Chapter 13 Neurogenic Dysphagia
Normal Swallowing
Swallowing is a surprisingly complicated and intricate phenomenon. It comprises a mixture of voluntary and reflex, or automatic, actions engineered and carried out by a combination of the 55 muscles of the oropharyngeal, laryngeal, and esophageal regions, along with five cranial nerves and two cervical nerve roots that in turn receive directions from centers within the central nervous system (Schaller et al., 2006). Reflex swallowing is coordinated and carried out at a brainstem level, where centers act directly on information received from sensory structures within the oropharynx and esophagus. Volitional swallowing is, not surprisingly, accompanied by additional activity that originates not only in motor and sensory cortices, but also in other cerebral structures (Hamdy et al., 1999; Zald and Pardo, 1999).
In the oral, or swallow-preparatory phase, food is taken into the mouth and, if needed, chewed. Saliva is secreted to provide both lubrication and the initial “dose” of digestive enzymes, and the food bolus is formed and shaped by the tongue. The tongue then propels the bolus backward to the pharyngeal inlet where, in a piston-like action, it delivers the bolus into the pharynx. This initiates the pharyngeal phase, in which a cascade of intricate, extremely rapid, and exquisitely coordinated movements seal off the nasal passages and protect the trachea while the cricopharyngeal muscle, which functions as the upper esophageal sphincter (UES), relaxes and allows the bolus to enter the pharynx. As an example of the intricacy of movements during this phase of swallowing, the UES, prompted in part by traction produced by elevation of the larynx, actually relaxes just prior to arrival of the food bolus, creating suction that assists in guiding the bolus into the pharynx. The bolus then enters the esophagus where peristaltic contractions usher it distally and, on relaxation of the lower-esophageal sphincter, into the stomach. Synchronization of swallowing with respiration such that expiration rather than inspiration immediately follows a swallow, thus reducing the risk of aspiration, is another example of the finely tuned coordination involved in the swallowing mechanism(Mehanna and Jankovic, 2010).
Neurophysiology of Swallowing
It is perhaps not surprising that the strongest activation in PET studies of volitional swallowing occurs in the lateral motor cortex within the inferior precentral gyrus, wherein lie the cortical representations of tongue and face. There is disagreement among investigators, however, in that some have noted bilaterally symmetrical activation of the lateral motor cortex (Zald and Pardo, 1999), whereas others have noted a distinctly asymmetrical activation, at least in a portion of subjects tested (Hamdy et al., 1999).
Some additional and perhaps somewhat surprising brain areas are also activated during volitional swallowing (Hamdy et al., 1999; Schaller et al., 2006; Zald and Pardo, 1999). The supplementary motor area may play a role in preparation for volitional swallowing, and the anterior cingulate cortex may be involved with monitoring autonomic and vegetative functions. Another area of activation during volitional swallowing is the anterior insula, particularly on the right. It has been suggested that this activation may provide the substrate that allows gustatory and other intraoral sensations to modulate swallowing. Lesions in the insula may also increase the swallowing threshold and delay the pharyngeal phase of swallowing (Schaller et al., 2006). PET studies also consistently demonstrate distinctly asymmetrical left-sided activation of the cerebellum during swallowing. This activation may reflect cerebellar input concerning coordination, timing, and sequencing of swallowing. Activation of putamen has also been noted during volitional swallowing, but it has not been possible to differentiate this activation from that seen with tongue movement alone.
Within the brainstem, swallowing appears to be regulated by central pattern generators that contain the programs directing the sequential movements of the various muscles involved. The dorsomedial pattern generator resides in the medial reticular formation of the rostral medulla and the reticulum adjacent to the nucleus tractus solitarius and is involved with the initiation and organization of the swallowing sequence (Schaller et al., 2006). A second central pattern generator, the ventrolateral pattern generator, lies near the nucleus ambiguus and its surrounding reticular formation (Prosiegel et al., 2005; Schaller et al., 2006). It serves primarily as a connecting pathway to motor nuclei such as the nucleus ambiguus and the dorsal motor nucleus of the vagus, which directly control motor output to the pharyngeal musculature and proximal esophagus.
Mechanical Dysphagia
Structural abnormalities, both within and adjacent to the mouth, pharynx, and esophagus, can interfere with swallowing on a strictly mechanical basis, despite fully intact and functioning nervous and musculoskeletal systems (Box 13.1). Within the mouth, macroglossia, temporomandibular joint dislocation, certain congenital anomalies, and intraoral tumors can impede effective swallowing and produce mechanical dysphagia. Pharyngeal function can be compromised by processes such as retropharyngeal tumor or abscess, cervical anterior osteophyte formation, Zenker diverticulum, or thyroid gland enlargement. An even broader array of structural lesions can interfere with esophageal function, including malignant or benign esophageal tumors, metastatic carcinoma, esophageal stricture from numerous causes, vascular abnormalities such as aortic aneurysm or aberrant origin of the subclavian artery, or even primary gastric abnormalities such as hiatal hernia or complications from gastric banding procedures. Gastroesophageal reflux can also produce dysphagia. Individuals with these problems, however, are more likely to be seen by the gastroenterologist rather than the neurologist.
Neuromuscular Dysphagia
A variety of neuromuscular disease processes of diverse etiology can involve the oropharyngeal and esophageal musculature and produce dysphagia as part of their broader neuromuscular clinical picture (Box 13.2). Certain muscular dystrophies, inflammatory myopathies, and mitochondrial myopathies all can display dysphagia, as can disease processes affecting the myoneural junction, such as myasthenia gravis.
Oculopharyngeal Muscular Dystrophy
Oculopharyngeal muscular dystrophy (OPMD) is a rare autosomal dominant disorder that has a worldwide distribution. It was initially described and is most frequently encountered in individuals with a French-Canadian ethnic background, although its highest reported prevalence is among the Bukhara Jews in Israel (Abu-Baker and Rouleau, 2007). It is the consequence of a GCG trinucleotide repeat expansion in the polyadenylate-binding protein, nuclear 1 gene (PABPN1; also known as poly(A)-binding protein 2 [PABP2]) on chromosome 14. OPMD is unique among the muscular dystrophies because of its appearance in older individuals, with symptoms typically first appearing between ages 40 and 60. It is characterized by slowly progressive ptosis, dysphagia, and proximal limb weakness. Because of the ptosis, patients with OPMD may assume an unusual posture characterized by raised eyebrows and extended neck.
Dysphagia in OPMD is due to impaired function of the oropharyngeal musculature. Although it evolves slowly over many years, OPMD can eventually result not only in difficulty or discomfort with swallowing, but also in weight loss, malnutrition, and aspiration. No specific treatment for the muscular dystrophy itself is available, but cricopharyngeal myotomy affords dysphagia relief in over 80% of treated individuals (Fradet et al., 1997). More recently, botulinum toxin injections have been successfully used to treat dysphagia in OPMD.
Myotonic Dystrophy
Myotonic dystrophy is an autosomal dominant disorder whose phenotypic picture includes not only skeletal muscle but also cardiac, ophthalmological, and endocrinological involvement. Mutations at two distinct locations have now been associated with the clinical picture of myotonic dystrophy (Turner and Hilton-Jones, 2010). Type 1 myotonic dystrophy is due to a CTG expansion in the myotonic dystrophy protein kinase (DMPK) gene on chromosome 19; type 2 is the consequence of a CCTG repeat expansion in the zinc finger protein 9 (ZNF9) gene on chromosome 3.
Gastrointestinal (GI) symptoms develop in more than 50% of individuals with the clinical phenotype of myotonic dystrophy. These may be the most disabling component of the disorder in 25% of individuals with type 1 myotonic dystrophy, and GI symptoms may actually antedate the appearance of other neuromuscular features (Turner and Hilton-Jones, 2010). Subjective dysphagia is one of the most prevalent GI features and has been reported in 37% to 56% of patients (Ertekin et al., 2001b). Coughing when eating, suggestive of aspiration, may occur in 33%. Objective measures paint a picture of even more pervasive impairment, demonstrating disturbances in swallowing in 70% to 80% of persons with myotonic dystrophy (Ertekin et al., 2001b). In one study, 75% of patients asymptomatic for dysphagia were still noted to have abnormalities on objective testing (Marcon et al., 1998).
A variety of abnormalities in objective measures of swallowing have been documented in myotonic dystrophy. Abnormal cricopharyngeal muscle activity is present in 40% of patients during electromyographic (EMG) testing (Ertekin et al., 2001b). Impaired esophageal peristalsis has also been noted in affected individuals studied with esophageal manometry. On videofluoroscopic testing, incomplete relaxation of the UES and esophageal hypotonia are the most frequently noted abnormalities (Marcon et al., 1998). Both muscle weakness and myotonia are felt to play a role in the development of dysphagia in persons with myotonic dystrophy (Ertekin et al., 2001b), and in at least one study, a correlation was noted between the size of the CTG repeat expansion and the number of radiological abnormalities in myotonic patients (Marcon et al., 1998).
Inflammatory Myopathies
Although dysphagia can develop in both conditions, it more frequently is present in dermatomyositis and when present is more severe. Dysphagia is present in 20% to 55% of individuals with dermatomyositis but in only 18% with polymyositis (Parodi et al., 2002). It is the consequence of involvement of striated muscle in the pharynx and proximal esophagus. Involvement of pharyngeal and esophageal musculature in polymyositis and dermatomyositis is an indicator of poor prognosis and can be the source of significant morbidity. A 1-year mortality rate of 31% has been reported in individuals with inflammatory myopathy and dysphagia (Williams et al., 2003), although other investigators have reported a 1-year survival rate of 89% (Oh et al., 2007).
Dysphagia in persons with inflammatory myopathy may be due to restrictive pharyngo-esophageal abnormalities such as cricopharyngeal bar, Zenker diverticulum, and stenosis. In fact, in one study of 13 patients with inflammatory myopathy, radiographic constrictions were noted in 9 (69%) individuals, compared with 1 of 17 controls with dysphagia of neurogenic origin (Williams et al., 2003). Aspiration was also more common in the patients with myositis (61% versus 41%). The resulting dysphagia can be severe enough to require enteral feeding. Acute total obstruction by the cricopharyngeal muscle has been reported in dermatomyositis, necessitating cricopharyngeal myotomy. Other investigators have reported improvement in 50% of individuals 1 month following cricopharyngeal bar disruption; improvement was still present in 25% at 6 months (Williams et al., 2003). The reason for the formation of restrictive abnormalities in inflammatory myopathy is uncertain, but it may be that long-standing inflammation of the cricopharyngeus muscle impedes its compliance and ability to open fully (Williams et al., 2003).
Dysphagia may also develop in inclusion body myositis. It may even be the presenting symptom (Cox et al., 2009). In the late stages of the disorder, the frequency of dysphagia may actually exceed that seen in dermatomyositis and polymyositis. In a group of individuals in whom inclusion-body myositis mimicked and was confused with motor neuron disease, dysphagia was present in 44% (Dabby et al., 2001). In another study, dysphagia was documented in 37 of 57 (65%) patients with inclusion-body myositis (Cox et al., 2009). Abnormal function of the cricopharyngeal sphincter, probably due to inflammatory involvement of the cricopharyngeal muscle, with consequently reduced compliance, was documented in 37%. A focal inflammatory myopathy involving the pharyngeal muscles and producing isolated pharyngeal dysphagia has also been described in individuals older than age 69. It has been suggested that this is a distinct clinical entity characterized by cricopharyngeal hypertrophy, although polymyositis localized to the pharyngeal musculature has also been reported.
Dysphagia in both dermatomyositis and polymyositis may respond to corticosteroids and other immunosuppressive drugs, and these remain the mainstay of treatment. Intravenous immunoglobulin therapy has produced dramatic improvement in dysphagia in individuals who were unresponsive to steroids. However, inclusion-body myositis typically responds poorly to these agents, and myotomy is often necessary (Ebert, 2010; Oh et al., 2007).
Myasthenia Gravis
Involvement of bulbar musculature, with resultant dysphagia, is relatively common in MG. In approximately 6% to 30% of patients, bulbar involvement is evident from the beginning (Koopman et al., 2004); with disease progression, most eventually develop bulbar symptoms such as dysphagia and dysarthria. Dysphagia in MG can be due to dysfunction at oral, pharyngeal, or even esophageal levels, and many patients experience it at multiple levels. In a study of 20 myasthenic patients experiencing dysphagia, abnormalities in the oral preparatory phase were evident in 13 individuals (65%), oral phase dysphagia in 18 (90%), and pharyngeal phase involvement in all 20 (100%) (Koopman et al., 2004). Oral phase involvement can be due to fatigue and weakness of the tongue or masticatory muscles. In MG patients with bulbar symptoms, repetitive nerve stimulation studies of the hypoglossal nerve have demonstrated abnormalities, as have studies utilizing EMG of the masticatory muscles recorded while chewing. Pharyngeal dysfunction is also common in MG patients who have dysphagia, as demonstrated by videofluoroscopy. Aspiration, often silent, may be present in 35% or more of these individuals (Colton-Hudson et al., 2002); in elderly patients the frequency of aspiration may be considerably higher. Bedside speech pathology assessment is not a reliable predictor of aspiration (Koopman et al., 2004). Motor dysfunction involving the striated muscle of the proximal esophagus has also been documented in MG. In one study that used testing with esophageal manometry, 96% of patients with MG demonstrated abnormalities such as decreased amplitude and prolongation of the peristaltic wave in this region. Cricopharyngeal sphincter pressure was also noted to be reduced.
It is important to remember that dysphagia can also precipitate myasthenic crisis in individuals with MG. In fact, in one study, dysphagia was considered to be a major precipitant of myasthenic crisis in 56% of patients (Koopman et al., 2004).
Neurogenic Dysphagia
A variety of disease processes originating in the central and peripheral nervous systems can also disrupt swallowing mechanisms and produce dysphagia. Processes affecting cerebral cortex, subcortical white matter, subcortical grey matter, brainstem, spinal cord, and peripheral nerves all can elicit dysphagia as a component of their clinical picture (Box 13.3).
In individuals with neurogenic dysphagia, prolonged swallow response, delayed laryngeal closure, and weak bolus propulsion combine to increase the risk of aspiration and the likelihood of malnutrition (Clavé et al., 2006)
Stroke
Dysphagia develops in 45% to 57% of individuals following stroke, and its presence is associated with increased likelihood of severe disability or death (Runions et al., 2004; Schaller et al., 2006). Aspiration is the most widely recognized complication of dysphagia following stroke, but undernourishment and even malnutrition occur with surprising frequency (Finestone and Greene-Finestone, 2003). Reported frequencies of nutritional deficits in patients with dysphagia following stroke range from 48% to 65%. The presence of dysphagia following stroke results in a threefold prolongation of hospital stay and increases the complication rate during hospitalization (Runions et al., 2004). It is also an independent risk factor for severe disability and death.
Finestone and Greene-Finestone (2003) have delineated a number of warning signs that can alert physicians to the presence of post-stroke dysphagia. Some are obvious, others more subtle. They include drooling, excessive tongue movement or spitting food out of the mouth, poor tongue control, pocketing of food in the mouth, facial weakness, slurred speech, coughing or choking when eating, regurgitation of food through the nose, wet or “gurgly” voice after eating, hoarse or breathy voice, complaints of food sticking in the throat, absence or delay of laryngeal elevation, prolonged chewing, prolonged time to eat or reluctance to eat, and recurrent pneumonia.
Although it is commonly perceived that the presence of dysphagia following stroke indicates a brainstem localization for the stroke, this is not necessarily so. Impaired swallowing has been documented in a significant proportion of strokes involving cortical and subcortical structures. The pharyngeal phase of swallowing is primarily impaired in brainstem infarction; in hemispheric strokes, the most striking abnormality often is a delay in initiation of voluntary swallowing. Strokes involving the right hemisphere tend to produce more impairment of pharyngeal motility, whereas left hemisphere lesions have a greater effect on oral stage function (Ickenstein et al., 2005). Dysphagia has been reported as the sole manifestation of infarction in both medulla and cerebrum.
Approximately 50% to 55% of patients with lesions in the posterior inferior cerebellar artery distribution, with consequent lateral medullary infarction (Wallenberg syndrome), develop dysphagia (Teasell et al., 2002). The fact that unilateral medullary infarction can produce bilateral disruption of the brainstem swallowing centers suggests that they function as one integrated center. Infarction in the distribution of the anterior inferior cerebellar artery can also result in dysphagia.
Aspiration is a potentially life-threatening complication of stroke. Studies have documented its occurrence in 30% to 55% of stroke patients. In one study, videofluoroscopic evidence of aspiration was observed in 36% of patients with unilateral cerebral stroke, 46% with bilateral cerebral stroke, 60% with unilateral brainstem stroke, and 50% with bilateral brainstem lesions. Other studies have suggested that the incidence of aspiration in brainstem strokes may be considerably higher—more than 80%—and that subcortical strokes may result in aspiration in 75% of cases. The risk of developing pneumonia is almost seven times greater in persons experiencing aspiration following stroke compared with those who do not. Individuals in whom aspiration occurs post stroke do not always experience clinical symptoms such as coughing or choking during food or liquid ingestion. Furthermore, an absent gag reflex does not help to differentiate those aspirating from those who are not (Finestone and Greene-Finestone, 2003). In a recent study, only 44% of patients with suspected oropharyngeal dysphagia following stroke had an impaired gag reflex, and only 47% coughed during oral feeding (Terre and Mearin, 2006). Therefore, the employment of objective testing measures to detect the presence and predict the risk of aspiration has been advocated. Modified barium swallow testing using videofluoroscopy is the standard method of diagnosis used, but simple bedside techniques such as a water swallowing test have also been advocated as practical, though somewhat less sensitive, alternatives.
Ickenstein and colleagues (2010) emphasize the value of a stepwise assessment of swallowing in patients admitted to the hospital with stroke, with the assessment beginning on the first day of admission. The first step is a modified swallowing assessment performed by the nursing staff on the day of admission; the second step is a clinical swallowing examination performed within 72 hours of admission by a swallowing therapist; the third step is performance of flexible transnasal swallowing endoscopy performed by a physician within 5 days of admission. Appropriate diet and treatment are then determined after each step. Employment of such a stepwise assessment of dysphagia resulted in a significant reduction in the rate of pneumonia and in antibiotic consumption in a stroke unit (Ickenstein et al., 2010).
Swallowing often improves spontaneously in the days and weeks after stroke. Improvement is more likely to occur after cortical strokes, compared with those of brainstem origin; the improvement is probably the result of compensatory reorganization of undamaged brain areas (Schaller et el., 2006). Nasogastric tube feeding can temporarily provide adequate nutrition and buy time until swallowing improves sufficiently to allow oral feeding, but it entails some risks itself, such as increasing the possibility of reflux with consequent aspiration. For individuals in whom significant dysphagia persists after stroke, percutaneous endoscopic gastrostomy (PEG) tube placement may become necessary. Ickenstein and colleagues (2005) documented this necessity in 77 of 664 (11.6%) stroke patients admitted to their rehabilitation hospital. Continued need for a PEG tube after discharge from the unit carried with it a somber prognosis. Various methods of behavioral swallowing therapy can be useful in managing persistent post-stroke dysphagia. Recent studies have provided some tantalizing hints that sensory pharyngeal stimulation and repetitive transcranial magnetic stimulation (rTMS) may improve some aspects of swallowing, but in a small percentage of individuals, placement of a PEG tube will be necessary.
Dysphagia is also a potential complication of carotid endarterectomy, not on the basis of stroke but due to laryngeal or cranial nerve injury. In one study, careful otolaryngologic examination demonstrated such deficits in almost 60% of patients postoperatively (Monini et al., 2005). Most deficits were mild and transient, but some persistent impairment was noted in 17.5% of those studied, and 9% required some rehabilitative procedures.
Multiple Sclerosis
Dysphagia is a frequent but often overlooked problem in MS. Survey studies have indicated the presence of dysphagia in 24% to 34% of individuals with MS (Calcagno et al., 2002; Prosiegel et al., 2004). The prevalence of dysphagia in MS rises with increasing disability; about 15% of individuals with mild disability may develop neurogenic dysphagia (Prosiegel et al., 2004), with the percentage escalating to 65% in the most severely affected. Individuals with severe brainstem involvement as part of their MS are especially likely to experience dysphagia.
Objective studies demonstrate a somewhat higher frequency of dysphagia than their survey study counterparts. In one study, approximately 50% of individuals with objective abnormalities were not aware of any difficulty swallowing; in another that used videofluoroscopic analysis, some alteration of swallowing efficiency or safety was present in over 80% of 23 patients studied (Terre-Boliart et al., 2004). Abnormalities in oral, pharyngeal, and even esophageal phases of swallowing have been documented. Rare instances of the anterior operculum syndrome with buccolinguofacial apraxia have been reported in MS. Abnormalities in the oral phase of swallowing are common in MS patients with mild disability, but additional pharyngeal phase abnormalities develop in those with more severe disability. Disturbances in both the sequencing of laryngeal events and function of the pharyngeal constrictor muscles are typically present in persons experiencing dysphagia. Pharyngeal sensory impairment may also play a role in the development of dysphagia in some patients.
Parkinson Disease
Dysphagia was first documented in PD by none other than James Parkinson himself in his original description of the illness in 1817. Recent survey studies have confirmed that dysphagia is indeed a frequent phenomenon in PD. Reported frequencies of dysphagia in these studies range from 30% to 82% (Pfeiffer, 2003), with the broad range probably reflecting differences in the detail within questionnaires. Objective testing indicates an even higher frequency of dysphagia in PD and has allowed its separation into two categories, oropharyngeal and esophageal.
Studies using modified barium swallow have demonstrated some abnormality in the oropharyngeal phase of swallowing in 75% to 97% of persons with PD (Pfeiffer, 2003). Even individuals asymptomatic for dysphagia frequently display abnormalities on modified barium swallow testing. Within the oral phase, difficulty with bolus formation, delayed initiation of swallowing, repeated tongue pumping, and other abnormalities have been described. Pharyngeal dysmotility and impaired relaxation of the cricopharyngeal muscle constitute examples of abnormalities noted in the pharyngeal phase. Individuals with PD are more likely to swallow during inspiration and also to inhale post swallow, both of which increase the risk of aspiration (Gross et al., 2008).
Esophageal dysfunction can also trigger dysphagia in PD. Esophageal manometry has demonstrated abnormalities in 61% to 73% of PD patients studied; videofluoroscopic studies show a broader range, with some abnormality reported in 5% to 86% of individuals (Pfeiffer, 2003). A wide variety of abnormalities of esophageal function has been described, including slowed esophageal transit, both segmental and diffuse esophageal spasm, ineffective or tertiary contractions, and even aperistalsis. Lower-esophageal sphincter dysfunction may also be present in PD and can produce not only symptoms of reflux but also dysphagia.
Aspiration has been noted to be present in 15% to 56% of patients with PD, and completely silent aspiration in 15% to 33% (Pfeiffer, 2003). Even more striking is a study in which vallecular residue, believed to indicate an increased risk of aspiration, was found to be present in 88% of PD patients without clinical dysphagia. Silent aspiration and laryngeal penetration of saliva have also been noted to occur in a significant percentage (10.7% and 28.6%, respectively) of individuals with PD who exhibit daily drooling (Rodrigues et al., 2010). In another study by the same group of investigators, a 9.75-fold increased risk of respiratory infection was documented in PD patients with daily drooling and silent aspiration or silent laryngeal penetration of food who were followed for 1 year (Nóbrega et al., 2008). Hypesthesia of laryngeal structures has also been noted in PD patients, possibly contributing to the risk of aspiration (Rodrigues et al., 2010).
Dysphagia demonstrates an inconsistent response to levodopa or dopamine agonist therapy. Objective improvement in swallowing, documented by modified barium swallow testing, has been observed in 33% to 50% of patients in some but not all studies. A recent meta-analysis, however, concluded that levodopa intake was not associated with improvement in swallowing (Menezes and Melo, 2009). In patients with cricopharyngeal muscle dysfunction, both cricopharyngeal myotomy and botulinum toxin injections have been used successfully. Behavioral swallowing therapy approaches are of benefit to some individuals. On rare occasions, PEG tube placement may be necessary.
Other Basal Ganglia Disorders
In the parkinsonism-plus syndromes, such as progressive supranuclear palsy (PSP), multiple system atrophy, corticobasal degeneration, and dementia with Lewy bodies (DLB), dysphagia is a frequent problem and, in contrast to PD, often develops relatively early in the course of the illness. The median latency to the development of dysphagia in PD is more than 130 months, whereas it is 67 months in multiple system atrophy, 64 months in corticobasal degeneration, 43 months in DLB, and 42 months in PSP (Muller et al., 2001). In fact, the appearance of dysphagia within 1 year of symptom onset virtually eliminates PD as a diagnostic possibility, although it does not help distinguish between the various parkinsonism-plus syndromes (Muller et al., 2001). In persons with PSP, the presence and severity of dysphagia does not correlate well with the presence and severity of dysarthria, so the decision to evaluate swallowing function should not be based on the presence or absence of speech impairment (Warnecke et al., 2010).
Dysphagia is also a well-documented complication of botulinum toxin injections for cervical dystonia, presumably as a consequence of diffusion of the toxin (Comella and Thompson, 2006). It should be noted, however, that 11% of patients with cervical dystonia experience dysphagia as part of the disease process itself, and 22% may display abnormalities on objective testing. Whether the dysphagia in individuals with cervical dystonia is mechanical or neurogenic has been the topic of debate. In a study of 25 patients with cervical dystonia, clinical assessment suggested the presence of dysphagia in 36%; electrophysiological evaluation demonstrated abnormalities in 72% (Ertekin et al., 2002). The electrophysiological abnormalities strongly suggested a neurogenic basis for the dysfunction.
Amyotrophic Lateral Sclerosis
Although dysphagia eventually develops in most individuals with ALS, bulbar symptoms can be the presenting feature in approximately 25% of patients. A sensation of solid food sticking in the esophagus may provide the initial clue to emerging dysphagia, but abnormalities in the oral phase of swallowing are most often evident in patients with early ALS. Impaired function of the lips and tongue (particularly the posterior portion of the tongue) due to evolving muscle weakness typically appears first, followed next by involvement of jaw and suprahyoid musculature, and finally by weakness of pharyngeal and laryngeal muscles. Lip weakness can result in spillage of food from the mouth; tongue weakness leads to impaired food bolus formation and transfer. Inadequate mastication due to the jaw muscle weakness adds to the difficulty with bolus formation, and the eventual development of pharyngeal and laryngeal weakness opens the door for aspiration. Neurophysiological testing in patients with ALS who have dysphagia demonstrates delay in, and eventual abolishment of, triggering of the swallowing reflex for voluntarily initiated swallows, with relative preservation of spontaneous reflexive swallows until the terminal stages of the disease (Ertekin et al., 2000). Although videofluoroscopy is the most precise means of evaluating dysphagia in individuals with ALS, scales such as the Norris ALS Scale provide an adequate venue to decide on the need for dysphagia treatment.
Spasm of the UES, with hyperreflexia and hypertonicity of the cricopharyngeal muscle, has been reported in ALS patients with bulbar dysfunction, presumably as a consequence of upper motor neuron involvement, and has been considered to be an important cause of aspiration (Ertekin et al., 2000; Ertekin et al., 2001a). This has prompted the employment of cricopharyngeal myotomy as a treatment measure in such patients, but this approach should be limited to those with objectively demonstrated UES spasm.
Cervical Spinal Cord Injury
Dysphagia may develop in individuals with cervical spinal cord injury, especially if the injury is associated with respiratory insufficiency. In a study of 51 persons with cervical spinal cord injury and respiratory insufficiency, 21 (41%) suffered from severe dysphagia with aspiration and another 20 (39%) had mild dysphagia (Wolf and Meiners, 2003). Individuals with higher spinal cord injury were statistically more likely to experience more prominent dysphagia after undergoing therapy, although this difference was not evident on admission. With treatment and time, most patients demonstrate improvement in their dysphagia.
Other Processes
Although rare in developed countries, rabies is encountered more frequently in developing nations. In endemic areas, approximately 10% of affected individuals do not report any prior exposure to animal bite (Kietdumrongwong and Hemachudha, 2005). Dysphagia, typically accompanying phobic spasms in the classic “furious” form of rabies, is a well-recognized feature of the human disease. A hyperactive gag reflex is usually also present in this situation. However, dysphagia may also develop in the “paralytic” form of rabies, which may be more difficult to diagnose because the classically recognized features are often absent.
Neurogenic oropharyngeal dysphagia has also been reported as a consequence of severe hypothyroid coma (Urquhart et al., 2001).
Evaluation of Dysphagia
Various diagnostic tests ranging from simple bedside analysis to sophisticated radiological and neurophysiological procedures have been developed to evaluate dysphagia (Box 13.4). Although most are actually performed by specialists other than neurologists, it is important for neurologists to have an awareness of them so that they can be employed when clinical circumstances are appropriate (Box 13.5).
Box 13.5 Dysphagia Testing
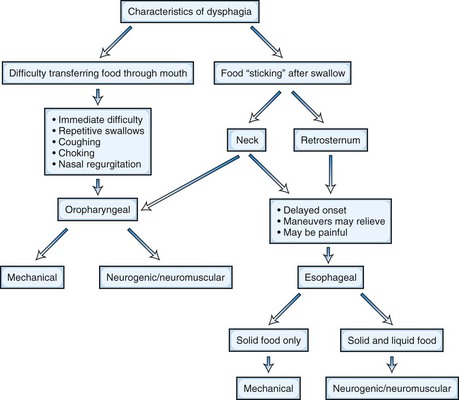
Clinical examination is somewhat limited because of the inaccessibility of some structures involved with swallowing, but both history and examination can provide useful clues to localization and diagnosis (Table 13.1). In fact, it has been reported that a good history will accurately identify the location and cause of dysphagia in 80% of cases (Cook, 2008). Difficulty initiating swallowing, the need for repeated attempts to succeed at swallowing, the presence of nasal regurgitation during swallowing, and coughing or choking immediately after attempted swallowing all suggest an oropharyngeal source for the dysphagia. A sensation of food “hanging up” in a retrosternal location implicates esophageal dysfunction, whereas a perception of the bolus “sticking” in the neck may indicate either pharyngeal or esophageal localization (Fig. 13.1). Individuals who report dysphagia for solid food but not liquids are more likely to have a mechanical obstruction, whereas equal dysphagia for both solids and liquids is more typical for an esophageal motility disorder. Lip and tongue function can be easily assessed during routine neurological examination, and both palatal and gag reflexes can be evaluated.
| Clue | Cause of Dysphagia |
|---|---|
| Difficulty initiating swallowing | Oropharyngeal dysfunction |
| Repetitive swallowing | Oropharyngeal dysfunction |
| Retrosternal “hanging-up” sensation | Esophageal dysfunction |
| Difficulty with solids but not liquids | Mechanical obstruction |
| Difficulty with both solids and liquids | Esophageal dysmotility |
| Regurgitation of undigested food | Zenker diverticulum |
| Halitosis | Zenker diverticulum |
The modified barium swallow test has become a standard method for assessing oropharyngeal dysphagia. Patients are observed via videofluoroscopy swallowing barium-impregnated food of differing consistencies (thin liquid, pudding, cookie). Both oral and pharyngeal function can be characterized and the presence of aspiration accurately documented; the response to corrective measures such as positioning techniques can also be evaluated. Increasing bolus viscosity typically improves swallowing function in individuals with neurogenic dysphagia (Clave et al., 2006).
Esophageal function can be assessed by endoscopy, esophageal manometry, and videofluoroscopy. Scintigraphic procedures can also be employed to evaluate oral, pharyngeal, and esophageal function but are not widely utilized. It has been suggested that scintigraphic examination with documentation of piecemeal deglutition and determination of the dysphagia limit may be particularly useful in centers where more sophisticated electrophysiological techniques are not available (Argon et al., 2004).
More sophisticated electrodiagnostic procedures have also been developed to study dysphagia. EMG recording of cricopharyngeal function and integrated submental activity has been useful in a research setting to characterize aspects of swallowing. Ertekin and colleagues (2002) have used EMG recordings to define an indicator of dysphagia they term the dysphagia limit. Normal subjects can swallow a 20-mL bolus of water in a single attempt, but persons with dysphagia must divide the bolus into two or more parts in order to complete the swallow. If individuals are administered stepwise increases in bolus volume, the volume of fluid at which the division of the bolus first occurs is labeled the dysphagia limit. The investigators consider a dysphagia limit of less than 20 mL as abnormal and indicative of dysphagia.
Abu-Baker A., Rouleau G.A. Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim Biophys Acta. 2007;1772:173-185.
Argon M., Secil Y., Duygun U., et al. The value of scintigraphy in the evaluation of oropharyngeal dysphagia. Eur J Nucl Med Mol Imaging. 2004;31:94-98.
Calcagno P., Ruoppolo G., Grasso M.G., et al. Dysphagia in multiple sclerosis: prevalence and prognostic factors. Acta Neurol Scand. 2002;105:40-43.
Clavé P., de Kraa M., Arreola V., et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24:1385-1394.
Colton-Hudson A., Koopman W.J., Moosa T., et al. A prospective assessment of the characteristics of dysphagia in myasthenia gravis. Dysphagia. 2002;17:147-151.
Comella C.L., Thompson P.D. Treatment of cervical Dystonia with botulinum toxins. Eur J Neurol. 2006;13(Suppl. 1):16-20.
Cook I.J. Diagnostic evaluation of dysphagia. Nat Clin Pract Gastroenterol Hepatol. 2008;5:393-403.
Cox F.M., Verschuuren J.J., Verbist B.M., et al. Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256:2009-2013.
Dabby R., Lange D.J., Trojaborg W., et al. Inclusion body myositis mimicking motor neuron disease. Arch Neurol. 2001;58:1253-1256.
Ebert E.C. Review article: the gastrointestinal complications of myositis. Aliment Pharmacol Ther. 2010;31:359-365.
Ertekin C., Aydogdu I., Secil Y., et al. Oropharyngeal swallowing in craniocervical dystonia. J Neurol Neurosurg Psychiatry. 2002;73:406-411.
Ertekin C., Yuceyar N., Aydogdu I., et al. Electrophysiological evaluation of oropharyngeal swallowing in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2001;70:363-371.
Ertekin C., Turman B., Tarlaci S., et al. Cricopharyngeal sphincter muscle responses to transcranial magnetic stimulation in normal subjects and in patients with dysphagia. Clin Neurophysiol. 2001;112:86-94.
Ertekin C., Aydogdu I., Yuceyar N., et al. Pathophysiological mechanisms of oropharyngeal dysphagia in amyotrophic lateral sclerosis. Brain. 2000;123:125-140.
Finestone H.M., Greene-Finestone L.S. Rehabilitation medicine: 2. Diagnosis of dysphagia and its nutritional management for stroke patients. CMAJ. 2003;169:1041-1044.
Fradet G., Pouliot D., Robichaud R., et al. Upper esophageal sphincter myotomy in oculopharyngeal muscular dystrophy: long-term clinical results. Neuromuscul Disord. 1997;7(Suppl. 1):S90-S95.
Gross R.D., Atwood C.W.Jr., Ross S.B., et al. The coordination of breathing and swallowing in Parkinson’s disease. Dysphagia. 2008;23:136-145.
Hamdy S., Rothwell J.C., Brooks D.J., et al. Identification of the cerebral loci processing human swallowing with H215O PET activation. J Neurophysiol. 1999;81:1917-1926.
Ickenstein G.W., Stein J., Ambrosi D., et al. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252:1510-1516.
Ickenstein G.W., Riecker A., Höhlig C., et al. Pneumonia and in-hospital mortality in the context of neurogenic oropharyngeal dysphagia (NOD) in stroke and a new NOD step-wise concept. J Neurol. 2010;257:1492-1499.
Kietdumrongwong P., Hemachudha T. Pneumomediastinum as initial presentation of paralytic rabies: a case report. BMC Infect Dis. 2005;5:92.
Koopman W.J., Wiebe S., Colton-Hudson A., et al. Prediction of aspiration in myasthenia gravis. Muscle Nerve. 2004;29:256-260.
Marcon M., Briani C., Ermani M., et al. Positive correlation of CTG expansion and pharyngoesophageal alterations in myotonic dystrophy patients. Ital J Neurol Sci. 1998;19:75-80.
Mehanna R., Jankovic J. Respiratory problems in neurologic movement disorders. Parkinsonism Relat Disord. 2010;16:628-638.
Menezes C., Melo A. Does levodopa improve swallowing dysfunction in Parkinson’s disease patients? J Clin Pharm Ther. 2009;34:673-676.
Monini S., Taurino M., Barbara M., et al. Laryngeal and cranial nerve involvement after carotid endarterectomy. Acta Otolaryngol. 2005;125:398-402.
Muller J., Wenning G.K., Verny M., et al. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol. 2001;58:259-264.
Nóbrega A.C., Rodrigues B., Melo A. Is silent aspiration a risk factor for respiratory infection in Parkinson’s disease patients? Parkinsonism Relat Disord. 2008;14:646-648.
Oh T.H., Brumfield K.A., Hoskin T.L., et al. Dysphagia in inflammatory myopathy: clinical characteristics, treatment strategies, and outcome in 62 patients. Mayo Clin Proc. 2007;82:441-447.
Parodi A., Caproni M., Marzano A.V., et al. Dermatomyositis in 132 patients with different clinical subtypes: cutaneous signs, constitutional symptoms and circulating antibodies. Acta Derm Venereol. 2002;82:48-51.
Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2:107-116.
Prosiegel M., Holing R., Heintze M., Wagner-Sonntag E., et al. The localization of central pattern generators for swallowing in humans – a clinical-anatomical study on patients with unilateral paresis of the vagal nerve, Avellis’ syndrome, Wallenberg’s syndrome, posterior fossa tumours and cerebellar hemorrhage. Acta Neurochir Suppl. 2005;93:85-88.
Prosiegel M., Schelling A., Wagner-Sonntag E. Dysphagia and multiple sclerosis. Int MS J. 2004;11:22-31.
Rodrigues B., Nóbrega A.C., Sampaio M., et al. Silent saliva aspiration in Parkinson’s disease. Mov Disord. 2010. PMID:21082717
Runions S., Rodrigue N., White C. Practice on an acute stroke unit after implementation of a decision-making algorithm for dietary management of dysphagia. J Neurosci Nurs. 2004;36:200-207.
Schaller B.J., Graf R., Jacobs A.H. Pathophysiological changes of the gastrointestinal tract in ischemic stroke. Am J Gastroenterol. 2006;101:1655-1665.
Teasell R., Foley N., Fisher J., et al. The incidence, management, and complications of dysphagia in patients with medullary strokes admitted to a rehabilitation unit. Dysphagia. 2002;17:115-120.
Terre R., Mearin F. Oropharyngeal dysphagia after the acute phase of stroke: predictors of aspiration. Neurogastroenterol Motil. 2006;18:200-205.
Terre-Boliart R., Orient-Lopez F., Guevara-Espinosa D., et al. Oropharyngeal dysphagia in patients with multiple sclerosis. Rev Neurol. 2004;39:707-710. [Article in Spanish]
Turner C., Hilton-Jones D. The myotonic dystrophies: diagnosis and management. J Neurol Neurosurg Psychiatry. 2010;81:358-367.
Urquhart A.D., Rea I.M., Lawson L.T., et al. A new complication of hypothyroid coma: neurogenic dysphagia: presentation, diagnosis, treatment. Thyroid. 2001;11:595-598.
Warnecke T., Oelenberg S., Teismann I., et al. Endoscopic characteristics and levodopa responsiveness of swallowing function in progressive supranuclear palsy. Mov Disord. 2010;25:1239-1245.
Williams R.B., Grehan M.J., Hersch M., et al. Biomechanics, diagnosis, and treatment outcome in inflammatory myopathy presenting as oropharyngeal dysphagia. Gut. 2003;52:471-478.
Wolf C., Meiners T.H. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord. 2003;41:347-353.
Zald D.H., Pardo J.V. The functional neuroanatomy of voluntary swallowing. Ann Neurol. 1999;46:281-286.