Chapter 209 Nontuberculous Mycobacteria
Clinical Manifestations
Lymphadenitis of the superior anterior cervical or submandibular lymph nodes is the most common manifestation of NTM infection in children (Table 209-1). Preauricular, posterior cervical, axillary, and inguinal nodes are involved occasionally. Lymphadenitis is most common in children 1-5 yr of age and has been related to their tendency to put objects contaminated with soil, dust, or standing water into their mouths. Given the constant environmental exposure to NTM, the occurrence of these infections might also reflect an atypical immune response of a subset of the infected children during or after their first contact with NTM.
Table 209-1 DISEASES CAUSED BY NONTUBERCULOUS MYCOBACTERIAL SPECIES
| CLINICAL DISEASE | COMMON SPECIES | LESS-COMMON SPECIES |
|---|---|---|
| Cutaneous infection | M. chelonae, M. fortuitum, M. abscessus, M. marinum | M. ulcerans* |
| Lymphadenitis | MAC | M. kansasii, M. haemophilum, M. malmoense† |
| Otologic infection | M. abscessus, MAC | M. fortuitum |
| Pulmonary infection | MAC, M. kansasii, M. abscessus | M. xenopi, M. malmoense,† M. szulgai, M. fortuitum, M. simiae |
| Catheter-associated infection | M. chelonae, M. fortuitum | M. abscessus |
| Skeletal infection | MAC, M. kansasii, M. fortuitum | M. chelonae, M. marinum, M. abscessus, M. ulcerans* |
| Disseminated | MAC | M. kansasii, M. genavense, M. haemophilum, M. chelonae |
MAC, Mycobacterium avium complex.
† Found primarily in Northern Europe.
From American Academy of Pediatrics: Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, p 703.
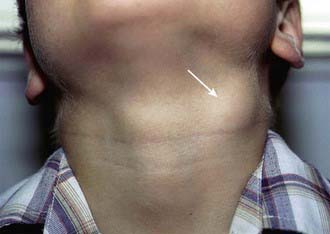
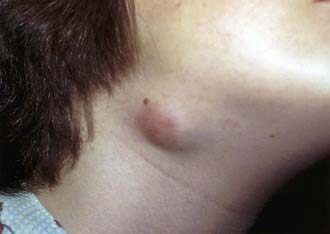
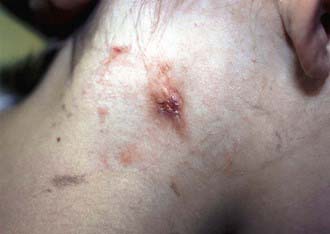
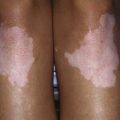
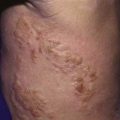
Affected children usually lack constitutional symptoms and present with a unilateral subacute and slowly enlarging lymph node or group of closely approximated nodes >1.5 cm that are firm, painless, freely movable, and not erythematous (Fig. 209-1). The involved nodes occasionally resolve without treatment, but most undergo rapid suppuration after several weeks (Fig. 209-2). The center of the node becomes fluctuant, and the overlying skin becomes erythematous and thin. Eventually, the nodes rupture and form cutaneous sinus tracts that drain for months or years, resembling the classic scrofula of tuberculosis (Fig. 209-3).
Cutaneous disease caused by NTM is rare in children (see Table 209-1). Infection usually follows percutaneous inoculation with fresh or salt water contaminated by M. marinum. Within 2-6 wk after exposure, an erythematous papule develops at the site of minor abrasions on the elbows, knees, or feet (swimming pool granuloma) and on the hands and fingers of fish tank owners, mostly inflicted during tank cleaning (fish tank granuloma). These lesions are usually nontender and enlarge over 3-5 wk to form violaceous plaques. Nodules or pustules can develop, and occasionally these lesions ulcerate, resulting in a serosanguineous discharge. The lesions sometimes resemble sporotrichosis, with satellite lesions near the site of entry, extending along the superficial lymphatics. Lymphadenopathy is usually absent. Although most infections remain localized to skin, penetrating M. marinum infections can result in tenosynovitis, bursitis, osteomyelitis, or arthritis.
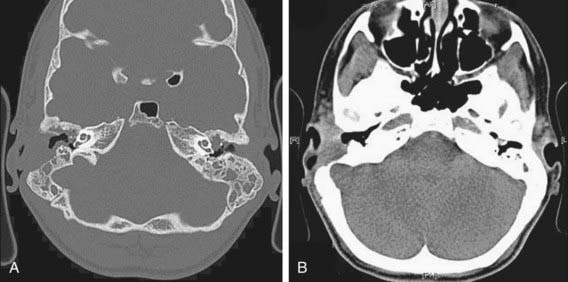
Otomastoiditis, or chronic otitis media, is a rare extrapulmonary NTM disease type that specifically affects children with tympanostomy tubes and a history of topical antibiotic or steroid use. M. abscessus is the most common causative agent, followed by M. avium complex (see Table 209-1). Patients present with painless, chronic otorrhea resistant to antibiotic therapy. CT imaging can reveal destruction of the mastoid bone with mucosal swelling (Fig. 209-4). Delayed or unsuccessful treatment can result in permanent hearing loss. In unusual circumstances, NTM causes other bone and joint infections that are indistinguishable from those produced by M. tuberculosis or other bacterial agents. Such infections usually result from operative incision or accidental puncture wounds. M. fortuitum infections from puncture wounds of the foot resemble infections caused by Pseudomonas aeruginosa and Staphylococcus aureus.
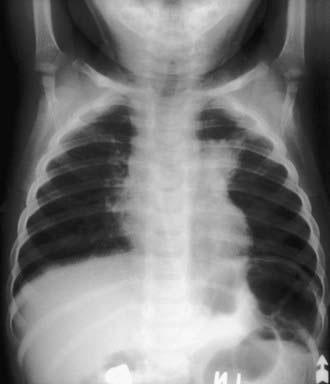
Pulmonary infections are the most common form of NTM illness in adults but are rare in children. M. avium complex bacteria, the most commonly identified organisms (see Table 209-1), are capable of causing acute pneumonitis, chronic cough, or wheezing associated with paratracheal or peribronchial lymphadenitis and airway compression in normal children. Associated constitutional symptoms such as fever, anorexia, and weight loss occur in 60% of these children. Chest radiographic findings are very similar to those for primary tuberculosis, with unilateral infiltrates and hilar lymphadenopathy (Fig. 209-5). Pleural effusion is uncommon. Rare cases of progression to endobronchial granulation tissue have been reported.
Disseminated disease in children without any apparent immunodeficiency is exceedingly rare.
Treatment
Therapy for NTM infections is long-term and cumbersome; expert consultation is advised. Therapy involves medical, surgical, or combined treatment (Chapter 206 and Table 206-3). Isolation of the infecting strain followed by drug susceptibility testing is ideal, because it provides a baseline for drug susceptibility. Important discrepancies exist between in vitro drug susceptibility and in vivo response to treatment, explained in part by synergism, mainly among first-line antituberculosis drugs. In vitro, slow growers (M. kansasii, M. marinum, M. xenopi, M. ulcerans, and M. malmoense) are usually susceptible to the first-line antituberculosis drugs rifampicin and ethambutol; M. avium complex bacteria are often resistant to these drugs alone but susceptible to the combination and have variable susceptibility to other antibiotics, most importantly the macrolides. Rapid growers (M. fortuitum, M. chelonae, M. abscessus) are highly resistant to antituberculosis drugs and often have inducible macrolide resistance mechanisms. Susceptibility to macrolides, aminoglycosides, carbapenems, tetracyclines, and glycylcyclines are most relevant for therapy guidance. In all NTM infections, multiple-drug therapy is essential to avoid development of resistance.
The preferred treatment of NTM lymphadenitis is complete surgical excision (Table 206-3). Nodes should be removed while still firm and encapsulated. Excision is more difficult if extensive caseation with extension to surrounding tissue has occurred, and complications of facial nerve damage or recurrent infection are more likely in such cases. Incomplete surgical excision is not advised, because chronic drainage can develop. If there is concern for possible M. tuberculosis infection, therapy with isoniazid, rifampin, ethambutol, and pyrazinamide should be administered until cultures confirm the cause to be NTM (Chapter 207). If for some reason surgery of NTM lymphadenitis cannot be performed, removal of infected tissue is incomplete, or recurrence or chronic drainage develops, a 3-6 mo trial of chemotherapy is warranted. Although there are no published controlled trials, several case reports and small series have reported successful treatment with chemotherapy alone or combined with surgical excision. Clarithromycin or azithromycin combined with rifabutin or ethambutol are the most commonly reported therapy regimens (Table 206-3).
Post-traumatic cutaneous NTM lesions in immunocompetent patients usually heal spontaneously after incision and drainage without other therapy (Table 206-3). M. marinum is susceptible to rifampin, amikacin, ethambutol, sulfonamides, trimethoprim-sulfamethoxazole, and tetracycline. Therapy with a combination of these drugs, particularly rifampin and ethambutol, may be given for 3-4 mo. Corticosteroid injections should not be used. Superficial infections with M. fortuitum or M. chelonae usually resolve after surgical incision and open drainage, but deep-seated or catheter-related infections require removal of infected central lines and therapy with parenteral amikacin plus cefoxitin or clarithromycin.
Albright JT, Pransky SM. Nontuberculous mycobacterial infections of the head and neck. Pediatr Clin North Am. 2003;50:503-514.
Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
Blyth CC, Best EJ, Jones CA, et al. Nontuberculous mycobacterial infection in children: a prospective national study. Pediatr Infect Dis. 2009;28:801-805.
Bruijnesteijn van Coppenraet LE, de Haas PE, Lindeboom JA, et al. Lymphadenitis in children is caused by Mycobacterium avium hominissuis and not related to “bird tuberculosis.”. Eur J Clin Microbiol Infect Dis. 2008;27:293-299.
Buijtels PC, van der Sande MA, de Graaff CS, et al. Nontuberculous mycobacteria, Zambia. Emerg Infect Dis. 2009;15:242-249.
Centers for Disease Control and Prevention. 2009 guidelines for the prevention and treatment of opportunistic infections among HIV-exposed and HIV-infected children. MMWR. 2009;58:1-166.
Dorman SE, Picard C, Lammas D, et al. Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. Lancet. 2004;364:2113-2121.
Esther CRJr, Henry MM, Molina PL, Leigh MW. Nontuberculous mycobacterial infection in young children with cystic fibrosis. Pediatr Pulmonol. 2005;40:39-44.
Falkinham JOIII. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107:356-367.
Forslow U, Geborek A, Hjelte L, et al. Early chemotherapy for non-tuberculous mycobacterial infections in patients with cystic fibrosis. Acta Paediatr. 2003;92:910-915.
Franklin DJ, Starke JR, Brady MT, et al. Chronic otitis media after tympanostomy tube placement caused by Mycobacterium abscessus: a new clinical entity? Am J Otol. 1994;15:313-320.
Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
Haverkamp MH, Arend SM, Lindeboom JA, et al. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin Infect Dis. 2004;39:457-458.
Horsburgh CRJr. Epidemiology of disease caused by nontuberculous mycobacteria. Semin Respir Infect. 1996;11:244-251.
Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, et al. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin Infect Dis. 2007;44:1057-1064.
Lindeboom JA, Prins JM, Bruijnesteijn van Coppenraet LE, et al. Cervicofacial lymphadenitis in children caused by Mycobacterium haemophilum. J Infect Dis. 2005;41:1569-1575.
Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med. 2002;23:553-567.
Mijs W, de Haas P, Rossau R, et al. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and M. avium subsp. hominissuis for the human/porcine type of M. avium. Int J Syst Evol Microbiol. 2002;52:1505-1518.
Nolt D, Michaels MG, Wald ER. Intrathoracic disease from nontuberculous mycobacteria in children: Two cases and a review of the literature. Pediatrics. 2003;112:e434-e439.
Olivier KN, Weber DJ, Wallace RJJr, et al. Nontuberculous mycobacteria I: multicenter prevalence study in cystic fibrosis. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group. Am J Respir Crit Care Med. 2003;167:828-834.
Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol. 2005;43:3467-3470.
Portaels F, Meyers WM, Ablordey A, et al. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl Trop Dis. 2008;2:e178.
Portaels F, Silva MT, Meyers WM. Buruli ulcer. Clin Dermatol. 2009;27:291-305.
Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Management of opportunistic mycobacterial infections: Joint Tuberculosis Committee guidelines 1999. Thorax. 2000;55:210-218.
Thoen CO, LoBlue PA. Mycobacterium bovis tuberculosis: forgotten, but not gone. Lancet. 2007;369:1236-1238.
van Ingen J, Bendien SA, de Lange WCM, et al. The clinical relevance of nontuberculous mycobacteria isolated in the Nijmegen-Arnhem region, the Netherlands. Thorax. 2009;64:502-506.
van Ingen J, Boeree MJ, Dekhuijzen PNR, van Soolingen D. Mycobacterial disease in patients with rheumatic disease. Nat Clin Pract Rheumatol. 2008;4:649-656.
Winthrop KL, Chang E, Yamashita S, et al. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15:1556-1561.