Chapter 4 Neurologic Examination of the Term and Preterm Infant
The Term Infant
Fetal monitoring during the labor process is difficult, but certain variables can be studied. The fetal biophysical profile includes fetal heart rate reactivity, fetal breathing movements, gross body movements, fetal tone (flexor-extensor movements and posture), and amniotic fluid volume. Ultrasonography has enabled this type of evaluation [Manning et al., 1998]. Other methods of evaluating the fetus are also utilized in various settings [Devoe, 2008]. Fetal central nervous system malformations detected by magnetic resonance imaging (MRI), performed when suspicion of malformations exists, are of value [Herman-Sucharska et al., 2009]. Injury of the developing brain may be offset by the plasticity inherent in the nervous system during the early stages of maturation [Johnston et al., 2009]. Assessment also routinely takes place by means of electronic fetal monitoring [Volpe, 2008a]. Alterations in fetal heart rate patterns may be valuable in assessing fetal status.
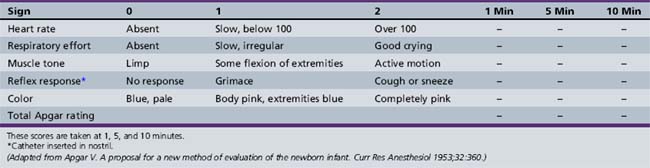
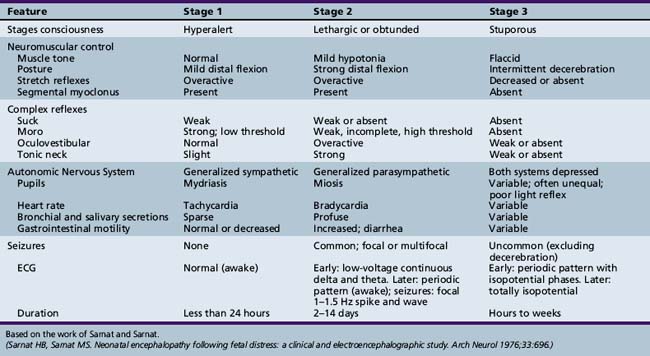
The term infant is examined, when possible, immediately after birth. Apgar scores are routinely obtained for term infants at the time of birth (Apgar, 1953). The categories for scoring are described in Table 4-1. Details of the scoring and the total scores often provide useful information concerning the newborn’s status for the examiner and subsequent health-care providers. Use of the Sarnat score is of value in assessing term infants who are encephalopathic at birth (Table 4-2) [Sarnat and Sarnat, 1976].
The neurologic examination of a term infant should be conducted in a quiet and evenly lit area that is suitably warmed so that the infant remains comfortable after removal of clothing and covering. When the infant is in a stable condition, a thorough examination during the first day is customary. If possible, another examination should be performed on the second or third day of life several hours after feeding so that the infant is optimally responsive. This examination is usually performed just before discharge. In emergent situations, the infant should be evaluated after stabilization has been achieved. It is often necessary to examine the infant on several occasions to confirm the presence and monitor the evolution of abnormal findings. Many protocols for the examination of the term infant (gestational age of 38–42 weeks) have been written [Amiel-Tison, 2002; Ashwal, 1995; Brazelton, 1973; Dubowitz and Dubowitz, 1981; Paine, 1960; Peiper, 1963; Prechtl, 1977]; some investigators have addressed the subject as part of the discussion of neonatal neurology [Fenichel, 2001; Volpe, 2008b]. Estimation of gestational age is discussed later in this chapter.
It is essential that the examination of the term infant be conducted in a systematic manner. Examination of the sick neonate may be difficult because of the presence of monitoring wires, sensors, catheters, eye shields, and infusion lines; however, systematic order in the sequence and extent of examination must be maintained to provide optimal information. These sick infants often must be examined on multiple occasions for sequential monitoring purposes and to complete portions of the examination not possible at the first encounter. The examiner should bear in mind that by 6 weeks post term, neurologic signs, particularly those related to muscle tone and posture, should reflect maturation of the nervous system [Guzzetta et al., 2005]. Furthermore, prediction of long-term development may be possible with the use of a combination of multiple complementary tools, including achieved milestones, neurological examination, and assessment of the quality of motor behavior [Heineman and Hadders-Algra, 2008].
Cranial Vault Evaluation
Among the most important facets of the examination is the measurement of the occipitofrontal (head) circumference. For the most part, this measurement is a reflection of brain growth. However, undue enlargement may be associated with cephalohematoma, subdural fluid collection, hydrocephalus, hydranencephaly, macrocephaly, or megalencephaly (Chapters 21–28). Serial measurements provide an index of brain growth in sick neonates. Microcephaly may be associated with many conditions, including intrauterine infection, hereditary abnormalities, maternal substance abuse, and poor nutrition.
The measurement of the occipitofrontal circumference should be performed carefully. An assistant may be necessary to stabilize the head during measurement. The measuring tape should be moved up and down the head until the largest circumference is obtained. The shape of the head influences the measure of the circumference. The nearer the head shape approximates a perfect circle, the smaller is the head circumference compared with the circumference of a noncircular head, despite the fact that the area of a plane through the maximal circumference and the brain volumes are the same. A similar relationship exists between a perfect sphere and the volume contained within it. The occipitofrontal circumference should be plotted on a graph standardized for gender, race, and gestational age to determine if the measurement falls within the normal range (i.e., two standard deviations above or below the mean) (Figure 4-1) [Braun et al., 2004]. On average, occipitofrontal circumference increases 2 cm during the first month of life, 6 cm during the first 4 months, 7 cm during the first 6 months, and 12 cm during the first 12 months of life [Fujimura and Seryu, 1977].
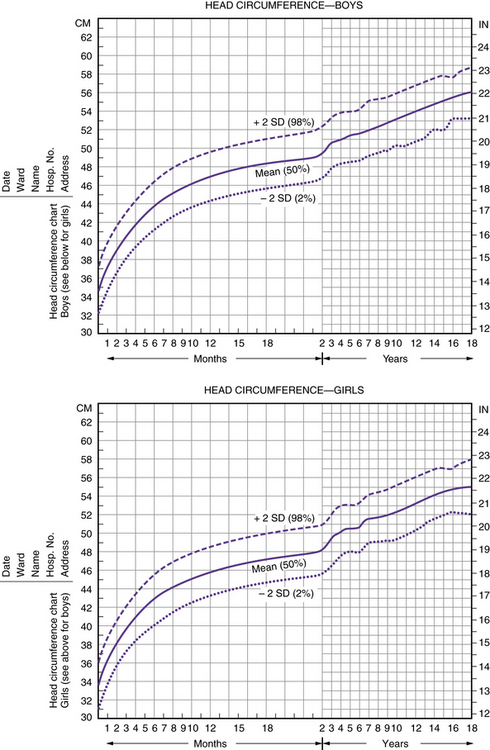
Fig. 4-1 Head circumference charts.
(From Nellhaus G. Composite international and interracial graphs. Pediatrics 1968;41:106.)
Infants delivered vaginally may have some deformity of the head because of scalp and subcutaneous edema with resulting caput succedaneum formation; vacuum extraction delivery often results in caput formation. Infants delivered by cesarean section usually have relatively round heads. The caput deformity, usually transient, produces an increased diameter and may confound accurate occipitofrontal circumference measurements. Cephalohematomas, which are delimited by the periosteum of the individual cranial bones, produce asymmetry of the head and increase the occipitofrontal circumference. Most occur over the parietal bones. A caput succedaneum, unlike a cephalohematoma, extends over two or more cranial bones and is not restricted to the subperiosteal (subgaleal) space. Subgaleal hematomas result from bleeding under the scalp aponeurosis and are often preceded by forceps or vacuum-assisted delivery space [Kilani and Wetmore, 2006]. The scalp may be edematous and boggy because of underlying blood. Although most subgaleal hematomas are benign, hypovolemic shock may ensue if a large amount of blood is sequestered in the subgaleal space.
The anterior fontanel, readily palpable at birth, is concave or flat in relation to the surrounding cranium. The fontanel should be assessed with the child held in the sitting position if there is any question of increased pressure. The fontanel may bulge during crying or in the presence of pathologic increased intracranial pressure. Unfortunately, the presence of normal conformation of the fontanel does not guarantee normal pressure; conversely, a bulging anterior fontanel strongly suggests increased intracranial pressure. The anterior fontanel varies in size but usually ranges from 1 to 3 cm in its longest dimension [Popich and Smith, 1972]. The fontanel pulsates synchronously with the infant’s pulse. The posterior fontanel in the neonate usually is open but admits only a fingertip. The presence of an enlarged posterior fontanel suggests the possibility of intrauterine increased intracranial pressure. From time to time, particularly in the presence of wormian bones, auxiliary fontanels may be palpable. A detailed discussion of the infant skull can be found in Chapter 28.
Auscultation over the infant skull, particularly the anterior fontanel and neck vessels, usually reveals a venous hum in a number of locations. Rarely, systolic-diastolic bruits, particularly those that are focal and asymmetric, indicate the presence of an arteriovenous malformation [Dodge, 1956]; however, these bruits may be heard in normal infants.
Developmental Reflexes
Developmental reflexes are primitive reflexes with complex responses, and largely reflect the integrity of the brainstem and spinal cord; the role of higher centers, although of importance, is not fully known. Many of these reflexes are present at birth and undergo modification during the first 6 months of life. Detailed discussion of these reflexes is presented in Chapter 3. Their persistence beyond the expected date of dissipation suggests maturational lag or impaired CNS function. This group includes the Moro, rooting, grasping, tonic neck, stepping, and placing reflexes. Generalized diminution of the manifestation of these reflexes suggests diffuse depression of brain function. Asymmetry indicates central or peripheral nervous system dysfunction that must be further localized. It is likely that infants born after breech presentation may have significant suppression of active movements when examined at the second and fourth days of life [Sekulic et al., 2009]. A stereotypic “elbowing” movement in newborns has been described. A curved wooden model of an ultrasonographic probe is gently used to exert pressure on the right and left subcostal regions. The newborn reacts with a particular defensive arm movement in which there is a three-phase response [Saraga et al., 2007].
Motor Function
Gentle manipulation of the infant’s limbs allows for assessment of muscle tone and strength. Tone is defined as resistance to passive movement (see Chapter 5). Tone at each large joint should be evaluated while the infant is at rest. Spontaneous movements and resistance of the infant to limb and trunk movement provide a measure of muscle strength. The examiner should recall any clues from the observation period suggesting muscle weakness and corroborating changes in tone and strength at this time. The infant should be supine with the head in the midposition while tone is evaluated so that the tonic neck reflex does not augment tone unilaterally.
Deep tendon reflexes are elicited using a reflex hammer and are often brisk in the newborn, although they may be normally absent [Critchley, 1968]. They may be inordinately enhanced by upper motor neuron abnormalities and are further facilitated by crying. CNS depression may be associated with reduced deep tendon reflexes. The examiner should confirm that the deep tendon reflexes are symmetric, because asymmetry may indicate central or peripheral nervous system impairment. If previous examination has suggested the possibility of hemiparesis, deep tendon reflexes should be carefully evaluated for asymmetry; they are usually increased on the affected side. Deep tendon reflex asymmetry in the arms may be associated with upper motor neuron abnormality, but asymmetrically absent deep tendon reflexes suggest peripheral involvement, possibly the result of brachial plexus injury. Nerve conduction studies in newborns may provide an index of neurologic maturity [Dubowitz et al., 1968].
Controversy remains over the significance of the plantar response in the newborn period in term infants. Although some investigators have reported that the Babinski sign is flexor and symmetric in the newborn period [Hogan and Milligan, 1971], this finding is more likely caused by obtaining a plantar grasp than a Babinski response if only the sole of the foot is used to elicit the response. The plantar response is extensor for at least the first month of life and usually through the first year of life. However, at all times, the response should always be bilaterally symmetric. Persistence of extensor toe-sign responses beyond infancy suggests corticospinal tract impairment and may be associated with alterations in tone and other deep tendon reflex abnormalities. Ankle clonus is frequently elicited in the newborn; rarely are there more than eight beats in normal infants. The clonus is enhanced during crying and may be facilitated during hyperexcitable states, such as those associated with metabolic abnormalities, infection, and subarachnoid hemorrhage. Sustained ankle clonus has the same significance in term newborns as in later life and suggests dysfunction of the corticospinal tracts.
A reflex akin to the plantar response has been described for the hand in term and preterm newborns. The examiner strokes the ulnar aspect of the infant’s palm with the thumb, beginning distally and stroking proximally from the small finger to the hypothenar eminence. The normal response is gradual extension of the fingers, beginning with the small finger and continuing to the middle fingers [Modanlou, 1988]. Lack of response or gross alteration of response may be observed in the presence of corticospinal tract dysfunction.
Cranial Nerve Examination
A more detailed discussion of the cranial nerve examination is found in Chapter 2. Cranial nerve I, the olfactory nerve, is infrequently tested but may be evaluated by the use of pleasant but definitive aromatic substances, such as cinnamon and cloves [Sarnat, 1978]. The infant usually manifests an arrest of activity, arousal, and sucking activity when exposed to these aromas. Virtually all neonates born after more than 32 weeks’ gestation respond [Sarnat, 1978].
Examination of the optic fundi may be difficult but is necessary. Numerous changes, including chorioretinitis (i.e., salt-and-pepper pigmentary changes), may be observed. Hemorrhages are commonly detected after vaginal delivery, even in the absence of traumatic delivery. The optic nerve may be hypoplastic, as manifested by a small, pearl-colored optic disc. The color of the optic disc in the newborn infant is grayish white. Retinal hemorrhages may be found in a large percentage of otherwise normal infants who have no history of abnormal delivery and who later prove to be neurologically normal [Besio et al., 1979]. Further discussion of funduscopic characteristics is presented in Chapter 6.
The newborn infant turns toward a light of moderate intensity and fixes on a bright object or the examiner’s face. Most often, the newborn’s eyes are symmetrically open or closed. If one eye is open and the other closed, there should be a shifting from one side to the other. Width of palpebral fissures should be equal; if not, the presence of ptosis should suggest an abnormality of cranial nerve III function, sympathetic innervation dysfunction, neuromuscular junction difficulty, weakness of the levator muscle of the lid, or abnormality of the lid connective tissue. Among the conditions to be considered are congenital myasthenia gravis, myotonic dystrophy, Horner’s syndrome (Figure 4-2), Möbius’ syndrome, congenital myopathies, and Duane’s syndrome. Occasionally, central or peripheral seventh nerve paresis may result in asymmetry of the palpebral fissure.
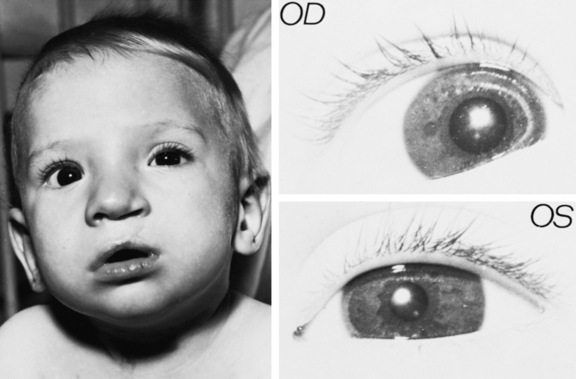
Fig. 4-2 Horner’s syndrome (left eye).
Miosis and ptosis are plainly evident.
(Courtesy of the Division of Pediatric Neurology, University of Minnesota Medical School.)
Extraocular movements should be monitored while a child is lying quietly. Slight lapses of conjugate gaze are common in the newborn period. Newborn visual acuity is difficult to assess, but black and white-patterned objects can be used. The examiner’s face is often the best “target.” The intended object of focus is moved slowly in the infant’s field of vision, less than a foot from the infant’s eyes. The infant slowly follows with eye movement, particularly in lateral directions. Prolonged gaze may occur in the newborn period [Brazelton et al., 1976]. Opticokinetic nystagmus may be elicited by using a striped, rotating drum or striped cloth strip, which is slowly pulled across the infant’s visual field in the vertical and horizontal directions. The response is the same as in older children (see Chapter 2).
Cranial nerve VII involvement may be the result of the position of the infant in the maternal pelvis and delivery by pressure incurred during forceps delivery, or by agenesis of the motor nucleus of cranial nerve VII. Facial movements are readily observed during crying; an asymmetry of mouth movement may indicate cranial nerve VII involvement. During crying, the angle of the mouth is depressed on the normal side. The syndrome referred to as asymmetric crying facies may manifest this way [Nelson and Eng, 1972]. This syndrome results from weakness of the lower lip caused by hypoplasia of the depressor muscle of the mouth angle. This phenomenon is a congenital abnormality and does not signal cranial nerve VII involvement. This condition also may be associated with somatic atrophy, vertebral and rib abnormalities, renal dysgenesis, and most importantly, cardiac defects (i.e., atrial or ventricular septal defect; cardiofacial syndrome) [Pape and Pickering, 1972].
If necessary, in the presence of olfactory, gustatory, visual, tactile, or auditory stimuli, sophisticated monitoring and scoring of body activity may be performed [Brazelton et al., 1976]. All such sensory stimuli produce habituation in the newborn [Lipsitt, 1977]. Lack of habituation or failure to respond to these stimuli is abnormal; however, the abnormality may be specific for the sensory mechanism or merely may be a reflection of generalized CNS depression.
The Preterm Infant
The designation of an infant as preterm is related primarily to length of gestation. Term gestation is 38–42 weeks from conception, and preterm therefore is any period less than 38 weeks, although most clinicians would not consider a baby preterm or premature between 36 and 38 weeks. Expected developmental milestones are based on gestation [Mercuri et al., 2003]. The clinician must estimate gestational age to facilitate interpretation of the observations and findings made during the neurologic examination. An infant whose birth weight is low compared with length of gestation (e.g., intrauterine growth retardation, small for gestational age) exhibits different growth patterns or neurologic findings than the infant whose weight is appropriate for gestational age. In a parallel fashion, preterm infants born of diabetic mothers may weigh more than 2500 g, but these infants manifest findings consistent with their preterm status during the neurologic evaluation.
The designation of extremely low birth weight infants has been assigned to those infants who weigh less than 1000 g at birth [Doyle et al., 2004; Kilbride, 2004]. Most of these infants are at 28 weeks’ gestation or less. The very low birth weight infants are those who weigh less than 1500 g at birth. In the absence of intrauterine growth retardation, most infants of this birth weight are born after 31–32 weeks’ gestation [Lubchenco et al., 1966]. The neurologic examination of these infants is reviewed in this portion of the chapter; the expected results of the examination should be based on the gestational age as determined from the various tables and illustrations.
General Examination
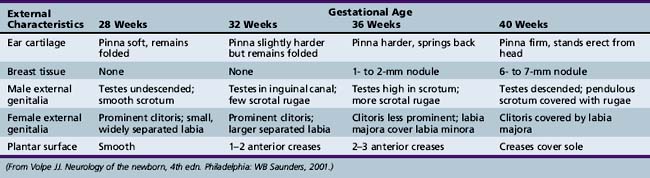
It is not possible with any great assurance to estimate gestational age from the date of the first day of the mother’s last menstrual period [Lubchenco, 1970]. Nevertheless, a number of physical findings evident during the examination can prove helpful in this evaluation [Farr et al., 1966a, 1966b; Lubchenco, 1970; Usher and McLean, 1969]. Among the most valuable findings are skin texture and color, quantity of breast tissue and ear cartilage, and the stage of development of the external genitalia. No single characteristic can determine the gestational age. The estimate should be based on the average of expected findings (Table 4-3). The combination of findings based on physical characteristics and neurologic examination has proved of value in the estimation of gestational age [Dubowitz et al., 1970]. This method is discussed with the specifics of the neurologic examination.
Electrophysiologic Assessment
Motor nerve conduction velocity studies of the ulnar and posterior tibial nerves may corroborate the clinical estimation of gestational age because nerve velocity becomes more rapid with maturation. Such studies also differentiate infants of short gestation from those with intrauterine growth retardation, including those at high risk and with very low birth weight [Dubowitz et al., 1968; Cruz-Martinez et al., 1983; Miller et al., 1983; Moosa and Dubowitz, 1972].
Electroencephalographic (EEG) patterns also appear to be a function of maturity. Comparison of EEG, anatomic, and clinical criteria provides one means of estimating gestational age [Scher and Barmada, 1987]. EEG correlates are listed in Box 4-1 [Scher and Barmada, 1987] and are further discussed in Chapter 12.
Neurologic Examination
Environmental Interaction
Periods of apparent wakefulness are rare before 28 weeks. Arousal by external stimulation is often necessary for the 28-week preterm infant [Illingworth, 1972]. Nevertheless, small preterm infants respond to environmental factors, such as temperature, light, and feeding. Periods of wakefulness and somnolence are relatively brief and change swiftly in the preterm infant. The waking periods of preterm infants of 25–30 weeks’ gestation are short compared with those of term infants [Fenichel, 1978, 1985]. A readily recognizable level of alertness during wakeful stages occurs even in infants of 31 weeks’ gestation [Hack et al., 1976]. In infants of 32 weeks’ gestation, external stimulation is usually unnecessary. Responsiveness increases with CNS maturation; suck rate increases, and sucking persists for longer periods. In those born after 37 weeks’ gestation, crying is commonly present during wakefulness. By 40 weeks’ gestational age, the preterm infant continues to be alert for reasonable periods and responds to visual, auditory, and tactile stimulation. Sleep and wakeful periods are easily identified. Sleep is discussed in detail in Chapter 66.
Formal Scale of Gestational Assessment
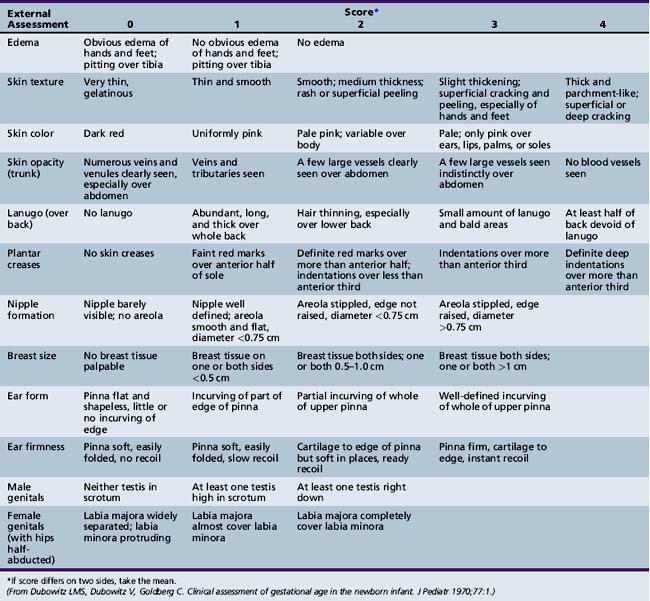
Using a systematic evaluation of body and neurologic characteristics, Dubowitz and colleagues [1970] were able to achieve a high correlation with gestational age, making this method of assessment most valuable. The evaluation was based on 10 neurologic and 11 external (e.g., skin texture, breast size, ear form) characteristics. The external evaluation was adapted from Farr and colleagues [1966a, 1966b]. The scoring scheme is detailed in Figure 4-3 and Figure 4-4 and in Table 4-4 [Dubowitz et al., 1970].
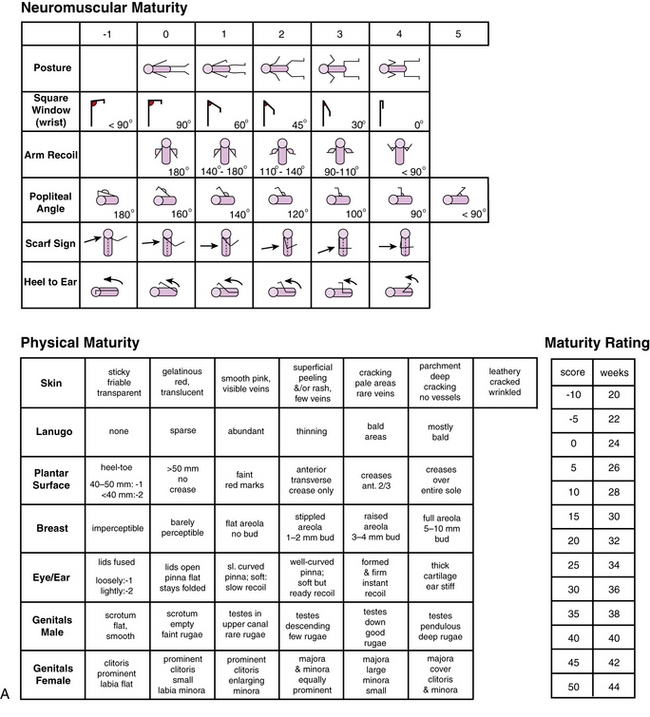
Fig. 4-3 A, Scoring system for neurologic criteria.
(From Dubowitz L, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr 1970;77:1.)

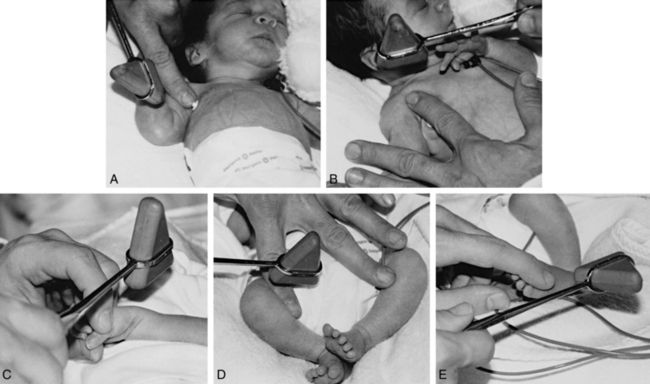
Fig. 4-3 B, Description of techniques used to assess neurologic signs.
(From Dubowitz L, Dubowitz V Goldberg. Clinical Assessment of gestational age in the newborn infant, J Pediatr 1970; 77:1.)
Deep Tendon Reflex Assessment
The assessment of deep tendon reflexes in preterm infants can be of value in the presence of many conditions, such as spinal cord anomalies, peripheral nerve injuries, congenital myopathies, and infantile spinal muscular atrophy. A standard method of deep tendon reflex examination for preterm infants has been proposed [Kuban et al., 1986]. The methods of elicitation are described in Table 4-5 and depicted in Figure 4-5 [Kuban et al., 1986]. Deep tendon reflexes differ in healthy and ill preterm infants of 33 weeks’ gestation; the elicitation rate and intensity of deep tendon reflexes vary with maturity (i.e., less than and greater than 33 weeks’ gestation) [Kuban et al., 1986]. In a study of preterm infants of more than 27 weeks’ postconceptional age, the pectoralis major reflex was elicited in all, regardless of maturity. In 98 percent of the infants of more than 33 weeks’ gestation, the Achilles, patellar, biceps, thigh adductor, and brachioradialis reflexes were obtained. Infants of less than 33 weeks’ gestation had decreased elicitation rates for patellar and biceps reflexes and had overall decreases in reflex intensity compared with their older counterparts. For all infants in the study, the following tendon reflexes were found to be present (in decreasing order): finger flexors, jaw, crossed adductors, and triceps. Contrary to conventional wisdom, head position had no effect on the reflexes [Kuban et al., 1986].
| Deep Tendon Reflexes | Innervation | Technique of Elicitation |
|---|---|---|
| Jaw | Cranial nerves V and VII | The point of the finger is placed over the chin so that the jaw is slightly open. The hammer strikes the index finger tip |
| Pectoralis major | Predominantly C7 and C8, lateral pectoral nerve | The examiner’s index finger is firmly placed in a caudad (or cephalad) direction on to the axilla over the pectoralis major |
| Biceps | C5 and C6, musculocutaneous nerve | The examiner’s index finger is placed on to the biceps tendon in the superomedial aspect of the antecubital fossa with the arm flexed at the elbow |
| Brachioradialis | C5, C6, radial nerve | The tendon of the muscle is struck directly over the distal third of the radius while slowly flexing and extending the arm |
| Triceps | C7, C8, radial nerve | The examiner’s finger is placed over the triceps tendon at its distal aspect proximal to the elbow. The triceps tendon also may be struck directly while flexing and extending the arm |
| Finger flexors | C8, T1, median nerve | The examiner’s finger is placed horizontally across the base of the infant’s fingers to elicit a partial grasp. The examiner’s finger is then struck |
| Patellar | L3, L4, femoral nerve | The reflex hammer strikes the examiner’s finger placed across the patellar tendon, or the latter is struck directly while flexing and extending the leg at the knee |
| Thigh adductors and crossed adductors | L3, L4, obturator nerve | The examiner’s index finger to be struck is placed diagonally across the medial aspect at the knee (thigh adductor) with the little finger placed on the contralateral leg to maintain a 45- to 60-degree angle (crossed adductor) |
| Achilles | L5, S1, tibial nerve | The examiner’s finger to be struck is placed horizontally across the plantar aspect of the infant’s foot, which is partially dorsiflexed |
(From Kuban KCK, Skouteli HN, Urion DK, et al. Deep tendon reflexes in premature infants. Pediatr Neurol 1986;2:266.)
Body Attitude
During maturation, preterm infants adopt typical postures that correspond to gestational age. These postures have been charted and are very useful for evaluation of gestational age [Dubowitz et al., 1970; Dubowitz and Dubowitz, 1981].
Muscle Tone
Assessment of muscle tone in the preterm infant is requisite to completion of a satisfactory neurologic evaluation [Paro-Panjan et al., 2005; Amiel-Tison, 1968, 2002; Saint-Anne Dargassies, 1966]. The muscle tone of small-for-gestational-age infants differs from that of infants with only a short gestation.
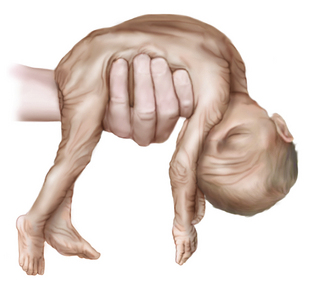
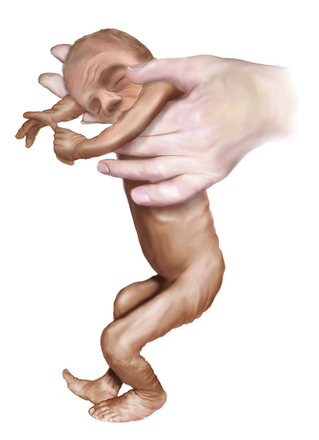
At 26–28 weeks’ gestation, the infant is extremely hypotonic. When held by the examiner in vertical suspension, the infant does not extend the head, limbs, or trunk (Figure 4-6). The change from the hypotonia of the preterm infant to the flexion posture of the term infant manifests first in the legs and then in the arms and head. At 34 weeks’ gestation, the infant lies in the frogleg position while supine; the legs are flexed at the hip and knee, but the arms remain extended and relatively hypotonic (Figure 4-7).
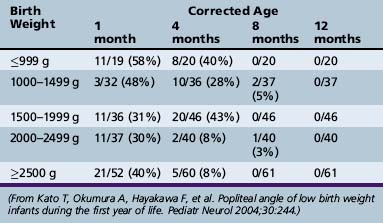
Measurement of various limb angles offers some objective evidence for the degree of tone. The popliteal angle, measured by maximum extension of the leg at the knee with the hip fully flexed, decreases from 180 degrees at 28 weeks’ gestation (Figure 4-8; see also Figure 4-14) to less than 90 degrees at term [Kato et al., 2005]. Similarly, the adductor and dorsiflexion angle of the foot diminishes to almost 0 degrees at term (Figure 4-9).

Fig. 4-14 Measurement of the popliteal angle in an infant who was 8 months old (corrected age).
(From Kato T, Okumura A, Hayakawa F, et al. Popliteal angle of low birth weight infants during the first year of life. Pediatr Neurol 2004;30:244.)
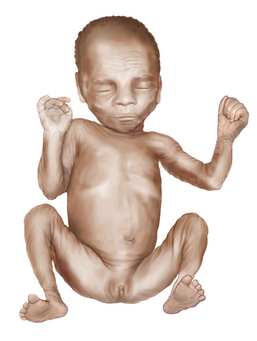
Fig. 4-9 The adductor angle of the thighs is almost 180 degrees in a preterm infant of 30 weeks’ gestation.
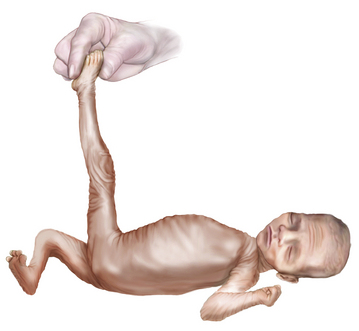
In small preterm infants, the scarf sign, which is elicited by folding the arm across the chest toward the opposite shoulder, is present if the elbow reaches the opposite shoulder (Figure 4-10). In term infants, the elbow cannot be brought beyond the midline.
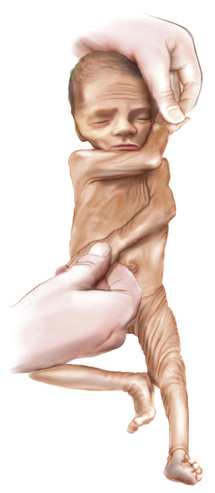
The extreme hypotonia of preterm infants permits the legs to be flexed at the hip so that the heel can be passively brought to the side of the face (i.e., heel-to-ear maneuver). Understandably, this positioning is restricted in the older infant because of increasing tone (Figure 4-11).
Tone may also be monitored while postural and righting reflexes are assessed. During the stepping maneuver, the 28-week preterm infant will not support weight (Figure 4-12). However, over the next few weeks, there is gradual support of weight, and by 34 weeks, a good supporting response is present. Tremors and even clonic movements manifest in the small preterm infant but are not normally discernible after 32 weeks’ gestation. Stretching movements of the limbs are common in small preterm infants while they are awake but somewhat less common during sleep. These movements may spread to include the trunk and head.
Serial measurements may indicate the likelihood of developing spasticity. A “tight” (<120 degrees) angle was found at 4 months in infants with birth weights ranging from less than 999 g up to 1999 g. Infants who had birth weights ranging from 2000 to more than 2500 g had only an 8 percent incidence of tight popliteal angle (Table 4-6).
Cranial Nerves
Some features of the preterm infant examination are different from features of the older infant’s examination. Head position is unpredictable in the small preterm infant, but by 35 weeks’ gestation, there is a preference for the head to be held to the right. By 39 weeks’ gestation, the head is held to the right approximately 80 percent of the time while the infant is at rest [Gardner et al., 1977].
The small preterm infant may cry in response to provocation [Fenichel, 1978], but crying often occurs when the infant is unprovoked. By 36–37 weeks’ gestation, the cry is more vigorous, frequent, and persistent, and it is easily elicited by noxious stimuli.
Although they may forcefully close their eyes when a bright light is directed toward them, infants of 28 weeks’ gestation or less do not turn in the direction of the light. By using a large target (e.g., large, red ball; hoop; handful of yarn), visual fixation and even rudimentary scanning and tracking may be evident in infants of 31–32 weeks’ gestation [Hack et al., 1976]. Associated with this response, there may be widening of the palpebral fissure. By 36–38 weeks’ gestation, the infant rotates the head toward a light and closes the eyes forcefully when a strong light stimulus is presented.
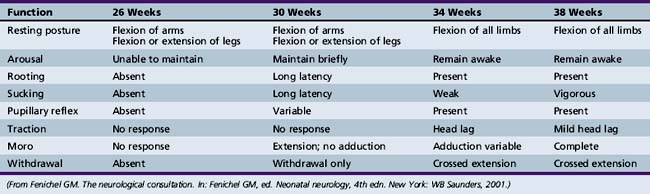
Developmental Reflexes
Observation and description of the major reflex changes peculiar to the preterm infant have been undertaken by many investigators (Table 4-7) [Amiel-Tison, 1968, 2002; Fenichel, 1985; Lubchenco, 1970; Saint-Anne Dargassies, 1966].
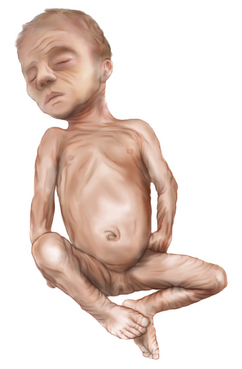
Ongoing neurologic examinations of the preterm infant are most important for the assessment of development and neurologic status. When the preterm infant reaches the equivalent of 40 weeks’ gestation, the neurologic examination results are not the same as those of a term newborn [Illingworth, 1972]. After reaching 40 weeks’ gestation the preterm infant lies with relatively less elevation of the pelvis, and so the prone body profile is flatter than that of the term newborn (Figure 4-13). The preterm infant continues toe-walking and, even at 40 weeks, manifests relative hypotonia, incomplete dorsiflexion of the foot, and a greater popliteal angle compared with the term newborn.

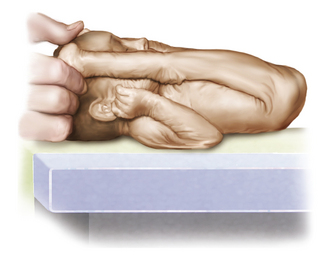
Fig. 4-13 The hips are abducted and the pelvis is low in a prone preterm infant of 32 weeks’ gestation.
Tone can also be evaluated by measurement of the popliteal angle in preterm infants. These data provide useful information in assessing tone (Kato et al., 2004). The angle is assessed as shown in Figure 4-14. Data documenting preterm infants and popliteal angle changes are found in Table 4-6.
Assessment of Head Growth Patterns
It is essential to measure and plot growth data to facilitate early detection of abnormal patterns. The shape of the head changes markedly with growth in preterm infants. The ratio of anteroposterior diameter to biparietal diameter increases rapidly in preterm infants during the first few months of life [Baum and Searls, 1971].
There are known differences between the extrauterine body growth patterns of preterm infants and the extrauterine body growth patterns of the term infant [Babson et al., 1970; Gardner and Pearson, 1971; Lubchenco et al., 1966; Usher and McLean, 1969]. The occipitofrontal circumference of preterm infants often shrinks during the first few days of life. Expected patterns of extrauterine head growth are reasonably well documented, making diagnosis of hydrocephalus or microcephaly in the preterm infant possible. Frequent serial occipitofrontal circumference measurements should be obtained to allow early diagnosis. A standard plotting curve must be used to monitor head growth in the preterm infant. A useful standard plot is depicted in Figure 4-15 [Babson and Benda, 1976]. Conventional symptoms and signs of hydrocephalus are not immediately evident, even though the presence of ventricular dilatation is documented with imaging techniques [Korobkin, 1975; Volpe et al., 1977].
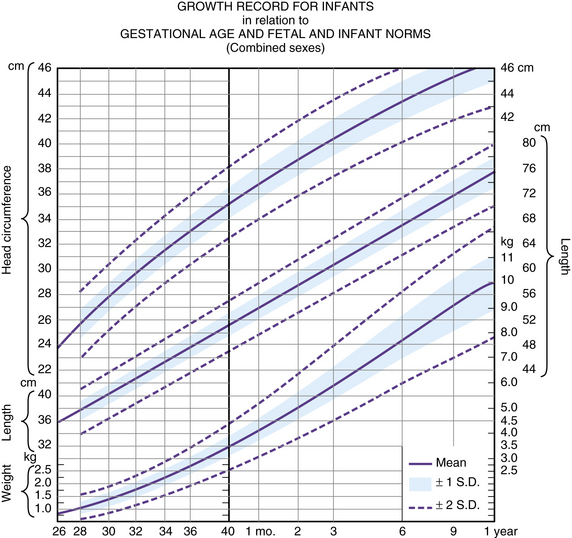
Fig. 4-15 A fetal-infant growth graph for infants of various gestational ages.
(From Babson SG, Benda GI. Growth graphs for the clinical assessment of infants of varying gestational age. J Pediatr 1976;89:814.)
The presence of certain characteristics should alert the clinician to the presence of hydrocephalus [Sher, 1982]; these include full fontanel with separation of the cranial sutures, abnormally high rate of occipitofrontal circumference increase, frontal bossing, scaphocephaly, and increased ratio of head size to body length.
The preterm infant’s state of health is a major determinant of head growth [Sher and Brown, 1975a, 1975b]. Mean occipitofrontal circumferences for small and large, healthy, preterm infants and for sick infants are graphed [Sher and Brown, 1975a] for comparison with the data of O’Neill [1961]. Irrespective of gestational age, sick infants were designated as those who were maintained with mechanical ventilation and intravenous therapy for various periods up to 2 weeks [Sher and Brown, 1975a, 1975b]. Infants with easily correctable metabolic abnormalities or minimal degrees of hyperbilirubinemia not requiring exchange transfusion were excluded from the sick group. On the basis of the study of Sher and Brown [1975a, 1975b], some conclusions are possible [Sher, 1982].
The rate of occipitofrontal circumference increase in the healthy preterm infant is approximately double (1.1 cm/week) that of the term infant in the first and second months after delivery (Figure 4-16). The rate of increase of occipitofrontal circumference in the healthy preterm infant is approximately equal (0.5 cm/week) to that of the term infant in the third and fourth months. The average rate of occipitofrontal circumference for ill preterm infants is 0.25 cm/week for the first 3 months.
When comparisons are made based on postconceptional age, the occipitofrontal circumference of preterm infants is greater than that of the term infant, at least for the first 5 postnatal months [Fujimura et al., 1977]. The maximum velocity of head growth in healthy, preterm infants with good caloric intake occurs shortly postpartum and decreases thereafter.
In general, the prognosis for normal development of low birth weight infants is much improved since the advent of focused obstetric practices related to the preterm infant and neonatal intensive care units [Hutson et al., 1986; Kitchen and Murton, 1985]; however, the increase in the number of very low birth weight infants has resulted in an increase in the prevalence of cerebral palsy in this population [Pharoah et al., 1990]. The clinician must be cognizant of the normal, rapid rate of head growth and avoid unnecessary procedures designed to diagnose hydrocephalus.
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Amiel-Tison C. Neurologic evaluation of the maturity of newborn infants. Arch Dis Child. 1968;43:89.
Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr Neurol. 2002;27:196.
Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesthesiol. 1953;32:360.
Ashwal S. Historical aspects of the neonatal neurological examination: Why child neurologists are not “little adult neurologists”. J Hist Neurosci. 1995;4:3.
Babson S.G., Behrman R.E., Lessel R. Fetal growth: Liveborn birth weights for gestational age of white middle class infants. Pediatrics. 1970;45:937.
Babson S.G., Benda G.I. Growth graphs for the clinical assessment of infants of varying gestational age. J Pediatr. 1976;89:814.
Baum J.D., Searls D. Head shape and size of newborn infants. Dev Med Child Neurol. 1971;13:576.
Besio R., Caballero C., Meerhoff E., et al. Neonatal retinal hemorrhages and influence of perinatal factors. Am J Ophthalmol. 1979;87:74.
Brazelton T.B. Neonatal behavioral assessment scale. London: Spastics International Medical Publications, 1973.
Brazelton T.B., Parker W.B., Zuckerman B. Importance of behavioral assessment of the neonate. Curr Probl Pediatr. 1976;2:49.
Braun L., Flynn D., Ko C.W., et al. Gestational age-specific growth parameters for infants born at US military hospitals. Ambul Pediatr. 2004;4:461.
Critchley E.M. The neurological examination of neonates. J Neurol Sci. 1968;7:427.
Cruz-Martinez A., Ferrer M.T., Martin M.J. Motor conduction velocity and H-reflex in prematures with very short gestational age. Electromyogr Clin Neurophysiol. 1983;23:13.
Devoe L.D. Antenatal fetal assessment: contraction stress test, non stress test, vibroacoustic stimulation, amniotic fluid volume, biophysical profile, and modified biophysical profile – an overview. Semin Perinatol. 2008;32:247.
Dodge H.W. Cephalic bruits in children. J Neurosurg. 1956;13:527.
Doyle L.W., for the Victorian Infant Collaborative Group. Changing availability of neonatal intensive care for extremely low birthweight infants in Victoria over two decades. Med J Aust. 2004;181:136.
Dubowitz L., Dubowitz V. The neurological assessment. In: Dubowitz L., Dubowitz V., editors. The neurological assessment of the preterm and full-term newborn infant. Clinics in developmental medicine. London: William Heinemann Medical Books, 1981. no. 79
Dubowitz L., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77:1.
Dubowitz W., Whittaker G.F., Brown B.H., et al. Nerve conduction velocity: An index of neurologic maturity of the newborn infant. Dev Med Child Neurol. 1968;10:741.
Farr V., Kerridge D.F., Mitchell R.G. The value of some external characteristics in the assessment of gestational age at birth. Dev Med Child Neurol. 1966;8:657.
Farr V., Mitchell R.G., Neligan G.A., et al. The definition of some external characteristics used in the assessment of gestational age in the newborn infant. Dev Med Child Neurol. 1966;8:507.
Fenichel G.M. Neurological assessment of the 25 to 30 week premature infant. Ann Neurol. 1978;4:92.
Fenichel G.M. The neurological consultation. In Fenichel G.M., editor: Neonatal neurology, ed 2, New York: Churchill Livingstone, 1985.
Fenichel G.M. Clinical pediatric neurology: A signs and symptoms approach, ed 4. Philadelphia: WB Saunders, 2001.
Fujimura M., Seryu J.I. Velocity of head growth during the prenatal period. Arch Dis Child. 1977;52:105.
Gardner D., Pearson J. A growth chart for premature and other infants. Arch Dis Child. 1971;46:783.
Gardner J., Lewkowicz D., Turkewitz G. Development of postural asymmetry in premature human infants. Dev Psychobiol. 1977;10:471.
Guzzetta A., Haataja L., Cowan F., et al. Neurological examination in healthy term infants aged 3–10 weeks. Biol Neonate. 2005;87(3):187-196. Epub 2004
Hack M., Mostow A., Miranda S.B. Development of attention in preterm infants. Pediatrics. 1976;58:669.
Heineman K.R., Hadders-Algra M. Evaluation of neuromotor function in infancy – A systematic review of available methods. J Dev Behav Pediatr. 2008;29:315.
Herman-Sucharska I., Bekiesinska-Figtowska M., Urbanik A. Fetal central nervous system malformations on MR images. Brain Dev. 2009;31:185.
Hogan G.R., Milligan J.E. The plantar reflex of the newborn. N Engl J Med. 1971;285:502.
Hutson J.M., Driscoll J.M., Fox H.E., et al. The effect of obstetric management on neonatal mortality and morbidity for infants weighing 700–1000 grams. Am J Perinatol. 1986;3:255.
Illingworth R.S. The development of the infant and young child, ed 5. Baltimore: Williams & Wilkins, 1972.
Johnston M., Ishida A., Nakajima Ishida W., et al. Plasticity and injury in the developing brain. Brain Dev. 2009;31:1.
Kato T., Okumura A., Hayakawa F., et al. Popliteal angle of low birth weight infants during the first year of life. Pediatr Neurol. 2004;30:244.
Kato T., Okumura A., Hayakawa F., et al. Popliteal angle in preterm infants with periventricular leukomalacia. Pediatr Neurol. 2005;32:84.
Kilani R.A., Wetmore J. Neonatal subgaleal hematoma: presentation and outcome-radiological findings and factors asssociated with mortality. Am J Perinatol. 2006;23:41.
Kilbride H.W. Effectiveness of neonatal intensive care for extremely low birth weight infants. Pediatrics. 2004;114:1374.
Kitchen W.H., Murton L.J. Survival rates of infants with birth weights between 501 and 1,000 gm: Improvement by excluding certain categories of cases. Am J Dis Child. 1985;139:470.
Korobkin R. The relationship between head circumference and the development of communicating hydrocephalus following intraventricular hemorrhage. Pediatrics. 1975;56:74.
Kuban K.C.K., Skouteli H.N., Urion D.K., et al. Deep tendon reflexes in premature infants. Pediatr Neurol. 1986;2:266.
Lipsitt L.P. The study of sensory and learning processes of the newborn. Clin Perinatol. 1977;4:170.
Lubchenco L.O. Assessment of gestational age and development at birth. Pediatr Clin North Am. 1970;17:125.
Lubchenco L.O., Hansman M., Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403.
Manning F.A., Bondaji N., Harman C.R., et al. Fetal assessment based on fetal biophysical profile scoring. VIII. The incidence of cerebral palsy in tested and untested perinates. Am J Obstet Gynecol. 1998;178:696.
Mercuri E., Guzzetta A., Laroche S., et al. Neurologic examination of preterm infants at term age: Comparison with term infants. Pediatrics. 2003;142:647.
Miller G., Heckmatt J.Z., Dubowitz L.M., et al. Use of nerve conduction velocity to determine gestational age in infants at risk and in very-low-birth-weight infants. J Pediatr. 1983;103:109.
Modanlou H. Extension reflex of fingers in the newborn. Pediatr Neurol. 1988;4:66.
Moosa A., Dubowitz V. Assessment of gestational age in newborn infants: Nerve conduction velocity versus maturity score. Dev Med Child Neurol. 1972;14:290.
Nelson K.B., Eng G.D. Congenital hypoplasia of depressor anguli oris muscle: Differentiation from congenital facial palsy. J Pediatr. 1972;81:16.
O’Neill E. Normal head growth and prediction of head size in infantile hydrocephalus. Arch Dis Child. 1961;36:241.
Paine R.S. Neurologic examination of infants and children. Pediatr Clin North Am. 1960;7:41.
Pape K.E., Pickering D. Asymmetrical crying facies: Index of other congenital anomalies. J Pediatr. 1972;81:21.
Peiper A. Cerebral function in infancy and childhood. New York: Consultants Bureau Enterprises, 1963.
Pharoah P.O.D., Cooke T., Cooke R.W.I., et al. Birthweight specific trends in cerebral palsy. Arch Dis Child. 1990;65:602.
Popich G.A., Smith D.W. Fontanels: Range of normal size. J Pediatr. 1972;80:749.
Prechtl H.F.R. The neurological examination of the full term newborn infant, ed 2. London: William Heinemann Medical Books, 1977.
Saint-Anne Dargassies S. Neurologic maturation of the premature infant of 28 to 41 weeks’ gestational age. In: Falkner F., editor. Human development. Philadelphia: WB Saunders, 1966.
Saraga M., Resić B., Krnić D., et al. A stereotypic “elbowing” movement, a possible new primitive reflex in newborns. Pediatr Neurol. 2007;36(2):84-87.
Sarnat H.B. Olfactory reflexes in the newborn infant. J Pediatr. 1978;92:624.
Sarnat H.B., Sarnat M.S. Neonatal encephalopathy following fetal distress. Arch Neurol. 1976;33:696.
Scher M.S., Barmada M.A. Estimation of gestational age by electrographic, clinical, and anatomic criteria. Pediatr Neurol. 1987;3:256.
Sekulic S., Zarkov M., Slankamenac P., et al. Decreased expression of the righting reflex and locomotor movements in breech-presenting newborns in the first days of life. Early Hum Dev. 2009;85:263.
Sher P.K. Neurologic examination of the premature infant. In: Swaiman K.F., Wright F.S., editors. The practice of pediatric neurology. St. Louis: CV Mosby, 1982.
Sher P.K., Brown S. A longitudinal study of head growth in preterm infants. I. Normal rates of head growth. Dev Med Child Neurol. 1975;17:705.
Sher P.K., Brown S. A longitudinal study of head growth in preterm infants. II. Differentiation between “catch-up” head growth and early infantile hydrocephalus. Dev Med Child Neurol. 1975;17:711.
Usher R., McLean F. Intrauterine growth of liveborn Caucasian infants at sea level: Standard obtained from infants born between 24 and 44 weeks’ gestation. J Pediatr. 1969;74:901.
Volpe J.J. Hypoxic-ischemic encephalopathy. In: Volpe J.J., editor. Neurology of the newborn. ed 5. Philadelphia: WB Saunders; 2008:247.
Volpe J.J. The neurological examination: normal and abnormal features. In: Volpe J.J., editor. Neurology of the newborn. ed 5. Philadelphia: WB Saunders; 2008:121.
Volpe J.J., Pasternak J.F., Allan W.C. Ventricular dilation preceding rapid head growth following neonatal intracranial hemorrhage. Am J Dis Child. 1977;131:1212.
Bacola E., Behrle F.C., deSchweinitz L., et al. Perinatal and environmental factors in late neurogenic sequelae. I. Infants having birth weights under 1500 grams. Am J Dis Child. 1966;112:359.
Bacola E., Behrle F.C., deSchweinitz L., et al. Perinatal and environmental factors in late neurogenic sequelae. II. Infants having birth weights from 1500 to 2500 grams. Am J Dis Child. 1966;112:369.
Beintema D.J. A neurological study of newborn infants. London: William Heinemann Medical Books, 1968.
Crosse V.M. The premature baby, ed 4. London: J&A Churchill, 1957.
Cruise M.O. A longitudinal study of the growth of low birth weight infants. I. Velocity and distance growth, birth to 3 years. Pediatrics. 1973;51:620.
Dekaban A. Examination. In: Dekaban A., editor. Neurology of early childhood. Baltimore: Williams & Wilkins, 1970.
Dodge P.R. Neurologic history and examination. In: Farmer T.W., editor. Pediatric neurology. New York: Harper & Brothers, 1964.
Heineman K.R., Hadders-Algra M. Evaluation of neuromotor function in infancy-A systematic review of available methods. J Dev Behav Pediatr. 2008;29:315.
Knobloch H., Pasamanick B. Predicting intellectual potential in infancy. Am J Dis Child. 1963;166:43.
Paine R.S. Neurologic conditions in the neonatal period: diagnosis and management. Pediatr Clin North Am. 1961;8:577.
Paine R.S. The evolution of infantile postural reflexes in the presence of chronic brain syndromes. Dev Med Child Neurol. 1964;6:345.
Paine R.S., Brazelton T.B., Donovan D.E., et al. Evolution of postural reflexes in normal infants and in the presence of chronic brain syndromes. Neurology. 1964;14:1036.
Paro-Panjan D., Sustersic B., Neubauer D. Comparison of two methods of neurologic assessment in infants. Pediatr Neurol. 2005;33:317.
Rawlings G., Reynolds E.O.R., Stewart A., et al. Changing prognosis for infants of very low birth weight. Lancet. 1971;1:516.
Robinson R.J. Assessment of gestational age by neurologic examination. Arch Dis Child. 1966;41:437.
Saint-Anne Dargassies S. Long-term neurological follow-up study of 286 truly premature infants. Dev Med Child Neurol. 1977;19:462.
Sustersic B., Paro-Panian D. Assessment of general movements in relation to neurologic signs at age two years. Pediatr Neurol. 2008;39:108.