Nonpulmonary Causes of Respiratory Failure
MECHANISMS UNDERLYING CHRONIC ALVEOLAR HYPOVENTILATION
CLINICAL RECOGNITION AND MANIFESTATIONS
Acute Respiratory Failure Caused by Neuromuscular Disease
Noninvasive Positive Pressure Ventilation
When to Use Noninvasive Ventilation in Nonpulmonary Causes of Respiratory Failure
Introduction
An estimated 20% of respiratory failure is a result of nonpulmonary causes such as disorders that affect the upper airway, chest wall, muscles of respiration, and nervous system. Hypoventilation is the primary pathophysiologic etiology of all of these disorders.1
Hypoventilation
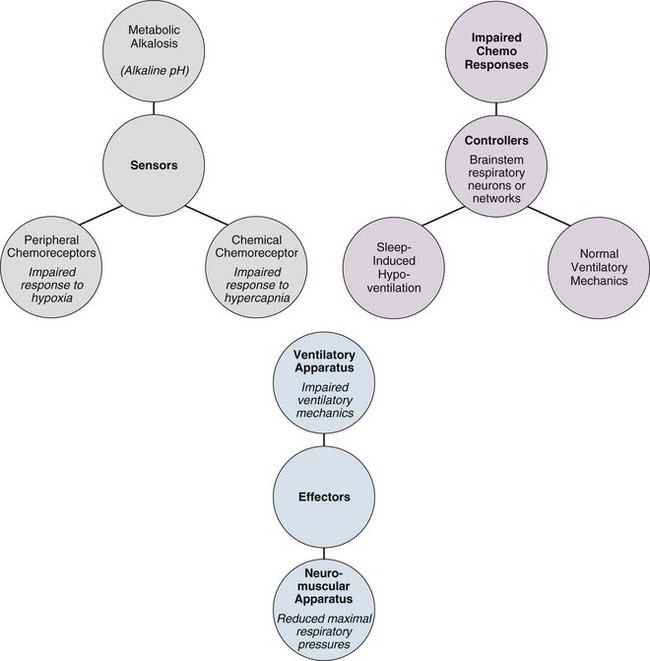
Pathophysiology (Fig. 41.1)
Hypoventilation is defined as alveolar ventilation that is inappropriately low for metabolic demands. Alveolar (A) and arterial (a) PaCO2 are elevated. Arterial pressure is decreased. Alveolar hypoventilation exists when arterial PCO2 increases above the normal range of 37 to 43 mm Hg.2–7
Hypoventilation is associated with arterial hypoxemia (when breathing room air oxygen) and a raised arterial PCO2. The rise in PaCO2 as a result of hypoventilation can be calculated using the alveolar ventilation equation.8 In a single-compartment lung model, alveolar PCO2 and PO2 are inversely related according to the alveolar air equation. An increase in alveolar PCO2 is associated with an obligatory fall in alveolar PO2. During hypercapnia, the alveolar minus arterial P(A – a)O2 difference predicted by this model (while breathing room air) is “normal.” Regarding the combination of hypercapnia and a normal P(A – a)O2 difference as a requirement to diagnose nonpulmonary disorders associated with hypoventilation is common clinical practice. However, these disorders are also commonly complicated by microatelectasis, retention of secretions, and bronchopneumonia, which cause abnormal ventilation/perfusion inequality and increase the P(A – a)O2 difference. Conversely, the P(A – a)O2 difference has been shown to be an unreliable index of abnormal gas exchange in the presence of substantial hypercapnia. For these reasons, a normal P(A – a)O2 difference is not helpful in differentiating pulmonary from nonpulmonary causes of respiratory failure. The alveolar partial pressure of oxygen is calculated with the formula:
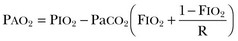
where FIO2 is the inspired oxygen fraction (0.21 in all calculations) in the dry gas, PIO2 is the inspired PO2, and R is the respiratory exchange ratio (assumed to be 0.8). In other words, the flow of CO2 molecules across the alveolar membrane per minute is divided by the flow of O2 molecules across the membrane per minute. PaCO2 is the ideal alveolar carbon dioxide tension.
This is true only if a steady state of alveolar ventilation and carbon dioxide production rate exists. In clinical practice it is common to consider the combination of hypercapnia and a normal P(A – a)O2 difference as a requirement to diagnose nonpulmonary disorders associated with hypoventilation.9 However, hypoventilation-induced atelectasis causing abnormal gas exchange will result in an increase in P(A – a)O2 difference.10 In the presence of substantial hypercapnia, an abnormal alveolar-to-arterial oxygen difference may not rule out a nonpulmonary cause of hypoventilation.
Mechanisms Underlying Chronic Alveolar Hypoventilation (see Fig. 41.1)
Defects in the metabolic control system result in hypoventilation when abnormalities in blood gases and cerebral acid-base status are not sensed or if sensed do not produce an appropriate change in motor output of the medullary respiratory neurons. Patients with such defects fail to breathe normally in response to metabolic respiratory stimuli, but because the behavioral control system, respiratory motor pathways, and ventilatory apparatus are intact, they are capable of voluntarily driving respiration. As a result, patients with defects in the metabolic control system typically demonstrate normal ventilatory mechanics, but they have impaired responses to metabolic respiratory stimuli and often hypoventilate severely during sleep, when ventilation is critically dependent on the metabolic control system.1,3,11–17 As a result of chronic hypoventilation, these patients have a primary respiratory acidosis leading to a secondary increase in extracellular bicarbonate ion concentration.18,19
In contrast, patients with a primary metabolic alkalosis may develop secondary hypoventilation as a compensatory response. This type of hypoventilation represents not a defect in respiratory control but rather an appropriate response of the metabolic control system to a disturbance in acid-base status. It is said that patients with metabolic alkalosis “shouldn’t” breathe, in contrast to those with control defects, who “won’t” breathe, and those with mechanical defects, who “can’t” breathe. However, the degree of hypoventilation that develops in response to metabolic alkalosis depends on several factors, including associated electrolyte disturbances and the sensitivity of the peripheral chemoreceptors to the accompanying hypoxemia.19,20 Therefore, patients with weak hypoxic responsiveness tend to hypoventilate more than do patients with brisk hypoxic responsiveness.
Chronic hypoventilation resulting from defects in effector elements of the respiratory system represents disturbances of ventilatory motor and mechanical function, and these defects do not in themselves mean that the metabolic control system is defective. Because the same effector elements also serve the behavioral control system, these patients are usually unable to breathe normally even when consciously attempting to do so. Hence, such defects are characterized either by reductions in the maximum inspiratory pressures that can be generated voluntarily or by impairment of lung volumes and flow rates.21 In the presence of such neural or mechanical defects, coexisting disturbances in respiratory control are often difficult to identify because the neuromuscular or mechanical defect may preclude normal responses to chemical respiratory stimuli even when the control system is intact.1
Etiologic Classification
Plum and Leigh22,23 described nonrespiratory causes of respiratory failure. Causes of hypoventilation include depression of the respiratory center by drugs such as morphine derivatives and barbiturates; diseases of the brainstem such as encephalitis; abnormalities of the spinal cord conducting pathways such as high cervical dislocation; anterior horn cell diseases including poliomyelitis that affect the phoenix nerves or supplying intercostal muscles; diseases of nerves to respiratory muscles (e.g., Guillain-Barré syndrome); diseases of the myoneural junction such as myasthenia gravis; diseases of the respiratory muscles themselves such as progressive muscular dystrophy; thoracic cage abnormalities (e.g., crushed chest); upper airway obstruction (e.g., thymoma); hypoventilation associated with extreme obesity (Pickwickian syndrome); and other miscellaneous causes such as metabolic alkalosis and idiopathic states.2 In all these conditions the lungs are normal.
Respiratory Failure from Neuromuscular Disease
Neuromuscular diseases are accompanied by variable degrees of involvement of the muscles of inspiration and expiration. The clinical manifestations reflect the compromise of both muscle groups.24 Disorders of respiratory control and those of peripheral neuromuscular disease are shown in Tables 41.1 and 41.2, respectively.
Table 41.1
Disorders of Ventilatory Control*
| Disorder | Associations | Mechanism |
| Metabolic alkalosis54 | Maintenance of metabolic alkalosis for any length of time means that renal homeostatic mechanisms for HCO3– excretion have been disrupted. | Impaired autonomic control of ventilation. Voluntary control remains intact. |
| Ondine’s curse55,56 (congenital or acquired central alveolar hypoventilation) | Usually caused by congenital hypoventilation syndrome but can be from surgical incisions into the second cervical segment to relieve intractable pain.57 Can also be seen in medullary infarction in an intermittent form.58–60 | Impaired automatic control of ventilation. Voluntary ventilation remains intact. Classically, the patient “forgets to breathe” when asleep. Patient maintains relatively normal blood gas while awake.3,61 |
| Carotid body resection62 | Introduced in Japan in the 1940s as a treatment for asthma.63,64 Also seen after bilateral endarterectomy for carotid vascular disease. | Depressed hypoxic ventilatory drive during exercise. Generally eucapnic at rest. Destruction of peripheral chemoreceptors.65,66 |
| Cheyne-Stokes respiration67,68 | Commonly associated with cardiac disease; can also be seen in neurologic disease sedation, sleep, altitude acclimatization.69 | Delay between changes in ventilation and detection of the resulting arterial PCO2 by the central chemoreceptors maintains a cyclic pattern of respiration. |
| Myxedema | In critically ill patients, laboratory differentiation between severe hypothyroidism and the euthyroid-sick syndromes is difficult and may require measurement of free hormone levels. | Respiratory muscle weakness. Depression of ventilatory drive.70–72 |
| Starvation | Nutritional intervention can return muscle ventilatory function to normal levels. Furthermore, it seems likely that the ventilatory drive can be influenced by dietary intake of amino acids and glucose. | Decreased ventilatory drive, decreased respiratory muscle function, alterations of lung parenchyma and depressed lung defense mechanisms. |
| Drug effects | Opiates, barbiturates, benzodiazepines | Should be used judiciously in patients with preexisting hypoventilation. |
| Medroxyprogesterone† | Increases ventilatory drive in normal males leading to about 5 mm fall in PaCO2. | |
| Used in obesity hypoventilation syndrome.73–75 | ||
| Theophylline† | Increases hypoxic ventilatory response and prevents the fall in hypoxic ventilatory response. | |
| Acetazolamide† | Efficacy and side effects of long-term use are unknown. Small, crossover study reduced central apnea in patients with congestive heart failure.76 |
*Studies cited in this table may be found in the complete list of references for this chapter provided online.
Table 41.2
Disorders of the Peripheral Neuromuscular System*
| Site of Disease | Disease | Type of Respiratory Failure |
| Spinal Cord | ||
| Space-occupying lesions | Syringomyelia | Chronic respiratory failure |
| Multiple sclerosis77 | Chronic respiratory failure | |
| Mass | Chronic respiratory failure | |
| Anterior horn cell lesions | Poliomyelitis5,16,78 | Chronic respiratory failure |
| Amyotrophic lateral sclerosis | Chronic respiratory failure | |
| Inhibiting neuronal blockade | Tetanus | Acute respiratory failure |
| Any level | Traumatic injury | Acute respiratory failure |
| Motor Nerves | ||
| Peripheral Neuropathy | ||
| Phrenic nerve injury54 | Acute/chronic respiratory failure | |
| Beriberi79 | Chronic respiratory failure | |
| Guillain-Barré80 | Chronic respiratory failure | |
| Critical illness polyneuropathy81,82 | Chronic respiratory failure | |
| Lyme disease83 | Chronic respiratory failure | |
| Diphtheria84 | Acute respiratory failure | |
| Neuromuscular Junction | ||
| Tick paralysis85 | ||
| Organophosphate poisoning | Acute respiratory failure | |
| Botulism86 | Acute respiratory failure | |
| Eaton-Lambert syndrome | Acute respiratory failure | |
| Myasthenia gravis5,31 | Acute/chronic respiratory failure | |
| Muscle Involvement | ||
| Dystrophies | Muscular dystrophy5,31,87–89 | Chronic respiratory failure |
| Myotonic dystrophy90,91 | Chronic respiratory failure | |
| Myopathy | Polymyositis, dermatomyositis, and other collagen vascular diseases92 | Chronic respiratory failure |
| Malnutrition93 | Chronic respiratory failure | |
| Thyroid, adrenal, pituitary glands | Chronic respiratory failure | |
| Metabolic acid-base, electrolyte4,18–20 | Acute respiratory failure | |
| Disorders of the Chest Wall | Obesity hypoventilation4,94–96 | Acute/chronic respiratory failure |
| Asphyxiating thoracic dystrophy97 | Acute/chronic respiratory failure | |
| Fibrothorax98 | Acute/chronic respiratory failure | |
| Thoracoplasty98 | Acute/chronic respiratory failure | |
| Ankylosing spondylitis98 | Acute/chronic respiratory failure | |
| Flail chest | Acute/chronic respiratory failure |
*Studies cited in this table may be found in the complete list of references for this chapter provided online.
Tests of Respiratory Muscle Strength
Respiratory muscle weakness is the hallmark of most neuromuscular disease. Simple tests measure maximal inspiratory and expiratory pressures (PImax and PEmax). These tests are conducted by measuring airway pressures while requiring the patient to make maximal inspiratory and expiratory efforts against a closed airway at low or high volumes, respectively.25 Respiratory muscle force is dependent on age, sex, and lung volume. PImax and PEmax of greater than 60 to 80 cm H2O exclude significant neuromuscular weakness. Abnormally low values are not diagnostic but indicate a need for further assessment.
Tests of Respiratory Control and Drive
Electromyography permits more direct measurements of respiratory muscle strength. Respiratory muscle fatigue is indicated by paradoxical respirations or respiratory alternans. The mechanism consists of intermittent decreases or cessation of the contribution of the diaphragm to the inspiratory effort. It has been assumed that this pattern of muscle recruitment indicates diaphragmatic fatigue.26
Clinical Recognition and Manifestations
Alveolar hypoventilation exists when arterial PCO2 increases above the normal range. In clinically important hypercarbia, PCO2 typically ranges from 50 to 70 mm Hg. Lethargy, confusion, and a depressed level of consciousness are seen as a result of hypercapnia. An increase in intracranial pressure and cerebral blood flow, as well as a decrease in myocardial contractility, occur.2 The oxyhemoglobin dissociation curve shifts to the right, which leads to an increase in the release of oxygen to the tissues.
In the acute form, progressive weakness of respiratory muscles leads to rapid reduction in vital capacity followed by respiratory failure with hypoxemia and hypercarbia. Symptoms are those of acute respiratory failure including dyspnea, tachypnea, and tachycardia. In the chronic form, impairment of the respiratory muscles affects mechanical properties of the lungs and chest wall, decreases the ability to clear secretions, and eventually may alter the function of the central respiratory centers. Symptoms include orthopnea, fatigue, disturbed sleep, and hypersomnolence.27
Significant hypoxemia may coexist with chronic hypercapnia or occur with the onset of additional acute pulmonary disorders. The administration of supplemental oxygen is expected to worsen hypercapnia. Major processes that contribute are worsening of ventilation perfusion mismatching caused by an attenuation of hypoxic pulmonary vasoconstriction, a decrease in the binding affinity of hemoglobin for CO2 (Haldane effect), and a decrease in minute ventilation. Importantly, the clinician must be aware that it is imperative to provide supplemental oxygen to maintain adequate tissue oxygenation. This precaution will avoid potentially life-threatening consequences; however, in the spontaneously breathing patient, the lowest FIO2 that produces acceptable oxygenation should be chosen. The major adverse effect seen in patients with hypercapnia with use of supplemental oxygen is an abrupt discontinuation of oxygen leading to hypoxemia.28–30
When hypoventilation is not caused by decreased central nervous system drive, neuromuscular weakness, or bellows dysfunction, tachypnea is a relatively early manifestation that can be seen in association with near-normal maximum respiratory pressures.31 Orthopnea that develops within seconds of lying down is a classic symptom of patients with diaphragmatic paralysis. The pathophysiology of dyspnea resulting from gravitational influences and the mechanical function of the diaphragm during water immersion, another manifestation of diaphragmatic paralysis, are discussed by McCool and Mead.32 Normal diaphragm contraction is associated with a downward movement of the diaphragm and outward movement of the abdomen. When subjects with diaphragmatic paralysis are observed in a supine posture, a paradoxical inward motion of the abdomen can be observed during inspiration. Because these patients are unable to develop transdiaphragmatic pressure gradients, the abdominal contents are drawn toward the chest by the inspiratory fall in pleural pressure generated by the accessory muscles of respiration.33
Patient Management
Respiratory Failure
• Acute hypercapnic respiratory failure: the patient will have no, or minor, evidence of preexisting respiratory disease, and arterial blood gas tensions will show a high PaCO2, low pH, and normal bicarbonate.
• Chronic hypercapnic respiratory failure: evidence of chronic respiratory disease, high PaCO2, near normal pH, high bicarbonate.
• Acute-on-chronic hypercapnic respiratory failure: an acute deterioration in an individual with significant preexisting hypercapnic respiratory failure, high PaCO2, low pH, high bicarbonate.34
Acute Respiratory Failure Caused by Neuromuscular Disease
Involvement of the inspiratory and expiratory muscle groups in neuromuscular disease occurs to variable degrees.24,35 Inspiratory muscle fatigue occurs as muscle weakness progresses or because of excessive ventilatory demands, as in systemic infection. Respiratory rate increases as a usual response to preserve minute ventilation. Work of breathing is increased in tachypnea due to increased dead-space ventilation and by decreased relative time spent in expiration. Blood flow to respiratory muscles is compromised because this occurs primarily during expiration.35 Weakness of inspiratory muscles predisposes to atelectasis because of reduced tidal volume and vital capacity. The elastic recoil of the stretched thoracic and lung tissue provides driving pressure for airflow. Therefore, expiratory muscle weakness has less impact on respiratory ventilation and mechanics. Essential function of the expiratory muscle is that of generation of an effective cough and clearance of secretions.
The risk of respiratory failure increases significantly when the vital capacity (VC) falls below 15 mL/kg, particularly if there is a clear downward trend. Serial measurements of vital capacity at the bedside are helpful in this situation.36,37 Careful assessments of ability to protect airway, oxygenation, ability to ventilate, and chest radiographs are essential in determining need for assistance with ventilation.38 Ideally, ventilatory support should be initiated in the setting of impending respiratory failure, when there has been a clear downward trend, rather than having to initiate after the development of cardiovascular instability. Noninvasive ventilation or endotracheal intubation with initiation of positive pressure ventilation is indicated at this time. An anticipatory approach avoids risks associated with emergent intubation and minimizes complications.39–41
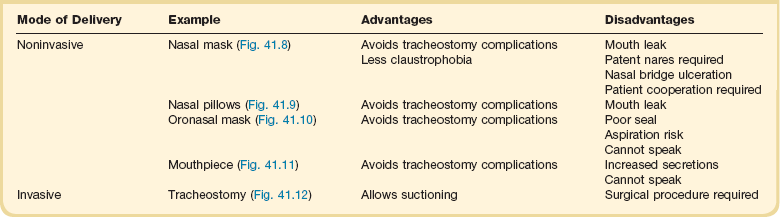
Noninvasive Positive Pressure Ventilation
Noninvasive ventilation (NIV) is widely used for acute and chronic respiratory failure via mask (Figs. 41.8, 41.9, 41.10, and 41.11). If arterial blood gas tensions do not improve, the level of support can be increased. If the aim is to abolish muscle effort completely, there is little to be gained by increasing the level of inspiratory pressure above 20 cm H2O (chest wall deformity) or 25 cm H2O (chronic obstructive pulmonary disease).42,43 NIV is widely considered to be an effective treatment in patients with chronic ventilatory failure caused by chest wall deformity and neuromuscular disease.44
When to Use Noninvasive Ventilation in Nonpulmonary Causes of Respiratory Failure45
Blood Gases
• Respiratory acidosis (PaCO2 > 50 mm Hg, pH < 7.35, or H+ > 45 nmol/L) that persists despite maximal medical treatment and appropriate controlled oxygen therapy (patients with pH < 7.25 or H+ > 56 nmol/L respond less well and should be managed in an intensive care unit)
• Low A-a oxygen gradient (patients with severe life-threatening hypoxemia are more appropriately managed by tracheal intubation)
Chronic Respiratory Failure
Prolonged mechanical ventilation is defined by the Centers for Medicare and Medicaid Services in the United States as greater than 21 days of mechanical ventilation for at least 6 hours per day.46 Most patients requiring prolonged mechanical ventilation will have a tracheostomy (Fig. 41.12) placed to facilitate comfort, communication, and chronic ventilator facility or home ventilation placement. Common problems among patients undergoing prolonged mechanical ventilation are similar to those on short-term ventilation including the following:47–50
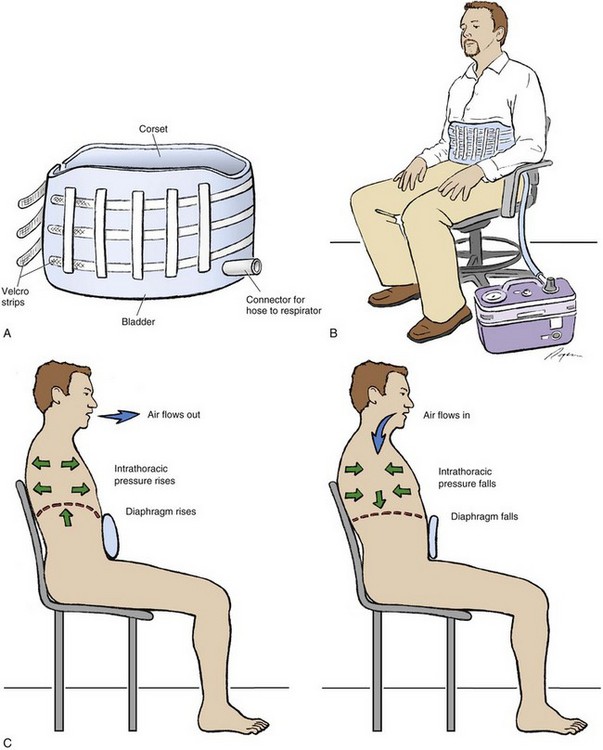
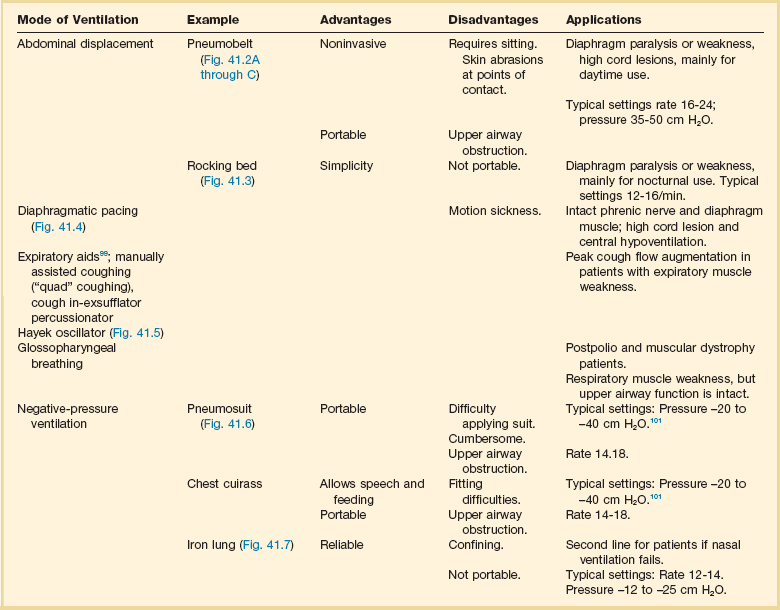
Chronic Ventilatory-Assist Devices
Methods of chronic mechanical ventilation include noninvasive and invasive techniques (Tables 41.3 and 41.4). Ventilatory-assist devices have been used for chronic respiratory failure for decades. Initially, negative-pressure ventilators were used, but this approach was soon replaced by the use of volume-cycled ventilation delivered by a tracheostomy. Because this patient population was in a terminal stage without other treatment options, tracheostomy and chronic ventilation became the standard of therapy if the patient wanted to continue aggressive care.
Table 41.3

*Studies cited in this table may be found in the complete list of references for this chapter provided online.
Figure 41.3 Rocking bed.
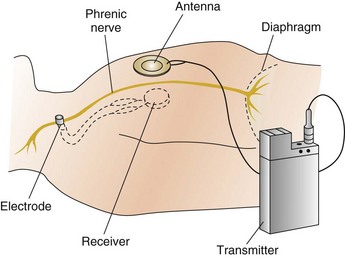
Figure 41.4 Diaphragmatic pacer.

Figure 41.5 Patients using cough assist devices.
Figure 41.6 Pneumosuit.

Figure 41.7 Iron lung.
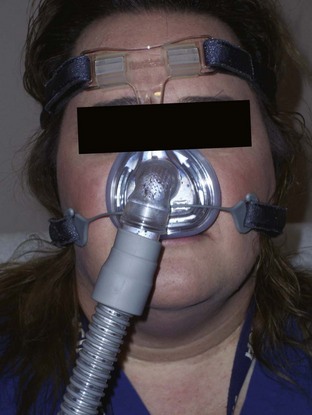
Figure 41.8 Nasal mask.
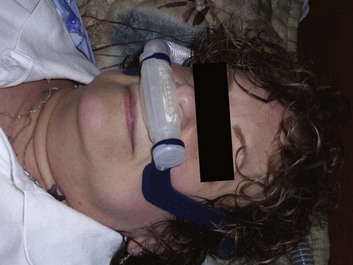
Figure 41.9 Nasal pillows.
Figure 41.11 Mouthpiece.
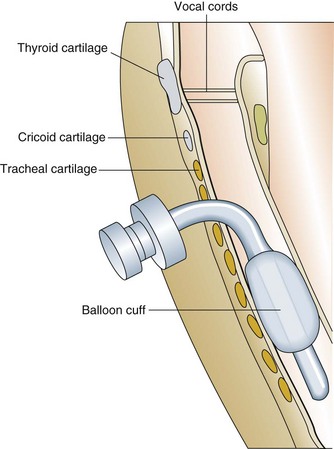
Figure 41.12 Completed tracheostomy.
Major ethical considerations surround the institution of chronic ventilatory support.51 This issue is evolving rapidly because of the widespread availability of relatively inexpensive technology. Guidelines have been published regarding clinical indications and management strategies for the use of noninvasive positive pressure ventilation, although they are based on inadequate data.52,53 The discussion about ventilatory assistance should occur before an emergent setting. Adequate and unbiased information should be given to patients so that they can make an informed decision. Different ventilatory methods and modes of delivery should be discussed with patients, including the possibility of foregoing any type of mechanical ventilation. Patient and caregiver quality of life and satisfaction have only recently been investigated in this population, and many unanswered questions remain. Management of the terminal phase can also be difficult.
Management of Airway Secretion Clearance
An understanding of neuromuscular respiratory pathophysiology and the modes of effective noninvasive cough support is key in the evaluation and management of neuromuscular diseases. A variety of cough-augmentation therapies or a combination of these therapies can be used to support cough.102 Here are some examples of clearance device categories:
References
1. Murray, M, Nadel, J. Murray and Nadel’s Textbook of Respiratory Medicine, 4th ed. Philadelphia: Elsevier; 2005.
2. Karnad, DR, Apte, SJ, Supe, AN. Effect of venous hypercarbia and hyperventilation on myocardial contractility in canine haemorrhagic shock. J Postgrad Med. 1993; 39:68–71.
3. Mellins, RB, Balfour, HH, Jr., Turino, GM, Winters, RW. Failure of automatic control of ventilation (Ondine’s curse). Medicine. 1970; 49:487–504.
4. Rochester, DF, Enson, Y. Current concepts in the pathogenesis of the obesity-hypoventilation syndrome. Am J Med. 1974; 57:402–420.
5. Davis, J, Goldman, M, Loh, L, et al. Diaphragm function and alveolar hypoventilation. Q J Med. 1976; 45:87–100.
6. Goldstein, R. Hypoventilation: Neuromuscular and chest wall disorders. Clin Chest Med. 1992; 13:507–521.
7. Newsom-Davis, J. The diaphragm and neuromuscular disease. Am Rev Respir Dis. 1979; 119:115–117.
8. Williams, MH, Jr. Ventilatory failure in COPD. Postgrad Med. 1973; 54:124–128.
9. Demers, RR, Irwin, R. Management of hypercapneic respiratory failure: A systematic approach. Respir Care. 1979; 24:328.
10. Gray, B, Blalock, JM. Interpretation of the alveolar oxygen difference in patients with hypercapnia. Am Rev Respir Dis. 1991; 143:4–8.
11. Hyland, RH, Jones, NL, Powles, AC, et al. Primary alveolar hypoventilation treated with nocturnal electrophrenic respiration. Am Rev Respir Dis. 1978; 117:165–172.
12. Rodman, T, Close, HP. The primary hypoventilation syndrome. Am J Med. 1959; 26:808–817.
13. Rhoads, GG, Brody, JS. Idiopathic alveolar hypoventilation: Clinical spectrum. Ann Intern Med. 1969; 71:271–278.
14. McNicholas, WT, Carter, JL, Rutherford, R, et al. Beneficial effect of oxygen in primary alveolar hypoventilation with central sleep apnea. Am Rev Respir Dis. 1982; 125:773–775.
15. Gozal, D, Marcus, CL, Shoseyof, D, et al. Peripheral chemoreceptor function in children with the congenital hypoventilation syndrome. J Appl Physiol. 1993; 74:379–387.
16. Hsu, AA, Staats, BA. “Postpolio” sequelae and sleep-related disordered breathing. Mayo Clin Proc. 1998; 73:216–224.
17. Spengler, CM, Gozal, D, Shea, SA. Chemoreceptive mechanisms elucidated by studies of congenital central hypoventilation syndrome. Respir Physiol. 2001; 129:247–255.
18. Schwartz, WB, Brackett, NC, Jr., Cohen, JJ. The response of extracellular hydrogen ion concentration to graded degrees of chronic hypercapnia: The physiologic limits of the defense of pH. J Clin Invest. 1965; 44:291–301.
19. Goldring, RM, Turino, GM, Heinemann, HO. Respiratory-renal adjustments in chronic hypercapnia in man. Am J Med. 1971; 51:772–784.
20. Heinemann, HO, Goldring, RM. Bicarbonate and the regulation of ventilation. Am J Med. 1974; 57:361–370.
21. Jackson, CE, Rosenfeld, J, Moore, DH, et al. A preliminary evaluation of a prospective study of pulmonary function studies and symptoms of hypoventilation in ALS/MND patients. J Neurol Sci. 2001; 191:75–78.
22. Plum, F, Leigh, RJ. Abnormalities of central mechanisms. In: Horbein TF, ed. Lung Biology in Health and Disease (vol. 17), Regulation of Breathing (part 2). New York: Marcel Dekker; 1981:989–1057.
23. Plum, F. Neurological integration of behavioral and metabolic control of breathing. In: Porter R, ed. Breathing. Hering-Breuer Centenary Symposium. London: Churchill, 1970.
24. Roussos, C, Macklem, PT. The respiratory muscles. N Engl J Med. 1982; 307:786–797.
25. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002; 166:518–624.
26. Zagelbaum, G, Gross, DA. Clinical manifestations of inspiratory muscle fatigue. Am J Med. 1982; 73:308–316.
27. Sivak, ED, Shefner, JM, Sexton, J. Neuromuscular disease and hypoventilation. Curr Opin Pulm Med. 1999; 5:355–362.
28. Dick, CR, Liu, Z, Sassoon, CS, et al. O2-induced change in ventilation and ventilatory drive in COPD. Am J Respir Crit Care Med. 1997; 155:609–614.
29. Sassoon, CS, Hassell, KT, Mahutte, CK. Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. Am Rev Respir Dis. 1987; 135:907–911.
30. Kelsen, SG, Fleegler, B, Altose, MD. The respiratory neuromuscular response to hypoxia, hypercapnia, and obstruction to airflow in asthma. Am Rev Respir Dis. 1979; 120:517–527.
31. Gibson, GJ, Pride, NB, Davis, JN, Loh, LC. Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis. 1977; 115:389–395.
32. McCool, FD, Mead, J. Dyspnea on immersion: Mechanisms in patients with bilateral diaphragm paralysis. Am Rev Respir Dis. 1989; 139:275–276.
33. Davis, J, Goldman, M, Loh, L, Casson, M. Diaphragm function and alveolar hypoventilation. Q J Med. 1976; 45:87–100.
34. Wyatt, J, Bellis, F. British Thoracic Society guidelines on non-invasive ventilation. Emerg Med J. 2002; 19:435.
35. Roussos, C, Koutsoukou, A. Respiratory failure. Eur Respir J. 2003; 47(suppl):3s–14s.
36. Rabinstein, AA, Wijdicks, EF. Warning signs of imminent respiratory failure in neurological patients. Semin Neurol. 2003; 23:97–104.
37. Ropper, AH. The Guillain-Barré syndrome. N Engl J Med. 1992; 326:1130–1136.
38. MacDuff, A, Grant, IS. Critical care management of neuromuscular disease, including long-term ventilation. Curr Opin Crit Care. 2003; 9:106–112.
39. Newton-John, H. Prevention of pulmonary complications in severe Guillain-Barré syndrome by early assisted ventilation. Med J Aust. 1985; 142:444–445.
40. Ropper, AH, Kehne, SM. Guillain-Barré syndrome: Management of respiratory failure. Neurology. 1985; 35:1662–1665.
41. Loh, L. Neurological and neuromuscular disease. Br J Anaesth. 1986; 58:190–200.
42. Tuggey, JM, Elliott, MW. Titration of non-invasive positive pressure ventilation in chronic respiratory failure. Respir Med. 2006; 100:1262–1269.
43. International Consensus Conferences in Intensive Care Medicine. Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 2001; 163:283–291.
44. Turkington, PM, Elliott, MW. Rationale for the use of non-invasive ventilation in chronic ventilatory failure. Thorax. 2000; 55:417–423.
45. Wyatt, J, Bellis, F. British Thoracic Society guidelines on non-invasive ventilation. Emerg Med J. 2002; 192:192–211.
46. MacIntyre, NR, Epstein, SK, Carson, S, et al. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest. 2005; 128:3937–3954.
47. Ding, LW, Wang, HC, Wu, HD, et al. Laryngeal ultrasound: A useful method in predicting post-extubation stridor. A pilot study. Eur Respir J. 2006; 27:384–389.
48. Chatila, WM, Criner, GJ. Complications of long-term mechanical ventilation. Respir Care Clin N Am. 2002; 8:631–647.
49. Kalb, TH, Lorin, S. Infection in the chronically critically ill: Unique risk profile in a newly defined population. Crit Care Clin. 2002; 18:529–552.
50. Chung, YH, Chao, TY, Chiu, CT, Lin, MC. The cuff-leak test is a simple tool to verify severe laryngeal edema in patients undergoing long-term mechanical ventilation. Crit Care Med. 2006; 34:409–414.
51. Polkey, MI, Lyall, RA, Davidson, AC, et al. Ethical and clinical issues in the use of home non-invasive mechanical ventilation for the palliation of breathlessness in motor neurone disease. Thorax. 1999; 54:367–371.
52. Robert, D, Willig, TN, Leger, P, Paulus, J. Long-term nasal ventilation in neuromuscular disorders: Report of a consensus conference. Eur Respir J. 1993; 6:599–606.
53. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: A consensus conference report. Chest. 1999; 116:521–534.
54. Tuller, MA, Mehdi, F. Compensatory hypoventilation and hypercapnia in primary metabolic alkalosis: Report of three cases. Am J Med. 1971; 50:281–290.
55. Weese-Meyer. Idiopathic congenital central hypoventilation syndrome: Diagnosis and management. American Thoracic Society. Am J Respir Crit Care Med. 1999; 160:368–373.
56. Fishman, AP. The syndrome of chronic alveolar hypoventilation. Bull Physiopathol Respir. 1972; 8:971–980.
57. Mullan, S, Hosobuchi, Y. Respiratory hazards in high cervical percutaneous cordotomy. J Neurosurg. 1968; 28:291–297.
58. Lassman, A, Mayer, SA. Paroxysmal apnea and vasomotor instability following medullary infarction. Arch Neurol. 2005; 62:1286–1288.
59. Bogousslavsky, J, Khurana, R, Deruaz, JP, et al. Respiratory failure and unilateral caudal brainstem infarction. Ann Neurol. 1990; 28:668–673.
60. Severinghaus, JW. Pathophysiologic aspects of the regulation of respiration. Bull Mem Acad R Med Belg. 1979; 134:261–271.
61. Severinghaus, JW. Respiration. Ann Rev Physiol. 1962; 24:421–470.
62. Wade, JG, Larson, CP, Jr., Hickey, RF, et al. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970; 282:823–829.
63. Winter, B. Bilateral carotid body resection for asthma and emphysema: A new surgical approach without hypoventilation or baroreceptor dysfunction. Int Surg. 1972; 57:458–466.
64. Winter, B. Carotid body resection. Controversy—confusion—conflict. Ann Thorac Surg. 1973; 16:648–659.
65. Lugliani, R, Whipp, BJ, Seard, C, Wasserman, K. Effect of bilateral carotid body resection on ventilatory control at rest and during exercise in man. N Engl J Med. 1971; 285:1105–1111.
66. Winter, B. Bilateral carotid body resection for asthma and emphysema. Respir Ther. 1972; 57:458–466.
67. Cherniack, NS, Longobardo, GS. Cheyne-Stokes breathing: An instability in physiologic control. N Engl J Med. 1973; 288:952–957.
68. Kasai, T, Narui, K, Dohi, T, et al. Efficacy of nasal bi-level positive airway pressure in congestive heart failure patients with Cheyne-Stokes respiration and central sleep apnea. Circ J. 2005; 69:913–921.
69. Cherniack, NS, Longobardo, G, Evangelista, CJ. Causes of Cheyne-Stokes respiration. Neurocrit Care. 2005; 3:271–279.
70. Zwillich, C, Peirson, DJ, Hofeldt, FD, et al. Ventilatory control in myxedema and hypothyroidism. N Engl J Med. 1975; 292:662–665.
71. Nordqvist, P, Dhuner, KG, Stenberg, K, et al. Myxoedema coma and carbon dioxide-retention. Acta Med Scand. 1960; 166:189–194.
72. Massumi, R, Winnacker, JL. Severe depression of the respiratory center in myxedema. Am J Med. 1964; 36:876.
73. Sutton, FD, Jr., Zwillich, CW, Creagh, CE, et al. Progesterone for outpatient treatment of Pickwickian syndrome. Ann Intern Med. 1975; 83:476–479.
74. Skatrud, JB, Dempsey, JA, Kaiser, DG. Ventilatory response to medroxyprogesterone acetate in normal subjects: Time course and mechanism. J Appl Physiol. 44, 1978. [393–344].
75. Kryger, M, McCullough, RE, Collins, D, et al. Treatment of excessive polycythemia of high altitude with respiratory stimulant drugs. Am Rev Respir Dis. 1978; 117:455–464.
76. Javaheri, S. Acetazolamide improves central sleep apnea in heart failure: A double-blind, prospective study. Am J Respir Crit Care Med. 2006; 173:234–237.
77. Berlin, L, Kurtzke, JF, Guthrie, TC. Acute respiratory failure in multiple sclerosis and its management. AMA Arch Neurol Psychiatry. 1953; 69:394–395.
78. Lane, DJ, Hazleman, B, Nichol, PJ. Late onset respiratory failure in patients with previous poliomyelitis. Q J Med. 1974; 43:551–568.
79. Fujita, I, Sata, T, Gondo, K, et al. Cardiac beriberi (shoshin beriberi) caused by excessive intake of isotonic drink. Acta Paediatr Jpn. 1992; 34:466–468.
80. Zifko, U, Chen, R. The respiratory system. Baillieres Clin Neurol. 1996; 5:477–495.
81. Dhand, UK. Clinical approach to the weak patient in the intensive care unit. Respir Care. 2006; 51:1024–1040.
82. Latronico, N, Peli, E, Botteri, M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005; 11:126–132.
83. Abbott, RA, Hammans, S, Margarson, M, Aji, BM. Diaphragmatic paralysis and respiratory failure as a complication of Lyme disease. J Neurol Neurosurg Psychiatry. 2005; 76:1306–1307.
84. Popova, LM, Avdiunina, IA, Alferova, VP, et al. Respiratory insufficiency in adults with diphtheric polyneuropathy. Anesteziol Reanimatol. 1996; Mar-April:9–13.
85. Grattan-Smith, PJ, Morris, JG, Johnston, HM, et al. Clinical and neurophysiological features of tick paralysis. Brain. 1997; 120:1975–1987.
86. FitzGerald, S, Lyons, R, Ryan, J, et al. Botulism as a cause of respiratory failure in injecting drug users. Ir J Med Sci. 2003; 172:143–144.
87. Coccagna, G, Mantovani, M, Parchi, C, et al. Alveolar hypoventilation and hypersomnia in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 1975; 38:977–984.
88. Inkley, SR, Oldenburg, FC, Vignos, JrPJ. Pulmonary function in Duchenne muscular dystrophy related to stage of disease. Am J Med. 1974; 56:297–306.
89. Hukins, CA, Hillman, DR. Daytime predictors of sleep hypoventilation in Duchenne muscular dystrophy. Am J Respir Crit Care Med. 2000; 161:166–170.
90. Denis, J, Cornu, P, Laffay, J, et al. Myotonic dystrophy and acute respiratory insufficiency. Sem Hop. 1977; 53:1683–1688.
91. Rimmer, KP, Golar, SD, Lee, MA, Whitelaw, WA. Myotonia of the respiratory muscles in myotonic dystrophy. Am Rev Respir Dis. 1993; 148:1018–1022.
92. Martyn, JB, Wong, MJ, Huang, SH. Pulmonary and neuromuscular complications of mixed connective tissue disease: A report and review of the literature. J Rheumatol. 1988; 15:703–705.
93. Saka, M, Balkan, A, Demirci, N, Sarikayalar, U. Pulmonary function and nutrition. Tuberk Toraks. 2003; 51:461–466.
94. Berger, KI, Ayappa, I, Chatr-Amontri, B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001; 120:1231–1238.
95. Lopata, M, Onal, E. Mass loading, sleep apnea, and the pathogenesis of obesity hypoventilation. Am Rev Respir Dis. 1982; 126:640–645.
96. Phipps, PR, Starritt, E, Caterson, I, Grunstein, RR. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002; 57:75–76.
97. Zizka, J, Rehulova, E, Juttnerova, V, Balicek, P. Asphyxiating thoracic dystrophy. Cesk Pediatr. 1979; 34:98–99.
98. Bergofsky, E. Respiratory failure in disorders of the thoracic cage. Am Rev Respir Dis. 1979; 119:643–669.
99. Bach, JR, Ishikawa, Y, Kim, H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997; 112:1024–1028.
100. Kasai, T, Narui, K, Dohi, T, et al. First experience of using new adaptive servo-ventilation device for Cheyne-Stokes respiration with central sleep apnea among Japanese patients with congestive heart failure: Report of four clinical cases. Circ J. 2006; 70:1148–1154.
101. Linton, DM. Cuirass ventilation: A review and update. Crit Care Resusc. 2005; 7:22–28.
102. Louis, J, Boitano MSc RRT. Management of airway clearance in neuromuscular disease. Respir Care. 2006; 51:913–922.
*NIV may be used despite the presence of these contraindications if it is to be the “ceiling” of treatment.