Perioperative Normothermia During Major Surgery: Is It Important?
Perioperative hypothermia (PH), usually defined as a temperature of less than 36.0°C during the perioperative period, can result from anesthesia-induced thermoregulatory inhibition combined with exposure to a cold operating room environment and is estimated to occur in 50% to 70% of patients undergoing anesthesia and major surgery [1]. Almost all anesthetics, including opioids, propofol, inhalational agents, and spinal/epidural anesthetics, have been shown to impair thermoregulatory mechanisms through their effects on the brain/hypothalamus, impairment of peripheral vasoconstriction, and the shivering response. As a result, patients (particularly the very young and the elderly) exposed to these agents become poikilothermic and body temperature decreases to less than 36.0°C in a cool operating room environment [2]. Return to normothermia often requires several hours, which in turn increases exposure to PH (and its attendant morbidities) beyond the immediate intraoperative period.
Although hypothermia has traditionally been used as a strategy to reduce cerebral and myocardial ischemic damage, recent studies suggest that PH may contribute to perioperative morbidity and mortality by increasing the risk of postoperative shivering, cardiac morbidity, coagulopathy, and postoperative wound infections. Despite growing awareness of the link between PH and poor perioperative outcomes, it is estimated that almost half of general surgery patients undergoing abdominal operations become hypothermic during surgery and a significant proportion are still hypothermic on arrival in the recovery room [3,4].
Pathophysiology of PH
In normal conditions, tonic thermoregulatory vasoconstriction maintains a significant core-to-peripheral temperature gradient. As a result, heat is not usually evenly distributed; instead, heat content is greater in the core than in peripheral tissues. General anesthesia impairs central thermoregulatory control (thus inhibiting normal tonic thermoregulatory vasoconstriction) and acts as a direct vasodilator. The resulting redistribution of core heat to the periphery often leads to a drop in the core temperature of 0.5°C to 1.5°C during the first hour following induction [5,6]. This rapid decrease in core temperature is usually followed by a slower, more linear reduction in the core temperature that often lasts several hours, after which a core temperature plateau is usually reached (which often remains unchanged for the remainder of the procedure) [7].
Although redistribution hypothermia can be difficult to treat, it can be prevented. Skin-surface warming before induction of epidural and general anesthesia does not increase core body temperature but does increase body heat content (particularly in the legs). As a result, redistribution hypothermia from subsequent anesthesia-induced inhibition of tonic thermoregulatory vasoconstriction is lessened because heat can only flow down a temperature gradient (which is now reduced) [8,9]. Although studies have shown the efficacy of prewarming in reducing redistribution hypothermia, it requires a significant amount of heat transfer and approximately 1 hour of moderate warming before surgery, which is not always feasible and often poorly tolerated by patients [10]. Alternatively, pharmacologic vasodilation with agents such as nifedipine can also reduce this temperature gradient and reduce redistribution hypothermia following induction [11]. Although impractical, both prewarming and drug-induced pharmacologic vasodilation can be so effective that, even without other warming interventions, they can help maintain normothermia for several hours following induction.
Adverse effect of hypothermia in the surgical patient
Shivering
One of the most common side effects of PH is postoperative shivering. Initially believed to result from dissociation of spinal reflexes from the cerebral cortex, it is now believed that most postoperative shivering is thermoregulatory in origin. Recent studies suggest that, on average, postoperative shivering increases oxygen consumption by approximately 40% [12]. Although there are few data to suggest that this increase in oxygen consumption is associated with perioperative morbidity, postoperative shivering can be uncomfortable for patients and can be easily treated with small doses of narcotics (particularly meperidine) [13]. In addition, skin-surface warmers can also be used to raise the skin temperature and optimize thermal comfort.
Cardiac morbidity
Perioperative cardiac events are associated with in-hospital mortality of up to 25% and increase length of stay by an average of 11 days [14]. PH is associated with increased serum catecholamine levels, vasoconstriction, and increased blood pressure, which, in turn, can lead to increased cardiac demand, ischemia, and morbidity. These cold-induced cardiovascular effects are more prominent after anesthesia once the adrenergic response is no longer suppressed, particularly in high-risk patients [15]. The relationship between PH and perioperative cardiac morbidity was analyzed in a trial by Frank and colleagues [16] in which 300 high-risk patients undergoing thoracic, abdominal, or peripheral vascular surgery were randomized to receive warmed intravenous (IV) fluids alone or warmed IV fluids and intraoperative/postoperative active warming via warm forced-air devices. Mean temperatures on arrival to the intensive care unit were significantly higher in the normothermia group (36.7°C) compared with the control group (35.4°C; P <.001). Although there was no difference in intraoperative cardiac events, the normothermia group had lower rates of electrophysiologic (7% vs 16%) and morbid cardiac events (1% vs 6%) during the subsequent 24-hour follow-up period. Although a subsequent trial comparing forced-air devices versus circulating-water blankets resulted in higher core temperatures in the forced-air device group (36.4°C vs 35.6°C), rates of postoperative cardiac events at 24 hours were similar [17]. Cardiac events were a secondary outcome, and, as such, the trial was not specifically powered to detect a difference.
Coagulopathy
It is well documented that PH can also adversely affect coagulation, resulting in surgical bleeding. Although PH can impair activity of various temperature-dependent factors in the coagulation cascade, it is often missed in the clinical setting because coagulation laboratory studies (ie, PT, PTT) are usually performed at a temperature of 37°C [18]. Hypothermia also impairs platelet function, likely because of reduced levels of thromboxane A2 [19]. Mild hypothermia was shown to increase perioperative bleeding and transfusion requirements in a recent randomized trial involving patients undergoing hip arthroplasty [20]. Perioperative blood loss was significantly higher in hypothermic patients (final intraoperative temperature 35.0°C) compared with normothermic patients: 2.2 L versus 1.5 L (P <.001). Seven out of 30 patients with hypothermia required blood transfusions, compared with only 1 normothermic patient. The investigators concluded that, in patients undergoing hip arthroplasty, typical PH increases perioperative blood loss by approximately 500 mL.
SSIs
SSIs are the third leading cause of nosocomial infections, accounting for 14% to 16% of all hospital-acquired infections, and are the leading cause of nosocomial infection among surgical patients [21]. SSIs are a major cause of postoperative morbidity and are associated with a twofold to 12-fold increased risk of postoperative mortality [22,23]. Furthermore, SSIs increase postoperative length of stay by an average of 4 days and result in attributable direct costs of up to $8000 per case [24], and, as such, represent a significant burden to the health care system.
Little is known about the direct effects of PH on the immune system. Incubation of leukocytes at low temperatures suppresses migration and the mitogenic response, whereas increases in temperature lead to enhanced interleukin (IL)-1 activity [25,26]. Hypothermic laboratory animals are more susceptible to bacterial infections compared with normothermic animals [27]. In patients having surgery, PH suppresses mitogen-induced activation of lymphocytes, reduces the production of IL-1 and IL-2, and impairs neutrophil oxidative killing in the intraoperative period [28]. PH has been hypothesized to predispose patients to SSIs by triggering thermoregulatory vasoconstriction, which may decrease partial pressure of oxygen in tissues, impair oxidative killing by neutrophils, and interfere with collage deposition resulting in impaired wound healing [29–35].
Several randomized controlled trials have analyzed the effect of active warming to prevent PH on SSI rates. In a seminal 1996 clinical trial, Kurz and colleagues [36] randomized 200 patients undergoing colorectal surgery to either active warming using forced-air warmers and intravenous fluid warmers to maintain patients’ core temperatures near 36.5°C (the normothermia group) or routine intraoperative thermal care (the hypothermia group). Six percent of patients in the normothermia group developed SSIs, compared with 19% of patients in the hypothermia group (P = .009). In addition, mean final intraoperative core temperature in the normothermia group was 36.6°C, compared with 34.7°C in the hypothermia group (P = .002). Although the data are compelling, serious concerns have been raised regarding some of the methods used in this trial [37,38]. Patients in the control arm received forced air at ambient temperature, which may have effectively cooled patients to lower than expected temperatures, increased the resulting difference in mean temperatures between study arms, and exaggerated the apparent effect of the active warming. In addition, patients in both groups received almost 4 days of postoperative systemic antibiotics, 35% of patients in the hypothermia group received intraoperative transfusions (a known risk factor for SSIs) compared with only 22% of patients in the normothermia group, and length of hospital stay was atypically long (approximately 2 weeks) in both groups [39–41]. Furthermore, SSI (the primary outcome of interest) was defined by positive aerobic or anaerobic cultures from pus that was aspirated or expressed from the surgical incision during the first 15 days after surgery (rather than by one of the more standard definitions of SSI used by the World Health Organization or the Centers for Disease Control and Prevention) [42].
More recently, Melling and colleagues [43] analyzed the effect of preoperative local warming (via noncontact radiant heat) or systemic warming (via warmed forced-air devices) versus routine perioperative thermal care on SSI rates in 421 patients undergoing clean surgery (ie, breast surgery, elective herniorrhaphy, or varicose vein surgery). Wound infections were defined as erythema or purulence within 6 weeks of surgery that lasted more than 5 days and required antibiotic therapy. SSI rates were significantly lower in patients who received preoperative local warming (4%) or systemic warming (6%) compared with nonwarmed patients (14%, P <.001). The study did not include patients having gastrointestinal surgery. More importantly, it did not report the resulting mean temperatures of the 3 study groups, and, as such, there was no direct evidence that the observed reduction in wound infection rates was caused by prevention of PH [44].
Although the trial data cited earlier suggest that active warming during surgery may reduce SSIs, there is limited evidence that perioperative normothermia, in and of itself, is associated with lower rates of SSI. Barone and colleagues [37] retrospectively reviewed the records of 150 consecutive patients who underwent colorectal surgery in a 30-month period. Although 1 or more warming modalities were used in all patients, approximately 33% of patients were hypothermic (defined as T <34.3°C) during the intraoperative or immediate postoperative period. Despite the extreme definition of hypothermia used in this study (even lower then the mean final intraoperative temperature [T <34.7°C] of the hypothermia group in the trial by Kurz and colleagues [36]), the rates of wound infections (defined as suppuration requiring removal of sutures) was identical at 6% in both the normothermia and hypothermic patient populations.
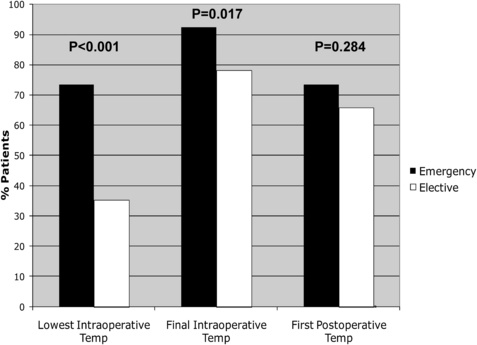
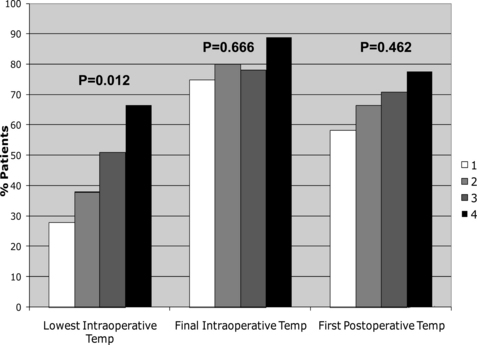
More recently, Lehtinen and colleagues used a nested, case-control study design to analyze the association between perioperative normothermia and incisional SSIs after gastrointestinal surgery [45]. Cases consisted of all patients having consecutive gastrointestinal surgery enrolled in a single institution’s American College of Surgeons National Surgical Quality Improvement Program database during a 3-year period who developed SSIs. When cases and control were compared with respect to recorded perioperative core temperatures, median lowest intraoperative temperatures and final intraoperative temperatures were, paradoxically, slightly higher in cases compared with controls. The percentage of patients with intraoperative normothermia was significantly higher among patients who underwent emergency surgery (Fig. 1), and the percentage of patients with lowest intraoperative temperature greater than 36°C also increased with increasing wound class (ie, patients with more contaminated wounds were more likely to have been normothermic during surgery; Fig. 2). Although cases and controls did not differ significantly with respect to median first postoperative temperature, cases were slightly more likely to be normothermic compared with controls (although this difference was not statistically significant). The investigators also compared cases and controls regarding rates of perioperative normothermia (defined as final intraoperative or first postoperative temperature ≥36°C). Overall, rates of perioperative normothermia were similar between cases and controls (91.5% vs 86.1%, respectively, P = .11), even when the analysis was stratified by colorectal versus noncolorectal surgery. To analyze the independent association of perioperative normothermia on postoperative SSI, perioperative normothermia was forced into multivariate models controlling for age, diabetes, American Society of Anesthesiologists class, significant alcohol use, surgery status (elective vs emergency), type of surgery, surgical approach (laparoscopic vs nonlaparoscopic), surgical complexity, wound class, length of surgery, and estimated blood loss. Perioperative normothermia was not independently associated with SSI (adjusted odds ratio, 1.05; 95% CI, 0.48–2.33; P = .90), even when controlling for potential negative confounders (elective vs emergency surgery status and wound class). There were also no apparent associations between final intraoperative normothermia (final intraoperative T ≥36°C) or immediate postoperative normothermia (first postoperative T ≥36°C) and SSI on additional multivariate analyses.
Previous studies have shown that elective surgery induces an increase in circulating IL-6 within 1 to 3 hours, and that the magnitude of the increase is related directly to tissue injury and postoperative septic morbidity [46–48]. Increased IL-6 levels are associated with epidural fever (even in the absence of maternal or fetal infection) and have been linked to postoperative sepsis after major cancer surgery and during the early postoperative period [49,50]. Although emergency surgery and increasing wound class were not associated with SSIs in the study by Lehtinen and colleagues, they were associated with higher rates of intraoperative normothermia. These differences were most pronounced at the lowest intraoperative time points, and less pronounced (or no longer significant) at the final intraoperative and/or first postoperative time points. The observed associations between normothermia, emergency surgery, and advanced wound class in that study suggested that the higher rates of normothermia in cases (patients with SSIs) may have been the result of higher rates of subclinical systemic inflammatory response syndrome in these patients. If so, higher rates of perioperative normothermia at a hospital level could paradoxically be associated with higher rates of SSIs (and other postoperative infections), rather than the converse. As such, the investigators concluded that compliance with pay-for-reporting measures focusing on perioperative normothermia (such as those endorsed in the Surgical Care Improvement Project) may be of limited value in preventing SSIs (at least after gastrointestinal surgery).
Temperature monitoring
Core temperature should be closely monitored during surgeries lasting more than 30 minutes, both to detect malignant hyperthermia and to help prevent PH. Although various devices are available to monitor perioperative temperatures, it is the site being monitored that really matters. The core thermal compartment is well perfused and its temperature higher and more uniform compared with the rest of the body, even during significant thermal disruptions such as cardiopulmonary bypass [51]. The tympanic membrane, pulmonary artery, nasopharynx, and esophagus are the sites that most accurately represent core temperatures, whereas the skin, axilla, bladder, and rectum represent more peripheral temperatures [52].
Infrared tympanic membrane, esophageal thermometers, and oral thermometers are the most practical devices to monitor intraoperative core temperatures. Although pulmonary artery catheters are the gold standard for monitoring core temperature, their cost and potential morbidity preclude their routine use for this indication. Lefrant and colleagues [53] compared axillary, esophageal, bladder, and rectal sites against pulmonary artery catheter temperature monitoring in 42 patients in intensive care units. Esophageal probes allowed for continuous monitoring and were the most accurate (they slightly underestimated core temperatures). In subsequent studies, oral temperatures were nearly as accurate as esophageal probes, although less practical [54,55]. Bladder and rectal probes can be dislodged or damaged during pelvic surgery and their performance can be compromised by exposure to peritoneal irrigation and ambient temperature in the operating room. In addition, rectal temperatures fail to increase rapidly during malignant hyperthermia and must be used with caution [56]. Although convenient, skin-surface thermometers (liquid crystal plastic strips that stick to the skin) are inaccurate and unreliable in detecting malignant hyperthermia [15].
Oral, axillary, and infrared tympanic membrane thermometers are the most practical means to monitor core temperatures during the postoperative period. Although axillary temperatures are often perceived as reasonable approximations of core temperatures, the accuracy and precision of axillary thermometry is far lower than that of oral thermometry [53–55]. Infrared tympanic membrane thermometers, widely used in postoperative care units, are the least accurate and almost half of measurements can vary significantly from core temperatures measured using the gold standard [51].
Prevention of PH
Airway heating/humidification
Less than10% of produced metabolic heat is lost via the respiratory tract, largely as a result of humidification of inspiratory gases [57]. Passive heating/humidification using heat exchangers and moisture exchangers may help maintain normal cilial function in the trachea and prevent bronchospasm, but are ineffective at maintaining core temperatures [51]. The contribution of active airway heating and humidification to core temperature is also trivial, and cutaneous warming is far more effective, even in infants and children [58,59].
Cutaneous warming
When heat loss to the environment exceeds metabolic heat production, mean body temperature decreases. It is estimated that approximately 90% of metabolic heat is lost through the skin [51]. During surgery, additional heat can also be lost through open surgical incisions.
Passive insulation
Cutaneous heat loss is proportional to the surface area of exposed skin. As such, the amount of skin covered is more important than which sites are covered. A single layer of passive skin insulation (eg, blankets, surgical drapes, plastic sheets/bags) reduces heat loss by almost 30%. Additional layers reduce further losses only slightly [60]. Although heat transfer by warmed blankets is admittedly minor and transient, it adds significantly to patient comfort, particularly in the setting of postoperative shivering [61].
Active warming
Circulating-water mattresses and warmed forced-air devices are the most commonly used systems for active cutaneous warming. Circulating-water mattresses are safer and more effective when placed over patients, rather than under patients [62]. When used directly under a patient, the combination of the patient’s weight and the heat from the mattress can lead to skin burns and necrosis [63]. However, when placed under the mattress covering the operating room table, these devices become ineffective.
Warmed forced-air devices are more widely used in operating rooms and consist of disposable perforated sheets (usually placed over the patient) through which warmed air is delivered. These devices can deliver up to 50 W across the skin surface and can be used to both maintain and raise body temperature [62]. Because skin-surface warming via these devices is more effective when patients are vasodilated, it is easier to maintain normothermia intraoperatively than to treat hypothermia postoperatively when patients are vasoconstricted [64]. Warmed forced-air devices have been shown to be superior to circulating-water mattresses in several studies and may optimize normothermia rates when combined with warmed IV fluids [65].
Warmed forced-air devices have been used during various phases of the perioperative period to prevent PH. As noted earlier, prewarming can reduce the heat gradient between the core and the periphery and help prevent redistribution hypothermia following induction [8,9]. Although shown to be effective, prewarming often requires application of warmed forced-air devices set at high temperatures (41°C) for long and impractical periods of time (up to 1 hour). The combination of preoperative warming with these devices with intraoperative warming (via various methods) has also been shown to be effective, although the relative benefit of the preoperative warming in these studies remains unclear [66,67].
Warmed intravenous fluids
Studies suggest that a liter of crystalloid solution administered at room temperature can decrease mean body temperature by approximately 0.25°C [50]. Heating intravenous fluids does not warm patients, but does prevent fluid-induced hypothermia in patients given large volumes of fluid. As such, IV fluid warmers should be used when administration of large volumes (ie, 2 L/h) of blood or IV fluids are anticipated. Although warmed IV fluids do not warm patients per se, they can help prevent fluid-induced hypothermia in these circumstances. The efficacy of warmed IV fluids to prevent PH (alone and in combination with other means of active warming) has been tested in several studies. Use of warmed fluids resulted in significantly higher intraoperative temperatures, and this benefit was maintained into the postoperative period [68,69].
Relative benefit of perioperative interventions to maintain normothermia
A myriad of perioperative techniques and devices are available to maintain normothermia and prevent PH. In the seminal study of active warming by Kurz and colleagues [36], the intervention consisted of both warmed forced-air devices and warmed IV fluids. In a recent study, Hedrick and colleagues [4] tested the efficacy of a standardized protocol to prevent PH in 132 patients undergoing elective colorectal surgery. Despite the combined use of warmed blankets, warmed forced-air devices, control of the operating room ambient temperature, and warmed IV fluids, the rate of normothermia improved during the study period was only 71%, compared with 64% during the baseline period [4].
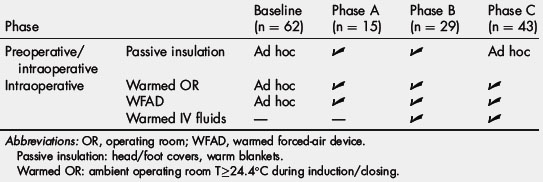
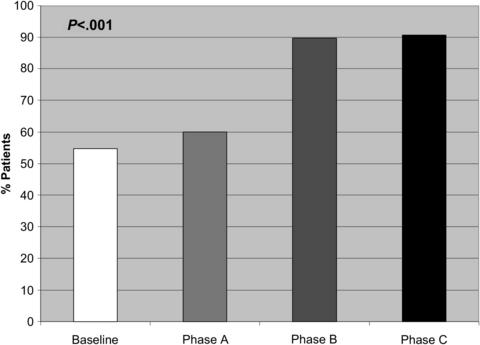
Our group recently conducted a study to determine the relative benefit of perioperative interventions to prevent PH [70]. Using a before-and-after study design, a multidisciplinary team of surgeons, anesthesiologists, and nurses incrementally tested various preoperative and intraoperative interventions for a period of 10 months in a sequential series of patients undergoing major oncologic surgery (Table 1). Results were compared with a baseline group of surgical patients who had received routine perioperative thermal care at the same institution during a previous period. During the first phase of the study (phase A), preoperative passive warming (using head/foot covers, warmed blankets) was combined with raising the ambient room temperature to greater than 24.4°C and intraoperative active warming with warmed forced-air blankets. As shown in Fig. 3, implementation of this protocol increased the rate of immediate postoperative normothermia from 54.8% at baseline to only 60% during phase A (P = .8). However, when warmed IV fluids were incorporated into the protocol during phase B, the rate of normothermia rose sharply to 89.7% (P <.001) and remained at this level through phase C after the preoperative passive warming measures were eliminated. On multivariate analysis, use of warmed IV fluids was independently associated with immediate postoperative normothermia. Length of surgery was also associated with higher rates of normothermia, even in patients who did not receive warmed IV fluids, suggesting that the benefit of the cutaneous active warming measures may require more time to become apparent.