CHAPTER 30 Nonvascularized Bone Grafting for the Treatment of Osteonecrosis of the Femoral Head
Indications
As with other treatment modalities, the successful employment of bone grafting is most dependent on the stage of the disease. Several classification systems, including the Ficat and Steinberg systems, have been used to define various stages of tissue involvement, as shown in Table 30-1. The goal of bone grafting is to preserve the structure of the bone and the articular cartilage, so the procedure is most useful during precollapse stages (Ficat and Steinberg stages I and II), especially when less than 30% of the femoral head is involved. Bone grafting may also be used for a limited number of patients who have smaller Ficat stage III lesions if the articular cartilage is mostly intact.
Table 30–1 Descriptions of the stages of two widely used systems for classifying osteonecrosis of the femoral head
| Stage | Ficat and Arlet | University of Pennsylvania* |
|---|---|---|
| 0 | No consistent findings on radiograph or bone scan. No symptoms. | No findings on radiographs, MRI, or bone scan. |
| I | No radiographic abnormality. Increased uptake on bone scan. | No radiographic abnormalities. Lesion present on MRI and/or bone scan. |
| II | Diffuse sclerosis and/or cystic lesions present on radiograph. | Diffuse sclerosis and/or lucent lesions present on radiograph. |
| III | Subchondral collapse (crescent sign present on radiograph, with or without femoral head flattening). | Subchondral collapse (crescent sign on radiograph without flattening of the femoral head). |
| IV | Femoral head flattening with acetabular involvement and joint destruction. | Flattening of the articular surface of the femoral head with a normal acetabulum. |
| V | N/A | Acetabular involvement (joint-line narrowing, sclerosis, lucencies, or osteophytes of the acetabulum). |
| VI | N/A | Advanced degeneration of the joint manifested by complete destruction of the joint line. |
* The University of Pennsylvania stages I-V are further subclassified into three grades: Grade A = mild, involving less than 15% of the femoral head. Grade B = moderate, involving 15 to 30% of the femoral head. Grade C = severe, involving greater than 30% of the femoral head. In Stage V, the grade is determined by averaging the extent of involvement of the femoral head and the acetabulum.
Surgical technique
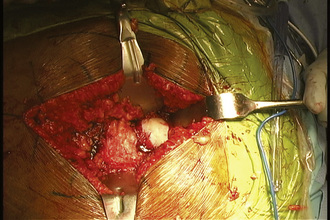
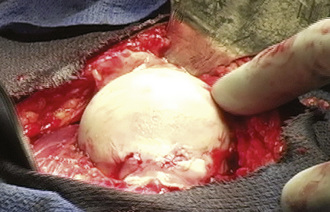
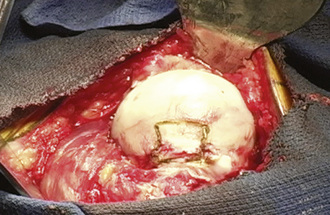
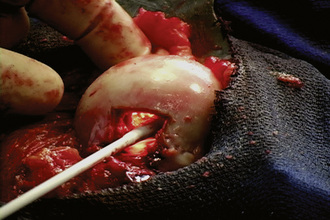
When performing a nonvascularized bone graft, the authors prefer to have the patient placed in the lateral decubitus position (Figures 30-1 through 30-6). The patient is secured to the operating table, pads and cushions are placed on the extremities, and the airway is controlled by the anesthesiologist. The direct lateral approach is the surgical approach of choice for the author, because the vasculature to the femoral head is more easily preserved. The posterior approach is also used, but greater care needs to be taken to avoid injury to the lateral epiphyseal vessel, which is the branch of the medial obturator vessel that supplies the femoral head. After the patient is appropriately positioned, prepared, and draped, an 8-cm to 14-cm incision is made overlying the center of the greater trochanter and brought proximally and distally. Sharp dissection is performed with the use of a No. 10 scalpel down to the level of the iliotibial band. The iliotibial band is then incised and retracted posteriorly and anteriorly. A blunt Hohmann retractor is placed posteriorly, again to avoid injury to the neurovascular structures located posterior to the greater trochanter. The bursa can be excised for the better identification of the vastus ridge, the greater trochanter, and the insertion site of the gluteus medius muscle. With the use of an electrocautery device, the anterior 40% of the gluteus medius muscle is elevated from the anterior neck of the proximal femur in conjunction with the gluteus minimus muscle. The bursa between the iliopsoas muscle and the hip capsule is explored and retracted anteriorly with the use of a Cobb elevator; this allows for the preservation of the muscle and avoids injury to the neurovascular structures anteriorly. Care is taken to avoid the excessive stripping of the hip capsule. The gluteus medius and minimus tendons are taken proximally and held in place with the use of a Taylor retractor. Care is taken to avoid injury to the neurovascular structures that innervate the gluteus medius and gluteus minimus tendons. Excessive dissection is not necessary posteriorly. If additional exposure is necessary, then a Cobb elevator is used to elevate some of the muscular attachments of the gluteus muscle onto the capsule. We prefer to place a Hohmann retractor anteriorly and a Taylor retractor superiorly, and this is followed by the use of a blunt Hohmann retractor posteriorly. These retractors allow for excellent exposure to the hip capsule. The hip capsule is incised from the femoral neck and extending to the acetabulum. It is important to avoid injury to the labrum of the acetabulum. The capsule incision is then extended from the base of the neck anteriorly and then posteriorly approximately 180 degrees around the circumference of the femoral neck. The posterior capsule is not violated. The capsule is also peeled off on the acetabular side approximately 180 degrees, thereby exposing the whole anterior lateral labrum. Next, the capsule incision is shaped like an “H,” which allows for the visualization of the neck–cartilage junction. The anterior neck is outlined with the use of a surgical marker. A 1.5-cm by 1.5-cm square area of bone is identified. The surgeon should take great care to avoid extending this window too far medially and thus compromising the neurovascular structures or too far laterally and thus creating a potential stress riser for a femoral neck fracture. An oscillating saw is used to remove the area of bone. The authors prefer to bevel the bony cuts to allow for the replacement of the bony window after the bone grafting procedure is completed. A high-speed burr is used to remove all of the necrotic bone from the femoral head. This procedure can successfully be performed without dislocation, but if dislocation is necessary, then we recommend partial dislocation to obtain better visualization of the femoral head. A full dislocation increases the risk of complete femoral head death. After the necrotic segment within the femoral head is removed, a light is placed into the femoral head to ensure that the entire necrotic segment has been removed, and the remaining bone is observed to ensure that bleeding is still present. The authors prefer to use fresh- frozen bone graft that has been milled and then mixed with bone morphogenic protein to maintain the osteoinductive and conductive properties of the bone. Autograft is also used, when available. The autograft can be harvested from small cores within the greater trochanter or from a secondary incision along the iliac crest. The femoral head is packed tightly with the substrate. If a large volume of the head has been denuded of bone, then small cortical strips can be added to the substrate to provide structural integrity. The cortical window is then placed back onto the femoral neck and fixed with the use of three resorbable pins.

Figure 30–1 The patient is in the direct lateral position, and the incision is centered over the trochanter.
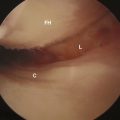
Figure 30–5 A light can be used to evaluate the amount of bone that has been removed from the femoral head.
Technical Pearls
Aldridge J.M.3rd, Urbaniak J.R. Avascular necrosis of the femoral head: etiology, pathophysiology, classification, and current treatment guidelines. Am J Orthop. 2004;33:327-332.
Ficat R.P. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3-9.
Hungerford D.S., Jones L.C. Asymptomatic osteonecrosis: should it be treated? Clin Orthop Relat Res. 2004;429:124-130.
Ko J.Y., Meyers M.H. Wenger DR. “Trapdoor” procedure for osteonecrosis with segmental collapse of the femoral head in teenagers. J Pediatr Orthop. 1995;15:7-15.
Mont M.A., Einhorn T.A., Sponseller P.D., Hungerford D.S. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998;80:56-62.
Mont M.A., Etienne G., Ragland P.S. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84-92.
Mont M.A., Hungerford D.S. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459-474.
Mont M.A., Jones L.C., Hungerford D.S. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117-1132.
Mont M.A., Ulrich S.D., Seyler T.M., Smith J.M., Marker D.R., McGrath M.S., Hungerford D.S., Jones L.C. Bone scanning of limited value for diagnosis of symptomatic oligofocal and multifocal osteonecrosis. J Rheumatol. 2008;35(8):1629-1634.
Petrigliano F.A., Lieberman J.R. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007;465:53-62.
Rosenwasser M.P., Garino J.P., Kiernan H.A., Michelsen C.B. Long term followup of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res. 1994;306:17-27.
Seyler T.M., Marker D.R., Ulrich S.D., Fatscher T., Mont M.A. Nonvascularized bone grafting defers joint arthroplasty in hip osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1125-1132.
Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34-41.