CHAPTER 174 Neurocritical Care in Children
Neurological Assessment in the Pediatric Intensive Care Unit
A variety of tools are available for monitoring neurological function in critically ill children and adults (for review, see Tisdall and Smith1 and Wartenberg and colleagues2). Such tools range from electroencephalographic (EEG) monitoring, which is well established as a modality essential for the detection of nonconvulsive seizures (NCSs) in the adult and pediatric intensive care unit (ICU),3,4 to intracranial pressure (ICP), transcranial Doppler,5 and tissue oxygen monitoring.1,6 Although thresholds for detection of cerebral ischemic injury have been proposed for brain oxygen tension7 and near-infrared spectroscopy,8 there is no consensus on normal values and age dependence of these end points, and there are only limited data from adult studies to show impact on outcome.9 Indeed, most of these studies focus on traumatic brain injury (TBI), and the application of these data to the quantification of primary neurological injury or detection of secondary insults in the context of critically ill children with stroke, refractory status epilepticus (RSE), or central nervous system (CNS) infections remains to be determined.
The importance of the bedside neurological examination for assessment of the initial neurological injury and progression of the insult, the need for serial examinations, and involvement of the entire medical team in this assessment cannot be overstated. It is clear that admission to a specialized neurosciences ICU,10 management by a neurocritical care team, or the presence of a neurointensivist11,12 is associated with reductions in length of stay and mortality in adults. Accordingly, the Glasgow Coma Scale (GCS) score is simple to determine and yields reproducible results among observers,13 but the GCS is not a neurological examination and does not assess brainstem (particularly pupil reactivity) function or the presence of focal findings.
Airway Management and Respiratory Failure
Respiratory decompensation creates additional challenges in critically ill children with neurological disorders. Given the impact that abnormalities in ventilation and oxygenation have on cerebral hemodynamics and metabolism, respiratory abnormalities can result in secondary insults to the brain. For example, hyperventilation can lead to hypocapnia and detrimental changes in cerebral blood flow—a 3% reduction in cerebral blood flow for each 1-mm Hg reduction in PCO2. Although targeted hyperventilation is considered a treatment option during periods of severe intracranial hypertension and cerebral herniation, unintentional hyperventilation has been independently associated with increased mortality.14 Avoidance of unintentional hyperventilation is especially important in the immediate postinjury period after TBI, when cerebral blood flow may already be markedly reduced or mismatch between oxygen demand and supply may be present.15,16 This principle should also be kept in mind when treating patients with other acute neurological disorders complicated by marginal brain perfusion.
Hypoventilation can lead to detrimental increases in PCO2. Hypercapnia will increase cerebral blood volume and can lead to severe intracranial hypertension in patients with decreased cerebral compliance. Causes of hypoventilation in the PICU include deterioration of neurological status as a result of side effects of narcotic therapy. Children younger than 6 months are at higher risk for respiratory depression related to narcotics because of their immature liver metabolism. For example, the mean half-life of morphine has been reported to be 5.4 ± 3.4 hours in infants 1 week to 2 months of age and 2.6 ± 1.7 hours in infants aged 2 to 6 months.17 Developmental differences in pharmacokinetics should be taken into consideration when determining doses of narcotics in the PICU to minimize the risk for respiratory depression.
Stroke in Children
There are a number of excellent reviews on stroke in children,18–27 including consensus statements that also address the controversies regarding treatment.28–31 This summary focuses on acute vascular insults, but it is important to consider that other disorders such as metabolic or mitochondrial disease, migraine, or seizures may mimic stroke.32 Timely and efficient evaluation for suspected stroke is therefore essential. The average interval between the onset of initial symptoms and medical evaluation is 34 hours.33
The incidence of ischemic stroke in non-neonates is 2 to 6 per 100,000 children per year, and in neonates it is 1 in every 5000 live births.26,34 The mortality and neurological morbidity associated with stroke in the young are substantial. Stroke is among the top 10 causes of death in children, and 60% of survivors have residual neurological deficits.20,25,35,36 Pooled data from patients with arterial ischemic stroke (AIS) in the United Kingdom and Canada who were not treated with antithrombotic drugs suggest a recurrence rate of nearly 50%.29 This rate may be reduced (although no controlled trials have been performed) because studies in which most children were treated with aspirin or anticoagulation drugs report recurrence rates of 5% to 25%.37,38 This question remains unresolved.
The causes of stroke in children differ substantially from those in adults, and large-artery atherosclerosis and small-vessel occlusive disease are not common pathologies in pediatric cerebrovascular disease (for review, see Lynch and colleagues26 and deVeber39). For AIS, the most common risk factors are congenital or acquired heart disease and, less frequently, hypercoagulable or autoimmune disorders and arteriopathies (arterial dissection, moyamoya syndrome, and vasculitis).21,26,40,41 In the United States, in the largest population-based study of AIS in children, the most common causes were cerebral arteriopathy (24%), infection (23%), and cardiac disease (12%), with sickle cell disease (SCD) accounting for just 3% of cases in this series.42 Among the arteriopathies, an association between antecedent varicella-zoster infection and increased risk for AIS has been reported in numerous studies,21 often involving the basal ganglia.43 Similarly, the frequency of cervicocephalic arterial dissection is relatively higher in the young, in whom it accounts for 8%44,45 to 20%35 of cases in different series. The causes of moyamoya disease are not known, but there is a robust association with progressive neurological dysfunction, which occurs in up to 60% of cases46 and may be reduced by revascularization surgery.47 In most published studies, up to 25% of children have no identifiable risk factor.
The contribution of thrombophilias to stroke in the young has been addressed in multiple studies.40,48–50 Coagulation abnormalities implicated in the increased risk for stroke in children include deficiencies in protein C, protein S, or antithrombin III; activated protein C resistance; factor V Leiden mutation; prothrombin gene mutation (G20210A); methylenetetrahydrofolate reductase TT677 mutation; and antiphospholipid antibody syndrome. The contribution of a single biochemical or genetic risk factor to elevated risk for stroke is not precisely defined and is likely, given the relative rarity of childhood stroke, to be low.48 In practice, this means that other factors should be investigated, even in the presence of a biochemical or genetically determined procoagulant state.28
There are limited data and no controlled trials addressing the management of hemorrhagic stroke in children. Intracerebral hemorrhage (ICH) in the young has symptoms similar to those in adults, most commonly (59%) headache or vomiting, and is associated with seizures in 37%.51 For nontraumatic ICH, the most common risk factor in this study was an arteriovenous malformation (AVM) or arteriovenous fistula, which occurred in 34% (23 of 68) of cases. This frequency of AVM is comparable to that reported (47%) in a smaller study of 34 children with nontraumatic ICH.52
Guidelines for the approach to diagnostic studies have been published.30,53 A high index of suspicion is the essential first step for considering stroke in the differential diagnosis of new neurological deficits in children. Imaging studies should begin with computed tomography (CT) or magnetic resonance imaging (MRI) to first establish the diagnosis of stroke, identify the presence of hemorrhage, and delineate the extent of infarction. Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) should be included if carotid or vertebral dissection is a possible mechanism. Venous sinus thrombosis is underdiagnosed, may be manifested by subtle signs, including seizures and subarachnoid hemorrhage or ICH,54,55 and therefore merits a low threshold for the inclusion of computed tomography venography (CTV) or magnetic resonance venography (MRV) in the imaging studies. If the stroke is not in a vascular distribution or mitochondrial disorders are a consideration for any reason, magnetic resonance spectroscopy should by performed. If the results of MRA are normal and dissection, small-vessel vasculitis, AVM, aneurysm, or moyamoya disease are suspected, conventional angiography should be performed. Cardiac echocardiography is indicated for the evaluation of new stroke and should include bubble contrast enhancement. Based on the index of suspicion, a transesophageal study may be needed. Laboratory studies should include measures of coagulation, platelets, homocysteine, fasting cholesterol, triglycerides, and lipoprotein (a). Screening for infection may include antibodies to varicella-zoster21,43 and Mycoplasma and, if indicated, Chlamydia, Helicobacter, and Borrelia titers. Studies of genetic and biochemical risk factors that have been implicated in childhood stoke, together with von Willebrand factor antigen and plasminogen, may be performed along with other screening studies for autoimmune disorders, including lupus anticoagulant, as well as the erythrocyte sedimentation rate. Because anticardiolipin antibodies may be abnormal acutely, studies need to be repeated 8 to 12 weeks after the stroke, along with any abnormal biochemical measures of coagulation. If a metabolic disorder is suspected, lactate, pyruvate, serum amino acids, and urine organic acids should be obtained during the acute phase of the stroke, together with measurement of lactate levels in cerebrospinal fluid if available.
Stroke in Children with Sickle Cell Disease
Children with SCD pose a separate set of challenges for diagnosis and management of acute neurological deficits (for review, see Kirkham56 and Switzer and associates57). By 45 years of age, 25% of patients with hemoglobin (Hb) SS disease and 10% of patients with HbSC disease have had a stroke.58 In children with HbSS disease, up to 25% have had a “silent” infarction without detectable neurological deficits by adolescence.59 The incidence of stroke in children with SCD (285 per 100,000 per year60) is at least 20 times higher than the incidence (2.3 to 13.0 per 100,000 children18) in the general pediatric population. Overall, stroke is the primary cause of death in 10% of patients with SCD.61 Importantly, stroke in children with SCD may be accompanied by a diverse range of symptoms from difficulty with rapid alternating movements to headache, seizures, or coma.62 In practice, therefore, stroke should be the leading differential diagnosis in children with SCD and new neurological findings.
Strokes in children with SCD may occur as a result of multiple mechanisms, including arterial ischemia, intracerebral or subarachnoid hemorrhage,63 aneurysm rupture, dissection, moyamoya syndrome,64 or venous sinus thrombosis.54 Thus, the imaging studies required for evaluation of acute neurological deficits in this population may include initial CT, MRI with diffusion weighting, MRA or CTA, and MRV or CTV. If imaging studies do not identify stroke as the mechanism, there should be a high index of suspicion for seizures, which occur 10 times more frequently in patients with SCD than in the general population.62
Specific risk factors that increase the risk for stroke in patients with SCD have been identified and include previous transient ischemic attack, low hemoglobin levels, elevated white blood cell count, hypertension, and acute chest syndrome.58 Indeed, children with acute chest syndrome are at significantly increased risk for stroke or stroke mimics, including posterior reversible leukoencephalopathy syndrome.65 In SCD patients with increased flow velocity (identified by transcranial Doppler) in the middle cerebral artery or distal internal carotid artery, the annual risk for stroke increases from 1% to 10%.66
The mainstay of stroke prevention in high-risk children with increased blood flow velocity and SCD is transfusion based on the 92% reduction in relative risk shown in the Stroke Prevention Trial in Sickle Cell Anemia (STOP) over a 2-year follow-up.67 Such therapy has limitations, however. In this study stroke developed in only 15% of nontransfused subjects with high blood flow velocity. Furthermore, chronic transfusion therapy may result in complications such as infection, iron overload, and alloimmunization. Continued long-term transfusion therapy is required even when velocities return to a normal range.68
For children with SCD and acute stroke, the initial focus of clinical management is exchange transfusion and hydration.28,56 Exchange transfusion is undertaken to reduce the percentage of HbS to less than 30% of total hemoglobin, increase the percentage of HbA, and raise hemoglobin levels to 10 to 12.5 g/dL.30 It should be noted that no controlled studies have proved the benefits of this approach, which is supported by class C evidence. Equally and again supported by class C evidence, children with acute ischemic stroke should receive adequate hydration and undergo correction of hypoxia and hypotension.28
Data on pharmacologic prophylaxis for prevention of stroke in patients with SCD are limited. Antiplatelet therapy with aspirin did not reduce stroke frequency.69 There is extensive evidence of the efficacy of statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) in the primary and secondary prevention of stroke in adults.70,71 However, there are no published data on their use in patients with SCD, and this merits further investigation.57,72
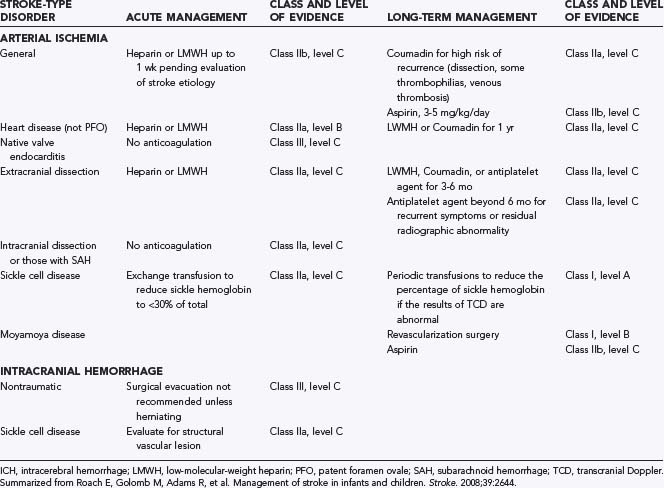
Stroke—Anticoagulation and Supportive Therapy
In contrast to adults, in whom there is evidence-based recommendations for the treatment of AIS,73 evidence-based data for children are more limited. There is universal consensus on the general goals of management: reestablishment of flow to the ischemic brain, minimization of secondary injury, and the principle that “time lost is brain lost.”28,74 There are, however, no published data showing that such management improves outcome in children with stroke, although the authors regard this as standard of care. Guidelines for the management of children with AIS and ICH are available28 and are summarized in Table 174-1. Notably, short-term anticoagulation may be considered for pediatric AIS, pending determination of the cause of the stroke. This is in contrast to the recommended practice in adults, for whom urgent anticoagulation is not recommended73 given the absence of benefit75 and increased risk for hemorrhage. In eligible adults within 3 hours of the onset of AIS, thrombolysis with tissue plasminogen activator (t-PA) improves outcome.76 Intravenous t-PA for the treatment of AIS has been used safely in small pediatric series77 and holds promise as therapy for pediatric stroke.
As for TBI, management of AIS or ICH in the ICU should seek to minimize secondary injury. Strategies that aggressively treat fever, maintain euglycemia and normotension, and prevent hypoxia are adapted from the management of adults with AIS.74 Although these recommendations for children are based on limited pediatric data (class I, level C evidence),28 there is broad agreement that such management is indicated.28,30,53
In the ICU, the challenges posed by children with stroke are threefold. First, there is the need to expedite diagnostic assessment of a child with suspected stroke so that therapy (including interventions to prevent secondary injury) can be started quickly if indicated. Second, there is the difficulty of identifying new cerebrovascular insults in critically ill children as a complication of other organ injury. This is often difficult in the sedated, intubated ICU patient population, so a high index of suspicion is warranted. Last, decisions about management of these patients will often need to be individualized because of limited or weak evidence-based guidelines for such management. Accordingly, decisions about anticoagulation, thrombolysis, blood pressure, temperature, and glucose management, as well as indications for ICP monitoring or hyperosmolar therapy, should be based on a consensus of the services involved. Criteria for escalation in therapy, up to and including thrombolysis and decompressive hemicraniectomy,78 should be discussed early in the hospital course if needed. Serial neurological examination and frequent communication among members of the treating team are essential for early recognition of evolution of the neurological injury or a new insult and a rapid, coordinated approach to diagnostic studies and therapeutic intervention.
Fluids, Electrolytes, and Nutrition
Children with acute brain injury and those recovering from neurosurgical procedures commonly require intravenous fluids early in the ICU course. Maintenance intravenous fluids (1500 L/m2 per day) are recommended to avoid dehydration and the consequent hemodynamic instability. The composition of maintenance intravenous fluids is tailored to avoid glucose and electrolyte abnormalities. Although hyperglycemia (blood glucose >180 mg/dL) is generally avoided because of its potential to worsen brain injury,79,80 care should be taken to avoid hypoglycemia, especially in neonates and infants. Even though older children generally tolerate glucose-free intravenous fluids, infants and neonates frequently need glucose supplementation to avoid severe hypoglycemia and its related consequences, including seizures, alterations in mental status, and brain damage. Hypotonic fluids such as lactated Ringer’s solution and 0.25 normal saline solution are avoided because of the risk for hyponatremia, which can lead to worsening brain edema and even herniation.81,82 In patients requiring fluid resuscitation to treat hypotension, normal saline solution is used as first-line therapy. The use of hypertonic saline (i.e., 3% saline solution) is considered a treatment option given its ability to restore hemodynamic stability and the added benefit of potentially reducing ICP.83,84 Limited information is available on the safety and efficacy of the use of hypertonic saline solutions in children with brain injury.85
Although the optimal time for initiation of enteral nutrition has not been established in children with brain injury, it is usually started as soon as possible and as tolerated by the patient’s neurological status. Guidelines for the management of severe TBI in children recommend replacement of 130% to 160% of resting metabolic expenditure, with nutritional support initiated by 72 hours after injury and full replacement by 7 days.86 Although gastric feeding is commonly well tolerated, transpyloric (jejunal) feeding is an option in children with brain injury who have gastric intolerance because of gastroparesis. It is important to note that transpyloric feeding does not decrease the risk for vomiting and aspiration in critically ill children or adult patients with brain injury.87–89 Additionally, vomiting can be a sign of neurological deterioration.
Fluid balance and electrolyte abnormalities can bee seen in children with acute brain injury and in the immediate postoperative period after neurosurgical procedures. Diabetes insipidus (DI) results from a deficiency of arginine vasopressin and can result in severe water and electrolyte imbalance.90 It develops in approximately 75% of patients after transcranial resection of a pituitary tumor and in 10% to 44% after transsphenoidal pituitary surgery.91,92 DI can also develop after other neurosurgical procedures such as ventricular fenestrations, as well as after TBI and cardiac arrest. In a few patients persistent DI will develop. Transient syndrome of inappropriate antidiuretic hormone secretion (SIADH) may develop in some patients as part of a triphasic manifestation (DI-SIADH-DI), or symptoms of cerebral salt wasting syndrome (CSWS) can develop.92–98 Diagnostic criteria for postoperative DI are summarized in Table 174-2.
TABLE 174-2 Diagnostic Criteria for Postoperative Diabetes Insipidus
Symptoms of DI can develop intraoperatively or in the immediate postoperative period (usually within the first 12 hours). The diagnostic criteria listed in Table 174-2 facilitate distinction between normal postoperative diuresis and DI. Management of children with DI can be complicated by overhydration or underhydration and electrolyte imbalance. These complications can be life-threatening when they result in hypotension and poor organ perfusion, severe hypernatremia, or hyponatremia. Hyponatremia can cause or worsen brain edema and seizures. These events can result in severe neurological injury.
Different treatment modalities for children with DI can be considered,91 including (1) aggressive free water replacement, (2) free water replacement with intermittent dosing of intranasal or oral vasopressin, and (3) free water replacement with continuous titration of intravenous vasopressin. The preferred approach is the one that would maximize patient safety and comfort. Although all three approaches have been used and can be considered, use of continuous, low-dose intravenous vasopressin with controlled fluid administration (1 L/m2 per day plus additional fluid as needed to maintain fluid balance and hemodynamic stability) has been shown to produce better results in the perioperative period in children with postoperative DI who cannot regulate their own oral intake. This approach facilitates safe and effective management of children with perioperative DI while minimizing fluctuations in serum sodium concentrations and avoiding complications that can result from delays in effective therapy and inadequate fluid administration. This approach will also avoid the need for intense fluid replacement (intravenously or orally) for excessive urine output, especially during the immediate postoperative period. Satisfaction may be improved by minimizing excessive thirst postoperatively in patients who may not be able to maintain adequate oral intake because of their age or neurological status.
Other disorders of fluid and electrolyte balance seen in the postoperative period and in patients with acute brain injury include SIADH. Patients with SIADH have decreased plasma osmolality (<275 mOsm/kg H2O), inappropriate urinary concentration (urine osmolality >100 mOsm/kg H2O with normal renal function), urinary sodium loss (>40 mEq/L) with normal salt and water intake, and clinical euvolemia. A less common but important disorder, CSWS is characterized by hyponatremia, hypovolemia, natriuresis, and diuresis.99 Severe hyponatremia can develop in patients with CSWS, and they require careful titration of fluid intake and sodium replacement. Given the detrimental effects that delayed diagnosis and treatment can have in patients with the disorders just described, our pediatric neurocritical care team has created a bedside document outlining diagnosis, monitoring, and treatment parameters for postoperative DI. This document is available in the Appendix. ![]()
Intracranial Hypertension
ICP monitoring techniques and treatment principles are described in Chapters 24 and 25. TBI and unique pediatric aspects are discussed in Chapter 214. The main indication for ICP monitoring in critically ill children is TBI. Children of all ages, including infants, are at risk for the development of intracranial hypertension and the consequent cerebral hypoperfusion and cerebral herniation. ICP monitoring in patients with medical conditions other than TBI is highly controversial. Examples of reversible brain pathology that results in increased ICP include meningitis and liver failure with encephalopathy. In patients with meningitis, ICP monitoring is used in an estimated 7% of patients.100 Mortality is higher in patients with meningitis and mean cerebral perfusion pressure (CPP) less than 50 mm Hg despite CPP-directed therapies.101 In patients with liver failure and encephalopathy, the potential benefit of ICP-directed therapy should be balanced against the risk for complications.102,103 Global ischemia from near drowning or cardiac arrest with consequent brain edema can result in increased ICP, but the incidence in children is not known, and because these patients are not routinely monitored, there are no data on the benefit of monitoring ICP and other parameters with the goal of optimizing cerebral perfusion and balancing cerebral metabolism. Limited reports suggest that ICP monitoring and interventions aimed at lowering ICP are not beneficial in patients after global ischemia.104 Overall, although ICP monitoring is feasible in conditions other than TBI, no information on the safety and efficacy of ICP-directed therapies is available.
Seizures
As is the case for stroke, time is of the essence for the treatment and prevention of SE. The duration of seizures required for the definition of SE has been shortened to 30 minutes,105 and a duration as short as 5 minutes has been proposed.106 This variation in operational definition has come about in response to important observations from animal studies that within 15 minutes repetitive seizures become self-sustaining and pharmacoresistant.107–109 A further refinement of the definition of SE has been proposed to include “impending” SE (continuous or intermittent seizures lasting more than 5 minutes without full recovery of consciousness between seizures) and “established” SE (clinical or electrographic continuous seizures lasting more than 30 minutes without full recovery of consciousness between seizures).110 Criteria for RSE include persistence of seizure activity despite appropriate medical and anticonvulsant drug (ACD) therapy, although the duration of seizures (1 to 2 hours)105,111 and the number of drugs (two or three)112 vary among studies.
SE and RSE are associated with a significant increase in mortality in adults and children.113–116 In a series of 22 children with RSE, 33% died and none of the previously healthy children recovered normal neurological function.115 Overall, a meta-analysis of pediatric RSE indicates a mortality of 16%.112
Early recognition of clinical or electrographic seizures is an important factor in enhancing the efficacy of ACDs to terminate the seizures and thereby prevent the additional metabolic stress that seizures impose on the injured brain. However, detection of seizures in the ICU is challenging. NCSs or SE may be difficult to diagnose and are often unrecognized in comatose patients117 in both adult and pediatric ICUs.81,84,85 Convulsive seizures or NCSs are reported in up to 50% of patients undergoing treatment in an ICU when examined by continuous EEG (cEEG) monitoring118–120; such patients often have nonconvulsive SE.121 Importantly, routine EEG recording misses NCSs in approximately 50% of critically ill patients whose NCSs were detected with cEEG monitoring.120,122 Where there is a high index of suspicion for NCSs, cEEG monitoring for at least 12 hours is required to detect NCSs.120,123,124
Protocols for the treatment of SE are well established (for review see Walker and Teach125 and Glauser and colleagues126). The typical protocol involves intravenous, rectal, or buccal administration of a short-acting benzodiazepine, commonly followed by (depending on age, current ACD use, and seizure type) phenytoin, phenobarbital, or valproic acid. A protocol in use at one of our (M.W.) institutions is shown in Figure 174-1. Key features of this approach in the ICU are the need for early identification of seizures, possibly requiring cEEG monitoring, the ready availability of ACDs, and attention to causes of the seizures in high-risk critically ill children.
Many patients in the ICU will meet the criteria for RSE. In contrast to SE, the approach to the management of RSE is not as well defined. Treatment protocols for adult127 and pediatric128 RSE have been proposed. After failure of first-line ACDs, treatment with midazolam followed by pentobarbital or other coma-inducing drugs is initiated. Other agents such as ketamine129 or topiramate130 have been shown in case series to be effective and may also be tried. Use of midazolam as the first-line agent for RSE in children is based on a number of studies showing efficacy131 (for review see Abend and Dlugos128). A protocol for the treatment of RSE in use in the ICU at one of our (M.W.) institutions is shown in Figures 174-3 and 174-4. This approach combines rapid escalation of the primary treatment (midazolam) with defined targeted physiologic parameters and selected diagnostic studies. This approach seeks to treat RSE in the context of requirements for maintaining cardiovascular function.
Infections
Encephalitis and bacterial meningitis are reviewed in detail in Chapter 45. Here, we focus on an overview of infectious issues most relevant to pediatric neurocritical care.
Mortality from bacterial meningitis in children has decreased to as low as 2%.132 In an Australian series of 122 children with pneumococcal meningitis, 15 died and 39 had permanent neurological deficits.133 In a U.S. series of 559 pediatric patients, 7% died in the PICU and mortality was higher in those in coma or shock on admission to the ICU.134 Treatment with dexamethasone improves outcome in children with all-cause bacterial meningitis, including both Haemophilus influenzae135,136 and pneumococcal meningitis.133 However, the use of adjunctive steroids for the treatment of pneumococcal meningitis is controversial, and practice varies in the United States.132
The fundamental principles of prevention of secondary neurological injury also apply to the management of meningitis. Essential in optimizing neurological outcome are maintenance of normal oxygenation and adequate CPP, prevention of hypoglycemia and hyponatremia, and early detection of seizures, AIS, ICH, and cerebral sinus venous thrombosis.137,138 The maximum elevation in ICP occurs in the first 24 to 48 hours after diagnosis.139 A retrospective cohort study of U.S. meningitis cases reported ICP monitoring in 7% of patients.140 There was no difference in mortality between groups, consistent with a previous series reported from 15 U.S. PICUs.134 Taken together, variations in practice and lack of demonstrated effect on outcome render impossible any recommendations for or against ICP monitoring in children with bacterial meningitis.140
Tuberculous meningitis (TBM) may occur as a reactivation of latent infection or as a new infection (for review, see Thwaites and Hien141). Early recognition is important to enhance the chance of good neurological outcome but is difficult given the nonspecific prodrome and, commonly, lack of fever.142 In one adult series, 28% of subjects reported a headache, 13% were febrile, and only 2% reported meningitis symptoms.143 Mortality and neurological morbidity are high, with a reported mortality of 13% and permanent neurological sequelae in 45% of 38 children in a British series.144 Strikingly, there were no deaths or neurological sequelae in children vaccinated with bacille Calmette-Guérin (BCG). The neurological complications of TBM are myriad, but hydrocephalus, stroke, and seizures are common and TBM should be considered in the differential diagnosis of any neurological deficits.145 Hydrocephalus is more frequent in children (80% to 90%) than adults (12%) with TBM.142 Accordingly, serial lumbar punctures or external ventricular drainage may be considered both to prevent the need for ventricular shunting and to increase the potential for benefit from shunt placement,146,147 despite the lack of data from controlled trials. The use of adjunctive corticosteroid treatment remains controversial, but cumulative evidence148 suggests that dexamethasone reduces morbidity but not mortality and should be administered to all patients regardless of age or disease severity.141
In the developing world, cysticercosis is the most common cause of adult-onset epilepsy.149 For patients from regions with endemic disease, neurocysticercosis should be considered in the differential diagnosis of brain calcifications and seizures. Criteria for treatment with anthelmintic agents and the optimal agents for such use have not been established (for review see Nash and colleagues150).
The neurological manifestations of viral encephalitis in children are protean and are most commonly manifested in the ICU setting as seizures. The most common agents are the nonpolio viruses, enteroviruses, respiratory viruses, and herpes group viruses, including Epstein-Barr virus, although a specific agent may be identified in just 40% of cases.151 In adults, the occurrence of seizures during the acute phase of a CNS infection significantly increases the risk for unprovoked seizures thereafter, with the risk being greatest in the first 5 years but remaining elevated (22%) over 20 years after recovery.152 In children, risk factors associated with a poor outcome after encephalitis include SE during the acute phase of encephalitis and herpes simplex as the etiologic agent.153 Neurological complications of the common respiratory syncytial virus are rare but include seizures, cardiac arrest, and postulated brainstem dysfunction.154 Finally, new CNS infection or reactivation of latent human herpesvirus 6 (HHV-6) should be considered in any immunosuppressed patient in the ICU setting with a change in neurological function.155 Limbic encephalitis in these patients may be manifested as an inability to sleep and short-term memory loss.156 A high index of suspicion is needed in any transplant recipient with these symptoms given the risk for long-term cognitive impairment associated with HHV-6 encephalitis.157
For any CNS infection requiring ICU care, seizures should be suspected either as a complication of infection or in the setting of deterioration of neurological function.141 Frequently, seizures in this population will be nonconvulsive, and cEEG monitoring will typically be necessary for detection of electrographic seizures in the ICU.120,158
Prevention of Secondary Insults to the Brain
Inadequate perfusion may have a direct and deleterious effect on the pathophysiology of brain injury. Cerebral perfusion is generally described in terms of CPP, which is calculated as the difference between mean arterial pressure (MAP) and mean ICP: CPP = MAP − ICP. Current recommended guidelines suggest that CPP be maintained around 70 mm Hg in adult patients. CPP between 40 and 65 mm Hg has been recommended for children with severe TBI.159 Based on data from adult patients, CPP of 60 to 70 mm Hg is recommended for adolescents. Children with CPP below these thresholds have worse outcomes. Optimal CPP levels for children younger than 2 years have not been established, but several studies have demonstrated that CPP of 40 mm Hg or less is associated with higher mortality and worse outcome in children of any age. We therefore consider 45 mm Hg a critical threshold for CPP in children younger than 2 years. Using normal MAP values for age and 10 mm Hg to estimate normal ICP, threshold levels of 50 mm Hg (2 to 6 years), 55 mm Hg (7 to 10 years), and 60 mm Hg (11 to 16 years) can be estimated. CPP values should be interpreted in the context of other determinants of brain tissue perfusion such as cerebral blood flow autoregulation.160–163 Adjustments in MAP to maintain CPP above critical thresholds may be necessary in children with disrupted autoregulation. Because ICP and blood pressure are commonly monitored in children after TBI, CPP values are usually available and help guide treatment. In critically ill children with other acute neurological disorders, ICP data are much less commonly available, thus making estimations of critical thresholds for brain perfusion difficult.
Even in the absence of increased ICP, low blood pressure has been shown to be a predictor of poor outcome in patients with brain injury.164 One of the difficulties we face while caring for children with a brain injury is determining an appropriate systolic blood pressure and MAP for a specific disease state. Additionally, normal systolic blood pressure and MAP values change with age (Table 174-3).165 The formula 70 mm Hg + (2 × age in years) allows easy calculation of the lower limit (fifth percentile) of systolic blood pressure for age.
| AGE (yr) | NORMAL MAP (mm Hg) |
|---|---|
| 1-2 | 53-59 |
| 3-4 | 61-65 |
| 5-6 | 67-69 |
| 7-8 | 70-72 |
| 9-10 | 73-75 |
| 11-12 | 75-77 |
| 13-14 | 77-79 |
| 15-17 | 80-84 |
Adapted from Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007;8:138.
Different definitions of hypotension have been used. A recent study using the 75th percentile for age-appropriate systolic blood pressure as the threshold for hypotension in children with TBI described an association between early hypotension and outcome.166 Maintenance of age-appropriate systolic blood pressure during the initial stabilization phase is important and may influence outcome in children with TBI and possibly other types of acute brain injury. As new technology becomes available, it may be possible to more effectively determine adequate brain perfusion thresholds in patients with nontraumatic brain injury. In the meantime, careful attention should be given to systemic hypotension.
Other factors that may result in secondary insults to the brain include hyperthermia, anemia, decreased cardiac output, seizures, pain and agitation, cerebral edema, and CNS thrombosis. Hyperthermia is known to correlate with worse outcome and longer ICU stay in patients with brain injury.167–169 Avoidance of hyperthermia is important during periods of brain ischemia. Although data in children are limited, both medications and external cooling methods to treat hyperthermia in ICU patients appear to be ineffective in a significant proportion of patients.170–172 Intravascular cooling methods are more effective but unavailable for children. Promising new external cooling technology may become available for children in the near future.172
Anemia and low cardiac output can aggravate brain injury and should be avoided. There is no clear threshold for transfusion in children, and although hemoglobin concentrations of 7 g/dL are well tolerated in stable children in the ICU,173 no data are available for children with marginal brain perfusion. Recent information has also raised concern about the effects of transfusion of stored blood on brain tissue perfusion.174
As for cardiac output, although coronary artery disease is much less common in children, pediatric patients can suffer from low cardiac output secondary to systemic hypoperfusion, severe brain injury, or the effect of medications such as pentobarbital, narcotics, and benzodiazepines. Propofol in particular has been reported to rarely result in refractory shock and metabolic acidosis. This medication is not recommended for continuous sedation in the PICU (http://www.fda.gov/medwatch/SAFETY/2001/safety01.htm#dipriv).
Pain and agitation resulting in hypermetabolism and increased ICP can also aggravate brain injury. The beneficial effects of sedatives and analgesics are balanced against the effects of these medications on the child’s neurological examination. In patients undergoing mechanical ventilation and ICP monitoring, continuous sedation and analgesia are more desirable than intermittent administration. In patients recovering from intracranial procedures such as resection of posterior fossa tumors, intermittent careful titration of analgesics is preferred, and consideration can be given to non-narcotic analgesics such as ketorolac in the absence of obvious contraindications.175–177 Ketorolac appears to be well tolerated in children after significant surgical procedures without increasing the risk for bleeding and renal dysfunction, but its safety and efficacy in neurosurgical patients have not been established. Ketorolac is typically prescribed for 48 hours or less.
Finally, although less common, deep venous thrombosis is possible in children with brain pathology.178 Risk factors include immobility, obesity, thoracic or abdominal surgery, need for central venous access, and procoagulant states.179,180 As children recover and tolerate external stimulation, physical and occupational therapy interventions will increase mobility and decrease the risk for venous thrombosis. Anticoagulation prophylaxis is indicated in selected cases. Treatment of cerebral venous thrombosis, a life-threatening condition in critically ill children, is rather complex and more studies are needed.181 The risk of bleeding should be balanced against the risk of expanding thrombosis, which can lead to severe brain injury.
Diagnosis of Brain Death in Children
Guidelines for determining death, including brain death, have developed over time at many institutions based on the Harvard criteria.182 In 1981, both national policy and state law regarding the determination of death were established.183 These directives indicate that as with irreversible failure of circulation and respiration, irreversible failure of brain function (including the brainstem) is death. Criteria for establishing brain death in children were published in 1987 in a statement issued by the American Academy of Pediatrics.184 These principles are consistent with the report by the Quality Standards Subcommittee of the American Academy of Neurology and address additional aspects of the determination of brain death that are unique to children.185
Before the determination of brain death, the patient must be known to have an irreversible disease that can cause brain death. Reversible disorders that may result in loss of brain function must be excluded. Examples of such recoverable disorders include drug poisoning, toxin, metabolic disorders, severe electrolyte disturbances, hypothermia, and shock. The degree of hypothermia that could interfere with a brain death examination is in general not well defined. While defining such a threshold at a given institution, consideration should be given to the fact that brainstem reflexes can be lost at 32°C.186 Additionally, a temperature of 36.5°C or lower can create difficulty in the interpretation of target carbon dioxide partial pressure (PCO2) values. Controversy surrounding the need to correct arterial blood gas values to the patient’s temperature is avoided at normothermia. Given the difficulty in achieving normothermia in children with loss of brain function and the paucity of data supporting a specific lower limit for temperature, a threshold of 35°C can be considered feasible and appropriate.
To establish brain death, there must be complete loss of brain function. The patient must have no spontaneous movement or vocalization and must not respond to noxious stimuli. There must be no decorticate or decerebrate posturing. Rudimentary spinal reflex responses may be present, such as deep tendon reflexes, plantar reflexes, triple flexion of the legs, and superficial abdominal reflexes. The pupils may be either widely dilated or midway between constriction and dilation, and stimulation with a bright light must fail to produce any pupillary constriction. The eyes must be motionless. Oculocephalic (doll’s eye) and oculovestibular (ice-water caloric) responses must be absent. The gag, cough, sucking, and rooting reflexes must be absent. Finally, to document the absence of spontaneous ventilatory efforts, an apnea test can be performed if there is no contraindication.187 Before interrupting the ventilator, the patient should be ventilated with 100% oxygen for at least 5 minutes to minimize the risk for hypoxia. Blood gas analysis should be performed to demonstrate that the patient has adequate acid-base balance and acceptable ventilation (PCO2 of 35 to 45 mm Hg) and oxygenation (PO2 of 100 mm Hg or higher). If any of these criteria are not met, the patient may not tolerate the apnea test without hypoxia developing. Although different methods have been described, a technique involving transition from the mechanical ventilator to a self-inflating resuscitation bag may reduce the risk for barotrauma and hypoxia.188 The bag is set for 100% oxygen and positive end-expiratory pressure (PEEP) that approximates the patient’s current mechanical ventilator settings. The patient is then observed constantly for 10 to 15 minutes. If no spontaneous effort at ventilation is observed at a PCO2 of 60 mm Hg or 20 mm Hg above baseline, the test is interpreted as consistent with the absence of brainstem function. If circulatory instability occurs at any time, the patient should be reconnected to the ventilator. Patients with lung disease or chronic CO2 retention may tolerate only a shorter period of observation, at least 2 to 3 minutes and preferably 5 minutes. Criteria for discontinuation of the apnea test include a decrease in oxygen saturation to less than 85% to 90%, hypotension, any arrhythmia associated with hemodynamic instability, and presence of respiratory effort.
Although a specific interval between the two required brain death evaluations is optional in patients 18 years and older, because of the difficulty in performing a brain death examination in younger patients, age-related observation periods between examinations have been recommended (Table 174-4). A single apnea test is typically conducted at the time of the second examination. The apnea test supports but is not required for the diagnosis of brain death.
TABLE 174-4 Age and Recommended Observation Periods for the Diagnosis of Brain Death
Ancillary tests such as a nuclear medicine perfusion scan, EEG recordings, cerebral angiography, and transcranial Doppler constitute options that can be used when deemed likely to be useful, but it is important to recognize that these ancillary tests have limitations.189,190 These tests support the diagnosis but are not required to diagnose brain death. A nuclear medicine perfusion scan may be preferred when drugs that may suppress EEG activity are present (i.e., pentobarbital).
Once the determination of brain death is made, appropriate documentation in the patient’s medical record and communication with the patient’s family are important.191 The use of a standardized documentation form may facilitate accurate and complete documentation of the brain death examination. One such form, used at our institution (J.P.), is available in the Appendix. At this point it may also be appropriate to discuss the recovery of organs for humanitarian purposes. Permission from the family may be requested for evaluation of the patient by an organ and tissue procurement agency.
Conclusion
Critically ill children with neurological conditions benefit from a structured approach that focuses on the prevention of secondary insults to the brain. Early recognition of new neurological insults is challenging in critically ill children but is an essential first step in improving neurological outcome. Pediatric neurocritical care teams can make meaningful contributions through a multidisciplinary approach to brain injury in critically ill children and the development of best clinical practice pathways and effective quality improvement efforts.192–194 Careful attention to the contribution of all organ systems to brain tissue preservation and recovery will lead to the best outcomes. As new technology becomes available, the contribution to outcome of poorly characterized physiologic variables such as cerebral blood flow autoregulation will be better understood. New strategies to optimize patient management will pave the way for future clinical trials of pharmacologic neuroprotective and rehabilitative interventions.
Abend N, Dlugos D. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38:377.
Adelson PD, Clyde B, Kochanek PM, et al. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26:200.
Amlie-Lefond C, Sebire G, Fullerton H. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008;7:425.
Bussmann Bussmann C, Bast T, Rating D. Hyponatraemia in children with acute CNS disease: SIADH or cerebral salt wasting? Childs Nerv Syst. 2001;17:58-62.
Curry R, Hollingworth W, Ellenbogen RG, et al. Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141.
Diringer M, Edwards D. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635.
Diringer MN, Zazulia AR. Osmotic therapy: fact and fiction. Neurocrit Care. 2004;1:219.
Fullerton H, Wu Y, Sidney S, et al. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495.
Goldstein L. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504.
Gubitz G, Counsell C, Sandercock P, et al. Anticoagulants for acute ischaemic stroke. Cochrane Database Systematic Rev. 2000;2:CD000024.
Hirsch J, Hirsch L. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660.
Jiménez Jiménez R, Casedo-Flores J, Nieto M, et al. Cerebral salt wasting syndrome in children with acute central nervous system injury. Pediatr Neurol. 2006;35:261-263.
Lehrnbecher Lehrnbecher T, Müller-Scholden J, et al. Perioperative fluid and electrolyte management in children undergoing surgery for craniopharyngioma. A 10-year experience in a single institution. Childs Nerv Syst. 1998;14:276-279.
Levine Levine JP, Steinicki E, Weiner HL, et al. Hyponatremia in the postoperative craniofacial pediatric patient population: a connection to cerebral salt wasting syndrome and management of the disorder. Plast Reconstr Surg. 2001;108:1501-1508.
Lynch J, Hirtz D, deVeber G, et al. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116.
Mathur M, Petersen L, Stadtler M, et al. Variability in pediatric brain death determination and documentation in southern California. Pediatrics. 2008;121:988.
Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508.
Palmer Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182-187.
Pokela ML, Olkkola KT, Seppala T, et al. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20:26.
Report of Special Task Force. Guidelines for the determination of brain death in children. American Academy of Pediatrics Task Force on Brain Death in Children. Pediatrics. 1987;80:298.
Roach E, Golomb M, Adams R, et al. Management of stroke in infants and children. Stroke. 2008;39:2644.
Sata Sata A, Hizuka N, Kawamata T, et al. Hyponatremia after transsphenoidal surgery for hypothalamo-pituitary tumors. Neuroendocrinology. 2006;83:117-122.
Smith Smith D, Finucane F, Phillips J, et al. Abnormal regulation of thirst and vasopressin secretion following surgery for craniopharyngioma. Clin Endocrinol (Oxf). 2004;61:273-279.
Shetty R, Singhi S, Singhi P, et al. Cerebral perfusion pressure–targeted approach in children with central nervous system infections and raised intracranial pressure: is it feasible? J Child Neurol. 2008;23:192.
Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805.
Stroke in childhood Stroke in childhood: Clinical guidelines for diagnosis, management and rehabilitation. Clinical Effectiveness & Evaluation Unit. London: Royal College of Physicians, 2004.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81.
Tisdall M, Smith M. Multimodal monitoring in traumatic brain injury: current status and future directions. Br J Anaesth. 2007;99:61.
Vavilala MS, Bowen A, Lam AM, et al. Blood pressure and outcome after severe pediatric traumatic brain injury. J Trauma. 2003;55:1039.
Verbalis Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471-503.
Wendon J, Lee W. Encephalopathy and cerebral edema in the setting of acute liver failure: pathogenesis and management. Neurocrit Care. 2008;9:97.
Wilson JD, Foster DW, Kronenberg HM, et al, eds. Williams Textbook of Endocrinology, ed 10. Philadelphia, Saunders, 2003.
Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children. J Neurosurg Anesthesiol. 2004;16:220.
Wise-Faberowski Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children. J Neurosurg Anesthesiol. 2004;16:220-225.
1 Tisdall M, Smith M. Multimodal monitoring in traumatic brain injury: current status and future directions. Br J Anaesth. 2007;99:61.
2 Wartenberg K, Schmidt J, Mayer S. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507.
3 Hirsch J, Hirsch L. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660.
4 Tay S, Hirsch L, Leary L, et al. Nonconvulsive status epilepticus in children: clinical and EEG characteristics. Epilepsia. 2006;47:1504.
5 Trabold F, Meyer P, Blanot S, et al. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004;30:108.
6 Nortje J, Gupta A. The role of tissue oxygen monitoring in patients with acute brain injury. Br J Anaesth. 2006;97:95.
7 van den Brink W, van Santbrink H, Steyerberg E, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000;46:868.
8 Al-Rawi P, Kirkpatrick P. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke. 2006;37:2720.
9 Stiefel MF, Spiotta A, Gracias VH, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005;103:805.
10 Suarez J, Zaidat O, Suri M, et al. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit Care Med. 2004;32:2311.
11 Diringer M, Edwards D. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635.
12 Varelas P, Eastwood D, Yun H, et al. Impact of a neurointensivist on outcomes in patients with head trauma treated in a neurosciences intensive care unit. J Neurosurg. 2006;104:713.
13 Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81.
14 Curry R, Hollingworth W, Ellenbogen RG, et al. Incidence of hypo- and hypercarbia in severe traumatic brain injury before and after 2003 pediatric guidelines. Pediatr Crit Care Med. 2008;9:141.
15 Adelson PD, Clyde B, Kochanek PM, et al. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26:200.
16 Sharples PM, Stuart AG, Matthews DS, et al. Cerebral blood flow and metabolism in children with severe head injury. Part 1. Relation to age, Glasgow coma score, outcome, intracranial pressure, and time after injury. J Neurol Neurosurg Psychiatry. 1995;58:145.
17 Pokela ML, Olkkola KT, Seppala T, et al. Age-related morphine kinetics in infants. Dev Pharmacol Ther. 1993;20:26.
18 Amlie-Lefond C, Sebire G, Fullerton H. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008;7:425.
19 Hogan A, Kirkham F, Isaacs E. Intelligence after stroke in childhood: review of the literature and suggestions for future research. J Child Neurol. 2000;15:325.
20 Ganesan V, Hogan A, Shack N, et al. Outcome after ischaemic stroke in childhood. Dev Med Child Neurol. 2000;42:455.
21 Ganesan V, Prengler M, McShane M, et al. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167.
22 Riela A, Roach E. Etiology of stroke in children. J Child Neurol. 1993;8:201.
23 deVeber G, Roach E, Riela A, et al. Stroke in children: recognition, treatment, and future directions. Semin Pediatr Neurol. 2000;7:309.
24 Roach E. Etiology of stroke in children. Semin Pediatr Neurol. 2000;7:244.
25 deVeber G, MacGregor D, Curtis R, et al. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316.
26 Lynch J, Hirtz D, deVeber G, et al. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116.
27 Kirkham F, Sebire G, Steinlin M, et al. Arterial ischaemic stroke in children. Thromb Haemost. 2004;92:697.
28 Roach E, Golomb M, Adams R, et al. Management of stroke in infants and children. Stroke. 2008;39:2644.
29 deVeber G. In pursuit of evidence-based treatments for paediatric stroke: the UK and Chest guidelines. Lancet Neurol. 2005;4:432.
30 Stroke in childhood: Clinical guidelines for diagnosis, management and rehabilitation. Clinical Effectiveness & Evaluation Unit. London: Royal College of Physicians, 2004.
31 Monagle P, Chan A, Massicotte P, et al. Antithrombotic therapy in children: the Seventh ACCP conference on antithrombotic and antithrombolytic therapy. Chest. 2004;126:645S.
32 Shellhaas R, Smith S, O’Tool E, et al. Mimics of childhood stroke: characteristics of a prospective cohort. Pediatrics. 2006;118:704.
33 Gabis L, Yangala R, Lenn N. Time lag to diagnosis of stroke in children. Pediatrics. 2002;110:924.
34 Lee J, Croen L, Backstrand K, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723.
35 Chabrier S, Husson B, Lasjaunias P, et al. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. J Child Neurol. 2000;15:290.
36 Lanthier S, Carmant L, David M, et al. Stroke in children: the coexistence of multiple risk factors predicts poor outcome. Neurology. 2000;54:371.
37 Sträter R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood—a 5-year follow-up study. Lancet. 2002;360:1540.
38 Ganesan V, Prengler M, Wade A, et al. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170.
39 deVeber G. Stroke and the child’s brain: an overview of the epidemiology, syndromes and risk factors. Curr Opin Neurol. 2002;15:133.
40 Nowak-Gottl U, Gunther G, Kurnik K, et al. Arterial ischemic stroke in neonates, infants and children: an overview of underlying conditions, imaging methods, and treatment modalities. Semin Thromb Hemost. 2003;29:405.
41 Kirkham F, Prengler M, Hewes D, et al. Risk factors for arterial ischemic stroke in children. J Child Neurol. 2000;15:299.
42 Fullerton H, Wu Y, Sidney S, et al. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495.
43 Gilden D, Kleinschmidt-DeMasters B, LaGuardia J, et al. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635.
44 Chabrier S, Lasjaunias P, Husson B, et al. Ischaemic stroke from dissection of the craniocervical arteries in childhood: report of 12 patients. Eur J Paediatr Neurol. 2003;7:39.
45 Rafay M, Armstrong D, deVeber G, et al. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol. 2006;21:8.
46 Kurokawa T, Tomita S, Ueda K, et al. Prognosis of occlusive disease of the circle of Willis (moyamoya disease) in children. Pediatr Neurol. 1985;1:274.
47 Fung L, Thompson D, Ganesan V. Revascularization surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst. 2005;21:358.
48 Lynch J, Han C, Nelson K. Prothrombotic factors in children with stroke or porencephaly. Pediatrics. 2005;116:447.
49 Strater R, Vielhaber H, Kassenbohmer R, et al. Genetic risk factors of thrombophilia in ischaemic stroke of cardiac origin: a prospective ESPED study. Eur J Pediatr. 1999;158:S122.
50 Nowak-Gottl U, Strater R, Heinecke A, et al. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999;94:3678.
51 Al-Jarallah A, Al-Rifai M, Riela A, et al. Nontraumatic brain hemorrhage in children: etiology and presentation. J Child Neurol. 2000;15:284.
52 Meyer-Heim A, Boltshauser E. Spontaneous intracranial haemorrhage in children: aetiology, presentation and outcome. Brain Dev. 2003;25:416.
53 Kirkham F. Stroke in childhood. Arch Dis Child. 1999;81:85.
54 Sebire G, Tabarki B, Saunders D, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477.
55 Adaletli I, Sirikci A, Kara B, et al. Cerebral venous sinus thrombosis presenting with excessive subarachnoid hemorrhage in a 14-year-old boy. Emerg Radiol. 2005;12:57.
56 Kirkham F. Therapy insight: stroke risk and its management in patients with sickle cell disease. Nat Clin Pract Neurol. 2007;3:254.
57 Switzer J, Hess D, Nichols F, et al. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurol. 2006;5:501.
58 Ohene-Frempong K, Weiner S, Sleeper L, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288.
59 Kinney T, Sleeper L, Wang W, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103:640.
60 Earley C, Kittner S, Feeser B, et al. Stroke in children and sickle-cell disease. Neurology. 1998;51:169.
61 Manci E, Culberson D, Yang Y, et al. for the Investigators of the Cooperative Study of Sickle Cell Disease: Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:3459.
62 Prengler M, Pavlakis S, Boyd S, et al. Sickle cell disease: ischemia and seizures. Ann Neurol. 2005;58:290.
63 Strouse J, Hulbert M, DeBaun M, et al. Primary hemorrhagic stroke in children with sickle cell disease is associated with recent transfusion and use of corticosteroids. Pediatrics. 2006;118:1916.
64 Dobson S, Holden K, Nietert P, et al. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002;99:3144.
65 Henderson J, Noetzel M, McKinstry R, et al. Reversible posterior leukoencephalopathy syndrome and silent cerebral infarcts are associated with severe acute chest syndrome in children with sickle cell disease. Blood. 2003;101:415.
66 Adams R, McKie V, Nichols F, et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605.
67 Adams R, McKie V, Hsu L, et al. Prevention of a first stroke by transfusion in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;338:5.
68 Adams R, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769.
69 Zago M, Costa F, Ismael S, et al. Treatment of sickle cell diseases with aspirin. Acta Haematol. 1984;72:61.
70 The Stroke Prevention by Aggressive Reduction in cholesterol levels (SPARCL) Investigators. High dose atorvastatin after stroke or transient ischaemic attack. N Engl J Med. 2006;355:549.
71 Ovbiagele B, Kidwell C, Saver J. Expanding indications for statins in cerebral ischemia: a quantitative study. Arch Neurol. 2005;62:67.
72 Eleftheriou D, Ganesan V. Treatment strategies for childhood stroke. Expert Opin Pharmacother. 2008;9:2955.
73 Adams H, del Zoppo G, Alberts M, et al. Guidelines for the management of adults with ischemic stroke. Stroke. 2007;38:1655.
74 Goldstein L. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504.
75 Gubitz G, Counsell C, Sandercock P, et al. Anticoagulants for acute ischaemic stroke. Cochrane Database Systematic Rev. 2000;2:CD000024.
76 Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581.
77 Carlson M, Leber S, Deveikis J, et al. Successful use of rt-PA in pediatric stroke. Neurology. 2001;57:157.
78 Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomized controlled trials. Lancet Neurol. 2007;6:215.
79 Chiaretti A, De Benedictis R, Langer A, et al. Prognostic implications of hyperglycaemia in paediatric head injury. Childs Nerv Syst. 1998;14:455.
80 Cochran A, Scaife ER, Hansen KW, et al. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:1035.
81 Moritz ML, Ayus JC. Preventing neurological complications from dysnatremias in children. Pediatr Nephrol. 2005;20:1687.
82 Carpenter J, Weinstein S, Myseros J, et al. Inadvertent hyponatremia leading to acute cerebral edema and early evidence of herniation. Neurocrit Care. 2007;6:195.
83 Diringer MN, Zazulia AR. Osmotic therapy: fact and fiction. Neurocrit Care. 2004;1:219.
84 Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144.
85 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 11. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S40.
86 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 18. Nutritional support. Pediatr Crit Care Med. 2003;4:S68.
87 Meert KL, Daphtary KM, Metheny NA. Gastric vs small-bowel feeding in critically ill children receiving mechanical ventilation: a randomized controlled trial. Chest. 2004;126:872.
88 Montejo JC, Grau T, Acosta J, et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit Care Med. 2002;30:796.
89 Spain DA, DeWeese RC, Reynolds MA, et al. Transpyloric passage of feeding tubes in patients with head injuries does not decrease complications. J Trauma. 1995;39:1100.
90 Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471.
91 Wise-Faberowski L, Soriano SG, Ferrari L, et al. Perioperative management of diabetes insipidus in children. J Neurosurg Anesthesiol. 2004;16:220.
92 Smith D, Finucane F, Phillips J, et al. Abnormal regulation of thirst and vasopressin secretion following surgery for craniopharyngioma. Clin Endocrinol (Oxf). 2004;61:273.
93 Bussmann C, Bast T, Rating D. Hyponatraemia in children with acute CNS disease: SIADH or cerebral salt wasting? Childs Nerv Syst. 2001;17:58.
94 Jimenez R, Casado-Flores J, Nieto M, et al. Cerebral salt wasting syndrome in children with acute central nervous system injury. Pediatr Neurol. 2006;35:261.
95 Lehrnbecher T, Muller-Scholden J, Danhauser-Leistner I, et al. Perioperative fluid and electrolyte management in children undergoing surgery for craniopharyngioma. A 10-year experience in a single institution. Childs Nerv Syst. 1998;14:276.
96 Levine JP, Stelnicki E, Weiner HL, et al. Hyponatremia in the postoperative craniofacial pediatric patient population: a connection to cerebral salt wasting syndrome and management of the disorder. Plast Reconstr Surg. 2001;108:1501.
97 Palmer BF. Hyponatremia in patients with central nervous system disease: SIADH versus CSW. Trends Endocrinol Metab. 2003;14:182.
98 Sata A, Hizuka N, Kawamata T, et al. Hyponatremia after transsphenoidal surgery for hypothalamo-pituitary tumors. Neuroendocrinology. 2006;83:117.
99 Berkenbosch JW, Lentz CW, Jimenez DF, et al. Cerebral salt wasting syndrome following brain injury in three pediatric patients: suggestions for rapid diagnosis and therapy. Pediatr Neurosurg. 2002;36:75.
100 Shetty R, Singhi S, Singhi P, et al. Cerebral perfusion pressure–targeted approach in children with central nervous system infections and raised intracranial pressure: is it feasible? J Child Neurol. 2008;23:192.
101 Odetola FO, Tilford JM, Davis MM. Variation in the use of intracranial-pressure monitoring and mortality in critically ill children with meningitis in the United States. Pediatrics. 2006;117:1893.
102 Raschke RA, Curry SC, Rempe S, et al. Results of a protocol for the management of patients with fulminant liver failure. Crit Care Med. 2008;36:2244.
103 Wendon J, Lee W. Encephalopathy and cerebral edema in the setting of acute liver failure: pathogenesis and management. Neurocrit Care. 2008;9:97.
104 Le Roux PD, Jardine DS, Kanev PM, et al. Pediatric intracranial pressure monitoring in hypoxic and nonhypoxic brain injury. Childs Nerv Syst. 1991;7:34.
105 Treatment of convulsive status epilepticus. Recommendations of the Epilepsy Foundation of America’s Working Group on Status Epilepticus. JAMA. 1993;270:854.
106 Meldrum B. The revised operational definition of generalized status-epilepticus in adults. Epilepsia. 1999;40:123.
107 Mazarati A, Bragin A, Baldwin R, et al. Epileptogenesis after self-sustaining status epilepticus. Epilepsia. 2002;43:74.
108 Sankar R, Shin D, Liu H, et al. Patterns of status epilepticus–induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8282.
109 Mazarati A, Wasterlain C, Sankar R, et al. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801:251.
110 Chen J, Wasterlain C. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5:246.
111 Lowenstein D, Alldredge B. Status epilepticus. N Engl J Med. 1998;338:970.
112 Gilbert D, Gartside P, Glauser T. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children. J Child Neurol. 1999;14:602.
113 Mayer S, Claassen J, Lokin J, et al. Refractory status epilepticus. Arch Neurol. 2002;59:205.
114 Claassen J, Hirsch L, Emerson R, et al. Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology. 2001;57:1036.
115 Sahin M, Menache C, Holmes G, et al. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461.
116 Kramer U, Shorer Z, Ben-Zeev B, et al. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol. 2005;20:184.
117 Towne A, Waterhouse E, Boggs J, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340.
118 Vespa P, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441.
119 Vespa P, Nuwer M, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750.
120 Claassen J, Mayer S, Kowalski R, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743.
121 Young G, Jordan K, Doig G. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83.
122 Pandian J, Cascino G, So E, et al. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit. Arch Neurol. 2004;61:1090.
123 Garcia P. Nonconvulsive seizures in the pediatric intensive care unit: out of sight, out of mind? Epilepsy Curr. 2007;7:70.
124 Jette N, Claassen J, Emerson R, et al. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750.
125 Walker D, Teach S. Update on the management of status epilepticus in children. Curr Opin Pediatr. 2006;18:239.
126 Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094.
127 Lowenstein D. The management of refractory status epilepticus: an update. Epilepsia. 2006;47(suppl 1):35.
128 Abend N, Dlugos D. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38:377.
129 Sheth R, Gidal B. Refractory status epilepticus: response to ketamine. Neurology. 1998;51:1765.
130 Perry M, Holt P, Sladky J. Topiramate loading for refractory status epilepticus in children. Epilepsia. 2006;47:1070.
131 Morrison G, Gibbons E, Whitehouse W. High-dose midazolam therapy for refractory status epilepticus in children. Intensive Care Med. 2006;32:2070.
132 Saez-Llorens X, McCracken G. Bacterial meningitis in children. Lancet. 2003;361:2139.
133 McIntyre P, MacIntyre C, Gilmour R, et al. A population based study of the impact of corticosteroid therapy and delayed diagnosis on the outcome of childhood pneumococcal meningitis. Arch Dis Child. 2005;90:391.
134 Odetola F, Bratton S. Characteristics and immediate outcome of childhood meningitis treated in the pediatric intensive care unit. Intensive Care Med. 2005;31:92.
135 Odio C, Faingezicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525.
136 Lebel M, Freij B, Syrogiannopoulos G, et al. Dexamethasone therapy for bacterial meningitis. Results of two double-blind, placebo-controlled trials. N Engl J Med. 1988;391:964.
137 Ashwal S, Perkin R, Thompson J, et al. Bacterial meningitis in children: current concepts of neurologic management. Curr Probl Pediatr. 1994;24:267.
138 Kaplan S. Adjunctive therapy in meningitis. Adv Pediatr Infect Dis. 1995;10:167.
139 Minns R, Engleman H, Stirling H. Cerebrospinal fluid pressure in pyogenic meningitis. Arch Dis Child. 1989;64:814.
140 Odetola F, Tilford J, Davis M. Variation in the use of intracranial-pressure monitoring and mortality in critically ill children with meningitis in the United States. Pediatrics. 2006;117:1893.
141 Thwaites G, Hien T. Tuberculous meningitis: many questions, too few answers. Lancet Neurol. 2005;4:160.
142 Thwaites G, Chau T, Mai N, et al. Neurological aspects of tropical disease: tuberculous meningitis. J Neurol Neurosurg Psychiatry. 2000;68:289.
143 Verdon R, Chevret S, Laissy J. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis. 1996;22:982.
144 Farinha N. Tuberculosis of the central nervous system in children: a 20-year survey. J Infect. 2000;41:61.
145 Leonard J, Des Prez R. Tuberculous meningitis. Infect Dis Clin North Am. 1990;4:769.
146 Palur R, Rajshekhar V, Chandy M, et al. Shunt surgery for hydrocephalus in tuberculous meningitis: a long-term follow up study. J Neurosurg. 1991;74:64.
147 Lamprecht D, Schoeman J, Donald P, et al. Ventriculoperitoneal shunting in childhood tuberculous meningitis. Br J Neurosurg. 2001;15:119.
148 Schoeman J, Van Zyl L, Laubscher J, et al. Effect of corticosteroids on intracranial pressure, computed tomographic findings and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99:226.
149 White A. Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101.
150 Nash T, Singh G, White A, et al. Treatment of neurocysticercosis. Neurology. 2006;67:1120.
151 Kolski H, Ford-Jones E, Richardson S, et al. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994-1995. Clin Infect Dis. 1998;26:398.
152 Annegers J, Hauser W, Beghi E, et al. The risk of unprovoked seizures after encephalitis and meningitis. Neurology. 1988;38:1407.
153 Chen Y, Fang P, Chow J. Clinical characteristics and prognostic factors of postencephalitic epilepsy in children. J Child Neurol. 2006;12:1047.
154 Millichap J, Wainwright M. Neurological complications of respiratory syncytial virus infection: case series and review of literature. J Child Neurol. 2009. Mar 4 [Epub ahead of print]
155 Seeley W, Marty F, Holmes T, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology. 2007;69:156.
156 Wainwright MS, Martin PL, Morse RP, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001;50:612.
157 Theodore W, Epstein L, Gaillard W, et al. Human herpes virus 6B: a possible role in epilepsy? Epilepsia. 2008;49:1828.
158 Saengpattrachai M, Sharma R, Hunjan A, et al. Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, and brain imaging findings. Epilepsia. 2006;47:1510.
159 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr Crit Care Med. 2003;4:S31.
160 Freeman SS, Udomphorn Y, Armstead WM, et al. Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology. 2008;108:588.
161 Tontisirin N, Armstead W, Waitayawinyu P, et al. Change in cerebral autoregulation as a function of time in children after severe traumatic brain injury: a case series. Childs Nerv Syst. 2007;23:1163.
162 Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225.
163 Vavilala MS, Tontisirin N, Udomphorn Y, et al. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9:45.
164 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4:S12.
165 Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med. 2007;8:138.
166 Vavilala MS, Bowen A, Lam AM, et al. Blood pressure and outcome after severe pediatric traumatic brain injury. J Trauma. 2003;55:1039.
167 Jonas RA. Cardiac surgery and neurological injury in children. Heart Lung Circ. 2000;9:16.
168 Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122:491.
169 Natale JE, Joseph JG, Helfaer MA, et al. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28:2608.
170 Brown JM, Udomphorn Y, Suz P, et al. Antipyretic treatment of noninfectious fever in children with severe traumatic brain injury. Childs Nerv Syst. 2008;24:477.
171 Carhuapoma JR, Gupta K, Coplin WM, et al. Treatment of refractory fever in the neurosciences critical care unit using a novel, water-circulating cooling device. A single-center pilot experience. J Neurosurg Anesthesiol. 2003;15:313.
172 Mayer SA, Kowalski RG, Presciutti M, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Crit Care Med. 2004;32:2508.
173 Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609.
174 Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, et al. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Crit Care Med. 2008;36:1290.
175 Gupta A, Daggett C, Ludwick J, et al. Ketorolac after congenital heart surgery: does it increase the risk of significant bleeding complications? Paediatr Anaesth. 2005;15:139.
176 Kokki H. Nonsteroidal anti-inflammatory drugs for postoperative pain: a focus on children. Paediatr Drugs. 2003;5:103.
177 Vitale MG, Choe JC, Hwang MW, et al. Use of ketorolac tromethamine in children undergoing scoliosis surgery. an analysis of complications. Spine J. 2003;3:55.
178 Levy ML, Granville RC, Hart D, et al. Deep venous thrombosis in children and adolescents. J Neurosurg. 2004;101:32.
179 Vu LT, Nobuhara KK, Lee H, et al. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg. 2008;43:1095.
180 Sandoval JA, Sheehan MP, Stonerock CE, et al. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. J Vasc Surg. 2008;47:837.
181 Sebire G, Tabarki B, Saunders DE, et al. Cerebral venous sinus thrombosis in children: risk factors, presentation, diagnosis and outcome. Brain. 2005;128:477.
182 A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337.
183 Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA. 1981;246:2184.
184 Report of Special Task Force. Guidelines for the determination of brain death in children. American Academy of Pediatrics Task Force on Brain Death in Children. Pediatrics. 1987;80:298.
185 Practice parameters for determining brain death in adults (summary statement). The Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1995;45:1012.
186 Wijdicks EF. The diagnosis of brain death. N Engl J Med. 2001;344:1215.
187 Rowland TW, Donnelly JH, Jackson AH. Apnea documentation for determination of brain death in children. Pediatrics. 1984;74:505.
188 Monterrubio-Villar J, Cordoba-Lopez A. Barotrauma during apnoea testing for brain death determination in a five-year-old boy. Anaesth Intensive Care. 2008;36:462.
189 Baron L, Shemie SD, Teitelbaum J, et al. Brief review: history, concept and controversies in the neurological determination of death. Can J Anaesth. 2006;53:602.
190 Young GB, Shemie SD, Doig CJ, et al. Brief review: the role of ancillary tests in the neurological determination of death. Can J Anaesth. 2006;53:620.
191 Mathur M, Petersen L, Stadtler M, et al. Variability in pediatric brain death determination and documentation in southern California. Pediatrics. 2008;121:988.
192 Bell MJ, Carpenter J, Au AK, et al. Development of a Pediatric Neurocritical Care Service. Neurocrit Care. 2009;10:4.
193 Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635.
194 Fakhry SM, Trask AL, Waller MA, et al. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma. 2004;56:492.
APPENDIX Guidelines for the Management of Postoperative Diabetes Insipidus in the Pediatric Intensive Care Unit
Background
Indications
Treatment Guidelines
Monitoring and Treatment Goals
Monitoring
*Alert the physician if UOP is greater than 3.5 or less than 1 mL/kg/hr.
Treatment Goals
 NS) with 20 mEq KCl/L at 1 L/m2/day ( _____ mL/hr).
NS) with 20 mEq KCl/L at 1 L/m2/day ( _____ mL/hr).
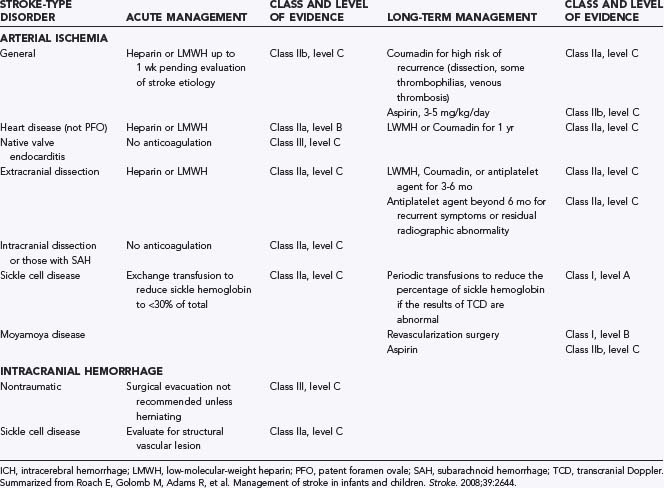
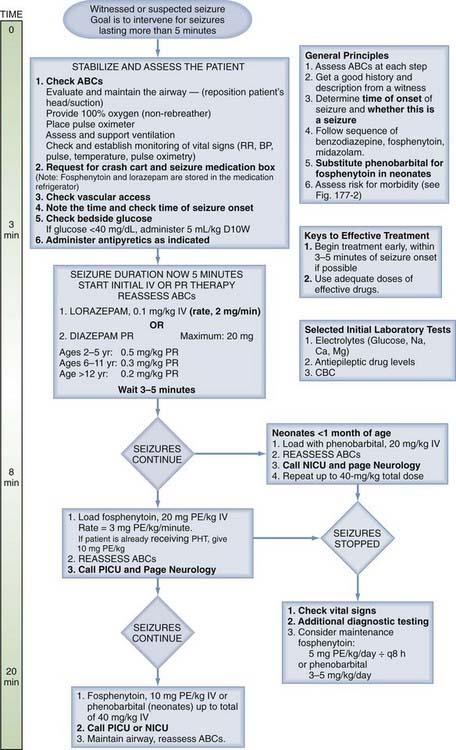
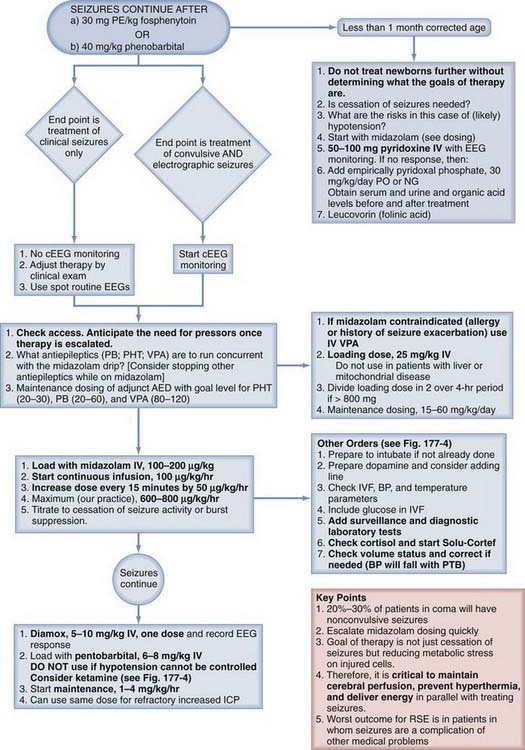

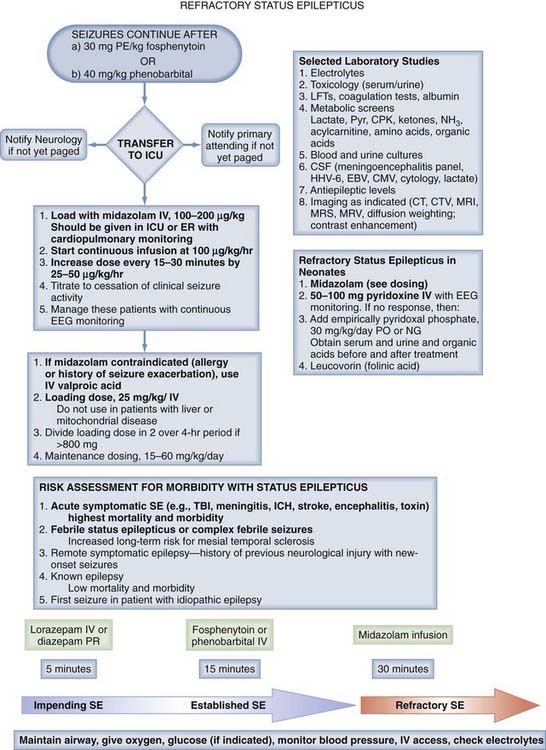
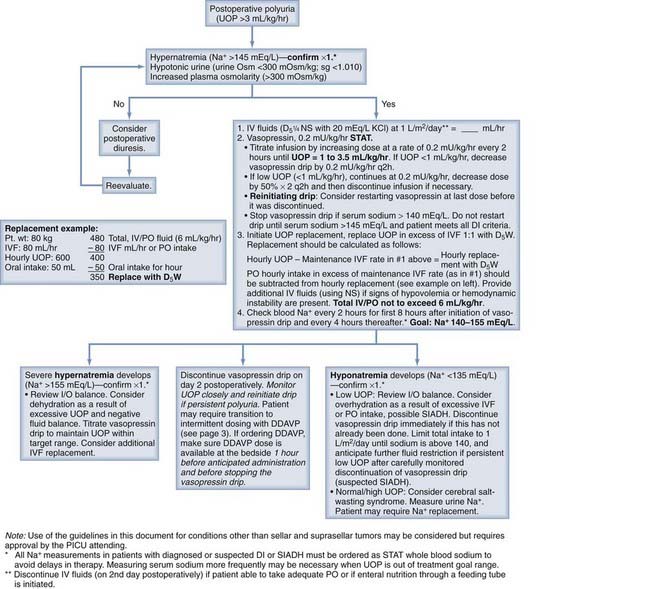
 ¼NS, 5% dextrose in one-quarter normal saline; D5W, 5% dextrose in water; I/O, intake/output; IV, intravenous; IVF, intravenous fluid; PICU, pediatric intensive care unit; PO, oral; sg, specific gravity; SIADH, syndrome of inappropriate antidiuretic hormone secretion; UOP, urinary output.
¼NS, 5% dextrose in one-quarter normal saline; D5W, 5% dextrose in water; I/O, intake/output; IV, intravenous; IVF, intravenous fluid; PICU, pediatric intensive care unit; PO, oral; sg, specific gravity; SIADH, syndrome of inappropriate antidiuretic hormone secretion; UOP, urinary output.