictors, such as Mallampati class III or IV,70 neck circumference of 40 cm or larger, limited mandibular protrusion,38 and severe OSA (AHI ≥40), to determine the need for awake intubation.
During awake intubation, sedatives and opioids, although desirable, should be minimized or totally avoided if possible, because airway obstruction can occur while the airway is being secured.6 Dexmedetomidine is a highly selective α2-adrenergic agonist with sedative, amnestic, analgesic, and sympatholytic properties that does not cause respiratory depression.71 It reduces salivary secretions through sympatholytic and vagomimetic effects, which should improve visualization during fiberoptic intubation and facilitate awake intubation. The addition of ketamine with dexmedetomidine may further improve a patient’s tolerance to awake intubation without impairing respiration.72
Topical anesthesia applied to facilitate awake intubation may compromise the airway patency.73,74 In the upper airway, topical anesthesia may impair neural compensatory mechanoreceptors necessary for the arousal response and cause narrowing of the pharyngeal cross-sectional area, which may induce and prolong apneic episodes.73,74 The spray-as-you-go technique for topicalization of the local anesthetic during awake intubation is often performed to minimize the time of obtundation of laryngeal reflexes.
C Endotracheal Intubation After Induction of Anesthesia
If endotracheal intubation is planned after induction of anesthesia, adequate preparation must be made for a difficult airway based on the ASA difficult airway management guidelines.75 Emergency airway equipment (e.g., video laryngoscopes, supralaryngeal airways, flexible fiberoptic bronchoscopes) and additional help must be immediately available. Video laryngoscopes offer superior viewing of the glottis and reduce the duration of performing endotracheal intubation, thereby preventing significant desaturation in morbidly obese patients.76–78 The laryngeal mask airway is an effective rescue device for the difficult airway or failed airway, even in obese patients.79,80 However, the increased intra-abdominal pressures associated with obesity may increase the risk of gastric aspiration with the use of the laryngeal mask airway, because it does not completely seal the airway. Restrictive pulmonary disease in obese patients can increase the peak inspiratory pressures and cause leaks around the laryngeal mask airway cuff, leading to hypoventilation and gastric insufflation.81,82
A short-acting muscle relaxant (e.g., succinylcholine) is recommended because it allows a rapid recovery, which may allow rapid return of spontaneous breathing. However, even with low-dose succinylcholine, recovery of breathing and pharyngeal patency may not occur before development of severe hypoxemia because morbidly obese patients can desaturate rapidly.61 A higher dose of succinylcholine has been recommended for optimal intubating conditions in morbidly obese patients.83,84
Use of high-dose rocuronium may allow rapid intubating conditions, but its longer duration of action may be detrimental during a DI or DMV. Sugammadex (not available in the United States) can rapidly reverse rocuronium-induced muscle relaxation in case of impossible bag-mask ventilation or endotracheal intubation to avoid disastrous consequences.85 However, it does not reverse unconsciousness induced by the intravenous anesthetic agent, and airway patency therefore may not be restored.
The need for rapid-sequence induction in the obese patient with no other risk factors (e.g., diabetes, history of significant reflux) is being questioned.5,86 Controlled induction of general anesthesia allows appropriate titration of intravenous anesthetic, prevents hemodynamic instability that can occur from a predetermined dose, allows adequate ventilation, and avoids hypoxia between induction and endotracheal intubation. However, these benefits should be weighed against the risks of DMV and DI with rapid development of severe O2 desaturation when faced with minimal risk of aspiration.
D Mechanical Ventilation
The aim of mechanical ventilation in obese patients is to prevent progressive atelectasis that is commonly seen in this patient population.87 Proposed ventilatory strategies for obese patients include the use of lower inspired oxygen concentrations (FIO2) to maintain physiologic oxygenation,88 low VT (6 to 8 mL/kg ideal body weight),89 application of PEEP (5 to 10 cm H2O), and inclusion of recruitment maneuvers (i.e., large manual or automatic lung inflations).90–93 Recruitment maneuvers to adequately open up the collapsed alveoli may require airway pressures of up to 55 cm H2O, which may have deleterious effects on hemodynamics and therefore should be performed only after hemodynamic stabilization and be maintained for only a short period.94 Use of pressure-controlled ventilation may allow improved distribution of gases and lower peak airway pressure.95,96 Hyperventilation should be avoided because hypocapnia may cause metabolic alkalosis and postoperative hypoventilation.87
IX Postoperative Considerations
There is a high risk of post-extubation airway obstruction in obese patients with OSA, which is further increased after airway surgery with subsequent nasal packing. The ASA Closed Claims Project database analysis of lawsuits involving loss of airway after tracheal extubation found that those cases resulted in higher incidences of brain death and death than loss of airway during induction of anesthesia.97 In 67% of the lawsuits, the patients were obese, and 28% had a history of OSA.97
The effects of obesity on respiratory mechanics, including a reduction in FRC, a decrease in lung compliance, expiratory flow limitation, and development of intrinsic PEEP, act together to elevate the work of breathing at rest and increase the risk of perioperative pulmonary complications.98,99 This situation may be compounded by the negative respiratory effects induced by sedative/hypnotics and muscle relaxants that can persist for several hours after anesthesia.100,101
Factors to consider when determining whether to leave the patient intubated after surgery include BMI, severity of OSA, associated cardiopulmonary disease, ease of bag-mask ventilation and intubation at induction of anesthesia, type and duration of surgical procedure, and the intraoperative course. Patients with OSA should be extubated in a semirecumbent position after they are fully awake (i.e., rational, oriented, and responding to commands in a quick and unambiguous manner) and after verification of complete reversal of neuromuscular blockade. A nasopharyngeal or oropharyngeal airway may prevent post-extubation airway obstruction.102
A Postoperative Noninvasive Positive-Pressure Ventilation
CPAP is the most commonly used form of noninvasive PPV in OSA patients. It works by acting as a pneumatic splint to prevent airway collapse during sleep, thereby reducing the work of breathing by counteracting auto-PEEP to reduce respiratory muscle load, improving lung function (particularly FRC), and improving gas exchange.103,104 Auto-CPAP, delivered by a self-titrating device, uses algorithms to detect variations in the degree of obstruction and adjusts to the pressure level to restore normal breathing, thereby compensating for factors modifying upper airway collapsibility, including stage of sleep, posture during sleep, and the use of drugs that affect upper airway muscle tone.105 However, it has not been confirmed that auto-CPAP is more effective than fixed CPAP.106,107
CPAP or BiPAP should be applied as soon as possible after surgery to patients who are receiving it preoperatively. It should be instituted when nausea, vomiting, post-extubation suctioning, level of consciousness, communication, facial edema, and drug depression issues are minimal or nonexistent.27 The use of CPAP immediately after tracheal extubation may improve postoperative pulmonary function.108
B Postoperative Disposition
Requirements for postoperative monitoring depend on patient-specific factors (e.g., high BMI, severe OSA, associated cardiopulmonary disease), invasiveness of the anesthetic technique, the type and duration of surgery, and the intraoperative course.31 Patients with severe OSA undergoing an extensive surgical procedure that requires significant opioid analgesia may require close monitoring in a monitored environment (e.g., intensive care unit, step-down unit). Creation of intermediate care (observational) units with higher nursing ratios has been suggested as the most rational solution for postoperative care of morbidly obese patients with OSA who have moderate disease and whose conditions are not severe enough to qualify for an intensive care unit.
The scientific literature regarding the safety of ambulatory surgery in OSA patients is sparse and of limited quality,5 and the suitability of these procedures remains controversial. The ASA practice guidelines propose a scoring system that may be used to determine the suitability for ambulatory surgery (see Box 43-2).27 Patients who are at significantly increased risk for perioperative complications (score ≥ 5) are not good candidates for ambulatory surgery.27 OSA patients should be monitored for a median of 3 hours longer than their non-OSA counterparts before discharge from the facility.27 Monitoring should continue for a median of 7 hours after the last episode of airway obstruction or hypoxemia while breathing room air in an unstimulated environment. Unfortunately, the recommendation for longer postoperative stays is not based on any scientific evidence and may be the major limitation on performing surgical procedures in an ambulatory setting.
XI Clinical Pearls
• Clinical diagnosis of obstructive sleep apnea (OSA) may be made by observing the components of the classic triad of sleep-disordered breathing (i.e., history or observation of apnea or snoring with hypopnea during sleep), arousal from sleep (i.e., extremity movement, turning, vocalization, or snorting), and daytime sleepiness (i.e., easily falling asleep during quiet times of the day) or fatigue.
• A high body mass index (BMI) is a weak but statistically significant predictor of difficult intubation (DI) or failed endotracheal intubation and of difficult mask ventilation (DMV). The distribution pattern of body fat, rather than the BMI value, may be a more relevant factor in a difficult laryngoscopy. Measuring the neck circumference at the level of the thyroid cartilage is a useful addition to the normal daily practice of measuring weight or BMI during preoperative airway evaluation.
• A lateral head and neck radiograph demonstrating caudal soft tissue displacement that shifts the hyoid bone caudally to increase the distance between the mandible and the hyoid bone to more than 20 mm suggests the presence of obstructive sleep apnea (OSA) and possible difficult airway.
• General anesthesia with a secure airway is preferable to deep sedation without an airway for superficial procedures and for OSA patients undergoing procedures involving the upper airway.
• Intubation under general anesthesia should be carried out with the patient fully preoxygenated to prevent hypoxia because the relatively low functional residual capacity (FRC) found in obese patients causes them to desaturate more rapidly.
• The head-elevated laryngoscopy position (HELP) significantly elevates the head, upper body, and shoulders above the chest so that an imaginary horizontal line connects the sternal notch with the external auditory meatus to create a better alignment among the three axes.
• Intravenous anesthetic agents, except for ketamine, significantly decrease inspiratory negative airway pressure by depressing the chemical drive. Ketamine has a less depressant effect on pharyngeal dilating muscle activity, but it increases pharyngeal secretions, thereby offsetting the beneficial effect.
• A short-acting muscle relaxant, such as succinylcholine, does not ensure recovery of muscle function or pharyngeal patency before development of severe hypoxemia in morbidly obese patients with significantly decreased FRC.
• Rapid-sequence intubation is often recommended in obese patients because of the high prevalence of lower esophageal sphincter hypotonia, which increases the prevalence of gastroesophageal reflux disease, but the benefits should be weighed against the risks of DMV or DI.
• The aim of mechanical ventilation in obese patients is to keep the lungs open during the entire respiratory cycle to counteract the negative effects of respiratory modifications induced by general anesthesia and paralysis, which can persist for a few days postoperatively.
• The endotracheal tube should be left in place, or extubation should be carried out over an airway exchange catheter if any doubt exists about the ability of the patient to breathe spontaneously or the ability of the practitioner to reintubate in an emergency.
• Tracheal extubation should occur in the semi-upright or head-up position only after the obese OSA patient regains full consciousness after general anesthesia and after confirming airway patency and verification of complete reversal of neuromuscular blockade. Continuous positive airway pressure (CPAP) should be applied as soon as possible after surgery in patients who are receiving it preoperatively, but it should be instituted when nausea, vomiting, post-extubation suctioning, level of consciousness, communication, facial edema, and drug depression issues are minimal or nonexistent.
• Obese OSA patients should be cared for in a monitored environment because of an increased risk of opioid-induced postoperative upper airway obstruction.
All references can be found online at expertconsult.com.
5 Joshi GP. The adult obese patient with sleep apnea for ambulatory surgery. ASA refresher courses in anesthesiology. Anesthesiology. 2007;35:97–106.
6 Benumof JL. Obesity, sleep apnea, the airway and anesthesia. Curr Opin Anaesthesiol. 2004;17:21–30.
7 Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489–1498.
8 Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563.
18 Tsuiki S, Isono S, Ishikawa T, et al. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108:1009–1015.
24 Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110:928–939.
25 Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821.
27 From Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: A report by the Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
68 Isono S. Obstructive sleep apnea of obese adults: Pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–921.
87 Gertler R, Joshi GP. Modern understanding of intraoperative mechanical ventilation in normal and diseased lungs. Adv Anesth. 2010;26:15–33.
1 O’Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–26.
2 Riley RW, Powell NB, Guilleminault C, et al. Obstructive sleep apnea surgery: Risk management and complications. Otolaryngol Head Neck Surg. 1997;117:648–652.
3 Gupta R, Parvizi J, Hanssen A, et al. Postoperative complications in patients with obstructive sleep apnea undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76:897–905.
4 Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235.
5 Joshi GP. The adult obese patient with sleep apnea for ambulatory surgery. ASA refresher courses in anesthesiology. Anesthesiology. 2007;35:97–106.
6 Benumof JL. Obesity, sleep apnea, the airway and anesthesia. Curr Opin Anaesthesiol. 2004;17:21–30.
7 Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489–1498.
8 Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563.
9 Bessesen D. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027–2034.
10 Herrara MF, Lozano-Salazar RR, Gonzalez-Barranco J, et al. Disease and problems secondary to massive obesity. Eur J Gastroenterol Hepatol. 1999;11:63–67.
11 Ogunnaike BO, Jones SB, Jones DB, et al. Anesthetic considerations for bariatric surgery. Anesth Analg. 2002;95:1793–1805.
12 Strollo PJ, Rogers RM. Obstructive sleep apnea. Current concepts. N Engl J Med. 1996;334:99–104.
13 Wiegand I, Zwillich CW, White DP. Collapsibility of the human airway during normal sleep. J Appl Physiol. 1989;66:1800–1808.
14 Morrell MJ, Arabi Y, Zahn B, et al. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med. 1998;158:1974–1981.
15 Schwab RI, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154:1106–1116.
16 Mortimore IL, Marshall I, Wraith PK, et al. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–283.
17 Kryger M, Felipe L, Holder D, et al. The sleep deprivation syndrome of the obese patient: A problem of periodic nocturnal upper airway obstruction. Am J Med. 1974;56:531–539.
18 Tsuiki S, Isono S, Ishikawa T, et al. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108:1009–1015.
19 Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: The predisposition to pharyngeal collapse. Am J Med. 2006;119:e9–e14.
20 Kimoff RJ, Cheong TH, Olha AE, et al. Mechanisms of apnea termination in obstructive sleep apnea: Role of chemoreceptor and mechanoreceptor stimuli. Am J Respir Crit Care Med. 1994;149:707–714.
21 Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300.
22 White DP. Pathophysiology of obstructive sleep apnea. Thorax. 1995;50:797–804.
23 McNicholas WT, Ryan S. Obstructive sleep apnea syndrome: Translating science to clinical practice. Respirology. 2006;11:136–144.
24 Ramachandran SK, Josephs LA. A meta-analysis of clinical screening tests for obstructive sleep apnea. Anesthesiology. 2009;110:928–939.
25 Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821.
26 Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for sleep apnea syndrome. Ann Intern Med. 1999;131:485–491.
27 Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: A report by the Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–1093.
28 Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–830.
29 Tsai WH, Remmers JE, Brant R, et al. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–1432.
30 Practice parameters for the indications for Polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–422.
31 Seet E, Chung F. Management of sleep apnea in adults: Functional algorithms for the perioperative period: Continuing professional development. Can J Anesth. 2010;57:849–864.
32 Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747.
33 Bond A. Obesity and difficult intubation. Anaesth Intensive Care. 1993;21:828–830.
34 Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of the fat deposits around the pharynx in obese patients with obstructive sleep apnea and weight matched controls. Eur Respir J. 1989;2:613–622.
35 Shelton KE, Gay SB, Woodson H, et al. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466.
36 Ezri T, Medalion B, Weisenberg M, et al. Increased body mass index per se is not a predictor of difficult laryngoscopy. Can J Anesth. 2003;50:179–183.
37 Lundstrom LH, Moller AM, Rosenstock C, et al. High body mass index is a weak predictor for difficult and failed tracheal intubation: A cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009;110:266–274.
38 Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–891.
39 Kheterpal S, Martin S, Shanks AM, et al. Prediction and outcomes of impossible mask ventilation: A review. Anesthesiology. 2009;110:891–897.
40 Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
41 Kim JA, Lee JJ. Preoperative predictors of difficult intubation in patients with obstructive sleep apnea syndrome. Can J Anesth. 2006;53:393–397.
42 Hoffstein V, Mateika S. Differences in abdominal and neck circumference in patients with and without obstructive sleep apnea. Eur Respir J. 1992;5:377–381.
43 Malhotra A, Huang Y, Fogel R, et al. The male predisposition to pharyngeal collapse: Importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395.
44 Whittle AT, Marshall I, Mortimore IL, et al. Neck soft tissue and fat distribution: Comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–328.
45 Brodsky JB, Lemmens HJM, Brock-Utne JG, et al. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–736.
46 Gonzalez H, Minville V, Delanoue K, et al. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg. 2008;106:1132–1136.
47 Kawaguchi Y, Fukumoto S, Inaba M, et al. Different impacts of neck circumference and visceral obesity on the severity of obstructive sleep apnea symdrome. Obesity. 2011;19:276–282.
48 Sakakibara H, Tong M, Matsushita K, et al. Cephalometric abnormalities in non-obese and obese patients with obstructive sleep apnoea. Eur Respir J. 1999;13:403–410.
49 Li KK, Kushida C, Powell NB, et al. Obstructive sleep apnea syndrome: A comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–1693.
50 Lowe AA, Santamaria ID, Fleetham JA, et al. Facial morphology and obstructive sleep apnea. Am J Orthod Dentofac Orthop. 1986;90:484–491.
51 Siyam MA, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome. Anesth Analg. 2002;95:1098–1102.
52 Ezri T, Gerwürtz G, Sessler DI, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia. 2003;58:1111–1114.
53 Ferguson KA, Ono T, Lowe AA, et al. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108:375–381.
54 Nuckton TJ, Glidden DV, Browner WS, et al. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29:903–908.
55 Cronin AJ, Keifer JC, Davies MF, et al. Postoperative sleep disturbance: Influences of opioids and pain in humans. Sleep. 2001;24:39–44.
56 Gögenur I, Wildschiøtz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100:45–49.
57 Knill RL, Moote CA, Skinner MI, et al. Anesthesia with abdominal surgery leads to intensive REM sleep during the first postoperative week. Anesthesiology. 1990;73:52–61.
58 Sabate JM, Jouet P, Merrouche M, et al. Gastroesophageal reflux in patients with morbid obesity: A role of obstructive sleep apnea syndrome? Obes Surg. 2008;18:1479–1484.
59 Hession PM, Joshi GP. Sedation: not quite that simple. Anesthesiology Clin. 2010;28:281–294.
60 Rusca M, Proietti S, Schnyder P, et al. Prevention of atelectasis formation during induction of general anesthesia. Anesth Analg. 2003;97:1835–1839.
61 Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
62 Rich JM. Use of an elevation pillow to produce the head-elevated laryngoscopy position for airway management in morbidly obese and large framed patients. Anesth Analg. 2004;98:264–265.
63 Cattano D, Melnikov V, Khalil Y, et al. An evaluation of the rapid airway management positioner in obese patients undergoing gastric bypass or laparoscopic gastric banding surgery. Obes Surg. 2010;20:1436–1441.
64 Cressey DM, Berthoud MC, Reilly CS. Effectiveness of continuous positive airway pressure to enhance pre-oxygenation in morbidly obese women. Anaesthesia. 2001;56:680–684.
65 El-Khatib MF, Kanazi G, Baraka AS. Noninvasive bilevel positive airway pressure for preoxygenation of the critically ill morbidly obese patient. Can J Anaesth. 2007;54:44–47.
66 Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: A randomized controlled study. Anesthesiology. 2005;102:1110–1115.
67 Coussa M, Proietti S, Schnyder P, et al. Prevention of atelectasis formation during the induction of general anesthesia in morbidly obese patients. Anesth Analg. 2004;98:1491–1495.
68 Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–921.
69 Hagberg CA, Vogt-Harenkamp C, Kamal J. A retrospective analysis of airway management in obese patients at a teaching institution. J Clin Anesth. 2009;21:348–351.
70 Voyagis G, Kyriakis K, Dimitriou V, et al. Value of oropharyngeal Mallampati classification in predicting difficult laryngoscopy among obese patients. Eur J Anaesthesiol. 1998;15:330–334.
71 Bergese SD, Candiotti KA, Bokesch PM, et al. A phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–595.
72 Corey SS, Melvin CG. Dexmedetomidine and low-dose ketamine provide adequate sedation for awake fibreoptic intubation. Can J Anesth. 2003;50:607–610.
73 Berry RB, Kouchi KG, Bower JL, et al. Effect of upper airway anesthesia on obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:1857–1861.
74 Cala SJ, Sliwinski P, Cosio MG, et al. Effect of topical upper airway anesthesia on apnea duration through the night in obstructive sleep apnea. J Appl Physiol. 1996;81:2618–2626.
75 Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–1277.
76 Maassen R, Lee R, Hermans B, et al. A comparison of three videolaryngoscopes: The Macintosh laryngoscope blade reduces, but does not replace, routine stylet use for intubation of morbidly obese patients. Anesth Analg. 2009;109:1560–1565.
77 Maassen R, Lee R, van Zundert A, et al. The videolaryngoscope is less traumatic than the classic laryngoscope for a difficult airway in an obese patient. J Anesth. 2009;23:445–448.
78 Ndoko SK, Amathieu R, Tual L, et al. Tracheal intubation of morbidly obese patients: A randomized trial comparing performance of Macintosh and Airtraq laryngoscopes. Br J Anaesth. 2008;100:263–268.
79 Frappier J, Guenoun T, Journois D, et al. Airway management using the intubating laryngeal mask airway for the morbidly obese patient. Anesth Analg. 2003;96:1510–1515.
80 Keller C, Brimacombe J, Kleinsasser A, et al. The laryngeal mask airway ProSeal(TM) as a temporary device in grossly and morbidly obese patients before laryngoscope-guided tracheal intubation. Anesth Analg. 2002;94:737–740.
81 Weiler N, Latorre F, Eberle B, et al. Respiratory mechanics, gastric insufflation pressure, and air leakage of the laryngeal mask airway. Anesth Analg. 1997;84:1025–1028.
82 Devitt JH, Wenstone R, Noel AG, et al. The laryngeal mask airway and positive pressure ventilation. Anesthesiology. 1994;80:550–555.
83 Lemmens HJ, Brodsky JB. The dose of succinylcholine in morbid obesity. Anesth Analg. 2006;102:438–442.
84 Calder I, Yentis SM. Could “safe practice” be compromising safe practice? Should anaesthetists have to demonstrate that face mask ventilation is possible before giving a neuromuscular blocker? Anaesthesia. 2008;63:113–115.
85 Naguib M. Sugammadex: Another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–581.
86 Freid EB. The rapid sequence induction revisited: Obesity and sleep apnea syndrome. Anesthesiol Clin North Am. 2005;23:551–564.
87 Gertler R, Joshi GP. Modern understanding of intraoperative mechanical ventilation in normal and diseased lungs. Adv Anesth. 2010;28:15–33.
88 Rothen HV, Sporre B, Engber G, et al. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology. 1995;82:832–842.
89 Bardoczky GI, Yernault JC, Houben JJ, et al. Large tidal volume ventilation does not improve oxygenation in morbidly obese patients during anesthesia. Anesth Analg. 1995;81:385–388.
90 Rothen HV, Sporre B, Engber G, et al. Reexpansion atelectasis during general anesthesia: A computed tomography study. Br J Anaesth. 1993;71:788–795.
91 Reinius H, Jonsson L, Gustafsson S, et al. Prevention of atelectasis in morbidly obese patients during general anesthesia and paralysis: A computerized tomography study. Anesthesiology. 2009;111:979–987.
92 Talab HF, Zabani IA, Abdelrahman HS, et al. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg. 2009;109:511–516.
93 Pelosi P, Ravagnan I, Giurati G, et al. Positive end-expiratory pressure improves respiratory function in obese patients but not in normal subjects during anesthesia and paralysis. Anesthesiology. 1999;91:1221–1231.
94 Whalen FX, Gajic O, Thompson GB, et al. The effects of alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth Analg. 2006;102:298–305.
95 Cadi P, Guenoun T, Journois D, et al. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br J Anaesth. 2008;100:709–716.
96 Soni N, Williams P. Positive pressure ventilation: What is the real cost? Br J Anaesth. 2008;101:446–457.
97 Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: A closed claims analysis. Anesthesiology. 2005;103:33–39.
98 Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87:654–660.
99 Rose DK, Cohen MM, Wigglesworth DF, et al. Critical respiratory events in the postanesthesia care unit: Patient, surgical, and anesthetic factors. Anesthesiology. 1994;81:410–418.
100 McConkey PP. Postobstructive pulmonary oedema—A case series and review. Anaesth Intensive Care. 2000;28:72–76.
101 Udeshi A, Cantie SM, Pierre E. Postobstructive pulmonary edema. J Crit Care. 2010;25:508.e1–508.e5.
102 Liang Y, Kimball WR, Kacmarek RM, et al. Nasal ventilation is more effective than combined oral-nasal ventilation during induction of general anesthesia in adult subjects. Anesthesiology. 2008;108:998–1003.
103 De Miguel K, Cabello J, Sanchez-Alarcos JM, et al. Long-term effects of treatment with nasal continuous positive airway pressure on lung function in patients with overlap syndrome. Sleep Breath. 2002;6:3–10.
104 Verbraecken J, Willemen M, De Cock W, et al. Continuous positive airway pressure and lung inflation in sleep apnea patients. Respiration. 2001;68:357–364.
105 Malhotra A, Trindler J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–1112.
106 Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: Results of a meta-analysis. Sleep. 2004;27:249–253.
107 Nolan GM, Ryan S, O’Connor TM, et al. Comparison of three auto-adjusting positive pressure devices in patients with sleep apnea. Eur Resp J. 2006;28:159–164.
108 Neligan PJ, Malhotra G, Fraser M, et al. Continuous positive airway pressure via the Boussignac system immediately after extubation improves lung function in morbidly obese patients with obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesthesiology. 2009;110:878–884.