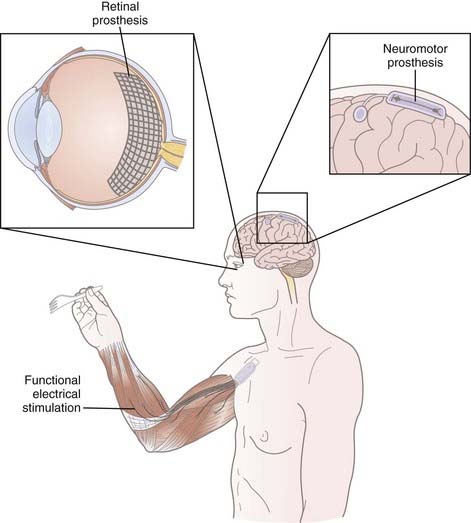
CHAPTER 94 Neuroprosthetics
Neural Interface Systems
The notion of establishing a connection between brain and machine for therapeutic benefit is not entirely new. By the early 1950s, stimulating and recording electrodes were placed intracranially in human cortex in the hope of alleviating psychosis.1 As technology advanced, it adapted to the growing understanding of neurological disorders. Recent multidisciplinary research has pursued the development of a fully integrated NIS to restore function and markedly improve quality of life. An NIS is an innovative means of coupling physical devices capable of interacting with the central nervous system (CNS) with devices that interact with sensory stimuli or motor prostheses. As a result, NISs have enormous potential to benefit the substantial patient population suffering from neurological disorders that leave the central structures intact, such as motor neuron disease, spinal cord injury, stroke, and most causes of congenital and acquired deafness and blindness. In fact, some neurotechnologies have already experienced clinical success. Cochlear implants have become a routine therapeutic measure for restoring audition to the hearing impaired, and deep brain stimulation (DBS) is increasingly being used as a treatment option for symptoms of Parkinson’s disease, dystonia, and even depression.2–5 These stimulating technologies are examples of devices generally included in the growing field of neuromodulation.
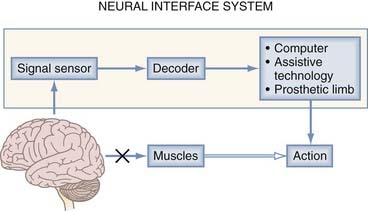
An NIS can may communicate information into or out of the nervous system. A neuromodulatory sensory prosthesis, or an input NIS, is a read-in system in which information is transduced from a physical device, such as a microphone or camera, to neural tissue. In contrast, an output NIS is designed to read out neural signals from the brain to provide movement signals or a readout of brain function. DBS is not included here because it is thoroughly covered in other chapters and is a different type of NIS (not a true sensory NIS). The standard components of both input and output NISs include a signal sensor to record neural or environmental signals (e.g., spikes, sound, or light), a signal-processing algorithm to decode information from the CNS or encode information from the environment, and a device (such as a stimulating electrode or assistive technology) to transform output signals into action (Fig. 94-1). Thus, one can think of these systems in the context of two broader categories: those that interpret signals and those that create them. An output NIS provides a new route for disconnected cortical motor signals that are unable to reach muscles so that computers, prosthetic limbs, and even the user’s own muscles can be controlled with functional electrical stimulation (FES). For an input NIS, such as cochlear implants and visual prostheses, information from the surrounding environment is encoded into patterned electrical stimulation of intact neural tissue.
Neuromotor Prostheses
Several hundred thousand people currently suffer from paralyzing disorders in which the brain areas responsible for volitional movement are preserved but the brain is disconnected from output pathways or the muscles necessary to enact movement.6 Paralysis of this sort can result from various sources at different levels of the motor system. Upper level motor areas are frequently preserved in cases of trauma, stroke, and neurodegenerative diseases of the descending motor tracts and spinal cord.7 Central motor control areas are also preserved when the absence of functional muscles results in immobilization, such as with amputation or muscular dystrophy. An NIS can replace or restore these lost motor functions by extracting movement-related neural signals from the cerebrum, decoding them, and using the information to accurately control an output device, such as a prosthetic limb.
Useful Neural Signals
To ensure precise control of NISs, movement-related neural signals must be information rich, have a high information transfer rate, be reliable, and be safely accessible by modern recording devices. One natural source for acquiring movement information is the frontal lobe motor areas where these commands are generated. The primary motor cortex (M1), which is located roughly along the posterior half of the precentral gyrus, and the premotor cortex and supplemental motor area, which extend anteriorly from M1, give rise to the neural signals responsible for planning, initiating, and generating volitional movement.8–10 Neuromotor prosthesis research has largely concentrated on restoring function to the upper extremities, where the somatotopic relationships for arm and hand motion have been particularly well defined by myriad neurophysiologic methods.11 There remains some disagreement in the field on which signal types originating from these regions are best for controlling neuromotor prosthetics, and this is an area of active exploration in basic research in nonhuman primates.
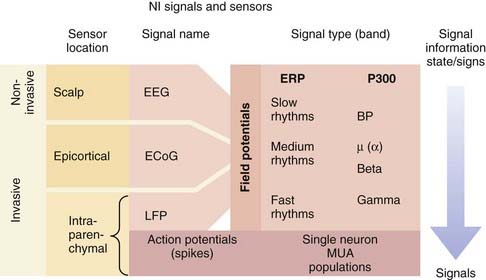
There are essentially two types of electrical signals in the brain: action potentials (spikes) and field potentials (FPs) (Fig. 94-2). Both categories, detected by different sensor technologies, are currently used as control signals for neuroprosthetic devices. NISs that use FPs are attractive because FPs can be acquired noninvasively with scalp electrodes whereas systems interpreting spiking activity require electrodes implanted in the brain parenchyma. As a result of the inherently invasive nature of decoding spike information, NISs using this approach have only recently been used in humans. Some NIS researchers are also developing systems that record FPs with epidural or subdural electrodes to obtain more reliable signals.
The action potential is the fundamental information-carrying unit of the nervous system.12 NISs can obtain rich movement-related information from the spike rate–based code of individual neurons. The basis for accurate control in NISs is that the firing patterns of neurons in the motor cortex reflect the direction of intended reach.13,14 In addition, firing activity in cortical motor neurons has been shown to reflect hand position, velocity, forces, joint angles, and target goals.15 Solely by recording and decoding these signals, the path of hand motion can be determined with decoding software and represented by an on-screen cursor or a prosthetic limb. Numerous experiments in nonhuman primates first illustrated that subjects could accurately modulate M1 neural output to drive computer cursor motion in two- and three-dimensional visuomotor tasks.16,17 These experiments served as the basis for trials in human subjects. The first implanted electrode for the purpose of driving an NIS consisted of two wires in a conical glass electrode.18 Even with this rudimentary implant, the patient was able to control a binary switch with the NIS output. In 2004, a team of Brown University researchers launched the BrainGate pilot trial, which demonstrated the feasibility of using an NIS in tetraplegic human participants. Important for the prospects of NIS development, this group found that directional tuning of neurons in M1 is present years after a patient has become paralyzed from spinal cord injury or brainstem stroke.19,20 Control of these signals was reportedly natural (cursor movement corresponding to intended hand or arm movement), required only a short period of training, and provided users with several degrees of freedom, including discrete functions similar to clicking a mouse button.21,22
The other type of neural electrical signal capable of controlling an NIS is FPs, many of which can be recorded noninvasively. FPs are much more complex signals than action potentials in that they represent the summed transmembrane currents across groups of neurons with different properties. Although this signal arises from a wide distribution of neurons, FPs have long been understood to carry significant information in their oscillations. Although spikes represent a discrete, binary neural signal, FPs carry both a neural activity–related signal and a brain state signal, which is dependent on higher cognitive functioning, alertness, and sleep stage.23 FPs can be classified according to their two characteristic signal band types: event-related potentials and rhythmic signals (see Fig. 94-2).20 Event-related potentials are the sweeping potential changes across a large population of neurons that are evoked by external events or volitionally. The P300 wave, which entails a signature voltage deflection approximately 300 msec after the stimulus event, is a prominent signal used for NISs. This signal is regarded as a sign of higher cognitive processing because it represents the rapid integration of sensory stimuli within the cortex. Rhythmic FPs can be further subdivided into fast, medium, and slow frequencies, all of which can serve as control signals for NISs. Humans can use these rhythms to control NISs by learning how to modulate the frequency or amplitude of these potentials. In the first successful human electroencephalography (EEG)-based NIS, participants modulated slow rhythms (less than 1 Hz), such as the Bereitschaftspotential, to achieve continuous, two-dimensional control.24,25 Despite this success, interest is shifting toward faster FP rhythms because they contain more information and are more easily modulated by the user. Some experiments have begun to evaluate medium rhythms, including alpha (8 to 12 Hz) and beta (12 and 20 Hz or greater) frequency bands. A more natural NIS control signal is generated because midrange rhythm amplitudes can be changed with the imagination of movements.25 FPs with the highest frequencies, fast beta rhythms and gamma rhythms (30 to 100 Hz), appear to contain distinct, specific information. For example, the beta rhythms in M1 in paralyzed humans and nondisabled monkeys have been found to indicate the transition from movement planning to execution.19,26 Gamma oscillations may provide additional dimensions to an FP NIS control signal inasmuch as they have been correlated with spiking activity in the parietal and visual regions of the cortex.27 Local FPs represent the highest frequency FPs and are thought to contain information about populations of spiking neurons.
Signal Sensors and Their Limitations
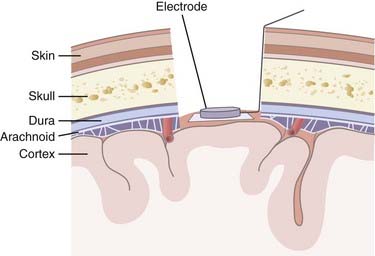
A range of sensors are available for capturing neuronal activity to use as an NIS control signal; each has certain advantages and limitations (Fig. 94-3). Noninvasive strategies use scalp electrodes and thereby avoid the risks associated with surgery. These external sensors record an EEG signal consisting of lower frequency oscillations. Although this EEG setup is relatively simple, the signals are not only attenuated by the skull and scalp but are also limited in their bandwidth. Higher frequency FPs and neural spiking activity are not accessible, which limits the NIS control signal. In contrast, by invasively recording epidurally or subdurally, an electrocorticography (ECoG) signal can be acquired, which has higher fidelity than EEG signals and is less prone to artifact. There is the potential for using ECoG in conjunction with subdural grid mapping in pre-resection epilepsy cases, where signals can be used from multiple electrodes. ECoG for NIS applications will require an invasive procedure, but it is hoped that the associated risks can be decreased as techniques improve and devices shrink. No ECoG-based NIS has yet been tested in humans with paralysis to determine whether ECoG signals from a limited area of cortex can serve as a reliable control signal for neural prosthesis applications.
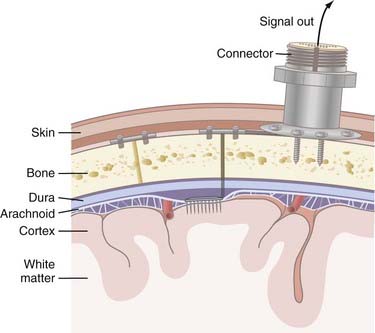
Invasive strategies are currently the only way to record spiking activity from individual neurons. However, one of the concerns with penetrating electrodes is the neuroimmune response elicited. Encapsulation is a common feature of the response as glia react to the foreign electrode. The degree of encapsulation is highly variable and may depend on the state of the patient’s immune system, the fabrication techniques of the penetrating electrodes, the insertion technique, and the materials with which the electrodes are constructed. Recording and stimulation with these electrodes are not necessarily precluded by gliosis; in fact, some electrode designs take advantage of the response from local cell populations. These electrodes are coated with neurotrophic factors that promote migration of neurites toward the electrode and thereby produce a longer-lasting and tighter coupling between stimulation and neuronal response.18 Another drawback of invasive recording techniques is the requirement for a percutaneous connection to external components (Fig. 94-4), which increases the risk for infection. Although biocompatibility is a major concern, preliminary studies in humans have shown that implanted electrodes are in fact safe and can provide signals for many years.21
Decoding Movement Signals
Many algorithms have been proposed for decoding spike-based data. Most firing rate–based decoding methods rely on data from a large population of neurons, or “units.” Population-based decoding more closely replicates the way we believe the nervous system naturally encodes movement information. Furthermore, ensemble-based decoding is preferred because it provides a more stable output signal; the population code is less susceptible to noise and extreme deviations in activity.28 Decoding then works by analyzing the specific multiunit pattern of activity across the neuronal population and comparing it with a known reference pattern distribution, which is usually generated at the beginning of a recording session. When the decoding algorithm is being built, the user is told to imagine moving a hand in a given manner, and the software learns to correlate that pattern of neural activity with the given motion. Decoding algorithms can use a linear filter or a recursive, bayesian inference decoding algorithm. In a linear filter system, individual units are assigned different weights, which correlate spiking with the direction and velocity of movement. In bayesian inference filters, such as the Kalman filter, the decoder is continuously updating by recursively incorporating position, velocity, and acceleration information about trained units to predict and control the movement kinematics of the output signal. In either case, the neural firing data on which the algorithm is trained must resemble the neural activity during user control tasks for stable neural cursor control to occur.
In an EEG-based NIS, decoding of FP signals relies on the user’s ability to learn to modulate slow cortical potentials or to selectively control a particular frequency band of the EEG at a particular electrode location (or locations).29 Decoding algorithms can be simple (linear) or complex (neural networks), but in either case a feature such as amplitude must be extracted from the signal synchronicity and correlated with a particular output action or direction.30 Other devices exploit the involuntary evoked potentials that appear in the presence of external stimuli (i.e., the P300). This state signal is extremely useful and can be decoded as a click signal.
Output Devices
Once a reliable movement output signal has been established, a wide range of prosthetic effector devices can be controlled. The goal of neuromotor prosthesis development is to create a system that restores, assists, or replaces the capabilities of natural movement to individuals disabled by a variety of nervous system disorders. Because the output signal from NISs is very versatile, it is possible that these implants could supply control signals for devices that provide unnatural abilities, such as control of remote robots.31
Assistive Technologies
Computer Cursor
Continuous point-and-click control of a computer cursor is possible in tetraplegic individuals by using intracortical NISs, and it offers nearly infinite possibilities for improving patient independence and quality of life. In the BrainGate NIS trial, multiunit spiking activity in M1 was modulated by the participants to control a cursor for opening e-mail, browsing the Internet, operating a television, using a drawing program, and playing video games.19 Importantly, this control was natural and required no learning. The participants simply imagined moving their arm or hand in the desired direction and the cursor moved with remarkable accuracy. Recently, one participant studied over the course of three sessions successfully reached and clicked 96% to 100% of specified targets.22 Some noninvasive methods have also been devised for point-and-click cursor control, but control is less intuitive (using different EEG signals) and reliable than with an intracortical NIS in humans. Furthermore, control of closed-loop spike-based NISs (i.e., visual feedback of cursor movement) can more easily be stabilized because the user can more intuitively modulate the intended movements to obtain accurate cursor control. As signal decoding improves, a tetraplegic patient will one day be able to operate all the computer software available to able-bodied individuals solely by using a neural control signal.
Robots
Robotic technology offers a way for NISs to replace lost motor function. The multidimensional data decoded from the intended movement of a paralyzed or missing limb (position, velocity, acceleration) is instead relayed to a prosthetic limb. Although the technology is still several years away from both performing naturally and being comfortably attachable to the human body, neural control of robotic devices has nevertheless been impressive. In the BrainGate pilot trial, a participant manipulated a robotic arm to retrieve a piece of candy and drop it in the hands of a technician.19 More recently, in a related experiment using a spike-based NIS, a nonparalyzed monkey used neural signals to feed itself with the control of a robot arm.32 In addition to the replacement of upper limb movement, replacement of locomotion is being actively pursued with the direct neural control of powered robotic wheelchairs. Using the same components of the neural output signal, the direction and velocity of particular intended movements are mapped to the chair. Advances in both these technologies will ensure that both upper limb and locomotive function can be surrogated with robotic devices in the future.
The Future: Restoring Movement with Functional Electrical Stimulation
Although advances in assistive and robotic devices will improve neural control of surrogate movement technologies, FES is intended to actually restore natural movement in paralyzed individuals by directly reconnecting brain to muscle. FES systems work by appropriately stimulating sets of motor nerves as they enter muscles, thereby causing muscle contraction and the resulting movement of the paralyzed limb. Currently, upper arm and upper leg FES systems are being tested in humans with spinal cord injuries.33 Specific patterns of muscle stimulation have restored basic movements to these patients. At present, FES is triggered in the muscles of study participants by either a mechanical switch or an automated signal. Recently, another study has shown that paralyzed individuals can use an EEG control signal to initiate FES to open and close the hand.34 Although generating this EEG control signal was considered tasking and represented only a triggering mechanism, this study nevertheless marked an important transition toward natural, thought-controlled generation of movements. Even though there were many problems with the EEG control signal, a multidimensional signal taking into account limb position, velocity, and acceleration could precisely guide limb movement. The next step toward achieving natural, user-guided control of their own limbs is to continue classifying complex FES patterns for the types of movements that they elicit and to correlate this with spiking activity in the motor cortex. With increased NIS and FES collaborative research, it is possible that paralysis will one day be reversed by a complex physical nervous system that reproduces movement with the fidelity of an intact biologic one.
Auditory Neuroprosthetics
The success of the implant depends on higher order signal pattern detection in the CNS. The elements of the linguistic pattern detection system are not innately organized. Like so many of the higher cognitive processes that the brain develops, the ability to comprehend spoken language relies on a stimulus-dependent critical period. When a cochlear implant is used to establish these central auditory pathways, the critical period begins shortly after birth and remains maximally plastic until 3.5 years of age.35 If the auditory cortex lacks input from the auditory thalamus and brainstem during this critical period, the processing capacity of the auditory cortex may be co-opted by other sensory modalities. If the brainstem and cortical structures are unable to properly analyze the salient features of the stimulus and cognitively assemble them into phonemes, words, and sentences, the neuroprosthesis cannot enable speech perception. For this reason, implantation candidates are most often adults who were deafened postlingually or prelingual children who are still within the critical period for establishing central auditory pattern detection.
Auditory Signal and Signal Processing
Stimulation of the Auditory Nerve
The first cochlear implant, the House/3M implant, consisted of a single electrode or channel. Sound was detected by a microphone, amplified, and processed through a 370- to 2700-Hz bandpass filter. The processed signal was used to modulate a 16-kHz carrier signal. The modulated signal was amplified again and sent to the implanted electrode through an external induction coil, which allowed transdermal transmission of the signal to an implanted coil. From the internal coil the signal was sent to the electrode, which sat inside the scala tympani. Because the stimulation provided such an impoverished representation of the auditory stimulus, implanted patients did not receive much benefit in context-free linguistic tests. When four patients were asked to identify a spoken consonant, the average correct score was 37%.36 Most patients were unable to identify monosyllabic words with the implants alone, and even exceptional users could not identify more than 5% of monosyllabic words. Later developments in processing strategies for single-electrode implants provided a more accurate representation of the temporal envelope of the auditory stimulus. Given this temporal cue about the nature of the stimulus, some of these patients were able to develop speech—one of the goals of the implant when used in prelingual children.
Further improvements in signal processing and stimulation led to the development of pulsed stimulation instead of continuous stimulation at the individual electrodes. As the number of channels on the implants has increased, the interelectrode distance has decreased: the Nucleus 24M implant has a center-to-center distance of 0.7 mm between adjacent electrodes. The CA approach requires that each of the channels be active simultaneously. With such little space between electrodes, the potential for distorting interaction among the channels is quite high. Channel interactions can adversely affect speech comprehension.37,38 The continuous interleaved sampling (CIS) approach sought to reduce this spatiotemporal interaction by activating each channel sequentially. The amplitude of each pulse is proportional to the amplitude of the output of each of the contiguous filters, as used in the CA approach. As expected, the CIS approach provided superior speech recognition.37
Both the CA and CIS approaches depended on representing a large swath of the frequency spectrum through a relatively small number of electrodes. Although these strategies capture the elements of the stimulus necessary for speech recognition, they also process a significant amount of information that may not be salient. The next development in speech processing used feature extraction to predict and analyze the formants in the stimulus. With the f0/f1/f2 approach, the fundamental, first, and second formants were estimated from the output of a number of bandpass filters. By spatially segregating where f1 and f2 were delivered, users were able to correctly identify 63% of words presented without syntactic or visual context.39 When more high-frequency information was included, consonant detection was significantly improved.40 As speech-processing strategies have become more refined, they have continued to increase the number of vowels, consonants, and words that users can correctly identify. Users of a single-channel implant would have been unable to use the telephone, but cochlear implant users today do so regularly. One of the largest challenges remaining in speech processing is the development of a strategy that works as well in natural, noisy conditions as it does in the controlled environment of the laboratory.
Future of Auditory Neuroprosthetics
There are a number of active research areas in the field of auditory neuroprosthetics. In addition to the work being done to improve speech-processing algorithms, a number of teams are engineering better implants. One of the major concerns with cochlear implants is the difficulty of inserting the electrode without damaging the spiral ligament or the basilar membrane. The original multichannel electrodes occupied an antimodiolar position after insertion because the arrays were pushed to the outside of the scala tympani during insertion. These electrodes may require a significant amount of potentially damaging force to be guided through the cochleostomy and into the apical cochlea.41 Newer implants are designed for perimodiolar positioning, which is accomplished by using a prestressed electrode array that naturally curls on itself. Perimodiolar placement allows greater proximity to the neurons of the spiral ganglion and potentially causes less damage to cochlear tissues during insertion. By closing the distance between electrode and neuron, perimodiolar implants may improve speech comprehension and decrease the amount of current needed to elicit a percept in the patient, thus reducing power consumption. Others are investigating the potential benefits of binaural implantation with the goal of increasing speech perception and restoring some degree of sound localization.
Auditory Brainstem Implants
An auditory brainstem implant (ABI) was developed to restore hearing to those who are not candidates for cochlear implantation, namely, those who lack a functioning auditory nerve, usually because of neurofibromatosis type 2 (NF2). The ABI implant consists of an array of button electrodes in contact with the brainstem cochlear nucleus, placed by direct exposure of the brainstem. ABI results have not matched those of more distal systems, however. Unlike the organ of Corti, the cochlear nucleus contains many cell types arranged in multiple dimensions of tonotopy. Activation of a single ABI electrode may excite a number of neurons, each of which may normally respond to a markedly different feature of the auditory stimulus and may therefore activate multiple pitches. Another ABI was developed with penetrating electrodes (PABI). By penetrating into the brainstem parenchyma, the electrodes may be better aligned to the tonotopic axes, but in NF2 patients, both PABI and ABI users were unable to perceive speech. These discouraging results may be the result of the patients’ underlying condition, however, because one report demonstrated that some non-NF2 ABI users were able to comprehend speech at the same level as cochlear implant users.42 One concern is that gross distortions in the brainstem from NF2 may make it difficult to place these arrays in ways that optimize activation of the target structures.
Visual Prostheses
Although visual implants have not yet achieved the same degree of success or popularity as auditory prostheses, significant progress has been made toward the development of a functional replacement for normal vision. These devices seek to return some degree of vision to patients with profound visual loss. As with cochlear implants, a variety of different visual deficits may benefit from a neural prosthesis, such as any pathology that spares an element of the retinofugal pathway, including macular degeneration and retinitis pigmentosa. Like the auditory system, the visual system organizes itself after birth during a critical period. Therefore, adult patients who have never had vision will not be suitable candidates for visual prostheses; however, once the sensory cortex has been wired correctly during postnatal development, a patient will retain the ability to perceive artificial visual percepts, called phosphenes, that are generated by electrical stimulation.43 The use of visual neuroprosthetics in presighted children has not been examined, so it is unclear whether implants can drive the organization of the visual cortex during the critical period.
An ideal visual implant would provide the patient with a sense of contrast, object orientation, object motion, and distances between objects, which can be described by acuity.44 Given these distinct cues, a patient could learn to navigate, recognize faces, and read.
Visual Signal Encoding
The first elements in many visual prostheses will be the camera and the signal processor. Advances in miniaturization and wireless communication have enabled researchers to develop eyeglasses and wearable image processors that contain all the necessary elements for encoding an electrical signal.45 Because the number of electrodes in an implant is many orders of magnitude smaller than the number of neurons in the primary visual cortex, image-processing strategies must encode the visual world as efficiently as possible.
Electrical stimulation of the retina, optic nerve, visual thalamus, and primary visual cortex will produce phosphenes.46 Thus, any neural structure normally involved in visual processing is a potential site for neuroprosthetic implantation, provided that the neurons there are excitable and maintain normal synaptic connections with higher visual cell populations. Each structure has unique anatomic, neurophysiologic, and cognitive features that demand close consideration as these devices are developed.
There are many advantages to implanting the device on the retina. Because the retina is easily accessed surgically, the implantation surgery for epiretinal prosthetics could ostensibly be developed as an outpatient procedure. A 16-electrode epiretinal implant prototype was engineered and implanted in six patients. A camera mounted in a pair of glasses captures images. The video is sent to a portable processor that translates the frames to electrical stimulation at each electrode. The 4 × 4 electrode grid is tacked to the retinal surface, where the leads make close contact with the retinal ganglion cells. In patients with retinitis pigmentosa, these ganglion cells remain viable and excitable. Excitation of these afferent fibers allows the transmission of information through the intact signal-processing and pattern detection elements of the lateral geniculate nucleus (LGN) and cortex. This device has been demonstrated to provide users with visual acuity that is proportional to the spacing between electrodes.47 Some patients who use this device are able to locate and count objects in a laboratory setting with 100% accuracy.48
Another strategy for retinal implants involves microphotodiode arrays.49 These diodes, which can be manufactured in dense arrays, produce an electrical potential when light is incident. Because this array is implanted into the space between the neural retina and the retinal pigment epithelium, it can easily stimulate bipolar, horizontal, and amacrine cells. These cell types normally make synaptic connections with each other and with the ganglion cells that constitute the output from the retina. By stimulating the cells that feed into the retinal ganglion cells, this approach takes advantage of another existing layer of innate processing in the nervous system. One advantage of this approach is that it provides the user with what is potentially the most natural interaction with the prosthetic and the visual world. Unlike images captured by a camera and sent to implanted electrodes after processing, the microphotodiode array simply replaces the damaged photoreceptors in the system. With a camera-based system, the image on the retina would not move as the eyes move in their orbits; users have to point their head toward the object that they wish to visually examine. With the microphotodiode approach, the image moves across the retina as it would in a normal eye. All systems that use software-based image processing and electrode stimulation will require significant learning on behalf of the patient and considerable time spent tuning the individual electrode stimulation parameters in therapy.
One drawback of the retinal implant strategy is that the evenly spaced electrodes or diodes in an implant will disproportionately stimulate the nonfoveal retina. Ideally, the bulk of image processing and stimulation would occur for images intended for the fovea because that area enjoys a disproportionately large share of processing in the cortex. Instead, these retina-based devices stimulate the peripheral retina more than they do the fovea. This problem may resolve itself as electrode array fabrication techniques develop, but there are problems with densely packing electrodes as well. As stimulating electrodes are placed closer together in an array, they have less surface contact with the target tissue. Consequently, these electrodes require greater current charge density to produce the same neural activity in the target neurons. This increased charge density can cause damage to surrounding cells.50 One problem that all visual prostheses encounter is the highly varied nature of phosphenes. Stimulation through the different electrodes in a single epiretinal array may produce phosphenes of different color, shape, size, and brightness.51 Thus, electrodes will not act as pixels representing the visual world. There will not be a direct correspondence between an object in visual space and current through an electrode. To successfully encode a visual scene as electrode activation, the encoding software must be programmed to predict the manner in which the brain will interpret a given stimulation. This prediction can become quite complex because neighboring electrodes produce currents with nonlinear interactions. As electrodes become more densely packed on arrays, the effects of electrode interaction will only increase and further confound image processing. Epiretinal implants also suffer from a fiber-of-passage problem. Current through a single electrode situated on top of the inner retina will stimulate all the nearby ganglion axons, even if those cells have their input on the other side of the retina. This problem becomes more pronounced at areas around the optic disc, the point at which all the retinal ganglion axons collect before leaving the eye. The result is a nonuniform “smearing” of stimulation, with some electrodes producing small, reproducible phosphenes and others producing largely variable percepts.
The visual cortex is an attractive target for prosthetics because the cortical magnification of foveal representation means that more of the stimulating electrodes will correspond to foveal space. Early experiments demonstrated that a blind patient could accurately locate a light source when given a photocell whose output was sent to electrodes implanted in the primary visual cortex.52 A more advanced array of surface electrodes allowed patients to identify 6-inch letters at 5 feet.53
Conclusion
NISs are nearing a threshold beyond which the potential to replace lost nervous system function may become a reality for patients suffering from a range of sensory and motor deficits. In many ways, the biotechnologic advances of NISs are following a trajectory much like that of cardiac pacemakers. The first cardiac pacemakers began appearing in the 1950s as a means of resolving cardiac arrhythmias by pacing the contractions of the heart. These pacemaker systems, like many current NISs, initially required a sizable external cart of electronics with a bundle of wires running percutaneously to the heart. Within several decades, these bulky and unsophisticated devices were transformed into miniaturized, fully implantable electronic systems that are used routinely.54 Although not nearly as refined as cardiac pacemakers, NISs are quickly advancing from their preliminary stages. Cochlear implants represent the first neuroprosthetic device to make this leap and testify to the power of NISs to transform people’s lives. Even current visual and neuromotor prostheses, despite still being rudimentary, can dramatically improve people’s quality of life by reconnecting them to their environment. Neuroprosthetic devices are still years away from re-creating “natural” sensory and motor function (Fig. 94-5); however, revolutionary advances in neurotechnology and our understanding of the brain strongly indicate that this may be realized in the not too distant future.
Birbaumer N, Weber C, Neuper C, et al. Physiological regulation of thinking: brain-computer interface (BCI) research. Prog Brain Res. 2006;159:369.
Caspi A, Dorn JD, McClure KH, et al. Feasibility study of a retinal prosthesis: spatial vision with a 16-electrode implant. Arch Ophthalmol. 2009;127:398.
Colletti V, Carner M, Miorelli V, et al. Auditory brainstem implant (ABI): new frontiers in adults and children. Otolaryngol Head Neck Surg. 2005;133:126.
Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032.
Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416.
Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249.
Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164.
Kim SP, Simeral JD, Hochberg LR, et al. Multi-state decoding of point-and-click control signals from motor cortical activity in a human with tetraplegia. Proc. 2007 IEEE/EMBS Int’l Conference on Neural Engineering, Kohala Coast, Hawaii: 486, 2007.
Lauer RT, Peckham PH, Kilgore KL. EEG-based control of a hand grasp neuroprosthesis. Neuroreport. 1999;10:1767.
Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327.
Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci U S A. 2007;104:7670.
Schmidt EM, Bak MJ, Hambrecht FT, et al. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain. 1996;119:507.
Schwartz AB, Taylor DM, Tillery SI. Extraction algorithms for cortical control of arm prosthetics. Curr Opin Neurobiol. 2001;11:701.
Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586:1217.
Serruya MD, Hatsopoulos NG, Paninski L, et al. Instant neural control of a movement signal. Nature. 2002;416:141.
Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272.
Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829.
Throckmorton CS, Collins LM. The effect of channel interactions on speech recognition in cochlear implant subjects: predictions from an acoustic model. J Acoust Soc Am. 2002;112:285.
Wilson BS, Finley CC, Lawson DT, et al. Better speech recognition with cochlear implants. Nature. 1991;352:236.
Yanai D, Weiland JD, Mahadevappa M, et al. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007;143:820.
Zrenner E. The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica. 2002;216(suppl 1):8.
1 Delgado JM, Hamlin H, Chapman WP. Technique of intracranial electrode implacement for recording and stimulation and its possible therapeutic value in psychotic patients. Confin Neurol. 1952;12:315.
2 Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol. 2008;13:193.
3 Arle JE, Alterman RL. Surgical options in Parkinson’s disease. Med Clin North Am. 1999;83:483.
4 Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008;242:3.
5 Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651.
6 Jackson AB, Dijkers M, Devivo MJ, et al. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil. 2004;85:1740.
7 Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249.
8 Kettner RE, Marcario JK, Clark-Phelps MC. Control of remembered reaching sequences in monkey. I. Activity during movement in motor and premotor cortex. Exp Brain Res. 1996;112:335.
9 Halsband U, Ito N, Tanji J, et al. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243.
10 Georgopoulos AP. Higher order motor control. Annu Rev Neurosci. 1991;14:361.
11 Burnod Y, Baraduc P, Battaglia-Mayer A, et al. Parieto-frontal coding of reaching: an integrated framework. Exp Brain Res. 1999;129:325.
12 Stevens CF, Zador A. Neural coding: the enigma of the brain. Curr Biol. 1995;5:1370.
13 Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416.
14 Schwartz AB, Taylor DM, Tillery SI. Extraction algorithms for cortical control of arm prosthetics. Curr Opin Neurobiol. 2001;11:701.
15 Scott SH. Inconvenient truths about neural processing in primary motor cortex. J Physiol. 2008;586:1217.
16 Serruya MD, Hatsopoulos NG, Paninski L, et al. Instant neural control of a movement signal. Nature. 2002;416:141.
17 Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829.
18 Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998;9:1707.
19 Hochberg LR, Serruya MD, Friehs GM, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164.
20 Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60:511.
21 Kim SP, Simeral JD, Hochberg LR, et al. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J Neural Eng. 2008;5:455.
22 Kim SP, Simeral JD, Hochberg LR, et al. Multi-state decoding of point-and-click control signals from motor cortical activity in a human with tetraplegia. Proc. 2007 IEEE/EMBS Int’l Conference on Neural Engineering, Kohala Coast, Hawaii: 486, 2007.
23 Bullock TH. Signals and signs in the nervous system: the dynamic anatomy of electrical activity is probably information-rich. Proc Natl Acad Sci U S A. 1997;94:1.
24 Birbaumer N, Weber C, Neuper C, et al. Physiological regulation of thinking: brain-computer interface (BCI) research. Prog Brain Res. 2006;159:369.
25 McFarland DJ, Wolpaw JR. Sensorimotor rhythm-based brain-computer interface (BCI): model order selection for autoregressive spectral analysis. J Neural Eng. 2008;5:155.
26 Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol. 2003;89:1941.
27 Womelsdorf T, Fries P. Neuronal coherence during selective attentional processing and sensory-motor integration. J Physiol Paris. 2006;100:182.
28 Serruya M, Hatsopoulos N, Fellows M, et al. Robustness of neuroprosthetic decoding algorithms. Biol Cybern. 2003;88:219.
29 Blankertz B, Muller KR, Curio G, et al. The BCI Competition 2003: progress and perspectives in detection and discrimination of EEG single trials. IEEE Trans Biomed Eng. 2004;51:1044.
30 Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032.
31 Cheng G, Fitzsimmons NA, Morimoto J, et al. Bipedal locomotion with a humanoid robot controlled by cortical ensemble activity. San Diego, CA, USA. Society for Neuroscience 37th Annual Meeting. 2007.
32 Velliste M, Perel S, Spalding MC, et al. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098.
33 Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327.
34 Lauer RT, Peckham PH, Kilgore KL. EEG-based control of a hand grasp neuroprosthesis. Neuroreport. 1999;10:1767.
35 Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272.
36 Rosen S, Walliker J, Brimacombe JA, et al. Prosodic and segmental aspects of speech perception with the House/3M single-channel implant. J Speech Hear Res. 1989;32:93.
37 Wilson BS, Finley CC, Lawson DT, et al. Better speech recognition with cochlear implants. Nature. 1991;352:236.
38 Throckmorton CS, Collins LM. The effect of channel interactions on speech recognition in cochlear implant subjects: predictions from an acoustic model. J Acoust Soc Am. 2002;112:285.
39 Dowell RC, Seligman PM, Blamey PJ, et al. Speech perception using a two-formant 22-electrode cochlear prosthesis in quiet and in noise. Acta Otolaryngol. 1987;104:439.
40 von Wallenberg EL, Battmer RD. Comparative speech recognition results in eight subjects using two different coding strategies with the Nucleus 22 channel cochlear implant. Br J Audiol. 1991;25:371.
41 Roland JTJr. A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325.
42 Colletti V, Carner M, Miorelli V, et al. Auditory brainstem implant (ABI): new frontiers in adults and children. Otolaryngol Head Neck Surg. 2005;133:126.
43 Schmidt EM, Bak MJ, Hambrecht FT, et al. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain. 1996;119:507.
44 Lepri BP. Is acuity enough? Other considerations in clinical investigations of visual prostheses. J Neural Eng. 2009;6:035003.
45 Tsai D, Morley JW, Suaning GJ, et al. A wearable real-time image processor for a vision prosthesis. Comput Methods Programs Biomed. 2009;95:258-269.
46 Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci U S A. 2007;104:7670.
47 Caspi A, Dorn JD, McClure KH, et al. Feasibility study of a retinal prosthesis: spatial vision with a 16-electrode implant. Arch Ophthalmol. 2009;127:398.
48 Yanai D, Weiland JD, Mahadevappa M, et al. Visual performance using a retinal prosthesis in three subjects with retinitis pigmentosa. Am J Ophthalmol. 2007;143:820.
49 Zrenner E. The subretinal implant: can microphotodiode arrays replace degenerated retinal photoreceptors to restore vision? Ophthalmologica. 2002;216(suppl 1):8.
50 McCreery DB, Yuen TG, Agnew WF, et al. Stimulus parameters affecting tissue injury during microstimulation in the cochlear nucleus of the cat. Hear Res. 1994;77:105.
51 Mokwa W, Goertz M, Koch C, et al. Intraocular epiretinal prosthesis to restore vision in blind humans. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5790.
52 Button J, Putnam T. Visual responses to cortical stimulation in the blind. J Iowa State Med Soc. 1962;52:17.
53 Dobelle WH, Mladejovsky MG, Evans JR, et al. “Braille” reading by a blind volunteer by visual cortex stimulation. Nature. 1976;259:111.
54 Kirk J. Machines in Our Hearts: The Cardiac Pacemaker, the Implantable Defibrillator, and American Health Care. Baltimore: Johns Hopkins University Press; 2001.