Chapter 101 Neurologic Disorders in Children with Heart Disease
Neurologic Conditions Associated with Congenital Heart Disease
Children with congenital heart disease (CHD) and its complications comprise one of the largest inpatient populations in major pediatric centers, and neurologic dysfunction is one of the most common extracardiac complications of CHD. The mortality of CHD has decreased markedly over the years, especially among the most critically ill infants at greatest risk of brain dysfunction. As neurodevelopmental integrity is a major factor determining quality of life, the prevention of neurologic dysfunction is central to the management of these children. Reliable estimates of both the incidence of CHD and the prevalence of neurologic dysfunction are difficult to obtain and vary widely across studies [Reller et al., 2008; Rosenthal, 1998]. This variation is due to differences in the diagnostic criteria and classification for both CHD and its neurologic complications. A recent review of 62 studies reported CHD incidences ranging from 4 to 75 per 1000 live births; the lower rates apply to moderate and severe CHD lesions, while the higher rates include even trivial CHD anomalies, such as small muscular ventricular septal defects [Hoffman and Kaplan, 2002]. The most reliable data are those for infants with “critical CHD” (i.e., requiring an interventional procedure during the first year of life) [Benson, 1989; Castaneda et al., 1974, 1989], which have an incidence around 1.5 per 1000 of all births [Reller et al., 2008].
Trends in Cardiac Surgery and Associated Neurologic Complications
The birth of pediatric cardiac surgery was in 1938, when Frey in Düsseldorf and Gross in Boston performed the first successful ligations of patent ductus arteriosus [Gross and Hubbard, 1984; Kaemmerer et al., 2004]. Over the subsequent seven decades, advances in CHD management have been associated with changes in the etiologic and clinical profile of neurologic dysfunction in children with CHD. In the years prior to cardiopulmonary bypass (CPB), neurologic complications of CHD were related, in large part, to the effects of right-to-left shunting and chronic cyanosis [Berthrong and Sabiston, 1951; Cottrill and Kaplan, 1973; Martelle and Linde, 1961; Phornphutkul et al., 1973; Terplan, 1976; Tyler and Clark, 1957a, 1957b]. The clinical application of CPB by Kirklin and Lillehei in 1955 [Moller et al., 2009] provided the extracorporeal circulatory support necessary for open-heart surgery. However, repair of complex CHD lesions in the newborn and young infant remained a major surgical challenge for almost two decades, due to the small heart size and the constricted surgical field. During this period, surgical options for complex CHD lesions were limited to repeated palliative procedures while awaiting sufficient physical growth to permit definitive corrective procedures. This delay to definitive cardiac repair exposed infants and children to the risk of cumulative brain injury from chronic hypoxia, polycythemia, and right-to-left shunts, as well as from the effects of repeated CPB and surgery [Newburger et al., 1984].
Primary neonatal correction of many complex CHD lesions became possible in the early 1970s, when Barrat-Boyes in Auckland and Castaneda in Boston began using intraoperative deep hypothermia to suppress cellular metabolism and oxygen demand. In this manner, deep hypothermia enabled maneuvers such as low-flow CPB and circulatory arrest to clear the surgical field of blood and cannulae, optimizing exposure during critical phases in the repair of small and complex CHD lesions. As a result of these techniques, neonatal heart repair has become commonplace in many major centers since the 1980s, significantly increasing the survival of infants with complex CHD lesions. In fact, the hospital mortality for neonatal cardiac procedures in most large centers has fallen to less than 3 percent [Wernovsky, 2006]. Unfortunately, these intraoperative support techniques were not without neurologic risk and, as a result, the prevalence of perioperative neurologic morbidity of infant cardiac surgery did not parallel the decrease in mortality. Although neonatal cardiac repair reduced exposure to earlier risk factors, such as chronic hypoxia, a paradoxical increase in other forms of neurologic morbidity was noted. First, severe and previously lethal CHD lesions often were associated with significant perioperative hemodynamic shock and acidosis, the long-term neurologic consequences of which were now becoming evident in the growing population of CHD survivors. Second, the intraoperative support techniques responsible for increasing survival had their own inherent risk for neurologic injury. Consequently, in a significant minority of infants, the increase in survival and decrease in cardiac morbidity associated with severe CHD was replaced by chronic, often lifelong, neurodevelopmental dysfunction. This unacceptable situation triggered a period of intense clinical and research focus on advancing intraoperative neuroprotection during these procedures. Based on a number of randomized clinical trials over the past two decades (discussed below), substantial changes have occurred in the perioperative management of CHD. Whether due directly to these clinical changes or to more general advances in the field, the incidence of major neurologic complications following neonatal cardiac surgery appears to be decreasing in recent years.
Neurologic Complications of Congenital Heart Disease Prior to Anatomic Intervention
Brain Anomalies of Fetal Onset Associated with CHD
Primary brain dysgenesis in congenital heart disease
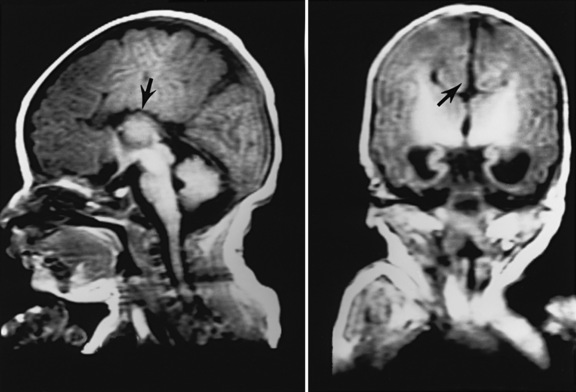
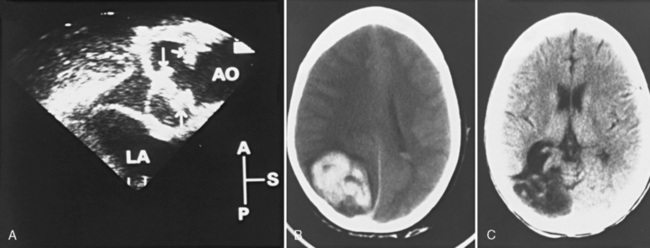
In earlier autopsy studies of children with CHD, the prevalence of brain malformations ranged from 10 to 29 percent [Glauser et al., 1990b; Jones, 1991; Miller and Vogel, 1999; Terplan, 1976] (Figure 101-1). The prevalence of dysgenetic brain lesions appears to be increased particularly in certain cardiac lesions [Glauser et al., 1990b; Jones, 1991; Terplan, 1976]. For example, a range of cerebral malformations, from microdysgenesis to gross malformations, such as holoprosencephaly, has been described at autopsy in infants with hypoplastic left heart syndrome (HLHS) [Glauser et al., 1990b]. Clinically, these malformations may present in the newborn period with seizures, alterations in level of consciousness, and abnormalities in motor tone, or may remain clinically silent until later infancy and childhood, when they present with developmental delay, epilepsy, and cerebral palsy. The in vivo diagnosis of cerebral dysgenesis is best confirmed by brain magnetic resonance imaging (MRI). Given the decreasing availability and inherent bias of autopsy studies, the increasing sophistication of MRI will be invaluable for the more precise delineation of the relationship between CHD and brain dysgenesis.
Children with certain chromosomal syndromes, particularly aneuploidy, are at increased risk for combined malformations of the heart and brain. Approximately 40 percent of children with trisomy 21 have CHD, typically endocardial cushion defects [Eskedal et al., 2004; Vergara et al., 2006]. The most common cardiac lesions in both trisomies 13 and 18 are ventricular septal defects and patent ductus arteriosus. Conversely, the spectrum of brain malformations in these trisomy syndromes is broad.
With a prevalence of 1:4000–5000, chromosome 22 deletion syndromes are the most common microdeletion disorders in man [Aggarwal and Morrow, 2008], and commonly have features of both cardiac anomalies and neurologic dysfunction.[Morrow et al., 1995] The phenotypic similarities across these syndromes have led to the acronym CATCH-22 spectrum (cardiac defect, abnormal facies, thymic hypoplasia, cleft palate, hypocalcemia, chromosome 22q11 deletions) [Demczuk et al., 1995; Driscoll, 1994; Lindsay et al., 1995; Morrow et al., 1995; Shprintzen et al., 1992]. This spectrum includes the DiGeorge’s and velocardiofacial (Shprintzen’s) syndromes. A second genetic locus for DiGeorge’s syndrome and velocardiofacial syndrome (DGS2) has also been identified on chromosome 10p [Bartsch et al., 2003; Daw et al., 1996].
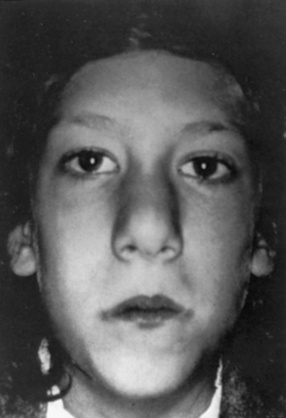
The most common cardiac defects in DiGeorge’s syndrome are conotruncal lesions, including interrupted aortic arch type B, truncus arteriosus, and tetralogy of Fallot. Features typical of the velocardiofacial syndrome include cleft palate or velopharyngeal insufficiency and a typical facial appearance (Figure 101-2), with a broad prominent nose and retrognathia. Ventricular septal defects and tetralogy of Fallot are the most common cardiac defects in the velocardiofacial syndrome. A range of brain anomalies has been reported [Zinkstok and van Amelsvoort, 2005], the more common of which are midline defects in corpus callosum, septum pellucidum, and vermis development, cerebral and cerebellar “atrophy,” and white-matter abnormalities, including hyperintensities and frontal periventricular cysts [Chow et al., 1999; van Amelsvoort et al., 2001]. In addition, under-opercularization and polymicrogyria (especially of the insula and adjacent cortex) also have been described in chromosome 22 deletion syndromes [Bingham et al., 1997, 1998; Ghariani et al., 2002; Sztriha et al., 2004; Worthington et al., 2000]. The neurologic dysfunction in these syndromes is broad and may evolve over time. These children may present initially with hypocalcemic seizures, and subsequently they develop neurologic or cognitive disturbances, including impaired attention, executive and visuospatial function, and memory [Antshel et al., 2008; Bearden et al., 2001; Moss et al., 1995; Simon et al., 2005]. Early life oromotor dysfunction and expressive language delay is common; however, over time, the most consistent developmental pattern is that of significant discrepancy between verbal IQ and significantly worse performance IQ [Moss et al., 1995]; the mean IQ is around 70. This nonverbal learning disability [Swillen et al., 1999] is in keeping with significant visuoperceptual deficits in many of these children. During childhood, a unusual and inappropriately blunt affect emerges [Golding-Kushner et al., 1985], evolving in some cases into an autistic spectrum disorder, and in adulthood into psychosis [Gothelf et al., 2004; Pulver et al., 1994; Shprintzen et al., 1992; Vorstman et al., 2006].
The most common cardiac lesions seen in Williams’ syndrome are supravalvar aortic stenosis, peripheral pulmonary stenosis, and ventricular/atrial septal defects. These patients are prone to neurologic dysfunction [Ardinger et al., 1994; Chapman et al., 1995; Kaplan, 1995; Soper et al., 1995] and usually have obvious cognitive impairment; the average IQ is around 55. This is compounded further by learning and social disabilities. Gross motor, fine motor, and oromotor dysfunction is common, as are visuospatial and constructional difficulties [Chapman et al., 1995]. Children with Williams’ syndrome also may develop an intracranial arteriopathy with subsequent risk of stroke [Ardinger et al., 1994; Kaplan, 1995; Soper et al., 1995].
Acquired brain lesions in the fetus with congenital heart disease
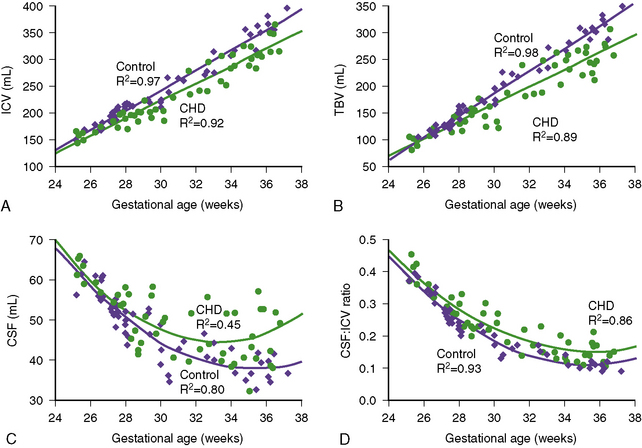
Recent studies have explored the impact of CHD on fetal brain development. In a retrospective review of aortic stenosis-HLHS spectrum cases [Hinton et al., 2008], midgestation (19–22 weeks) fetal ultrasound measurements were compared with neonatal measurements. Several important observations were made in these subjects. First, more than half of these infants had a birth head circumference below the 10th percentile. Second, there was significant decrease in head circumference percentile over the third trimester. Third, fetuses with conditions associated with greater limitation of aortic arch blood flow documented greater impairment of head growth. Finally, there was a tendency for greater restriction of head growth than somatic growth [Hinton et al., 2008]. The only prospective MRI study to date to examine in vivo brain growth in fetuses with CHD versus control fetuses used volumetric measures of brain size and measures of fetal brain metabolism using 1H-MR spectroscopy [Limperopoulos et al., 2010]. This study revealed a progressive impairment in third-trimester brain growth and metabolism in fetuses with CHD (Figure 101-3). Reduced brain volume and brain N-acetyl aspartate (NAA)/choline ratios were most common in fetuses with HLHS and transposition of the great arteries (TGA); these CHD diagnoses were also responsible for the 20 percent of fetuses with elevated cerebral lactate, suggestive of anaerobic cerebral metabolism. The effects of these CHD diagnoses on cerebral volumetric growth also may underlie the increased incidence of disturbed late-gestation developmental processes in brain development, seen in children with HLHS and TGA. For example, incomplete insular closure, sometimes with polymicrogyria, both late-gestation events, has been described in close to 90 percent of newborns with TGA and HLHS [Licht et al., 2009]. In addition, a high prevalence of disturbances in the third-trimester events of cortical organization has been described in HLHS autopsy studies [Glauser et al., 1990a]. In summary, the findings of these studies suggest that brain growth might be most impaired in those fetuses with CHD resulting in decreased cerebral blood flow and/or hypoxemia. Other studies in newborns with CHD have described decreased pyramidal tract maturation (by diffusion tensor MRI tractography) [Partridge et al., 2006] and biochemical immaturity of the developing white matter (i.e., lower NAA/choline ratio using 1H-MR spectroscopy) [Miller et al., 2007], suggesting a fetal origin for these brain anomalies.
Acquired Neurologic Injury Between Birth and Anatomic Intervention
The increase in antenatal diagnosis of CHD has been associated with an improved overall outcome [Montana et al., 1996; Tworetzky et al., 2001; Verheijen et al., 2001], including a decreased rate of neonatal acidosis [Eapen et al., 1998; Kumar et al., 1999] and improved short-term neurologic outcome. This increase is, in large part, due to planned delivery and immediate neonatal care, especially for serious lesions such as HLHS [Mahle et al., 2001]. Furthermore, the advances in neonatal heart repair have reduced the delay to correction, thereby decreasing the brain’s exposure to the deleterious effects of an uncorrected cardiovascular anatomy (e.g., chronic hypoxia). However, while enabling surgical repair in increasingly smaller infants with immature cerebrovascular structure and function, these advances paradoxically have increased the risk of prematurity-related hemorrhagic (e.g., germinal matrix-intraventricular hemorrhage) and ischemic (e.g., periventricular leukomalacia) brain injury.
Preoperative Neurologic Complications
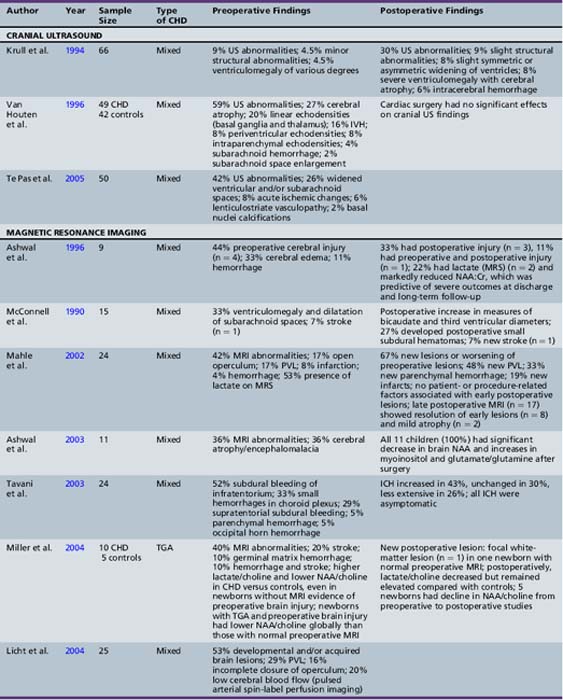
Preoperative neurologic dysfunction has been described in newborns with CHD, in some studies involving more than half of cases [Clancy et al., 2001; Fuller et al., 2009b; Limperopoulos et al., 1999a, 2000, 2001; Newburger et al., 1993; Robertson et al., 2004]. Clinical abnormalities described include muscle tone abnormalities, jitteriness, motor asymmetries, abnormal behavioral state regulation, decreased sensory (visual and auditory) responsiveness, and feeding difficulties. In one study, neurologic compromise was more prominent among newborns with acyanotic CHD [Limperopoulos et al., 1999a]. Congenital microcephaly, despite appropriate birth weight, has been reported in one-third or more of neonates with CHD, suggesting fetal-onset impaired brain growth [Licht et al., 2004; Limperopoulos et al., 1999b; Manzar et al., 2005; Shillingford et al., 2007]. Likewise, preoperative electroencephalographic (moderate, diffuse, background disturbances) and evoked potential abnormalities have been reported in over 40 percent of newborns with CHD [Limperopoulos et al., 1999b, 2000].
Neuroimaging studies in newborns with CHD, using either cranial ultrasound or MRI, have depicted a significantly increased prevalence of brain injury, largely clinically silent. Cranial ultrasound studies performed preoperatively and postoperatively in a cohort of infants undergoing cardiac surgery identified a 24 percent incidence of new lesions after cardiac surgery, with new hemorrhagic lesions in 6 percent of cases [Krull et al., 1994]. Preoperative cranial ultrasound abnormalities, including cerebral atrophy (41 percent), enlarged cerebrospinal fluid (CSF) spaces (26 percent), linear deep gray-matter echodensities (20 percent), intraventricular hemorrhage (16 percent), and parenchymal echodensities (16 percent), have been reported in 40–60 percent of newborns with CHD [Te Pas et al., 2005; van Houten et al., 1996]. The incidence of these cranial ultrasound abnormalities is greatest in newborns with aortic coarctation (especially with ventricular septal defect) or HLHS, ranging from 63 percent [Te Pas et al., 2005] to 71 percent [van Houten et al., 1996].
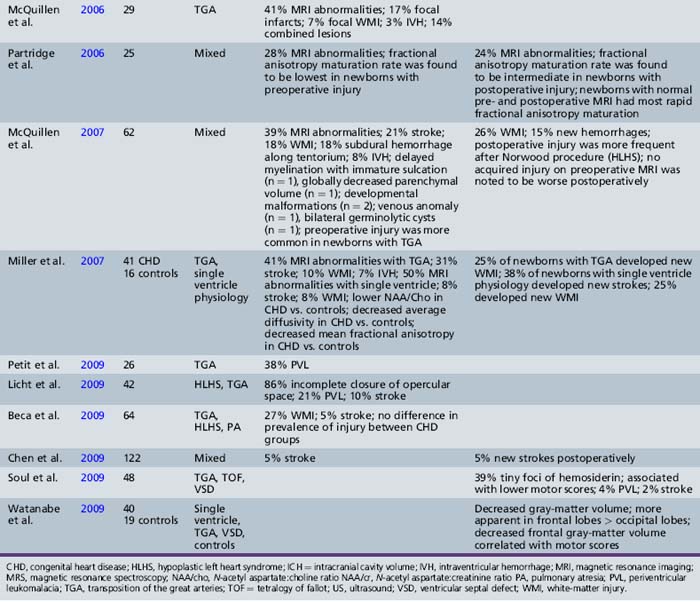
Recent MRI studies have confirmed this high prevalence of preoperative brain injury [Mahle et al., 2002; McConnell et al., 1990; McQuillen et al., 2006, 2007; Miller et al., 2004; Tavani et al., 2003], especially in infants with certain CHD lesions, including HLHS and TGA, as well as those with low preoperative oxygenation and longer time to surgery [Cheng, 2006; McQuillen et al., 2006]. In an MRI study of infants with HLHS, 23 percent documented preoperative evidence of focal or diffuse brain injury [Dent et al., 2005]. Procedures such as preoperative balloon-atrial septostomy also have been associated with evidence of infarction on MRI in some [Cheng, 2006; McQuillen et al., 2006], but not all, studies [Beca et al., 2009; Petit et al., 2009]. Prospective MRI studies have also increased the detection of periventricular leukomalacia (PVL) [Mahle et al., 2002; McConnell et al., 1990; McQuillen et al., 2006, 2007; Miller et al., 2004; Tavani et al., 2003], with some studies reporting a prevalence as high as 20 percent [Galli et al., 2004; Mahle et al., 2002]. PVL characteristically is a lesion of the premature infant, mediated by an interplay between the vulnerability of immature oligodendrocytes and insults such as cerebral ischemia. The fact that the preoperative PVL described in infants with CHD has been in term or near-term newborns has led to speculation that oligodendrocyte maturation is delayed in infants with CHD [du Plessis, 1997; Licht et al., 2009; Miller et al., 2007]. The notion of cerebral hypoperfusion in the newborn with CHD has been supported by studies indicating decreased cerebral perfusion (by arterial MRI spin-labeling) [Licht et al., 2004], as well as elevated cerebral lactate (by preoperative 1H-MR spectroscopy) in up to 53 percent of newborns with CHD – most commonly, those with TGA and single ventricle physiology [Ashwal et al., 1996, 2003; Mahle et al., 2002; Miller et al., 2004, 2007]. Table 101-1 summarizes the prevalence and type of preoperative brain injury in infants with CHD.
Neurologic Complications Following Anatomic Intervention
Two decades ago, the frequency of neurologic complications developing in the postoperative period following deep hypothermic cardiac surgery began to draw increasing attention. At that time, the prevalence of such complications was estimated to be as high as 25 percent in some centers [Ferry, 1987]. This finding led to a period of intense basic and clinical research into the mechanisms of intraoperative brain injury and its prevention, including several large clinical trials. Over the intervening years, the incidence of these complications appears to be decreasing in some [Menache et al., 2002], but not all, reports [Fallon, 1995; Kirkham, 1998]. In fact, it has been suggested that intraoperative (“procedure-specific”) factors are no longer major determinants of long-term outcome [Gaynor et al., 2007]. There is a clear need for a comprehensive and multicenter review of neurologic complications in the current era.
Mechanisms of Neurologic Injury during Deep Hypothermic Cardiac Surgery
Regardless of its frequency, intraoperative brain injury remains a major concern for children undergoing surgery for severe CHD. Intra- and perioperative hypoxic-ischemic/reperfusion (HI/R) insults generally are considered the principal cause of postoperative neurologic dysfunction. During open-heart surgery, a multitude of complex, dynamic, and interrelated phenomena may impact simultaneously on cerebral oxygen delivery and demand (Box 101-1) during the core cooling, low or no-flow bypass, and rewarming phases of deep hypothermic cardiac surgery. In individual infants sustaining intraoperative brain injury, the precise role and contribution of each of these phenomena are often unclear. The potential mechanisms for the development of HI/R are multiple and include both global (Box 101-1) and focal insults (Box 101-2). In adults undergoing cardiac surgery, the predominant form of brain injury is focal embolic ischemia, resulting when atheromatous debris dislodged from the aorta enters the arteriosclerotic cerebrovascular bed. Conversely, in the young infant undergoing open-heart surgery, the cerebral HI/R insults are mediated primarily by global hypoperfusion. Normally, the delicate balance between cerebral oxygen supply and utilization is controlled by intrinsic cerebral autoregulatory systems. These systems are likely overwhelmed by the major hemodynamic and metabolic changes induced during infant open-heart surgery. During the intraoperative period, it becomes the responsibility of the anesthesiologist-perfusionist team to maintain a positive balance of cerebral energy supply and demand. Guidelines for this extrinsic regulation of cerebral oxygenation remain based largely on theoretical safety parameters of aspects, such as duration of circulatory arrest, as well as rate and depth of cooling. The complex and dynamic intraoperative changes in both oxygen substrate demand and supply, as well as the lack of reliable and validated intraoperative monitoring techniques, make it challenging in the individual patient to identify imminent cerebral hypoxia-ischemia before injury occurs [du Plessis et al., 1995b; Sakamoto et al., 2001, 2004; Wardle et al., 1998].
Box 101-1 Intraoperative Determinants of Global Cerebral Oxygen Delivery and Use
Over the last two decades, there has been intense research into techniques that reduce the risk of intraoperative hypoxia-ischemia. Clinical trials have focused on strategies for optimizing intraoperative cerebral oxygen availability, which is most tenuous during periods of low-flow cardiopulmonary bypass (LFB) or deep hypothermic circulatory arrest (DHCA). Since longer periods of both DHCA and LFB have their own attendant risks, the neurologic outcome associated with LFB and DHCA was compared in a randomized clinical trial of 171 infants with TGA undergoing neonatal cardiac repair. Based on the findings of this trial, in which DHCA was associated with higher perioperative morbidity [Newburger et al., 1993] and worse developmental outcome at age 1 year, most centers adopted a strategy that minimized DHCA. The longer-term outcomes of this study are discussed below [Bellinger et al., 1997, 1999b, 2003].
Deep hypothermia has significant acid–base effects, and different intraoperative management strategies have been used to manipulate these acid–base changes with the goal of optimizing tissue metabolism. Both the more alkalotic alpha-stat strategy and the more acidotic pH-stat strategy have theoretical advantages. The alpha-stat approach is based on the natural alkaline shift in tissue that accompanies hypothermia and is thought to optimize enzyme function at low temperatures. Conversely, the pH-stat technique utilizes the addition of CO2 to counter this alkaline shift and is used to enhance tissue oxygen delivery. The more hypercarbic perfusate increases cerebral perfusion and increases oxygen release in tissues by countering the increased oxygen-hemoglobin affinity associated with hypothermia. A randomized clinical trial comparing these two pH management strategies suggested that infants managed by the pH-stat strategy had less perioperative morbidity and a shorter time to recovery of electrocortical activity [du Plessis et al., 1997]. As a result, the pH-stat strategy has become incorporated into the intraoperative acid–base management protocols of many centers, particularly during the induction of hypothermia on CBP.
Hemodilution is used widely in CBP to improve blood flow in the microcirculation. However, the benefits of improved perfusion are countered by the risks of reduced oxygen-carrying capacity of the circulation. A randomized clinical trial of different hematocrit levels in infants undergoing hypothermic CBP found that hemodilution was associated with greater perioperative hemodynamic instability and worse psychomotor development at age 1 year [Jonas et al., 2003]. However, at longer-term follow-up, the neurodevelopmental outcomes were similar in both groups [Newburger et al., 2008].
Besides global HI/R insults, several other mechanisms have been implicated in intraoperative brain injury. Compared with those in adults, vaso-occlusive insults play a lesser role in intraoperative brain injury during cardiac surgery in infants. CPB and the surgical field may generate particulate (e.g., platelet, synthetic, or fat) (Figure 101-4) or gaseous (e.g., air) emboli [Boyajian et al., 1993; Fish, 1988; Moody et al., 1990; Padayachee et al., 1987]. Inflammatory mechanisms triggered during CPB also have been investigated for their role in intraoperative brain injury, an interest stimulated by the growing body of evidence for cytokine-mediated neurotoxicity in animal studies [Back, 2006; Back et al., 2007; Den et al., 2009]. A post-bypass systemic inflammatory syndrome [Kirklin et al., 1983; Westaby, 1987], with systemic vascular injury manifesting with marked edema, is common, particularly in young infants. This syndrome is related to the relatively large blood volume required for priming bypass pumps and the prolonged exposure of this blood to artificial surfaces. The elevated levels of cytokines (interleukins-6 and 8, tumor necrosis factor-α, and endothelin-1) measured in bypass blood [Bando et al., 1998; Elliott, 1999; Elliott and Finn, 1993; Jansen et al., 1992; Journois et al., 1996; Kirklin et al., 1983; Millar et al., 1993; Steinberg et al., 1993] potentially may mediate vascular, as well as cellular, injury. Ultrafiltration of blood during or after CPB reduces both cytokine levels and excess free plasma water [Gaynor, 2003]. Ultrafiltration has been associated with improved postoperative hemodynamics [Bando et al., 1998] and improved myocardial function [Chaturvedi et al., 1999; Davies et al., 1998]. It is not known whether the beneficial effects of ultrafiltration result primarily from a reduction in postoperative cytokine levels or from hemoconcentration. To date, the potential contribution of this systemic inflammatory response to intraoperative brain injury has not been demonstrated convincingly in clinical trials with long-term follow-up.
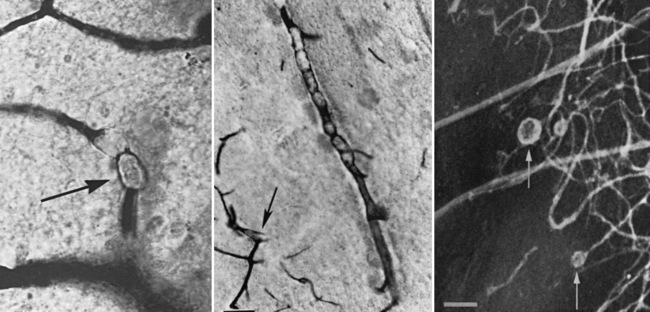
Fig. 101-4 Aneurysmal dilatations (arrows) in the cerebral microvasculature after cardiopulmonary bypass.
(From Moody D, Bell M, Challa VR, et al: Brain microemboli during cardiac surgery or aortography, Ann Neurol 28:477–486, 1990. Copyright © 1990 Wiley–Liss, Inc., a Wiley Company. Reproduced with permission of John Wiley & Sons, Inc.)
Mechanisms of Neurologic Injury with Extracorporeal Membrane Oxygenation
Extracorporeal oxygenation-circulation devices are used increasingly in children with critical heart disease as a “bridge” to recovery, surgery, or transplantation. Extracorporeal life-support devices were introduced into clinical practice by Kirklin and Lillehei in the mid-1950s for the CPB support of patients undergoing open-heart surgery [Lillehei, 1982; Moller et al., 2009]. On-going technological advances expanded the applications of these devices, and in the 1980s they emerged from the operating room to become established for the intensive care management of severe but potentially reversible cardiorespiratory failure. In the early years, the principal target population in children was the term newborn with respiratory failure due to meconium aspiration and persistent pulmonary hypertension, and subsequently congenital diaphragmatic hernia [Bartlett et al., 1977]. Since its inception, an estimated 20,000 infants have had extracorporeal membrane oxygenation (ECMO) for respiratory failure [Lequier et al., 2008]. The advent of nitric oxide pulmonary vasodilators obviated the need for ECMO in many cases of respiratory failure due to pulmonary hypertension [Christou et al., 2000; Clark et al., 2000]. Coinciding with its waning use for respiratory failure, ECMO became used increasingly for critical but potentially reversible circulatory failure. There are two broad indications for so-called cardiac ECMO in children. Most commonly, cardiac ECMO is used to support children with severe cardiovascular failure, for which spontaneous recovery or effective intervention is possible. Included in this group are newborn infants who fail to transition effectively from a fetal to an extrauterine circulation; children with intractable cardiac failure awaiting transplant; and children who fail to wean off CPB after cardiac surgery. Another indication for cardiac ECMO, employed in some centers, is cardiorespiratory arrest refractory to conventional resuscitative measures (cardiopulmonary resuscitation [CPR]-ECMO) [del Nido et al., 1992]. The use of CPR-ECMO has been recommended by the American Heart Association for in-hospital cardiac arrest that is potentially reversible or amenable to transplant.
ECMO may injure the brain through multiple mechanisms that promote either ischemic or hemorrhagic injury. Carotid ligation causes an immediate reduction in blood flow to the ipsilateral middle cerebral artery, which may be restored only partially by collateral flow through the circle of Willis and the external carotid artery. This reduced flow may cause direct ischemia and may interfere with cerebral autoregulation, rendering that hemisphere vulnerable to systemic hypotension. Jugular vein ligation can result in cerebral venous hypertension, which may predispose to venous infarction, and which further decreases the cerebral arteriovenous pressure gradient. Systemic heparinization increases the risk of intracranial hemorrhage, which may be either primary or due to hemorrhagic transformation of an ischemic lesion. Finally, ECMO causes elevated levels of inflammatory cytokines, which may contribute to the development of PVL [Fortenberry et al., 1996; Kinney and Back, 1998].
The Neurologic Outcome of Children Undergoing Extracorporeal Membrane Oxygenation
An important determinant of long-term neurologic outcome following ECMO is the underlying indication for its use. Given the significant clinical instability and illness severity preceding ECMO, and the difficulty obtaining definitive brain imaging acutely, it remains difficult to distinguish between brain injury associated with the ECMO technique itself and that due to the underlying critical illness. For a number of reasons, the outcome after cardiac ECMO is less favorable than for respiratory ECMO. This finding is perhaps not surprising since cerebral ischemia is known to be a more potent cause of brain injury than is cerebral hypoxemia, a notion supported by the fact that the severity of arterial metabolic acidosis prior to ECMO is a significant predictor of adverse neurologic outcome [Cengiz et al., 2005].
Intracranial hemorrhage and infarction are the primary neurologic complications of ECMO, with previous estimates ranging from 25 to 50 percent overall, and severe lesions in 10–20 percent of infants [Volpe, 2001]. The most common sites of intracranial hemorrhage are into the lateral ventricles and posterior fossa structures. Cerebral ischemia may be global or focal, and may manifest as PVL [Jarjour and Ahdab-Barmada, 1994; Kinney and Back, 1998]. Clinical seizures occur in approximately 10 percent of both cardiac and respiratory ECMO cases [Dalton, 2004]. However, given the overall morbidity and the use of sedating-paralyzing drugs, it is not surprising that electrographic seizures have been detected in up to 70 percent of cases. [Kim and Stolar, 2000]. Despite this high risk for acute seizures, the risk for subsequent epilepsy is low. Sensorineural hearing impairment, often reversible, is reported in 4–15 percent of neonates after ECMO [Desai et al., 1997; Hofkosh et al., 1991; Schumacher et al., 1991]. ECMO survivors are at significant risk for neurocognitive delays; between 10 and 29 percent have developmental quotients below 70, the greatest risk being in the smaller infants [Hamrick et al., 2003; Revenis et al., 1992].
The short-term benefit of respiratory (VV) ECMO was established early, with multicenter survival rates around 75–80 percent; of note, a major criterion for ECMO eligibility was a predicted mortality of greater than 80 percent [Dalton et al., 2005; Stolar et al., 1991]. However, reports of neurologic injury in infants undergoing ECMO soon emerged, and this occurrence remains a major complication among survivors of ECMO. The reported long-term neurologic morbidity has ranged from 15 to 30 percent [Hanekamp et al., 2006; McNally et al., 2006; Robertson et al., 1995].
There is a relative paucity of data for the long-term outcome of cardiac (VA) ECMO. Overall survival is lower than for respiratory ECMO, and ranges from 30 to 55 percent [Balasubramanian et al., 2007; Chen et al., 2008; Taylor et al., 2007], but varies with the specific underlying indication. Children with dilated cardiomyopathy or myocarditis requiring ECMO may have survival rates as high as 80–90 percent [Duncan et al., 2001; McMahon et al., 2003]. Neurologic deficits have been described in up to 60 percent of cardiac ECMO survivors [Chow et al., 2004; Hamrick et al., 2003; Ibrahim et al., 2000], with mental retardation in 30–50 percent of survivors, depending on the indication [Hamrick et al., 2003; Lequier et al., 2008].
Not surprisingly, survival is significantly higher when ECMO is started before cardiac arrest than after, CPR-ECMO having the highest mortality and morbidity risks. While conventional CPR lasting 30 minutes or more has a survival of 5 percent or less [de Mos et al., 2006; Morris et al., 2004], survival after CPR-ECMO in patients who had failed conventional CPR is around 30–40 percent [Alsoufi et al., 2007; Chan et al., 2008; Lequier et al., 2008; Thiagarajan et al., 2007]. Risk factors for death in CPR-ECMO cases are severe metabolic acidosis (pH ≤ 7.00) prior to ECMO, persistent metabolic acidosis despite CPR-ECMO, and the development of multisystem organ failure [Kolovos et al., 2003; Kulik et al., 1996; Thiagarajan et al., 2007]. In a recent study of outcome following cardiac ECMO, including CPR-ECMO, survival to discharge was 46 percent, but more than 50 percent of survivors had mental retardation at long-term follow-up, with time to normalization of circulating lactate and the degree of inotropic support needed being significant predictors [Lequier et al., 2008].
Early Postoperative Manifestations of Neurologic Dysfunction
Twenty years ago, as many as 25 percent of children undergoing open heart operations demonstrated evidence of early postoperative neurologic dysfunction [Ferry, 1987, 1990; Newburger et al., 1993]. On-going advances in management, including those based on the randomized clinical trials discussed above, appear to be associated with a decrease in the incidence of postoperative neurologic dysfunction [Menache et al., 2002]. These early postoperative neurologic complications are often transient, and initially were considered of little long-term consequence. However, subsequent follow-up studies suggested that these early complications may be associated with long-term adverse outcome.[Bellinger et al., 1995].
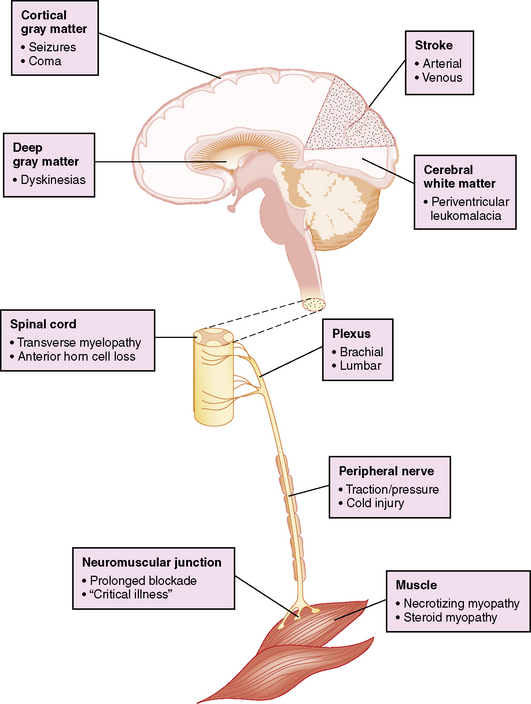
Postoperative neurologic manifestations may result from pre-, intra-, and postoperative injury to the nervous system at any or multiple levels of the neuroaxis (Figure 101-5). However, the bedside clinical diagnosis of neurologic dysfunction in these critically ill infants may be complicated by the subtle manifestations of acute cerebrovascular injury, and further obscured by the widespread use of sedating or paralyzing medications. These confounding factors have delayed the accurate diagnosis of perioperative brain dysfunction and limited the formulation of rational management strategies.
Delayed Recovery of Consciousness
Prolonged delay in the recovery of consciousness after cardiac surgery, anesthesia, and sedation is not uncommon. The standard diagnostic guidelines [Plum and Posner, 1985] for the evaluation of altered mental status should be applied to these patients, although the precise mechanism(s) remains unclear in most cases. Ultimately, some of these children demonstrate clinical and neuroimaging features suggestive of cerebral HI/R injury. However, because intraoperative events specific for HI/R often are lacking, other potentially reversible etiologies should be excluded, including postoperative hepatic or renal impairment with accumulation of toxic metabolites, or impaired metabolism or excretion of sedating drugs. Prolonged use of neuromuscular blocking agents for ventilatory management may be associated with delayed recovery of motor function [Gooch et al., 1991; Partridge et al., 1990; Waitling and Dasta, 1994], and, in severe cases, may mimic impaired consciousness. Neuromuscular blockade may be excluded with a bedside peripheral nerve stimulator or nerve conduction studies. A significant minority of infants develop postoperative seizures, which may recur serially and often are clinically silent [Newburger et al., 1993]. In the absence of other causes to explain persistent impairment of consciousness, occult seizures or a prolonged postictal state should be considered.
Postoperative Seizures
Seizures are among the most common manifestations of neurologic dysfunction after infant open-heart surgery. Past series have reported clinical seizures in up to 15 percent of infants in the early postoperative period [Ehyai et al., 1984; Miller et al., 1995; Newburger et al., 1993], whereas clinically silent, electrographic seizures were detected by continuous electroencephalographic (EEG) monitoring in up to 20 percent of infants [Helmers et al., 1997]. More recent studies from large pediatric cardiac surgery centers report widely disparate postoperative seizure rates ranging from 1.2 to 17.7 percent [Clancy et al., 2003; Menache et al., 2002]. Risk factors for postoperative seizures include the use of DHCA, prolonged DHCA times, the presence of a ventricular septal defect, and pre-existing genetic conditions [Clancy et al., 2003; Helmers et al., 1997]. The recent decline in seizure incidence observed in some centers also may be attributable to refinements in extracorporeal perfusion support, such as the minimization of DHCA.
Two forms of early postoperative seizures may be distinguished, i.e., seizures with a readily identifiable cause and those without (so-called “postpump seizures”). The usual etiologies of seizures should be excluded, particularly reversible causes such as hypoglycemia and electrolyte disturbances. Cyclosporine A toxicity is a common cause of seizures in heart transplant recipients [Menache et al., 2002].
Postpump seizures
Postpump seizures (i.e., those without an identifiable etiology) were the most common form of postoperative seizures in previous years, but their incidence appears to be decreasing. Postpump seizures tend to have certain characteristic clinical features and are confined almost exclusively to young infants. Conversely, postoperative seizures in older infants and children usually are associated with an identifiable cause. Postpump seizures often are assumed to reflect intraoperative HI/R injury; however, they differ from other post-HI/R (e.g., birth asphyxia) seizures in several ways. First, the onset of postpump seizures (including EEG seizures) is typically delayed until 24–48 hours postoperatively. This is significantly later than postasphyxial seizures, which often present within the first 12 hours after birth. Second, although not always benign, the long-term outcome of postpump seizures [Bellinger et al., 1995] is markedly better than the 50 percent incidence of adverse neurologic outcome seen after postasphyxial seizures [Andre et al., 1990; Bergman et al., 1983; Volpe, 2001].
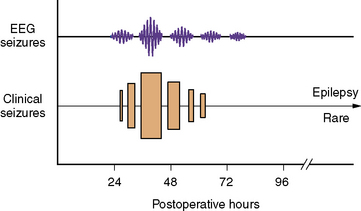
The temporal predilection for postpump seizures is relatively circumscribed to a “window” of several days (Figure 101-6). During this period, seizures tend to recur and, not uncommonly, status epilepticus develops. After several days, the tendency for further seizures decreases rapidly. The clinical manifestations of postpump seizures may be subtle, especially in infants receiving sedating or paralyzing drugs. Behavioral changes may be confined to paroxysmal changes in autonomic function, such as tachycardia, hypertension, and pupillary dilatation. Convulsive activity, when evident, is often focal or multifocal. Bedside EEG helps distinguish true epileptic phenomena from other behavioral or autonomic changes [Mizrahi, 1987; Scher and Painter, 1990]. Furthermore, the ability of EEG to detect clinically occult seizure activity helps guide anticonvulsant drug therapy. Finally, highly focal EEG abnormalities may reflect an underlying structural lesion (e.g., stroke), indicating the need for neuroimaging.
The therapeutic approach to postpump seizures is dictated best by their typical clinical course. After reversible etiologies, such as hypoglycemia, hypomagnesemia, and hypocalcemia, [Lynch and Rust, 1994; Satur et al., 1993] have been excluded, anticonvulsant therapy should commence. In view of the tendency toward repeated seizures and status epilepticus, the initial goal is rapid achievement of therapeutic anticonvulsant levels by an intravenous route. The vast majority of postoperative seizures are controlled by standard anticonvulsant regimens used in infants. Careful cardiorespiratory monitoring is essential when using potentially cardiotoxic anticonvulsants during the postoperative period, when myocardial dysfunction or arrhythmias are prevalent. Specifically, in infants with established myocardial dysfunction, cautious administration of phenobarbital is advisable, whereas pre-existent conduction disturbances, particularly bradyarrhythmias, necessitate careful monitoring of phenytoin therapy [Cranford et al., 1978]. The apparently circumscribed window of susceptibility for these postpump seizures and the rarity of subsequent epilepsy allow successful early withdrawal of anticonvulsant agents, often before hospital discharge.
Prognosis of postoperative seizures
The prognosis of postoperative seizures depends on the underlying etiology. Postpump seizures without an obvious etiology previously were considered benign, transient events. Although a significant association between postoperative seizures and worse neurodevelopmental outcome and MRI abnormalities has been demonstrated in a large prospective study, [Bellinger et al., 1995; Rappaport et al., 1998], this has not been the case in other studies [Gaynor et al., 2006]. Epilepsy is a rare sequel to typical postpump seizures [Ehyai et al., 1984], although West’s syndrome has been described in occasional survivors of intractable postoperative seizures [du Plessis et al., 1994a]. The long-term outcome of infants with an identified cause for their postoperative seizures is related to the specific etiology. Seizures in infants with brain dysgenesis are associated with an almost universal poor long-term outcome, and epilepsy is a common complication. Stroke in young infants commonly presents with seizures. The overall risk for subsequent epilepsy in these infants ranges from 20 to 30 percent [Lanska et al., 1991; Yang et al., 1995], and relates to age at stroke and the post-stroke latency to first seizure. Specifically, the risk for later epilepsy is lowest when stroke occurs in the newborn infant [Levy et al., 1985] and when seizures are an early manifestation of stroke [Yang et al., 1995]. These features should be considered when planning maintenance antiepileptic drug therapy.
Stroke in the Early Postoperative Period
Vaso-occlusive stroke in children with CHD diagnosed in the early postoperative period likely has an etiologic profile that differs from stroke occurring prior to or remote from cardiac surgery. The latter category of stroke is discussed later in this chapter. Since strokes diagnosed in the early postoperative period are likely to be clinically silent during the operation and early postoperative recovery [Chen et al., 2009], their incidence is likely underestimated. A recent 10-year review described a 5.4 per 1000 incidence of arterial and venous occlusive brain injury within the first 3 days of cardiac surgery [Domi et al., 2008]. Another study using MRI in the first 14 days after surgery found a 10 percent incidence of stroke that included both vaso-occlusive and global hypoperfusion (“watershed”) injuries; vaso-occlusive lesions comprised less than half these “strokes” [Chen et al., 2009] (Figure 101-7). Of interest, the vast majority of these lesions were clinically silent in the acute phase.
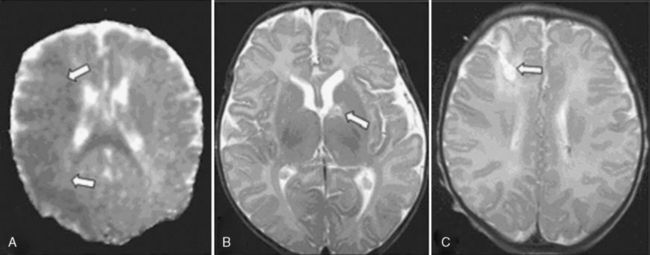
Fig. 101-7 Illustrative magnetic resonance imaging findings of infarcts (arrows).
(From Chen J, Zimmerman RA, Jarvik GP, et al: Perioperative stroke in infants undergoing open heart operations for congenital heart disease, Ann Thorac Surg 88:823, 2009, Figure 1. Reprinted with permission.)
Certain features of open-heart surgery and the immediate postoperative period may predispose to stroke and warrant brief review. During the intraoperative period, both embolic and thrombotic mechanisms may cause cerebral vaso-occlusive injury. Particulate and gaseous emboli originating from the CPB apparatus or surgical field bypass the normal pulmonary filtration system during CPB and thus enter the systemic circulation directly [Boyajian et al., 1993; Moody et al., 1990; Padayachee et al., 1987; Solis et al., 1975]. Improvements in bypass circuits, particularly the replacement of bubble oxygenators by membrane oxygenators, have decreased the incidence of macroembolic injury [Nussmeier and McDermott, 1988]. The impact of these advances on the incidence of microvascular injury [Fish, 1988] is unclear. The marked inflammatory response triggered by the extensive and prolonged exposure between bypass blood and artificial surfaces [Kirklin et al., 1983; Millar et al., 1993; Steinberg et al., 1993] is known to trigger complex cascades, including endothelial-leukocyte interactions [del Zoppo, 1994; Elliott and Finn, 1993; Feuerstein et al., 1994; Lucchesi, 1993]. The resulting microvascular dysfunction may predispose to intravascular thrombosis or thromboembolism, manifesting with vaso-occlusive brain injury.
In the early postoperative period, several factors predispose to cardiogenic stroke. First, vascular stasis may result from localized areas of low flow within the heart [du Plessis et al., 1995a; Rosenthal et al., 1995a, 1995b], or global ventricular dysfunction. Prolonged postoperative immobility predisposes to systemic venous stasis and thrombosis. Transient pulmonary hypertension is common after CPB, elevating venous pressure and decreasing the rate of blood flow through the right heart chambers and central veins. Injury to native tissue or the presence of prosthetic tissue alters vascular surfaces in contact with the circulation. A number of these stroke risk factors may be present after the Fontan procedure, the most common final operation for CHD with a single ventricle physiology [Day et al., 1995; Dobell et al., 1986; du Plessis et al., 1995a; Hutto et al., 1991; Mathews et al., 1986; Rosenthal et al., 1995a, 1995b], and is discussed in more detail below.
Movement Disorders After Cardiac Surgery
For many years, movement disorders have been considered an uncommon but dreaded complication of deep hypothermic cardiac surgery [Barrat-Boyes, 1990; Bergouignan et al., 1961; Bjork and Hultquist, 1962; Brunberg et al., 1974; Chaves and Scaltsas-Persson, 1988; Curless et al., 1994; DeLeon et al., 1990; Donaldson et al., 1990; Huntley et al., 1993; Medlock et al., 1993; Robinson et al., 1988; Wical and Tomasi, 1990; Wong et al., 1992]. Although less commonly described in recent years, these postoperative movement disorders are often dramatic, frequently intractable, and ultimately debilitating, with severe forms associated with substantial mortality.
Choreoathetosis is the most frequently reported dyskinesia after cardiac surgery, but a spectrum of movement disorders, including oculogyric crises [du Plessis et al., 1994b], akathisia, and parkinsonian syndromes [Straussberg et al., 1993], may be encountered. These dyskinesias are likely under-diagnosed and under-reported, making the true incidence difficult to ascertain; however, in reported case series, the incidence of postoperative choreoathetosis has ranged from 0.5 percent [Wessel and du Plessis, 1995] to 19 percent [Brunberg et al., 1974].
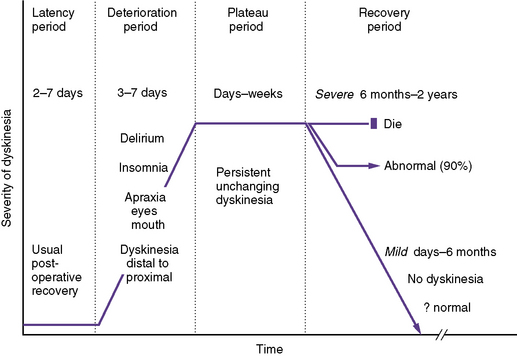
Postoperative choreoathetosis has a fairly stereotypic presentation and clinical course (Figure 101-8). The temporal course of choreoathetosis is relatively stereotypic after cardiac surgery, with subacute mental status changes, including often severe insomnia and irritability, emerging after a usually unremarkable period of 2–7 days; these are followed by “restlessness,” progressing to frankly abnormal involuntary movements, even ballismus, which typically commence in the distal extremities and orofacial muscles and progress proximally to the girdle muscles and trunk. The dyskinesia is confined to wakefulness, increases with stress, and resolves during the brief and fitful periods of sleep. Oculomotor, faciomotor, and oromotor apraxia may be prominent, raising concerns of “blindness” because these children appear not to “look at” or show recognition of familiar faces; in addition, oral feeding and expressive language may be disrupted. The involuntary movements intensify over days to a week, and then plateau over 1–2 weeks, before a gradual and variable recovery may begin. Mild dyskinesia usually resolves completely over days to weeks, and by 6 months at the latest. Severe postoperative dyskinesia may gradually improve for up to 2 years, after which further meaningful recovery is unlikely.
Diagnosis of these postoperative hyperkinetic syndromes is essentially clinical. Currently available adjunctive neurodiagnostic techniques have been useful only in so far as they exclude other disorders. Neuroimaging studies may be normal or depict nonspecific and nonfocal changes, most commonly diffuse cerebral atrophy [Medlock et al., 1993; Robinson et al., 1988; Wong et al., 1992]. Single-photon emission computer tomography (SPECT) functional brain imaging studies document a high incidence of both cortical and subcortical perfusion defects [du Plessis et al., 1994b]. EEG is usually normal or documents diffuse slowing. Neuropathologic findings have been limited and inconsistent, and have not elucidated the mechanisms of injury [Chaves and Scaltsas-Persson, 1988; Kupsky et al., 1995]. The basal ganglia do not undergo infarction; selective neuronal loss and gliosis may be seen in the external globus pallidus, an uncharacteristic region for HI/R injury, at least at normothermia [Kupsky et al., 1995]. Interestingly, an animal model of deep hypothermic circulatory arrest [Johnston et al., 1995; Redmond et al., 1994] has suggested that, when HI/R occurs at deep hypothermia, selective neuronal necrosis is prominent in the globus pallidus.
The prognosis of these conditions depends largely on their initial severity. Based on the severity and persistence of the dyskinesias, two forms of postpump choreoathetosis may be distinguished: a mild transient form and a severe persistent form [Wong et al., 1992]. In the mild transient form, involuntary movements are confined to the distal extremities or face, and tend to resolve over several weeks to months in virtually all cases [du Plessis et al., 2002]. However, a significant minority of these children have persistent disturbances in gait, fine motor function, and language. Severe postpump choreoathetosis has a much worse prognosis, with a mortality approaching 40 percent (due to aspiration pneumonia, massive caloric consumption, and infection); amongst survivors, long-standing dyskinesias, pervasive behavioral and cognitive disturbances, and even mental retardation are common [du Plessis et al., 2002]. Furthermore, these severe cases are associated with older age (beyond early infancy) at surgery, cyanotic heart disease, and shorter time from onset of CPB to DHCA (Wong et al., 1992). [Wong et al., 1992].
Dyskinesias also have been associated with the use of fentanyl and midazolam, widely used postoperative analgesics and sedatives. These medication-associated forms of movement disorder have a good prognosis, with less prominent sensorium changes and mild involuntary movements that resolve over days to weeks [Bergman et al., 1991; Lane et al., 1991; Petzinger et al., 1995].
Spinal Cord Injury
Spinal cord injury is fortunately a rare complication of cardiac surgery in infants and children, and is most commonly, but not exclusively [Puntis and Green, 1985], seen after aortic coarctation repair, occurring in 0.4 percent [Brewer et al., 1972] to 1.5 percent [Lerberg et al., 1982; Pennington et al., 1979] of such cases. During aortic surgery, spinal cord injury results from a watershed-type of HI/R insult. In the spinal cord, end-zone or watershed territories of arterial supply are located transversely at a lower thoracic level, and longitudinally between the supply territories of the anterior and posterior spinal arteries. Transverse ischemia at the thoracic level is the usual form of cord injury seen after coarctation repair. The highly variable, inconsistent collateral arterial supply of the distal cord may predispose to transverse cord ischemia during clamping of the aorta distal to the subclavian arteries, resulting in postoperative paraplegia with pyramidal features, with or without a thoracolumbar sensory level and bladder and bowel dysfunction.
The longitudinal cord syndrome is rare and occurs after systemic hypotension, as occurs with birth asphyxia [Sladky and Rorke, 1986] or with cardiac defects [Rousseau et al., 1993]. Injury is maximal in the ventral gray matter with selective anterior horn cell loss. Unlike the predominant pyramidal features of transverse cord ischemia, longitudinal cord injury causes a prominent lower motor neuron syndrome, with hypotonia (acute and long-term) and weakness with decreased or absent reflexes; posterior column sensory function is relatively spared. Intraoperative somatosensory-evoked potentials sometimes are used to monitor spinal cord function during coarctation surgery [Guerit et al., 1997; Laschinger et al., 1983, 1988], but, since these evaluate the posterolateral spinal pathways, rather than the more vulnerable anterior horn cells, this approach has not been adopted universally [Laschinger et al., 1988].
Peripheral Neuromuscular Injury
Plexopathies
Plexopathies, particularly brachial, are not uncommon following cardiac procedures [Kent et al., 1994; Lederman et al., 1982]. The brachial plexus is vulnerable to traction injury during the intraoperative retraction of a sternal thoracotomy incision; this form of plexopathy is more common in older patients. Cardiac catheterization, particularly newer interventional techniques such as radiofrequency ablation of aberrant conduction pathways and the placement of devices to occlude septal defects, may require prolonged shoulder hyper-abduction in the sedated patient. This may result in traction injury, usually to the lower brachial plexus, resulting in post-catheterization weakness of the wrist and finger extensors, the intrinsic muscles of the hand, and, occasionally, a Horner’s syndrome. As the lesion is usually neuropraxic, the weakness usually resolves over time [Dawson and Fischer, 1977]. Conversely, upper plexus lesions most commonly follow direct injury or extravasated blood associated with indwelling central venous catheters in the neck vessels, and although variable, weakness most commonly affects the shoulder abductors and external rotators. The prognosis for recovery of these direct traumatic lesions is more guarded.
Catheterization of the femoral vessels may be complicated by a localized hematoma or false aneurysm formation, the compressive and inflammatory effects of which may injure the lumbar plexus (with retroperitoneal hematomas) or the femoral nerve and its branches (with inguinal hematomas). Recovery from these injuries is usually complete [Kent et al., 1994].
Mononeuropathies
Pressure palsies may develop at a variety of dependent sites, most commonly the peroneal and ulnar nerves. Hypothermic cardiac surgery also may be complicated by phrenic nerve injury with diaphragmatic paralysis and postoperative ventilator dependence. Recent reports suggest an incidence of diaphragmatic palsy due to phrenic nerve injury complicating between 1.4 and 5.9 percent of cardiac operations [Akay et al., 2006; Lemmer et al., 2006]. The precise mechanism of injury often is unclear, but is thought to reflect direct cold injury from ice packed around the heart, or inadvertent transection of the nerve as it courses alongside the heart [Dunne et al., 1991; Mok et al., 1991; Watanabe et al., 1987]. The diagnosis may be established by ultrasound or fluoroscopy of the diaphragm, or more recently, by bedside phrenic nerve conduction and diaphragmatic electromyography [Bolton, 1993; Swenson and Rubenstein, 1992]. Prolonged mechanical ventilation and intensive care unit and hospital stays are common [Lemmer et al., 2006]. Although most phrenic nerve palsies are transient and presumably neuropraxic in origin, this is not always the case [Akay et al., 2006; Lemmer et al., 2006]. In cases of permanent phrenic palsy, diaphragmatic plication or, rarely, diaphragmatic pacing may be required [Weese-Mayer et al., 1992].
Polyneuropathy and myopathy
A prolonged neuromuscular syndrome may follow withdrawal of nondepolarizing neuromuscular blocking drugs [Gooch et al., 1991; Waitling and Dasta, 1994], such as vecuronium and pancuronium, commonly used to facilitate post-thoracotomy ventilatory management. Renal and hepatic failures increase the risk of this syndrome. Administration of acetylcholinesterase inhibitors may result in improved strength, but this effect is unpredictable and usually transient. The neuropathology of this syndrome is highly variable, ranging from an axonal motor neuropathy to myopathic changes [Danon and Carpenter, 1991; Subramony et al., 1991]. The neuropathy associated with neuromuscular blockers may be difficult to distinguish clinically from “critical illness polyneuropathy” [Sheth and Bolton, 1995]. This syndrome occurs in patients with sepsis and multiorgan failure, conditions that may complicate CHD and surgery.
Prolonged neuromuscular blockade, especially in conjunction with high-dose steroids, may occasionally result in severe myopathy [Hirano et al., 1992]. Unlike the neuropathies described previously, muscle weakness associated with these myopathic syndromes usually is maximal in proximal muscles. Deep tendon reflexes are reduced or absent, and sensory function is preserved. Two forms of myopathy may complicate neuromuscular blockade: an acute necrotizing myopathy and an acute myosin deficiency myopathy [Al-Lozi et al., 1994; Hirano et al., 1992]. These myopathies may be distinguished by electromyography.
Late Postoperative Manifestations of Neurologic Dysfunction
The delayed neurologic manifestations of cardiac surgery may be considered in three categories. First, there are the developmental and cognitive sequelae of the often-transient acute postoperative neurologic complications. For example, infants who develop postpump seizures rarely develop later epilepsy, but appear to be at increased risk for long-term neurodevelopmental deficits [Bellinger et al., 1995]. Second, neurologic injury may remain clinically silent in the early perioperative period, but may present as later neurodevelopmental impairment. Examples include lesions such as stroke and PVL, described in prospective neuroimaging studies. In the third category are those complications that develop ab initio, remote from surgical procedures. This section focuses primarily on the latter two categories.
Late Postoperative Stroke
The overall annual incidence of childhood stroke ranges from 2.5 [Schoenberg et al., 1978] to 7.9 per 100,000 children [Giroud et al., 1995]. CHD is a major risk factor for stroke in childhood, being present in 25–30 percent of cases [Lanska et al., 1991; Riela and Roach, 1993; Schoenberg et al., 1978]. Stroke in the child with CHD, including stroke in the late postoperative period, is predominantly thromboembolic in origin. Less commonly, arteriopathic or stenotic lesions of the intracranial vasculature may be seen in certain congenital heart lesions. Both these forms of stroke are discussed.
Thromboembolic cardiogenic stroke
Although thromboembolic stroke may complicate CHD at any time in the preoperative, intraoperative, and early postoperative periods, the majority of cardiogenic strokes occur remote from surgery. Risk factors for cardiogenic stroke in earlier years (e.g., polycythemia and right-to-left shunt) [Berthrong and Sabiston, 1951; Cottrill and Kaplan, 1973; Martelle and Linde, 1961; Phornphutkul et al., 1973; Terplan, 1976; Tyler and Clark, 1957a, 1957b] have decreased with the surgical correction of heart lesions in early infancy. Autopsy studies from earlier decades described cerebrovascular ischemic lesions in up to 20 percent of children with CHD [Berthrong and Sabiston, 1951; Terplan, 1976]; given the paucity of recent neuropathologic studies in this population, the current rate of strokes at autopsy is unknown.
In patients who survive cyanotic CHD into adulthood, the risk for stroke persists and likely increases. In a study of patients older than 18 years with cyanotic CHD, a stroke incidence of 1/100 patient-years was described [Ammash and Warnes, 1996]. Similar to earlier studies in children [Cottrill and Kaplan, 1973; Linderkamp et al., 1979], the strongest risk factor for stroke in this cyanotic population was microcytosis. The proposed mechanism is a procoagulant shift resulting from an increased blood viscosity caused by the microcytic erythrocytosis.
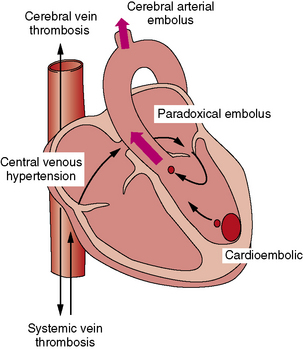
The major physiologic and anatomic risk factors for stroke, including the three elements of Virchow’s triad (i.e., altered vascular surface, stasis [du Plessis et al., 1995a; Rosenthal et al., 1995a, 1995b] and hypercoagulability [Cromme-Dijkhuis et al., 1990; Komp and Sparrow, 1970; Linderkamp et al., 1979]), are commonly present in children with CHD. The presence of paradoxical vascular pathways between the systemic venous system and the cerebral arteries constitutes a further major risk factor for cardiogenic stroke. In broad terms, cardiogenic stroke may be mediated by three mechanisms (Figure 101-9). First, arterial emboli may emanate from an intracardiac embolic source (cardioembolic stroke). Second, emboli may arise from a systemic venous or right-heart source and bypass the pulmonary circulation through a right-to-left shunt (paradoxic embolic stroke). Finally, cerebral venous thrombosis may result from a combination of central venous hypertension, venous stasis, and polycythemia. In earlier autopsy reports, venous thrombosis was the most common form of cerebral vaso-occlusive lesion, usually occurring around 2–3 years of age and particularly in the setting of iron-deficiency anemia [Cottrill and Kaplan, 1973].
Thromboembolic events, including stroke, are known long-term complications of the Fontan operation [Day et al., 1995; Dobell et al., 1986; du Plessis et al., 1995a; Hutto et al., 1991; Mathews et al., 1986; Rosenthal et al., 1995a, 1995b]. The Fontan procedure redirects venous blood returning to the right atrium into the pulmonary arterial system, thereby bypassing the single ventricle, which then serves as the systemic perfusion pump. Consequently, right atrial pressure is often elevated, slowing flow through this chamber and the central veins supplying it. The procoagulant effect of this slow venous flow is enhanced further by the use of a prosthetic “tunnel” or baffle within the atrium to redirect flow. These factors combine to predispose to thrombus formation within the right atrium and central veins. In the fenestrated Fontan modification, a right-to-left interatrial defect is created in the baffle or “tunnel.” This fenestrated modification, which has markedly reduced the mortality of the Fontan operation, essentially connects the thrombogenic right atrium to the cerebral arterial supply. Protein-losing enteropathy, a complication of the Fontan physiology, may result in a decrease in circulating protein C and S, as well as antithrombin, particularly in the setting of infection.
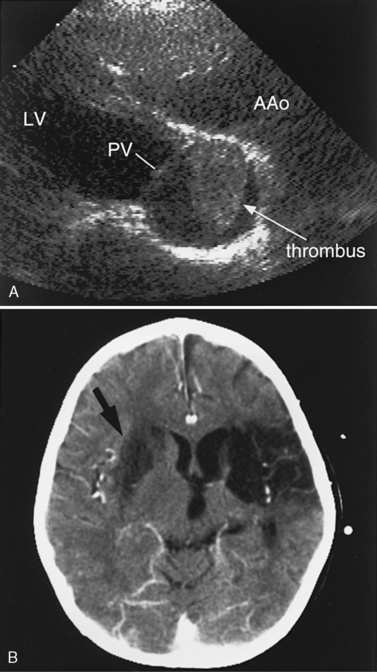
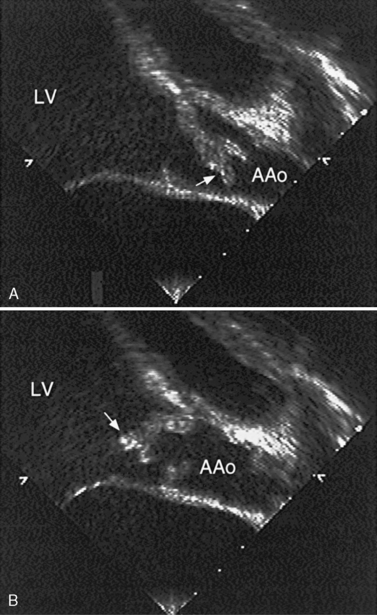
Another potential area of thrombus formation after the Fontan procedure results when the main pulmonary artery is ligated, leaving a blind-ending, low-flow stump. Thrombi in this location (Figure 101-10A) are capable of generating emboli directly into the aorta and cerebral circulation (Figure 101-10B). Retrospective studies of post-Fontan patients confirm the increased stroke risk, with a prevalence ranging from 2.6 percent [du Plessis et al., 1995a] to 8.8 percent [Day et al., 1995]. This risk for stroke after the Fontan operation persists for up to 3 [du Plessis et al., 1995a] to 15 years [Rosenthal et al., 1995b] after the procedure.
Stroke therapies may be considered in broad terms as rescue or preventive strategies. Rescue strategies aim to limit the extent of stroke by salvaging injured but potentially viable brain. Rescue therapies include thrombolytic agents [del Zoppo et al., 1988; Mori et al., 1988] or agents directed at limiting the injurious biochemical cascades triggered by HI/R insults [Gerlach et al., 1995; Vannucci, 1990]. Preventive therapies for stroke may be primary (i.e., treatment of high-risk patients to prevent a first stroke) or secondary (i.e., aimed at preventing stroke recurrence) [Anderson, 1991]. A review of the various preventive and rescue therapies for stroke, including that in children with CHD, is provided elsewhere in this text.
Arteriopathic stroke
Various forms of progressive steno-occlusive vasculopathy involving the intracranial circulation have been reported in patients with CHD. Although these conditions usually are not related to cardiac surgery, they are discussed here because they should be included in the differential diagnosis of stroke in CHD. Several cardiac lesions have been associated with intracranial arteriopathy and may be grouped into two broad categories: conotruncal defects and obstructive lesions of the aorta or pulmonary arteries. Patients with CHD who developed moyamoya disease, with repeated strokes and seizures, have been described [Lutterman et al., 1998]. Coarctation of the aorta has been associated with cerebral aneurysms [Orsi et al., 1993] of the circle of Willis and spinal arteries [Ling and Bao, 1994], as well as arteriovenous malformations [LeBlanc et al., 1968; Shearer et al., 1970; Tomlinson et al., 1992; Young et al., 1982]. Children with aortic coarctation who develop hypertension may be particularly vulnerable to intracranial hemorrhage from these intracranial vascular defects. After coarctation repair, patients who develop stroke symptoms (particularly when referable to the posterior circulation) should be evaluated for a subclavian steal syndrome [Saalouke et al., 1978].
The multifocal vasculopathy in Williams’ syndrome of the supravalvar aorta, as well as the carotid, vertebral, and intracranial arteries, may be associated with stroke. The stenotic lesions result from a medial fibroelastic dysplasia, hypertrophied smooth muscle cells, and deficient disorganized elastin fibers [Ardinger et al., 1994; Ewart et al., 1993; Kaplan, 1995; Soper et al., 1995]. The relationship between these various angiopathic syndromes associated with CHD needs further study.
Chronic Headache
In general, headache is mediated by stimulation of nerve endings in the dura and blood vessels, by processes as diverse as inflammation and tension/pressure. As many of the physiologic changes capable of stimulating these structures (e.g., vasodilatation and intracranial hypertension) occur in CHD, it is not surprising that headaches are a common complication of CHD. For example, both decreased cerebral oxygen delivery and hypercarbia cause vasodilatation. Elevated cerebral venous pressure may cause headaches by venous distention and, if sustained, by causing communicating hydrocephalus due to impaired CSF absorption [Rosman and Shands, 1978]. Headaches are particularly common in adolescent and adult survivors of earlier palliative procedures, in whom progressive pulmonary hypertension, central venous hypertension, and polycythemia develop over time. Certain surgical procedures (e.g., the Fontan and Glenn operations) are prone to central venous hypertension and commonly are complicated by headaches. In patients with venous hypertension and polycythemia, the development of headaches may herald dural vein thrombosis. The intensity and frequency of headaches associated with progressive polycythemia tend to parallel the rising hematocrit and respond to erythropheresis.
Headache is the most common initial complaint in the often-subtle clinical presentation of brain abscess [Aicardi, 1992b]. Consequently, it is mandatory that a diagnosis of brain abscess be excluded before the direct measurement of CSF pressure when doing a lumbar puncture in CHD patients with papilledema. Sudden-onset severe headaches should always raise concern about subarachnoid hemorrhage, particularly in patients with infective endocarditis [Bohmfalk et al., 1978; Jones and Sieker, 1989] and in hypertensive patients with coarctation of the aorta [LeBlanc et al., 1968; Shearer et al., 1970; Tomlinson et al., 1992; Young et al., 1982].
Long-Term Neurodevelopmental Dysfunction
Although children with acute postoperative neurologic manifestations are at risk for long-term neurodevelopmental dysfunction, it is likely that, in many cases, structural brain injury in early (even fetal) life and the acute perioperative period remains clinically silent until the later functional consequences are detected. Examples include the delayed neurodevelopmental impact of neonatal stroke and PVL. The few prospective studies to examine the neurodevelopmental outcome of infants with CHD longitudinally over time have been, for the most part, in the context of randomized clinical trials. Most studies have been cross-sectional in design and have focused primarily on two high-risk populations: namely, children with TGA and HLHS. However, others have reported increased risk for long-term developmental disabilities in all CHD subtypes severe enough to require open-heart surgery in early life [Fuller et al., 2009a; Gaynor et al., 2007; Limperopoulos et al., 2001, 2002; Majnemer et al., 2006, 2008, 2009; Massaro et al., 2008]. Children at greatest risk for long-term neurodevelopmental disability appear to be those with complex CHD requiring palliative procedures and staged repair, including patients with HLHS [Dittrich et al., 2003; Limperopoulos et al., 2002; McCusker et al., 2007].
Previously, outcome studies focused primarily on cognitive scores (i.e., intelligence quotients), the neuromotor examination in survivors of open-heart surgery, or both. Recent studies have used more comprehensive testing instruments to examine a broader spectrum of long-term outcome, including performance in motor, learning, behavior, socialization, functional skills in day-to-day activities, and quality of life. Not surprisingly, children with CHD exhibit a wide range of long-term developmental outcomes overall. Mean IQ scores at follow-up generally are in the low-average range [Bellinger et al., 1999a, 2009; Majnemer et al., 2008], while neuromotor abnormalities, such as hypotonia and motor delay, are reported in up to 50 percent of subjects and may persist well into school age [Bellinger et al., 1999a, 2009; Limperopoulos et al., 2002; Majnemer et al., 2006]. Conversely, severe neuromotor impairments (e.g., hemiplegia, restricted mobility) are less common (<5 percent) [Majnemer et al., 2006, 2009]. Increasingly reported are attentional problems, executive function deficits, and visual-spatial and visual motor impairments, as well as speech and language delays (e.g., oromotor apraxia) [Bellinger et al., 1999a; Mahle and Wernovsky, 2004]. Behavioral problems have been described in up to 27 percent of subjects [Bellinger et al., 2009; Majnemer et al., 2008]. Functional limitations in socialization, communication, adaptive behavior skills, and daily living skills are also described in close to 20 percent [Majnemer et al., 2008]. These studies suggest that children with CHD are at increased risk for learning difficulties and decreased social participation at school age. Recent reports suggest that a significant proportion of survivors of open-heart surgery for CHD are at risk for psychological maladjustment and impaired quality of life, including autonomy and motor, social, and emotional functioning [Landolt et al., 2008; Latal et al., 2009].
Of great interest in the few longitudinal studies published to date is the evolving neurodevelopmental profile in survivors of CHD surgery. For example, in the Boston Circulatory Arrest Study comparing outcome in infants with TGA randomized to intraoperative strategies of DHCA versus LFB, testing at 1 year of age revealed that infants assigned to DHCA were at higher risk of delayed motor development and neurologic abnormalities [Bellinger et al., 1995, 1997]. However, subsequent evaluations in this cohort at 4 and 8 years indicated no difference between the two groups in IQ scores, neurological status, academic achievement, memory, problem solving, or visual-motor integration [Bellinger et al., 1999a, 2009]. Assignment to DHCA was associated with lower gross and fine motor function and more severe speech abnormalities, while assignment to LFB was associated with a more impulsive response style and worse behavior. Risk factors for behavioral problems in middle childhood included postoperative seizures and intraoperative management with severe hemodilution and an alpha-stat acid–base management strategy [Bellinger et al., 2009]. The authors concluded that both the DHCA and LFB strategies are associated with increased risk of neurodevelopmental vulnerabilities. The evolving nature of neurodevelopmental function in these infants exposed to early-life CHD repair is of great interest, especially when considered in the context of other studies in premature infants that document similar evolving patterns of long-term outcome over time [Ment et al., 2003].
Over the past decades, the search for predictors of long-term neurodevelopmental impairment in survivors of CHD surgery has focused largely on intraoperative factors (see above). More recently emerging data suggest that predictors of adverse neurodevelopmental outcome are multifactorial, and include preoperative, intraoperative, and postoperative factors. In fact, several studies have reported that the strongest predictors of worse neurodevelopmental outcome are patient-specific factors that outweigh intraoperative management-related factors [Gaynor, 2003; Gaynor et al., 2007; Zeltser et al., 2008]. Such patient-specific factors have included genetic syndromes, low birth weight, gender, ethnicity, age at surgery, socioeconomic status, and parental factors such as mental health and IQ, amongst others [Ballweg et al., 2007; Forbess et al., 2002; Hovels-Gurich et al., 2006; Limperopoulos et al., 2002; McCusker et al., 2007; Newburger et al., 1993; Wernovsky et al., 2000]. In addition, preoperative factors, such as Apgar score, birth weight, microcephaly, and neurologic status, amongst others, also have been identified as predictors of long-term outcome [Fuller et al., 2009a; Limperopoulos et al., 2002; Majnemer et al., 2006, 2008; Neufeld et al., 2008; Robertson et al., 2004].
Of particular interest is a series of reports from prospective studies in infants with CHD undergoing brain MRI during the pre- and early postoperative periods [Licht et al., 2004, 2009; Mahle et al., 2002; McQuillen et al., 2006, 2007; Miller et al., 2004; Tavani et al., 2003]. These studies have shown a significant and previously under-recognized prevalence of structural brain abnormalities that, for the most part, have been clinically silent in the perioperative period. Among the most common findings have been focal infarctions, micro-hemorrhages, and PVL [Licht et al., 2009; McQuillen et al., 2006; Miller et al., 2006, 2007; Tavani et al., 2003]. Early postoperative MRI studies have depicted a striking prevalence of periventricular white-matter injury, particularly after neonatal cardiac surgery. In some studies, the prevalence of PVL on early postoperative MRI exceeded 50 percent in newborns following cardiac surgery [Galli et al., 2004; Mahle et al., 2002] (Figure 101-11), and was associated with hypotension and hypoxemia in the first 48 hours postoperatively [Galli et al., 2004]. Unfortunately, the long-term neurodevelopmental impact of these perioperative MRI findings has yet to be reported. Two recent reports from prospective studies in which MRI was performed after the early postoperative period have evaluated the functional associations of specific structural brain lesions. Volumetric MRI performed several months following open-heart infant cardiac surgery indicated a significant reduction in cerebral cortical gray matter (but interestingly, not white matter), particularly in the frontal lobes, which was associated with impaired psychomotor development on later testing [Watanabe et al., 2009]. Another MRI study 1 year after infant cardiac surgery described a 38 percent incidence of subtle hemorrhagic lesions, which were associated with lower motor developmental scores at age 1 year [Soul et al., 2009]. Further studies of the long-term significance of perioperative MRI lesions are needed before this imaging technique can be used for meaningful prognostication.
Neurologic Conditions Associated with Acquired Heart Disease
Infectious and Parainfectious Conditions
Infective Endocarditis
Neurologic injury complicates between 20 and 40 percent of infective endocarditis cases [Derex et al., 2009; Francioli, 1991; Hart et al., 1990; Horstkotte et al., 2004; Kanter and Hart, 1991; Saiman et al., 1993; Salgado et al., 1989; Thuny et al., 2007]. The neurologic manifestations of infective endocarditis are protean and include meningitis, brain abscess, seizures, and, most commonly, cerebrovascular injury. Systemic embolization occurs in up to 50 percent of patients with infective endocarditis [Lutas et al., 1986; Pelletier and Petersdorf, 1977; Roy et al., 1976], with as many as 65 percent of embolic events targeting the brain, usually the middle cerebral artery territory [Pruitt et al., 1978] (Figure 101-12).
Cerebrovascular complications are not only the most common form of neurologic injury, but also the most lethal [Pruitt et al., 1978]. Stroke complicates up to 40 percent of left-sided endocarditis [Derex et al., 2009; Hart et al., 1990; Horstkotte et al., 2004; Kanter and Hart, 1991; Salgado et al., 1989; Thuny et al., 2007]. For these reasons, anticoagulant therapy has been controversial to date for children with infective endocarditis. Strokes occur as a result of arterial infected embolus or mycotic aneurysm. The risk of stroke is highest in the first week of antibiotic treatment and decreases rapidly thereafter. Risk factors for embolization and stroke include vegetations over 10 mm in size, especially on the anterior mitral leaflet, and certain organisms such as Staphylococcus aureus or Staph. bovis, Enterococcus, or Candida albicans [Pruitt et al., 1978]. Although cerebral hemorrhage complicates only 5 percent of infective endocarditis, the mortality of this complication exceeds 50 percent [Hart et al., 1990; Horstkotte et al., 2004; Kanter and Hart, 1991; Salgado et al., 1989; Thuny et al., 2007]. The mechanisms of hemorrhage include arteritis, hemorrhagic transformation of a stroke, or rupture of a mycotic aneurysm. In children with endocarditis who develop neurologic symptoms, these risks for intracranial hemorrhage have long been considered a contraindication for anticoagulation. In addition, the benefit of anticoagulation has never been demonstrated in cases of native valve endocarditis, but may be beneficial for mechanical valve vegetations [Baddour et al., 2005].
Cerebral mycotic aneurysms are an infrequent but often lethal complication of infective endocarditis. Although mycotic aneurysms complicate only 1.2–5 percent of cases of infective endocarditis, the overall mortality of this lesion approaches 60 percent [Bohmfalk et al., 1978]. Two-thirds of cerebral mycotic aneurysms occur at the more distal bifurcations, particularly the middle cerebral artery. The remaining aneurysms are more centrally located at the branches of the circle of Willis [Corr et al., 1995]. Angiographic studies have determined that, in approximately 30 percent of cases, multiple aneurysms are present [Corr et al., 1995]. The clinical presentation is highly variable, with features ranging from those of sudden catastrophic hemorrhage (Figure 101-13), a mass lesion, or an embolic lesion. Presentation and outcome depend primarily on the location and integrity of the aneurysm. The mortality rate in patients with intact aneurysms is about 30 percent, and increases to 90 percent if the aneurysm ruptures [Bohmfalk et al., 1978; Jones and Sieker, 1989]. Aneurysms that leak rather than rupture are associated with a sterile meningeal syndrome, with elevations of CSF protein and of red cell and white cell counts.
Mycotic aneurysms should be excluded in all cases of infective endocarditis complicated by severe headaches, “sterile” meningitis, strokelike events, or cranial nerve abnormalities. Currently, the screening technique of choice is high-resolution, contrast-enhanced CT, because it is capable of identifying both the presence of cerebral hemorrhage and the location of mycotic aneurysms. The definitive diagnostic test remains four-vessel angiography. MR angiography techniques are insensitive to aneurysms smaller than 5 mm [Huston et al., 1994].
Management of mycotic aneurysms is complex and includes both medical and surgical approaches [D’Angelo et al., 1995; Elowiz et al., 1995; Utoh et al., 1995]. A 6-week course of antimicrobial therapy directed at the organism responsible for the underlying endocarditis should be completed. Hereafter, follow-up angiography is indicated to assess the response to treatment and to detect enlarging or new aneurysms [Corr et al., 1995]. Subsequent treatment is based on these angiographic findings. When angiograms are repeated after a full course of appropriate antimicrobial therapy, up to 20 percent indicate enlargements, 17–29 percent decrease in size, and one third to 50 percent indicate complete resolution [Bingham, 1977; Corr et al., 1995]. Up to 50 percent of patients experience full clinical recovery of their neurologic deficits after a 6-week course of treatment [Corr et al., 1995]. Indications for surgical intervention remain controversial [Wilson et al., 1982], but include the presence of single or multiple aneurysms distal to the first middle cerebral artery bifurcation that persist, enlarge, or bleed, despite appropriate antibiotic therapy [Corr et al., 1995; Scotti et al., 1996]. The usual surgical approach to these aneurysms is direct excision or clip application. More recently, endovascular procedures [Scotti et al., 1996] have been used to manage selected, severe cases of deep or distal mycotic aneurysms.
Brain Abscess
Brain abscess has become a rare complication of CHD in childhood [Ghosh et al., 1988]. However, among those children diagnosed with brain abscess, almost half have CHD [Aebi et al., 1991]. In children with CHD, brain abscess is rare before the age of 2 years, with a peak incidence between 4 and 7 years of age [Kagawa et al., 1983]. The risk is highest in cyanotic CHD; earlier studies reported that brain abscess developed in 2–6 percent of patients [Shu-yuan, 1989]. The risk for brain abscess is highest in children with polycythemia and right-to-left shunts [Tyler and Clark, 1957a], particularly tetralogy of Fallot [Aebi et al., 1991]. The incidence, morbidity, and mortality of brain abscess in CHD are correlated inversely with circulating arterial oxygenation [Shu-yuan, 1989]. Infants with higher oxygen saturations are less likely to develop brain abscess and more likely to recover. During periods of systemic illness and dehydration, polycythemia may disturb cerebral microvascular perfusion critically, with subsequent localized areas of ischemia. A right-to-left shunt allows organisms to bypass the pulmonary filtration system and thus to gain direct access to the brain. In areas of necrosis, organisms breach the disrupted blood–brain barrier, passing into necrotic areas to form focal septic cerebritis that may evolve to frank cerebral abscess. The recent marked decrease in incidence of brain abscess among children with CHD is likely due to a number of factors, including earlier corrective surgery, decreased exposure to polycythemia and hypoxia, and more aggressive rehydration.
Clinical manifestations of brain abscess are determined by the degree of intracranial hypertension, focal neurologic injury, and sepsis. The location of the abscess influences the neurologic presentation. A total of 75 percent of brain abscesses are supratentorial. Posterior fossa abscesses are less common but more dangerous because they often remain clinically silent until the onset of rapid deterioration from tonsillar herniation and brainstem compression (Figure 101-14). Cerebral abscesses are multifocal in about 20 percent of patients.
The presentation of brain abscess is usually subtle (with the exception of seizures) and slowly progressive. The most common presenting features are headache and vomiting [Aicardi, 1992a]. Up to 75 percent of patients are afebrile and have minimal peripheral leukocytosis. Diagnosis of brain abscess is best established by contrast-enhanced CT or MRI scan. With CT scan, brain abscess presents as an area of hypodensity with contrast ring enhancement. The lesion is often surrounded by marked cerebral edema. Once brain imaging has excluded significant mass effect, CSF should be obtained. Typically, the CSF contains elevated protein but only mild leukocytosis.
Optimal management of brain abscess remains controversial [Dodge and Pomeroy, 1992]. Surgery is still considered the definitive first-line treatment in many centers, either by direct resection or CT-guided aspiration. Advances in both antibiotic therapy and neuroimaging surveillance have allowed a more conservative approach [Berg et al., 1978; Rosenblum et al., 1980]. This approach remains controversial, but may prove effective in early cases of focal cerebritis without rapid progression. Whether combined with surgery or not, high-dose antibiotic therapy should be maintained for at least 6 weeks.
Initial antimicrobial management should be directed at the most common causative organisms (i.e., mixed aerobic and anaerobic streptococci and staphylococci) [Ghosh et al., 1988]. Third-generation cephalosporins, used in combination with antistaphylococcal and anaerobic agents, have superseded earlier antibiotic regimens largely. Subsequent antibiotic treatment should be guided by microbial culture results. In immunosuppressed patients (e.g., after cardiac transplantation), other lower-virulence organisms, as well as fungi (e.g., Aspergillus) and parasites (e.g., Toxoplasma), should be considered. As the clinical presentation of brain abscess may closely resemble stroke in up to 30 percent of patients [Kurlan and Griggs, 1983], it has been suggested that children with cyanotic CHD and a strokelike presentation should be managed with antibiotic therapy until a brain abscess is excluded [Kurlan and Griggs, 1983].
Diagnostic and therapeutic advances have reduced the mortality of brain abscess from 40 percent [Kagawa et al., 1983] to 10 percent [Dodge and Pomeroy, 1992]. However, in survivors, the prevalence of neurologic sequelae largely has remained unchanged at about 35–45 percent [Aebi et al., 1991; Dodge and Pomeroy, 1992]. Epilepsy may develop, often years later, in up to 30 percent of survivors [Aebi et al., 1991].
Rheumatic Heart Disease
Sydenham’s chorea, the major neurologic complication of acute rheumatic fever [Special Writing Group of the Committee on Rheumatic Fever Endocarditis and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association, 1992] was, in earlier years, the most common form of acquired chorea in childhood [Eschel et al., 1993]. For several decades, there has been a global decline in the incidence of rheumatic fever and in the incidence of chorea as a complication of rheumatic fever [Eschel et al., 1993]. Possible reasons for this decline are an alteration in the virulence or epitopes of rheumatogenic streptococci and a decrease in antigen cross-reactivity with the basal ganglia. However, outbreaks of rheumatic fever in the United States [Ayoub, 1992] and elsewhere [Karademir et al., 1994] focused attention on this condition.
Chorea complicates rheumatic fever in only 10–25 percent of patients [Eschel et al., 1993]. Chorea may emerge from 1 week to 8 months after the onset of rheumatic fever, and then persist for 1–6 months [Eschel et al., 1993]. Sydenham’s chorea is rare under the age of 3 years and occurs more commonly in girls than in boys. In some patients, chorea may be the only clinical manifestation of rheumatic fever. The onset of this disorder is often subtle, and the abnormal movements tend to be preceded by psychoemotional symptoms, such as anxiety, emotional lability, distractibility, the emergence or exacerbation of attention-deficit hyperactivity disorder, obsessive-compulsive symptoms, and sleep disturbances [Swedo, 1994; Swedo and Kiessling, 1994]. On occasion, both the psychoemotional prodrome and the dyskinesia may be explosive in onset. The motor activity, which at first is described as “fidgety,” soon becomes choreiform, evolving from initial brief, myoclonic-like proximal muscle jerks to more complex, writhing distal movements. The handgrip often has a rippling character due to inconsistently sustained finger pressure (“milkmaid’s grip”). The chorea may be asymmetric and, in some cases, pure hemichorea develops. Muscle hypotonia and mild to moderate weakness often are present. Patients may have difficulty initiating and sustaining spontaneous motor activity. Speech and oral motor activity often are affected; an explosive dysarthria may develop, evolving in severe cases to complete mutism. These symptoms usually begin to subside after about 2–6 months, but may recur in the setting of subsequent illnesses, particularly streptococcal infections, pregnancy (chorea gravidarum), or oral contraceptive use.
The diagnosis of acute rheumatic fever is based on specific (Jones’) criteria [Special Writing Group of the Committee on Rheumatic Fever Endocarditis and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association, 1992], which may be major or minor, depending on their diagnostic importance. A patient with two major criteria, or one major and two minor criteria, has a high probability of rheumatic fever. The major criteria are carditis, polyarthritis, chorea, erythema marginatum, and subcutaneous nodules. The minor criteria are based on clinical features (fever, arthralgia, previous rheumatic fever) or laboratory features (acute-phase reaction, elevated erythrocyte sedimentation rate, leukocytosis, C-reactive protein, and prolonged P–R interval on electrocardiogram). Additional laboratory findings, such as an elevated antistreptolysin O titer or positive throat cultures for group A beta hemolytic streptococci, may be helpful. Diagnosis of chorea, on the other hand, is purely clinical. Despite the causal relationship to streptococcal infection, the onset of chorea is often delayed. Consequently, serologic evidence of streptococcal disease may be absent in 25 percent of patients. Rheumatogenic strains of group A streptococci contain particular (M-type) proteins that share antigenic determinants with neurons in the basal ganglia, particularly in the caudate and subthalamic nuclei. Autoantibodies against these neurons (antineuronal antibodies) have been detected in up to 90 percent of patients in some studies [Swedo, 1994; Swedo et al., 1997]. As this condition is seldom fatal, neuropathologic data are limited. However, neuronal loss, with vascular and perivascular inflammatory changes, has been described in the caudate and putamen, as well as the frontoparietal cortex. The topography of these earlier neuropathologic findings has been supported by MRI [Giedd et al., 1995; Heye et al., 1993] and SPECT [Heye et al., 1993]. These techniques have also demonstrated disturbances in the blood–brain barrier, with localized edema, presumably resulting from vasculitis [Heye et al., 1993].
Current management of the chorea includes dopamine-blocking agents (e.g., haloperidol, 0.5–1 mg twice a day) for 2–6 months and then gradually withdrawn. Other agents used include carbamazepine and valproic acid [Aicardi, 1992b; Swedo et al., 1993]. These agents often diminish the intensity of the chorea, but fail to control it completely. In severe cases, prednisone has been used with limited success. Prophylaxis against group A streptococcal infections with penicillin or monthly intramuscular benzathine penicillin is recommended because even asymptomatic future infections may precipitate recurrence of chorea. The high incidence of antineuronal [Swedo, 1994; Swedo and Kiessling, 1994] and anticardiolipin antibodies [Diniz et al., 1994; Figueroa et al., 1992] in these patients has led to trials of plasmapheresis and intravenous immunoglobulin therapy; results from these studies are not yet available.
Inherited Disorders of Heart, Muscle, and Nervous System
Inborn Errors of Metabolism
Cardiac dysfunction may be a prominent feature and often the cause of death in several inherited metabolic disorders [Lyon et al., 1996]. The inheritance of these conditions is usually recessive (autosomal or, rarely, X-linked) or mitochondrial. The cardiac dysfunction results from a hypertrophic or dilated cardiomyopathy due to myocardial infiltration or energy failure, rather than from a primary structural lesion. The cardiac involvement in these conditions manifests clinically with myocardial failure, valvular insufficiency, arrhythmias, or coronary insufficiency (in storage disorders) with myocardial ischemia. Neurologic manifestations may be due primarily to the underlying enzyme defect or, conversely, may be secondary to global or focal cerebral hypoperfusion caused by diminished cardiac output.
Disorders of Energy Production
Mitochondrial fatty acid oxidation defects
These conditions include a group of autosomal-recessive disorders that may present with combined cardiac and neurologic dysfunction (see Chapter 37). These conditions present with several clinical syndromes, including recurrent metabolic crises, depressed mental status, or ataxia. Primary systemic carnitine deficiency results from a defect in carnitine transport and is the only disorder of carnitine metabolism with prominent cardiomyopathic features. Children with long-chain acyl-CoA dehydrogenase (LCAD) deficiency commonly develop a hypertrophic or dilated cardiomyopathy owing to myocardial fat deposition. Infants with the neonatal-onset form of multiple acyl-CoA dehydrogenase deficiency (glutaric aciduria type II) may present with dysmorphic features, a “sweaty feet” odor, and a severe, often catastrophic, illness within the first days of life. Neuronal loss and gliosis lead to caudate and putaminal atrophy [Chow et al., 1989], with a prominent ataxic and dyskinetic syndrome. The cardiomyopathy in this condition is severe and usually lethal within weeks to months.
Disorders of oxidative phosphorylation
These disorders result from enzyme defects in pyruvate metabolism and the mitochondrial electron transport chain. Brain and muscle are the most commonly involved systems. Cardiac dysfunction may be prominent, with the presenting features related to the age at onset. In young infants, a rapidly progressive and usually fatal hypertrophic cardiomyopathy develops, whereas older patients with later-onset cardiac involvement are more likely to develop dysrhythmias and conduction defects [Guenthard et al., 1995]. Certain respiratory chain enzyme defects, such as complex I (nicotinamide adenine dinucleotide [NADH] CoQ reductase) [Rustin et al., 1994] and complex IV (cytochrome c oxidase) [Zeviani and Van Dyke, 1986] deficiencies (often in combination) [Nagai et al., 1993], are more prone to cardiac dysfunction. Complex III (reduced CoQ-cytochrome c reductase) deficiency may cause an isolated cardiomyopathy. Cytochrome c oxidase deficiency may present in the newborn or young infant as a severe, rapidly fatal condition, or as a benign, reversible form. Initially, the benign form may be indistinguishable from the lethal form; in fact, the lactic acidemia may be more severe in the benign form [DiMauro et al., 1990]. As infants with the benign form may recover fully with supportive management, an aggressive approach is warranted until the clinical distinction becomes evident. Kearns–Sayre syndrome usually is associated with complex I or IV deficiencies, and causes retinal degeneration and chronic progressive external ophthalmoplegia. Cardiac involvement results in atrioventricular heart block, with syncopal spells or sudden death occurring between late childhood and adulthood.
Storage Disorders of the Heart and Nervous System
Glycogen storage diseases
Among the different forms of glycogen storage disease, combined cardiac and neurologic dysfunction is confined largely to the early-infantile form of type II glycogen storage disease owing to acid maltase (acid α-glucosidase) deficiency (see Chapter 34). Later-onset type II glycogen storage disease presenting after age 2 years is not associated with cardiac dysfunction. Glycogen deposition in the early-infantile form causes macroglossia, a hypertrophic cardiomyopathy, and a rapidly progressive diffuse skeletal myopathy. In the nervous system, glycogen deposition is confined to the anterior horn cells of the spinal cord and brainstem [Martin et al., 1973], sparing cerebral cortical neurons. The progressive obstructive cardiomyopathy and respiratory weakness owing to myopathy and anterior horn cell involvement usually culminate in cardiorespiratory death before 1 year of age.
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Aebi C., Kaufmann F., Schaad U. Brain abscess in childhood: Long-term experiences. Eur J Pediatr. 1991;150:282.
Aggarwal V.S., Morrow B.E. Genetic modifiers of the physical malformations in velo-cardio-facial syndrome/DiGeorge syndrome. Dev Disabil Res Rev. 2008;14:19.
Aicardi J.. Postnatally acquired infectious diseases. vol 115. Diseases of the Nervous System in Childhood. London: Mac Keith Press; 1992. (118)
Aicardi J.. Para-infectious and other inflammatory disorders of immunological origin. Diseases of the Nervous System in Childhood. vol 115. London: Mac Keith Press; 1992. (118)
Akay T.H., Ozkan S., Gultekin B., et al. Diaphragmatic paralysis after cardiac surgery in children: incidence, prognosis and surgical management. Pediatr Surg Int. 2006;22:341.
Al-Lozi M., Pestronk A., Yee W., et al. Rapidly evoving myopathy with myosin-deficient muscle fibers. Ann Neurol. 1994;35:273.
Alsoufi B., Al-Radi O.O., Nazer R.I., et al. Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg. 2007;134:952.
Ammash N., Warnes C. Cerebrovascular events in adult patients with cyanotic congenital heart disease. J Am Coll Cardiol. 1996;28:768.
Anderson D. Cardioembolic stroke: Primary and secondary prevention. Postgrad Med. 1991;90:67.
Andre M., Matisse N., Vert P. Prognosis of neonatal seizures. In: Wasterlain C.G., Vert P., editors. Neonatal Seizures. New York: Raven Press; 1990:61.
Antshel K.M., Fremont W., Kates W.R. The neurocognitive phenotype in velo-cardio-facial syndrome: a developmental perspective. Dev Disabil Res Rev. 2008;14:43.
Ardinger R., Goertz K., Matteoli L. Cerebrovascular stenosis with cerebral infarction in a child with Williams syndrome. Am J Med Genet. 1994;51:200.
Ashwal S., Holshouser B.A., del Rio M.J., et al. Serial proton magnetic resonance spectroscopy of the brain in children undergoing cardiac surgery. Pediatr Neurol. 2003;29:99.
Ashwal S., Holshouser B., Schell R., et al. Proton magnetic resonance spectroscopy in the evaluation of children with congenital heart disease and acute central nervous system injury. J Thorac Cardiovasc Surg. 1996;112:403.
Ayoub E. Resurgence of rheumatic fever in the United States. Postgrad Med. 1992;92:133.
Back S.A. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129.
Back S.A., Riddle A., McClure M.M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724.
Baddour L.M., Wilson W.R., Bayer A.S., et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394.
Balasubramanian S.K., Tiruvoipati R., Amin M., et al. Factors influencing the outcome of paediatric cardiac surgical patients during extracorporeal circulatory support. J Cardiothorac Surg. 2007;2:4.
Ballweg J.A., Wernovsky G., Gaynor J.W. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2007;28:126.
Bando K., Turrentine M.W., Vijay P., et al. Effect of modified ultrafiltration in high-risk patients undergoing operations for congenital heart disease. Ann Thorac Surg. 1998;66:821.
Barrat-Boyes B. Choreoathetosis as a complication of cardiopulmonary bypass. Ann Thorac Surg. 1990;50:693.
Bartlett R.H., Gazzaniga A.B., Huxtable R.F., et al. Extracorporeal circulation (ECMO) in neonatal respiratory failure. J Thorac Cardiovasc Surg. 1977;74:826.
Bartsch O., Nemeckova M., Kocarek E., et al. DiGeorge/velocardiofacial syndrome: FISH studies of chromosomes 22q11 and 10p14, and clinical reports on the proximal 22q11 deletion. Am J Med Genet. 2003;117A:1.
Bearden C.E., Woodin M.F., Wang P.P., et al. The neurocognitive phenotype of the 22q11.2 deletion syndrome: selective deficit in visual-spatial memory. J Clin Exp Neuropsychol. 2001;23:447.
Beca J., Gunn J., Coleman L., et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807.
Bellinger D.C., Jonas R.A., Rappaport L.A., et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549.
Bellinger D.C., Newburger J.W., Wypij D., et al. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86.
Bellinger D.C., Rappaport L.A., Wypij D., et al. Patterns of developmental dysfunction after surgery during infancy to correct transposition of the great arteries. J Dev Behav Pediatr. 1997;18:75.
Bellinger D.C., Wypij D., du Plessis A.J., et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385.
Bellinger D.C., Wypij D., Kuban K.C., et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526.
Bellinger D.C., Wypij D., Kuban K.C., et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526.
Benson D. Changing profile of congenital heart disease. Pediatrics. 1989;83:790.
Berg B., Franklin G., Cuneo R., et al. Nonsurgical cure of brain abscess: Early diagnosis and follow-up with computerized tomography. Ann Neurol. 1978;3:474.
Bergman I., Painter M., Hirsch R., et al. Outcome in neonates with convulsions treated in an intensive care unit. Ann Neurol. 1983;14:642.
Bergman I., Steeves M., Burckart G., et al. Reversible neurologic abnormalities associated with prolonged intravenous midazolam and fentanyl administration. J Pediatr. 1991;119:644.
Bergouignan M., Fontan F., Trarieux M. Syndromes choreiformes de l’enfant au de’cours d’interventions cardio-chirurgicales sous hypothermic profounde. Rev Neurol. 1961;105:48.
Berthrong M., Sabiston D. Cerebral lesions in congenital heart disease. Bull Johns Hopkins Hosp. 1951;89:384.
Bingham P.M., Lynch D., McDonald-McGinn D., et al. Polymicrogyria in chromosome 22 delection syndrome. Neurology. 1998;51:1500.
Bingham P.M., Zimmerman R.A., McDonald-McGinn D., et al. Enlarged sylvian fissures in infants with interstitial deletion of chromosome 22q11. Am J Med Genet. 1997;75:538.
Bingham W. Treatment of mycotic intracranial aneurysms. J Neurosurg. 1977;46:428.
Bjork V., Hultquist G. Contraindications to profound hypothermia in open-heart surgery. J Thorac Cardiovasc Surg. 1962;44:1.
Bohmfalk G., Story J., Wissinger J., et al. Bacterial intracranial aneurysm. J Neurosurg. 1978;48:369.
Bolton C. Clinical neurophysiology of the respiratory system. Muscle Nerve. 1993;16:809.
Boyajian R.A., Sobel D.F., DeLaria G.A., et al. Embolic stroke as a sequela of cardiopulmonary bypass. J Neuroimag. 1993;3:1.
Brewer L., Fosburg R., Mulder G., et al. Spinal cord complications following surgery for coarctation of the aorta. J Thorac Cardiovasc Surg. 1972;64:68.
Brunberg J., Doty D., Reilly E. Choreoathetosis in infants following cardiac surgery with deep hypothermia and circulatory arrest. J Pediatr. 1974;84:232.
Castaneda A.R., Lamberti J., Sade R.M., et al. Open-heart surgery during the first three months of life. J Thorac Cardiovasc Surg. 1974;68:719.
Castaneda A.R., Mayer J.E., Jonas R.A., et al. The neonate with critical congenital heart disease: repair – a surgical challenge. J Thorac Cardiovasc Surg. 1989;98:869.
Cengiz P., Seidel K., Rycus P.T., et al. Central nervous system complications during pediatric extracorporeal life support: incidence and risk factors. Crit Care Med. 2005;33:2817.
Chan T., Thiagarajan R.R., Frank D., et al. Survival after extracorporeal cardiopulmonary resuscitation in infants and children with heart disease. J Thorac Cardiovasc Surg. 2008;136:984.
Chapman C., du Plessis A., Pober B. Neurologic findings in children and adults with Williams Syndrome. J Child Neurol. 1995;10:63.
Chaturvedi R.R., Shore D.F., White P.A., et al. Modified ultrafiltration improves global left ventricular systolic function after open-heart surgery in infants and children. Eur J Cardiothorac Surg. 1999;15:742.
Chaves E., Scaltsas-Persson I. Severe choreoathetosis (CA) following congenital heart disease (CHD) surgery (abstr). Neurology. 1988;38:284.
Cheng T.O. That balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries is another powerful argument in favor of therapeutic closure of every patent foramen ovale. Am J Cardiol. 2006;98:277.
Chen J., Zimmerman R.A., Jarvik G.P., et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. 2009;88:823.
Chen Y.S., Yu H.Y., Huang S.C., et al. Extracorporeal membrane oxygenation support can extend the duration of cardiopulmonary resuscitation. Crit Care Med. 2008;36:2529.
Chow C., Frerman F., Goodman S., et al. Striatal degeneration in glutaric aciduria type II. Acta Neuropathol. 1989;77:554.
Chow E.W., Mikulis D.J., Zipursky R.B., et al. Qualitative MRI findings in adults with 22q11 deletion syndrome and schizophrenia. Biol Psychiatry. 1999;46:1436.
Chow G., Koirala B., Armstrong D., et al. Predictors of mortality and neurological morbidity in children undergoing extracorporeal life support for cardiac disease. Eur J Cardiothorac Surg. 2004;26:38.
Christou H., Van Marter L.J., Wessel D.L., et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med. 2000;28:3722.
Clancy R.R., McGaurn S.A., Goin J.E., et al. Allopurinol neurocardiac protection trial in infants undergoing heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2001;108:61.
Clancy R.R., McGaurn S.A., Wernovsky G., et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111:592.
Clark R.H., Kueser T.J., Walker M.W., et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469.
Corr P., Wright M., Handler L. Endocarditis-related cerebral aneurysms: radiologic changes with treatment. Am J Neuroradiol. 1995;16:745.
Cottrill C., Kaplan S. Cerebral vascular accidents in cyanotic congenital heart disease. Am J Dis Child. 1973;125:484.
Cranford R., Leppik I., Patrick B., et al. Intravenous phenytoin: Clinical and pharmacological aspects. Neurology. 1978;28:874.
Cromme-Dijkhuis A., Henkens C., Bijleveld C., et al. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet. 1990;336:1087.
Curless R.G., Katz D.A., Perryman R.A., et al. Choreoathetosis after surgery for congenital heart disease. J Pediatr. 1994;124:737.
Dalton H.J. Fighting the flu: the rise of the machine. Pediatr Crit Care Med. 2004;5:415.
Dalton H.J., Rycus P.T., Conrad S.A. Update on extracorporeal life support 2004. Semin Perinatol. 2005;29:24.
D’Angelo V., Fiumara E., Gorgoglione L., et al. Surgical treatment of a cerebral mycotic aneurysm using the stereo-angiographic localizer. Surg Neurol. 1995;44:263.
Danon M., Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve. 1991;14:1131.
Davies M.J., Nguyen K., Gaynor J.W., et al. Modified ultrafiltration improves left ventricular systolic function in infants after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1998;115:361.
Daw S.C., Taylor C., Kraman M., et al. A common region of 10p deleted in DiGeorge and velocardiofacial syndromes. Nat Genet. 1996;13:458.
Dawson D., Fischer E. Neurologic complications of cardiac catheterization procedures. Neurology. 1977;27:496.
Day R., Boyer R., Tait V., et al. Factors associated with stroke following the Fontan procedure. Pediatr Cardiol. 1995;16:270.
DeLeon S., Ilbawi M., Arcilla R., et al. Choreoathetosis after deep hypothermia without circulatory arrest. Ann Thorac Surg. 1990;50:714.
del Nido P.J., Dalton H.J., Thompson A.E., et al. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86:II300.
del Zoppo G. Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev. 1994;6:47.
del Zoppo G., Ferbert A., Otis S., et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke: A pilot study. Stroke. 1988;19:307.
Demczuk S., Levy A., Aubry M., et al. Excess of deletions of maternal origin in the DiGeorge/Velo-cardio-facial. A study of 22 new patients and review of the literature. Hum Genet. 1995;96:9.
de Mos N., van Litsenburg R.R., McCrindle B., et al. Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med. 2006;34:1209.
Dent C.L., Spaeth J.P., Jones B.V., et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:1523.
Den U., Favrais G., Plaisant F., et al. Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: Key role of TNFalpha pathway and protection by etanercept. Brain Behav Immun. 2009.
Derex L., Bonnefoy E., Delahaye F. Impact of stroke on therapeutic decision making in infective endocarditis. J Neurol. 2009.
Desai S., Kollros P.R., Graziani L.J., et al. Sensitivity and specificity of the neonatal brain-stem auditory evoked potential for hearing and language deficits in survivors of extracorporeal membrane oxygenation. J Pediatr. 1997;131:233.
DiMauro S., Lombes A., Nakase H., et al. Cytochrome c oxidase deficiency. Pediatr Res. 1990;28:536.
Diniz R., Goldenberg J., Andrade L., et al. Antiphospholipid antibodies in rheumatic fever chorea. J Rheumatol. 1994;21:1367.
Dittrich H., Buhrer C., Grimmer I., et al. Neurodevelopment at 1 year of age in infants with congenital heart disease. Heart. 2003;89:436.
Dobell A., Trusler G., Smallhorn J., et al. Atrial thrombi after the Fontan operation. Ann Thorac Surg. 1986;42:664.
Dodge P., Pomeroy S.. Parameningeal infections (including brain abscess, epidural abscess, subdural empyema). Feigin R., Cherry J., editors. Textbook of Pediatric Infectious Diseases. vol. II. Philadelphia: WB Saunders Co; 1992:455.
Domi T., Edgell D.S., McCrindle B.W., et al. Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122:1292.
Donaldson D., Fullerton D., Gollub R., et al. Choreoathetosis in children after cardiac surgery. Neurology. 1990;40:337.
Driscoll D.A. Genetic basis of DiGeorge and velocardiofacial syndromes. Curr Opin Pediatr. 1994;6:702.
Duncan B.W., Bohn D.J., Atz A.M., et al. Mechanical circulatory support for the treatment of children with acute fulminant myocarditis. J Thorac Cardiovasc Surg. 2001;122:440.
Dunne J.W., Reutens D.C., Newman M., et al. Phrenic nerve injury in open heart surgery. Muscle Nerve. 1991;14:883.
du Plessis A., Chang A., Wessel D., et al. Cerebrovascular accidents following the Fontan procedure. Pediatr Neurol. 1995;12:230.
du Plessis A.J. Neurologic complications of cardiac disease in the newborn. Clin Perinatol. 1997;24:807.
du Plessis A.J., Bellinger D.C., Gauvreau K., et al. Neurologic outcome of choreoathetoid encephalopathy after cardiac surgery. Pediatr Neurol. 2002;27:9.
du Plessis A.J., Jonas R.A., Wypij D., et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114:991.
du Plessis A.J., Newburger J., Jonas R.A., et al. Cerebral oxygen supply and utilization during infant cardiac surgery. Ann Neurol. 1995;37:488.
du Plessis A., Kramer U., Jonas R., et al. West syndrome following deep hypothermic cardiac surgery. Pediatr Neurol. 1994;11:246.
du Plessis A., Treves S., Hickey P., et al. Regional cerebral perfusion abnormalities after cardiac operations. J Thorac Cardiovasc Surg. 1994;107:1036.
Eapen R.S., Rowland D.G., Franklin W.H. Effect of prenatal diagnosis of critical left heart obstruction on perinatal morbidity and mortality. Am J Perinatol. 1998;15:237.
Ehyai A., Fenichel G., Bender H. Incidence and prognosis of seizures in infants after cardiac surgery with profound hypothermia and circulatory arrest. JAMA. 1984;252:3165.
Elliott M. Modified ultrafiltration and open heart surgery in children. Paediatr Anaesth. 1999;9:1.
Elliott M., Finn A. Interaction between neutrophils and endothelium. Ann Thorac Surg. 1993;56:1503.
Elowiz E., Johnson W., Milhorat T. Computerized tomography (CT) localized stereotactic craniotomy for excision of a bacterial intracranial aneurysm. Surg Neurol. 1995;44:265.
Eschel G., Lahat E., Azizi E., et al. Chorea as a manifestation of rheumatic fever: A 30 year survey (1960-1990). Eur J Pediatr. 1993;152:645.
Eskedal L., Hagemo P., Eskild A., et al. A population-based study of extra-cardiac anomalies in children with congenital cardiac malformations. Cardiol Young. 2004;14:600.
Ewart A., Morris C., Atkinson D., et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature Genet. 1993;5:11.
Fallon P., Aparicio J.M., Elliot M.J., et al. Incidence of neurological complications of surgery for congenital heart disease. Arch Dis Child.. 1995 May;72(5):418-422.
Ferry P.C. Neurologic sequelae of cardiac surgery in children. Am J Dis Child. 1987;141:309.
Ferry P.C. Neurologic sequelae of open-heart surgery in children. An ‘irritating question’. Am J Dis Child. 1990;144:369.
Feuerstein G., Liu T., Barone F. Cytokines, inflammation, and brain injury: Role of tumor necrosis factor-a. Cerebrovasc Brain Metab Rev. 1994;6:341.
Figueroa F., Berrios X., Gutierrez M., et al. Anticardiolipin antibodies in acute rheumatic fever. J Rheumatol. 1992;19:1175.
Fish K.J. Microembolization: etiology and prevention. In: Hilberman M., editor. Brain Injury and Protection during Cardiac Surgery. Boston: Martinus Nijhoff; 1988:67.
Forbess J.M., Visconti K.J., Hancock-Friesen C., et al. Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation. 2002;106:I95.
Fortenberry J.D., Bhardwaj V., Niemer P., et al. Neutrophil and cytokine activation with neonatal extracorporeal membrane oxygenation. J Pediatr. 1996;128:670.
Francioli P. Central nervous complications of infective endocarditis. In: Scheld W., Whiteley R., Durack D., editors. Infections of the Central Nervous System. New York: Raven Press; 1991:515.
Fuller S., Nord A.S., Gerdes M., et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36:40.
Fuller S., Nord A.S., Gerdes M., et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009.
Galli K.K., Zimmerman R.A., Jarvik G.P., et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692.
Gaynor J.W. The effect of modified ultrafiltration on the postoperative course in patients with congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003;6:128.
Gaynor J.W., Jarvik G.P., Bernbaum J., et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:181.
Gaynor J.W., Wernovsky G., Jarvik G.P., et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344.
Gerlach M., Riederer P., Youdim M. Neuroprotective therapeutic strategies: Comparison of experimental and clinical results. Biochem Pharmacol. 1995;50:1.
Ghariani S., Dahan K., Saint-Martin C., et al. Polymicrogyria in chromosome 22q11 deletion syndrome. Eur J Paediatr Neurol. 2002;6:73.
Ghosh S., Chandy M., Abraham J. Brain abscess and congenital heart disease. J Ind Med Assoc. 1988;88:312.
Giedd J., Rapoport J., Kruesi M., et al. Sydenham’s chorea: Magnetic resonance imaging of the basal ganglia. Neurology. 1995;45:2199.
Giroud M., Lemesle M., Gouyon J.-B., et al. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: A study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48:1343.
Glauser T., Rorke L., Weinberg P., et al. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:991.
Glauser T., Rorke L., Weinberg P., et al. Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85:984.
Golding-Kushner K., Weller G., Shpintzen R. Velo-cardio-facial syndrome: Language and psychological profiles. J Craniofac Genet. 1985;5:259.
Gooch J.L., Suchyta M.R., Balbierz J.M., et al. Prolonged paralysis after treatment with neuromuscular junction blocking agents. Crit Care Med. 1991;19:1125.
Gothelf D., Presburger G., Zohar A.H., et al. Obsessive-compulsive disorder in patients with velocardiofacial (22q11 deletion) syndrome. Am J Med Genet B Neuropsychiatr Genet. 2004;126B:99.
Gross R.E., Hubbard J.P.. Landmark article Feb 25, 1939: Surgical ligation of a patent ductus arteriosus. Report of first successful case. By Robert E. Gross and John P. Hubbard JAMA. 1984;251:1201.
Guenthard J., Wyler F., Fowler B., et al. Cardiomyopathy in respiratory chain disorders. Arch Dis Child. 1995;72:223.
Guerit J.M., Witdoeckt C., Rubay J., et al. The usefulness of the spinal and subcortical components of the posterior tibial nerve SEPs for spinal cord monitoring during aortic coarctation repair. Electroencephalogr Clin Neurophysiol. 1997;104:115.
Hamrick S.E., Gremmels D.B., Keet C.A., et al. Neurodevelopmental outcome of infants supported with extracorporeal membrane oxygenation after cardiac surgery. Pediatrics. 2003;111:e671.
Hanekamp M.N., Mazer P., van der Cammen-van Zijp M.H., et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. 2006;10:R127.
Hart R.G., Foster J.W., Luther M.F., et al. Stroke in infective endocarditis. Stroke. 1990;21:695.
Helmers S.L., Wypij D., Constantinou J.E., et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. 1997;102:27.
Heye N., Jergas M., Hotzinger H., et al. Sydenham chorea: Clinical, EEG, MRI and SPECT findings in the early stages of the disease. J Neurol. 1993;240:121.
Hinton R.B., Andelfinger G., Sekar P., et al. Prenatal Head Growth and White Matter Injury in Hypoplastic Left Heart Syndrome. Pediatr Res. 2008.
Hirano M., Ott B., Raps E., et al. Acute quadriplegic myopathy: A complication of treatment with steroids, nondepolarizing blocking agents, or both. Neurology. 1992;42:2082.
Hoffman J.I., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890.
Hofkosh D., Thompson A.E., Nozza R.J., et al. Ten years of extracorporeal membrane oxygenation: neurodevelopmental outcome. Pediatrics. 1991;87:549.
Horstkotte D., Follath F., Gutschik E., et al. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J. 2004;25:267.
Hovels-Gurich H.H., Konrad K., Skorzenski D., et al. Long-term neurodevelopmental outcome and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann Thorac Surg. 2006;81:958.
Huntley D., Al-Mateen M., Menkes J. Unusual dyskinesia complicating cardiopulmonary bypass surgery. Dev Med Child Neurol. 1993;35:631.
Huston J.I., Nichols D., Luetmer P., et al. Blinded retrospective evaluation of the sensitivity of MR angiography to known intracranial aneurysms: importance of aneurysm size. Am J Neuroradiol. 1994;15:1607.
Hutto R., Williams J., Maertens P., et al. Cerebellar infarct: Late complication of the Fontan procedure. Pediatr Neurol. 1991;7:293.
Ibrahim A.E., Duncan B.W., Blume E.D., et al. Long-term follow-up of pediatric cardiac patients requiring mechanical circulatory support. Ann Thorac Surg. 2000;69:186.
Jansen N.J., van Oeveren W., Gu Y.J., et al. Endotoxin release and tumor necrosis factor formation during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:744.
Jarjour I.T., Ahdab-Barmada M. Cerebrovascular lesions in infants and children dying after extracorporeal membrane oxygenation. Pediatr Neurol. 1994;10:13.
Johnston M.V., Redmond J.M., Gillinov A.M., et al. Neuroprotective strategies in a model of selective neuronal necrosis from hypothermic circulatory arrest. In: Moskowitz M.A., Caplan L.R., editors. Cerebrovascular Diseases. Boston: Butterworth-Heinemann; 1995:165.
Jonas R.A., Wypij D., Roth S.J., et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126:1765.
Jones H., Sieker R. Neurologic manifestations of infective endocarditis. Brain. 1989;122:1295.
Jones M. Anomalies of the brain and congenital heart disease: a study of 52 necropsy cases. Pediatr Pathol. 1991;11:721.
Journois D., Israel-Biet D., Pouard P., et al. High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology. 1996;85:965.
Kaemmerer H., Meisner H., Hess J., et al. Surgical treatment of patent ductus arteriosus: a new historical perspective. Am J Cardiol. 2004;94:1153.
Kagawa M., Takeshita M., Yato S., et al. Brain abscess in congenital heart disease. J Neurosurg. 1983;58:913.
Kanter M.C., Hart R.G. Neurologic complications of infective endocarditis. Neurology. 1991;41:1015.
Kaplan P. Cerebral artery stenoses in Williams syndrome cause strokes in childhood. J Pediatr. 1995;126:943.
Karademir S., Demirceken F., Atalay S., et al. Acute rheumatic fever in children in the Ankara area in 1990-1992 and comparison with a previous study in 1980-1989. Acta Paediatr. 1994;83:862.
Kent K., Moscucci M., Gallagher S., et al. Neuropathy after cardiac catheterization: incidence, clinical patterns, and long-term outcome. J Vasc Surg. 1994;19:1008.
Kim E.S., Stolar C.J. ECMO in the newborn. Am J Perinatol. 2000;17:345.
Kinney H.C., Back S.A. Human oligodendroglial development: relationship to periventricular leukomalacia. Semin Pediatr Neurol. 1998;5:180.
Kirkham F.J. Recognition and prevention of neurological complications in pediatric cardiac surgery. Pediatr Cardiol. 1998 Jul-Aug;;19(4):331-345.
Kirklin J.K., Westaby S., Blackstone E.H., et al. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1983;86:845.
Kolovos N.S., Bratton S.L., Moler F.W., et al. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg. 2003;76:1435.
Komp D., Sparrow A. Polycythemia in cyanotic heart disease – a study of altered coagulation. J Pediat. 1970;76:231.
Krull F., Latta K., Hoyer P., et al. Cerebral ultrasonography before and after cardiac surgery in infants. Pediat Cardiol. 1994;15:159.
Kulik T.J., Moler F.W., Palmisano J.M., et al. Outcome-associated factors in pediatric patients treated with extracorporeal membrane oxygenator after cardiac surgery. Circulation. 1996;94:II63.
Kumar R.K., Newburger J.W., Gauvreau K., et al. Comparison of outcome when hypoplastic left heart syndrome and transposition of the great arteries are diagnosed prenatally versus when diagnosis of these two conditions is made only postnatally. Am J Cardiol. 1999;83:1649.
Kupsky W.J., Drozd M.A., Barlow C.F. Selective injury of the globus pallidus in children with post-cardiac surgery choreic syndrome. Dev Med Child Neurol. 1995;37:135.
Kurlan R., Griggs R. Cyanotic congenital heart disease with suspected stroke. Should all patients receive antibiotics. Arch Neurol. 1983;40:209.
Landolt M.A., Valsangiacomo Buechel E.R., Latal B. Health-related quality of life in children and adolescents after open-heart surgery. J Pediatr. 2008;152:349.
Lane J.C., Tennison M.B., Lawless S.T., et al. Movement disorder after withdrawal of fentanyl infusion. J Pediatr. 1991;119:649.
Lanska M., Lanska D., Horwitz S., et al. Presentation, clinical course and outcome of childhood stroke. Pediatr Neurol. 1991;7:333.
Laschinger J., Cunningham J., Isom O., et al. Definition of the safe lower limits of aortic resection during procedures on the thoracolumbar aorta: Use of somatosensory evoked potentials. J Amer Coll Cardiol. 1983;2:959.
Laschinger J., Owen J., Rosenbloom M., et al. Direct noninvasive monitoring of the spinal cord motor function during thoracic aortic occlusion: use of motor evoked potentials. J Vasc Surg. 1988;7:161.
Latal B., Helfricht S., Fischer J.E., et al. Psychological adjustment and quality of life in children and adolescents following open-heart surgery for congenital heart disease: a systematic review. BMC Pediatr. 2009;9:6.
LeBlanc F., Charrette E., Dobell A., et al. Neurological complications of aortic coarctation. Can Med Assn J. 1968;99:299.
Lederman R.J., Breuer A.C., Hanson M.R., et al. Peripheral nervous system complications of coronary artery bypass graft surgery. Ann Neurol. 1982;12:297.
Lemmer J., Stiller B., Heise G., et al. Postoperative phrenic nerve palsy: early clinical implications and management. Intensive Care Med. 2006;32:1227.
Lequier L., Joffe A.R., Robertson C.M., et al. Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg. 2008;136:976.
Lerberg D., Hardesty R., Siewers R., et al. Coarctation of the aorta in infants and children: 25 years of experience. Ann Thorac Surg. 1982;33:159.
Levy S.R., Abroms I.F., Marshall P.C., et al. Seizures and cerebral infarction in the full-term newborn. Ann Neurol. 1985;17:366.
Licht D.J., Shera D.M., Clancy R.R., et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529.
Licht D.J., Wang J., Silvestre D.W., et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg. 2004;128:841.
Lillehei C.W. A personalized history of extracorporeal circulation. Trans Am Soc Artif Intern Organs. 1982;28:5.
Limperopoulos C., Majnemer A., Rosenblatt B., et al. Multimodality evoked potential findings in infants with congenital heart defects. J Child Neurol. 1999;14:702.
Limperopoulos C., Majnemer A., Shevell M.I., et al. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. 2001;108:1325.
Limperopoulos C., Majnemer A., Shevell M.I., et al. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J Pediatr. 2000;137:638.
Limperopoulos C., Majnemer A., Shevell M.I., et al. Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics. 1999;103:402.
Limperopoulos C., Majnemer A., Shevell M.I., et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141:51.
Limperopoulos C., Tworetzky W., McElhinney D., et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26.
Linderkamp O., Klose H., Betke K., et al. Increased blood viscosity in patients with cyanotic congenital heart disease and iron deficiency. J Pediatr. 1979;95:567.
Lindsay E., Goldberg R., Jurecic V., et al. Velo-cardio-facial syndrome: Frequency and extent of 22q11 deletions. Am J Med Genet. 1995;57:514.
Ling F., Bao Y. Myelopathy and multiple aneurysms associated with aortic arch interruption: case report. Neurosurgery. 1994;35:310.
Lucchesi B. Complement activation, neutrophils, and oxygen radicals in reperfusion injury. Stroke. 1993;24:I 41.
Lutas E., Roberts R., Devereux R., et al. Relation between the presence of echocardiographic vegetations and the complication rate in infective endocarditis. Am Heart J. 1986;112:107.
Lutterman J., Scott M., Nass R., et al. Moyamoya syndrome associated with congenital heart disease. Pediatrics. 1998;101:57.
Lynch B., Rust R. Natural history and outcome of neonatal hypocalcemic and hypomagnesemic seizures. Pediatr Neurol. 1994;11:23.
Lyon G., Adams R., Kolodny E. Neurology of Inherited Metabolic Diseases of Children. New York: McGraw-Hill; 1996.
Mahle W.T., Clancy R.R., McGaurn S.P., et al. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107:1277.
Mahle W.T., Tavani F., Zimmerman R.A., et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109.
Mahle W.T., Wernovsky G. Neurodevelopmental outcomes in hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:39.
Majnemer A., Limperopoulos C., Shevell M., et al. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153:55.
Majnemer A., Limperopoulos C., Shevell M., et al. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72.
Majnemer A., Limperopoulos C., Shevell M.I., et al. A new look at outcomes of infants with congenital heart disease. Pediatr Neurol. 2009;40:197.
Manzar S., Nair A.K., Pai M.G., et al. Head size at birth in neonates with transposition of great arteries and hypoplastic left heart syndrome. Saudi Med J. 2005;26:453.
Martelle R., Linde L. Cerebrovascular accidents with the Tetralogy of Fallot. Am J Dis Child. 1961;101:206.
Martin J.J., De Barsy T., van Hoof F., et al. Pompe’s disease: An inborn lysosomal disorder with storage of glycogen. A study of brain and striated muscle. Act Neuropathol (Berl). 1973;23:229.
Massaro A.N., El-Dib M., Glass P., et al. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev. 2008.
Mathews K., Bale J., Clark E., et al. Cerebral infarction complicating Fontan surgery for cyanotic congenital heart disease. Pediatr Cardiol. 1986;7:161.
McConnell J.R., Fleming W.H., Chu W.K., et al. Magnetic resonance imaging of the brain in infants and children before and after cardiac surgery. A prospective study. Am J Dis Child. 1990;144:374.
McCusker C.G., Doherty N.N., Molloy B., et al. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch Dis Child. 2007;92:137.
McMahon A.M., van Doorn C., Burch M., et al. Improved early outcome for end-stage dilated cardiomyopathy in children. J Thorac Cardiovasc Surg. 2003;126:1781.
McNally H., Bennett C.C., Elbourne D., et al. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics. 2006;117:e845.
McQuillen P.S., Barkovich A.J., Hamrick S.E., et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736.
McQuillen P.S., Hamrick S.E., Perez M.J., et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280.
Medlock M.D., Cruse R.S., Winek S.J., et al. A 10-year experience with postpump chorea. Ann Neurol. 1993;34:820.
Menache C.C., du Plessis A.J., Wessel D.L., et al. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002;73:1752.
Ment L.R., Vohr B., Allan W., et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289:705.
Millar A.B., Armstrong L., van der Linden J., et al. Cytokine production and hemofiltration in children undergoing cardiopulmonary bypass. Ann Thorac Surg. 1993;56:1499.
Miller G., Eggli K., Contant C., et al. Postoperative neurologic complications after open heart surgery on young infants. Arch Pediatr Adolesc Med. 1995;149:764.
Miller G., Vogel H. Structural evidence of injury or malformation in the brains of children with congenital heart disease. Semin Pediatr Neurol. 1999;6:20.
Miller S.P., Mayer E.E., Clyman R.I., et al. Prolonged indomethacin exposure is associated with decreased white matter injury detected with magnetic resonance imaging in premature newborns at 24 to 28 weeks’ gestation at birth. Pediatrics. 2006;117:1626.
Miller S.P., McQuillen P.S., Hamrick S., et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928.
Miller S.P., McQuillen P.S., Vigneron D.B., et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg. 2004;77:1698.
Mizrahi E. Neonatal seizures: Problems in diagnosis and classification. Epilepsia. 1987;28:S46.
Mok Q., Ross-Russell R., Mulvey D., et al. Phrenic nerve injury in infants and children undergoing cardiac surgery. Br Heart J. 1991;65:287.
Moller J.H., Shumway S.J., Gott V.L. The first open-heart repairs using extracorporeal circulation by cross-circulation: a 53-year follow-up. Ann Thorac Surg. 2009;88:1044.
Montana E., Khoury M.J., Cragan J.D., et al. Trends and outcomes after prenatal diagnosis of congenital cardiac malformations by fetal echocardiography in a well defined birth population, Atlanta, Georgia, 1990-1994. J Am Coll Cardiol. 1996;28:1805.
Moody D., Bell M., Challa V., et al. Brain microemboli during cardiac surgery or aortography. Ann Neurol. 1990;28:477.
Mori E., Tabuchi M., Yoshida T., et al. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke. 1988;19:802.
Morris M.C., Wernovsky G., Nadkarni V.M. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5:440.
Morrow B., Goldberg R., Carlson C., et al. Molecular definition of the 22q11 deletions in velo-cardio-facial syndrome. Am J Human Genet. 1995;56:1391.
Moss E., Wang P., McDonald-McGinn D., et al. Characteristic cognitive profile in patients with a 22q11.2 deletion: verbal IQ exceeds nonverbal IQ. Am J Hum Genet. 1995;57:A20.
Nagai T., Tuchiya Y., Taguchi Y., et al. Fatal infantile mitochondrial encephalomyopathy with complex I and IV deficiencies. Pediatr Neurol. 1993;9:151.
Neufeld R.E., Clark B.G., Robertson C.M., et al. Five-year neurocognitive and health outcomes after the neonatal arterial switch operation. J Thorac Cardiovasc Surg. 2008;136:1413.
Newburger J., Silbert A., Buckley L., et al. Cognitive function and age at repair of transposition of the great arteries in children. New Eng J Med. 1984;310:1495.
Newburger J.W., Jonas R.A., Soul J., et al. Randomized trial of hematocrit 25 percent versus 35 percent during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135:347.
Newburger J.W., Jonas R.A., Wernovsky G., et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Eng J Med. 1993;329:1057.
Nussmeier N., McDermott J. Macroembolization: prevention and outcome modification. In: Hilberman M., editor. Brain Injury and Protection during Cardiac Surgery. Boston: Martinus Nijhoff; 1988:85.
Orsi P., Rosa G., Liberatori G., et al. Repair of two unruptured intracranial aneurysms in the presence of coarctation of the aorta-anesthetic implications and management. J Neurosurg Anesthes. 1993;5:48.
Padayachee T., Parsons S., Theobold R., et al. The detection of microemboli in the middle cerebral artery during cardiopulmonary bypass: A transcranial Doppler ultrasound investigation using membrane and bubble oxygenators. Ann Thorac Surg. 1987;44:298.
Partridge B.L., Abrams J.H., Bazemore C., et al. Prolonged neuromuscular blockade after long-term infusion of vecuronium bromide in the intensive care unit. Crit Care Med. 1990;18:1177.
Partridge S.C., Vigneron D.B., Charlton N.N., et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640.
Pelletier L., Petersdorf R. Infective endocarditis: a review of 125 cases from the University Washington Hospitals, 1963-1972. Medicine. 1977;6:287.
Pennington D., Liberthson R., Jacobs M., et al. Critical review of experience with surgical repair of coarctation of the aorta. J Thorac Cardiovasc Surg. 1979;77:217.
Petit C.J., Rome J.J., Wernovsky G., et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709.
Petzinger G., Mayer S.A., Przedborski S. Fentanyl-induced dyskinesias. Movement Disord. 1995;10:679.
Phornphutkul C., Rosenberg A., Nadas A., et al. Cerebrovascular accidents in infants and children with cyanotic congenital heart disease. Am J Cardiol. 1973;32:329.
Plum F., Posner J. Multifocal, diffuse, and metabolic brain diseases causing stupor or coma. In The Diagnosis of Stupor and Coma. Philadelphia: F.A. Davis Company; 1985.
Pruitt A., Rubin R., Karchmer A., et al. Neurologic complications of bacterial endocarditis. Medicine. 1978;57:329.
Pulver A., Nestadt G., Goldberg R., et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Mental Dis. 1994;182:476.
Puntis J., Green S. Ischemic spinal cord injury after cardiac surgery. Arch Dis Child. 1985;60:517.
Rappaport L.A., Wypij D., Bellinger D.C., et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773.
Redmond J., Gillinov A., Zehr K., et al. Glutamate excitotoxicity: a mechanism of neurologic injury associated with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1994;107:776.
Reller M.D., Strickland M.J., Riehle-Colarusso T., et al. Prevalence of congenital heart defects in metropolitan Atlanta, 1998-2005. J Pediatr. 2008;153:807.
Revenis M.E., Glass P., Short B.L. Mortality and morbidity rates among lower birth weight infants (2000 to 2500 grams) treated with extracorporeal membrane oxygenation. J Pediatr. 1992;121:452.
Riela A., Roach E. Etiology of stroke in children. J Child Neurol. 1993;8:201.
Robertson C.M., Finer N.N., Sauve R.S., et al. Neurodevelopmental outcome after neonatal extracorporeal membrane oxygenation. Cmaj. 1995;152:1981.
Robertson C.M., Joffe A.R., Sauve R.S., et al. Outcomes from an interprovincial program of newborn open heart surgery. J Pediatr. 2004;144:86.
Robinson R., Samuels M., Pohl K. Choreic syndrome after cardiac surgery. Arch Dis Child. 1988;63:1466.
Rosenblum M., Hoff J., Norman D., et al. Nonoperative treatment of brain abscess in selected high-risk patients. J Neurosurg. 1980;52:217.
Rosenthal D.N., Bulbul Z.R., Friedman A.H., et al. Thrombosis of the pulmonary artery stump after distal ligation. J Thorac Cardiovasc Surg. 1995;110:1563.
Rosenthal D.N., Friedman A.H., Kleinman C.S., et al. Thromboembolic complications after Fontan operations. Circulation. 1995;92(Suppl II):287.
Rosenthal G.R. Prevalence of congenital heart disease. In Science and Practice of Pediatric Cardiology. Baltimore, MD: Williams & Wilkins; 1998.
Rosman P., Shands K. Hydrocephalus caused by increased intracranial venous pressure: a clinicopathological study. Ann Neurol. 1978;3:445.
Rousseau S., Metral S., Lacroix C., et al. Anterior spinal artery syndrome mimicking infantile spinal muscular atrophy. Am J Perinatol. 1993;10:316.
Roy P., Tajik A., Giuliani E., et al. Spectrum of echocardiographic findings in bacterial endocarditis. Circulation. 1976;53:474.
Rustin P., Lebidois J., Chretien D., et al. Endomyocardial biopsies for early detection of mitochondrial disorders in hypertrophic cardiomyopathies. J Pediatr. 1994;124:224.
Saalouke M., Perry L., Breckbill D., et al. Cerebrovascular abnormalities in postoperative coarctation of the aorta. Four cases demonstrating left subclavian steal on aortography. Am J Cardiol. 1978;42:97.
Saiman L., Prince A., Gersony W. Pediatric infective endocarditis in the modern era. J Pediatr. 1993;122:847.
Sakamoto T., Jonas R.A., Stock U.A., et al. Utility and limitations of near-infrared spectroscopy during cardiopulmonary bypass in a piglet model. Pediatr Res. 2001;49:770.
Sakamoto T., Zurakowski D., Duebener L.F., et al. Interaction of temperature with hematocrit level and pH determines safe duration of hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2004;128:220.
Salgado A., Furlan A., Keys T., et al. Neurologic complications of endocarditis: a 12 year experience. Neurology. 1989;32:173.
Satur C., Jennings A., Walker D. Hypomagnesemia and fits complicating pediatric cardiac surgery. Ann Clin Biochem. 1993;30:315.
Scher M.S., Painter M.J. Electroencephalographic diagnosis of neonatal seizures: Issues of diagnostic accuracy, clinical correlation, and survival. In: Wsaterlain C., Vert P., editors. Neonatal Seizures. New York: Raven Press; 1990:15.
Schoenberg B., Mellinger J., Schoenberg D. Cerebrovascular disease in infants and children: A study of incidence, clinical features, and survival. Neurology. 1978;28:763.
Schumacher R.E., Palmer T.W., Roloff D.W., et al. Follow-up of infants treated with extracorporeal membrane oxygenation for newborn respiratory failure. Pediatrics. 1991;87:451.
Scotti G., Li M., Righi C., et al. Endovascular treatment of bacterial intracranial aneurysms. Neuroradiology. 1996;38:186.
Shearer W., Rutman J., Weinberg W., et al. Coarctation of the aorta and cerebrovascular accident: A proposal for early corrective surgery. J Pediatr. 1970;77:1004.
Sheth R.D., Bolton C.F. Neuromuscular complications of sepsis in children. J Child Neurol. 1995;10:346.
Shillingford A.J., Ittenbach R.F., Marino B.S., et al. Aortic morphometry and microcephaly in hypoplastic left heart syndrome. Cardiol Young. 2007;17:189.
Shprintzen R., Goldberg R., Golding-Kushner K., et al. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42:141.
Shu-yuan Y. Brain abscess associated with congenital heart disease. Surg Neurol. 1989;31:129.
Simon T.J., Bearden C.E., Mc-Ginn D.M., et al. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005;41:145.
Sladky J.T., Rorke L.B. Perinatal hypoxic/ischemic spinal cord injury. In Pediatric Pathology. New York: Hemisphere Publishing Corp; 1986.
Solis R., Kennedy P., Beall A., et al. Cardiopulmonary bypass: Microembolization and platelet aggregation. Circulation. 1975;52:103.
Soper R., Chaloupka J.C., Fayad P.B., et al. Ischemic stroke and intracranial multifocal cerebral arteriopathy in Williams syndrome. J Pediatr. 1995;126:945.
Soul J.S., Robertson R.L., Wypij D., et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J Thorac Cardiovasc Surg. 2009;138:374.
Special Writing Group of the Committee on Rheumatic Fever Endocarditis and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. JAMA. 1992;268:2069.
Steinberg J.B., Kapelanski D.P., Olson J.D., et al. Cytokine and complement levels in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1993;106:1008.
Stolar C.J., Snedecor S.M., Bartlett R.H. Extracorporeal membrane oxygenation and neonatal respiratory failure: experience from the extracorporeal life support organization. J Pediatr Surg. 1991;26:563.
Straussberg R., Shahar E., Gat R., et al. Delayed parkinsonism associated with hypotension in a child undergoing open-heart surgery. Devel Med Child Neurol. 1993;35:1007.
Subramony S.H., Carpenter D.E., Raju S., et al. Myopathy and prolonged neuromuscular blockade after lung transplant. Crit Care Med. 1991;19:1580.
Swedo S.E. Sydenham’s chorea: a model for childhood autoimmune neuropsychiatric disorders. Jama. 1994;272:1788.
Swedo S.E., H.L. L., Mittleman B.B., et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiat. 1997;154:110.
Swedo S.E., Kiessling L.S. Speculations on antineuronal anti-body mediated neuropsychiatric disorders. Pediatrics. 1994;93:323.
Swedo S.E., Leonard H.L., Schapiro M.B., et al. Sydenham’s chorea: physical and psychological symptoms of St Vitus Dance. Pediatrics. 1993;91:706.
Swenson M., Rubenstein R. Phrenic nerve conduction studies. Muscle Nerve. 1992;15:597.
Swillen A., Vandeputte L., Cracco J., et al. Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychol. 1999;5:230.
Sztriha L., Guerrini R., Harding B., et al. Clinical, MRI, and pathological features of polymicrogyria in chromosome 22q11 deletion syndrome. Am J Med Genet A. 2004;127:313.
Tavani F., Zimmerman R.A., Clancy R.R., et al. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology. 2003;45:253.
Taylor A.K., Cousins R., Butt W.W. The long-term outcome of children managed with extracorporeal life support: an institutional experience. Crit Care Resusc. 2007;9:172.
Te Pas A.B., van Wezel-Meijler G., Bokenkamp-Gramann R., et al. Preoperative cranial ultrasound findings in infants with major congenital heart disease. Acta Paediatr. 2005;94:1597.
Terplan K.L. Brain changes in newborns, infants and children with congenital heart disease in association with cardiac surgery. Additional observations. J Neurol. 1976;212:225.
Thiagarajan R.R., Laussen P.C., Rycus P.T., et al. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693.
Thuny F., Avierinos J.F., Tribouilloy C., et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: a prospective multicentre study. Eur Heart J. 2007;28:1155.
Tomlinson F., Piepgras D., Nichols D., et al. Remote congenital cerebral arteriovenous fistulae associated with aortic coarctation. J Neurosurg. 1992;76:137.
Tworetzky W., McElhinney D.B., Reddy V.M., et al. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103:1269.
Tyler R., Clark D.B. Cerebrovascular accidents in patients with congenital heart disease. Arch Neurol Psychiatry. 1957;77:483.
Tyler R., Clark D.B. Incidence of neurologic complications in congenital heart disease. Arch Neurol Psychiatry. 1957;77:17.
Utoh J., Miyauchi Y., Goto H., et al. Endovascular approach for an intracranial mycotic aneurysm associated with infective endocarditis. J Thorac Cardiovasc Surg. 1995;110:557.
van Amelsvoort T., Daly E., Robertson D., et al. Structural brain abnormalities associated with deletion at chromosome 22q11: quantitative neuroimaging study of adults with velo-cardio-facial syndrome. Br J Psychiatry. 2001;178:412.
van Houten J., Rothman A., Bejar R. High incidence of cranial ultrasound abnormalities in full-term infants with congenital heart disease. Am J Perinatol. 1996;13:47.
Vannucci R. Current and potentially new management strategies for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1990;85:961.
Vergara P., Digilio M.C., De Zorzi A., et al. Genetic heterogeneity and phenotypic anomalies in children with atrioventricular canal defect and tetralogy of Fallot. Clin Dysmorphol. 2006;15:65.
Verheijen P.M., Lisowski L.A., Stoutenbeek P., et al. Prenatal diagnosis of congenital heart disease affects preoperative acidosis in the newborn patient. J Thorac Cardiovasc Surg. 2001;121:798.
Volpe J.J. Neurology of the Newborn. Philadelphia: WB Saunders; 2001.
Vorstman J.A., Morcus M.E., Duijff S.N., et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104.
Waitling S., Dasta J. Prolonged paralysis in intensive care unit patients after use of neuromuscular blocking agents: a review of the literature. Crit Care Med. 1994;22:884.
Wardle S.P., Yoxall C.W., Weindling A.M. Cerebral oxygenation during cardiopulmonary bypass. Arch Dis Child. 1998;78:26.
Watanabe K., Matsui M., Matsuzawa J., et al. Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiovasc Surg. 2009;137:146.
Watanabe T., Trusler G.A., Williams W.G., et al. Phrenic nerve paralysis after pediatric cardiac surgery. Retrospective study of 125 cases. J Thorac Cardiovasc Surg. 1987;94:383.
Weese-Mayer D.E., Hunt C.E., Brouillette R.T., et al. Diaphragm pacing in infants and children. J Pediatr. 1992;120:1.
Wernovsky G. Current insights regarding neurological and developmental abnormalities in children and young adults with complex congenital cardiac disease. Cardiol Young. 2006;16(Suppl 1):92.
Wernovsky G., Stiles K.M., Gauvreau K., et al. Cognitive development after the Fontan operation. Circulation. 2000;102:883.
Wessel D.L., du Plessis A.J. Choreoathetosis. In: Jonas R.A., Newburger J.W., Volpe J.J., editors. Brain Injury and Pediatric Cardiac Surgery. Boston MA: Butterworth-Heinemann; 1995:353.
Westaby S. Organ dysfunction after cardiopulmonary bypass: a systemic inflammatory reaction by the extracorporeal circuit. Intensive Care Med. 1987;13:89.
Wical B., Tomasi L. A distinctive neurologic syndrome after profound hypothermia. Pediatr Neurol. 1990;6:202.
Wilson W., Giuliani E., Danielson G., et al. Management of complications of infective endocarditis. Mayo Clin Proc. 1982;57:162.
Wong P.C., Barlow C.F., Hickey P.R., et al. Factors associated with choreoathetosis after cardiopulmonary bypass in children with congenital heart disease. Circulation. 1992;86:118.
Worthington S., Turner A., Elber J., et al. 22q11 deletion and polymicrogyria – cause or coincidence? Clin Dysmorphol. 2000;9:193.
Yang J., Park Y., Hartlage P. Seizures associated with stroke in childhood. Pediatr Neurol. 1995;12:136.
Young R., Liberthson R., Zalneraitis E. Cerebral hemorrhage in neonates with coarctation of the aorta. Stroke. 1982;13:491.
Zeltser I., Jarvik G.P., Bernbaum J., et al. Genetic factors are important determinants of neurodevelopmental outcome after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2008;135:91.
Zeviani M., Van Dyke D. Myopathy and fatal cardiopathy due to cytochrome c oxidase deficiency. Arch Neurol. 1986;43:1198.
Zinkstok J., van Amelsvoort T. Neuropsychological profile and neuroimaging in patients with 22Q11.2 Deletion Syndrome: a review. Child Neuropsychol. 2005;11:21.