Nocardia, Streptomyces, Rhodococcus, and Similar Organisms
1. Describe the general characteristics of the aerobic actinomycetes, including their Gram stain morphology, microscopic morphology, colonial morphology, and biochemical reactions.
2. Describe the habitats of actinomycetes and the routes of transmission.
3. Describe the three types of skin infections caused by Nocardia spp. in immunocompromised individuals.
4. List the laboratory tests used to differentiate the clinically relevant aerobic actinomycetes.
5. List the laboratory tests used to differentiate the pathogenic Nocardia spp.
6. Describe the chemical structures required for an organism to be classified as acid-fast.
7. List the virulence factors associated with Nocardia asteroides.
8. Define mycetoma and actinomycetoma.
9. List the various selective media used to isolate aerobic actinomycetes and describe their usefulness in achieving optimal recovery.
These organisms are aerobic, facultatively anaerobic, or obligately anaerobic; only the aerobic actinomycetes are discussed in this chapter. Aerobic actinomycetes belong to the order Actinomycetales. Actinomycetes comprise more than 40 genera, but only the clinically relevant aerobic actinomycetes genera are considered here (Table 19-1). In this chapter, only aerobic actinomycetes that exhibit branching and/or partial acid-fastness are addressed. Although both the Corynebacterium and Mycobacterium genera belong to the order Actinomycetales, Corynebacterium spp. do not usually exhibit branching filaments or partial acid-fastness, and Mycobacterium spp. do not exhibit branching and are strongly (acid-alcohol) acid-fast; for these reasons, the Corynebacteriaceae and Mycobacteriaceae are addressed in Chapters 17 and 43, respectively. Another clinically significant aerobic actinomycete is Tropheryma whipplei; because this organism has not been cultured on artificial media, it is reviewed in Chapter 44. For purposes of discussion, the remaining genera of aerobic actinomycetes are divided into the two large groups: those with cell walls that contain mycolic acid and are therefore partially acid-fast and those with cell envelopes that do not contain mycolic acid and therefore are non–acid-fast.
TABLE 19-1
Clinically Relevant Aerobic Actinomycetes*
| Cell Wall Containing Mycolic Acid | Genus |
| Present | |
| Absent |
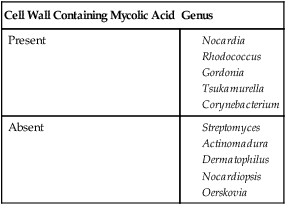
*The genera Williamsia, Skermania, and Dietzia are also aerobic actinomycetes but to date are not clinically relevant.
General Characteristics
Partially Acid-Fast Aerobic Actinomycetes
Nocardia spp.
Currently, the taxonomy in the genus Nocardia is changing rapidly. Recognition and description of new species continue and remain controversial regarding the number of validly described species; recent publications cite 22 to 30 valid species. Of significance, Cloud et al.1 reported that the most commonly identified species was Nocardia cyriacigeorgica, not N. asteroides, as determined by partial 16S rRNA DNA sequencing, followed by N. farcinica, N. nova, N. africana, and N. veterana. The species considered human pathogens or that have been implicated as human pathogens are listed in Box 19-1. N. asteroides, N. nova, N. farcinica, N. brasiliensis, N. otitidiscaviarum (formerly N. caviae), N. pseudobrasiliensis, and N. transvalensis account for most of the diseases in humans caused by Nocardia spp.
Rhodococcus, Gordonia, Tsukamurella spp.
Organisms belonging to the Rhodococcus, Gordonia, and Tsukamurella genera are similar to Nocardia spp. in that they are gram-positive, aerobic, catalase-positive, partially acid-fast, branching, filamentous bacteria that can fragment into rods and cocci. The extent of acid-fastness depends on the amount and complexity of mycolic acids in the organism’s cell envelope and on culture conditions. The differentiation of these three genera, as well as species identification, is difficult. In particular, the genus Rhodococcus consists of a very diverse group of organisms in terms of morphology, biochemical characteristics, and ability to cause disease. As previously mentioned, the taxonomy of these organisms continues to evolve; species included in these three genera, as of this writing, are summarized in Table 19-2.
TABLE 19-2
Species Included in the Genera Rhodococcus, Gordonia, and Tsukamurella
| Genus | Species |
| Rhodococcus | equi, erythropolis, rhodnii, rhodochrous (other species of unknown significance include globerulus, marinonascens, and ruber) |
| Gordonia | aichiensis, bronchialis, polyisoprenivorans, rubripertincta, sputi, terrae (remaining species isolated from environmental sources) |
| Tsukamurella | paurometabola, pulmonis, tyrosinosolvens, strandjordae (T. ichonensis, T. wratislaviensis isolated from nature) |
Data compiled from Brown JM et al: In Murray PR, Baron EJ, Pfaller MA et al, editors: Manual of clinical microbiology, ed 10, Washington, DC, 2003, American Society for Microbiology; Goodfellow M, Chun J, Stubbs S et al: Lett Appl Microbiol 19:401, 1994; Klatte S, Rainey FA, Kroppenstedt RM: Int J Syst Bacteriol 44:769, 1994; Lasker BA, Brown JM, McNeil MM: Clin Infect Dis 15:233, 1992; Maertens J et al: Clin Microbiol Infect 4:51, 1998; Riegel P et al: J Clin Microbiol 34:2045, 1996; Yassin AF, Rainey FA, Burrghardt J et al: Int J Syst Bacteriol 47:607, 1997; Arenskötter M et al: Appl Environ Microbiol 70:3195, 2004
Non–acid-Fast Aerobic Actinomycetes: Streptomyces, Actinomadura, Dermatophilus, Nocardiopsis, and the Thermophilic Actinomycetes
The non–acid-fast aerobic actinomycetes (i.e., Streptomyces, Actinomadura, Dermatophilus, Nocardiopsis, and the thermophilic actinomycetes) are gram-positive, branching filaments that do not contain mycolic acids in their cell envelopes and are therefore non–acid-fast. This group of actinomycetes is heterogeneous and is encountered infrequently in the clinical laboratory. Only the non–acid-fast actinomycetes associated with human disease are addressed (Table 19-3).
TABLE 19-3
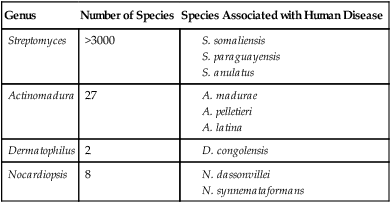
Non–Acid-Fast Aerobic Actinomycetes Associated with Human Disease
| Genus | Number of Species | Species Associated with Human Disease |
| Streptomyces | >3000 |
Epidemiology and Pathogenesis
Partially Acid-Fast Aerobic Actinomycetes
Nocardia spp.
Non–acid-Fast Aerobic Actinomycetes: Streptomyces, Actinomadura, Dermatophilus, Nocardiopsis, and the Thermophilic Actinomycetes
Aspects of the epidemiology of the non–acid-fast aerobic actinomycetes are summarized in Table 19-4. Little is known about how these agents cause infection.
TABLE 19-4
Epidemiology of the Non–Acid-Fast Aerobic Actinomycetes
| Organism | Habitat (Reservoir) | Distribution | Routes of Primary Transmission |
| Streptomyces somaliensis | Sandy soil | Africa, Saudi Arabia, Mexico, South America | Penetrating wound/abrasions in the skin |
| S. anulatus | Soil | Most common isolate in United States | Penetrating wound/abrasions in the skin |
| Actinomadura madurae | Soil | Tropical and subtropical countries | Penetrating wound/abrasions in the skin |
| A. pelletieri, A. latina | Unknown, possibly soil | Tropical and subtropical countries | Penetrating wound/abrasions in the skin |
| Dermatophilus congolensis | Unknown; skin commensal or saprophyte in soil(?) | Worldwide, but more prevalent in humid, tropical, and subtropical regions | Trauma to the epidermis caused by insect bites and thorns; contact with tissues of infected animals through abrasions in the skin |
| Nocardiopsis dassonvillei* | Unknown | Unknown | Unknown |
| Thermophilic actinomycetes | Ubiquitous; water, air, soil, compost piles, dust, hay | Worldwide | Inhalation |
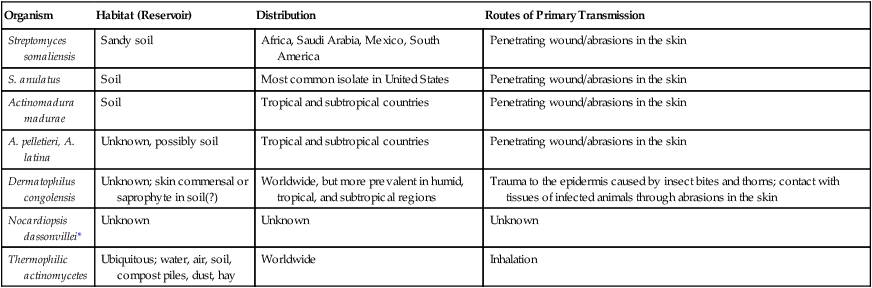
*Only a few cases of infection identified in the literature.
Spectrum of Disease
Partially Acid-Fast Aerobic Actinomycetes
The partially acid-fast actinomycetes cause various infections in humans.
Rhodococcus, Gordonia, Tsukamurella spp.
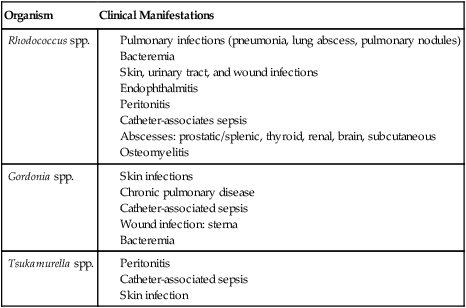
The types of infections caused by Rhodococcus, Gordonia, and Tsukamurella spp. are listed in Table 19-5. For the most part, these organisms are considered opportunistic pathogens, because most infections occur in immunocompromised individuals.
TABLE 19-5
Infections Caused by Rhodococcus, Gordonia, and Tsukamurella spp.
| Organism | Clinical Manifestations |
| Rhodococcus spp. |
Non–acid-Fast Aerobic Actinomycetes: Streptomyces, Actinomadura, Dermatophilus, Nocardiopsis, and the Thermophilic Actinomycetes
Except for the thermophilic actinomycetes, most of these agents have rarely been associated with other types of infections (Table 19-6). These nonmycetomic infections have occurred in immunosuppressed patients, such as those infected with HIV.
TABLE 19-6
Clinical Manifestations of Infections Caused by Non–Acid-Fast Aerobic Actinomycetes
| Organism | Clinical Manifestations |
| Streptomyces spp. (S. somaliensis and other species such as S. anulatus and S. albus) | Actinomycetoma Other (rare): pericarditis, bacteremia, and brain abscess |
| Actinomadura spp. (A. madurae, A. pelletieri, and A. latina) | Actinomycetoma Other (rare): peritonitis, wound infection, pneumonia, and bacteremia |
| Dermatophilus congolensis | Exudative dermatitis with scab formation (dermatophilosis) |
| Nocardiopsis dassonvillei | Actinomycetoma and other skin infections |
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Appropriate specimens should be collected aseptically from affected areas. For the most part, no special requirements are needed for specimen collection, transport, or processing of the organisms discussed in this chapter (refer to Table 5-1 for general information). When nocardiosis is clinically suspected, multiple specimens should be submitted for culture, because smears and cultures are simultaneously positive in only a third of the cases. The significance of random isolation of Nocardia spp. from the respiratory tract is questionable, because these organisms are so widely distributed in nature. Some of the actinomycetes tend to grow as a microcolony in tissues, leading to the formation of granules. Most commonly, these granules are formed in actinomycetomas, such as those caused by Nocardia, Streptomyces, Nocardiopsis, and Actinomadura spp. Therefore, material from draining sinus tracts is an excellent specimen for direct examination and culture.
Direct Detection Methods
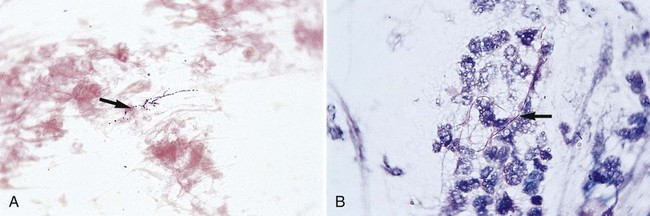
Direct microscopic examination of Gram-stained preparations of clinical specimens is of utmost importance in the diagnosis of infections caused by the aerobic actinomycetes. Often, the demonstration of gram-positive, branching or partially branching beaded filaments provides the first clue to the presence of an aerobic actinomycete (Figure 19-1). Unfortunately, the actinomycetes do not always exhibit such characteristic morphology; many times these organisms are not seen at all or appear as gram-positive cocci, rods, or short filaments. Nevertheless, if gram-positive, branching or partially branching organisms are observed, a modified acid-fast stain should be performed (i.e., 1% sulfuric acid rather than 3% hydrochloric acid as the decolorizing agent) (see Procedure 19-1 on the Evolve site). The modified acid-fast stain is positive in only about half of these smears showing gram-positive beaded, branching filaments subsequently confirmed as Nocardia sp. Histopathologic examination of tissue specimens using various histologic stains, such as Gomori’s methenamine-silver (GMS) stain, can also detect the presence of actinomycetes.
Molecular Diagnostics
Amplification techniques (i.e., polymerase chain reaction [PCR]) involving the 16srRNA sequence have been used to examine the relatedness among the genera and species within the non–acid-fast aerobic actinomycetes and thermophilic actinomycetes. When the MicroSeq System was used for identification (see Chapter 8), almost 15% of isolates were identified as Nocardia spp., but no definitive species were given. PCR paired with restriction endonuclease analysis has been used to identify commonly isolated Nocardia spp. Housekeeping heat shock protein genes coupled with the 16srRNA sequence are used in this assay. DNA sequencing of several genes, including the 16srRNA, a heat shock protein gene, and a housekeeping gene are referred to as secA1. These methods currently are not available in the clinical laboratory; they are predominantly used for taxonomic, epidemiologic, and research studies.
Cultivation
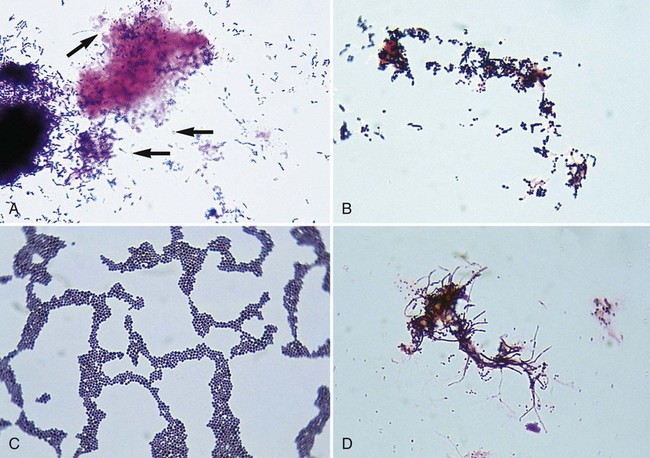
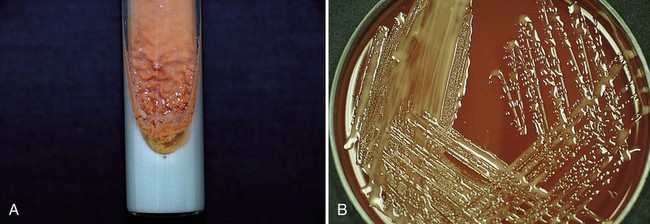
If other aerobic actinomycetes are considered, a selective medium, such as brain-heart infusion agar with chloramphenicol and cycloheximide, is recommended in addition to routine agar to enhance isolation from contaminated specimens. Although most aerobic actinomycetes grow at 35°C, recovery is increased at 30°C. Therefore, selective and nonselective agars should be incubated at 35°C and 30°C. Plates should be incubated for 2 to 3 weeks. The typical Gram-stain morphology and colonial appearance of the aerobic actinomycetes are summarized in Table 19-7. Examples of Gram stains and cultures of different aerobic actinomycetes are shown in Figures 19-2 and 19-3.
TABLE 19-7
Typical Gram-Stain Morphology and Colonial Appearance
| Organism | Gram Stain* | Colonial Appearance on Routine Agar |
| Nocardia spp. | Branching, fine, delicate filaments with fragmentation | Extremely variable; adherent; some isolates are beta-hemolytic on sheep blood agar; wrinkled; often dry, chalky-white appearance to orange-tan pigment; crumbly |
| Rhodococcus spp. | Diphtheroid-like with minimal branching or coccobacillary; colonial growth appears as coccobacilli in zigzag configuration | Nonhemolytic; round; often mucoid with orange to red, salmon-pink pigment developing within 4 to 7 days (pigment may vary widely) |
| Gordonia spp. | Nonmotile, short rods | Somewhat pigmented; G. sputi: smooth, mucoid and adherent to media; G. bronchialis: dry and raised |
| Tsukamurella spp. | Mostly long rods that fragment, no spores or aerial hyphae | May have rhizoid edges, dry, white to creamy to orange |
| Streptomyces spp. | Extensive branching with chains and spores; does not fragment easily | Glabrous or waxy heaped colonies; variable morphology |
| Actinomadura spp. | Moderate, fine, intertwining branching with short chains of spores, fragmentation | White-to-pink pigment, mucoid, molar tooth appearance after 2 weeks’ incubation |
| Dermatophilus sp. | Branched filaments divided in transverse and longitudinal planes; fine, tapered filaments | Round, adherent, gray-white colonies that later develop orange pigments; often beta-hemolytic |
| Nocardiopsis sp. | Branching with internal spores | Coarsely wrinkled and folded with well-developed aerial mycelium |
*Aerobic actinomycetes are gram-positive organisms that are often beaded in appearance.
Data compiled from Brown JH, Mcneil MM: In Murray PR, Baron EJ, Pfaller MA et al, editors: Manual of clinical microbiology, ed 10, Washington, DC, American Society for Microbiology, 2003; McNeil MM, Brown JM: Clin Microbiol Rev 7:357, 1994.
Approach to Identification
If Gram-stain morphology or colonial morphology suggests a possible actinomycetes (see Table 19-7), an acid-fast stain should be performed first to rule out rapidly growing mycobacteria (see Chapter 43), followed by a modified acid-fast stain (see Procedure 19-1). If the modified acid-fast stain results are positive, the isolate is a probable partially acid-fast aerobic actinomycete (i.e., Nocardia, Rhodococcus, Tsukamurella, or Gordonia sp). If the acid-fast stain result is negative, these organisms still are not completely ruled out because of the variability of acid-fastness among isolates belonging to this group. Aerobic actinomycetes can be initially placed into major groupings by considering the following:
• Gram-stain morphology (see Figures 19-1 and Figure 19-2)
• Modified acid-fast stain results
• Presence or absence of aerial hyphae when grown on tap water agar
• Growth or no growth in nutrient broth containing lysozyme (250 µg/mL (Figure 19-4) (see Procedure 19-2 on the Evolve site)
• Other tests: urea hydrolysis, nitrate reduction, and ability to grow anaerobically
Table 19-8 summarizes the key characteristics of aerobic actinomycetes.
TABLE 19-8
Preliminary Grouping of the Clinically Relevant Aerobic Actinomycetes
| Characteristics | Nocardia spp. | Rhodococcus spp. | Gordonia spp. | Tsukamurella spp. | Streptomyces spp. | Actinomadura spp. | Dermatophilus sp. | Nocardiopsis spp. |
| Partially acid-fast | + | ± | ± | ± | − | − | − | − |
| Appearance on tap water agar*: branching/aerial hyphae | Extensive/+ | Minimal/− | Minimal/− | Minimal/− | Extensive/+ | Variable/sparse | Branching | Extensive/+ |
| Lysozyme resistance | + | ± | − | + | − | − | − | − |
| Urea hydrolysis | + | ± | + | + | ± | − | + | + |
| Nitrate reduction | ± | ± | + | − | ± | + | − | + |
| Growth anaerobically | − | − | − | − | − | − | − | − |
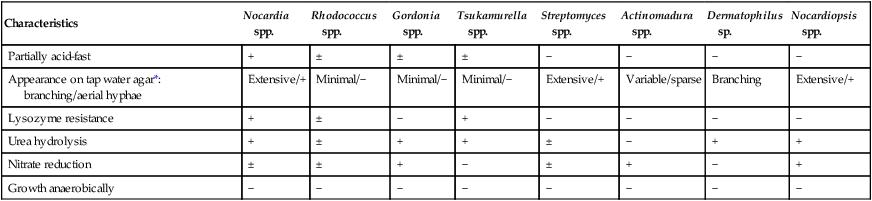
+, predominantly positive; −, predominantly negative; ±, mostly positive with some negative isolates.
*Tap water agar: Bacto agar (Difco Laboratories, Detroit, Mich.) is added to 100 mL of tap water, sterilized, and then poured into plates.Two plates are lightly inoculated using a single streak and incubated at 30°C for up to 7 days and examined daily.
Modified from Brown JM, McNeil MM: In Murray PR, Baron EJ, Pfaller MA et al, editors: Manual of clinical microbiology, ed 10, Washington, DC, 2003, American Society for Microbiology.
Accurate identification of Nocardia to the species level is important, because differences among the species have emerged in terms of virulence, antibiotic susceptibility, and epidemiology. However, identification of the pathogenic nocardiae to the species level can be problematic, because no single method can identify all Nocardia isolates, and the methods used are time-consuming, often requiring 2 weeks. Useful phenotypic tests include the use of casein, xanthine, and tyrosine hydrolysis; growth at 45°C; acid production from rhamnose; gelatin hydrolysis; opacification of Middlebrook agar; and antimicrobial susceptibility patterns. Some of these reactions with the nocardial pathogens are summarized in Table 19-9.
TABLE 19-9
Key Tests for Differentiation of the Pathogenic Nocardia spp.
| Test | N. asteroides sensu stricto | N. farcinica* | N. nova | N. travalensis (N. asteroides type IV) | N. transvalensis sensu stricto | N. brasiliensis | N. otitidiscaviarum | N. pseudobrasiliensis |
| Hydrolysis of: | ||||||||
| Casein | − | − | − | − | −/+ | + | − | + |
| Xanthine | − | − | − | − | −/+ | − | − | − |
| Tyrosine | − | − | − | − | −/+ | + | −/+ | + |
| Growth at 42°C after 3 days | ± | + | − | − | − | − | ± | − |
| 14-day arylsulfatase | − | − | + | NT | − | − | − | − |
| Acid from rhamnose | ± | ± | − | − | − | − | − | − |
| Gelatin hydrolysis | − | − | − | − | − | + | − | − |
| Opacification of Middlebrook agar 4 | − | + | − | − | − | − | −/+ | − |
| Api 20C assimilation: | ||||||||
| Galactose | − | − | − | + | + | + | − | + |
| Glycerol | + | + | − | + | + | + | + | + |
| Trehalose | − | − | − | + | + | + | + | + |
| Adonitol | − | − | − | − | + | − | − | − |
| Sensitivity by Kirby Bauer disk diffusion†: | ||||||||
| Gentamicin | S | R | S/R | R/S | R/S | S | S | S |
| Tobramycin | S/R | R | S/R | R | R | S | S/R | S |
| Amikacin | S | S | S | S/R | R | S | S | S |
| Erythromycin | R | S/R | S | R | R | R | R | R |
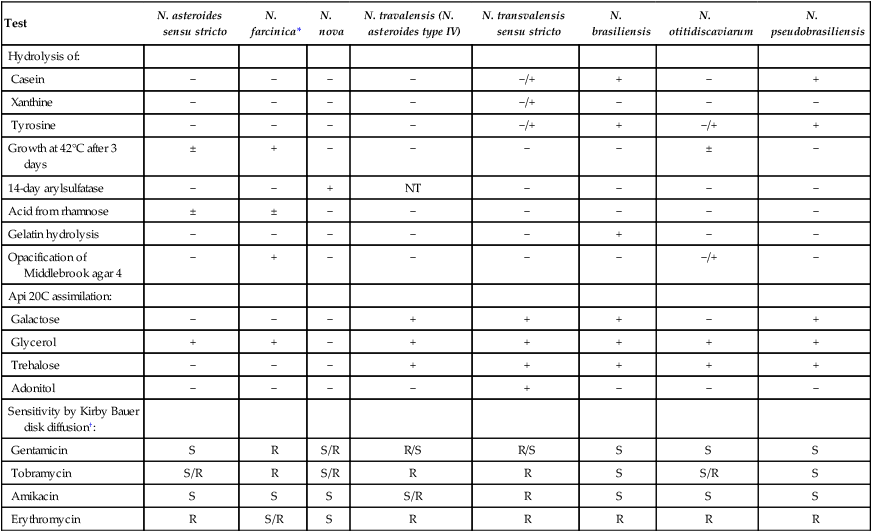
NT, Not tested; +, predominantly positive; −, predominantly negative; ±, mostly positive with some negative isolates; −/+, mostly negative with some positive isolates.
†Sensitive (S) or resistant (R) as determined by Kirby Bauer disk diffusion.
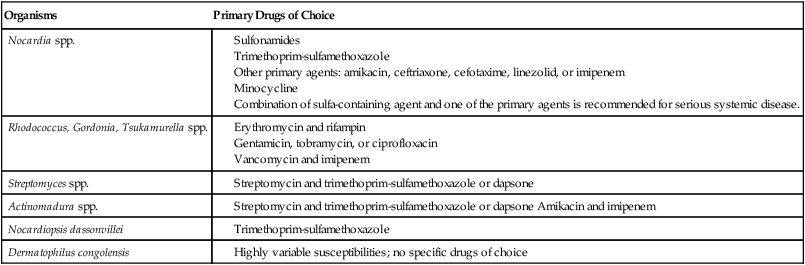
Antimicrobial Susceptibility Testing and Therapy
The primary drugs of choice against the aerobic actinomycetes are shown in Table 19-10; no effective antimicrobial therapy is available for hypersensitivity pneumonitis caused by the thermophilic actinomycetes.