CHAPTER 79 Neurotrophic Arthritis
INTRODUCTION
As noted in prior editions of this book, little has occurred in recent years to change the fact that neurotrophic arthritis of the elbow is distinctly unusual, and poorly understood and treated. Although a large number of causes of neurotrophic arthritis are recognized (Box 79-1), diagnosis is made simpler by the fact that only five or six of these causes affect the upper extremity joints, and only three occur with any frequency at all. In fact, a Medline literature search has revealed that only two case reports have appeared in the literature since 1996.48,69,73 In this section, the causes and pathogenesis of neurogenic arthropathy of the elbow are discussed, along with laboratory investigations and differential diagnosis. Arthropathy of lower limb joints and the spine will not be considered, but references cited in Box 79-1 are provided to direct the interested reader to other sources of information. A recent excellent review of the subject is available.56
PATHOGENESIS
Debate about the pathogenesis of neurotrophic arthritis began with Charcot’s paper in 1868,14 and today our understanding of the pathophysiology remains incomplete. Charcot, observing the posterior column demyelination of tabes dorsalis, suggested the loss of a trophic function protecting joints as a pathophysiologic mechanism. This view was soon challenged by those who believed that the disorder resulted from trauma. A major advance in our understanding followed the often-quoted work of Eloesser,26 who sectioned the posterior roots of the hindlimb of cats. Deafferentation combined with thermocautery of the joint cartilage led to the rapid development of neurogenic arthropathy, bone fractures, and dislocations, emphasizing the role of insensitivity and trauma. The results of chemical analysis and tests of bone strength and elasticity were normal–evidence against Charcot’s view that the joints and bones wasted from an abnormality of trophic nerves. The roles of insensitivity, activity, and trauma were further emphasized by Corbin and Hinsey,19 who found that cats with loss of sensation developed a Charcot joint only if they were allowed to roam freely in the cage, whereas those kept in a restricted space did not. These researchers were impressed by the importance of proprioceptive loss, which allowed an abnormal range of joint movement. It is also recognized that limbs rendered immobile by spasticity rarely develop an arthropathy. An important observation of Harris and Brand36 was that joint breakdown in the neuropathy of leprosy can be minimized or prevented by proper protection. Johnson37 emphasized the importance of unrecognized and untreated fractures, especially stress fractures, in the development of neurogenic arthropathy.
LOSS OF NOCICEPTION
The most widely held view on the pathogenesis of Charcot joints, then, involves a loss of protective joint nociception and position sense that subjects the joint to repeated trauma that is not recognized by the affected individual. The neurologically normal patient with an injured joint presents quickly with development of pain and loss of function, leading to immobilization and the search for appropriate medical treatment. In the neurologically impaired person, on the other hand, unrecognized injury is untreated and leads to a vicious circle of compounding injuries. This explanation is intuitively reasonable, particularly in the presence of severe superficial and deep loss of pain perception. It is more difficult to invoke when joint destruction evolves, but sensory loss is minimal or difficult to demonstrate. For example, the author has studied patients with subclinical inherited neuropathy with severe neurotrophic arthropathy of various joints and recurring fractures who had no sensory symptoms or findings on clinical examination.24 Even with sophisticated tests, including computer-assisted sensory examination, periosteal nociception, and morphometric and graded teased fiber evaluation of cutaneous nerves, only a mild neuropathic abnormality was found. Methods of measuring joint capsule and articular bone pain perception are not available; however, cortical bone is known to contain both unmyelinated and myelinated fibers, and presumably some of these subserve nociception.18 Joint capsules, particularly in their external layers, contain several types of formed corpuscles and naked nerve endings. Synovium has not been observed to contain nerve endings. Although pain sense is carried by both small myelinated and unmyelinated fibers, the relative importance of these two systems in normal protective joint sensation is unknown. In those puzzling patients in whom sensation appears to be normal, a new method of measuring joint pain perception or afferent nerve fiber activity is needed.
NEUROVASCULAR THEORY
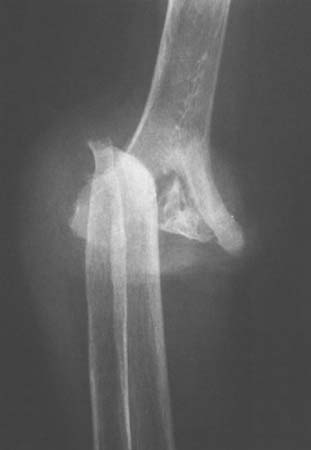
First, a proportion of patients with Charcot joints are said to have no neurologic disease when joint destruction develops, making it difficult to blame joint insensitivity. Second, there are instances in which neurotrophic joints develop in bedridden patients in whom weight bearing is impossible and repeated severe joint trauma unlikely. Third, a few patients suffer joint destruction and resorption of articular structures in a matter of weeks to a few months, making pure mechanical destruction hard to accept as the sole mechanism.18 Fourth, some neurotrophic arthropathies are associated with long bone fractures that seemingly occur spontaneously or without unusual trauma. Although histologic examination is reported to show pathologically increased vascularity, it is also a fact that acutely damaged joints become hot and swollen from increased blood flow and exudation as part of the body’s normal repair process, making pathologic vascularity difficult to prove. Hindlimb sympathectomy might be expected to increase blood flow; however, in experimental animals, at least, it does not lead to joint damage or abnormalities in the chemical composition of bone.19 It is still possible, however, that local hyperemia is exaggerated in some patients, leading to rapid bony softening and resorption, and it is not possible to discard this hypothesis out of hand (Fig. 79-1).
INFECTION
Although infection may coexist but cannot be offered as a cause of this problem, tuberculosis of the elbow, on the other hand, can cause resorptive and destructive changes similar to those in a neurotrophic joint (Fig. 79-2).
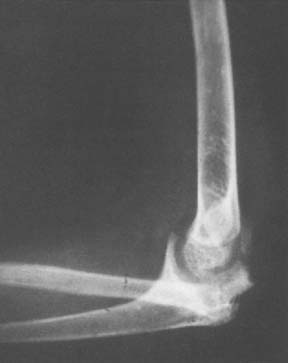
FIGURE 79-2 Gross destruction of an elbow joint involved with tuberculous arthritis. Note that both sides of the joint are involved. A distinction between this entity and a neurotrophic arthropathy in the early stages is complicated by the possibility that the two conditions coexist.47
(Courtesy of Dr. Richard Marks, Cape Town, South Africa.)
ENVIRONMENT
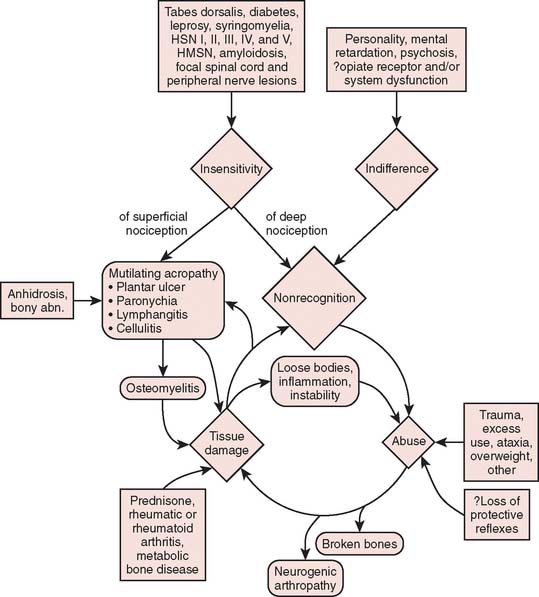
As is being more commonly recognized today, intra-articular administration of local anesthetic is associated with chondrocyte cell death.15,33 For some, it is occasionally followed by rapid joint disintegration. Patients reported with this complication have not had any neurologic disease, and therefore, the joint destruction cannot be called a neurotrophic arthropathy. The interplay of multiple factors in the genesis of bone and joint complications is emphasized in Figure 79-3.
CONDITIONS CAUSING NEUROTROPHIC ARTHROPATHY OF THE ELBOW
SYRINGOMYELIA
In contrast to tabes dorsalis, 80% of the joints involved in syringomyelia are in the upper extremities.30 It is estimated that approximately 25% of these affected joints will develop joint breakdown. Neuropathic disease involves the shoulder most commonly, followed by the elbow and wrist. Degenerative changes in the cervical spine are not uncommon. The process evolves gradually with paresthesias of the hands, followed by progressive weakness and wasting of the small hand muscles and then atrophy of arm and shoulder muscles. Loss of pain and temperature sense affects the arms and upperthoracic segments, often in a cape distribution. As the syrinx enlarges, long tract signs appear, and a Horner syndrome is noted. The Arnold-Chiari malformation is commonly associated and is related to the development of syringomyelia.
Post-traumatic syringohydromyelia presenting as a neuropathic arthropathy of the elbow and of the shoulder has been reported.51 Morrey has seen one additional instance of an acquired neurotrophic elbow joint after injury to the cervical spine (Fig. 79-4). The mechan-ism is also presumably that of a traumatic syringohydromyelia.
Electromyography and somatosensory evoked potentials can indicate the presence of a spinal cord lesion and the need for an imaging study.70 Radiographic examination of the cervical spine may show widening of the spinal canal, but the diagnostic procedure of choice is magnetic resonance imaging, which demonstrates both the syrinx and any associated malformation.41 Computed tomography with metrizamide contrast enhancement of the cerebrospinal fluid may also demonstrate spinal cord enlargement and cavitations in some instances.
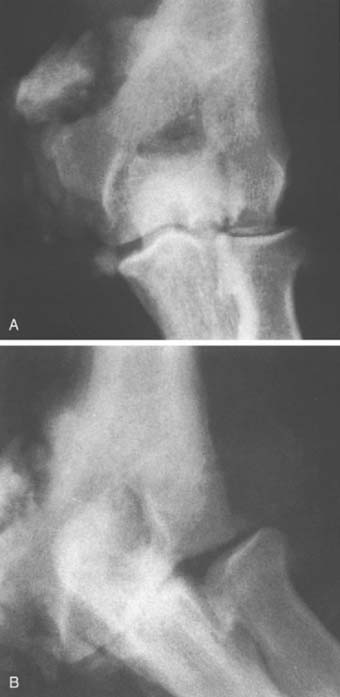
Elbow arthropathy may follow neurologic symptoms but is sometimes a presenting complaint.4 A history of trauma is usually lacking. Joint swelling due to effusion may be marked, and pain is experienced in some cases. Atrophic changes in the bone, particularly of the shoulder, are more common than in tabes dorsalis.67 Although extremely rapid destruction of the shoulder may occur with resorption of the humeral head, the elbow seems less likely to be affected in this fashion.51,68
The radiograph of the elbow shows resorption of bone ends and often of the entire joint (Fig. 79-5). Reparative callus is evident, along with gross deformity and instability. The youngest patient documented with elbow involvement was recently reported related to a myelomeningocele.73
DIABETES MELLITUS
Today the rate of diabetes mellitus as the leading cause of neurotrophic arthropathy in the lower extremity is well recognized. The arthropathy usually develops in diabetics who have had the disease for some time and who have suffered the additional complication of a symmetrical sensorimotor polyneuropathy. In diabetic “pseudotabes,” severe sensory and autonomic impairment leads to an ataxic gait, pupillary abnormalities, neurogenic bladder, and lightning pains reminiscent of tabes dorsalis. Diabetic arthropathy affects primarily the joints of the feet, with less frequent involvement of the ankles and knees.6,17,46,52,59 The distal predominance of the arthropathy is in keeping with the stocking pattern of sensory loss, which is maximal in the feet. Bony abnormalities in the upper extremities of diabetics are uncommon, but involvement of the shoulder, elbow, and wrist has been recorded.28 Campbell and Feldman11 presented a radiograph of the elbow in a 59-year-old patient that showed marked disorganization of the joint with destruction of the articular surfaces, numerous bone fragments, and periosteal new bone formation typical of a Charcot joint.
TABES DORSALIS
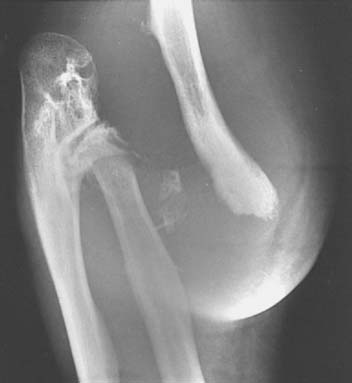
Although syphilis used to be responsible for up to 90% of neurotrophic joints, tabes dorsalis is now a rare disease. Approximately 10% of all tabetics are said to develop a Charcot joint, the majority occurring between the ages of 40 and 60 years, approximately 20 years or more after the primary infection. In approximately 78% of cases, the lower limbs are affected,30 with the knee most frequently involved. In the upper limbs, the shoulder, elbow, hands, and wrists are affected.3,20,61,66,69 Polyarticular involvement occurs in up to 40%. Clinical features include the presence of lightning pains, neurogenicbladder disturbances, optic neuritis, and visceral crises. Most patients have pupillary abnormalities, and loss of pupillary reflexes to light (Argyll Robertson pupils) occurs in up to 62%. Vibration and position senses are reduced, and deep pain sensation may be absent. Pain perception may be delayed. Although the Venereal Disease Research Laboratory (VDRL) test may be negative, rapid plasma reagin (RPR) and fluorescent treponemal antibody absorption (FTA-ABS) test results are usually positive. Elbow movement may be restricted, or the elbow may be subluxed and hypermobile with marked instability (Fig. 79-6). Spontaneous fractures can occur. Radiographic examination may show extensive destruction of the bone ends, disintegration of the joint, abundant periosteal callus formation, valgus deformity, and free bodies in the articulation.
CONGENITAL INDIFFERENCE AND INSENSITIVITY TO PAIN
The concept of indifference to pain implies that these patients perceive pain stimuli in a normal fashion but fail to react in the usual defensive manner, a kind of asymbolia for pain, whereas insensitivity denotes an inability to receive a painful stimulus because of a neurologic deficit. Most patients with the former disorder have not had detailed electrophysiologic, biomechanical, and pathologic examinations, and it is likely that previously reported patients have had a congenital sensory and autonomic neuropathy of type IV or V.23 Nevertheless, occasionally patients in this group may develop a neurotrophic arthropathy or fracture that may affect the elbow.29,44,58 Involvement of the elbow in Charcot-Marie-Tooth disease has also been recorded.8
HEREDITARY SENSORY AND AUTONOMIC NEUROPATHY TYPE II
Case Report
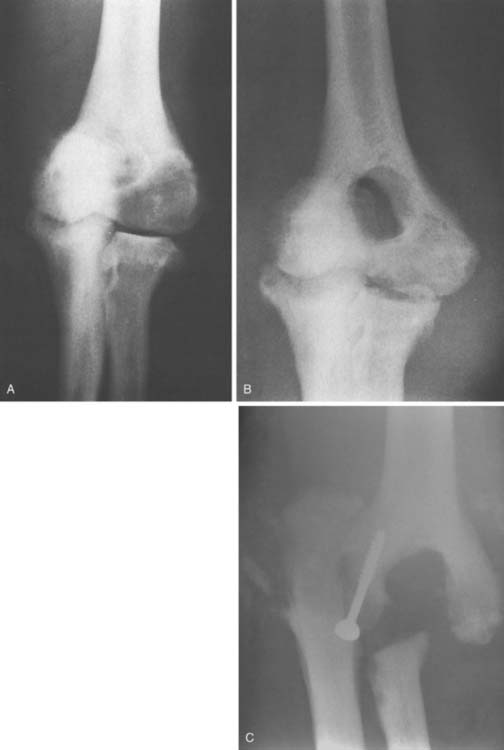
Radiographs showed medial subluxation of the left ulna and radius, with marked destructive joint changes and osseous debris in the periarticular soft tissues (Fig. 79-7). There was marked soft tissue swelling about the elbow, especially in the region of the olecranon bursa. The findings were consistent with a neuropathic joint. Efforts to stabilize the joint failed.
Commercial testing to define genetic abnormality is now available for many varieties of inherited neuropathy.40a
SURGICAL DENERVATION
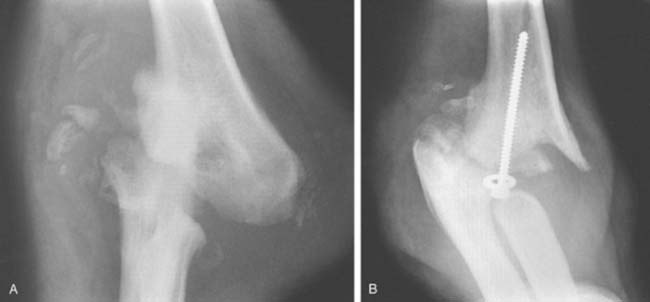
In recent years, there is evidence that aggressive surgical release of contracted elbow joints that causes marked stripping of the bone may cause severance of joint innervation. Two of Morrey’s patients have developed destructive resorption of the elbow after fascial arthroplasty following an aggressive surgical release of the soft tissue around the joint (Fig. 79-8).45 Several years later, one was diagnosed with congenital indifference to pain. The other appears to have occurred possibly from the aggressive soft tissue release.
MISCELLANEOUS
A case of idiopathic arthropathy of the elbow was reported by Meyn and Yablon43 in 1973; however, syringomyelia was not excluded because the patient refused myelography. A second example of “idiopathic” arthropathy of the elbow may also have been syringomyelia.5 In a poorly studied patient reported by Karten38 with the CRST* variant of systemic sclerosis and multiple neuropathic joints including the elbows, hysterical indifference to pain was suggested to be a major factor. This seems an unlikely explanation, however, because muscle weakness and atrophy and absence of deep pain were present. Another unusual patient, described by Alajouanine and Boudin,1 had elbow arthropathy associated with distal muscular atrophy, subcutaneous and vascular calcification, and abnormal calcium and phosphorus metabolism. There were no sensory changes, and the cause of the arthropathy is obscure.
INVESTIGATION AND DIAGNOSIS
Laboratory investigations that my colleagues and I have found useful in the investigation of neurogenic arthropathy, in addition to “routine tests,” are listed in Box 79-2. We have found the computer-assisted sensory examination and electromyography and nerve conduction studies helpful in detecting disease in minimally affected patients and family members. In a few patients, nerve biopsy, including teased fiber preparations, quantitative morphometry, electron microscopy, histochemistry, analysis of myelin lipids, the in vitro nerve action potential, and studies of axonal transport may be needed for complete evaluation of the specimen. The differential diagnosis of neurogenic arthropathy is listed in Box 79-3.
TREATMENT
Two stages of the disease process should be identified for treatment. During the early stages of development, the neurotrophic joint should be protected with a long-arm cast or molded splint. The appropriate diagnosis is obviously important during this stage. Thus, some knowledge of the existence of this entity as well as the etiologic factors is important (see Box 79-1). As the active process resolves, the residual dysfunction should be assessed. Pain is rare. Hence, stability is the primary consideration in most clinical settings. This residual is usually treated with an orthoplast splint with hinges to retain function. Surgical fusion is extremely difficult and should be considered rarely only as a procedure of last resort.60
MAYO EXPERIENCE
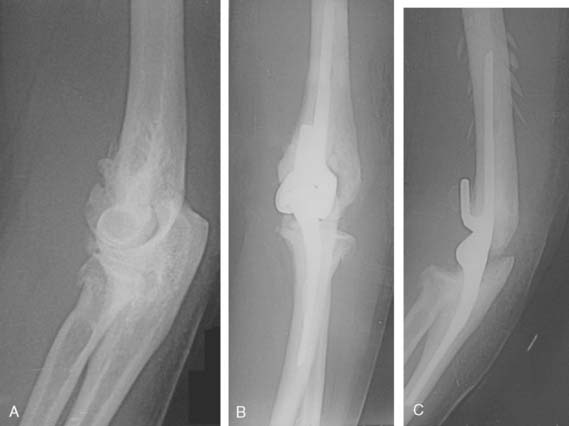
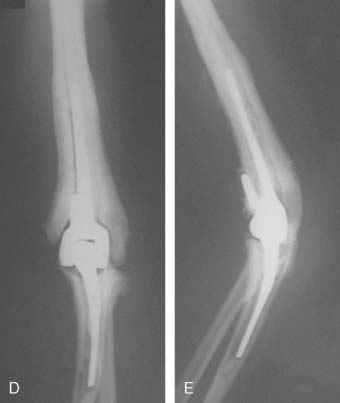
We have recently reviewed the management of six patients treated at Mayo and followed a mean of 51 months. All three treated by efforts to stabilize the joint failed, and the treatment failed miserably in two (see Fig. 79-8C). There is little experience with prosthetic replacement. We have concerns about this as a long-term option (Fig. 79-9). Today, our recommendation is to reaffirm the classic: protect and splint; do not operate.
1 Alajouanine T., Boudin G. Sur un complexus clinique caractérisé par une atrophie musculaire, myelopathique de type distal avec grosses déformations des pieds, arthropathies du caude et de la colonne vertébrale nodosités calcaires sous-cutanées et artérité calcaire avec perturbation du métabolisme phosphocalcique. Rev. Neurol. 1945;77:193.
2 Alarcon-Segovia D., Ward L.E. Charcot-like arthropathy in rheumatoid arthritis: Consequences of overuse of a joint repeatedly injected with hydrocortisone. J. A. M. A. 1965;193:1052.
3 Beetham W.P., Kaye R.L., Polley H.F. Charcot’s joints: A case of extensive polyarticular involvement, and discussion of certain clinical and pathologic features. Ann. Intern. Med. 1963;58:1002.
4 Bhaskaran R.K., Suresh K., Iyer G.V. Charcot’s elbow. J. Postgrad. Med. 1981;27:194.
5 Blanford A.T., Keane S.P., McCarty D.J., Albers J.W. Idiopathic Charcot joint of the elbow. Arthritis Rheum. 1978;21:923.
6 Boehm H.J. Diabetic Charcot joint: Report of a case and review of the literature. N. Engl. J. Med. 1962;267:185.
7 Bruckner F.E. Double Charcot’s disease. Br. Med. J. 1968;2:603.
8 Bruckner F.E., Howell A. Neuropathic joints. Semin. Arthritis Rheum. 1972;2:47.
9 Bruckner F.E., Kendall B.E. Neuroarthropathy in Charcot-Marie-Tooth disease. Ann. Rheum. 1969;28:577.
10 Brunt P.W. Unusual cause of Charcot joints in early adolescence (Riley-Day syndrome). Br. Med. J. 1967;4:277.
11 Campbell W.L., Feldman F. Bone and soft tissue abnormalities of the upper extremity in diabetes mellitus. A.J.R. 1975;124:7.
12 Carr T.L. The orthopaedic aspects of one hundred cases of spina bifida. Postgrad. Med. J. 1956;32:201.
13 Chandler G.N., Jones D.T., Wright V., Hartfall S.J. Charcot’s arthropathy following intra-articular hydrocortisone. Br. Med. J. 1959;1:952.
14 Charcot J.M. Sur quelques arthropathies qui paraissent dépendre d’une lesion cerveau ou de la moelle épinieère. Arch. Physiol. Normale Pathol. 1868;1:161.
15 Chu C.R., Izzo N.J., Papas N.E., Fu F.H. In vitro expsoure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693.
16 Cleveland M., Smith A. Fusion of the knee in cases of Charcot’s disease: Report of four cases. J. Bone Joint Surg. 1931;13:849.
17 Clouse M.E., Gramm H.F., Legg M., Floyd T. Diabetic osteoarthropathy: Clinical and roentgenographic observations in 90 cases. A.J.R. 1974;121:22.
18 Cooper R.R. Nerves in cortical bone. Science. 1968;160:327.
19 Corbin K.B., Hinsey J.C. Influence of the nervous system on bone and joints. Anat. Rec. 1939;75:307.
20 Das P.C., Banerji A., Roy A., Basu S. Neurogenic arthropathies (Charcot’s joints). J. Indian Med. Assoc. 1979;54:368.
21 Daughaday W.H. Extreme gigantism. N. Engl. J. Med. 1977;297:1267.
22 Dyck P.J. Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neurons. In: Dyck P.J., Thomas P.K., Griffin J.W., Low P.A., Poduslo J.F., editors. Peripheral Neuropathy. 3rd ed. Philadelphia: W. B. Saunders; 1993:1065.
23 Dyck P.J., Mellinger J.F., Reagan T.J., Horwitz S.J., McDonald J.W., Litch W.J., Daube J.R., Fealey R.D., Gio V.L., Kao P.C., Brimijoin W.S., Lambert E.H. Not “indifference to pain,” but varieties of hereditary sensory and autonomic neuropathy. Brain. 1983;106:373.
24 Dyck P.J., Stevens J.C., O’Brien P.C., Oviatt K.F., Lais A.C., Coventry M.B., Beabout J.W. Neurogenic arthropathy and recurring fractures with subclinical inherited neuropathy. Neurology. 1983;33:357.
25 Eibel P. Painless arthropathy complicated by massive hemorrhagic effusion. Clin. Orthop. 1968;60:149.
26 Eloesser L. On the nature of neuropathic affections of the joints. Ann. Surg. 1917;66:201.
27 Faget G.H., Mayoral A. Bone changes in leprosy: A clinical and roentgenologic study of 505 cases. Radiology. 1944;42:1.
28 Feldman M.J., Becker K.L., Reefe W.E., Longo A. Multiple neuropathic joints including the wrist, in a patient with diabetes mellitus. J. A. M. A. 1969;209:1690.
29 Fitzgerald J.A.W. Neuropathic arthropathy secondary to atypical congenital indifference to pain. Proc. R. Soc. Med. 1968;61:663.
30 Floyd W., Lowell W., King R.E. The neuropathic joint. South. Med. J. 1959;52:563.
31 Foster J.B., Hudgson P. Syringomyelia. In: Barnett H.J.M., Foster J.B., Hudgson P., editors. Traditional Concepts of Syringomyelia. Philadelphia: W. B. Saunders, 1973.
32 Goldberg M.F., Payne J.W., Brunt P.W. Ophthalmologic studies of familial dysautonomia: The Riley-Day syndrome. Arch. Ophthalmol. 1968;80:732.
33 Gomoll A.H., Kang R.W., Williams J.M., Bach B.R., Cole B.J. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813.
34 Greitemann B., Schuling S., Baumgartner R. Charcot-Ellenbogengelenk bei syringomyelie. Seitschrift Fur Orthopadie Und Ihre Grenzgebiete. 1992;130:5515.
35 Halonen P.I., Jarvinen A.J. On the occurrence of neuropathic arthropathies in pernicious anemia. Ann. Rheum. Dis. 1948;7:152.
36 Harris J.R., Brand P.W. Patterns of disintegration of the tarsus in the anaesthetic foot. J. Bone Joint Surg. 1966;48B:4.
37 Johnson J.T.H. Neuropathic fractures and joint injuries. J. Bone Joint Surg. 1967;49A:1.
38 Karten I. CRST syndrome and “neuropathic” arthropathy. Arthritis Rheum. 1969;12:636.
39 Kernwein G., Lyon W.F. Neuropathic arthropathy of the ankle joint resulting from complete severance of the sciatic nerve. Ann. Surg. 1942;115:261.
40 Key J.A. The treatment of tabetic arthropathies. Urol. Cutan. Rev. 1945;49:161.
40a Klein C.J. The inherited neuropathies. Neurol. Clin. 2007;25:173.
41 Kokmen E., Marsh W.R., Baker H.L.Jr. Magnetic resonance imaging in syringomyelia. Neurosurgery. 1985;17:267.
42 Kwon Y.W., Morrey B.F. Neuropathic elbow arthropathy: A review of six cases. J. Shoulder Elbow Surg. 2006;15:378.
43 Meyn M., Yablon I.G. Idiopathic arthropathy of the elbow. Clin. Orthop. 1973;97:90.
44 Mooney V.M.H.J. A case of congenital insensitivity to pain with neuropathic arthropathy. Arthritis Rheum. 1966;9:820.
45 Morrey B.F. Post-traumatic contracture of the elbow: Operative treatment including distraction arthroplasty. J. Bone Joint Surg. 1990;72A:601.
46 Muggia F.M. Neuropathic fracture: Unusual complication in a patient with advanced diabetic neuropathy. J. A. M. A. 1965;191:336.
47 Nissenbaum M. Neurotrophic arthropathy of the shoulder secondary to tuberculous arachnoiditis. Clin. Orthop. Relat. Res. 1976;118:169.
48 Nozawa S., Miyamoto K., Nishimoto H., Sakaguchi Y., Hosoe H., Shimizu K. Charcot joint in the elbow associated with syringomyelia. Orthopedics. 2003;26:731.
49 Peitzman S.J., Miller J.L., Ortega L., Schumacher H.R., Fernandez P.C. Charcot arthropathy secondary to amyloid neuropathy. J. A. M. A. 1976;235:1345.
50 Petrie J.G. A case of progressive joint disorders caused by insensitivity to pain. J. Bone Joint Surg. 1953;35B:399.
51 Rhoades C.E., Neff J.R., Rengachary S.S., Batnitsky S., Ketcherside J., Price H.I., Jacobs R.R. Diagnosis of post-traumatic syringohydromyelia presenting as neuropathic joints: Report of two cases and review of the literature. Clin. Orthop. Relat. Res. 1983;180:182.
52 Robillard R., Gagnon P.A. Diabetic neuroarthropathy: Report of four cases. Can. Med. Assoc. J. 1964;91:795.
53 Russell W.R., Garland H.G. Progressive hypertrophic polyneuritis with case reports. Brain. 1930;53:376.
54 Samilson R.L., Sankaran B., Bersani F.A., Smith A. Orthopedic management of neuropathic joints. Arch. Surg. 1959;78:115.
55 Scott R.B., Elmore S., Brackett N.C., Harris W.O., Still W.J.S. Neuropathic joint disease (Charcot joints) in Waldenström’s macroglobulinemia with amyloidosis. Am. J. Med. 1973;54:535.
56 Sequeira W. The neuropathic joint. Clin. Exp. Rheumatol. 1994;12:325.
57 Shands A.R. Neuropathies of the bones and joints: Report of a case of an arthropathy of the ankle due to a peripheral nerve lesion. Arch. Surg. 1930;20:615.
58 Silverman F.N., Gilden J.J. Congenital insensitivity to pain: A neurologic syndrome with bizarre skeletal lesions. Radiology. 1959;72:176.
59 Sinha S., Munichoodappa C.S., Kozak G.P. Neuro-arthopathy (Charcot joints) in diabetes mellitus. Medicine. 1972;51:191.
60 Smith F.M. Surgery of the Elbow. Philadelphia: W. B. Saunders, 1972.
61 Soto-Hall R., Haldeman K.O. The diagnosis of neuropathic joint disease (Charcot joint). J. A. M. A. 1940;114:2076.
62 Sprenger H.R., Foley C.J. Hip replacement in a Charcot joint. Clin. Orthop. Relat. Res. 1982;82:191.
63 Steinberg C., Duthie R.B., Piva A.E. Charcot-like arthropathy following intra-articular hydrocortisone. J. A. M. A. 1962;181:851.
64 Steindler A. The tabetic arthropathies. J. A. M. A. 1931;29:250.
65 Stepanek V., Stepanek P. Changes in the bones and joints of paraplegics. Radiol. Clin. North Am. 1960;29:28.
66 Storey G. Charcot joints. Br. J. Vener. Dis. 1964;40:109.
67 Storey G. Charcot joints. Ann. Rheum. Phys. Med. 1970;10:312.
68 Storey G.A., Stein J., Poppel M.H. Rapid osseous changes in syringomyelia. Radiology. 1957;69:415.
69 Unnanuntana A., Saranatra W. Neuropathic arthropathy of the elbow. A report of two cases. J. Med. Assoc. Thailand. 2006;89:533.
70 Veilleux M., Stevens J.C. Syringomyelia: Electrophysiologic aspects. Muscle Nerve. 1987;10:449.
71 Wirth C.R., Jacobs R.L., Rolander S.D. Neuropathic spinal arthropathy: A review of the Charcot spine. Spine. 1980;5:558.
72 Wolfgang G.L. Neurotrophic arthropathy of the shoulder: A complication of progressive adhesive arachnoiditis. Clin. Orthop. Relat. Res. 1972;87:217.
73 Zimmerman A., Law C., Blount J., Gilbert S. Neuropathic arthropathy of the elbow in a pediatric patient with myelomeningocele. Dev. Neurorehabil. 2007;10:261.