17 Neurological Complications of Radiation Therapy
Introduction
Radiation therapy (RT) plays a key role in neuro-oncology but is associated with significant, sometimes life-threatening, neurotoxicity. The development of new techniques (such as radiosurgery or brachytherapy) has widened the indications of RT to include more benign conditions (e.g., trigeminal neuralgia or vascular malformations), and has increased rates of long-term survivors who may develop late toxicity. Thus, complications of RT have gained increasing interest. Nervous tissue tolerance depends on several currently well-defined factors such as volume, total dose, dose per fraction, and duration of irradiation. In addition, other factors that may increase the risk of radiation-induced toxicity are older age, concurrent diseases (such as diabetes, hypertension), vascular disease, adjuvant chemotherapy, and probably genetic predisposition.1,2 The adverse neurological effects of radiotherapy are usually classified according to the time course in relation to irradiation and include acute disorders (days to weeks), early-delayed complications (1 to 6 months) and late-delayed complications (more than 6 months) (Table 17-1). Radiation damage may be direct to the central or peripheral nervous system, or secondary to vascular or endocrine lesions or to the development of a radiation-induced tumor (Figure 17-1).
HISTOPATHOLOGY
Due to the difficulty in obtaining irradiated human tissue, histopathological studies of irradiated brains in human are scarce. However, a few autopsy studies in adults and children are available and show similar features of vascular and demyelinating lesions at light microscopic level. Lai et al.3 studied the brains of five adult patients with primary CNS lymphoma who were in complete remission, but who died after combined modality therapy with WBRT and chemotherapy. The MRIs showed cerebral atrophy, ventricular dilatation, and white matter hyperintensity on FLAIR and T2 images. Occasional enhancing lesions were observed. The corresponding histopathological lesions were myelin and axonal loss, spongiosis, gliosis in white matter, fibrotic thickening of small blood vessels in the deep white matter, and atherosclerosis of the large vessels in the circle of Willis. All patients but one were older than 60, and symptoms of neurotoxicity developed within 3 months of completion of treatment. An autopsy study was performed in 34 children with gliomas, 22 of whom had undergone CNS radiation therapy.4 Causes of death were not detailed in this study and the cognitive status of the children before they died was unknown. Lesions such as demyelination, focal necrosis, cortical atrophy, endothelial proliferation, vascular thrombosis, and vascular thickening were more frequently observed in irradiated brains, whereas neuronal degeneration, cerebral edema, and gliosis were common in irradiated and nonirradiated brains. Demyelination was observed at all time points from 6 months but was more frequent 9 months after radiotherapy. Vascular changes appeared as a late effect of radiation injury. Panagiotakos et al.5 performed an original and extensive study on human normal and irradiated white matter brain samples from surgical biopsies of glial tissue around tumor. Histological assessments of human tissue were completed by ultrastructural analysis and immunohistochemistry. Samples from irradiated patients exhibited persistent loss of oligoprogenitors starting as early as 2 months after radiation, whereas the decline of more mature oligodendrocytes only started beyond a year after irradiation. Early and transitory endothelial cell loss was noted. Myelin sheaths showed signs of degradation. Signs suggesting axonal damage were only seen late after irradiation. Neuronal cell bodies seemed to be spared from radiation injury.
Radiation-induced histolopathological lesions in brain were studied in rats in a few prospective studies, using either only light microscopy analysis or more sophisticated methods such as immunocytochemical and ultrastructural analysis. Kamiryo et al.6 studied histological changes in the rat brain within 1 year of radiation at doses of 50, 75, and 120 Gy, mimicking therapeutic radiosurgery. The authors observed time-dependent and dose-dependent changes. The first lesions to appear were morphological changes in astrocytes, followed by vasodilation and fibrin deposition in capillary walls for all the studied doses. BBB leakage (Evans blue permeability assay) was observed only after 75 and 120 Gy, and necrosis only after 120 Gy. The time course of these lesions varied dramatically with radiation dose: after 120 Gy, the first lesions were observed only 3 days after irradiation and the whole set of lesions occurred within 4 weeks, whereas after 50 Gy, first lesions were only seen at 3 months and developed over 12 months.
An earlier study by Calvo et al.7 focused on radiation-induced damage in the choroid plexus of the rat brain after a single dose of 17.5 to 25 Gy. They observed early morphological changes in the epithelial cells, followed by interstitial fibrosis associated with degenerative changes of arterioles and thrombi. Severity of lesions was dose and time dependent. The same authors later showed that necrosis was more frequent and occurred earlier in the fimbria than in the internal capsule and corpus callosum. They found a correlation between the incidence of necrosis in the white matter, seen after a latent interval of 26 weeks, and earlier changes in the vasculature such as blood vessel dilatation, blood vessel wall thickening, endothelial cell nuclear enlargement, and hypertrophy of perivascular astrocytes.
Fractionated (eight fractions) WBRT (40 Gy) was delivered to adult rats by Yoneoka et al.,8 more closely modeling therapeutic irradiation in humans. Though rats showed radiation-induced cognitive dysfunction, no histological abnormalities were found in irradiated brains over 12 months after irradiation. This study suggested that cognitive dysfunction can either precede morphological changes in the brain, or even arise without them. It is worthy of note that only light microscopic examination was performed. Vascular permeability was not assessed.
Iradiation of the spinal cord led to progressive abnormalities in the white matter, beginning at 19 weeks, for doses greater than 17 Gy. The spinal cord of paralyzed animals showed areas of necrosis and demyelination, but neither gross vascular lesion nor inflammatory infiltrate was found.9 Occasional vessel dilation was observed.
PATHOPHYSIOLOGY
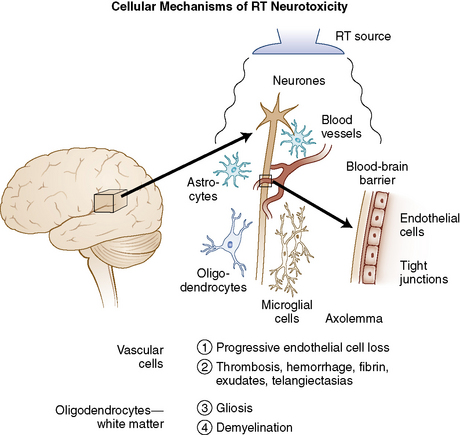
The pathophysiology of white matter abnormalities potentially leading to necrosis are not fully understood, but several hypotheses have been proposed. Because histological examination usually shows vascular lesions and demyelination, vessels and glial cells have often been considered as the primary target of radiation injury (Figure 17-1). However, the situation is probably more complicated: recently, the interactions between radiation and other cell types have been studied more closely, as well as the regenerative abilities of the CNS.10 A short discussion of the main aspects of the pathophysiology of RT-related injuries is described in the following sections.
Vascular Damage
Transient disruption of the blood-brain barrier, possibly initiated by sphingomyelinase-mediated endothelial apoptosis, is thought to be responsible for the acute or early-delayed, steroid-responsive forms of radiation toxicity.11–13 A vascular theory has also been advocated to explain radiation necrosis. Necrosis would be a consequence of ischemia secondary to blood vessel damage. Vascular damage is indeed prominent during radionecrosis, including thrombosis, hemorrhage, fibrinous exudates, telangiectasias, vascular fibrosis/hyalinization with luminal stenosis, and fibrinoid vascular necrosis. Furthermore, a progressive endothelial cell loss and vessel rarefaction seems to precede white matter necrosis.12 Thus, a cascade (involving signaling molecules among others) could be initiated at the time of irradiation, producing gradual cell loss throughout the clinically silent period.3 At the molecular level, VEGF seems to play a pivotal role in the endothelial cell loss.9 This progressive loss would eventually lead to overt necrosis.14 Despite these findings, several points argue against a purely ischemic model: first, neurons are very sensitive to ischemia and should be prominently damaged if the lesion was primarily vascular, whereas neurons are in fact largely spared during radionecrosis.15 Second, vascular damage is not always present in radionecrosis.16 Other cellular mechanisms may be involved and perpetuate vascular damage or contribute to edema, gliosis, and demyelination in the brain, such as upregulation of diverse adhesion molecules,17 production of cytokines,18,19 or cumulative oxidative stress in endothelial cells.
Oligodendrocytes
The demyelinating lesions observed after RT underscore the putative role of oligodendrocytes as a target of radiation damage. Oligodendrocytes are responsible for the production of the myelin sheath in the CNS and derive from progenitor cells such as O-2A. CNS radiation induces depletion of oligodendrocytes and suppresses, at least transiently, the production of oligodendrocyte progenitors,20–22 possibly through a p53-dependent pathway.23–25 However, the contribution of demyelination in tissue destruction remains questionable since severely demyelinating conditions such as multiple sclerosis do not lead to overt necrosis.15
Other CNS Cell Types
Several studies over the past few years have focused on the potential role of neurons, astrocytes, and microglia in the development of radiation-induced lesions, either as primary targets or through alterations of their regulatory capacity.26–28 Particularly noteworthy is the possible role of microglia29 that may enhance radiation injury through persistent oxidative stress.30,31 Radiation damage to the subventricular zone, a tissue containing glial and neural stem cells, was reported more than 25 years ago.32 Recent studies have found a dose-dependent reduction of neural stem cells of the subependyma after irradiation in this region.33,34 Their implication in radiation injury thus seems probable but remains to be elucidated.
Sequelae of Radiation Therapy to the Brain
ACUTE ENCEPHALOPATHY
Acute encephalopathy usually appears within 2 weeks after the beginning of cranial RT, often a few hours after delivery of the first fraction. The patient presents with nausea and vomiting, drowsiness, headache, dysarthria, and a worsening of preexisting neurological deficits, sometimes associated with fever. The clinical course is usually favorable, but herniation and death have been reported in patients with large tumors who have already presented with intracranial hypertension (e.g., in multiple metastases, posterior fossa tumors, or intraventricular tumors). Large doses per fraction (usually >3 Gy/fraction) are the main risk factor: Young et al.35 reported acute radiation damage in 50% of patients with brain metastases treated with 15 Gy in two fractions, and Hindo et al.36 reported four deaths within 48 hours of a 10 Gy RT given in one fraction. As these large doses are no longer in use, this syndrome is rarely seen. However, a minor form of this condition occurs in many patients, consisting of nausea and moderate headache occurring within hours following cranial irradiation. Steroids may help in preventing or limiting the consequences of acute encephalopathy, especially in patients with large primary or secondary brain tumors or with considerable edema, particularly those at risk of herniation. In such patients, daily doses of steroids of at least 16 mg dexamethasone should be prescribed 48 to 72 hr before the first fraction; a limitation of dose per fraction (2 Gy or less per fraction) is also recommended in this situation.37,38 The pathophysiology of acute complications supposedly results from radiation-induced blood-brain barrier (BBB) disruption, accounting for a rise in intracranial pressure.39 To prevent this complication, surgical debulking should, ideally, be carried out before starting RT treatment.
EARLY-DELAYED COMPLICATIONS OF RT
Several early-delayed clinical patterns have been described (Table 17-1). They occur 2 weeks to 6 months after RT. The pathophysiology is thought to be due to a transient demyelinating process triggered by BBB disruption and/or selective oligodendrocyte dysfunction.
Somnolence Syndrome
This condition was first described in the late 1920s in children receiving low-dose RT for scalp ringworm. Several studies reported cases of children who developed somnolence syndrome 5 to 8 weeks after prophylactic cranial RT for leukemia40,41; other reports have shown that it also occurs in adults. The incidence of somnolence syndrome varies greatly (with figures ranging from 8%41 to 84%42; this difference is related to various factors including tumor types, radiation dose, fractionation, and diagnostic criteria).43 Prominent symptoms include drowsiness, hypersomnolence, nausea, and anorexia. Headache and/or fever may also be reported. Friends or family often notice irritability, attentional deficits, and short-term memory impairment. The clinical severity of this complication is variable, with extremes ranging from minimal disorders to sleep periods of over 20 hours a day.41 MRI studies are not contributory. Electroencephalographic abnormalities include nonspecific diffuse slow waves.43 Most studies report a monophasic course of symptoms, usually with a favorable outcome within a few weeks. However, a prospective study on 19 adults treated for primary brain tumors with cranial RT (45 to 55 Gy) reported a biphasic pattern of symptoms with two critical periods (from the 11th to the 21st day and from the 31st to the 35th day) following RT.44 Furthermore, in this study, an accelerated fractionation led to significantly more severe drowsiness and fatigue than a conventional scheme. Some authors advocate the use of steroids during and after radiotherapy as prophylactic or symptomatic treatment.45 A prospective double-blind randomized trial in leukemic children found that a dose of 4 mg/m2 of dexamethasone during cranial radiotherapy reduced the incidence of somnolence syndrome (17.6% vs. 64.3%) as compared to a dose of 2 mg/m2.46
Worsening of Preexisting Symptoms, or Tumor Pseudoprogression
In patients under treatment for malignant brain tumors, a worsening of preexisting neurological focal deficits leads to concerns over tumor progression, especially when observed in association with features of the somnolence syndrome and with transitory cognitive impairment. This complication arises within 6 weeks to 3 months after RT, and is clinically impossible to differentiate from progressive disease. Neuroimaging may be normal or show edema and contrast enhancement within the tumor bed, a situation that does not allow this syndrome to be differentiated from tumor recurrence and explains why inclusion of patients in experimental regimens for “recurrence” is not indicated during this period47; it is worth noting that this radiological pattern can also be associated with no clinical worsening—“pseudoprogression” rates can reach 20% after a concomitant temozolomide and radiotherapy regimen used to treat glioblastoma and may be associated with better tumor response.48 Improvement usually follows within a few weeks or months and a close followup of CT or MR scan will show spontaneous regression within 4 to 8 weeks. As in the somnolence syndrome, the treatment lies in supportive care with steroids. Improvements in imaging techniques such as magnetic resonance spectroscopy (MRS) may help in distinguishing between pseudoprogression and true progression but are limited by the fact that residual tumor is often still present within the irradiated area.
Transient Cognitive Decline
A transient cognitive decline can be observed within the first 6 months after cranial RT, mainly affecting attention and recent memory, and may sometimes be associated with a somnolence syndrome. Armstrong et al.49 prospectively followed five patients treated for primary brain tumors: memory impairment was conspicuous in all patients 1.5 months after focal cranial RT (43 to 63 Gy), but complete regression was observed after 2.5 to 10.5 months. In another prospective study by Vigliani et al.50 comparing 17 patients treated with focal cranial RT (54 Gy) for good-prognosis gliomas and 14 matched control patients who did not undergo RT, 36% of the patients had a significant early-delayed impairment of their reaction test, with a return to normal baseline performance 12 months after RT. This test result was correlated with their occupational status: 69% of the patients could not work at 6 months, whereas 73% had continued or resumed their jobs at 1 year. In our experience, informing patients about this possible difficulty in returning to a normal life (particularly work), at least during the first 6 months following radiotherapy, is useful. Although severe and, in some cases, persistent early-delayed symptoms have been occasionally reported,51 it is of note that transient cognitive impairment does not appear to be a clear-cut prognostic factor predicting the further development of long-term cognitive disorders.
Subacute Rhombencephalitis
Distinct from brainstem radionecrosis, which occurs later, early-delayed subacute rhombencephalitis may be observed about 1 to 3 months after RT using portals involving the brainstem, as in ocular, pituitary, or head and neck tumors. The clinical picture includes ataxia, dysarthria, diplopia, and/or nystagmus as well as auditory loss. In some cases, the cerebrospinal fluid analysis shows inflammatory changes. MRI may demonstrate white matter abnormalities appearing as grossly round or more extensive T1-weighted hypointensities and T2-weighted hyperintensities affecting the brainstem and the cerebellar peduncles; the lesions may enhance after gadolinium injection.52,53 The condition usually improves progressively over a few weeks to a few months, either spontaneously or with steroids, but coma and death have been reported in rare cases.54,55
LATE-DELAYED COMPLICATIONS OF RT
Focal Brain Radionecrosis
Focal radionecrosis constitutes a challenging complication of radiation therapy because it often mimics tumor recurrence and its functional consequences can be devastating. This complication may occur not only in patients who received local RT for a primary or metastasic brain tumor but also in patients without brain lesions with a history of RT for extraparenchymal lesions, in whom normal brain was included in the radiation field (head and neck or pituitary tumors, meningiomas, skull osteosarcomas). A classical example is the bilateral medial temporal lobe necrosis that results from RT for pituitary or nasopharyngeal tumors. This once frequent complication of conventional RT has become rarer over the last 20 years, as a consequence of the generalized use of safer irradiation protocols. It has been shown that the upper limits of a “safe dose” were defined by a total dose of 55 to 60 Gy administered to a focal field with fractions of 1.8 to 2 Gy per day. Vascular risk factors such as diabetes, old age, and associated chemotherapy may also favor the potential development of radionecrosis. However, patients without any particular risk factors may develop radiation necrosis, probably because of an individual, unpredictable sensitivity to irradiation.56 The focal delivery of a single large radiation fraction during “radiosurgery” may also lead to focal necrosis of the brain adjacent to the irradiated lesion. In arteriovenous malformations (AVM), the incidence of brain necrosis ranges from less than 5% to 20% of cases, with location and volume found to be the main risk factors.57–59 After standard RT, radiation necrosis generally occurs within 1 to 2 years,60 but it has also been observed after several decades. As described above, shorter latencies (as short as 3 months) have also been reported, especially in patients treated with interstitial brachytherapy61 or radiosurgery. Patients may experience seizures (first symptom in about 50% of cases), intracranial hypertension, and/or focal neurological deficits.62–64 Such symptoms closely mimic tumor recurrence or progression.
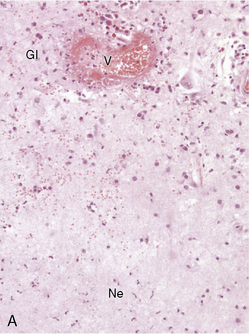
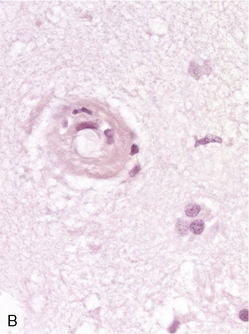
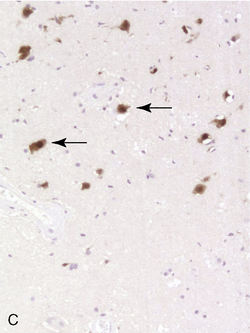
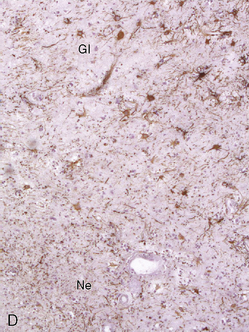
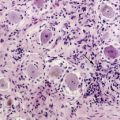
Diagnosing radiation necrosis is often a challenge. In many cases, CT and MR scans show a tumor-like pattern, often indistinguishable from tumor progression or recurrence.65 As diagnostic assessment based on clinical and standard neuroimaging data is difficult, many studies have tried to address the problem of noninvasive differentiation between tumor recurrence/progression and radionecrosis. Positron emission tomography (PET) with 18F-fluorodeoxyglucose66 or 11C-methionine, single photon emission computed tomography (SPECT) with 201thallium or marked methoxy-isobutyl-isonitrile (99mTc-MIBI),67 and, more recently, the use of 3-[123]iodo-alpha-methyl-l-tyrosine (IMT)68,69 have been used to assess the nature of the lesions. In typical cases, radionecrosis is characterized by hypometabolism and tumoral growth by hypermetabolism. The more recent development of magnetic resonance spectroscopy (MRS) seems promising70: spectral analysis of necrosis areas shows an overall, harmonious decrease of metabolite peaks, and a possible increase of lipids corresponding to cellular necrosis; no lactate peak is observed.71,72 However, none of these techniques offers 100% sensitivity or specificity.73,74 One of the reasons for limitations is the frequent coexistence of radiation-induced necrosis with viable tumor tissue within the same area, a situation that obviously renders clear cut distinction impossible.37 In some cases, angiography may provide further information, with an avascular mass seen in patients with radionecrosis; however, the risks and benefits of this procedure must be carefully weighed. While neuropathological examination remains the diagnostic standard (Figure 17-2),75 even pathological analysis may be difficult because of the frequent mixture of both residual/recurrent tumor and radiation necrosis within the lesion.13
Resection of necrotic foci is often the best treatment in symptomatic cases. Steroids are generally used, with possible long-term improvement37; however, steroid dependence does occur. Other treatments have been reported in this setting, but their efficacy has not yet been addressed in large studies: anticoagulants have been prescribed by Glantz et al.76 in eight glioma patients (seven with histological evidence of necrosis) after the failure of steroids, leading to improvement in five patients. In our experience, anticoagulants have been somewhat disappointing. Further assessment of this therapy is required to confirm those results. Some authors have advocated the use of hyperbaric oxygen (HBO), with the rationale that HBO increases the tissue pO2 and enhances angiogenesis. Chuba et al.77 treated ten patients with CNS radionecrosis (proved by biopsy in eight cases) with 100% oxygen at 2.0 to 2.4 atm for 90 to 120 minutes/session in at least 20 sessions. All patients were stabilized or improved initially, and the six surviving patients showed durable improvement after 3 to 36 months. However, most patients were given steroids, and the respective effect of each treatment is not clear. HBO treatment was also reported to improve radionecrosis due to radiosurgery.78,79 The possible role of HBO in radiation-induced neurotoxicity needs to be evaluated in prospective trials.80 Other drugs or combinations such as pentoxifylline, alpha-tocopherol,81 low-iron diet, desferrioxamine, and pentobarbital1 have also been proposed occasionally without definite evidence of efficacy. The usefulness of radioprotective agents such as difluoromethylornithine (DMO),82 U-74389G (a 21 aminosteroid83), or others84–87 in reducing the risk of necrosis remains to be determined. Preclinical studies have suggested that embryonic stem cell-derived glial precursors could be used as myelinating transplants in demyelinated postradiation experimental lesions.88
Several assays have been developed to identify individuals at risk for radiation sensitivity, such as analysis of the survival fraction of cultured skin fibroblasts after 2 Gy irradiation,56 study of radiation-induced chromosomal aberrations, search for the ataxia-telangectasia mutation, and the G2 cell-cycle phase delay analysis.1,89 However, to date, such assessments have not reached the clinical setting.
Cognitive Dysfunction and Leukoencephalopathy
Considering cognitive dysfunction as a consequence of RT alone would result in a considerable overestimation of the incidence of radiation-induced sequelae. Cognitive impairment may be the consequence of complex interactions90 between preexisting cognitive abnormalities (especially in the case of brain tumors), brain tumor growth, concomitant treatments (such as chemotherapy, antiepileptic91 or psychotropic drugs), paraneoplastic encephalomyelitis, and endocrine dysfunction. In practice, this should lead to a precise individual workup in patients with potential radiation-induced cognitive impairment, but also to a careful and critical interpretation of the higher cognitive dysfunction rates found in the literature. Interestingly, a study comparing 195 low-grade glioma patients (104 had undergone RT during the previous years) to low-grade hematological patients and controls, showed that cognitive dysfunction was mostly tumor-related; patients treated with doses per fraction over 2 Gy were the only ones to develop RT-linked memory impairment.92 A more recent study of these patients followed up for 12 years has shown a progressive cognitive decline in attentional and executive tasks in patients treated with radiotherapy fractions less than 2 Gy/fraction compared with unirradiated patients, which was not apparent after 6 years follow-up.92a
Recent studies seem to confirm that RT plays a limited part in cognitive decline when using modern irradiation procedures.93–95 Nevertheless, several factors have been clearly linked to an increased risk of leukoencephalopathy (Figure 17-3):
Methotrexate (MTX) is clearly implicated in combined toxicity; neurotoxic by itself, it is responsible for frequent cognitive dysfunction when associated with RT. In children, most studies to date have concluded that the combination of cranial irradiation and intrathecal MTX was associated with declines in both IQ and achievement scores.103 In adults treated with WBRT (40 Gy + 14 Gy boost) and a combination of intravenous and intrathecal MTX for CNS lymphoma, the incidence of severe progressive cognitive impairment increases with age, reaching 83% in patients over 60 years.104,105 The timing of MTX chemotherapy is important, because the cognitive dysfunction rate is higher whenever MTX is prescribed during or after RT. This drug should therefore be given before irradiation. Data about the neurotoxicity of other combinations are sparse, but agents such as nitrosourea, cisplatin, etoposide, cytarabine, or actinomycin D are also suspected to increase radiation-induced cognitive toxicity. Multidrug regimens or high-dose chemotherapy combined with WBRT are probably associated with a higher risk of neurotoxicity.106 Although there is a progressive continuum between mild to moderate cognitive impairment and severe fatal dementia, we will consider the two conditions separately.
Radiation-Induced Mild to Moderate Cognitive Impairment
A mild to moderate cognitive dysfunction is more frequent in long-term survivors than real dementia. The features of this condition are not perfectly defined, as results greatly vary according to the studies, probably due to neuropsychological evaluation procedures, duration of follow-up, and population discrepancies.107
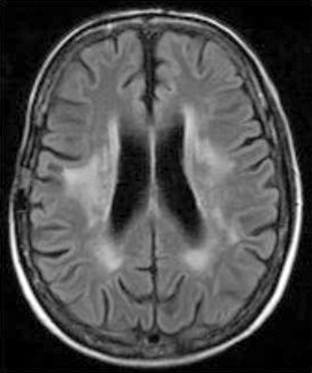
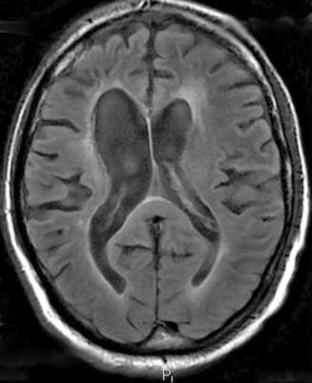
In most reported cases, cognitive impairment affects mainly attention and short-term memory, while intellectual functions are generally preserved as assessed by neuropsychological evaluations. Nevertherless, most patients have to decrease or even discontinue their professional activities. CT scan may be abnormal, showing periventricular hypodensities, an increase in the normal interface between white and gray matter, and ventricular enlargement. However, there seems to be no correlation between CT-scan abnormalities and the degree of cognitive impairment. MRI shows variable degrees of T2-weighted hyperintensities in the white matter, with a gross correlation between neuropsychological status and white matter lesions (Figures 17-3 and 17-4).108
The course of the disease is difficult to predict: some patients deteriorate slowly while the majority apparently remains stable. Progression to dementia is seldom reported. There is no recognized treatment for this syndrome although some authors have advocated the use of methylphenidate for symptomatic relief.109 More recently, anticholinesterase drugs have been used with encouraging results.110 Data from animal studies have also shown that the administration of erythropoietin (EPO) may prevent cognitive impairment.111
Free-radical scavengers, such as amifostin or angiotensin-converting enzyme inhibitor (ACEi) could have a protective effect.111b Amifostin is already under clinical investigation with controversial results,111c but to date, despite encouraging preclinical data, no trial has been conducted with ACEi.
Radiation-Induced Dementia
The incidence of this devastating complication varies widely in the literature (from 0% to more than 60%) according to the series. In a large review of several studies comprising 748 adult patients, the incidence of severe cognitive impairment compatible with dementia was at least 12.3%.112 More recent studies and clinical practice provide less cause for alarm.30,113
The clinical picture is characterized by a “subcortical dementia” pattern that probably reflects the consequences of diffuse white matter injury, occuring in 69% of patients within 2 years of radiotherapy.107
Patients present with progressive memory and attention deficits, intellectual loss, gait abnormalities, emotional lability, apathy, and fatigue.114 The absence of hallucinations or delirium and the very unusual occurrence of aphasia, agnosia, or apraxia (deficits suggesting cortical involvement) are important clinical features for narrowing the differential diagnosis, especially in elderly patients. Depression is frequent, but antidepressants do not improve cognitive function. Eventually, patients may develop gait ataxia, incontinence, and sometimes a picture of akinetic mutism. Nonspecific features such as seizures, pyramidal or extrapyramidal signs, or tremor are also frequently encountered in the course of the disease. Neuroimaging always shows diffuse white matter lesions, best seen on MRI as T2-weighted hyperintensities, associated with cortical and subcortical atrophy as well as ventricular enlargement (Figure 17-4). When performed, the lumbar puncture usually shows normal to moderately elevated (< 1 g/l) CSF protein levels.
No specific treatment is currently able to cure radiation-induced dementia. However, as the clinical features are similar to those of normal-pressure hydrocephalus, some authors have advocated ventriculoperitoneal shunting; this procedure does improve the quality of life in a few selected patients.30,115,116
Deterioration occurs in about 80% of cases, leading to the death of the patient; stabilization is possible (18% of cases). Lasting improvement is exceptional. Death generally occurs within 1 to 48 months after the onset of the disorder.102
Radiation-Induced Brain Tumors
The precise role of RT in the development of a tumor is difficult to determine and cannot be assessed with certainty, in great part because these tumors have no distinctive features compared to unirradiated patients. However, data from animal and epidemiological studies indicate that irradiated patients or animals are more likely to develop a second brain tumor than would have been expected from the control data.117
The relative risk of developing a radiation-induced tumor has been studied in several large studies. A study by Ron et al. of 10,834 patients treated with low-dose cranial and cervical irradiation for Tinea capitis (mean dose to neural tissue: 1.5 Gy) showed a relative risk of developing a tumor of 6.9; the risk for glioma was 2.6.118
In another study of 10,106 survivors of childhood cancers by the British Childhood Cancer Research Group,119 the relative risk of developing a secondary CNS tumor was 7. Most other studies have found similar results.120,121 However, the risk is probably higher in patients treated for acute lymphoblastic leukemia (ALL): a large retrospective cohort study of 9720 children122 found a relative risk of developing a nervous system tumor as high as 22. In another setting, a large study on second brain tumors in 426 patients with pituitary adenoma treated with surgery and radiotherapy showed a 2.4% risk at 20 years.121
Criteria for a radiation-induced tumor include: (a) long interval between radiotherapy and the occurrence of the second tumor (the mean onset delay is 12 years, with cases ranging from 1 to 40 years); (b) tumor growth within the radiation portal or at its margins; (c) a different histological subtype. After stereotactic radiosurgery, radiation-induced neoplasms are extremely rare, with only four reported cases in the literature.123 Three types of tumors have been reported to be linked with cranial irradiation: meningioma in about 70% of cases, glioma in 20%, and sarcoma in fewer than 10%. More than 300 cases of radiation-induced meningiomas have been reported in the literature; female predominance is less prominent than in spontaneous meningiomas.124 The risk of occurrence correlates with radiation dose: low-dose RT induced a relative risk of 9.5 in one study,118 whereas high-dose RT was linked to a relative risk of 37.120 The tumor emerges after a long latency period: a review found extremes ranging from 2 to 63 years (mean 18.7 years) after high-dose RT.125
Most patients (68%) had been irradiated during childhood, and the interval between RT and the onset of the tumor was shorter in younger patients; however, the radiation dose did not influence the latency. Radiation-induced meningiomas are often multiple and recurrent with malignant histological features.126,127 Unlike sporadic meningiomas, NF2 gene inactivation and chromosome 22q deletions seem to be less frequent in radiation-induced meningiomas, while other chromosomal lesions (especially loss of 1p) possibly induced by irradiation, may be more important in the development of these tumors.128,129
Radiation-induced gliomas are much less frequent. Since 1960, about 120 cases have been reported in the English literature;130,131 fewer than half of them were glioblastomas. In the group of patients treated with RT for acute leukemia, multifocality occurred in 20% of cases. The median delay of onset ranges from 6 to 9 years.131 The prognosis of these tumors is poor: intrinsic resistance to treatment as well as previously received aggressive therapies considerably limits the applicable therapies.
Molecular alterations are apparently the same in sporadic and radiation-induced gliomas.132 Fewer than 40 cases of sarcomas have been reported to date, different and they consist of several histological types (e.g., gliosarcomas, meningiosarcomas, neurofibrosarcomas).117
Radiation Vasculopathy
Large and Medium Intracranial and Extracranial Arterial Injury
An arteriopathy affecting the large cervical blood vessels, especially the carotid artery,133 may be a complication of cervical radiation therapy, usually administered for lymphomas or head and neck cancers. Intracranial vessels may also be affected. The main early-delayed vascular complication is carotid rupture,134,135 which usually follows a few weeks after cervical RT and surgery for head and neck tumors. Associated skin lesions such as necrosis or wound infection are common. The outcome of this exceptional complication is, of course, very poor.
Late-delayed complications are more frequent, and generally occur many years after RT (median time about 20 years for extracranial, 7 years for intracranial artery lesions). The lesions are similar to those induced by atherosclerosis, but are often located in unusual places for common atherosclerosis and occur in an accelerated fashion. It has been observed that the larger the diameter of an irradiated artery, the longer the latency between RT and the onset of vasculopathy, a fact that might explain the shorter latency of RT-induced vasculopathy in children. Shorter latencies have also been reported with interstitial radiotherapy.136 The dose required to induce vascular lesions usually exceeds 50 Gy, but the type of irradiation, fractionation, and portal differs greatly from one case to another. The lesions consist of one or more stenoses or occlusions in the arteries included within the radiation portal. The diagnosis, suspected when a cervical murmur is heard in the immediate vicinity of radiation-induced skin lesions, relies on magnetic resonance angiography, ultrasound examination and arteriography.
The treatment is similar to that of usual atherosclerotic lesions; in the event of carotid stenoses, endarterectomy may be appropriate. However, surgery may be more difficult than in unirradiated patients because of vascular fibrosis and skin lesions, with higher postoperative risk of infection or healing problems. In other patients, antiplatelet agents may be prescribed if there is no contraindication. Some authors have advocated lowering serum cholesterol levels to prevent the development of such lesions in patients at risk.137
Radiation-Induced Vasculopathy with Moyamoya Pattern
Intracranial vasculopathy leading to a progressive occlusive disease and a moyamoya pattern (characterized by abnormal anastomoses and netlike blood vessels), accounting for focal seizures, strokes, or transient ischemic attacks, may follow intracranial irradiation, especially in very young children. This complication is particularly frequent in children treated for optic chiasm glioma, a condition often associated with neurofibromatosis type 1 (NF-1, which is a risk factor for vasculopathy itself). It may also occur with other tumors such as brainstem glioma and craniopharyngioma.138 In a series139 of 69 children (11 with NF-1) treated for optic pathway glioma with RT (median dose 55 Gy), 13 (19%) developed clinical and radiological signs of vasculopathy after a median latency of 36 months. The strong association between NF-1 and moyamoya is one of the reasons why radiation has been replaced with chemotherapy in younger children.113 The treatment focuses on preventing further strokes through surgical revascularization techniques; calcium blockers such as flunarizine have been advocated by some authors.140–143 The role of antiplatelet agents has not been defined in this setting.
Silent Lacunar Lesions
One report144 described a rare pattern of silent cerebral lacunes occurring in children treated for brain tumors. In this study reviewing 524 consecutive children, 5 of 421 treated with RT and chemotherapy had lacunes. Patients were a median of 4.5 years old at the time of the diagnosis and RT, and developed lacunes after a median latency of 2 years (ranging from 0.26 to 6 years). This pattern was associated with no further clinical deficit or neuropsychological impairment when compared to patients without lacunes. This condition is probably linked to delayed radiation-induced capillary and small vessel lesions.
Radiation-Induced Cavernomas, Angiomatous Malformations, and Aneurysms
Brain vascular malformations such as telangiectasias and cavernomas124,145,146 have been rarely observed following RT. Ocular telangiectasias may also occur.147 When present, their main risk is intracranial bleeding.
Several cases of multiple radiation-induced cavernous angiomas have also been reported,148 occurring 18 months to 23 years after RT.
Finally, fewer than 15 cases of radiation-induced intracranial aneurysms have been described in the literature.149,150 The median age of the patients was 37.5 years (ranging from 11 to 65 years) and a latency of 10 months to 21 years, with no correlation between the onset of aneurysms and the radiation dose. This represents a rare but potentially severe problem, as rupture is always possible; six of nine ruptured aneurysms proved fatal. A growing aneurysm can also mimic tumor recurrence. Aneurysms are sometimes detected preclinically with the usual imaging procedures for tumors (CT scan and MRI), as was stressed by Azzarelli et al.,151 and particular attention should be drawn to evaluating the onset of such lesions during imaging follow-up. When an aneurysm is detected on CT or MR scan, or if the clinical history strongly suggests its presence, cerebral angiography is required for delineation.
Endocrine Dysfunction
Frequently underestimated,113,152 endocrine disorders can be the consequence of direct irradiation of a gland (e.g., the thyroid gland, with about 50% of patients developing hypothyroidism within 20 years following radiotherapy for Hodgkin disease or certain head and neck cancers) or result from hypothalamic-pituitary dysfunction secondary to cranial irradiation (several authors believe that the hypothalamus is more radiosensitive than the pituitary gland).153 We will focus on the second type of disorder, which can be induced by cerebral or nasopharyngeal tumor irradiation.
There is a positive correlation between radiation dose and the incidence of endocrine complications. In a prospective study of 268 patients treated with different RT schemes to the brain, Littley et al. found that, 5 years after RT, the incidence of TSH deficiency was 9% after treatment with 20 Gy, 22% with 35 to 37 Gy, and 52% with 42 to 45 Gy.154 A hormonal deficit can appear at any time after RT, but may arise more rapidly in patients treated with higher radiation doses.155
In children, varied endocrine deficits may result from cranial RT (administered for brain tumors or during prophylactic irradiation in acute lymphoblastic leukemia). Growth hormone (GH) is usually the first, and in many cases the only, anterior pituitary deficit in young patients. This complication affects about 50% of children treated with prophylactic cranial RT for acute lymphoblastic leukemia.156
According to a recent Danish study of 73 children treated with RT for a primary brain tumor (not involving the hypothalamo-pituitary axis directly) and with a long follow-up (median 15 years), 80% of patients manifested growth hormone deficiency; the median biological effective dose (BED) in the hypothalamo-pituitary area was higher in GH-deficient children than in patients without GH deficiency.157 Administration of GH is recommended in children with growth hormone deficiency but, as it has no effects on vertebral bodies, long-term survivors acquire a typical “spiderlike” physical appearance with long extremities and short trunk.113 A subtle central hypothyroidism is common in children and should be treated with thyroxine replacement therapy in order to limit the potential for thyroid carcinoma as well as to improve longitudinal growth.158,159
In adults, a recent study160 evaluating 31 long-term brain tumor survivors, followed 1.5 to 11 years after RT with a mean total dose of 62.3 ± 2.8 Gy, compared with 31 age- and sex-matched controls, found hypothalamic hypothyroidism in 26% of patients, hypothalamic hypogonadism in 32% of patients, hyperprolactinemia in 29% of patients, and panhypopituitarism in one patient. Low adrenal hormone levels were found in most patients, but without apparent clinical consequence. In the control group, only 6% had a baseline hormonal concentration outside the normal range. None of the controls had two or more hormonal abnormalities, while 42% of the patients had multiple deficits. Only 23% of patients had normal thyroid, gonadal, and adrenal baseline levels; this result is consistent with another earlier study by Taphoorn et al. reporting hypothalamic-pituitary dysfunction in 10 of 13 (77%) long-term survivors irradiated for supratentorial low-grade glioma.161 Another study of patients treated for nasopharyngeal cancer found secondary hypothyroïdism in 27% of cases (of hypothalamic origin in 19% and pituitary origin in 8%).162
Secondary hypogonadism is an important concern especially in male patients, responsible for a decrease in libido and sometimes impotence, and impacting negatively on quality of life. Hyperprolactinemia of hypothalamic origin is a notable concern in women who develop oligo-menorrhea and galactorrhea163; in men, it may result in gynecomastia and a decrease in libido.
The follow-up consultations are a good place for a regular clinical endocrine evaluation; the precise biological follow-up scheme is debated and is adapted according to the emerging deficits, but long-term assessment should be the rule. The treatment of hormonal deficits lies in replacement therapy, and usually leads to an improvement in the patient’s condition. Bromocriptine has been utilized with success in patients with symptomatic hyperprolactinemia.117
SEQUELAE OF RADIOTHERAPY TO THE SPINAL CORD
Damage to the spinal cord may be the consequence of RT administered for spinal cord tumors, Hodgkin disease, mediastinal or head and neck cancers. Early descriptions in the 1940s164 were followed by numerous descriptions of postradiation myelopathy delineating the main clinical patterns, that is, early-delayed myelopathy and several types of late-delayed complications including progressive myelopathy, lower motor neuron disorder, and spinal hemorrhage. There is no clear clinical or experimental evidence of acute spinal cord toxicity due to RT, and a sudden worsening during irradiation should lead to a search for intratumoral hemorrhage or tumor progression.37
EARLY-DELAYED (TRANSIENT) RADIATION MYELOPATHY
The onset of this complication occurs from 6 weeks to 6 months after RT, and improvement follows in most cases within 2 to 9 months,165 though persistence of the symptoms for a longer time is possible in rare cases. It usually follows radiation to the cervical or thoracic spinal cord. After mantle RT for Hodgkin disease, early-delayed myelopathy occurred in 15% of cases.166 In another study, Fein et al. found a global incidence of 3.6% (40 cases among 1112 patients receiving 30 Gy or more). The incidence was 8% in the group of patients receiving 50 Gy or more, 3% after doses of 45 to 49.9 Gy, 4% after doses of 40 to 44.9 Gy, and 2% after doses of 30 to 39.9 Gy. The risk was also increased with a fraction size over 2 Gy.167
The clinical pattern first described by Esik et al.168 generally consists of Lhermitte phenomenon, triggered by neck flexion, and characterized by brief unpleasant sensations of numbness, tingling, and/or often electric shock–like feelings from the neck to the spine and extremities. There are no MRI changes associated with this condition. This symptom is nonspecific, and other causes should be considered in a patient with cancer,169 including chemotherapy (cisplatin or docetaxel), spinal tumor, vitamin B12 deficiency, herpes zoster, or even multiple sclerosis (which may be aggravated by irradiation).
The presumed pathophysiology of early-delayed myelopathy is transient demyelination, probably secondary to a loss of oligodendroglial cells following RT.170,171 There is no specific treatment for this condition, and none is required, as recovery occurs in most cases. Early-delayed spinal cord disorder is not predictive of evolution to the much more serious progressive myelopathy.
LATE-DELAYED RADIATION-INDUCED SPINAL CORD DISORDERS
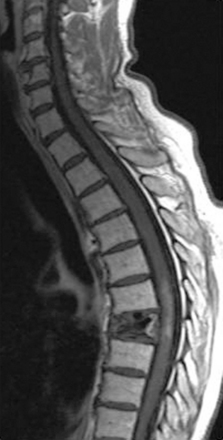
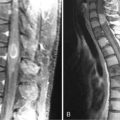
Spinal radionecrosis (Figure 17-5) (with features similar to its cerebral counterpart), progressive myelopathy, and spinal hemorrhage have been described as late complications of spine radiation.
Progressive Myelopathy, or Delayed Radiation Myelopathy (DRM)
This complication occurs 6 months to 10 years after exposure to RT. Risk factors include advancing age, large radiation doses and fractions, previous irradiation especially in childhood, and large portals involving thoracic or lumbar spinal cord.165 Chemotherapy may increase the risk of delayed radiation myelopathy,172 but data are still unclear on this point.
The generally accepted tolerance for the spinal cord is 45 Gy in 22 to 25 daily fractions, with a risk of less than 1% for a dose of 50 Gy, increasing to 5% for a dose of 60 Gy delivered in 1.8 to 2 Gy fractions.173
The diagnosis of delayed radiation myelopathy implies—as was underlined as early as 1961 by Pallis et al.174—that the site of the main lesion is within the radiation-exposed area of the spinal cord and that all other potential causes of myelopathy have been carefully reviewed and eliminated.
Spinal cord MRI is helpful, though nonspecific. The initial description of Wang et al.175,176 has been confirmed in several subsequent studies177–179: the initial MRI may be normal if performed during the first weeks of the disease, but a slightly delayed examination usually reveals a swollen cord with T1-weighted hypointensity and T2-weighted hyperintensity. Lesions enhance in about 50% of cases after gadolinium injection (Figure 17-5).180,181 In contrast, late examinations, performed years after the onset of the disease, may show spinal cord atrophy without any signal abnormality; a case of cystic formation in late delayed radiation myelopathy has also been reported.
Corticosteroids may improve some patients, probably because of their action on the inflammatory and edematous components of the disorder; however, patients often become steroid-dependent and only a few experience long-term improvement. There is no current proven long-term treatment for delayed radiation myelopathy. However, Angibaud et al. have reported the efficacy of hyperbaric oxygen in stabilizing or improving six out of nine patients with DRM,182 and Calabro et al. recently reported a similar case.183 Anticoagulation has also been tried, with improvement in one patient with myelopathy treated for over 3 months with full anticoagulation, and stabilization in another treated with coumarin.76
Late-Delayed Spinal Hematoma
This rare complication has only been described in a few cases, following spinal radiotherapy by 6 to 30 years, and occurring within the radiation portal but outside the location of the primary tumor184; acute-onset leg weakness and back pain rapidly lead to paraparesis or tetraparesis. The diagnosis relies on MRI demonstration of hemorrhage.
Sequelae of Radiotherapy on the Cranial Nerves
Apart from acute reversible radiation toxicity, any of the cranial nerves may be involved in late-delayed radiation-induced complications if included in the radiation portal. These complications are rare, probably arising in fewer than 1% of cases after conventional radiotherapy (60 Gy, 2 Gy per daily fraction). Large daily radiation fractions increase this risk. However, a recent study in brain tumors survivors found that 17% of patients developed neurosensory impairment and that RT exposure greater than 50 Gy to the posterior fossa was associated with a higher likelihood of developing any hearing impairment.185 The main complications are described below:
OLFACTORY NERVE INJURY
During cranial radiation, patients may describe reversible sensations of smelling an odor.186 This may be due to direct acute stimulation of the olfactory neurons. Anosmia has also been described in some patients,187,188 often associated with taste disorders.189
OPTIC NEUROPATHY
Probably facilitated by preexisting lesions (e.g., in diabetic patients), optic neuropathy may occur 6 months to 14 years after radiation therapy for a tumor of the orbital, pituitary, or suprasellar regions.190 In one study, the incidence of retrobulbar optic neuropathy was 3.8% after a conventional radiation scheme for head and neck cancer.191 Optic neuropathy can also overshadow the prognosis of patients treated with high-energy electron beam therapy for age-related macular degeneration in up to 19% of cases.192
Proton beam irradiation, currently proposed in the treatment of several tumors including meningioma and choroidal melanoma, may also induce this complication.193 The classical pattern consists of progressive or sometimes acute-onset visual loss, leading to monocular or binocular blindness with optic atrophy.194 This disorder is painless. In the case of anterior lesions, the ocular fundus usually shows papilledema and prepapillary and premacular hemorrhage, sometimes associated with radiation-induced retinal lesions. In contrast, fundoscopy may be normal if the lesions are posterior. In those cases, brain MRI may be useful, demonstrating enlargement of the optic nerve and chiasma, with T2-weighted hyperintensities and contrast enhancement.
Demyelination, axonal loss, gliosis, and modifications of the vessel walls characterize these lesions histologically; endothelial cell loss has recently been stressed in this setting, with more significant abnormalities in patients treated with high-dose (55 to 70 Gy) compared with those treated with low-dose (10 Gy or less) radiation therapy.195 These lesions are irreversible in many patients. Steroids and anticoagulants186 have been advocated in chiasmatic lesions, with inconsistent results, while the use of hyperbaric oxygen in optic neuropathy remains controversial.197–199 Optic nerve sheath fenestration has been attempted with some success in a few patients.200
ACEi may have a protective role in the optic nerve independent of its antihypertensive effect. ACEi given 2 weeks after stereotactic brain irradiation of rats significantly reduced radiation-induced optic neuropathy electrophysiologically in terms of the visual evoked-potential response to light and morphologically in terms of quantitative changes in myelin contents and axons in the optic nerves and chiasm.200b
OCULAR MOTOR NERVE INJURY
Rarely reported, the involvement of ocular motor nerves may be associated with optic neuropathy. The most frequent of these palsies affects the abducens nerve.37 Transient ocular motor palsies have been reported following radiation schemes focused on the pituitary tract, but permanent palsies have also been described after radiation therapy of nasopharyngeal carcinoma. Possible regression suggests that a demyelinating process may be involved, rather than progressive fibrosis.
Neuromyotonia is a late-delayed complication following RT to the sella turcica or the cavernous sinus region by several years, and is characterized by spontaneous spasms of the eye muscles responsible for episodes of transitory painless diplopia, usually lasting a few seconds; these episodes can occur up to several times an hour.201 Membrane stabilizers such as phenytoin or carbamazepine may improve this disorder. Radiation-induced hyperexcitability of the nerve fibers may underlie the pathophysiology.
TRIGEMINAL NERVE DYSFUNCTION
Involvement of the trigeminal nerve is quite rare. Neuromyotonia in the trigeminal distribution is exceptionally encountered; treatment with carbamazepine is effective in this condition.202,203 After gamma knife radiosurgery for trigeminal neuralgia, the main reported complication to this day is mild facial numbness occuring in 2.7% to 14% of patients.204–206 Trigeminal neuropathy can also result from radiosurgery for vestibular schwannoma.207
FACIAL NERVE INJURY
The different branches of the facial nerve are not equally affected by radiation. Taste dysfunction is usual and many patients complain of aguesia, a symptom that may be permanent in up to 50% of patients irradiated with 50 to 60 Gy for head and neck tumors.208 However, taste disturbances are a common feature in cancer patients, and chemotherapy may play a part.209
Motor deficit is almost never a consequence of fractionated RT and should prompt a search for tumoral invasion.37 In contrast, radiosurgery has been reported to account for possible facial palsy; however, the relatively high initial figures of facial weakness after treatment for vestibular schwannoma210,211 seem to have much decreased, being currently under 5% of cases.206,212
ACOUSTIC NERVE DYSFUNCTION
The diagnosis is often easy, as otoscopic examination reveals fluid behind the tympanic membrane. Usually spontaneously regressive, otitis media can, in some cases, require myringotomy for symptom alleviation. In most cases, relief can be obtained by prescribing nasal vasoconstriction agents. Late-delayed hearing loss may result from damage to the organ of Corti with subsequent acoustic nerve atrophy; however, a report underlines the relative resistance of the organ of Corti to radiation. The precise histological pattern of these disorders is not known; however, in previous studies, the labyrinth has been shown to be damaged.213,214
The consequences to hearing of radiosurgery for vestibular schwannoma have been better assessed over the last few years.212,215–218 In a recent report,219 14% of patients with measurable hearing before treatment became deaf after radiosurgery, and 42% of patients had an elevation of their pure tone threshold of 20 dB or more. The risk factors for hearing loss in this study included neurofibromatosis type 2 (NF-2), history of prior surgical resection, and tumor size.
LOWER CRANIAL NERVE INVOLVEMENT
These nerves (glossopharyngeal, vagus, spinal accessory, and hypoglossal nerves) can be damaged after head or neck radiation therapy with large doses. Complications occur earlier the larger the radiation dose and typically arise months to years after the treatment. The pathophysiology is likely radiation fibrosis. The hypoglossal nerve is the most commonly involved lower cranial nerve220; the patient may present with unilateral, often asymptomatic tongue paralysis,221–223 or with bilateral and disabling paralysis. This complication may occur many years after RT.222
Longstanding paralysis is responsible for tongue atrophy, with asymmetry that may be associated with fatty infiltration or edema-like changes on MRI.224,225 Paralysis of the vagus nerve leads to unilateral paralysis of the vocal cord and of the palate, responsible for difficulties in swallowing.226 Horner syndrome may be associated with these disorders, resulting from injury to the sympathetic fibers.37 Lesions of the spinal accessory nerve lead to shoulder drop which is diagnosed during the clinical examination. Patients may also present with multiple lower cranial nerve palsies.227 Cranial nerve palsies may occur during skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma.228
Consequences of Radiotherapy on the Peripheral Nervous System
DROPPED HEAD SYNDROME
Dropped head syndrome has been recently described as a potential late-delayed complication of RT.229 These patients usually develop weakness of neck extensors several years after irradiation involving the cervical region, for example, mantle irradiation for Hodgkin lymphoma. Clinical examination reveals an amyotrophic deficit of neck muscles, often extending to other muscles innervated by upper cervical roots, without any impairment of sensation. The differential diagnoses include myasthenia gravis, ALS, and inflammatory myopathies. The mechanism and precise location of the causative lesions are unclear.
BRACHIAL PLEXOPATHY
Early-Delayed Brachial Plexopathy
This complication occurs a median of 4.5 months after RT, with a range of 2 to 14 months. Its incidence is about 1% to 2% after irradiation for breast cancer.230,231 The clinical pattern includes paresthesia in the hand with a distal motor deficit; amyotrophy and fasciculations may be present at later stages. Axillary pain is reported in about 60% of cases; always moderate, it may be spontaneous or occur with movement. Although a progressive course towards paralysis of the brachial plexus is possible, complete improvement is the rule, in most cases after 3 to 6 months. Electrophysiological investigations show a decrease in nerve conduction velocities. The pathophysiology of this condition is not fully understood; a direct radiation toxicity on the Schwann cells inducing demyelination has been invoked.232
Late-Delayed Brachial Plexopathy
Delayed radiation-induced brachial plexopathy appears after a median time of 40 months (up to 20 years).233 Its incidence varies widely in the literature, with figures ranging from 14% to 73%,234 the most important risk factors being total radiation dose (> 60 Gy) and fraction size (> 2 Gy). Overlap of radiation fields has also been incriminated as well as combined radio-chemotherapy. For example, Olsen et al. found a definite or probable radiation plexopathy in 42% of patients treated with chemotherapy (cyclophosphamide, tamoxifen, or a combination of cyclophosphamide, methotrexate and 5-fluorouracil) and RT, versus 26% in patients treated with RT alone.235
The pathophysiology is unclear and may have biphasic conponents: during the first phase, direct radiation damage to the nerves may cause electrophysiological and histochemical changes; later on, injury to the small vessels and fibrosis around the nerves may account for severe nerve injury.236
The disorder is usually progressive. Initial symptoms include distal paresthesias (typically, pins-and-needles or numbness of the thumb and first finger) and mild sensory deficit on clinical examination, often with no clear radicular topography. The other initial findings consist of some degree of amyotrophy and an early abolition of reflexes. Proximal weakness is found in about a quarter of cases.237,238 Visible myokymia, when present, is quite suggestive of the diagnosis. The examination may also show local complications of radiotherapy, such as radiation dermatitis, painful induration of the axillary region, and/or lymphedema. During the later course of late-delayed plexopathy, a generally progressive motor deficit may be observed (in a few cases, an apoplectic onset has been reported, sometimes after physical effort). Pain is quite uncommon at diagnosis and is usually a relatively minor feature. The severity of the condition is variable, from a simple discomfort to an almost complete paralysis of the limb.
The differential diagnosis must necessarily eliminate a neoplastic invasion of the brachial plexus. Some clinical signs may be important clues. In a large retrospective study on 100 cases of brachial plexus lesions, including 22 radiation plexopathies and 78 metastatic brachial plexopathies (34 in irradiated patients and 44 in nonirradiated patients), Kori et al.237 found several factors as indicators of neoplastic invasion: (i) pain, especially when severe, is an important feature, present in 89% of irradiated patients with neoplastic infiltration of the plexus (versus 18% of patients with radiation plexopathy); (ii) Horner’s syndrome was present in 56% of patients with tumor infiltration (versus 14%). On the contrary, the following signs argue for a radiation-induced disorder: (i) dysesthesia, present in 55% of radiation plexopathies (versus 6% of plexopathies linked with tumor infiltration); (ii) lymphedema, reported in 73% of cases (versus 15%). These results are consistent with those of other authors.239
Motor conduction velocities are usually normal or slightly decreased in radiation plexopathies. Sensory conduction velocities are rarely altered. The F waves can be absent or delayed. Electromyography is always abnormal, with fasciculations, fibrillation, and slow denervation potentials. The most important finding in favor of radiation-induced plexopathy is the presence of myokymic discharge, present in about two thirds of patients; this feature is quite rare (< 5%) in patients with an infiltrating tumor. Myokymic discharges are often located in the abductor pollicis brevis and pronator quadratus muscles.240
The main aim of imaging is to differentiate between radiation plexopathy and neoplastic invasion. CT scan was the first noninvasive examination to be useful241,242; this imaging technique may show a distortion of the tissue planes and fat or may be normal. MRI is superior to CT scan in this indication243; furthermore, bone artifacts do not impair the interpretation of MRI, which also allows a study of the cervical spine in search of epidural or cervical root secondary lesions. Radiation fibrosis is responsible for a thickening of the components of the brachial plexus, sometimes with contrast enhancement.243 Tumor invasion is diagnosed when a mass lesion is visible along the roots of the branches of the brachial plexus. Nevertheless, a retrospective study at the Mayo Clinic of 71 patients with cancer and brachial plexopathy who had an MRI yielded a 21% (15 patients) discordance rate between imaging and eventual diagnosis.186,239
A recent study of 50 breast cancer patients,244 using an association of body-coil and surface-coil techniques, suggests a major role for MRI to assess neoplastic recurrence. This technique allowed a correct diagnosis of tumor recurrence in 26 of 27 patients, directly related to the brachial plexus in 17 of them, and associated with cervical spine degenerative lesions in seven cases. Exclusion of a malignant disease was accurate in 20 of 21 cases. These results corresponded to a sensitivity of the MR criteria for tumor detection of 96% and a specificity of 95%, with similar positive and negative predictive values. Positron emission tomography using 18-fluoro-2 deoxyglucose (18FDG-PET) may also be helpful to differentiate tumor infiltration from radiation-induced plexopathy.245
The treatment of pain is often challenging, and includes analgesic drugs, tricyclic antidepressants, and/or anticonvulsants. Steroids can also be helpful. Anticoagulants have also been reported to be beneficial in radiation-induced neuropathies.246 Techniques such as transdermal electrical nerve stimulation and dorsal column roots stimulation have also been proposed. Neurolysis, with or without omentoplasty, has been performed in radiation-induced brachial plexopathy, but the benefit of this approach is questionable. Prudent physiotherapy may be indicated.238
LUMBOSACRAL PLEXOPATHY
Late-Delayed Lumbosacral Plexopathy
This disorder shares similar features with brachial plexopathy but is much less frequently reported. The onset follows initial RT by 1 to 30 years (median 5 years). The clinical pattern is characterized by a progressive, usually asymmetric, and bilateral motor deficit of the lower limbs associated with less-marked sensory deficits. As in brachial plexopathy, pain is generally mild or absent. The course of the disease leads to a slow worsening of the motor deficit. The patient may stabilize after several months or years.248
Treatment of pain is identical to that of brachial plexopathy. Two patients treated with anticoagulation remained stable with an improvement of pain.76
LOWER MOTOR NEURON SYNDROME
A lower motor neuron syndrome can be a consequence of pelvic irradiation for testicular tumors, lumbosacral RT, or craniospinal RT for medulloblastoma, and begins 3 months to 25 years after RT. Maier et al.249 reported 15 cases of this syndrome out of 343 patients who had undergone a lumboaortic irradiation scheme. About 30 cases have been reported since that period with various radiation schemes.
The patient presents with a progressive proximal and distal, often bilateral, and more-or-less symmetrical weakness of the inferior limbs; muscle atrophy and fasciculations may be associated with this deficit. Physical examination confirms a flaccid motor deficit and areflexia, but no sensory loss appears during the early stages. Sensory deficit may appear after several years, as well as sphincter disturbance characterized by lack of bladder sensation and incontinence.250 Variable patterns of progression and associated disability can be seen. MRI may be normal, but contrast enhancement of the roots of the cauda equina has been described.250
It is unclear whether the lesions localize to the anterior horn cells of the spinal cord or the proximal part of the nerve roots. Some reports advocate an anterior horn cell disorder, as no sensory signs were reported and electrophysiological data were compatible with pure motor neuron syndrome.251
In a study of six patients treated with RT (mean dose 45 Gy) for testicular cancer and including neuropathological examination of one case, Bowen et al.250 found strong arguments favoring radiculopathy, including (i) the presence of late-delayed sensory and sphincter disturbances, appearing 4 to 8 years after the motor symptoms; (ii) MRI abnormalities showing contrast enhancement of the lumbosacral roots of the cauda equina in two out of three patients; (iii) no lesion in the cord at necropsy but thickening of the roots of the cauda equina with focal areas of hemorrhagic discoloration, fibrosis, and axonal loss; the roots included abnormal dilated vessels with thickened and hyalinized walls. Thus, Wohlgemuth et al.252 suggest that a better term for this syndrome would be “postirradiation cauda equina syndrome.”
There is no recognized treatment of this condition. A patient has been reported to improve while on warfarin and steroids.253
RADIATION-INDUCED PERIPHERAL NERVE SHEATH TUMORS
A few dozen cases of radiation-induced nerve sheath tumors have been reported.254,255 Patients with neurofibromatosis type 1 (NF-1) have an increased risk of developing this complication. In a retrospective study on radiation-induced peripheral nerve sheath tumors, three patients out of nine (33%) had familial and/or clinical signs of NF-1256; in this series, the latency between RT and the onset of the secondary tumor ranged from 4 to 41 years.
Pain followed by the development of a sensorimotor deficit typifies the clinical presentation. The differential diagnosis of a local recurrence of the primary tumor generally requires biopsy. The treatment of these nerve sheath tumors relies principally on aggressive surgery with tumor-free margins; amputation of a limb, when performed, does not significantly change overall survival.257 Neither chemotherapy nor radiotherapy have shown any clear benefit has in terms of survival yet in those tumors.258
1. P. New. Radiation injury to the nervous system. Curr Opin Neurol. 2001;14:725-734.
2. M.H. Swennen. Delayed radiation toxicity after focal or whole brain radiotherapy for low-grade glioma. J Neurooncol. 2004;66:333-339.
3. R. Lai, L.E. Abrey, M.K. Rosenblum, L.M. DeAngelis. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology. 2004;62:451-456.
4. S. Oi, T. Kokunai, A. Ijichi, S. Matsumoto, A.J. Raimondi. Radiation-induced brain damage in children—histological analysis of sequential tissue changes in 34 autopsy cases. Neurol Med Chir. 1990;30:36-42.
5. G. Panagiotakos, G. Alshamy, B. Chan, R. Abrams, E. Greenberg, A. Saxena, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE. 2007;2:e588.
6. T. Kamiryo, N.F. Kassell, Q.A. Thai, M.B. Lopes, K.S. Lee, L. Steiner. Histological changes in the normal rat brain after gamma irradiation. Acta Neurochir. 1996;138:451-459.
7. W. Calvo, J.W. Hopewell, H.S. Reinhold, T.K. Yeung. Radiation induced damage in the choroid plexus of the rat brain: a histological evaluation. Neuropathol Appl Neurobiol. 1986;12:47-61.
8. Y. Yoneoka, M. Satoh, K. Akiyama, K. Sano, Y. Fujii, R. Tanaka. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999;72:1196-1201.
9. R.A. Nordal, A. Nagy, M. Pintilie, C.S. Wong. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342-3353.
10. C. Belka, W. Budach, R.D. Kortmann, et al. Radiation-induced CNS toxicity: molecular and cellular mechanisms. Br J Cancer. 2001;85:1233-1239.
11. Y.Q. Li, P. Chen, A. Haimovitz-Friedman, et al. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950-5956.
12. N. Lyubimova, J.W. Hopewell. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br J Radiol. 2004;77:488-492.
13. A. Perry, R.E. Schmidt. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol (Berl). 2006;111:197-212.
14. J.A. Coderre, G.M. Morris, P.L. Micca, et al. Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res. 2006;166:495-503.
15. P.J. Tofilon, J.R. Fike. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357-370.
16. T.E. Schultheiss, L.E. Kun, K.K. Ang, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093-1112.
17. S. Quarmby, P. Kumar, S. Kumar. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82:385-395.
18. G. Eissner, F. Kohlhuber, M. Grell, et al. Critical involvement of transmembrane tumor necrosis factor-alpha in endothelial programmed cell death mediated by ionizing radiation and bacterial endotoxin. Blood. 1995;86:4184-4193.
19. J.L. Daigle, J.H. Hong, C.S. Chiang, et al. The role of tumor necrosis factor signaling pathways in the response of murine brain to irradiation. Cancer Res. 2001;61:8859-8865.
20. R.W.M. Van der Maazen, I. Berhagen, B.J. Kleiboer, et al. Radiosensitivity of glial progenitor cells of the perinatal and adult rat optic nerve studied by an in vitro clonogenic assay. Radiother Oncol. 1991;20:258-264.
21. R.W.M. Van der Maazen, B.J. Kleiboer, I. Berhagen, et al. Irradiation in vitro discriminates between different O-2A progenitor cell subpopulations in the perinatal central nervous system of rats. Radiat Res. 1991;128:64-72. [Abstract]
22. R.W.M. Van der Maazen, B.J. Kleiboer, I. Berhagen, et al. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int J Radiat Biol. 1993;63:661-666.
23. B.M. Chow, Y.Q. Li, C.S. Wong. Radiation-induced apoptosis in the adult central nervous system is p53-dependent. Cell Death Differ. 2000;7:712-720.
24. S.L. Atkinson, Y.Q. Li, C.S. Wong. Apoptosis and proliferation of oligodendrocyte progenitor cells in the irradiated rodent spinal cord. Int J Radiat Oncol Biol Phys. 2005;62:535-544.
25. S. Hornsey, R. Myers, P.G. Coultas, et al. Turnover of proliferative cells in the spinal cord after X-irradiation and its relation to time-dependent repair of radiation damage. Br J Radiol. 1981;54:1081-1085.
26. Y. Enokido, T. Araki, K. Tanaka, et al. Involvement of p53 in DNA strand break-induced apoptosis in postmitotic CNS neurons. Eur J Neurosci. 1996;8:1812-1821.
27. G.T. Gobbel, M. Bellinzona, A.R. Vogt, et al. Response of postmitotic neurons to X-irradiation: implications for the role of DNA damage in neuronal apoptosis. J Neurosci. 1998;18:147-155.
28. C.S. Chiang, W.H. McBride, H.R. Withers. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother Oncol. 1993;29:60-68.
29. W.E. Thomas. Brain macrophages: evaluation of microglia and their function. Brain Res Rev. 1992;B17:61-74.
30. A.M. Omuro. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595-1600.
31. R. Rola, Y. Zou, T.T. Huang, K. Fishman, J. Baure, S. Rosi, et al. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 2007;42:1133-1145.
32. J.W. Hopewell, J.B. Cavanagh. Effects of X-irradiation on the mitotic activity of the subependymal plate of rats. Br J Radiol. 1972;45:461-465.
33. E. Tada, E. Yang, G.T. Gobbel, K.R. Lamborn, et al. Long-term impairment of subependymal repopulation following damage by ionizing irradiation. Exp Neurol. 1999;160:66-77.
34. M. Bellinzona, G.T. Gobbel, C. Shinohara, et al. Apoptosis is induced in the subependyma of young adult rats by ionizing irradiation. Neurosci Lett. 1996;208:163-166.
35. D.F. Young, J.B. Posner, F. Chu, et al. Rapid-course radiation therapy of cerebral metastases: results and complications. Cancer. 1974;34:1069-1076.
36. W.A. Hindo, F.A. DeTranaIII, M.S. Lee, et al. Large dose increment irradiation in treatment of cerebral metastases. Cancer. 1970;26:138-141.
37. J.B. Posner. Side effects of radiation therapy. In: Posner J.B., editor. Neurologic Complications of Cancer. Philadelphia: F.A. Davis Company; 1995:311-337.
38. F. Keime-Guibert, M. Napolitano, J.Y. Delattre. Neurological complications of radiotherapy and chemotherapy. J Neurol. 1998;245:695-708.
39. P.C. Phillips, J.Y. Delattre, C.A. Berger, et al. Early and progressive increases in regional brain capillary permeability following singleand fractionated-dose cranial radiation in rat. Neurology. 1987;37(Suppl. 1):301.
40. J.E. Freeman, P.G. Johnston, J.M. Voke. Somnolence after prophylactic cranial irradiation in children with acute lymphoblastic leukaemia. Br Med J. 1973;4:523-525.
41. P. Littman, J. Rosenstock, G. Gale, et al. The somnolence syndrome in leukemic children following reduced daily dose fractions of cranial radiation. Int J Radiat Oncol Biol Phys. 1984;10:1851-1853.
42. L.T. Ch’ien, R.J. Aur, S. Stagner, et al. Long-term neurological implications of somnolence syndrome in children with acute lymphocytic leukemia. Ann Neurol. 1980;8:273-277.
43. E. Chow, L. Davis, L. Holden, et al. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18-23.
44. S. Faithfull, M. Brada. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin Oncol. 1998;10:250-254.
45. L.R. Mandell, R.W. Walker, P. Steinherz, et al. Reduced incidence of the somnolence syndrome in leukemic children with steroid coverage during prophylactic cranial radiation therapy: results of a pilot study. Cancer. 1989;63:1975-1978.
46. D. Uzal, E. Ozyar, M. Hayran, et al. Reduced incidence of the somnolence syndrome after prophylactic cranial irradiation in children with acute lymphoblastic leukemia. Radiother Oncol. 1998;48:29-32.
47. M.C. De Wit, H.G. de Bruin, W. Eijkenboom, et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535-537.
48. A.A. Brandes, A. Tosoni, F. Spagnolli, G. Frezza, M. Leonardi, F. Calbucci, E. Franceschi. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: Pitfalls in neurooncology. Neuro Oncol. 2008;26:2192-2197.
49. C. Armstrong, J. Ruffer, B. Corn, et al. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: neuropsychologic outcome and proposed mechanisms. J Clin Oncol. 1995;13:2263-2271.
50. M.C. Vigliani, N. Sichez, M. Poisson, et al. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int J Radiat Oncol Biol Phys. 1996;35:527-533.
51. L.Y. Chak, L.M. Zatz, P. Wasserstein, et al. Neurologic dysfunction in patients treated for small cell carcinoma of the lung: a clinical and radiological study. Int J Radiat Oncol Biol Phys. 1986;12:385-389.
52. A. Creange, D. Felten, I. Kiesel, et al. Leucoencéphalopathie subaiguë du rhombencéphale après radiothérapie hypophysaire. Rev Neurol (Paris). 1994;150:704-708.
53. T. Song, B.L. Liang, S.Q. Huang, et al. Magnetic resonance imaging manifestations of radiation injury in brain stem and cervical spinal cord of nasopharyngeal carcinoma patients after radiotherapy. Ai Zheng. 2005;24:357-361. [Abstract]
54. P. Lampert, M.I. Tom, W.D. Rider. Disseminated demyelination of the brain following Co60 radiation. Arch Pathol. 1959;68:322-330.
55. S. Ochi, Y. Takahashi, S. Yokoyama. Fulminating midbrain irradiation injury of pediatric brain tumor. No To Shinke. 2005;57:800-805. [Abstract]
56. S. Malone, G.P. Raaphorst, R. Gray, et al. Enhanced in vitro radiosensitivity of skin fibroblasts in two patients developing brain necrosis following AVM radiosurgery: a new risk factor with potential for a predictive assay. Int J Radiat Oncol Biol Phys. 2000;47:185-189.
57. J.C. Flickinger, D. Kondziolka, L.D. Lunsford, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000;46:1143-1148.
58. M. Schlienger, D. Atlan, D. Lefkopoulos, et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys. 2000;46:1135-1142.
59. L. Miyawaki, C. Dowd, W. Wara, et al. Five-year results of LINAC radiosurgery for arteriovenous malformations: outcome for large AVMS. Int J Radiat Oncol Biol Phys. 1999;44:1089-1106.
60. G.M. Morris, J.A. Coderre, P.L. Micca, et al. Central nervous system tolerance to boron neutron capture therapy with p-boronophenylalanine. Br J Cancer. 1997;76:1623-1629.
61. J.H. Oppenheimer, M.L. Levy, U. Sinha, et al. Radionecrosis secondary to interstitial brachytherapy: correlation of magnetic resonance imaging and histopathology. Neurosurgery. 1992;31:336-343.
62. R. Van Effenterre, A.L. Boch. Radionécrose du chiasma. Neurochirurgie. 1993;39:75-84.
63. K.M. Coghlan, P. Magennis. Cerebral radionecrosis following the treatment of parotid tumours: a case report and review of the literature. Int J Oral Maxillofac Surg. 1999;28:50-52.
64. C. Cirafisi, F. Verderame. Radiation-induced rhombencephalopathy. Ital J Neurol Sci. 1999;20:55-58.
65. A.J. Kumar, N.E. Leeds, G.N. Fuller, et al. Malignant gliomas: MR imaging spectrum of radiation therapy-and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377-384.
66. T.J. Janus, E.E. Kim, R. Tilbury, et al. Use of [18F] fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol. 1993;33:540-548.
67. C. Lamy-Lhullier, F. Dubois, S. Blond, et al. Intérêt de la tomoscintigraphie cérébrale au sestamibi marqué au technétium dans le diagnostic différentiel récidive tumorale-radionécrose des tumeurs gliales sus-tentorielles de l’adulte. Neurochirurgie. 1999;45:110-117.
68. M. Henze, A. Mohammed, H. Schlemmer, et al. Detection of tumour progression in the follow-up of irradiated low-grade astrocytomas: comparison of 3-[123I]-iodo-alpha-methyl-l-tyrosine and 99mTc-MIBI SPET. Eur J Nucl Med Mol Imaging. 2002;29:1455-1461.
69. P. Matheja, M. Weckesser, Ch Rickert, et al. I-123-lodo-alpha-methyl tyrosine SPECT in nonparenchymal brain tumours. Nuklearmedizin. 2002;41:191-196.
70. D. Galanaud, F. Nicoli, D. Figarella-Branger, et al. MR spectroscopy of brain tumors. J Radiol. 2006;87:822-832.
71. H.P. Schlemmer, P. Bachert, K.K. Herfarth, et al. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am J Neuroradiol. 2001;22:1316-1324.
72. M.P. Lichy, P. Bachert, F. Hamprecht, et al. Application of1 MR spectroscopic imaging in radiation oncology: choline as a marker for determining the relative probability of tumor progression after radiation of glial brain tumors. Rofo. 2006;178:627-633. [Abstract]
73. P.E. Ricci, J.P. Karis, J.E. Heiserman, et al. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol. 1998;19:407-413.
74. P. Matheja, C. Rickert, M. Weckesser, et al. Scintigraphic pitfall: delayed radionecrosis: case illustration. J Neurosurg. 2000;92:732.
75. P.A. Forsyth, P.J. Kelly, T.L. Cascino, et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? J Neurosurg. 1995;82:436-444.
76. M.J. Glantz, P.C. Burger, A.H. Friedman, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020-2027.
77. P.J. Chuba, P. Aronin, K. Bhambhani, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80:2005-2012.
78. K.A. Leber, H.G. Eder, H. Kovac, et al. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70:229-236.
79. K. Kohshi, H. Imada, S. Nomoto, et al. Successful treatment of radiation-induced brain necrosis by hyperbaric oxygen therapy. J Neurol Sci. 2003;209:115-117.
80. J.J. Feldmeier, N.B. Hampson. A systematic review of the literature reporting the application of hyperbaric oxygen prevention and treatment of delayed radiation injuries: an evidence based approach. Undersea Hyperb Med. 2002;29:4-30. [Abstract]
81. A.S. Chan, M.C. Cheung, S.C. Law, et al. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. 2004;100:398-404.
82. J.R. Fike, G.T. Gobbel, L.J. Marton, et al. Radiation brain injury is reduced by the polyamine inhibitor alpha-difluoromethylornithine. Radiat Res. 1994;138:99-106.
83. D. Kondziolka, Y. Mori, A.J. Martinez, et al. Beneficial effects of the radioprotectant 21-aminosteroid U-74389G in a radiosurgery rat malignant glioma model. Int J Radiat Oncol Biol Phys. 1999;44:179-184.
84. L.R. Guelman, M.A. Zorrilla Zubilete, H. Rios, et al. WR-2721 (amifostine, ethyol) prevents motor and morphological changes induced by neonatal X-irradiation. Neurochem Int. 2003;42:385-391.
85. A.D. Sasse, L.G. Clark, E.C. Sasse, et al. Amifostine reduces side effects and improves complete response rate during radiotherapy: results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2006;64:784-791.
86. N. Lyubimova, P. Coultas, K. Yuen, et al. In vivo radioprotection of mouse brain endothelial cells by Hoechst 33342. Br J Radiol. 2001;74:77-82.
87. L. Guelman, Z. Zorilla, H. Rios, et al. GM1 ganglioside treatment protects against long-term neurotoxic effects of neonatal X-irradiation on cerebellarcortex cytoarchitecture and motor functions. Brain Res. 2000;858:303-311.
88. O. Brustle, K.N. Jones, R.D. Learish, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:650-651.
89. D. Christie, M. Lavin, L. Tan. Clinical application of in vitro radiohypersensitivity testing. Australas Radiol. 2000;44:333-335.
90. M.J. Taphoorn, M. Klein. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159-168.
91. M. Klein, N.H. Engelberts, H.M. van der Ploeg, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54:514-520.
92. M. Klein, J.J. Heimans, N.K. Aaronson, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360:1361-1368.
92a. L. Douw, M. Klein, S.S.A.A. Fagel, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8:810-818.
93. C.L. Armstrong, J.V. Hunter, G.E. Ledakis, et al. Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology. 2002;59:40-48.
94. I.J. Torres, A.J. Mundt, P.J. Sweeney, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113-1118.
95. M. Klein, J.J. Heimans, N.K. Aaronson, et al. Impaired cognitive functioning in low-grade glioma patients: relationship to tumor localisation, radiotherapy and the use of anticonvulsants. J Clin Oncol. 2004;22:966-967.
96. A. Asai, M. Matsutani, T. Kohno, et al. Subacute brain atrophy after radiation therapy for malignant brain tumor. Cancer. 1989;63:1962-1974.
97. J.P. Imperato, N.A. Paleologos, N.A. Vick. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28:818-822.
98. M.C. Vigliani, C. Duyckaerts, J.Y. Delattre. Radiation-induced cognitive dysfunction in adults. Vecht C.J., editor. Handbook of Clinical Neurology, vol. 23. Amsterdam: Elsevier. 1997:371-388.
99. B. Fisher, W. Seiferheld, C. Schultz, et al. Secondary analysis of Radiation Therapy Oncology Group study (RTOG) 9310: an intergroup phase II combined modality treatment of primary central nervous system lymphoma. J Neurooncol. 2005;74:201-205.
100. Y.M. Archibald, D. Lunn, L.A. Ruttan, et al. Cognitive functioning in long-term survivors of high-grade glioma. J Neurosurg. 1994;80:247-253.
101. P.W. Lee, B.K. Hung, E.K. Woo, et al. Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry. 1989;52:488-492.
102. L.M. DeAngelis, J.Y. Delattre, J.B. Posner. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
103. P.K. Duffner. Long-term effects of radiation on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293-310.
104. L.M. DeAngelis, J. Yahalom, H.T. Thaler, et al. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10:635-643.
105. L.E. Abrey, J. Yahalom, L.M. DeAngelis. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144-3150.
106. M.W. Wassenberg, J.E. Bromberg, T.D. Witkamp, et al. White matter lesions and encephalopathy in patients treated for primary central nervous system lymphoma. J Neurooncol. 2001;52:73-80.
107. C.L. Armstrong, B.W. Corn, J.E. Ruffer, et al. Radiotherapeutic effects on brain function: double dissociation of memory systems. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13:101-111.
108. T.J. Postma, M. Klein, C.C. Verstappen, et al. Radiotherapy-induced cerebral abnormalities in patients with low-grade glioma. Neurology. 2002;59:121-123.
109. C.A. Meyers, M.A. Weitzner, A.D. Valentine, et al. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998;16:2522-2527.
110. E.G. Shaw, R. Rosdhal, R.B. D’AgostinoJr, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415-1420. Abstract.
111. N. Senzer. Rationale for a phase III study of erythropoietin as a neurocognitive protectant in patients with lung cancer receiving prophylactic cranial irradiation. Semin Oncol. 2002;29:47-52.
111b. M.E. Robbins, D.I. Diz. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6-12.
111c. C. Nieder, N.H. Andratschke, N. Wiedenmann, M. Molls. Prevention of radiation-induced central nervous system toxicity: a role for amifostine? Anticancer Res. 2004;24:3803-3809.
112. J.R. Crossen, D. Garwood, E. Glatstein, et al. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627-642.
113. R.K. Mulhern, T.E. Merchant, A. Gajjar, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399-408.
114. P.D. Brown, J.C. Buckner, J.R. O’Fallon, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination. J Clin Oncol. 2003;21:2519-2524.
115. B. Thiessen, L.M. DeAngelis. Hydrocephalus in radiation leukoencephalopathy: results of ventriculoperitoneal shunting. Arch Neurol. 1998;55:705-710.
116. P. Perrini, A. Scollato, F. Cioffi, et al. Radiation leukoencephalopathy associated with moderate hydrocephalus: intracranial pressure monitoring and results of ventriculoperitoneal shunting. Neurol Sci. 2002;23:237-241.
117. B.K. Kleinschmidt-Demasters, J.S. Kang, K.O. Lillehei. The burden of radiation-induced central nervous system tumors: a single institutions experience. J Neuropathol Exp Neurol. 2006;65:204-216.
118. E. Ron, B. Modan, J.D. BoiceJr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033-1039.
119. M.M. Hawkins, G.J. Draper, J.E. Kingston. Incidence of second primary tumours among childhood cancer survivors. Br J Cancer. 1987;56:339-347.
120. M. Brada, D. Ford, S. Ashley, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304:1343-1346.
121. G. Minniti, D. Traish, S. Ashley, et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after further 10 years. J Clin Endocrinol Metab. 2005;90:800-804.
122. J.P. Neglia, A.T. Meadows, L.L. Robison, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325:1330-1336.
123. X. Muracciole, D. Cowen, J. Regis. Radiosurgery and brain radio-induced carcinogenesis: update. Neurochirurgie. 2004;50:414-420.
124. A. Amirjamshidi, K. Abbassioun. Radiation-induced tumors of the central nervous system occurring in childhood and adolescence: four unusual lesions in three patients and a review of the literature. Childs Nerv Syst. 2000;16:390-397.
125. P. Strojan, M. Popovic, B. Jereb. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. 2000;48:65-73.
126. B.S. Musa, I.K. Pople, B.H. Cummins. Intracranial meningiomas following irradiation: a growing problem? Br J Neurosurg. 1995;9:629-637.
127. A. De Tommasi, M. Occhiogrosso, C. De Tommasi, et al. Radiation-induced intracranial meningiomas: review of six operated cases. Neurosurg Rev. 2005;28:104-114.
128. Y. Shoshan, O. Chernova, S.S. Juen, et al. Radiation-induced meningioma: a distinct molecular genetic pattern? J Neuropathol Exp Neurol. 2000;59:614-620.
129. E. Rajcan-Separovic, J. Maguire, T. Loukianova, et al. Loss of 1p and 7p in radiation-induced meningiomas identified by comparative genomic hybridization. Cancer Genet Cytogene. 2003;144:6-11.
130. E.E. Mack. Radiation-induced tumors. In: Berger M.S., Wilson C.B., editors. The Gliomas. Philadelphia: WB Saunders; 1999:724-735.
131. M. Salvati, A. Frati, N. Russo, et al. Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol. 2003;60:60-67.
132. D.J. Brat, C.D. James, A.E. Jedlicka, et al. Molecular genetic alterations in radiation-induced astrocytomas. Am J Pathol. 1999;154:1431-1438.
133. K.E. Murros, J.F. Toole. The effect of radiation on carotid arteries: a review article. Arch Neurol. 1989;46:449-455.
134. S. Gupta. Radiation-induced carotid artery blow out: a case report. Acta Chir Belg. 1994;94:299-300.
135. R.A. McCready, G.L. Hyde, B.A. Bivins, et al. Radiation-induced arterial injuries. Surgery. 1983;93:306-312.
136. M. Bernstein, M. Lumley, G. Davidson, et al. Intracranial arterial occlusion associated with high-activity iodine-125 brachytherapy for glioblastoma. J Neurooncol. 1993;17:253-260.
137. M.H. Werner, P.C. Burger, E.R. Heinz, et al. Intracranial atherosclerosis following radiotherapy. Neurology. 1988;38:1158-1160.
138. M. Bitzer, H. Topka. Progressive cerebral occlusive disease after radiation therapy. Stroke. 1995;26:131-136.
139. J. Grill, D. Couanet, C. Cappelli, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45:393-396.
140. Y. Suzuki, M. Negoro, M. Shibuya, et al. Surgical treatment for pediatric moyamoya disease: use of the superficial temporal artery for both areas supplied by the anterior and middle cerebral arteries. Neurosurgery. 1997;40:324-329.
141. R.C. Dauser, G.F. Tuite, C.W. McCluggage. Dural inversion procedure for moyamoya disease: technical note. J Neurosurg. 1997;86:719-723.
142. I.B. Ross, M.I. Shevell, J.L. Montes, et al. Encephaloduroarteriosynangiosis (EDAS) for the treatment of childhood moyamoya disease. Pediatr Neurol. 1994;10:199-204.
143. S.K. Kim, K.C. Wang, I.O. Kim, et al. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal)synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88-96.
144. M. Fouladi, J. Langston, R. Mulhern, et al. Silent lacunar lesions detected by magnetic resonance imaging of children with brain tumors: a late sequela of therapy. J Clin Onco. 2000;18:824-831.
145. J.E. Baumgartner, J.L. Ater, C.S. Ha, et al. Pathologically proven cavernous angiomas of the brain following radiation therapy for pediatric brain tumors. Pediatr Neurosurg. 2003;39:201-207.
146. S. Heckl, A. Aschoff, S. Kunze. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002;94:3285-3291.
147. M. Mauget-Faysse, M. Vuillaume, M. Quaranta, et al. Idiopathic and radiation-induced ocular telangiectasia: the involvement of the ATM gene. Invest Ophthalmol Vis Sci. 2003;44:3257-3262.
148. P.M. Novelli, D.H. Reigel, G.P. Langham, et al. Multiple cavernous angiomas after high-dose whole-brain radiation therapy. Pediatr Neurosurg. 1997;26:322-325.
149. F.K. Jensen, A. Wagner. Intracranial aneurysm following radiation therapy for medulloblastoma: a case report and review of the literature. Acta Radiol. 1997;38:37-42.
150. E. Louis, N. Martin-Duverneuil, A.F. Carpentier, et al. Anévrysme post-radique de la carotide intra-caverneuse. Rev Neurol (Paris). 2003;159:319-322.
151. B. Azzarelli, J. Moore, R. Gilmor, et al. Multiple fusiform intracranial aneurysms following curative radiation therapy for suprasellar germinoma: case report. J Neurosurg. 1984;61:1141-1145.
152. L.S. Constine, P.D. Woolf, D. Cann, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87-94.
153. M.D. Littley, S.M. Shalet. Beardwell CG, et al. Radiation and hypothalamic-pituitary function. Baillieres Clin Endocrinol Metab. 1990;4:147-175.
154. M.D. Littley, S.M. Shalet, C.G. Beardwell, et al. Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf). 1989;31:363-373.
155. P.E. Clayton, S.M. Shalet. Dose dependency of time of onset of radiation-induced growth hormone deficiency. J Pediatr. 1991;118:226-228.
156. R. Rappaport, R. Brauner. Growth and endocrine disorders secondary to cranial irradiation. Pediatr Res. 1989;25:561-567.
157. M. Schmiegelow, S. Lassen, H.S. Poulsen, et al. Cranial radiotherapy of childhood brain tumours: growth hormone deficiency and its relation to the biological effective dose of irradiation in a large population based study. Clin Endocrinol (Oxf). 2000;53:191-197.
158. G. Stevens, S. Downes, A. Ralston. Thyroid dose in children undergoing prophylactic cranial irradiation. Int J Radiat Oncol Biol Phys. 1998;42:385-390.
159. E.A. Livesey, P.C. Hindmarsh, C.G.D. Brook, et al. Endocrine disorders following treatment of childhood brain tumours. Br J Cancer. 1990:61622-61625.
160. W. Arlt, U. Hove, B. Muller, et al. Frequent and frequently overlooked: treatment-induced endocrine dysfunction in adult long-term survivors of primary brain tumors. Neurology. 1997;49:498-506.
161. M.J. Taphoorn, J.J. Heimans, E.A. van der Veen, et al. Endocrine functions in long-term survivors of low-grade supratentorial glioma treated with radiation therapy. J Neurooncol. 1995;25:97-102.
162. N.A. Samaan, R. Vieto, P.N. Schultz, et al. Hypothalamic, pituitary and thyroid dysfunction after radiotherapy to the head and neck. Int J Radiat Oncol Biol Phys. 1982;8:1857-1867.
163. T. Petterson, I.A. MacFarlane, P.M. Foy, et al. Hyperprolactinemia and infertility following cranial irradiation for brain tumours: successful treatment with bromocriptine. Br J Neurosurg. 1993;7:571-574.
164. H. Ahlbom. Results of radiotherapy of hypopharyngeal cancer at Radium-Hemmet, Stockholm. Acta Radiol. 1941;22:155-171.
165. R. Rampling, P. Symonds. Radiation myelopathy. Curr Opin Neurol. 1998;11:627-632.
166. J.A. Word, U.P. Kalokhe, B.S. Aron, et al. Transient radiation myelopathy (L’hermitte’s sign) in patients with Hodgkin’s disease treated by mantle irradiation. Int J Radiat Oncol Biol Phys. 1980;6:1731-1733.
167. D.A. Fein, R.B. MarcusJr, J.T. Parsons, et al. L’hermitte’s sign: incidence and treatment variables influencing risk after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1993;27:1029-1033.
168. O. Esik, T. Csere, K. Stefanits, et al. A review on radiogenic L’hermitte’s sign. Pathol Oncol Res. 2003;9:115-120.
169. C.R. Lewanski, J.A. Sinclair, J.S. Stewart. L’hermitte’s sign following head and neck radiotherapy. Clin Oncol (R Coll Radiol). 2000;12:98-103.
170. Y.Q. Li, V. Jay, C.S. Wong. Oligodendrocytes in the adult rat spinal cord undergo radiation-induced apoptosis. Cancer Res. 1996;56:5417-5422.
171. Z. Lengyel, G. Reko, K. Majtenyi, et al. Autopsy verifies demyelination and lack of vascular damage in partially reversible radiation myelopathy. Spinal Cord. 2003;41:577-585.
172. M.W. Chao, A. Wirth, G. Ryan, et al. Radiation myelopathy following transplantation and radiotherapy for non-Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 1998;41:1057-1061.
173. T.E. Schultheiss, L.C. Stephens. Invited review: permanent radiation myelopathy. Br J Radiol. 1992;65:737-753.
174. C.A. Pallis, S. Louis, R.L. Morgan. Radiation myelopathy. Brain. 1961;84:460-479.
175. P.Y. Wang, W.C. Shen, J.S. Jan. MR imaging in radiation myelopathy. AJNR Am J Neuroradiol. 1992;13:1049-1055.
176. P.Y. Wang, W.C. Shen, J.S. Jan. Serial MRI changes in radiation myelopathy. Neuroradiology. 1995;37:374-377.
177. T. Yasui, H. Yagura, M. Komiyama, et al. Significance of gadolinium-enhanced magnetic resonance imaging in differentiating spinal cord radiation myelopathy from tumor: case report. J Neurosurg. 1992;77:628-631.
178. H. Komachi, K. Tsuchiya, M. Ikeda, et al. Radiation myelopathy: a clinicopathological study with special reference to correlation between MRI findings and neuropathology. J Neurol Sci. 1995;132:228-232.
179. P.J. Koehler, H. Verbiest, J. Jager, et al. Delayed radiation myelopathy: serial MR-imaging and pathology. Clin Neurol Neurosurg. 1996;98:197-201.
180. M. Michikawa, Y. Wada, M. Sano, et al. Radiation myelopathy: significance of gadolinium-DTPA enhancement in the diagnosis. Neuroradiology. 1991;33:286-289.
181. E.R. Alfonso, M.A. De Gregorio, P. Mateo, et al. Radiation myelopathy in over-irradiated patients: MR imaging findings. Eur Radiol. 1997;7:400-404.
182. G. Angibaud, J.L. Ducasse, G. Baille, et al. Potential value of hyperbaric oxygenation in the treatment of post-radiation myelopathies. Rev Neurol (Paris). 1995;151:661-666.
183. F. Calabro, J.R. Jinkins. MRI of radiation myelitis: a report of a case treated with hyperbaric oxygen. Eur Radiol. 2000;10:1079-1084.
184. J.C. Allen, D.C. Miller, G.N. Budzilovich, et al. Brain and spinal cord hemorrhage in long-term survivors of malignant pediatric brain tumors: a possible late effect of therapy. Neurology. 1991;41:148-150.
185. R.J. Packer, J.G. Gurney, J.A. Punyko, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255-3261.
186. S.M. Sagar, R.J. Thomas, L.T. Loverock, et al. Olfactory sensations produced by high-energy photon irradiation of the olfactory receptor mucosa in humans. Int J Radiat Oncol Biol Phys. 1991;20:771-776.
187. K.A. Carmichael, A.S. Jennings, R.L. Doty. Reversible anosmia after pituitary irradiation. Ann Intern Med. 1984;100:532-533.
188. D. Ophir, A. Guterman, R. Gross-Isseroff. Changes in smell acuity induced by radiation exposure of the olfactory mucosa. Arch Otolaryngol Head Neck Surg. 1988;114:853-855.
189. Q. Qiu, S. Chen, C. Meng, et al. Observation on the changes in nasopharyngeal carcinoma patients’ olfactory before and after radiotherapy. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2001;15:57-58. [Abstract]
190. S. Lessell. Friendly fire: neurogenic visual loss from radiation therapy. J Neuroophthalmol. 2004;24:243-250.
191. J.T. Parsons, F.J. Bova, C.R. Fitzgerald, et al. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755-763.
192. C. Valanconny, F. Koenig, M. Benchaboune, et al. Complications de la radiothérapie des néovaisseaux de la dégénérescence maculaire liée à l’âge. J Fr Ophtalmol. 2000;23:151-157.
193. A. Meyer, C. Levy, J. Blondel, et al. Neuropathie optique après protonthérapie pour mélanome malin de la choroïde. J Fr Ophtalmol. 2000;23:543-553.
194. R. Piquemal, J.P. Cottier, S. Arsene, et al. Radiation-induced optic neuropathy 4 years after radiation: report of a case followed up with MRI. Neuroradiology. 1998;40:439-441.
195. L.A. Levin, E.S. Gragoudas, S. Lessell. Endothelial cell loss in irradiated optic nerves. Ophthalmology. 2000;107:370-374.
197. F.X. Borruat, N.J. Schatz, J.S. Glaser, et al. Visual recovery from radiation-induced optic neuropathy. The role of hyperbaric oxygen therapy. J Clin Neuroophthalmol. 1993;13:98-101.
198. D. Roden, T.M. Bosley, B. Fowble, et al. Delayed radiation injury to the retrobulbar optic nerves and chiasm. Clinical syndrome and treatment with hyperbaric oxygen and corticosteroids. Ophthalmology. 1990;97:346-351.
199. M. Boschetti, M. De Lucchi, M. Giusti, et al. Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing disease. Eur J Endocrinol. 2006;154:813-818.
200. I.G. Mohamed, W. Roa, D. Fulton, et al. Optic nerve sheath fenestration for a reversible optic neuropathy in radiation oncology. Am J Clin Oncol. 2000;23:401-405.
200b. J.H. Kim, S.L. Brown, A. Kolozsvary, K.A. Jenrow, S. Ryu, M.L. Rosenblum, et al. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137-142.
201. S. Lessell, I.M. Lessell, J.F. RizzoIII. Ocular neuromyotonia after radiation therapy. Am J Ophthalmol. 1986;102:766-770.
202. J. Marti-Fabregas, J. Montero, D. Lopez-Villegas, et al. Post-irradiation neuromyotonia in bilateral facial and trigeminal nerve distribution. Neurology. 1997;48:1107-1109.
203. J.M. Diaz, E.S. Urban, J.S. Schiffman, et al. Post-irradiation neuromyotonia affecting trigeminal nerve distribution: an unusual presentation. Neurology. 1992;42:1102-1104.
204. S. Maesawa, C. Salame, J.C. Flickinger, et al. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94:14-20.
205. C.L. Rogers, A.G. Shetter, J.A. Fiedler, et al. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47:1013-1019.
206. S.E. Combs, C. Thilmann, J. Debus, et al. Long-term outcome of stereotactic radiosurgery (SRS) in patients with acoustic neuromas. Int J Radiat Oncol Biol Phys. 2006;64:1341-1347.
207. S. McClelland3rd, B.J. Gerbi, P.D. Higgins, J.B. Orner, W.A. Hall. Safety and efficacy of fractionated stereotactic radiotherapy for acoustic neuromas. J Neurooncol. 2008;86:191-194.
208. W.L. Giese, T.J. Kinsella. Radiation injury to peripheral and cranial nerves. In: Gutin P.H., Leibel S.A., Sheline G.E., editors. Radiation Injury to the Nervous System. New York: Raven; 1991:383-403.
209. W.D. DeWys, K. Walters. Abnormalities of taste sensation in cancer patients. Cancer. 1975;36:1888-1896.
210. G. Noren, D. Greitz, A. Hirsch, et al. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993;58:104-107.
211. R.L. Foote, R.J. Coffey, J.W. Swanson, et al. Stereotactic radiosurgery using the gamma knife for acoustic neuromas. Int J Radiat Oncol Biol Phys. 1995;32:1153-1160.
212. M.T. Selch, A. Pedroso, S.P. Lee, et al. Stereotactic radiotherapy for the treatment of acoustic neuromas. J Neurosurg. 2004;101:362-372.
213. A.G. Gibb, K.S. Loh. The role of radiation in delayed hearing loss in nasopharyngeal carcinoma. J Laryngol Otol. 2000;114:139-144.
214. L.W. McDonald, M.P. Donovo, R.G. Plantz. Radiosensitivity of the vestibular apparatus of the rabbit. Radiat Res. 1966;27:510-511.
215. D. Kondziolka, N. Nathoo, J.C. Flickinger, et al. Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery. 2003;53:815-821.
216. D. Kondziolka, L.D. Lunsford, M.R. McLaughlin, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426-1433.
217. R. Spiegelmann, Z. Lidar, J. Gofman, et al. Linear accelerator radiosurgery for vestibular schwannoma. J Neurosurg. 2001;94:7-13.
218. A. Niranjan, L.D. Lunsford, J.C. Flickinger, et al. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery. 1999;45:753-762.
219. K. Ito, M. Shin, M. Matsuzaki, et al. Risk factors for neurological complications after acoustic neurinoma radiosurgery: refinement from further experiences. Int J Radiat Oncol Biol Phys. 2000;48:75-80.
220. P.S. Berger, J.P. Bataini. Radiation-induced cranial nerve palsy. Cancer. 1977;40:152-155.
221. V.S. Cheng, M.D. Schultz. Unilateral hypoglossal nerve atrophy as a late complication of radiation therapy of head and neck carcinoma: a report of four cases and a review of the literature on peripheral and cranial nerve damages after radiation therapy. Cancer. 1975;35:1537-1544.
222. E.F. Johnston, A.J. Hammond, J.G. Cairncross. Bilateral hypoglossal palsies: a late complication of curative radiotherapy. Can J Neurol Sci. 1989;16:198-199.
223. M.Y. Kang, J.M. Holland, K.R. StevensJr. Cranial neuropathy following curative chemotherapy and radiotherapy for carcinoma of the nasopharynx. J Laryngol Otol. 2000;114:308-310.
224. A.D. King, A. Ahuja, S.F. Leung, et al. MR features of the denervated tongue in radiation-induced neuropathy. Br J Radiol. 1999;72:349-353.
225. A.D. King, S.F. Leung, P. Teo, et al. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Head Neck. 1999;21:614-619.
226. Y. Stern, G. Marshak, T. Shpitzer, et al. Vocal cord palsy: possible late complication of radiotherapy for head and neck cancer. Ann Otol Rhinol Laryngol. 1995;104:294-296.
227. T. Takimoto, Y. Saito, M. Suzuki, et al. Radiation-induced cranial nerve palsy: hypoglossal nerve and vocal cord palsies. J Laryngol Otol. 1991;105:44-45.
228. X.M. Huang, Y.Q. Zheng, X.M. Zhang, et al. Diagnosis and management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope. 2006;116:1626-1631.
229. J. Rowin, G. Cheng, S.L. Lewis, M.N. Meriggioli. Late appearance of dropped head syndrome after radiotherapy for Hodgkin’s disease. Muscle Nerve. 2006;34:666-669.
230. A.L. Salner, L.E. Botnick, A.G. Herzog, et al. Reversible brachial plexopathy following primary radiation therapy for breast cancer. Cancer Treat Rep. 1981;65:797-802.
231. S.M. Pierce, A. Recht, T.I. Lingos, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915-923.
232. F. Vega, L. Davila, J.Y. Delattre, et al. Experimental carcinomatous plexopathy. J Neurol. 1993;240:54-58.
233. P.F. Pradat, M. Poisson, J.Y. Delattre. Neuropathies radiques. Rev Neurol (Paris). 1994;150:664-677.
234. B.A. Stoll, J.T. Andrews. Radiation-induced peripheral neuropathy. BMJ. 1966;11:834-837.
235. N.K. Olsen, P. Pfeiffer, K. Mondrup, et al. Radiation-induced brachial plexus neuropathy in breast cancer patients. Acta Oncol. 1990;29:885-890.
236. E.L. Gillette, P.A. Mahler, B.E. Powers, et al. Late radiation injury to muscle and peripheral nerves. Int J Radiat Oncol Biol Phys. 1995;31:1309-1318.
237. S.H. Kori, K.M. Foley, J.B. Posner. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31:45-50.
238. S.H. Kori. Diagnosis and management of brachial plexus lesions in cancer patients. Oncology (Huntingt). 1995;9:756-760.
239. D. Thyagarajan, T. Cascino, G. Harms. Magnetic resonance imaging in brachial plexopathy of cancer. Neurology. 1995;45:421-427.
240. C.M. HarperJr, J.E. Thomas, T.L. Cascino, et al. Distinction between neoplastic and radiation-induced brachial plexopathy, with emphasis on the role of EMG. Neurology. 1989;39:502-506.
241. J. Cooke, S. Powell, C. Parsons. The diagnosis by computed tomography of brachial plexus lesions following radiotherapy for carcinoma of the breast. Clin Radiol. 1988;39:602-606.
242. E.K. Fishman, J.N. Campbell, J.E. Kuhlman, et al. Multiplanar CT evaluation of brachial plexopathy in breast cancer. J Comput Assist Tomogr. 1991;15:790-795.
243. H. Wouter van Es, A.M. Engelen, T.D. Witkamp, et al. Radiation-induced brachial plexopathy: MR imaging. Skeletal Radiol. 1997;26:284-288.
244. A. Qayyum, A.D. MacVicar, A.R. Padhani, et al. Symptomatic brachial plexopathy following treatment for breast cancer: utility of MR imaging with surface-coil techniques. Radiology. 2000;214:837-842.
245. A. Ahmad, S. Barrington, M. Maisey, et al. Use of positron emission tomography in evaluation of brachial plexopathy in breast cancer patients. Br J Cancer. 1999;79:478-482.
246. O. Soto. Radiation-induced conduction block: resolution following anticoagulant therapy. Muscle Nerve. 2005;31:642-645.
247. J.M. Gerard, N. Franck, Z. Moussa, et al. Acute ischemic brachial plexus neuropathy following radiation therapy. Neurology. 1989;39:450-451.
248. J.E. Thomas, T.L. Cascino, J.D. Earle. Differential diagnosis between radiation and tumor plexopathy of the pelvis. Neurology. 1985;35:1-7.
249. J.G. Maier, R.H. Perry, W. Saylor, et al. Radiation myelitis of the dorsolumbar spinal cord. Radiology. 1969;93:153-160.
250. J. Bowen, R. Gregory, M. Squier, et al. The post-irradiation lower motor neuron syndrome neuronopathy or radiculopathy? Brain. 1996;119(Pt 5):1429-1439.
251. C. Lamy, J.L. Mas, B. Varet, et al. Post-radiation lower motor neuron syndrome presenting as monomelic amyotrophy. J Neurol Neurosurg Psychiatry. 1991;54:648-649.
252. W.A. Wohlgemuth, K. Rottach, G. Jaenke, et al. Radiogenic amyotrophy: cauda equina lesion as a late radiation sequel. Nervenarzt. 1998;69:1061-1065. [Abstract]
253. T. Anezaki, T. Harada, I. Kawachi, et al. A case of post-irradiation lumbosacral radiculopathy successfully treated with corticosteroid and warfarin. Rinsho Shinkeigaku. 1999;39:825-829. [Abstract]
254. C.J. Hussussian, S.E. Mackinnon. Post-radiation neural sheath sarcoma of the brachial plexus: a case report. Ann Plast Surg. 1999;43:313-317.
255. D.C. Adamson, T.J. Cummings, A.H. Friedman. Malignant peripheral nerve sheath tumor of the spine after radiation therapy for Hodgkin’s lymphoma. Clin Neuropathol. 2004;23:245-255.
256. K.M. Foley, J.M. Woodruff, F.T. Ellis, et al. Radiation-induced malignant and atypical peripheral nerve sheath tumors. Ann Neurol. 1980;7:311-318.
257. M.H. Shiu, B.S. Hilaris, L.B. Harrison, et al. Brachytherapy and function-saving resection of soft tissue sarcoma arising in the limb. Int J Radiat Oncol Biol Phys. 1991;21:1485-1492.
258. J.E. Wanebo, J.M. Malik, S.R. VandenBerg, et al. Malignant peripheral nerve sheath tumors: a clinicopathologic study of 28 cases. Cancer. 1993;71:1247-1253.