Chapter 69 Nonoperative Management and Treatment of Spine Injuries
At the Scene
According to Advanced Trauma Life Support (ATLS) protocol, life-threatening compromise to airway, breathing, and circulation should be promptly addressed. Although the greatest risk for spinal cord injury occurs at the time of high-energy impact, neurologic deficits can develop thereafter during treatment. In 1983 Podolsky et al. reported that up to 25% of spinal cord injuries had been caused by or aggravated after the patient had come under medical care.1 Immobilization of the injured spine is the key to preventing such catastrophic decline.
At the Hospital
The patient should arrive in the emergency department on a backboard with a cervical collar in place. In the face of a global instability, motion can still occur in spite of all attempts at rigid immobilization. The patient should be moved on and off the backboard as few times as possible until the stability of the spine can be adequately assessed. For most injuries the collar provides an increased level of stability. However, it does not provide complete immobilization.2 With a complete ligamentous disruption, the collar has minimal effect. The person stabilizing the spine is much more significant in restricting motion.3
The risk of decubitus ulceration is directly proportional to the length of time on a backboard—8 hours on a backboard is associated with a 100% likelihood of a decubitus ulcer.4 The patient should be moved from the board as soon as possible. Appropriate spine immobilization must be continued at all times.
Contrary to all available evidence suggesting that the log roll is an ineffective and potentially dangerous technique for spine immobilization, it is still almost universally used. In fact, studies conducted prior to 2004 showed dramatic and unacceptable motion with a log roll.5 Recently, many studies have reevaluated this controversial subject. Compared with any other method of transfer, the log roll maneuver has been shown to cause more segmental motion at the level of the unstable, injured segment.6–13 Lift and slide techniques are far superior since they tend to create less motion at the injured segment.10
Imaging Studies
Following review of the initial CT scan, another assessment of spinal stability can be performed. When a closed reduction of a dislocated segment is needed, it should be performed expediently in the awake and alert patient. Serial neurologic examinations are performed during such a reduction maneuver. If the patient is obtunded or reliable neurologic examinations are impossible, then an emergent MRI should be obtained prior to attempting reduction to rule out significant disc herniation.14
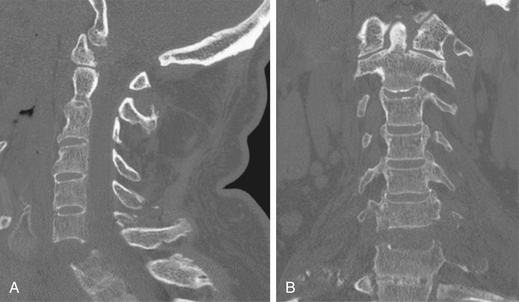
Anteroposterior and lateral radiographs of the cervical, thoracic, lumbar, and sacral spine are standard imaging studies obtained in cases of high-energy impact with suspected spinal injury. Distracting injuries can often mask symptoms secondary to significant spine injury, and meticulous assessment of the spine must always be performed (Figs. 69-1 to 69-3). It is necessary to image the entire spinal column due to a 10% incidence of noncontiguous spinal injuries.15 Specific injury mechanisms and fracture patterns should prompt the treating team to search for commonly associated nonspinal injuries. Flexion-distraction injuries are highly associated with potentially life-threatening intra-abdominal injuries, so these must be ruled out. Patients with transverse process fractures at L5 have a 61% incidence of a pelvic fracture.16 Falls from a height with resulting burst fractures are often associated with significant lower-extremity fractures, in particular those of the tibia and calcaneus.
Closed Reduction of the Cervical Spine
Decompression of the spinal cord through closed reduction should be performed as soon as the patient can medically tolerate it. Closed reduction is a means of reducing cervical spine deformity, indirectly decompressing neural elements, and providing stability. It has been shown to be safe and can dramatically improve neurologic status if performed within the first few hours following injury. In animal studies, Carlson et al. showed that decompression within 3 hours showed better and quicker neurologic recovery.17 A small series of patients with no cord function who received reduction in an emergent manner immediately began to recover.18 Although this is anecdotal, it is extremely important. The timing question is answered after consideration of the risk-benefit ratio between waiting to obtain a prereduction MRI and proceeding directly with reduction.
Considerable controversy surrounds the order of the reduction and obtainment of an MRI in an acute cervical spine dislocation. Eismont et al. reported on a series of six patients who roentgenographically demonstrated herniation of an intervertebral disc with marked protrusion of disc material into the spinal canal following subluxation or dislocation of a cervical facet. For the first patient in this series, no myelogram or CT scan was performed, and the patient awoke with complete quadriplegia following dorsal open reduction and internal fixation. Following a myelogram and ventral decompression surgery, the cause was identified as an extruded intervertebral disc. The authors recommended obtaining an MRI in all patients prior to attempted closed reduction and definitive surgery.19–21 Several other cases with neurologic deficits after open reduction under general anesthesia have been reported.20,21 There have also been reports of progression of neurologic deficits during traction while the patient was awake and participating that later resolved.22
The group from Thomas Jefferson University advocates performing immediate closed reduction in the patient that is awake and can reliably participate in serial neurologic examinations. Vaccaro et al. published their series of 11 patients who underwent successful awake closed reduction without any neurologic worsening. In their series, two patients had herniated discs prior to reduction, and another five had herniated discs following reduction.14 Darsaut et al. attempted to determine the effect of disc herniation during closed reduction in a series of 17 patients who had a cervical dislocation that was reduced under MRI monitoring. They demonstrated that at least as a research tool this technique was possible.23
A commonly used algorithm is based on the patient’s neurologic status. If the patient is unable to participate reliably in the physical examination, then an MRI is obtained as expediently as possible. In cases of mild radicular deficit, MRI has the principal disadvantage of requiring vital time. If the patient has a significant spinal cord injury, the risk is that the cord will remain compressed for a longer time. Therefore, if the patient has minimal or no neurologic dysfunction, an MRI should be obtained. If the patient has an American Spinal Injury Association (ASIA) A, B, or C injury and is able to cooperate with the reduction and serial neurologic examinations, consideration should be given to an immediate, rapid reduction. In this situation, the MRI would be obtained after reduction.
Reduction Techniques
Reduction of a cervical spine dislocation must be performed under image guidance and in a very controlled manner. Gardner-Wells tongs are applied with the pins placed 1 cm above the pinna of the ear just below the equator of the head. Pins are tightened to 3.6 kg of pressure. This is indicated once the precalibrated indicator pins protrude a measured amount. If using weights over 25 kg, titanium pins and MRI-compatible tongs are insufficient. Weights of over 60 kg have been used safely for closed reduction of such injuries.24 In such instances, stainless steel pins or two sets of tongs must be used. Another option includes the use of a halo that provides four titanium pins to distribute the forces over more pins. The major disadvantage of stainless steel pins is their MRI incompatibility.
Timing of Surgical Intervention
Debate continues over the appropriate timing of traction or surgery in cases of acute spinal cord injury. It is difficult to demonstrate improvement in neurologic function from acute surgical intervention.25 Complete cord injuries and neurologically intact patients are very likely to remain neurologically unchanged with appropriate surgical or nonoperative care. Incomplete lesions typically improve with either surgical or nonsurgical care. Late surgery with decompression of the spinal canal in incomplete cord injuries has been shown to improve neurologic function even several years following the traumatic event.26,27 In the acute setting, there is sparse and unconvincing evidence supporting early surgery. Surgery for the purpose of spinal canal decompression in a neurologically intact patient is difficult to defend considering that several series have shown dramatic spinal canal remodeling over time in patients with and without surgery.28–35
Upper Cervical Injuries
Occipitocervical injuries are most often fatal and typically found postmortem. When encountering a patient with such an injury, the treating physician must be vigilant about the diagnosis to ensure the patient’s survival. Initially, such patients should be meticulously immobilized on a backboard with a collar and the head secured with sandbags and tape. Atlanto-occipital disassociation is then stabilized with a halo vest until definitive surgical stabilization is performed.36 It should be noted that traction for type II injuries (axial distraction) can be catastrophic and is strictly contraindicated. The injury is treated with dorsal occipital cervical fusion with at least 3 months of halo vest immobilization. Occipital condyle fractures and Jefferson or atlas ring fractures are typically managed with an orthosis or a halo.37
Odontoid fractures depend on the injury itself. Many can be treated with a rigid orthosis or halo vest. Type I and type III fractures typically heal uneventfully and have a good prognosis without surgery. Transverse type II fractures through the waist of the odontoid have a high associated nonunion rate and are therefore the ideal fractures for surgical treatment. A dorsally displaced odontoid fracture is more likely to be treated with surgery.38–43 Polin reported on a series of patients treated with a rigid collar as opposed to a halo. More nonunions were associated with the type II fractures. However, there was no statistically significant difference between the orthoses used.43 Chronic odontoid nonunions in the elderly can often be followed and may not require surgical intervention. In a series of persistent nonunions, no progression of atlantoaxial instability or neurologic deficit was noted or myelopathic symptoms during the follow-up period.44
At later reassessment of stability, transverse ligament ruptures can be managed in an orthosis if a bony avulsion occurs.45,46 If successful, this avoids the significant loss of motion following an atlantoaxial arthrodesis. Dickman et al. demonstrated a 100% failure to heal in complete ligamentous disruptions. These injuries often result in a significant incidence of neurologic injury, and there is frequent association with other upper cervical injuries. They should be treated with C1-2 arthrodesis.47
The vast majority of other axis injuries can be stabilized with an orthosis or a halo vest. Traumatic spondylolisthesis of the axis most commonly occurs secondary to a hyperextension and axial load mechanism. Neurologic deficit rarely occurs, with the exception for the atypical fracture that occurs ventral to the dorsal vertebral body cortex.48 These atypical fractures may require surgery to prevent neurologic decline. Severe hangman’s fractures with instability through the C2-3 disc space require surgery. Most other axis injuries can be managed successfully nonoperatively.37,40,49–52
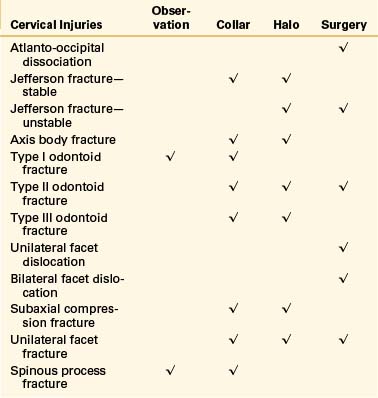
Table 69-1 summarizes the treatment options for cervical fractures.
Thoracic and Thoracolumbar Fractures
Transverse process fractures that are isolated require no treatment and can be mobilized as tolerated. With multiple transverse process fractures, a thorough assessment must be made to determine instability or a fracture-dislocation. An L5 transverse process fracture has a 61% association with a pelvic or sacral fracture.16 These should be critically evaluated with a pelvic CT scan to rule out an associated pelvic injury.
Compression fractures without injury to the dorsal ligamentous complex can be mobilized as tolerated with an orthosis for comfort. Ohana et al. have even suggested that an orthosis may not be necessary.56 Multiple-level compression fractures in a young healthy individual suggest a high-energy mechanism. Particular attention must be paid to the dorsal ligamentous complex at all injured levels. If a dorsal ligamentous injury is present, surgical treatment is necessary.
Burst Fractures
Most lumbar and thoracolumbar burst fractures can be managed nonsurgically (Table 69-2). Several excellent studies have shown comparable functional outcome to surgery, with less morbidity from nonsurgical treatment. The key to establishing stability is the intact dorsal ligamentous complex. Wood et al. demonstrated in a prospective, randomized series comparable or better outcomes than in those patients treated surgically.57 Although Denis et al. in 1984 reported a 17% increase in neurologic deficit with nonoperative treatment, this has not since been reported.58 There are multiple reports with minimal complications and almost no progression of neurologic deficits. There appears to be little correlation to long-term pain and disability with degree of kyphosis.28,29,31,32,57–87
Although the dorsal ligamentous complex is the key to deciding on operative versus nonoperative treatment, a definitive way of defining stability can be elusive in many cases. A palpable interspinous gap or pain that occurs with deep palpation is a good indicator of dorsal ligamentous involvement. Although it has been popularized that kyphosis can be an indicator of dorsal injury, this has not been conclusive. Many utilize MRI to assess the dorsal ligaments, although the findings can often be ambiguous.83 Lee et al. reported that a fat-suppressed T2 sagittal MRI was the most reliable way to assess the dorsal ligamentous complex. In their series of 34 burst fractures they identified 30 patients with dorsal ligamentous instability,88 which seems to be an extremely high number of unstable fractures. Instead, it may be that MRI changes may not necessarily correlate with competency. Oner et al. did a similar study and was unable to correlate the MRI to an Arbeitsgemeinschaft für Osteosynthesefragen (AO) classification to determine treatment.89 The treating physician must therefore exercise a great deal of judgment in deciding which fractures require surgical intervention.
Multiple-Level and Noncontiguous Injuries
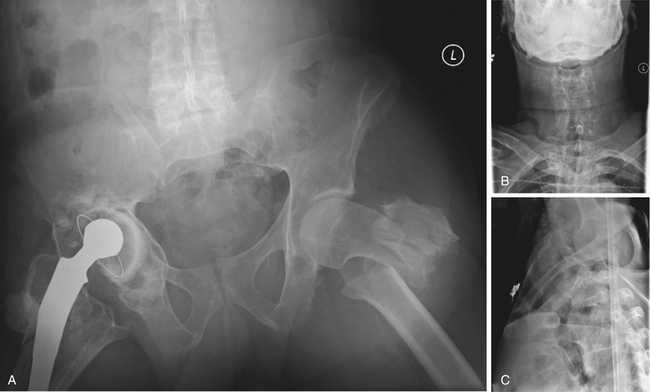
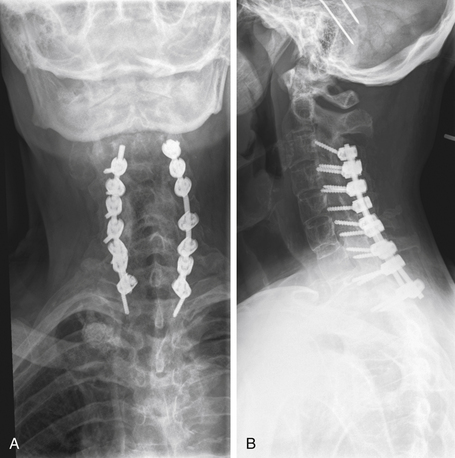
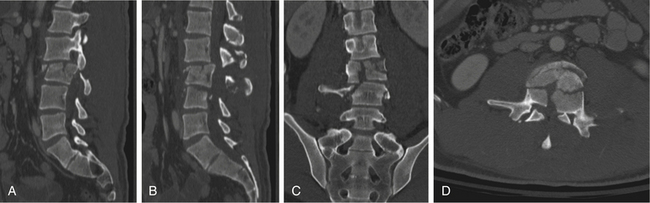
In cases of multiple-level injuries, particularly noncontiguous injuries, bear in mind that surgical intervention requires multiple-level fusions at multiple levels of the spine or one long fusion mass. This fact has dramatic effects on the final range of motion, function, and long-term outcome. Avoiding fusion, therefore, greatly benefits this patient population (Figs. 69-4 and 69-5). Another alternative is the Jacobs rod long and fuse short technique (i.e., using long rods to reduce fractures while fusing the minimum number of segments). In this technique, the instrumentation is later removed to restore motion at the unfused segments.90
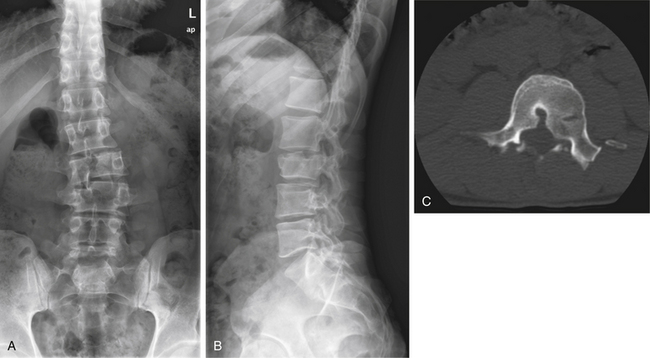
FIGURE 69-5 The same patient as in Figure 69-4 returned to work 3 months following injury, after being treated conservatively in a RotoRest bed. Anteroposterior (A) and lateral (B) radiographs at 2-year follow-up demonstrating adequate alignment and healing of the fractures. C, Axial CT image showing canal remodeling at 2 years.
Kinetic Therapy
Whether used as a temporary measure or for definitive treatment, kinetic therapy is a useful adjunct to the management of spinal injury. A specially designed hospital bed achieves kinetic therapy by continually rotating the patient through a minimum of 40 degrees. The Kinetic Concepts, Inc. (KCI, San Antonio, TX) RotoRest bed stabilizes the spine while providing kinetic therapy. The Hill-Rom (Batesville, IN) SPO2RT and KCI TriaDyne beds provide patient rotation but are not adequate for spinal stabilization. Use of kinetic therapy has demonstrated shorter ICU stays, decreased time on a ventilator, better pulmonary outcomes, and a lower incidence of adult respiratory distress syndrome (ARDS) in trauma patients.91–103
Initial Emergency Department Stabilization
A trauma patient who cannot be immediately mobilized can be treated very effectively with kinetic therapy. Until the spine is stabilized surgically or sufficient time passes for bony healing, the KCI RotoRest bed can provide 24-hour-a-day mobilization and pulmonary benefit as well as maintaining spine stabilization.5,104,105 Even after spine stabilization surgery, kinetic therapy with a SPO2RT or TriaDyne bed can minimize postoperative pneumonia and ARDS.
Short-Term Treatment
In the setting of multiple-level noncontiguous fractures, the patient can be maintained on a RotoRest bed or in traction to allow for initial osseous healing of some of the fractures.106 Cancellous vertebral body fractures heal rapidly if the patient is receiving adequate nutrition. A period of 2 to 3 weeks of initial healing may allow for single-level fusion or no surgery at all instead of a multiple-level procedure.
Long-Term Definitive Treatment
For polytrauma patients with multiple fractures and life-threatening associated injuries, surgery is not an attractive solution and in many instances is contraindicated. Four to six weeks of recumbency in a kinetic therapy bed can allow for enough healing to mobilize the patient in a brace.79
Neurologic Complete Cord Injuries
Although many advocate immediate stabilization for earlier rehabilitation in a patient with a complete cord injury, there is a dramatically increased risk of infection in the long term. In the neurologically complete patient, the incidence of acute wound infection has been reported to be as high as 25% to 40%.107 Furthermore, these patients experience multiple urinary tract infections over the course of their lifetimes, and these transient episodes of bacteremia or septicemia can lead to late infections.
Penetrating Injuries and Gunshot Wounds
Low-velocity missile wounds to the spine rarely require surgical stabilization. Exploration of missile tracts for vessel or hollow viscous injuries should be determined by the general surgery trauma team. Removal of foreign bodies for spinal canal decompression is not routinely warranted at spinal cord levels. Some investigators report improved neurologic function with canal decompression in the region of the cauda equina. The risk of such acute complications as spinal fluid fistulas, infections, and wound dehiscence is increased with surgical intervention.108–111 Blood levels can be monitored over time if there is concern of plumbism. In the setting of increased serum lead levels, the bullet fragments can be removed later. This is an uncommon occurrence.112–114
Osteoporosis Fractures
All osteoporotic fractures should be treated nonsurgically if possible. Short-term bedrest, bracing, or cement augmentation is usually preferable to surgical stabilization. Constructs that are usually effective in the nonosteoporotic spine may not hold in soft bone. The difference in stiffness between the instrumentation and bone presents a problem at the bone-implant interface, with a high likelihood of implant pullout or failure. Adjacent segment fractures also pose a significant problem due to the stiff instrumented segment producing a stress riser and the presence of osteoporotic bone.115,116
Keys to Nonoperative Care
The spine must be accurately assessed and then reassessed to ensure the accurate determination of spinal stability. Stability is reevaluated when the patient is able to be mobilized and examined upright. Upright radiographs in the orthosis or definitive method of treatment provide another chance to determine the level of stability and effectiveness of treatment. Mehta et al. reported that in 25% of their patients, the upright radiographs resulted in a change in treatment.117
Brunette D.D., Rockswold G.L. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27(4):445-457.
Denis F., Armstrong G.W., Searls K., Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142-149.
McGuire R.A., Neville S., Green B.A., et al. Spinal instability and the log-rolling maneuver. J Trauma. 1987;27(5):525-531.
Pape H.C., Remmers D., Weinberg A., et al. Is early kinetic positioning beneficial for pulmonary function in multiple trauma patients? Injury. 1998;29(3):219-225.
Podolsky S., Baraff L.J., Simon R.R., et al. Efficacy of cervical spine immobilization methods. J Trauma. 1983;23(6):461-465.
Rechtine G.R.2nd, Cahill D., Chrin A.M. Treatment of thoracolumbar trauma: comparison of complications of operative versus nonoperative treatment. J Spinal Disord. 1999;12(5):406-409.
1. Podolsky S., Baraff L.J., Simon R.R., et al. Efficacy of cervical spine immobilization methods. J Trauma. 1983;23(6):461-465.
2. Del Rossi G., Heffernan T.P., Horodyski M., et al. The effectiveness of extrication collars tested during the execution of spine-board transfer techniques. Spine J. 2004;4(6):619-623.
3. Rechtine G.R., Del Rossi G., Conrad B.P., et al. Motion generated in the unstable spine during hospital bed transfers. J Trauma. 2004;57(3):609-611. discussion 611–612
4. Curry K., Casady L. The relationship between extended periods of immobility and decubitus ulcer formation in the acutely spinal cord-injured individual. J Neurosci Nurs. 1992;24(4):185-189.
5. McGuire R.A., Neville S., Green B.A., et al. Spinal instability and the log-rolling maneuver. J Trauma. 1987;27(5):525-531.
6. Bearden B.G., Conrad B.P., Horodyski M., et al. Motion in the unstable cervical spine: comparison of manual turning and use of the Jackson table in prone positioning. J Neurosurg Spine. 2007;7(2):161-164.
7. Conrad B.P., Horodyski M., Wright J., et al. Log-rolling technique producing unacceptable motion during body position changes in patients with traumatic spinal cord injury. J Neurosurg Spine. 2007;6(6):540-543.
8. Del Rossi G., Horodyski M., Conrad B.P., et al. Transferring patients with thoracolumbar spinal instability: are there alternatives to the log roll maneuver? Spine (Phila Pa 1976). 2008;33(14):1611-1615.
9. Del Rossi G., Horodyski M., Heffernan T.P., et al. Spine-board transfer techniques and the unstable cervical spine. Spine (Phila Pa 1976). 2004;29:E134-E138.
10. Del Rossi G., Horodyski M.H., Conrad B.P., et al. The 6-plus-person lift transfer technique compared with other methods of spine boarding. J Athl Train. 2008;43(1):6-13.
11. DiPaola C.P., DiPaola M.J., Conrad B.P., et al. Comparison of thoracolumbar motion produced by manual and Jackson-table-turning methods. Study of a cadaveric instability model. J Bone Joint Surg [Am]. 2008;90(8):1698-1704.
12. DiPaola M.J., DiPaola C.P., Conrad B.P., et al. Cervical spine motion in manual versus Jackson table turning methods in a cadaveric global instability model. J Spinal Disord Tech. 2008;21(4):273-280.
13. Rechtine G.R., Conrad B.P., Bearden B.G., et al. Biomechanical analysis of cervical and thoracolumbar spine motion in intact and partially and completely unstable cadaver spine models with kinetic bed therapy or traditional log roll. J Trauma. 2007;62(2):383-388. discussion 388
14. Vaccaro A.R., Falatyn S.P., Flanders A.E., et al. Magnetic resonance evaluation of the intervertebral disc, spinal ligaments, and spinal cord before and after closed traction reduction of cervical spine dislocations. Spine (Phila Pa 1976). 1999;24(12):1210-1217.
15. Gupta A., el Masri W.S. Multilevel spinal injuries. Incidence, distribution and neurological patterns. J Bone Joint Surg [Br]. 1989;71(4):692-695.
16. Nork S.E., Jones C.B., Harding S.P., et al. Percutaneous stabilization of U-shaped sacral fractures using iliosacral screws: technique and early results. J Orthop Trauma. 2001;15(4):238-246.
17. Carlson G.D., Minato Y., Okada A., et al. Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J Neurotrauma. 1997;14(12):951-962.
18. Brunette D.D., Rockswold G.L. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27(4):445-447.
19. Eismont F.J., Arena M.J., Green B.A. Extrusion of an intervertebral disc associated with traumatic subluxation or dislocation of cervical facets. Case report. J Bone Joint Surg [Am]. 1991;73(10):1555-1560.
20. Jeanneret B., Magerl F., Ward E.H., Ward J.C. Posterior stabilization of the cervical spine with hook plates. Spine (Phila Pa 1976). 1991;16(Suppl 3):S56-S63.
21. Olerud C., Jonsson H.Jr. Compression of the cervical spine cord after reduction of fracture dislocations. Report of 2 cases. Acta Orthop Scand. 1991;62(6):599-601.
22. Wimberley D.W., Vaccaro A.R., Goyal N., et al. Acute quadriplegia following closed traction reduction of a cervical facet dislocation in the setting of ossification of the posterior longitudinal ligament: case report. Spine (Phila Pa. 1976). 2005;30(15):E433-E438.
23. Darsaut T.E., Ashforth R., Bhargava R., et al. A pilot study of magnetic resonance imaging-guided closed reduction of cervical spine fractures. Spine (Phila Pa 1976). 2006;31(18):2085-2090.
24. Cotler J.M., Herbison G.J., Nasuti J.F., et al. Closed reduction of traumatic cervical spine dislocation using traction weights up to 140 pounds. Spine (Phila Pa 1976). 1993;18(3):386-390.
25. McKinley W., Meade M.A., Kirshblum S., et al. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85(11):1818-1825.
26. Bohlman H.H., Anderson P.A. Anterior decompression and arthrodesis of the cervical spine: long-term motor improvement. Part I—Improvement in incomplete traumatic quadriparesis. J Bone Joint Surg [Am]. 1992;74(5):671-682.
27. Bohlman H.H., Kirkpatrick J.S., Delamarter R.B., et al. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res. 1994;300:24-29.
28. Dai L.Y. Remodeling of the spinal canal after thoracolumbar burst fractures. Clin Orthop Relat Res. 2001;382:119-123.
29. Mumford J., Weinstein J.N., Spratt K.F., et al. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine (Phila Pa 1976). 1993;18(8):955-970.
30. Yazici M., Atilla B., Tepe S., et al. Spinal canal remodeling in burst fractures of the thoracolumbar spine: a computerized tomographic comparison between operative and nonoperative treatment. J Spinal Disord. 1996;9(5):409-413.
31. Cantor J.B., Lebwohl N.H., Garvey T., et al. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine (Phila Pa 1976). 1993;18(8):971-976.
32. Shen W.J., Shen Y.S. Nonsurgical treatment of three-column thoracolumbar junction burst fractures without neurologic deficit. Spine (Phila Pa 1976). 1999;24(4):412-415.
33. Wessberg P., Wang Y., Irstam L., et al. The effect of surgery and remodelling on spinal canal measurements after thoracolumbar burst fractures. Eur Spine J. 2001;10(1):55-63.
34. Sandor L., Barabas D. [Spontaneous “regeneration” of the spinal canal in traumatic bone fragments after fractures of the thoraco-lumbar transition and the lumbar spine]. Unfallchirurg. 1994;97(2):89-91.
35. Sjostrom L., Jacobsson O., Karlstrom G., et al. Spinal canal remodelling after stabilization of thoracolumbar burst fractures. Eur Spine J. 1994;3(6):312-317.
36. Fisher C.G., Sun J.C., Dvorak M. Recognition and management of atlanto-occipital dislocation: improving survival from an often fatal condition. Can J Surg. 2001;44(6):412-420.
37. Hadley M.N., Dickman C.A., Browner C.M., et al. Acute traumatic atlas fractures: management and long term outcome. Neurosurgery. 1988;23(1):31-35.
38. Hanssen A.D., Cabanela M.E. Fractures of the dens in adult patients. J Trauma. 1987;27(8):928-934.
39. van Holsbeeck E., Stoffelen D., Fabry G. Fractures of the odontoid process. Conservative and operative treatment. Prognostic factors. Acta Orthop Belg. 1993;59(1):17-21.
40. Fujimura Y., Nishi Y., Kobayashi K. Classification and treatment of axis body fractures. J Orthop Trauma. 1996;10(8):536-540.
41. Wang G.J., Mabie K.N., Whitehill R., et al. The nonsurgical management of odontoid fractures in adults. Spine (Phila Pa 1976). 1984;9(3):229-230.
42. Bettini N., Cervellati S., Di Silvestre M., et al. The nonsurgical treatment of fractures of the dens epistrophei. Chir Organi Mov. 1991;76(1):17-24.
43. Polin R.S., Szabo T., Bogaev C.A., et al. Nonoperative management of Types II and III odontoid fractures: the Philadelphia collar versus the halo vest. Neurosurgery. 1996;38(3):450-456. discussion 456–457
44. Hart R., Saterbak A., Rapp T., et al. Nonoperative management of dens fracture nonunion in elderly patients without myelopathy. Spine (Phila Pa 1976). 2000;25(11):1339-1343.
45. Pennington R.G., Gnanalingham K.K., Van Dellen J.R. Unilateral avulsion fracture of the transverse atlantal ligament: successful treatment in a rigid cervical collar. Br J Neurosurg. 2004;18(4):382-384.
46. Lo P.A., Drake J.M., Hedden D., et al. Avulsion transverse ligament injuries in children: successful treatment with nonoperative management. Report of three cases. J Neurosurg. 2002;96(Suppl 3):338-342.
47. Dickman C.A., Mamourian A., Sonntag V.K., et al. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial instability. J Neurosurg. 1991;75(2):221-227.
48. Starr J.K., Eismont F.J. Atypical hangman’s fractures. Spine (Phila Pa 1976). 1993;18(14):1954-1957.
49. Seljeskog E.L., Chou S.N. Spectrum of the hangman’s fracture. J Neurosurg. 1976;45(1):3-8.
50. German J.W., Hart B.L., Benzel E.C. Nonoperative management of vertical C2 body fractures. Neurosurgery. 2005;56(3):516-521. discussion 521
51. Greene K.A., Dickman C.A., Marciano F.F., et al. Acute axis fractures. Analysis of management and outcome in 340 consecutive cases. Spine (Phila Pa 1976). 1997;22(16):1843-1852.
52. Hadley M.N., Dickman C.A., Browner C.M., et al. Acute axis fractures: a review of 229 cases. J Neurosurg. 1989;71(5 Pt 1):642-647.
53. Cotler H.B., Cotler J.M., Alden M.E., et al. The medical and economic impact of closed cervical spine dislocations. Spine (Phila Pa 1976). 1990;15(6):448-452.
54. Beyer C.A., Cabanela M.E., Berquist T.H. Unilateral facet dislocations and fracture-dislocations of the cervical spine. J Bone Joint Surg [Br]. 1991;73(6):977-981.
55. Beyer C.A., Cabanela M.E. Unilateral facet dislocations and fracture-dislocations of the cervical spine: a review. Orthopedics. 1992;15(3):311-315.
56. Ohana N., Sheinis D., Rath E., et al. Is there a need for lumbar orthosis in mild compression fractures of the thoracolumbar spine? A retrospective study comparing the radiographic results between early ambulation with and without lumbar orthosis. J Spinal Disord. 2000;13(4):305-308.
57. Wood K., Buttermann G., Mehbod A., et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg [Am]. 2003;85(5):773-781.
58. Denis F., Armstrong G.W., Searls K., et al. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142-149.
59. Anderson P.A. Nonsurgical treatment of patients with thoracolumbar fractures. Instr Course Lect. 1995;44:57-65.
60. Capen D.A., Gordon M.L., Zigler J.E., et al. Nonoperative management of upper thoracic spine fractures. Orthop Rev. 1994;23(10):818-821.
61. Chan D.P., Seng N.K., Kaan K.T. Nonoperative treatment in burst fractures of the lumbar spine (L2-L5) without neurologic deficits. Spine (Phila Pa 1976). 1993;18(3):320-325.
62. Chow G.H., Nelson B.J., Gebhard J.S., et al. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine (Phila Pa 1976). 1996;21(18):2170-2175.
63. Clark P., Letts M. Trauma to the thoracic and lumbar spine in the adolescent. Can J Surg. 2001;44(5):337-345.
64. Dai L.Y., Yao W.F., Cui Y.M., et al. Thoracolumbar fractures in patients with multiple injuries: diagnosis and treatment-a review of 147 cases. J Trauma. 2004;56(2):348-355.
65. Dall B.E., Stauffer E.S. Neurologic injury and recovery patterns in burst fractures at the T12 or L1 motion segment. Clin Orthop Relat Res. 1988;233:171-176.
66. Davies W.E., Morris J.H., Hill V. An analysis of conservative (non-surgical) management of thoracolumbar fractures and fracture-dislocations with neural damage. J Bone Joint Surg [Am]. 1980;62(8):1324-1328.
67. Donovan W.H. Operative and nonoperative management of spinal cord injury. A review. Paraplegia. 1994;32(6):375-388.
68. Folman Y., Gepstein R. Late outcome of nonoperative management of thoracolumbar vertebral wedge fractures. J Orthop Trauma. 2003;17(3):190-192.
69. Hitchon P.W., Torner J.C., Haddad S.F., et al. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49(6):619-626. discussion 626–627
70. Huang T.J., Chao E.K., Chen Y.J., et al. [Complete fracture-dislocation of the thoracic spine with spontaneous neurologic decompression: a case report]. Changgeng Yi Xue Za Zhi. 1995;18(4):387-391.
71. Islinger R.B., Kuklo T.R., Polly D.W.Jr. Spine fractures in active duty soldiers and their return to duty rate. Mil Med. 1998;163(8):536-539.
72. Kim N.H., Lee H.M., Chun I.M. Neurologic injury and recovery in patients with burst fracture of the thoracolumbar spine. Spine (Phila Pa 1976). 1999;24(3):290-293. discussion 294
73. Knight R.Q., Stornelli D.P., Chan D.P., et al. Comparison of operative versus nonoperative treatment of lumbar burst fractures. Clin Orthop Relat Res. 1993;293:112-121.
74. Krompinger W.J., Fredrickson B.E., Mino D.E., et al. Conservative treatment of fractures of the thoracic and lumbar spine. Orthop Clin North Am. 1986;17(1):161-170.
75. Lalonde F., Letts M., Yang J.P., et al. An analysis of burst fractures of the spine in adolescents. Am J Orthop. 2001;30(2):115-120.
76. Mick C.A., Carl A., Sachs B., et al. Burst fractures of the fifth lumbar vertebra. Spine (Phila Pa 1976). 1993;18(13):1878-1884.
77. Place H.M., Donaldson D.H., Brown C.W., et al. Stabilization of thoracic spine fractures resulting in complete paraplegia. A long-term retrospective analysis. Spine (Phila Pa 1976). 1994;19(15):1726-1730.
78. Rechtine G.R. Nonsurgical treatment of thoracic and lumbar fractures. Instr Course Lect. 1999;48:413-416.
79. Rechtine G.R.2nd, Cahill D., Chrin A.M. Treatment of thoracolumbar trauma: comparison of complications of operative versus nonoperative treatment. J Spinal Disord. 1999;12(5):406-409.
80. Reid D.C., Hu R., Davis L.A., et al. The nonoperative treatment of burst fractures of the thoracolumbar junction. J Trauma. 1988;28(8):1188-1194.
81. Shapiro S., Abel T., Rodgers R.B. Traumatic thoracic spinal fracture dislocation with minimal or no cord injury. Report of four cases and review of the literature. J Neurosurg. 2002;96(Suppl 3):333-337.
82. Shen W.J., Liu T.J., Shen Y.S. Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine (Phila Pa 1976). 2001;26(9):1038-1045.
83. Terk M.R., Hume-Neal M., Fraipont M., et al. Injury of the posterior ligament complex in patients with acute spinal trauma: evaluation by MR imaging. AJR Am J Roentgenol. 1997;168(6):1481-1486.
84. Vaccaro A., Kim D., Brodke D., et al. Diagnosis and management of thoracolumbar spine fractures. J Bone Joint Surg [Am]. 2003;85:2456-2470.
85. Vanichkachorn J.S., Vaccaro A.R. Nonoperative treatment of thoracolumbar fractures. Orthopedics. 1997;20(10):948-953. quiz 954–955
86. Weinstein J.N., Collalto P., Lehmann T.R. Long-term follow-up of nonoperatively treated thoracolumbar spine fractures. J Orthop Trauma. 1987;1(2):152-159.
87. Weinstein J.N., Collalto P., Lehmann T.R. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine (Phila Pa 1976). 1988;13(1):33-38.
88. Lee H.M., Kim H.S., Kim D.J., et al. Reliability of magnetic resonance imaging in detecting posterior ligament complex injury in thoracolumbar spinal fractures. Spine (Phila Pa 1976). 2001;25(16):2079-2084.
89. Oner F.C., van Gils A.P., Dhert W.J., et al. MRI findings of thoracolumbar spine fractures: a categorisation based on MRI examinations of 100 fractures. Skeletal Radiol. 1999;28(8):433-443.
90. Akbarnia B.A., Crandall D.G., Burkus K., et al. Use of long rods and a short arthrodesis for burst fractures of the thoracolumbar spine. A long-term follow-up study. J Bone Joint Surg [Am]. 1994;76(11):1629-1635.
91. Hildebrand F., Giannoudis P.V., Griensven M., et al. Management of polytraumatized patients with associated blunt chest trauma: a comparison of two European countries. Injury. 2005;36(2):293-302.
92. Powers J., Daniels D. Turning points: implementing kinetic therapy in the ICU. Nurs Manage. 35(5), 2004. suppl 1–7quiz 8
93. Cedidi C., Hierner R., Pichlmaier M., et al. Survival of severe ARDS with five-organ system failure following burns and inhalation injury in a 15-year-old patient. Burns. 2003;29(4):389-394.
94. Stiletto R., Gotzen L., Goubeaud S. [Kinetic therapy for therapy and prevention of post-traumatic lung failure. Results of a prospective study of 111 polytrauma patients]. Unfallchirurg. 2000;103(12):1057-1064.
95. Pape H.C., Remmers D., Weinberg A., et al. Is early kinetic positioning beneficial for pulmonary function in multiple trauma patients? Injury. 1998;29(3):219-225.
96. Bein T., Reber A., Metz C., et al. Acute effects of continuous rotational therapy on ventilation-perfusion inequality in lung injury. Intensive Care Med. 1998;24(2):132-137.
97. Pape H.C., Regel G., Borgmann W., et al. The effect of kinetic positioning on lung function and pulmonary haemodynamics in posttraumatic ARDS: a clinical study. Injury. 1994;25(1):51-57.
98. Gentilello L., Thompson D.A., Tonnesen A.S., et al. Effect of a rotating bed on the incidence of pulmonary complications in critically ill patients. Crit Care Med. 1988;16(8):783-786.
99. Ahrens T., Kollef M., Stewart J., et al. Effect of kinetic therapy on pulmonary complications. Am J Crit Care. 2004;13(5):376-383.
100. Dodek P., Keenan S., Cook D., et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141(4):305-313.
101. Heyland D.K., Cook D.J., Dodek P.M. Prevention of ventilator-associated pneumonia: current practice in Canadian intensive care units. J Crit Care. 2002;17(3):161-167.
102. Kocan M.J. Pulmonary considerations in the critical care phase. Crit Care Nurs Clin North Am. 1990;2(3):369-374.
103. Shabot M.M., Wittman M. Kinetic treatment tables. West J Med. 1989;150(4):466.
104. Green B.A., Green K.L., Klose K.J. Kinetic nursing for acute spinal cord injury patients. Paraplegia. 1980;18(3):181-186.
105. Green B.A., Green K.L., Klose K.J. Kinetic therapy for spinal cord injury. Spine (Phila Pa 1976). 1983;8(7):722-728.
106. Tannoury T.Y., Zmurko M.G., Tannoury C.A., et al. Multiple unstable cervical fractures with cord compromise treated nonoperatively: a case report. Spine (Phila Pa 1976). 2004;29(11):E234-E238.
107. Rechtine G.R., Bono P.L., Cahill D., et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma. 2001;15(8):566-569.
108. Heiden J.S., Weiss M.H., Rosenberg A.W., et al. Penetrating gunshot wounds of the cervical spine in civilians. Review of 38 cases. J Neurosurg. 1975;42(5):575-579.
109. Heary R.F., Vaccaro A.R., Mesa J.J., et al. Thoracolumbar infections in penetrating injuries to the spine. Orthop Clin North Am. 1996;27(1):69-81.
110. Kumar A., Wood G.W.2nd, Whittle A.P. Low-velocity gunshot injuries of the spine with abdominal viscus trauma. J Orthop Trauma. 1998;12(7):514-517.
111. Bono C.M., Heary R.F. Gunshot wounds to the spine. Spine J. 2004;4(2):230-240.
112. Scuderi G.J., Vaccaro A.R., Fitzhenry L.N., et al. Long-term clinical manifestations of retained bullet fragments within the intervertebral disk space. J Spinal Disord Tech. 2004;17(2):108-111.
113. Isiklar Z.U., Lindsey R.W. Gunshot wounds to the spine. Injury. 1998;29(Suppl 1):SA7-SA12.
114. Yoshida G.M., Garland D., Waters R.L. Gunshot wounds to the spine. Orthop Clin North Am. 1995;26(1):109-116.
115. Nguyen H.V., Ludwig S., Gelb D. Osteoporotic vertebral burst fractures with neurologic compromise. J Spinal Disord Tech. 2003;16(1):10-19.
116. Rhyne A.3rd, Banit D., Laxer E., et al. Kyphoplasty: report of eighty-two thoracolumbar osteoporotic vertebral fractures. J Orthop Trauma. 2004;18(5):294-299.
117. Mehta J.S., Reed M.R., McVie J.L., et al. Weight-bearing radiographs in thoracolumbar fractures: do they influence management? Spine (Phila Pa 1976). 2004;29(5):564-567.