CHAPTER 114 NEUROLOGY OF GASTROENTEROLOGY AND HEPATOLOGY
The enteric nervous system within the gut wall, replete with its neuronal networks and numerous neurotransmitters, mirrors many aspects of the central nervous system (CNS) and intimately interfaces with the CNS via the autonomic nervous system. Although seldom recognized, the number of neurons in the enteric nervous system is actually comparable to the number of neurons in the spinal cord, leading some authorities to refer to the enteric nervous system as the “little brain.”1,2 The striking similarities and intricate interaction between the neurological and gastrointestinal systems have been highlighted by the emergence of “neurogastroenterology” as a field of growing research and clinical interest.3
The boulevard connecting the two disciplines is a two-way street. Gastrointestinal manifestations of some neurological diseases are well known; the sometimes prominent gastrointestinal manifestations of Parkinson’s disease serve as a ready example.4 However, the potential presence of neurological dysfunction in the setting of gastrointestinal disease is often overlooked. Although neurological dysfunction in the setting of hepatic failure is very well known to both neurologists and gastroenterologists, the potential for neurological dysfunction in other gastrointestinal disease processes often eludes recognition. This chapter highlights several of these disease processes and also addresses some neurological aspects of hepatic failure.
GASTROINTESTINAL DISEASE
Celiac Disease
Celiac disease, also known as nontropical sprue, celiac sprue, or gluten-sensitive enteropathy, has historically been conceptualized as a disorder of the small intestine that in its adult-onset form is characterized clinically by steatorrhea, intermittent diarrhea, abdominal bloating, flatulence, malabsorption, weight loss, and aphthous stomatitis. Pathological features include villous atrophy and crypt hyperplasia. The proximal small intestine, especially the jejunum, is most prominently involved. Exposure to gluten, the protein fraction of wheat, appears to trigger an autoimmune response that in turn produces the intestinal mucosal damage characteristic of celiac disease. There are two distinct proteins in gluten (glutenin and gliadin), and it is the production of immunoglobulin A antibodies to gliadin that has been traditionally held to be the vehicle for the mucosal damage, although it has also been suggested that these antibodies, although diagnostic markers of the disease, are not crucial to its pathogenesis.5 Other similar proteins, called prolamins, present in barley, rye, and oats, may induce a similar response.
Most reports have estimated the prevalence of celiac disease at approximately 1% of the white population; one study indicated that 1 per every 120 to 300 persons in the United States and Europe is affected.6 Celiac disease appears to be most prevalent in northwestern Europe. Two peaks of clinical appearance have been identified: the first during infancy and the second between the ages of 30 and 50. A genetic component is presumably present, inasmuch as approximately 90% of individuals with diagnosed celiac disease carry the human leukocyte antigen DQ2 haplotype.7,8 Possible susceptibility loci have also been identified on chromosomes 2 and 5,9 but specific genetic mutations have not been delineated.
Not all individuals with celiac disease manifest clinical symptoms. Serological tests and even intestinal biopsy may demonstrate the presence of the pathological process in completely asymptomatic individuals. Clinicians have also realized that celiac disease is not merely a gastrointestinal disease but is, in fact, a multisystemic disorder. Dermatitis herpetiformis is characterized primarily by a blistering skin rash, but patients with this disorder also demonstrate evidence of gluten-induced intestinal pathology, the presence of gluten-induced antibodies, and a response to dietary gluten restriction, which suggests that the two disorders may be part of a pathological spectrum.10 Osteoporosis and infertility have been reported as complications of celiac disease,6 as has psychiatric dysfunction.11 Individuals with celiac disease are also at increased risk for developing certain types of cancer, including lymphoma and carcinomas of the small intestine, esophagus, and pharynx.12 A particular variety of T cell lymphoma, now designated enteropathy-associated T cell lymphoma, has been specifically associated with celiac disease. Associations with other autoimmune diseases, such as autoimmune thyroid disease, type I diabetes mellitus, Sjögren’s syndrome, and primary biliary cirrhosis have also been described.13
Neurological dysfunction has also been identified in the context of celiac disease (Table 114-1). Neurological complications may, in fact, develop in 6% to 12% of persons with celiac disease.14–16 CNS disorders have undergone the most intense investigation, but peripheral nervous system dysfunction has also been recognized.
Ataxia
Ataxia is the neurological complication that has received the most interest and attention in the setting of celiac disease. In 1966, Cooke and Smith described 16 patients with celiac disease who had also developed progressive ataxia (along with other neurological abnormalities) that was unresponsive to gluten restriction.17 It was not until 1996, however, that the idea that ataxia might be a direct consequence of gluten sensitivity was proposed by Hadjivassiliou and colleagues when they published the first of a series of reports that delineated the presence of antigliadin antibodies in individuals with sporadic adult-onset ataxia of unknown etiology and subsequently created the term gluten ataxia for the condition.7,8,18,19 They later documented the presence of antigliadin antibodies (immunoglobulin G and/or immunoglobulin A) in 41% (54) of 132 individuals with sporadic idiopathic ataxia, in comparison with only 15% (5) of 33 persons with clinically probable multiple system atrophy, 14% (8) of 59 patients with familial ataxia, and 12% (149) of 1200 normal controls.19 In the same report, a second group of 44 patients with sporadic idiopathic ataxia from another clinic was also studied; antigliadin antibodies were present in 32% (14) of this group. Other investigators have also reported elevations of antigliadin antibodies in patients with sporadic idiopathic ataxia but with lower frequencies, ranging from 11% to 27%.14,20–22 This finding has not been universal, however. Some investigators have not encountered this elevation in their study populations.23,24
Gluten ataxia, as currently recognized by its proponents, has no particularly distinguishing clinical characteristics. Gait ataxia is, by definition, present in all individuals with gluten ataxia. Limb ataxia, dysarthria and ocular signs are present in the vast majority. Cerebellar atrophy is evident on magnetic resonance imaging (MRI) in 79% of patients, whereas white matter hyperintensities are evident in only 19%.19 Evidence of classical celiac disease was found in only 24% (12) of 51 individuals with gluten ataxia who underwent gastroscopy and duodenal biopsy. In another report by the same group of investigators, 26 of 43 patients placed on a gluten-free diet were able to maintain the diet for 1 year; all 26 experienced improvement in the ataxia, regardless of whether an enteropathy was present.25
Some investigators are not convinced that gluten ataxia exists as a distinct entity.5,23 They point to the nonspecificity of antigliadin antibodies, as reflected in the fact that antigliadin antibodies are present in significant numbers of normal controls. Moreover, antigliadin antibodies have been noted to be present in 44% (23) of a group of 52 patients with Huntington’s disease, which prompted speculation that antigliadin antibodies might simply be an epiphenomenon in certain neurodegenerative diseases and that gluten ataxia may not be a distinct clinical entity.26 The absence of any clearly defined pathophysiological mechanism that might account for the cerebellar dysfunction in affected individuals has been another criticism of the concept of gluten ataxia as a distinct clinical entity. However, a chronic, immune-mediated inflammatory process has been proposed to be responsible for the cerebellar damage,27 and autopsy examination in several affected individuals has demonstrated Purkinje cell loss and lymphocytic infiltration within the cerebellum and posterior columns of the spinal cord.8
Hadjivassiliou and colleagues (2003) suggested that screening for gluten sensitivity be undertaken in all individuals who present with adult-onset ataxia without any other obvious cause.19 There is, however, no universal agreement regarding the specific screening test that should be employed. Antireticulin, antiendomysial and antitissue transglutaminase antibodies may actually be identical,5 and their presence is quite specific for celiac disease. In contrast, antigliadin antibody, especially the immunoglobulin G isotype, is nonspecific and actually present in more than 10% of healthy blood donors. This diminishes the usefulness of antigliadin antibody testing as a screening test for celiac disease, but Hadjivassiliou and colleagues believed that immunoglobulin G antigliadin antibodies are the best diagnostic marker for gluten ataxia in that they mark the whole spectrum of gluten sensitivity and not just gluten enteropathy alone.19 It thus appears that research on gluten ataxia is currently unfinished.
Epilepsy
Some investigators have suggested that the prevalence of epilepsy is increased among individuals with celiac disease. Epilepsy has been reported to be present in 3.5% to 5.5% of individuals with celiac disease.28,29 Examining the converse, Cronin and associates studied a group of patients with epilepsy and found the frequency of celiac disease, diagnosed from the presence of endomysial antibody, to be 2.3%, in comparison with 0.4% in a control group.30 A specific syndrome of epilepsy, bilateral occipital calcification, and celiac disease has also been described.31 These reports have been largely from Italian investigators, but reports from other parts of the globe have also been published.32 The mechanism for such an association is obscure. It also is not entirely clear whether a gluten-free diet improves seizure control in patients with celiac disease and epilepsy, although some investigators have reported that it does.32 An association between celiac disease and epilepsy has not been found by all investigators.33,34 Moreover, in one study, the presence of celiac disease–associated antibodies (antigliadin, antiendomysial, and antitissue transglutaminase) did not differ between 968 patients with epilepsy and a reference group of 584 individuals.35
Migraine
An association between celiac disease and migraine has also been proposed. In a report by Gabrielli and colleagues, 4.4% (4) of 90 patients with migraine were found to have serological evidence of celiac disease; subsequent jejunal biopsy confirmed the presence of celiac disease in all four of these.36 Migraine severity improved in all individuals on a gluten-free diet. Abnormalities of regional cerebral blood flow, which were noted on single photon emission computed tomographic scanning in all patients with the combination of celiac disease and migraine, also improved in all individuals. Additional case reports have described resolution of migraine with treatment of celiac disease.37 Low serotonin levels have been proposed to be a common link between the two disorders.38
Learning Disabilities
Learning disabilities, such as attention deficit/hyperactivity disorder, have been shown to occur with increased frequency in the setting of celiac disease.27 The co-occurrence of dyslexia and celiac disease has also been reported.39
Peripheral Neuropathy and Myopathy
Both peripheral nerve involvement and myopathy have been described in celiac disease. In a retrospective chart review, Vaknin and associates found that peripheral neuropathy accounted for 17% of the neurological abnormalities present in a group of patients with celiac disease.16 Another group of investigators identified chronic axonal sensorimotor neuropathy in 23% (6) of 26 patients with celiac disease and abnormalities on neurophysiological testing in 31% (8).40 These findings were present despite the fact that all 26 individuals studied had been on gluten-free diets for a median of 3 years (range, 2 to 28 years). Sural nerve biopsy, when performed, has also demonstrated the presence of axonal injury.41
Inflammatory myopathy has also been described in the setting of celiac disease and in at least one instance has been associated with vitamin E deficiency; reversal of both clinical symptoms and muscle biopsy abnormalities was documented in that individual after treatment with vitamin E and institution of a gluten-free diet.42
Other Processes
A number of other neurological manifestations of celiac disease have also been reported but less extensively evaluated. Examples include myoclonic ataxia,43 progressive multifocal leukoencephalopathy,44 chorea,45 autonomic neuropathy,46 and neuromyelitis optica.47 The significance of these reported associations is uncertain. Lymphoma within the CNS, with the immunophenotype of enteropathy-associated T cell lymphoma, has also been reported in celiac disease.12
Inflammatory Bowel Disease
Two similar but distinct disease entities, ulcerative colitis and Crohn’s disease (regional enteritis, granulomatous colitis), are the most widely recognized members of a group of conditions collectively labeled inflammatory bowel disease (IBD). An autoimmune etiology, characterized by a dysregulated mucosal immune response to antigens normally present within the intestinal lumen, is suspected in both.48,49 During the latter half of the 20th century, a significant rise in the incidence of Crohn’s disease, but not ulcerative colitis, was noted, especially in North America and northern Europe.50 The explanation for this increased incidence, which has appeared to stabilize since about 1980, is uncertain, but suspicion has fallen on a variety of environmental factors. Genetic factors also appear to play a role in the generation of the inappropriate immune response. This has been most clearly identified in Crohn’s disease, in which the NOD2/CARD15 gene, which is involved in the immune detection of bacterial products, has been identified as a susceptibility gene for Crohn’s disease.51,52 Despite many similarities, the clinical features and pathological profiles of the two conditions also demonstrate decided differences (Tables 114-2, 114-3, and 114-4). Neurological dysfunction has been described in both.
TABLE 114-3 Peripheral Nervous System Dysfunction in Inflammatory Bowel Disease
TABLE 114-4 Gastrointestinal Features of Ulcerative Colitis
Ulcerative colitis is characterized clinically by urgent, bloody diarrhea (see Table 114-4). Its course is typically marked by exacerbations and remissions. The pathological hallmark of ulcerative colitis is diffuse inflammation of the mucosa and superficial submucosa of the colon, extending a variable distance proximally from the rectum but not beyond the colon.53
The primary clinical characteristics of Crohn’s disease, in contrast, consist of abdominal pain and nonbloody, less urgent diarrhea (see Table 114-2). Weight loss is also common. Scarring and stricture formation can lead to partial intestinal obstruction, and fistula formation is also frequent. As with ulcerative colitis, the clinical course of Crohn’s disease often entails exacerbations and remissions. Although Crohn’s disease may involve virtually all levels of the gastrointestinal tract, it demonstrates a distinct predilection for the distal small intestine and proximal colon. In contrast to ulcerative colitis, gastrointestinal tract involvement in Crohn’s disease is patchy or segmental and extends deeply, often transmurally. Noncaseating granulomas may form but are not invariably present.
Extraintestinal manifestations of both Crohn’s disease and ulcerative colitis are surprisingly common, with reported frequencies ranging from approximately 25% to more than 50% of affected individuals.54–57 Some of the extraintestinal manifestations, such as involvement of joints, skin, mouth, and eyes, seem to be correlated with the presence of active colonic inflammation and are observed in both Crohn’s disease and ulcerative colitis. Other clinical processes, such as gallstones and renal calculi, are correlated more directly with small intestinal involvement and are observed primarily in Crohn’s disease.58 Primary sclerosing cholangitis is a particularly important extraintestinal complication of ulcerative colitis that may progress to hepatic failure, independent of the severity of the ulcerative colitis itself.
In comparison with many of the systemic manifestations of IBD, neurological involvement (myopathy and myasthenia gravis) occurs less frequently in both ulcerative colitis and Crohn’s disease. Lossos and colleagues (1995) reported the presence of neurological involvement in 3% (19) of 638 persons they studied with either ulcerative colitis or Crohn’s disease.59 In their comprehensive review, they described four categories of neurological involvement in Crohn’s disease and ulcerative colitis: peripheral neuropathy, myopathy or myoneural junction dysfunction, cerebrovascular disease, and myelopathy. Other groups of investigators have also documented seizures and encephalopathy in patients with IBD. Treatment for both Crohn’s disease and ulcerative colitis involves potent medications; thus, a number of neurological complications of treatment for these diseases have also been reported. Although neurological impairment often becomes evident during periods of disease activity, it can also emerge when the disease process is quiescent.
Peripheral Nervous System Involvement in Inflammatory Bowel Disease
Peripheral Neuropathic Disease
The peripheral nervous system is the dominant site of neurological dysfunction in ulcerative colitis and Crohn’s disease, accounting for 31.5% of neurologically affected patients in the experience of Lossos and colleagues.59 A rather extensive array of peripheral neuropathic processes has received recognition (see Table 114-3). Acute inflammatory demyelinating neuropathy (Guillain-Barré syndrome), axonal sensorimotor neuropathy, mononeuropathy, brachial plexopathy, mononeuritis multiplex, multiple compressive neuropathies, and cranial neuropathies have all been described in the setting of Crohn’s disease and ulcerative colitis.59–62
Gondim and associates performed a retrospective study of 18 patients with Crohn’s disease and 15 with ulcerative colitis who had developed peripheral neuropathy with no other identifiable etiology.63 The neuropathy was demyelinating in nature in slightly fewer than 30% of the patients, small- or large-fiber axonal sensory in about 30%, and large-fiber axonal sensorimotor in approximately 40%. Small-fiber axonal sensory neuropathy was more frequently present in younger patients, whereas large-fiber axonal neuropathy tended to be present in older patients. Although both axonal and demyelinating neuropathies often responded to immunotherapy, the response was more consistent and robust in patients with demyelinating neuropathy.
Two specific and unusual processes consisting of cranial nerve involvement have been noted in individuals with IBD. Melkersson-Rosenthal syndrome is characterized by the clinical constellation of recurrent facial nerve palsy, intermittent orofacial swelling, and fissuring of the tongue (lingua plicata). Not only has this syndrome been noted in the presence of Crohn’s disease, but also its pathological hallmark—noncaseating granuloma formation—is observed in Crohn’s disease. This has led some to suggest that Crohn’s disease and Melkersson-Rosenthal syndrome are actually part of the same pathological spectrum.64 Acute sensorineural hearing loss has been described, primarily in persons with ulcerative colitis.65–68 The presumption of an autoimmune basis for the hearing loss is based in part on reported responses to steroid administration. Chronic, subclinical hearing loss, discovered on audiometric screening, has also been documented in ulcerative colitis.69
Muscle and Myoneural Junction Disease
Myopathy has been described in the setting of both ulcerative colitis and Crohn’s disease, but it is present more frequently in Crohn’s disease. It accounted for 16% of the cases of neurological dysfunction in the series compiled by Lossos and colleagues.59 Dermatomyositis, polymyositis, rimmed vacuole myopathy, and granulomatous myositis have all been noted in this setting. As with peripheral nerve involvement, an autoimmune basis is presumed to be present. Localized myositis involving the gastrocnemius muscles has also been reported in Crohn’s disease and is known as gastrocnemius myalgia syndrome.70 In approximately 50% of cases, the appearance of myopathic pathology seems to be correlated with disease activity in the bowel. Myasthenia gravis, another autoimmune disorder, has also been reported in the setting of both Crohn’s disease and ulcerative colitis, although reports of this association are quite sparse.
Central Nervous System Involvement in Inflammatory Bowel Disease
CNS involvement in inflammatory bowel disease can assume many guises. Cerebrum, brainstem, and spinal cord can all be involved (Table 114-5).
TABLE 114-5 Central Nervous System Involvement in Inflammatory Bowel Disease
Myelopathic and Motor Neuron Disease
Chronic, slowly progressive myelopathy is yet another neurological manifestation of inflammatory bowel disease, accounting for 26% of the patients with neurological involvement in the series of Lossos and colleagues.59 Most of these individuals were suffering from Crohn’s disease rather than ulcerative colitis. An inflammatory basis was suspected, and oligoclonal banding was noted in one patient. Other investigators have suggested a possible association between ulcerative colitis and multiple sclerosis,71,72 and it has been reported that the incidence of multiple sclerosis is threefold greater in persons with ulcerative colitis than in the general population.71 Transverse myelitis has been reported in an individual with ulcerative colitis who also was found to have anti–Jo-1 antibody (antisynthetase) syndrome.73 Whether cases such as these are causal or coincidental is uncertain. A more definite causal relationship can be drawn with the occurrence of spinal empyema in the setting of Crohn’s disease secondary to fistula formation.74
There has been one case report of the coexistence of ulcerative colitis with motor neuron disease, although the issue of cause versus coincidence cannot be escaped in this instance either.75
Cerebrovascular Disease
Vascular complications are well-documented extraintestinal manifestations of inflammatory bowel disease. In a massive undertaking, Talbot and coworkers reviewed the records of 7199 patients with either Crohn’s disease or ulcerative colitis and noted the presence of vascular complications in 1.3%.76 Deep venous thrombosis and pulmonary embolus were the most common sites of involvement, whereas cerebrovascular events accounted for only 9.8% of the total vascular complications (in 9 of 92 patients). Vascular complications occur more frequently in ulcerative colitis than in Crohn’s disease; the reason for this difference is uncertain.77 Hypercoagulability has been presumed to be responsible for the thromboembolic events, and elevations of factors V and VIII and fibrinogen levels, along with decreased antithrombin III levels, have been noted.78 Anticardiolipin antibodies, thrombopoietin, and homocysteine levels have also been shown to be elevated in individuals with ulcerative colitis and Crohn’s disease, but the elevations are not correlated with increased risk of thromboembolic events.79–81 In fact, no single or specific abnormality has been identified as the hypercoagulable culprit, and no consistent abnormality of the routinely recognized coagulopathy susceptibility factors has been discovered.82
A variety of cerebrovascular events has been reported in ulcerative colitis and Crohn’s disease. Both large artery and lacunar infarcts have been described.83,84 Cerebral vasculitis has been identified in both Crohn’s disease and ulcerative colitis, which led to the proposal that an autoimmune basis is responsible for the cerebrovascular events. However, responses to both immunosuppressive therapy (corticosteroids and azathioprine) and anticoagulation have been reported, which suggests that a single explanation is improbable and that both hypercoagulable and autoimmune processes may have roles in different individuals.85–87 Both dural and cortical venous sinus thrombosis have been the subjects of numerous case reports in individuals with inflammatory bowel disease, predominantly but not exclusively in ulcerative colitis.77,88 Such events have occurred both during active exacerbations and during periods of disease quiescence. As with other cerebrovascular events, the mechanism of the coagulopathy is not entirely clear.
Seizures and Encephalopathy
Seizures are an infrequently reported neurological complication of inflammatory bowel disease. They may occur as a complication of the surgical management of these diseases, presumably precipitated by factors such as fluid overload, electrolyte imbalance, hypoxia, and steroid administration or withdrawal.89 Seizures have also been reported as a complication of cyclosporine treatment in an individual with Crohn’s disease.90 Status epilepticus, with long-term sequelae, has been described in the setting of ulcerative colitis; the genesis for this has not been clearly delineated.91
Diffuse encephalopathy with altered consciousness may also develop in individuals with ulcerative colitis or Crohn’s disease. This may occur in the context of cerebral vasculitis85 but also as a consequence of nutritional deficiencies. Both Wernicke’s encephalopathy and possible selenium-induced encephalopathy have been described in individuals with Crohn’s disease receiving total parenteral nutrition.92,93 Wernicke’s encephalopathy has also been reported in an individual with clinically inactive Crohn’s disease.94
Whipple’s Disease
Although originally described as a gastrointestinal disease, it has become abundantly clear that Whipple’s disease is a multisystem disorder (Table 114-6) that may also demonstrate joint, dermatological, lymphatic, cardiac, pulmonary, ocular, and neurological dysfunction.95 Thus, in addition to diarrhea, weight loss, and abdominal pain, individuals with Whipple’s disease may display migratory polyarthritis, generalized lymphadenopathy, anemia, fever, generalized malaise, chronic cough, pseudo-addisonian skin pigmentation, congestive heart failure, hypotension, pericardial friction rub, splenomegaly, focal glomerulitis, visual changes, uveitis, retinitis and a variety of neurological manifestations.95,96
Whipple’s disease affects primarily middle-aged men of European heritage, with an average age at symptom onset of approximately 50; the male-to-female ratio appears to have diminished since the 1990s from 8 : 1 to 4-5 : 1.95 By all accounts, Whipple’s disease is a very rare condition, even though it may be more prevalent than commonly recognized. There is some evidence that farmers have an increased risk for developing Whipple’s disease.96 Although the presence of rod-shaped organisms was already described by Whipple in his 1907 report, it was only as recently as 2001 that the organism responsible for Whipple’s disease, Tropheryma whippelii, was identified and characterized as a member of the actinomycete family.95 The natural habitat of the organism and the route of infection remain obscure, although it has been suggested that T. whippelii may be a soil-dwelling organism, which might explain the increased incidence of infection in farmers.
Because Whipple’s disease is so rare, no extensive literature covering the CNS manifestations of the condition exists. In fact, in a review of 12 patients from their own clinical practice, Gerard and associates were able to gather information from the literature on only an additional 122 individuals with CNS manifestations of Whipple’s disease.97 Clinical CNS involvement develops in 10% to 43% of patients with Whipple’s disease, but postmortem examinations demonstrate CNS lesions in over 90% of both symptomatic and asymptomatic individuals.95,98 Neurological dysfunction may be the presenting feature in approximately 5% of persons with Whipple’s disease (Table 114-7).98 Cognitive changes are the most frequently observed neurological manifestation of Whipple’s disease, appearing in 71% of individuals; psychiatric symptoms such as depression and personality or behavioral changes often accompany the cognitive dysfunction.99,100 Symptoms indicative of hypothalamic involvement, such as insomnia, hypersomnia, hyperphagia, polyuria, and polydipsia are less common.95,99,101 Cerebellar dysfunction with gait and balance impairment is said to develop in approximately 20% of persons with Whipple’s disease; pyramidal tract abnormalities may occur.99,100 Peripheral neuropathy, ostensibly caused by malabsorption with consequent nutritional deficiency, has also been reported.100,102
TABLE 114-7 Neurological Features of Whipple’s Disease
Vertical gaze impairment develops in approximately 50% of patients and may lead to diagnostic confusion with progressive supranuclear palsy.100 Abnormalities of cranial nerves III, IV, and VI; internuclear ophthalmoplegia; ptosis; and pupillary abnormalities do occur but are unusual in Whipple’s disease.100,103
Approximately 20% of individuals with CNS manifestations of Whipple’s disease develop a unique type of involuntary movement, oculomasticatory myorhythmia.99,104 These movements consist of the combination of pendular convergence nystagmus and concurrent slow, rhythmic synchronous contractions of the masticatory muscles, and they are invariably accompanied by a supranuclear vertical gaze paresis. Sometimes the muscle contractions also involve the extremities, hence the term oculo-facial-skeletal myorhythmia. These movements (oculomasticatory myorhythmia and oculo-facial-skeletal myorhythmia) have been held to be pathognomonic for Whipple’s disease.
Confirmation of the diagnosis of Whipple’s disease has typically depended on identification of periodic acid–Schiff stain–positive inclusions in macrophages present in duodenal biopsy specimens. However, both false-negative and false-positive errors may occur. Polymerase chain reaction analysis appears to be a more sensitive method of diagnosis, but there is some evidence that T. whippelii DNA may be present in healthy individuals without Whipple’s disease.95,98
In individuals with CNS symptoms, brain biopsy, when performed, yields positive results in more than 80%.99 Cerebrospinal fluid analysis may also be useful, often demonstrating an inflammatory cell response that sometimes contains periodic acid–Schiff stain–positive macrophages.101 Polymerase chain reaction analysis of the cerebrospinal fluid may also be positive in 80% of patients with Whipple’s disease and neurological symptoms.105
Prompt diagnosis of Whipple’s disease is important because effective treatment is available. The rarity of Whipple’s disease has precluded formal clinical trials, but empirical evidence suggests that an initial 2-week course of parenteral therapy with either a combination of penicillin G and streptomycin or with a third-generation cephalosporin (e.g., ceftriaxone), followed by a 1-year course of oral trimethoprim-sulfamethoxazole, is an effective treatment approach.95 The prolonged course of trimethoprim-sulfamethoxazole, which crosses the blood-brain barrier, is intended to treat potential or identified CNS involvement. This is especially important because CNS relapses carry a poor prognosis and a high mortality rate.
HEPATIC DISEASE
Minimal Hepatic Encephalopathy
A change in nomenclature has evolved for the stage of hepatic encephalopathy in which routine clinical neurological and mental status examination findings are normal but subtle deficits can be documented on detailed neuropsychological testing. Earlier terms, such as subclinical hepatic encephalopathy or latent hepatic encephalopathy, have now yielded to the label minimal hepatic encephalopathy (MHE). Despite superficially normal neurological and neuropsychological functioning, individuals with MHE have identifiable deficits in occupational and psychosocial functioning, activities of daily living, and overall quality of life.106 Complex activities, such as planning a trip or handling family finances, may be affected.107 One study has also confirmed that individuals with MHE have impaired ability to drive an automobile.108 Estimates of the prevalence of MHE in individuals with cirrhosis range from 30% to 84%.109 In addition to the severity of hepatic functional impairment, other risk factors for the development of MHE include age, alcohol as the etiology of the liver failure, prior episodes of overt hepatic encephalopathy, presence of esophageal varices, and transjugular intrahepatic or surgical portosystemic shunts.107 MHE may also develop in individuals with congenital portosystemic shunts or shunts that develop as a result of portal thrombosis.110 A diagnosis of MHE has traditionally been based on formal neuropsychological testing, but a multimodal approach employing spectral electroencephalography and determination of partial pressure of ammonia has also been advocated.111 Although the functional deficits present in MHE often improve with treatment of the underlying hepatic disease, the long-term treatment benefits are uncertain. There is some evidence that, even after liver transplantation, some subtle impairment may persist.112
Chronic Hepatic Encephalopathy
Subtle changes in attention span, memory, personality, concentration, and reaction time typically constitute the earliest indications of insidiously emerging encephalopathy. With time and disease progression, these subtle features may evolve into stages of more overt cognitive impairment, characterized by increasing degrees of drowsiness, confusion, and disorientation, with the eventual evolution of stupor and coma. These levels of increasing neurological and cognitive dysfunction are traditionally divided into four stages and are known as the West Haven Criteria (Table 114-8).113
TABLE 114-8 Hepatic Encephalopathy: Clinical Stages, West Haven Criteria
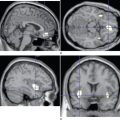
Although the diagnosis of CHE is ultimately based on clinical examination findings, both neurophysiological and neuroimaging procedures can provide useful information. Although neither specific for nor universally present in CHE, the classic electroencephalographic finding in CHE is the presence of triphasic waves. MRI may also demonstrate distinctive changes in individuals with hepatic failure. Hyperintensity within the globus pallidus on T1-weighted images can be prominent, but these changes are not limited to patients with overt CHE and are present in more than 75%,114 and perhaps up to 95%,115 of persons with cirrhosis, regardless of whether neurological symptoms are present. These pallidal abnormalities on MRI have been attributed to manganese deposition.114,116 Abnormalities on diffusion-weighted MRI, magnetic resonance spectroscopy, and on fluorine 18–fluorodeoxyglucose positron emission tomography have also been reported in CHE115,117 but are not employed in the diagnosis of CHE in routine clinical practice.
The etiology of CHE has been the subject of long-running controversy. In particular, the role of ammonia in the generation of the CNS dysfunction has been the subject of much discussion and dispute.118 Oral protein loading and gastrointestinal bleeding are well-recognized precipitants of CHE that produce elevations of blood ammonia levels. Electrolyte imbalance, infection, and deteriorating liver function may also precipitate CHE. Although elevated blood ammonia levels are not universal, they are present in most individuals with CHE. Moreover, considerable overlap has been documented in ammonia levels between different grades of CHE severity, and this overlap has diluted and rendered controversial any correlation between the two.118 Thus, the usefulness of single blood ammonia determinations is severely limited in diagnosing CHE.119,120 Kundra and colleagues reported that plasma ammonia elevations mirrored the severity of encephalopathy in individuals with acute liver failure but not in persons with CHE.118
It has been suggested that venous ammonia levels, in particular, are unreliable and that arterial sampling is necessary for accurate assessment, but this was not borne out in one study.119 Proper handling of the blood specimen, however, is important, because failure to keep the sample on ice, delay in getting the sample to the laboratory, and use of tourniquets before blood sampling can all produce false elevations of ammonia level.120
Current evidence suggests that elevated blood ammonia level alone is not the sole determinant of CHE; rather, a multifactorial pathogenesis is more likely.121,122 It has been suggested that hyperammonemia leads to increased production of glutamine within astrocytes and that this, in turn, produces osmotic stress and astrocytic edema that culminates in altered neuropsychological function.123,124 “Untimely” activation of N-methyl-D-aspartate receptors within the CNS may also be involved in the evolution of CHE,125 as may increased oxidative/nitrosative stress.122 In a cell culture experiment, the combination of ammonia and manganese was lethal to a high percentage of cultured astrocytes, although neither substance alone was toxic; moreover, both the antioxidant superoxide dismutase and a nitric oxide inhibitor blocked the cell death.126
The genesis of CHE may also involve alterations in various neurotransmitter systems within the brain. Involvement of the γ-amino butyric acid receptor complex in CHE, particularly the benzodiazepine receptor site, has been the object of particular scrutiny. In an animal model of hepatic encephalopathy, increased messenger RNA expression levels of γ-amino butyric acid–A receptor subunits in the basal ganglia and hippocampus were described.127 Evidence for the presence of endogenous benzodiazepine receptor ligands, perhaps produced by gut bacteria, has been documented in CHE, and the plasma concentration of these ligands is correlated approximately with the degree of CHE.128
Ahboucha and associates documented increases up to 13-fold in concentrations of the inhibitory neurosteroid allopregnanolone in the brains of 11 patients who died in hepatic coma, in comparison with 11 controls without hepatic, renal, or neurological diseases.129 Many neurosteroids possess potent sedative-hypnotic properties, and the authors suggested that the elevations present may be of pathophysiological significance.129
Activation of the serotonergic system and increased serotonin turnover has also been suggested to play a role in the pathogenesis of CHE, particularly its later stages.130,131
Standard treatment of CHE has traditionally consisted of measures to reduce ammonia levels. Identification and appropriate treatment of precipitating causes, restriction of dietary protein, and removal of sources of ammonia production within the gastrointestinal tract have been the bastions of treatment for CHE.128 Protein restriction is employed to reduce protein intake, and nonabsorbable disaccharides, such as lactulose and lactilol, are administered to acidify colonic contents and thus diminish absorption of both ammonia and endogenous benzodiazepine receptor ligands. Antibiotics, such as neomycin, ampicillin, and rifaximin, may also be employed to eliminate ammonia-producing bacteria from the gut, although they are not considered to be first-line treatment approaches, at least partly because of tolerability issues.132 Furthermore, the importance of colonic bacteria in the production of ammonia in the setting of CHE has been questioned, and the role of the small intestine as a generator of ammonia via glutamine uptake has been emphasized instead by some investigators.133
Controversy regarding the use of nonabsorbable disaccharides in CHE has surfaced in a Cochrane database review that questioned their beneficial effects in CHE and concluded that there currently is insufficient evidence to support their use.134,135 Some investigators have concurred with this assessment133,136; others have cautioned that the deficiencies in prior clinical trials of these substances should not be equated with dismissal of their use in clinical practice.137,138
The benzodiazepine receptor antagonist flumazenil produces short-term improvement in CHE but has not yet been shown to improve recovery or survival.139,140 The use of flumazenil in the treatment of CHE remains quite limited, primarily to situations of suspected pharmacological intoxication.141
Other modalities that have been proposed for the treatment of CHE include L-carnitine,142 branched-chain amino acids,143 and L-ornithine–L-aspartate.133 Artificial support systems are being investigated in CHE but have been targeted primarily toward individuals with more acute hepatic decompensation.
Fulminant Hepatic Failure
Rapidly progressive fulminant hepatic failure (FHF), evolving over a period of days to weeks in individuals without any history of liver dysfunction, is a dreaded and often dramatic manifestation of liver disease. A variety of processes can trigger FHF, but drug reactions and viral hepatitis appear to be the most frequent culprits. In one report, acetaminophen overdose was the cause of FHF in 39% of 308 patients, idiosyncratic drug reactions were responsible in 13%, and viral hepatitis (hepatitis A and B combined) in 12%.144 More rare reported causes of FHF include senna laxative toxicity,145 carbamazepine toxicity,146 parvovirus infection,147 and dengue hemorrhagic fever.148 In many cases, however, the cause of the FHF remains unknown.
The hallmark and defining characteristic of the encephalopathy that develops in FHF is cerebral edema. The cerebral edema, with consequent increased intracranial pressure, can develop so rapidly in FHF that papilledema may not have time to even develop, and the increased intracranial pressure eventually may reach levels that compromise cerebral perfusion. The pathogenesis of the cerebral edema is not entirely understood. Suspicion has focused primarily on increased astrocytic glutamine concentrations, with consequent cytotoxic edema, as the primary driver of cerebral edema in FHF, but some investigators propose that reductions in other brain osmolytes, specifically myoinositol and taurine, and increased lactate production may also be important.149 Alterations in cerebral blood flow with cerebral hyperemia may also be a contributing factor to the malignant cerebral edema that characterizes FHF.149,150
Reported mortality rates of FHF demonstrate considerable variability. Before the emergence of liver transplantation as a treatment option, mortality rates ranged from 80% to 85%. In a more recent large study of 295 patients with FHF, however, the rate of overall mortality at 1 year was 43%.151 Of the 295 patients, 25% survived with medical management, 41% underwent liver transplantation, and 34% died without transplantation. The rate of 1-year survival among the patients who received transplants was 76%.
Numerous difficult issues complicate the management of FHF. Coagulopathy, hypoglycemia, and multiple organ failure present dauntingly difficult and complex treatment challenges for the hepatologist. For the neurologist, the management of cerebral edema and the recognition and treatment of seizures are equally difficult problems. Electroencephalographic monitoring, if available, provides invaluable assistance in the early recognition of subclinical seizure activity, especially in comatose or sedated patients. Intracranial pressure monitoring provides the most accurate assessment of cerebral edema but may also precipitate significant complications, such as intracranial hemorrhage, in 4% to 22% of patients.152 Mannitol or hypertonic saline administration may provide short-term reduction in intracranial pressure.153 The use of mild to moderate hypothermia is also undergoing evaluation as a means of treatment for cerebral edema in FHF.150,154 However, survival for most patients with FHF is ultimately dependent on liver transplantation. Artificial and bioartificial extracorporeal liver support devices are currently undergoing evaluation as bridging measures to sustain patients awaiting liver transplantation.155 One of these devices, the Molecular Adsorbent Recirculating System, has been used clinically since 1993 in more than 4000 patients.156 However, these systems are still considered experimental, and their efficacy in FHF has not yet been generally accepted.
Chronic Acquired Hepatocerebral Degeneration
Although van Woerkom, in 1914, was probably the first to describe what is now recognized as chronic acquired (nonwilsonian) hepatocerebral degeneration (CAHD),157–160 it was the report in 1965 by Victor and colleagues, in which they described 27 patients with the disorder, that clearly established CAHD as a distinct clinical entity.158 CAHD is generally considered rare, although Victor and colleagues maintained that it is “much more frequent” than Wilson’s disease.158 Nevertheless, Chen and associates, in a case report and review of the literature that covered the years 1981 to 2003, were able to identify only 36 additional cases.161 It is possible that many cases of CAHD go unrecognized in individuals with chronic liver disease, in whom neurological features, especially if mild, might simply be overlooked or attributed to hepatic encephalopathy. The observations of parkinsonism in 11 (21.6%) of 52 study subjects with cirrhosis by one group of investigators114 and of extrapyramidal features in over 50% of individuals with MHE by another group106 could conceivably reflect this lack of recognition.
The clinical picture of CAHD typically emerges in individuals with advanced liver disease who have experienced repeated episodes of hepatic encephalopathy. However, this is not invariably the case, and CAHD has been documented in individuals who have never displayed any evidence of hepatic encephalopathy.159 It can also develop in persons who have not actual liver disease but rather other processes, such as portal vein thrombosis, that result in shunting of blood around the liver.158,162 In the series of Victor and colleagues, hepatic disease preceded the appearance of neurological disease in 81% of patients,158 but the severity and frequency of episodes of hepatic encephalopathy are not necessarily predictive of the ultimate neurological deficit.159
In general, CAHD is characterized by the combination of cognitive impairment and abnormalities of movement, but the clinical presentation can encompass considerable variability (Table 114-9). The cognitive features typically consist of executive dysfunction, apathy, slowness in responding, and reduced attention and concentration. Cortical findings, such as aphasia or apraxia, are generally not part of the clinical picture.158,159,163 Victor and colleagues reported cognitive impairment to be present in 80% of the patients they studied.158
TABLE 114-9 Neurological Features of Chronic Acquired Hepatocerebral Degeneration
Abnormalities of movement in CAHD reflect a combination of basal ganglia and cerebellar dysfunction. Dysarthria, ataxia, tremor, parkinsonism, chorea, myoclonus, asterixis, and dystonia all may appear, although dystonia is uncommon.158,159 Action, typically postural, tremor is the most frequently observed type of tremor, whereas rest tremor is distinctly less common.158,159 Mild pyramidal tract findings may be evident in a minority of individuals, but frank myelopathy is rare.158,159
Although the clinical setting and neurological features of CAHD are very similar to those of Wilson’s disease, the two can be distinguished by the absence of family history, the absence of Kayser-Fleischer rings, and the absence of abnormalities of copper metabolism in CAHD.158,159 The hallmark MRI feature of CAHD consists of bilaterally symmetrical hyperintense signal changes on T1-weighted images, most prominently in the globus pallidus, although involvement of the putamen, mesencephalon, and even cerebellum has been reported.159,160,164,165 Manganese deposition is believed to be responsible for these changes.164
Victor and colleagues believed that the course and clinical features of CAHD were progressive and largely irreversible.158 However, both responsiveness to levodopa and resolution of dysfunction after liver transplantation have been reported.114,163
CONCLUSION
Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii.”. Clin Microbiol Rev. 2001;14:561-583.
Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685-691.
Lewis M, Howdle PD. The neurology of liver failure. Q J Med. 2003;96:623-633.
Lossos A, River Y, Eliakim A, et al. Neurologic aspects of inflammatory bowel disease. Neurology. 1995;45:416-421.
Quigley EMM, Pfeiffer RF, editors. Neurogastroenterology. Philadelphia: Butterworth Heinemann, 2004.
1 Camilleri M, Bharucha AE. An overview of gastrointestinal dysfunction in diseases of central and peripheral nervous systems. In: Quigley EMM, Pfeiffer RF, editors. Neurogastroenterology. Philadelphia: Butterworth-Heinemann; 2004:35-57.
2 Quigley EMM, Conklin JL. The “big” brain and the “little” brain: interactions between the enteric and central nervous systems in the regulation of gut function. In: Quigley EMM, Pfeiffer RF, editors. Neurogastroenterology. Philadelphia: Butterworth-Heinemann; 2004:3-14.
3 Quigley EMM, Pfeiffer RF, editors. Neurogastroenterology. Philadelphia: Butterworth-Heinemann, 2004.
4 Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107-116.
5 Wills AJ, Unsworth DJ. The neurology of gluten sensitivity: separating the wheat from the chaff. Curr Opin Neurol. 2002;15:519-523.
6 Farrel RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180-188.
7 Hadjivassiliou M, Boscolo S, Davies-Jones GAB, et al. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58:1221-1226.
8 Hadjivassiliou M, Grünewald RA, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582-1585.
9 Ryan AW, Thornton JM, Brophy K, et al. Chromosome 5q candidate genes in coeliac disease: genetic variation at IL4, IL5, IL9, IL13, IL17 and NR3C1. Tissue Antigens. 2005;65:150-155.
10 Wills AJ, Turner B, Lock RJ, et al. Dermatitis herpetiformis and neurological dysfunction. J Neurol Neurosurg Psychiatry. 2002;72:259-261.
11 De Santis A, Addolorato G, Romito A, et al. Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J Intern Med. 1997;242:421-423.
12 Tutt ANJ, Brada M, Sampson SA. Enteropathy associated T cell lymphoma presenting as an isolated CNS lymphoma three years after diagnosis of coeliac disease: T cell receptor polymerase chain reaction studies failed to show the original enteropathy to be a clonal disorder. Gut. 1997;40:801-803.
13 Pengiran Tengah DSNA, Wills AJ, Holmes GKT. Neurological complications of coeliac disease. Postgrad Med J. 2002;78:393-398.
14 Pellecchia MT, Scala R, Filla A, et al. Idiopathic cerebellar ataxia associated with celiac disease: lack of distinctive neurological features. J Neurol Neurosurg Psychiatry. 1999;66:32-35.
15 Lagerqvist C, Ivarsson A, Juto P, et al. Screening for adult coeliac disease—which serological markers to use? J Intern Med. 2001;250:241-248.
16 Vaknin A, Eliakim R, Ackerman Z, et al. Neurological abnormalities associated with celiac disease. J Neurol. 2004;251:1393-1397.
17 Cooke WT, Smith WT. Neurological disorders associated with adult coeliac disease. Brain. 1966;89:683-722.
18 Hadjivassiliou M, Gibson A, Davies-Jones GA, et al. Does cryptic gluten sensitivity play a part in neurological illness? Lancet. 1996;347:369-371.
19 Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685-691.
20 Bürk K, Bösch S, Müller CA, et al. Sporadic cerebellar ataxia associated with gluten sensitivity. Brain. 2001;124:1013-1019.
21 Bushara KO, Goebel SU, Shill H, et al. Gluten sensitivity in sporadic and hereditary cerebellar ataxia. Ann Neurol. 2001;49:540-543.
22 Luostarinen LK, Collin PO, Peraaho MJ, et al. Coeliac disease in patients with cerebellar ataxia of unknown origin. Ann Med. 2001;33:445-449.
23 Abele M, Schöls L, Schwartz S, et al. Prevalence of antigliadin antibodies in ataxia patients. Neurology. 2003;60:1674-1675.
24 Combarros O, Infante J, Lopez-Hoyos M, et al. Celiac disease and idiopathic cerebellar ataxia. Neurology. 2000;54:2346.
25 Hadjivassiliou M, Davies-Jones GA, Sanders DS, et al. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74:1221-1224.
26 Bushara KO, Nance M, Gomez CM. Antigliadin antibodies in Huntington’s disease. Neurology. 2004;62:132-133.
27 Zelnick N, Pacht A, Obeid R, et al. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004;113:1672-1676.
28 Chapman RWG, Laidlow JM, Colin-Jones D, et al. Increased prevalence of epilepsy in coeliac disease. BMJ. 1978;2:250-251.
29 Holmes GK. Non-malignant complications of coeliac disease. Act Paediatr Suppl. 1996;412:68-75.
30 Cronin CC, Jackson LM, Feighery C, et al. Coeliac disease and epilepsy. Q J Med. 1998;91:303-308.
31 Magaudda A, Dalla Bernardina B, De Marco P, et al. Bilateral occipital calcification, epilepsy and coeliac disease: clinical and neuroimaging features of a new syndrome. J Neurol Neurosurg Psychiatry. 1993;56:885-889.
32 Pfaender M, D’Souza WJ, Trost N, et al. Visual disturbances representing occipital lobe epilepsy in patients with cerebral calcifications and coeliac disease: a case series. J Neurol Neurosurg Psychiatry. 2004;75:1623-1625.
33 Hanly JG, Stassen W, Whelton M, et al. Epilepsy and coeliac disease. J Neurol Neurosurg Psychiatry. 1982;45:729-730.
34 Pengiran Tengah DS, Holmes GK, Wills AJ. The prevalence of epilepsy in patients with celiac disease. Epilepsia. 2004;45:1291-1293.
35 Ranua J, Luoma K, Auvinen A, et al. Celiac disease-related antibodies in an epilepsy cohort and matched reference population. Epilepsy Behav. 2005;6:388-392.
36 Gabrielli M, Cremonini F, Fiore G, et al. Association between migraine and celiac disease: results from a preliminary casecontrol and therapeutic study. Am J Gastroenterol. 2003;98:625-629.
37 Serratrice J, Disdier P, de Roux C, et al. Migraine and coeliac disease. Headache. 1998;38:627-628.
38 Roche Herrero MC, Arcas Martinez J, Martinez-Bermejo A, et al. [The prevalence of headache in a population of patients with coeliac disease]. Rev Neurol. 2001;32:301-309.
39 Knivsberg AM. Urine patterns, peptide levels and IgA/IgG antibodies to food proteins in children with dyslexia. Pediatr Rehabil. 1997;1:25-33.
40 Luostarinen L, Himanen S-L, Luostarinen M, et al. Neuromuscular and sensory disturbances in patients with well treated celiac disease. J Neurol Neurosurg Psychiatry. 2003;74:490-494.
41 Chin RL, Sander HW, Brannagan TH, et al. Celiac neuropathy. Neurology. 2003;60:1581-1585.
42 Kleopa KA, Kyriacou K, Zamba-Papanicolaou E, et al. Reversible inflammatory and vacuolar myopathy with vitamin E deficiency in celiac disease. Muscle Nerve. 2005;31:260-265.
43 Bhatia KP, Brown P, Gregory R, et al. Progressive myoclonic ataxia associated with celiac disease. The myoclonus is of cortical origin but the pathology is in the cerebellum. Brain. 1995;118:1087-1093.
44 Kepes JJ, Chou SM, Price LW. Progressive multifocal leukoencephalopathy with 10-year survival in a patient with nontropical sprue. Neurology. 1975;25:1006-1012.
45 Pereira AC, Edwards MJ, Buttery PC, et al. Choreic syndrome and coeliac disease: a hitherto unrecognised association. Mov Disord. 2004;19:478-482.
46 Gibbons CH, Freeman R. Autonomic neuropathy and coeliac disease. J Neurol Neurosurg Psychiatry. 2005;76:579-581.
47 Jacob S, Zarei M, Kenton A, et al. Gluten sensitivity and neuromyelitis optica: two case reports. J Neurol Neurosurg Psychiatry. 2005;76:1028-1030.
48 Lim WC, Hanauer SB. Emerging biologic therapies in inflammatory bowel disease. Rev Gastroenterol Disord. 2004;4:66-85.
49 Ahmad T, Tamboli CP, Jewell D, et al. Clinical relevance of advances in genetics and pharmacogenetics of IBD. Gastroenterology. 2004;126:1533-1549.
50 Loftus EVJr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517.
51 Philpott DJ, Viala J. Towards an understanding of the role of NOD2/CARD15 in the pathogenesis of Crohn’s disease. Best Pract Res Clin Gastroenterol. 2004;18:555-568.
52 Mathew CG, Lewis CM. Genetics of inflammatory bowel disease: progress and prospects. Hum Mol Genet. 2004;13:161-168.
53 Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991;325:928-937.
54 Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29-34.
55 Christodoulou DK, Katsanos KH, Kitsanou M, et al. Frequency of extraintestinal manifestations in patients with inflammatory bowel disease in northwest Greece and review of the literature. Dig Liver Dis. 2002;34:781-786.
56 Novotny DA, Rubin RJ, Slezak FA, et al. Arterial thromboembolic complications of inflammatory bowel disease. Report of three cases. Dis Colon Rectum. 1992;35:193-196.
57 Rogler G, Scholmerich J. [Extraintestinal manifestations of inflammatory bowel disease]. Med Klin (Munich). 2004;99:123-130.
58 Greenstein AJ, Janowitz HD, Sachar DB. The extraintestinal complications of Crohn’s disease and ulcerative colitis: a study of 700 patients. Medicine. 1976;55:401-412.
59 Lossos A, River Y, Eliakim A, et al. Neurologic aspects of inflammatory bowel disease. Neurology. 1995;45:416-421.
60 Coert JH, Dellon AL. Neuropathy related to Crohn’s disease treated by peripheral nerve decompression. Scand J Plast Reconstr Surg Hand Surg. 2003;37:243-244.
61 Demarquay JF, Caroli-Bose FX, Buckley M, et al. Right-sided sciatalgia complicating Crohn’s disease. Am J Gastroenterol. 1998;93:2296-2298.
62 Greco F, Pavone P, Falsaperla R, et al. Peripheral neuropathy as first sign of ulcerative colitis in a child. J Clin Gastroenterol. 2004;38:115-117.
63 Gondim FA, Brannagan TH3rd, Sander HW, et al. Peripheral neuropathy in patients with inflammatory bowel disease. Brain. 2005;128:867-879.
64 Lloyd DA, Payton KB, Guenther L, et al. Melkersson-Rosenthal syndrome and Crohn’s disease: one disease or two? Report of a case and discussion of the literature. J Clin Gastroenterol. 1994;18:213-217.
65 Summers RW, Harker L. Ulcerative colitis and sensorineural hearing loss: is there a relationship? J Clin Gastroenterol. 1982;4:251-252.
66 Kumar BN, Walsh RM, Wilson PS, et al. Sensorineural hearing loss and ulcerative colitis. J Laryngol Otol. 1997;111:277-278.
67 Weber RS, Jenkins HA, Coker NJ. Sensorineural hearing loss associated with ulcerative colitis. A case report. Arch Otolaryngol. 1984;110:810-812.
68 Hollanders D. Sensorineural deafness—a new complication of ulcerative colitis? Postgrad Med J. 1986;62:753-755.
69 Kumar BN, Smith MS, Walsh RM, et al. Sensorineural hearing loss in ulcerative colitis. Clin Otolaryngol. 2000;25:143-145.
70 Christopoulos C, Savva S, Pylarinou S, et al. Localised gastrocnemius myositis in Crohn’s disease. Clin Rheumatol. 2003;22:143-145.
71 Rang EH, Brooke BN, Hermon-Taylor J. Association of ulcerative colitis with multiple sclerosis. Lancet. 1982;2:555.
72 Pandian JD, Pawar G, Singh GS, et al. Multiple sclerosis in a patient with chronic ulcerative colitis. Neurol India. 2004;52:282-283.
73 Ray DW, Bridger J, Hawnaur J, et al. Transverse myelitis as the presentation of Jo-1 antibody syndrome (myositis and fibrosing alveolitis) in long-standing ulcerative colitis. Br J Rheumatol. 1993;32:1105-1108.
74 Hershkowitz S, Link R, Ravden M, et al. Spinal empyema in Crohn’s disease. J Clin Gastroenterol. 1990;12:67-69.
75 Rajendra S, Kadir ZA, Karim N, et al. Ulcerative colitis and motor neurone disease: causal or coincidental? Singapore Med J. 2003;44:423-425.
76 Talbot RW, Heppell J, Dozois RR, et al. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140-145.
77 Derdeyn CP, Powers WJ. Isolated cortical venous thrombosis and ulcerative colitis. AJNR Am J Neuroradiol. 1998;19:488-490.
78 Musio F, Older SA, Jenkins T, et al. Case report: cerebral venous thrombosis as a manifestation of acute ulcerative colitis. Am J Med Sci. 1993;305:28-35.
79 Aichbichler BW, Petritsch W, Reicht GA, et al. Anticardiolipin antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1999;44:852-856.
80 Papa A, Danese S, Piccirillo N, et al. Thrombopoietin serum levels in patients with inflammatory bowel disease with and without previous thromboembolic events. Hepatogastroenterology. 2003;50:132-135.
81 Oldenburg B, Fijnheer R, van der Griend R, et al. Homocysteine in inflammatory bowel disease: a risk factor for thromboembolic complications? Am J Gastroenterol. 2000;95:2825-2830.
82 Jackson LM, O’Gorman PJ, O’Connell J, et al. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. Q J Med. 1997;90:183-188.
83 Younes-Mhenni S, Derex L, Berruyer M, et al. Large-artery stroke in a young patient with Crohn’s disease. Role of vitamin B6 deficiency–induced hyperhomocysteinemia. J Neurol Sci. 2004;221:113-115.
84 Fukuhara T, Tsuchida S, Kinugasa K, et al. A case of pontine lacunar infarction ulcerative colitis. Clin Neurol Neurosurg. 1993;95:159-162.
85 Druschky A, Heckmann JG, Druschky K, et al. Severe neurological complications of ulcerative colitis. J Clin Neurosci. 2002;9:84-86.
86 Gobbele R, Reith W, Block F. [Cerebral vasculitis as a concomitant neurological illness in Crohn’s disease]. Nervenarzt. 2000;71:299-304.
87 Schluter A, Krasnianski M, Krivokuca M, et al. Magnetic resonance angiography in a patient with Crohn’s disease associated cerebral vasculitis. Clin Neurol Neurosurg. 2004;106:110-113.
88 Johns DR. Cerebrovascular complications of inflammatory bowel disease. Am J Gastroenterol. 1991;86:367-370.
89 Schulak JA, Moossa R, Block GE, et al. Convulsions complicating colectomy in inflammatory bowel disease. JAMA. 1977;237:1456-1458.
90 Rosencrantz R, Moon A, Raynes H, et al. Cyclosporine-induced neurotoxicity during treatment of Crohn’s disease: lack of correlation with previously reported risk factors. Am J Gastroenterol. 2001;96:2778-2782.
91 Akhan G, Andermann F, Gotman MJ. Ulcerative colitis, status epilepticus and intractable temporal seizures. Epileptic Disord. 2002;4:135-137.
92 Hahn JS, Berquist W, Alcorn DM, et al. Wernicke encephalopathy and beriberi during total parenteral nutrition attributable to multivitamin infusion shortage. Pediatrics. 1998;101:E10.
93 Kawakubo K, Iida M, Matsumoto T, et al. Progressive encephalopathy in a Crohn’s disease patient on long term total parenteral nutrition: possible relationship to selenium deficiency. Postgrad Med J. 1994;70:215-219.
94 Eggspuhler AW, Bauerfeind P, Dorn T, et al. Wernicke encephalopathy—a severe neurological complication in a clinically inactive Crohn’s disease. Eur Neurol. 2003;50:184-185.
95 Dutly F, Altwegg M. Whipple’s disease and “Tropheryma whippelii.”. Clin Microbiol Rev. 2001;14:561-583.
96 Weiner SR, Utsinger P. Whipple disease. Semin Arthritis Rheum. 1986;15:157-167.
97 Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81:443-457.
98 Peters G, du Plessis DG, Humphrey PR. Cerebral Whipple’s disease with a stroke-like presentation and cerebrovascular pathology. J Neurol Neurosurg Psychiatry. 2002;73:336-339.
99 Louis ED, Lynch T, Kaufmann P, et al. Diagnostic guidelines in central nervous system Whipple’s disease. Ann Neurol. 1996;40:561-568.
100 Franca MCJr, de Castro R, Balthazar ML, et al. Whipple’s disease with neurological manifestations. Arq Neuropsiquiatr. 2004;62:342-346.
101 Perkin GD, Murray-Lyon I. Neurology and the gastrointestinal system. J Neurol Neurosurg Psychiatry. 1998;65:291-300.
102 Topper R, Gartung C, Block F. [Neurologic complications in inflammatory bowel diseases]. Nervenarzt. 2002;73:489-499.
103 Chan RY, Yannuzzi LA, Foster CS. Ocular Whipple’s disease: earlier definitive diagnosis. Ophthalmology. 2001;108:2225-2231.
104 Schwartz MA, Selhorst JB, Ochs AL, et al. Oculomasticatory myorhythmia: a unique movement disorder occurring in Whipple’s disease. Ann Neurol. 1986;20:677-683.
105 von Herbay A, Ditton HJ, Schuhmacher F, et al. Whipple’s disease: staging and monitoring by cytology and polymerase chain reaction analysis of cerebrospinal fluid. Gastroenterology. 1997;113:434-441.
106 Jover R, Compañy L, Gutiérrez A, et al. Minimal hepatic encephalopathy and extrapyramidal signs in patients with cirrhosis. Am J Gastroenterol. 2003;98:1599-1604.
107 Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42(Suppl 1):S45-S53.
108 Wein C, Koch H, Popp B, et al. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739-745.
109 Quero JC, Schalm SW. Subclinical hepatic encephalopathy. Semin Liver Dis. 1996;16:321-328.
110 Ortiz M, Córdoba J, Alonso J, et al. Oral glutamine challenge and magnetic resonance spectroscopy in three patients with congenital portosystemic shunts. J Hepatol. 2004;40:552-557.
111 Senzolo M, Amodio P, D’Aloiso MC, et al. Neuropsychological and neurophysiological evaluation in cirrhotic patients with minimal hepatic encephalopathy undergoing liver transplantation. Transplant Proc. 2005;37:1104-1107.
112 Mechtcheriakov S, Graziadei IW, Mattedi M, et al. Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Transpl. 2004;10:77-83.
113 Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. A controlled, double blind clinical trial. Am J Dig Dis. 1978;23:398-406.
114 Burkhard PR, Delavelle J, Du Pasquier R, et al. Chronic parkinsonism associated with cirrhosis. A distinct subset of acquired hepatocerebral degeneration. Arch Neurol. 2003;60:521-528.
115 Stewart CA, Reivich M, Lucey MR, et al. Neuroimaging in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3:197-207.
116 Hauser RA, Zesiewicz TA, Rosemurgy AS, et al. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871-875.
117 Lodi R, Tonon C, Stracciari A, et al. Diffusion MRI shows increased water apparent diffusion coefficient in the brains of cirrhotics. Neurology. 2004;62:762-766.
118 Kundra A, Jain A, Banga A, et al. Evaluation of plasma ammonia levels in patients with acute liver failure and chronic liver disease and its correlation with the severity of hepatic encephalopathy and clinical features of raised intracranial tension. Clin Biochem. 2005;38:696-699.
119 Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:188-193.
120 Wang V, Saab S. Ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114:237-238.
121 Lewis M, Howdle PD. The neurology of liver failure. Q J Med. 2003;96:623-633.
122 Haussinger D, Schliess F. Astrocyte swelling and protein tyrosine nitration in hepatic encephalopathy. Neurochem Int. 2005;47:64-70.
123 Jalan R, Shawcross D, Davies N. The molecular pathogenesis of hepatic encephalopathy. Int J Biochem Cell Biol. 2003;35:1175-1181.
124 Balata S, Olde Damink SWM, Ferguson K, et al. Induced hyperammonemia alters neuropsychology, brain MR spectroscopy and magnetization transfer in cirrhosis. Hepatology. 2003;37:931-939.
125 Izumi Y, Izumi M, Matsukawa M, et al. Ammonia-mediated LTP inhibition: effects of NMDA receptor antagonists and L-carnitine. Neurobiol Dis. 2005;20:615-624.
126 Jayakumar AR, Rama Rao KV, Kalaiselvi P, et al. Combined effects of ammonia and manganese on astrocytes in culture. Neurochem Res. 2004;29:2051-2056.
127 Li XQ, Dong L, Liu ZH, et al. Expression of gamma-aminobutyric acid A receptor subunits α1, β1, γ2 mRNA in rats with hepatic encephalopathy. World J Gastroenterol. 2005;11:3319-3322.
128 Fitz JG. Hepatic encephalopathy, hepatopulmonary syndromes, hepatorenal syndrome, coagulopathy, and endocrine complications of liver disease. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger & Fordtran’s Gastrointestinal and Liver Disease. Philadelphia: WB Saunders; 2002:1543-1565.
129 Ahboucha S, Layrargues GP, Mamer O, et al. Increased brain concentrations of a neuroinhibitory steroid in human hepatic encephalopathy. Ann Neurol. 2005;58:169-170.
130 Lozeva V, Montgomery JA, Tuomisto L, et al. Increased brain serotonin turnover correlates with the degree of shunting and hyperammonemia in rats following variable portal vein stenosis. J Hepatol. 2004;40:742-748.
131 Yano M, Adachi N, Liu K, et al. Flumazenil-induced improvement of the central dopaminergic system in rats with acute hepatic failure. J Neurosurg Anesthesiol. 2005;17:69-74.
132 Maddrey WC. Role of antibiotics in the management of hepatic encephalopathy. Rev Gastroenterol Disord. 2005;5(Suppl 1):3-9.
133 Shawcross D, Jalan R. Dispelling myths in the treatment of hepatic encephalopathy. Lancet. 2005;365:431-433.
134 Als-Nielsen B, Gluud L, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. (2):2004. CD003044
135 Als-Nielsen B, Gluud LL, Gluud C. Nonabsorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046-1050.
136 Shawcross DL, Jalan R. Treatment of hepatic encephalopathy. It’s not lactulose. BMJ. 2004;329:112.
137 Blei AT. Treatment of hepatic encephalopathy. Lancet. 2005;365:1383-1384.
138 Morgan MY, Amodio P. Treatment for hepatic encephalopathy: tips from TIPS? Hepatol. 2005;42:626-628.
139 Dursun M, Caliskan M, Canoruc F, et al. The efficacy of flumazenil in subclinical to mild hepatic encephalopathic ambulatory patients. Swiss Med Wkly. 2003;133:118-123.
140 Als-Nielsen B, Gluud L, Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy. Cochrane Database Syst Rev. (2):2004. CD002798
141 Córdoba J, Mínguez B, Vergara M. Treatment of hepatic encephalopathy. Lancet. 2005;365:1384-1385.
142 Malaguarnera M, Pistone G, Astuto M, et al. L-Carnitine in the treatment of mild or moderate hepatic encephalopathy. Dig Dis. 2003;21:271-275.
143 Mascarenhas R, Mobarhan S. New support for branched-chain amino acid supplementation in advanced hepatic failure. Nutr Rev. 2004;62:33-38.
144 Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954.
145 Vanderperren B, Rizzo M, Angenot L, et al. Acute liver failure with renal impairment related to the abuse of senna anthraquinone glycosides. Ann Pharmacother. 2005;39:1353-1357.
146 Syn WK, Naisbitt DJ, Holt AP, et al. Carbamazepine-induced acute liver failure as a part of the DRESS syndrome. Int J Clin Pract. 2005;59:988-991.
147 Dutta U, Mittal S, Ratho RK, et al. Acute liver failure and severe hemophagocytosis secondary to parvovirus B19 infection. Indian J Gastroenterol. 2005;24:118-119.
148 Subramanian V, Shenoy S, Joseph AJ. Dengue hemorrhagic fever and fulminant hepatic failure. Dig Dis Sci. 2005;50:1146-1147.
149 Zwingmann C, Chatauret N, Rose C, et al. Selective alterations of brain osmolytes in acute liver failure: protective effect of mild hypothermia. Brain Res. 2004;999:113-118.
150 Jalan R. Pathophysiological basis of therapy of raised intracranial pressure in acute liver failure. Neurochem Int. 2005;47:78-83.
151 Schiodt FV, Atillasoy E, Shakil AO, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29-34.
152 Blei AT, Olafsson S, Webster S, et al. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet. 1993;341:157-158.
153 Murphy N, Auzinger G, Bernel W, et al. The effect of hypertonic sodium chloride on intracranial pressure in patients with acute liver failure. Hepatology. 2004;39:464-470.
154 Blei AT. The pathophysiology of brain edema in acute liver failure. Neurochem Int. 2005;47:71-77.
155 Liu JP, Gluud LL, Als-Nielsen B, et al. Artificial and bioartificial support systems for liver failure. Cochrane Database Syst Rev. (1):2004. CD003628
156 Mitzner S, Klammt S, Stange J, et al. [Extracorporeal blood purification in severe liver failure with the albumin dialysis MARS—impact on relevant intensive care parameters]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2005;40:199-206.
157 Van Woerkom W. La cirrhose hepatique avec alteration dans les centres nerveux evoluant chez des sujets d’age moyen. Nouvelle Iconographie de la Salpetriere. Clin Maladies Systeme Nerveux. 1914;7:41-51.
158 Victor M, Adams RD, Cole M. The acquired (nonwilsonian) type of chronic hepatocerebral degeneration. Medicine (Baltimore). 1965;44:345-396.
159 Jog MS, Lang AE. Chronic acquired hepatocerebral degeneration: case reports and new insights. Mov Disord. 1995;10:714-722.
160 Wijdicks EF, Wiesner RH. Acquired (nonwilsonian) hepatocerebral degeneration: complex management decisions. Liver Transpl. 2003;9:993-994.
161 Chen W-X, Wang P, Yan S-X, et al. Acquired hepatocerebral degeneration: a case report. World J Gastroenterol. 2005;11:764-766.
162 Saporta MAC, André C, Bahia PRV, et al. Acquired hepatocerebral degeneration without overt liver disease. Neurology. 2004;63:1981-1982.
163 Stracciari A, Guarino M, Pazzaglia P. Acquired hepatocerebral degeneration: full recovery after liver transplantation. J Neurol Neurosurg Psychiatry. 2001;70:136-137.
164 Cakirer S, Karaarslan E, Arslan A. Spontaneously T1-hyperintense lesions of the brain on MRI: a pictorial review. Curr Probl Diagn Radiol. 2003;32:194-217.
165 Park SA, Heo K. Prominent cerebellar symptoms with unusual magnetic resonance imaging findings in acquired hepatocerebral degeneration. Arch Neurol. 2004;61:1458-1460.