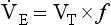NERVOUS CONTROL OF BREATHING
Introduction
This chapter is closely related to Chapter 9, which described chemical control of breathing. This division of the subject of control is a semantic one, designed to make learning easier. All chemical control involves neural sensory mechanisms, and it is neural mechanisms that determine and bring about breathing, which in turn plays such an important part in the homeostatic control of the chemical composition of the body. The difference between chemical and neural control is really a matter of time: chemical control, which we have dealt with, takes place over seconds or minutes; neural control reacts in fractions of a second to influence breathing breath by breath.
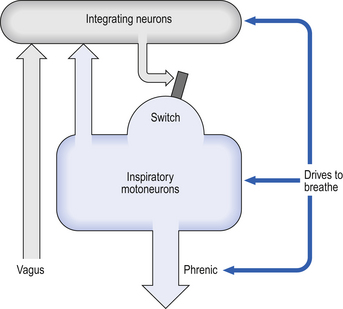
Minute ventilation 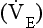 is described by the equation:
is described by the equation:
where VT and f are the tidal volume of each individual breath and the frequency of breathing, respectively. The equation tells us that a particular minute ventilation can be made up of an infinite variety of volumes and frequencies, from high frequencies and small volumes to low frequencies and large volumes. How and why we unconsciously ‘choose’ a particular pattern is the province of neural control of breathing.
Economy of energy is an evolutionary advantage, and the pattern of breathing chosen by our bodies is aimed at minimizing the amount of work we have to do to produce a particular minute ventilation. This work is directly related to the force exerted by the respiratory muscles, and it may be that we aim to minimize the tension in our respiratory muscles and/or minimize work. It seems that we get our respiratory systems to ‘resonate’. The resonant frequency for any particular minute ventilation depends on the value of that minute ventilation and the mechanical properties of the lungs (which have been dealt with in Chapters 3 and 4).
Breathing originates in the brainstem (Fig. 10.1) which is made up of the medulla (which means marrow, as in bone marrow) and the pons (which means bridge), which connects the medulla to the rest of the brain. The neural basis of breathing within the brainstem is the central pattern generator, whose output to the respiratory muscles is modulated by numerous afferent inputs. Nerve impulses leave the central nervous system via the phrenic and intercostal nerves to bring about breathing by contraction of the respiratory muscles, mainly the diaphragm and intercostals. Other nerves to accessory muscles (e.g. of the larynx) synchronize their contractions with the phases of breathing.
The rhythm generator
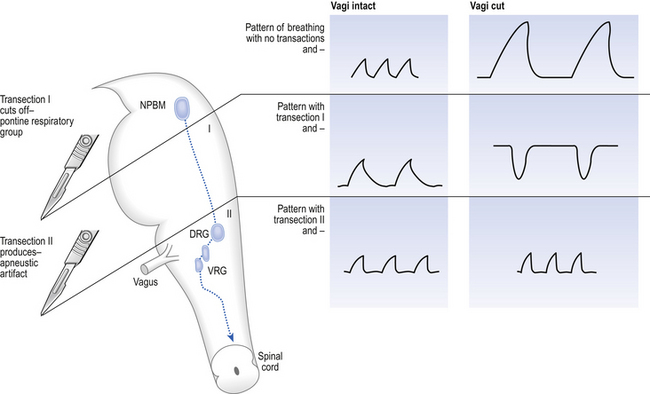
The basic pattern of breathing originates in that part of the brainstem which joins the spinal cord to the midbrain and cerebellum, shown in Figure 10.1 and in more detail in Figure 10.5. This region consists of the medulla and pons. If the brain above the medulla is removed (as by Transection II in Figure 10.5) the breathing pattern is remarkably normal. Breathing only ceases when connections between the medulla and spinal cord are cut. (There is some evidence that there are rhythm generators capable of producing breathing movements in the spinal cord itself, but this is a very minor effect occurring under very limited conditions.) The major generator of basic respiratory rhythm is situated in the medulla and is influenced by higher regions of the brain and by activity from receptors in other parts of the body.
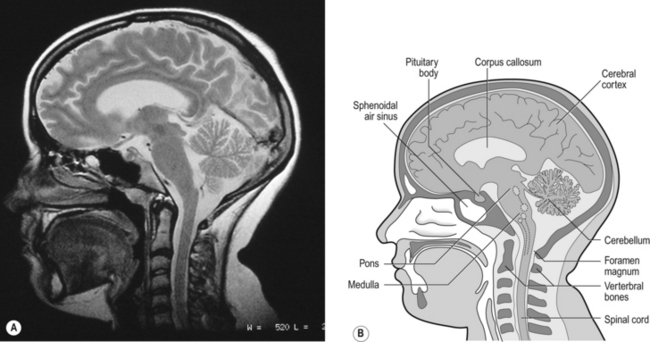
The idea that oscillating activity in the phrenic nerve could originate in a group of neurons that simply increase and decrease their activity is untenable because there is no reason why such a system should not simply ‘stick’ in the on or off position, which would result in the subject being ‘stuck’ in inspiration or expiration. This argument also applies to the old but persistent idea of two groups of neurons that produce either inspiration or expiration and reciprocally inhibit each other (Fig. 10.2).
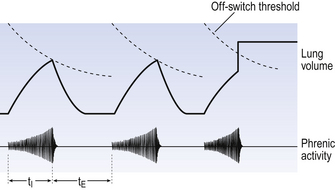
All plausible models of the central pattern generator start with inspiratory neurons, because to generate a pattern of quiet breathing that is all that is required, expiration in quiet breathing being passive. Most of these models involve some sort of self-limiting negative feedback in the medulla, which operates an ‘off switch’ that limits inspiration (Fig. 10.3).
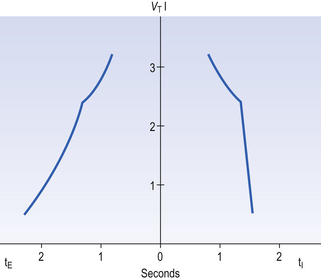
The durations of the two phases of breathing (inspiratory duration, tI, and expiratory duration, tE) are under independent control and so can change independent of each other, or both can change at the same time. Both are influenced by the volume of the lungs. Thus when breathing is accelerated tE is the first to be shortened as VT increases, with tI remaining fairly constant until a threshold is reached, after which it begins to shorten significantly (Fig. 10.4).
• All brainstem neurons involved in inspiration are linked and all brainstem neurons involved in expiration are linked by self-exciting connections which synchronize their activity.
• On the other hand, there are self-inhibiting connections between all the neurons of the inspiratory group and all the neurons of the expiratory group, which limit the duration of action of each group.
• If there is any activity in the expiratory neuron group during eupnoea (quiet breathing) it does not reach a level that activates the expiratory muscles of the abdominal wall, expiration is passive in normal quiet breathing.
Pattern of breathing in COPD
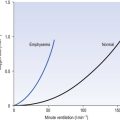
The relationship between VT, tI and tE shown in Figure 10.4 is disrupted in disease. In chronic obstructive pulmonary disease, for example, overall minute ventilation (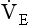 ), which can be thought of as the physical expression of the neural drive to breathe, increases as the disease progresses. This offsets to some extent the decrease in efficiency caused by
), which can be thought of as the physical expression of the neural drive to breathe, increases as the disease progresses. This offsets to some extent the decrease in efficiency caused by 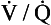 mismatching and changes in lung mechanics.
mismatching and changes in lung mechanics.
Within this increase in 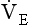 the pattern of breathing also changes. There is initially an increase in VT, but as airways resistance increases as the disease progresses VT decreases below normal. Frequency of breathing increases throughout the progress of the disease. The relationship of inspiratory duration to tidal volume (tI to VT) reflects the drive to breathe. The relationship between inspiratory duration and the total breath duration (tI to tTot) reflects the way a single breath is divided up into the time to fill the lungs (tI) and the time to empty back to the start position (tE). These two phases are of course governed by the mechanical properties of the lungs and airways. Central drive to breathe must increase as airflow limitation progresses, reaching a maximum with respiratory failure. This increased drive effectively increases VT until the increased work of breathing resulting from airflow limitation overpowers it and actually causes a fall in VT. The only way the patient can now increase his minute ventilation is to increase his frequency of breathing. The problem with this strategy is that the expiratory airflow limitation produced by the disease demands that a greater proportion of each breath be devoted to expiration, and the fraction of each breath devoted to inspiration has to be reduced. Air trapping and a subjective sensation of relief when breathing at high volume (which holds the airways open in what has been termed ‘auto-PEEP’) causes the patient with severe COPD to breathe with a rapid shallow pattern at increased lung volumes. This is an inefficient pattern because, in addition to the airflow obstruction, it places the respiratory muscles at a mechanical disadvantage to such an extent that the increased work of breathing can exhaust the patient.
the pattern of breathing also changes. There is initially an increase in VT, but as airways resistance increases as the disease progresses VT decreases below normal. Frequency of breathing increases throughout the progress of the disease. The relationship of inspiratory duration to tidal volume (tI to VT) reflects the drive to breathe. The relationship between inspiratory duration and the total breath duration (tI to tTot) reflects the way a single breath is divided up into the time to fill the lungs (tI) and the time to empty back to the start position (tE). These two phases are of course governed by the mechanical properties of the lungs and airways. Central drive to breathe must increase as airflow limitation progresses, reaching a maximum with respiratory failure. This increased drive effectively increases VT until the increased work of breathing resulting from airflow limitation overpowers it and actually causes a fall in VT. The only way the patient can now increase his minute ventilation is to increase his frequency of breathing. The problem with this strategy is that the expiratory airflow limitation produced by the disease demands that a greater proportion of each breath be devoted to expiration, and the fraction of each breath devoted to inspiration has to be reduced. Air trapping and a subjective sensation of relief when breathing at high volume (which holds the airways open in what has been termed ‘auto-PEEP’) causes the patient with severe COPD to breathe with a rapid shallow pattern at increased lung volumes. This is an inefficient pattern because, in addition to the airflow obstruction, it places the respiratory muscles at a mechanical disadvantage to such an extent that the increased work of breathing can exhaust the patient.
The respiratory ‘centres’
Disconnecting the upper pons from the brainstem (Transection I in Fig. 10.5) removes the effect of the pontine respiratory group (PRG). The neurons that make up this centre are found in and around the nucleus parabranchialis medialis (NPBM).
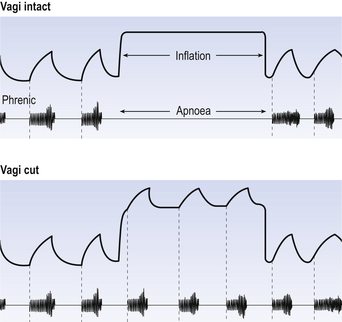
When Transection II (Fig. 10.5) is made, with the vagi cut, breathing becomes slower and deeper. This rate-controlling, volume-limiting effect of the PRG is probably a result of the inspiratory neuron group of the medulla stimulating the PRG during inspiration. When stimulated in this way the PRG, after a short delay, sends inhibitory impulses back to the inspiratory neurons, cutting short their activity (and hence phrenic nerve discharge to the diaphragm) in a classic negative-feedback arrangement. Shortening one breath in this way means that the next breath can start earlier, that is, the frequency of breathing is increased and the volume of breaths reduced. It has been suggested that the PRG evolved as a means of mediating rapid shallow breathing (panting), initiated for thermal or emotional reasons by higher parts of the brain.
The medullary groups
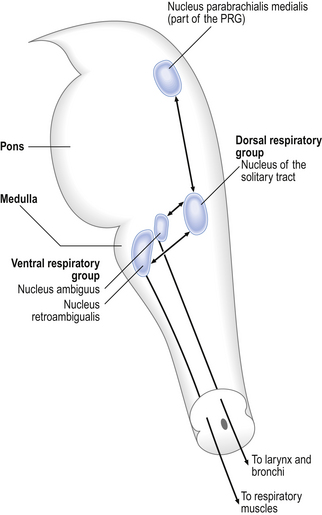
The pons connects the medulla to the higher regions of the brain. From direct electrical recording and observations of the clock-like unmodulated rhythms (slow deep patterns of breathing and apneusis) seen when these regions are damaged two functionally distinct groups of neurons in the medulla have been identified (Fig. 10.6).
The dorsal respiratory group (DRG)
This is made up of exclusively inspiratory neurons in the region of the nucleus of the tractus solitarius (solitary tract). These neurons integrate information from chemoreceptors and mechanoreceptors related to breathing. The nerve cells of the DRG have a spontaneous rhythm of discharge, similar to that of breathing and synchronized with inspiration, which can be altered by environmental changes that alter breathing rate. This suggests that the neurons of the DRG may be the origin of the most basic respiratory rhythm.
Conscious control of breathing
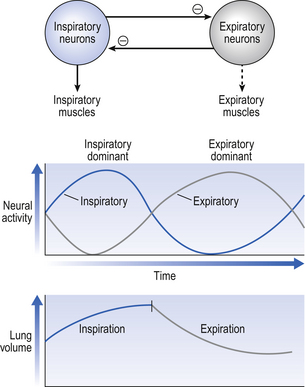
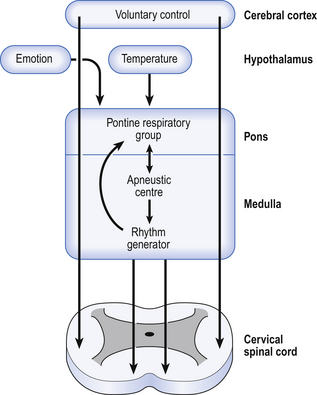
The control of breathing described so far is automatic and independent of the brain above the pons. In unanaesthetized humans, however, the higher parts of the brain affect breathing both involuntarily, during emotion, hyperthermia, and probably during exercise, but also during conditions that require voluntary control of our respiratory muscles. These conditions may be primarily respiratory, speaking, blowing, whistling; or be for other purposes, such as when we lock the ribcage to provide a framework against which the arms can act, or when we increase intra-abdominal pressure during defecation. In all cases voluntary control is always bilateral: we cannot contract one half of our diaphragm or one half of our larynx. The origin of this control is probably the motor cortex, and the voluntary pathways bypass the PRG and rhythm generator in the brainstem to descend in the pyramidal tracts (Fig. 10.7).
These voluntary pathways can be destroyed independently of the involuntary pathways, for example by a stroke. Patients with such a stroke breathe normally, respond to reflex and chemical stimuli, but cannot voluntarily change their pattern of breathing; they will cough if their larynx is stimulated, but cannot cough on command. Very rarely, the opposite situation is seen, where the automatic pathways have been destroyed but the pyramidal tracts are left intact. The patient can breathe deliberately but not automatically, as in sleep. This condition is called ‘Ondine’s curse’ after the folk-tale of the mortal man who formed a liaison with an ondine or water-nymph. Her father, the king of the ondines, objected to this relationship (the way fathers do) and put a curse on the man that if he did not remember to keep his vital functions, such as heartbeat and breathing, going, they would stop. Of course, when he fell asleep he died. Fortunately, this condition, and ondines, are very rare.
Respiratory muscle innervation
The larynx exhibits movements synchronized with breathing. These movements are brought about by the muscles of the larynx, which are innervated by the superior laryngeal and recurrent laryngeal nerves. These are branches of the vagus nerve. During inspiration the vocal folds are pulled apart and this reduces the resistance to airflow into the lungs. During expiration the vocal folds come together, slowing down expiratory flow. These movements are automatic – we are not conscious of them, and the nerve impulses that cause them arise from the rostral part of the ventral respiratory group in the nucleus ambiguus. We can, of course, consciously control our larynx, as in vocalization. The larynx is therefore almost a model of the control of respiration, with an automatic rhythm that can be consciously overridden.
The abdominal muscles only become involved when forced expiration is required during exercise or coughing. As exercise becomes more intense, or breathing becomes more laboured, as in disease, more accessory muscles, for example those of the shoulder girdle, are recruited. The muscles of the abdomen and chest are also involved in posture, locomotion, and in the movement of the arms in lifting heavy weights. During almost all of these activities, if not too extreme, it is possible to breathe and vocalize, which involves well controlled modification of airflow. This ability reaches its peak in opera singers, who seem to be able to sing in the most unusual positions.
Neuromuscular disorders
• Central nervous system. Trauma to the brain and spinal cord can result in partial or total loss of respiratory function, depending on the degree and site of the lesion. Frequently, as a result of trauma, vasoconstriction, hypertension, mucus secretion and oedema result from increased uncontrolled activity of airways innervation.
Hemispheric strokes interfere with the voluntary pathways of breathing. Brainstem strokes that affect the dorsal medullary centres (see Fig. 10.6) cause fatal apnoea.
• Poliomyelitis. This disease is now rare owing to the advent of vaccines. About 25% of those infected require mechanical ventilation during the acute stage. Many recover respiratory muscle strength thanks to the reinnervation of denervated fibres.
• Diphtherial. Corynebacterium diphtheriae produces an exotoxin that provokes a demyelinating neuropathy, which results in respiratory failure if the respiratory muscles are involved. Antitoxin is the only specific therapy.
• Botulism. The anaerobe Clostridium botulinum, which can be foodborne, colonizes the gut of infants or infects wounds and produces a toxin which blocks the release of acetylcholine at the neuromuscular junction. When innervation of the respiratory muscles becomes involved artificial ventilation is required. Even then 10% of patients may die. The innervation of the respiratory muscles seems particularly vulnerable, and ventilation may be required for several months. Treatment includes debridement of infected wounds, penicillin, and antitoxin in the early stages.
• Duchenne’s muscular dystrophy. This X-linked recessive genetic disorder affects the gene for the production of protein dystrophin. From the age of 10 vital capacity declines inexorably in these patients. They first develop nocturnal hypoxaemia and commonly die from respiratory failure secondary to pulmonary infection at about 20 years of age. Mechanical ventilation is the only option to relieve respiratory failure.
Vagal reflexes
• Slowly adapting pulmonary stretch receptors (PSR)
• Rapidly adapting (sometimes called irritant) receptors (RAR)
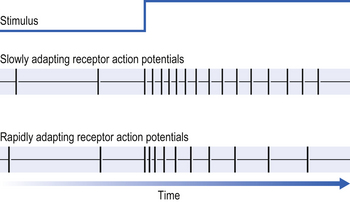
If a constant stimulus is applied to a receptor it ‘gets used to it’ and the frequency of the receptor discharge (in action potentials per second) decreases even though the stimulus does not change (Fig. 10.8).
Slowly adapting pulmonary receptors (pulmonary stretch receptors)
These receptors are situated in the smooth muscle which makes up a large part of the walls of the trachea and bronchi, and so are affected by factors that influence bronchial tone (tension). Their specific stimulus is tension. The tension in the airways walls is influenced by lung volume: the more the lung is inflated the greater will be the stretch of the structures within it, including the airways. Thus pulmonary stretch receptors signal the volume of the lungs at any instant. This signal is in the form of action potentials: the greater the volume the higher the frequency of action potentials. During inspiration the lungs increase in volume and the frequency of discharge increases. It is thought that when the frequency reaches a certain ‘threshold’ it operates some kind of neural ‘off switch’ which switches off inspiration. This ‘threshold’ falls during each inspiration (the off switch becomes more sensitive (Fig. 10.9)) and is reset back to its original sensitivity during expiration while the inspiratory neurons are not active.
The effect of stretch receptors on pattern of breathing is most dramatically seen in the Hering–Breuer Inflation Reflex. When the lungs of an anaesthetized animal are kept inflated it ceases to make inspiratory efforts for some time (Fig. 10.10). Students are sometimes confused by the demonstration of this reflex into thinking breathing is being physically obstructed by the inflating pressure, rather like preventing someone breathing by putting your arms round their chest and squeezing. Not so: inflating the lungs is producing a reflex. The subject is not even trying to breathe, as demonstrated by the absence of phrenic activity during the lung inflation. Eventually, however, CO2 builds up in the blood and forces breathing to restart.
Pulmonary stretch receptors are still active while the lungs are deflating during the start of expiration, and some are active throughout the respiratory cycle. It is important to look at what stretch receptors do to both phases of breathing. They are generally described as terminating inspiration and limiting tidal volume. Equally importantly, they extend expiratory time. This is what you see in the Hering–Breuer inflation reflex. This aspect of their action is important because the expiratory phase of breathing is usually the longest at rest, and so is the most important phase in determining rate of breathing.
Rapidly adapting (irritant) receptors
These mechanoreceptors respond to a sustained physical stimulus with a discharge whose frequency rapidly returns to the rest level (see Fig. 10.8). For this reason, in breathing where the stimulus is constantly changing, their discharge is highly erratic and difficult to quantify. These receptors can be powerfully stimulated by the inhalation of irritating gases and vapours such as ammonia or cigarette smoke, and this gives rise to the name irritant receptors, one of their many alternative names. They are also stimulated by procedures that distort the lung, such as pneumothorax (air in the pleural space, which causes lung collapse), and have occasionally been called ‘deflation receptors’. These are obviously not the physiological stimuli the receptors would encounter under normal conditions, and therefore rapidly adapting receptors is a more suitable name. The physiological stimulus of these receptors is not lung volume, as in the case of stretch receptors, but probably the rate of change of lung volume, which of course is related to rate of airflow into or out of the lungs.
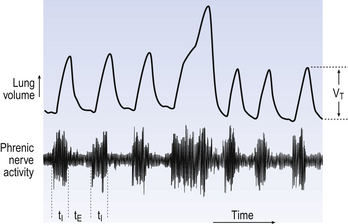
1. Rapid shallow breathing, resulting mainly from a shortening of expiration.
2. Long deep augmented breaths, in which inspiration is about twice as long as normal (Fig. 10.11).
These augmented breaths are periodically taken by mammals (every 5–20 minutes in resting humans) to reverse the slow collapse of the lungs that takes place during quiet breathing.
Other reflexes
The nose and pharynx
Sneezing is usually provoked by the stimulation of bare nerve endings in the nasal mucosa which send their information to the brain in the trigeminal nerves. A sneeze is a rather stereotypical response, consisting of a deep inspiration followed by closure of the glottis, against which pressure builds up until rapid opening of the glottis and expiratory effort produces airflow whose velocity can approach the speed of sound and ejects the offending stimulation. The sneeze has many features in common with the cough, but it is interesting to note that, unlike a cough, a sneeze is difficult to mimic and almost impossible to suppress. Sneezing can be provoked by stimuli applied to regions of the body other than the nose. In some people it is provoked by bright light, and in an unfortunate few by sexual orgasm!